Abstract
Alzheimer’s disease (AD) is a neurodegenerative disorder, which starts as mild cognitive impairment (MCI) several years before its full-blown clinical manifestation. In the last two decades, optical coherence tomography (OCT) has been used to measure a significant loss in peripapillary retinal nerve fiber layer (RNFL) and in macular thickness and volume in patients affected by a form of mild to severe dementia. These morphological abnormalities correlate to some extent with the severity of the disease as evaluated with neuropsychological tests. Furthermore, these structural measures correlate with electrophysiological parameters of pattern electroretinogram, reflecting integrity of the innermost retinal layers, but not with those of the visual evoked potentials, reflecting activity of the post-chiasmatic visual pathway. The latter evidence suggests that RNFL thickness reduction is related to neuronal degeneration in the ganglion cell layer and not to a retrograde degeneration from changes in the post-chiasmatic visual pathway. These data suggest a possible role of OCT in monitoring the progression of AD and in assessing the effectiveness of purported AD treatments.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Alzheimer’s disease
- Optical coherence tomography
- Mild cognitive impairment
- Electroretinogram
- Psychometric testing
- Retina
13.1 Introduction
Alzheimer’s disease (AD) is the most common cause of dementia with an incidence that increases with age exponentially. According to the World Health Organization the worldwide prevalence of Alzheimer’ disease (AD) is increasing yearly and receives growing attention because it affects quality of life, and also because has a significant economic impact, being a major public-health problem [1] and a substantial financial burden on families [2]. The recent publication of the Global Burden of Disease survey 2016 shows that among neurological conditions, the global number of individuals who lived with dementia has doubled compared to 1990 and dementia was the fifth leading cause of death globally [3].
AD is a brain degenerative disorder, where inherited susceptibility and environmental factors are probably interrelated [4, 5]. The clinical evolution of AD is a slowly progressive and episodic memory loss as the predominant symptom, with various accompanying signs including aphasia, apraxia, and agnosia, and general cognitive symptomology, such as impaired judgement, decision-making, and orientation [4]. Because of the slowness of the disease’s progression, the neurodegenerative processes are likely to start 20–30 years before the clinical manifestations of AD. This transitional phase is recognized as mild cognitive impairment (MCI), which has some memory deficit, preservation of general cognitive and functional abilities, and absence of diagnosed dementia as cardinal features [6]. The amnestic type of MCI (aMCI) shows the highest annual incidence conversion rate to AD [7]. Nonetheless MCI can be comorbid with other neurological disorders, such as nondemented Parkinson’s disease (PD) patients and increased the risk of progressive cognitive decline and dementia [8].
Despite great advances in the understanding of AD pathophysiology in the last few years, the exact pathogenesis of AD and its precursor MCI is still not comprehensively understood.
At the histopathologic level, neuronal loss, beta-amyloid plaques, neurofibrillary tangles made of hyperphosphorylated tau and neuritic plaques, and granulovacuolar degeneration characterize the brain of AD [4]. These lesions have been seen in the hippocampus and other limbic structures, as well as in the visual associative areas [9, 10], the primary visual cortex [11, 12], and in subcortical structures including the lateral geniculate nuclei and the superior colliculi [13,14,15], and within the retina [16,17,18].
AD patients may be affected by various visual disturbances, such as deficits in contrast sensitivity [19,20,21], motion perception [22, 23], and color discrimination [24,25,26]. Historically, these visual dysfunctions in AD have been attributed to damage in the primary visual cortex and to degenerative processes in primary and associative visual cortical areas, but cortical dysfunction alone cannot explain the pattern of observed defects. Multiple forms of evidence points toward the involvement of retinal ganglion cells and their axons in the optic nerve as a basis of the visual dysfunction in AD [16, 27]. This peripheral involvement in AD and MCI could be independent from the central one [28].
