Abstract
Abiotic stresses are emerging environmental factors limiting agricultural productivity around the world. Among these stresses, salt stress is a serious threat affecting crop production especially in arid and semiarid regions of the world. Development of strategies to ameliorate deleterious effects of salt stress on plants has received considerable attention. In this scenario, the use of salt-tolerant plant growth-promoting microorganisms to enhance salinity resilience in crops is encouraged due to their vital interactions with crop plants. Bacteria are widely used to diminish deleterious impacts of high salinity on crop plants because they possess various direct and indirect plant growth-promoting characteristics. This chapter focuses on the effect of salt stress on plants, plant growth-promoting bacterial survival in saline conditions, and their mechanisms to mitigate salt stress at genetic level.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
7.1 Introduction
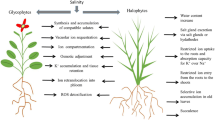
Salinity is one of the major abiotic stresses which negatively affects crop growth and yields and puts down crop production. The presence of high sodium chloride concentration has been reported to cause reduction in microbial flora in the soil (Ibekwe et al. 2010). Most of the world’s plateaus confined to the tropics and Mediterranean regions have potential salinity problems (Cordovilla et al. 1994). It is accounted for the presence of naturally high salt levels, salt accumulation during irrigation, or the application of chemical fertilizers. High salinity owing to its toxic effects inhibits the growth of plants by affecting cellular growth and entry of ions across the root system by slowing down the water uptake of plants. Sodium chloride is the most disparaging salt that affects the growth of plants. Saline habitats are frequently inhabited by an abundance of microbial communities adapted to these ecosystems (Zahran et al. 1992). Halophilic bacteria which flourish in hypersaline habitats may retain their potential to express various types of plant growth-promoting activities such as phosphate solubilization, nitrogen fixation, or phytohormone production. These PGPRs offer promise as potential biofertilizers for improvement of plant growth under stress conditions by reducing the impact of salinity on plant growth and its productivity. Salt-tolerant bacterial species of Bacillus, Pseudomonas, Azotobacter, and Enterobacter have been isolated from salt-affected soil and were found to be efficient plant growth promoters (Gopalkrishnan et al. 2012; Allam et al. 2018; Nakbanpote et al. 2014; Kapoor et al. 2017). It is well drafted that indigenous strains have better potential to multiply under stress conditions as compared to the exotic strains. These facts are important while selecting the microbial inoculants for a specific environment. This chapter emphasizes on the assessment of plant growth-promoting rhizobacteria (PGPR) approaches for the alleviation of salinity stress with a brief overview of adaptation mechanism and genetic variability of salt-tolerant strains facilitating them to grow in saline environments.
7.2 Diversity of Salt-Tolerant Bacteria
Salinity affects the structure and species composition of the rhizospheric communities. Saline environments harbor taxonomically diverse bacterial groups such as Enterobacter, Pseudomonas, Vibrio, and a few Gram-positive bacterial species, e.g., Bacillus, Micrococcus, and Salinicoccus, which exhibit modified physiological and structural characteristics under the prevailing saline conditions (DasSarma and DasSarma 2012). Salt-tolerant bacteria have been isolated from different sources such as salt lakes (Hedi et al. 2009), river water (Tiquia et al. 2007), rhizosphere (Hasnain and Taskeen 1989), root nodules (Gal and Choi 2003), and soil samples (Takashina et al. 1994). Gram-negative bacteria including nodulating bacteria have been reported to colonize the saline soil (Zahran et al. 1992). Nodulating bacteria such as Rhizobium have been reported in association with the salt marsh grass (Whiting et al. 1986). Among free-living bacteria, those belonging to genus Azospirillum, Bacillus, Enterobacter, and Azotobacter play a crucial role in different stressed conditions (Sahoo et al. 2014). In fact, inoculation with Azotobacter has been found to exert several beneficial effects on plant yields as possess various plant growth promoting traits and also found to produce exopolysaccharides under saline conditions (De la Vega et al. 1991; Mrkovacki et al. 1996). Consequently, it has been implicit that isolating bacteria with PGP traits from naturally saline environments would give indigenous isolates to improve the effect of salt stress on plants.
7.3 Plant Growth-Promoting Activities of Salt-Tolerant Bacteria
7.3.1 Nitrogen Fixation Under Salt Stress
Nitrogen is the essential macronutrient required for plant growth. Bacteria inhabiting under saline conditions alter some of their activities and pathways to adapt themselves. One of the sensitive activities is the nitrogenase activity, which is affected by extreme saline conditions. Nitrogen fixation was found to be decreased in saline soils as salt stress adversely affects the nitrogenase enzyme activity (Gao et al. 2014). The extent of effects of salinity on denitrification process is dependent on the type of nitrogen compound present in the soil (El-Shinnawi et al. 1982). These stressed conditions disrupt the nitrogen cycle and lead to the disappearance of nitrate (NO3−) from saline soil through denitrification process, resulting in alteration of enzymatic processes (Azhar et al. 1989). Biological nitrogen fixation (BNF) involves the enzymatic reduction of nitrogen to ammonia (NH3), which acts as the precursor molecule for the biosynthesis of amino acids and other nitrogen-containing biomolecules. Islam et al. (2010) studied the free-living culturable diazotrophic bacteria of paddy soils under salt stress conditions and found that 32 bacteria were positive for acetylene reduction assay (ARA) and the values ranged from 1.8 to 2844.7 nmol ethylene h−1 mg−1 protein. The study carried out by Chowdhury et al. (2007) on diazotrophic bacterial isolates showed that the predominance of Gram-negative bacteria from the surface-sterilized roots of Lasiurus scindicus were capable of fixing nitrogen. Nitrogen-fixing Bacillus strains were also obtained from saline lands of Egypt; these strains reduced acetylene in pure culture at 5% NaCl (Zahran et al. 1992).
7.3.2 Phosphate Solubilization
Phosphorus is one of the key nutrients for plants, but a major portion of it is available in insoluble form. Microorganisms play a vital role in solubilizing phosphorous and in increasing the availability of phosphorous to plants. Phosphate-solubilizing microorganisms belonging to genera Klebsiella, Erwinia, Rhizobium, Achromobacter, Aerobacter, Enterobacter, Pseudomonas, Micrococcus, and Bacillus have been reported earlier. However, strains belonging to Pseudomonads and Bacillus are deliberated as the most proficient phosphate solubilizers (Villegas and Fortin 2002), whereas fungal species such as Aspergillus, Penicillium, and Curvularia and yeast are widely reported to solubilize various forms of inorganic phosphates (Das et al. 2013).
Several researchers have isolated phosphate-solubilizing microorganisms from various niches of saline soils (Sharan et al. 2008; Park et al. 2010; Srinivasan et al. 2012). A salt-tolerant, nitrogen-fixing, and phosphate-solubilizing species Swaminathania salitolerans has been isolated from the rhizosphere, roots, and stems of salt-tolerant mangrove associated with wild rice (Loganathan and Nair 2004). In another study, phosphate-solubilizing bacteria Alteromonas sp. and Pseudomonas aeruginosa have been isolated from salt-affected soils. These isolates were found to solubilize phosphate under saline conditions, i.e., up to 2M NaCl concentration (Srinivasan et al. 2012). Rosado et al. (1998) and Nautiyal (1999) observed increased phosphate-solubilizing activity of bacteria in the presence of 10% NaCl, but the solubilizing activity decreased with increase in NaCl concentration.
7.3.3 Siderophore Production
Iron is the fourth abundant and essential growth element for all living organisms and perhaps the most important micronutrient used by bacteria for their metabolism. To confiscate and solubilize ferric iron, many microorganisms utilize low-molecular-weight (<1000 Da) compounds with high iron affinity known as “siderophores.” Siderophores are produced by rhizospheric bacteria to enhance the growth and development of plants by increasing the availability of iron. Siderophore-producing microorganisms prevailing in the rhizosphere suggest that plants would all become iron deficient in the absence of iron-chelating siderophores (Kloepper et al. 1980). Nine halophilic archaea were isolated from marine salterns for siderophore production (Dave and Desai 2006). Ramadoss et al. (2013) found that Bacillus halodenitrificans and Halobacillus sp. isolated from saline habitats exhibited siderophore-producing activity.