In the last two decades, several studies have considered the retinal involvement in AD pathophysiology. Sophisticated imaging techniques have been used, such as optical coherence tomography (OCT), scanning laser polarimetry, and pattern electroretinography (PERG), to assess the morphologic and functional changes of the retina in AD [29].
Optical Coherence Tomography (OCT) permits the objective quantification in vivo of the retinal nerve fiber layer (RNFL) that consists of axons that form the optic nerve and contributes partially to the retinal thickness. This method consists of a non-invasive technology allowing cross-sectional imaging of the eye [30, 31]. The implementation of Spectral-Domain OCT provides advantages in signal-to-noise ratio, permitting faster signal acquisition [32, 33]. Taking in mind that the human eye is an embryological protrusion of the brain, and the nerves and axons of the retinal nerve fiber layer (RNFL) are similar to those in the brain, it is not surprising that OCT has been widely employed in assessing RNFL thickness in several neurologic as well as neuro-ophthalmologic disorders [34,35,36,37,38].
The intent of this chapter is to provide a comprehensive overview of the results provided by the OCT technique as used to understand morphological retinal changes that occur due to the degenerative processes associated with Alzheimer’s disease and other dementing disorders.
13.2 Retina and Alzheimer’s Disease
As an integrated part of the nervous system, the retina is physiologically under senescence processes, the majority of them still under intense scrutiny. Therefore, in order to better evaluating AD patients, it is important to quantify the degree of thinning arising with age in the absence of pathological processes. It is also important to determine whether age-related loss is uniformly distributed across the optic disc or has any tendency to be sectorial and might therefore be a confounding factor for a correct evaluation of disease-related processes.
In the quest for understanding the degenerative changes accompanying AD, researchers performed both histological and in-vivo studies on healthy subjects.
Using immunohistochemical techniques, Löffler and colleagues examined the presence and expression of amyloid-related proteins, the same that have been found in specimens of AD patients’ brains, in the human retina collected from cadaver eyes at various ages [17]. They did not find any abnormal deposits or immunoreactivity changes in the outer neural retina. On the other hand, they demonstrated the presence of tau proteins and amyloid precursor protein in the inner retina layers, including retinal ganglion cells and nerve fiber layer, of the human retina in older persons. Small amounts of beta-amyloid were detected in the sub-retinal pigment epithelium space [17]. Intracellular and extracellular Aβ deposits were recently detected in immunoreactive melanopsin retinal ganglion cells (mRGC) in AD retinas with abnormal morphology [18].
The analysis of normal in-vivo human retinal nerve fiber layer thickness, as measured by time-domain OCT, has been repeatedly confirmed as an age-related reduction of RNFL thickness in normal subjects [39,40,41,42]. This thinning mainly involved the superior and inferior optic disc areas [43,44,45].
13.2.1 Animal Model of AD
One of the most recognised theories for the genesis of AD, the “amyloid cascade hypothesis”, postulates that APP dysmetabolism and beta-amyloid deposition are the primary events that facilitate the disease process of AD [46].
In the past decade, through the use of genetic engineering techniques, transgenic (Tg) animal models over-expressing mutant forms of amyloid precursor protein (APP) and/or presenilin 1 (PS1) have been generated to mimic various aspects of AD pathology, including Aβ deposition, cognitive deficits, inflammation, and synaptic dysfunction, to test newly proposed therapeutic agents [47]. Recent publications have shown the accumulation of Aβ-plaques within the retina [48,49,50,51] and its microvasculature [52], retina ganglion cell death [53], and local neuroinflammation [52] in different Tg animal models. Nevertheless, these transgenic models only mimic part of the pathological features of AD.