7.3.4 Indole Acetic Acid (IAA) Production
Some PGPR strains enhance plants’ growth and development by modulating the concentration of known phytohormones. Among plant hormones, auxins and ethylene play an essential role in root system development and crop yield. Indole-3-acetic acid (IAA) is the common natural auxin that extensively affects plant physiology. Diverse microbial groups are capable of producing physiological active auxins, which exert pronounced effects on plant growth and its establishment. In order to produce auxin, bacteria use tryptophan as a precursor molecule and convert it into IAA (Etesami et al. 2009). In plants, saline stress often affects the production of IAA and makes them imbalance. Thus, it is important to study IAA-producing rhizobacteria in saline conditions which could facilitate plant growth under salt stress. It has been reported that pre-sowing seeds with phytohormones alleviated the growth-restricted effect of salt stress (Ramadoss et al. 2013). Zahir et al. (2010) isolated IAA-producing halophilic Rhizobium phaseoli strains from the mung bean nodules and evaluated their growth parameters in the presence and absence of tryptophan under salt stress conditions. Growth promotion effects were observed, and this might be due to higher auxin production and mineral uptake in rhizosphere, which reduced the adverse effect of salinity.
7.3.5 Lytic Enzyme Production
Lytic enzyme production is one of the indirect approaches for plant growth promotion. A wide array of organisms have been obtained from harsh environments that produce many active and stable enzymes including proteases (Durham et al. 1987), amylases (Horikoshi 1971), lipases (Watanable et al. 1977), etc. Lytic enzymes produced by biocontrol organisms mediate defense against the pathogens and improve plant growth (Vivekananthan et al. 2004). Enzymes that are stable and active at extreme saline conditions are very much in demand for various industrial processes. Shaheen et al. (2008) reported the protease enzyme production by Bacillus subtilis at different concentrations of salt (0–6% NaCl). Sivaprakasam et al. (2011) obtained salt-tolerant alkaline protease from P. aeroginosa that was capable of enzymatic degradation.
7.4 Mechanism of Salt Tolerance
Salt stress reduces microbial population in the rhizosphere. Microbes that inhabit hypersaline environments experience intense osmotic pressure and thus use “compatible solute strategy” or the “salt-in strategy” to resist salt stress (Etesami and Beattie 2017). Bacteria accumulate compatible solutes and other amino acids under saline conditions (Brown 1976). Some salt-tolerant bacteria can use salt in strategy mechanism and accumulate electrolytes, e.g., K+ glutamate. Furthermore, enzymes, ribosomes, and transport proteins of these bacteria require high level of potassium for stability and activity. Organic solutes increase the intracellular osmotic strength and stabilize the cellular macromolecules (Lippert and Galinski 1992).
Specific genetic induction is required to accumulate compatible organic solutes in salt-tolerant bacteria (Plemenitas et al. 2014). Intracellular proline was found to increase rapidly in Bacillus in response to osmotic stress by NaCl, and the corresponding genes were detected as proB, proA, and proC encoding γ-glutamyl kinase (γ-GK), γ-glutamyl-phosphate reductase (γ-GPR), and pyrroline-5-carboxylate (P5C) reductase, respectively (Chen et al. 2007). Various genes encoding L-aspartokinase (Ask), L-2,4-diaminobutyric acid transaminase (EctB), L-2,4- diaminobutyric acid acetyltransferase (EctA), and L-ectoine synthase (EctC) have been located and found to be involved in the biosynthesis of ectoine in Halobacillus dabanensis (Nada et al. 2011). Four genes, viz., betI, betC, betB, and beta, were found to be essential for oxidation of choline or choline-O-sulfate to glycine betaine organized in one operon (Sevin and Sauer 2014). Other antiporter genes that have been reported in salt-tolerant bacteria are also essential for maintaining the balance of Na+ and K+ ions in the cell in order to attain an osmotic equilibrium. This mechanism is accompanied by certain physiological modifications which are required to protect all the metabolic and regulatory functions at high salinity (Eisenberg and Wachtel 1987). Na+/H+ antiporters are membrane proteins involved in pH and Na+ homeostasis in cells that exchange Na + for H+ (Inaba et al. 2001). The genes that are proved to be involved in halotolerance in bacteria either through knockout studies or through overexpression studies are given in Table 7.1.
7.4.1 Genetic Variations Based on Nha
Sodium hydrogen antiporters transport Na+ or Li+ in exchange for H+ across the cytoplasmic membrane of cell (Alkoby et al. 2014) and maintain intracellular pH homeostasis, detoxification of cells from Na+ ions, regulation of cell volume, and establishment of an electrochemical potential of Na+ ions (Padan 2014). Various Na+/H+ antiporters such as nhaA, nhaB, nhaC, nhaD, nhaP, chaA, tetA(L), and napA have been identified in Gram-positive and Gram-negative bacteria (Padan et al. 2001; Majernik et al. 2001). NhaA gene responsible for salt tolerance in Enterobacter sp. has been reported previously (Kapoor et al. 2017). The primary structure of all the abovementioned genes exhibits very weak or no significant homology. This indicates that different transport systems coupling H+ and Na+ circulation have developed during evolution. Several genes encoding Na+/H+antiporters from different microorganisms have been shown variability by replacing nhaA of Escherichia coli e.g., nhaA of Vibrio alginolyticus (Nakamura et al. 1994), Vibrio parahaemolyticus (Kuroda et al. 1994), Bacillus aquimaris and Enterobacter cloacae (Kapoor 2014), Enterobacter ludwigii (Kapoor et al. 2017), as well as nhaB of V. parahaemolyticus (Nozaki et al. 1996), nhaD of V. parahaemolyticus (Nozaki et al. 1998), nhaP of Pseudomonas aeruginosa (Utsugi et al. 1998), nhaC of Bacillus pseudofirmus OF4 (Ivey et al. 1993), napA of Enterococcus hirae (Strausak et al. 1993), and mnh of Staphylococcus aureus (Hiramatsu et al. 1998).
Amino acid residues Asp-133, Asp-163, and Asp-164 (Inoue et al. 1995) and His-225 were proposed to be involved in pH sensitivity in E. coli for binding of sodium ions (Gerchman et al. 1993). Furthermore, amino acid residues Gly-14, Gly-166, Phe-267, Leu-302, Gly-303, Cys-335, Ser-342, and Ser-369 located in the cell membrane were identified by Nuomi et al. (1997) and found to be essential for the activity of nhaA in E. coli. In general, 111 amino acid residues were found to be fully conserved in the nhaA gene products from different bacteria (Inoue et al. 1995; Vimont and Berche 2000). Our previous study showed that specific insertions/deletions caused major variations of amino acids in salt-tolerant strains (Kapoor et al. 2017). However, these types of alleles mined the rare mutation among the salt- and non-salt-tolerant strains, and little information is available on allele mining of genes responsible for salt tolerance.
7.5 Conclusions
The salt tolerance mechanism of plants modulated by rhizosphere bacteria opens up new prospects to understand plant–microbe interaction. PGPR strains have been conventionally used as biofertilizers to augment the growth and yield of different crops under salt stress conditions. The variability in salt tolerance behavior of bacteria can be explored by targeting the genes involved in salt tolerance mechanism. Genetic and genomic studies used to determine allele mining in gene sequences among salt- and non-salt-tolerant strains are yet to be explored. Gene silencing approach can be used to study the precise function of specific gene in salt-tolerant strains.
References
Alkoby D, Rimon A, Burdak M, Patino-Ruiz M, Calinescu O (2014) NhaA Na+/H+ antiporter mutants that hardly react to the membrane potential. PLoS One 9:e93200
Allam NG, Kinany R, El-Refai E, Ali WY (2018) Potential use of beneficial salt tolerant bacteria for improving wheat productivity grown in salinized soil. J Microbiol Res 8:43–53. https://doi.org/10.5923/j.microbiology.20180802.03
Azhar E, Van Cleemput O, Verstraete W (1989) The effect of sodium chlorate and nitrapyrin on the nitrification mediated nitrosation process in soils. Plant Soil 116:133–139
Brown AD (1976) Microbial water stress. Bacteriol Rev 40:803–846
Chen M, Wei H, Cao J, Liu R, Wang Y, Zheng C (2007) Expression of Bacillus subtilis proBA genes and reduction of feedback inhibition of proline synthesis increases proline production and confers osmotolerance in transgenic Arabidopsis. J Biochem Mol Biol 40:396–403
Chowdhury SP, Schmid M, Hartmann A, Tripathi AK (2007) Identification of diazotrophs in the culturable bacterial community associated with roots of Lasiurus sindicus, a perennial grass of Thar Desert, India. Microb Ecol 54:82–90
Cordovilla MP, Ligero F, Lluch C (1994) The effect of salinity on N fixation and assimilation in Vicia faba. J Exp Bot 45:1483–1488
Das A, Dutta BK, Barooah AK (2013) In vitro solubilization of inorganic phosphate by phosphate solubilizing fungi isolated from tea agroecosystem soil of Barak Valley, Southern Assam. Int J Microbiol Res 4:336–341
DasSarma S, DasSarma P (2012) Halophiles. In: eLS. Wiley, Chichester
Dave SR, Desai HB (2006) Microbial diversity at marine salterns near Bhavnagar, Gujarat, India. Curr Sci 90:497–500
De la Vega MG, Cejudo FJ, Paneque A (1991) Production of exocellular polysaccharide by Azotobacter chroococcum. Appl Biochem Biotechnol 30:273–284
Durham DR, Stewart DB, Stellwag EJ (1987) Novel alkaline and heat stable proteases from alkalophilic Bacillus sp. Strain GX 6638. J Bacteriol 169:2262–2768
Eisenberg H, Wachtel EJ (1987) Structural studies of halophilic proteins, ribosomes, and organelles of bacteria adapted to extreme salt concentrations. Annu Rev Biophys Biophys Chem 16:69–92
El-Shinnawi MM, Omran MS, Abo El-Naga SA (1982) Denitrification in soil saturated with saline water. Appl Microbiol Biotechnol 43:1099–1106
Etesami H, Beattie GA (2017) Plant-microbe interactions in adaptation of agricultural crops to abiotic stress conditions. In: Kumar V, Kumar M, Sharma S, Prasad R (eds) Probiotics and plant health. Springer, Singapore, pp 163–200
Etesami H, Alikhani HA, Akbari A (2009) Evaluation of plant growth hormones production (IAA) ability by Iranian soils rhizobial strains and effects of superior strains application on wheat growth indexes. World Appl Sci J 6:1576–1584
Gal SW, Choi YJ (2003) Isolation and characterization of salt tolerance Rhizobia from Acacia root nodules. Agric Chem Biotechnol 46:58–62
Gao H, Bail J, He X, Zhao Q, Lu Q, Wang J (2014) High temperature and salinity enhance soil nitrogen mineralization in a tidal freshwater marsh. PLoS One 9:e95011
Gerchman Y, Olami Y, Rimon A, Taglicht D, Schuldiner S, Padan E (1993) Histidine-226 is part of the pH sensor of NhaA, a Na+/H+ antiporter in Escherichia coli. PNAS 90:1212–1216
Gopalkrishnan S, Upadhyaya HD, Vadlamudi S, Humayun P, Vidya MS, Alekhya G, Singh A, Vijyabharathi Bhimineni RK, Seema M, Rathore RO (2012) Plant growth promoting traits of biocontrol potential bacteria isolated from rice rhizosphere. Springerplus 1:71–76
Hasnain S, Taskeen N (1989) Characterization of salt tolerant bacteria isolated from the rhizosphere of Leptochloa fusca and Atritplex rhocodoidaes. Pak J Pharm Sci 2:55–57
Hedi A, Sadfi N, Fardeau M-L, Rebib H, Cayol J-L, Ollivier B, Boudabous A (2009) Studies on the biodiversity of halophilic microorganisms isolated from El-Djerid salt lake (Tunisia) under aerobic conditions. Int Microbiol 9:731786
Hiramatsu T, Kodama K, Kuroda T, Mizushima T, Tsuchiya T (1998) A putative multisubunit Na+/H+ antiporter from Staphylococcus aureus. J Bacteriol 180:6642–6648
Horikoshi K (1971) Production of alkaline enzyme by alkalophilic microorganisms. Part II. Alkaline amylase produced by Bacillus No. A-40-2. Agric Biol Chem 35:1783–1791
Ibekwe AM, Poss JA, Grattan SR, Grieve CM, Suarez D (2010) Bacterial diversity in cucumber (Cucumis sativus) rhizosphere in response to salinity, soil pH, and boron. Soil Biol Biochem 42:567–575
Inaba M, Sakamoto A, Murata N (2001) Functional expression in Escherichia coli of low-affinity and high-affinity Na +(Li+)/H+ antiporters of Synechocystis. J Bacteriol 183:1376–1384
Inoue H, Nuomi T, Tsuchiya T, Kanzawa H (1995) Essential aspartic acid residues, Asp-133, Asp-163 and Asp-164, in the transmembrane helices of a Na+/H+antiporter (NhaA) from Escherichia coli. FEBS Lett 363:264–268
Islam R, Trivedi P, Madhaiyan M, seshadre S, Lee G, Yang J, Kim Y, Kim M, Han G, Chauhan PS, Sa T (2010) Isolation, enumeration, and characterization of diazotrophic bacteria from paddy soil sample under long term fertilizer management experiment. Biol Fertil Soils 46:261–269
Ivey DM, Guffanti AA, Zemsky J (1993) Cloning and characterization of a putative Ca2+/H+ antiporter gene from Escherichia coli upon functional complementation of Na+/H+ antiporter-deficient strains by the overexpressed gene. J Biol Chem 268:11296–11303
Kapoor R (2014) Bacterial diversity of salt tolerant nitrogen fixers around the salt mines of Himachal Pradesh. PhD Thesis. CSKHPKV, Palampur (HP)
Kapoor R, Gupta MK, kumar N, Kanwar SS (2017) Analysis of nhaA gene from salt tolerant and plant growth promoting Enterobacter ludwigii. Rhizosphere 4:62–69. https://doi.org/10.1016/j.rhisph.2017.07.002
Klahn S, Marquardt DM, Rollwitz I, Hagemann M (2009) Expression of the ggpPS gene for glucosylglycerol biosynthesis from Azotobacter vinelandii improves the salt tolerance of Arabidopsis thaliana. J Exp Bot 60(6):1679–1689
Kloepper JW, Leong J, Teintze M, Schroth MN (1980) Enhanced plant growth by siderophores produced by plant growth promoting rhizobacteria. Nature 286:885–886
Kuroda T, Shimamoto T, Inaba K, Tsuda M, Tsuchiya T (1994) Properties and sequence of the NhaA Na1/H1 antiporter of Vibrio parahaemolyticus. J Biochem 116:1030–1038
Lippert K, Galinski EA (1992) Enzyme stabilization by ectoine-type compatible solutes: protection against heating, freezing and drying. Appl Microbiol Biotechnol 37:61–65
Loganathan P, Nair S (2004) Swaminathania salitolerans gen. nov. sp. nov., a salt-tolerant nitrogen-fixing and phosphate solubilizing bacterium from wild rice (Porteresia coarctata Tateoka). Int J Syst Evol Microbiol 54:1185–1190
Majernik A, Gottschalk G, Daniel R (2001) Screening of environmental DNA libraries for the presence of genes conferring Na+(Li+)/H+antiporter activity on Escherichia coli: characterization of the recovered genes and the corresponding gene products. J Bacteriol 183:6645–6653
Miller KJ, Wood JM (1996) Osmoadaptation by rhizosphere bacteria. Annu Rev Microbiol 50:101–136
Mrkovacki N, Mezei S, Kovacev L (1996) Effect of Azotobacter inoculation on dry matter mass and nitrogen content in the hybrid varieties of sugar beet. A Periodical of Scientific Research on Field and Vegetable Crops 25:107–113
Nada AMK, Refaat MH, Abdel-Sabour MS, Hassan AM, Abd El Kader MM (2011) Molecular studies on EctC gene (Ectoine) in some halophilic bacterial isolates. Researcher 3:34–42
Nakamura T, Komano Y, Itaya E, Tsukamoto K, Tsuchiya T, Unemoto T (1994) Cloning and sequencing of an Na+/H+ antiporter gene from the marine bacterium Vibrio alginolyticus. Biochim Biophys Acta 1190:465–468
Nakbanpote W, Panitlurtumpai N, Sangdee A, Sakulpone N, Sirisom P, Pimthong A (2014) Salt-tolerant and plant growth-promoting bacteria isolated from Zn/Cd contaminated soil: identification and effect on rice under saline conditions. J Plant Interact 9:379–387
Nautiyal CS (1999) An efficient microbiological growth medium for screening phosphate solubilizing microorganisms. FEMS Microbiol Lett 170:265–270
Nogales J, Campos R, BenAbdelkhalek H, Olivares J, Lluch C, Sanjuan J (2002) Rhizobium tropici genes involved in free-living salt tolerance are required for the establishing of efficient nitrogen-fixing symbiosis with Phaseolus vulgaris. Mol Plant-Microbe Interact 15:225–232
Nozaki K, Inaba K, Kuroda T, Tsuda M, Tsuchiya T (1996) Cloning and sequencing of the gene for Na+/H+ antiporter of Vibrio parahaemolyticus. Biochem Biophys Res Commun 222:774–779
Nozaki K, Kuroda T, Mizuschima T, Tsuchiya T (1998) A new Na+/H+ antiporter, NhaD, of Vibrio parahaemolyticus. Biochim Biophys Acta 1369:213–220
Nuomi TH, Inoue T, Tsuchiya ST, Kanzawa H (1997) Identification and characterization of functional residues in a Na+/H+ antiporter (NhaA) from Escherichia coli by random mutagenesis. J Biochem 121:661–670
Padan E (2014) Functional and structural dynamics of NhaA, a prototype for Na+ and H+ antiporters, which are responsible for Na+ and H+ homeostasis in cells. Biochim Biophys Acta 1837:1047–1062
Padan E, Venturi M, Gerchman Y, Dover N (2001) Na(+)/H(+) antiporters. Biochim Biophys Acta 1505:144–157
Park KH, Lee OM, Jung HI, Jeong JH, Jeon YD, Hwang DY, Lee CY, Son HJ (2010) Rapid solubilization of insoluble phosphate by a novel environmental stress-tolerant Burkholderia vietnamiensis M6 isolated from ginseng rhizospheric soil. Appl Microbiol Biotechnol 86:947–955
Plemenitas A, Lenassi M, Konte T, Kejzar A, Zajc J, Gostincar C, Cimerman NG (2014) Adaptation to high salt concentrations in halotolerant/Halophilic fungi: a molecular prospective. Front Microbiol 5:199
Pocard JA, Vincent N, Boncompagni E, Smith LT, Poggi MC, DLe R (1997) Molecular characterization of the bet genes encoding glycine betaine synthesis in Sinorhizobium meliloti 102F34. Microbiology 143:1369–1379
Ramadoss D, Lakkineni VK, Bose P, Ali S, Annapurna K (2013) Mitigation of salt stress in wheat seedlings by halotolerant bacteria isolated from saline habitats. Springerplus 2:6
Rosado AS, de Azevedo FS, da Cruz DW, van Elsasand JD, Seldin L (1998) Phenotypic and genetic diversity of Paenibacillus azotofixans strains isolated from the rhizoplane or rhizosphere soil of different grasses. J Appl Microbiol 84:216–226
Sahoo RM, Ansari MW, Pradhan M, Dangar TK, Mihanty S, Tuteja M (2014) A novel Azotobacter vinellandii (SRIAz3) functions in salinity stress tolerance in rice. Plant Signal Behav 9:e29377
Sevin DC, Sauer U (2014) Ubiquinone accumulation improves osmotic-stress tolerance in Escherichia coli. Nat Chem Biol 10:266–227
Shaheen M, Shah AA, Hameed A, Hasan F (2008) Influence of culture conditions on production and activity of protease from Bacillus subtilis bs1. Pak J Bot 40:2161–2169
Sharan A, Shikha DNS, Gaur R (2008) Xanthomonas compestris, a novel stress tolerant, phosphate solubilizing bacterial strain from saline –alkali soils. World J Microbiol Biotechnol 24:753–759
Sivaprakasam S, Dhandapani B, Mahadevan S (2011) Optimization studies on production of a salt-tolerant protease from Pseudomonas aeruginosa strain bc1 and its application on tannery saline wastewater treatment. Braz J Microbiol 42:1506–1515
Srinivasan R, Alagawadi AR, Mahesh S, Meena KK, Saxena AK (2012) Characterization of phosphate solubilizing microorganisms from salt-affected soils of India and their effect on growth of sorghum plants Sorghum bicolor (L.). Moench. Ann Microbiol 62:93–105
Strausak D, Waser M, Solioz M (1993) Functional expression of the Enterococcus hirae NaH-antiporter in Escherichia coli. J Biol Chem 268:26334–26337
Takashina T, Otozati K, Hamamoto T, Horikoshi K (1994) Isolation of halophilic and halotolerant bacteria from a Japanese salt field and comparison of the partial 16S rRNA gene sequence of an extremely halophilic isolate with those of other extreme halophiles. Biodivers Conserv 3:632–642
Tiquia SM, Davis D, Hadid H, Kasparian S, Ismail M, Sahly R, Shim J, Singh S, Murray KS (2007) Halophilic and halotolerant bacteria from river waters and shallow groundwater along the Rouge river of southeastern Michigan. Environ Technol 28:297–230
Utsugi J, Inaba K, Kuroda T, Tsuda M, Tsuchiya T (1998) Cloning and sequencing of a novel Na+/H+ antiporter gene from Pseudomonas aeruginosa. Biochim Biophys Acta 1398:330–334
Villegas J, Fortin JA (2002) Phosphorous solubilization and pH changes as a result of the interactions between soil bacteria and arbuscular mycorrhizal fungi on a medium containing NO3 − as nitrogen source. Can J Bot 80:571–576
Vimont S, Berche P (2000) NhaA, an Na1/H1 antiporter involved in environmental survival of Vibrio cholera. J Bacteriol 182:2937–2944
Vivekananthan R, Ravi M, Ramanathan A, Samiyappan R (2004) Lytic enzymes induced by Pseudomonas fluorescene and other biocontrol organisms mediated defence against the anthracnose pathogen in mango. World J Microbiol Bioltechnol 20:235–244
Watanable N, Ota Y, Minoda Y, Yomada K (1977) Isolation and identification of alkaline lipase producing microorganisms, cultural conditions and some properties of crude enzymes. Agric Biol Chem 41:1353–1358
Wei W, Jiang J, Yang SS (2004) Mutagenesis and complementation of relA from Sinorhizobium meliloti 042BM as a salt tolerance involvement gene. Ann Microbiol 54:317–324
Whiting GJ, Gandy EL, Yoch DC (1986) Tight coupling of root-associated nitrogen fixation and plant photosynthesis in the salt marsh grass Spartina alterniflora and carbon dioxide enhancement of nitrogenase activity. Appl Environ Microbiol 52:108–113
Zahir ZA, Shah KM, Naveed M, Akhter JM (2010) Substrate dependent auxin production by Rhizobium phaseoli improves the growth and yield of Vignaradiata L. under salt stress conditions. J Microbiol Biotechnol 20:1288–1294
Zahran HH, Moharram AM, Mohammad HA (1992) Some ecological and physiological studies on bacteria isolated from salt affected soils of Egypt. J Basic Microbiol 32:405–413
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Kapoor, R., Kanwar, S.S. (2019). Plant Growth-Promoting Bacterial Life at High Salt Concentrations: Genetic Variability. In: Varma, A., Tripathi, S., Prasad, R. (eds) Plant Biotic Interactions . Springer, Cham. https://doi.org/10.1007/978-3-030-26657-8_7
Download citation
DOI: https://doi.org/10.1007/978-3-030-26657-8_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-26656-1
Online ISBN: 978-3-030-26657-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)