An interesting Tg AD rat model, TgF344-AD, expressing mutant human amyloid precursor protein (APPsw) and presenilin 1 genes, seems to more faithfully encapsulate most of the features of AD. This animal model is characterized by an otherwise healthy long lifespan, but the rats suffer from later age cognitive decline, age-dependent cerebral amyloidosis, taupathy, gliosis, and frank neuronal loss that seems to parallel those in humans [54]. Tsai and colleagues investigated a group of TgF344-AD rats and age-matched wild type rats [55]. When compared with age-matched controls, the retinas of Tg rats demonstrated the presence of Aβ plaques, hypertrophic retinal pigment epithelial cells, signs of neuroinflammation, and a significant thinning of retinal choroid [55].
In mice models, non-mutant human Tau (hTau) gene expression may be sufficient to initiate AD and may thus be a more relevant model to study the disease [56]. In hTau mice at 5 and 17 months of age researchers examined functional, histological and molecular changes occurring in the visual system. They unexpectedly observed that hTau expression is not toxic for retinal cells at least in-so-far as the electroretinogram response was not altered in hTau compared with mTKO littermates, but even more pronounced in amplitude in hTau than in KO mice deprived of Tau retinae, suggesting a protective role of hTAU in visual physiology [57].
13.2.2 Histological Studies in Post-Mortem AD Eyes
In 1986, histological examination in a human post-mortem study revealed depletion of axons in the optic nerves from AD patients [16]. Sadun and colleagues were the first to demonstrate this primary AD-associated optic neuropathy and further, to characterize, by morphometric analysis, that the predominant effect was on the largest retinal ganglion cells (M-cells) that contribute their large caliber fibers to the optic nerve [27]. Other studies [58, 59] failed to confirm the selective large axon losses. This discrepancy has been attributed, at least in part, to methodological biases such as different post-mortem delays, different techniques in axonal counting and/or difficulties in obtaining well-preserved myelinated axons.
Extensive retinal ganglion cell loss in the central retina in AD was also observed in histological studies [16] or by other methods of evaluating the RNFL in vitro. Morphometric analysis of the central retina (fovea/foveola/parafoveal retina) in eyes from 9 AD and 11 age-matched controls revealing an overall decrease of 25% in total numbers of neurons in the ganglion cell layer (GCL) (fovea/parafoveal retina) in AD as compared with control eyes [60]. Moreover, the most severe decrease was observed in the foveal region (−43%) over the far temporal region. Similar results were obtained in AD patients with the evaluation of the peripheral retina: the neuronal loss was more pronounced in the superior and inferior quadrants (50–59%) and a significantly increased ratio of astrocytes to neurons was detected when compared with control eyes [61]. Moreover, histological signs of degeneration in the retinal ganglion cells (RGCs) were described to include a vacuolated, “frothy” appearance of the cytoplasm in the absence of neurofibrillary tangles within the RGCs, or of neuritic plaques or amyloid angiopathy in the retinas or optic nerves in AD [62].
Koronyo-Hamaoui and colleagues recently identified retinal Aβ plaques in post-mortem eyes from 8 AD patients and in 5 suspected early stage cases, but not in five age-matched non-AD individuals [50].
Plaque-like structures were seen in two retinas from two AD patients, with thickening of retinal choroid and no changes in the retinal pigment epithelial cells [55]. Fibrillar tau or Aβ aggregates were seen in post-mortem retinas from the same AD patients that had been previously observed by using an in-vivo imaging technique [63].
In a recent post-mortem study [18], researchers identified mRGCs with antibody targeting melanopsin from 14 AD patients with preserved retinal anatomy, and 13 controls. In AD patients, they revealed preserved retinal layers as compared to controls, but smaller somas and thinner dendrites, focal attenuations of melanopsin immunostaining, signs of optic neuropathy with axonal loss predominantly affecting the larger fibers in the superior quadrant. Melanopsin RGC number was significantly correlated with age in controls but not in AD patients, meaning that the degenerative processes at the basis of AD are, at least in part, independent from age related changes. Finally, in a subgroup of subjects, they found evidence of consistent accumulation of Aβ inside immunoreactive mRGCs soma and extracellular/abluminal areas in AD patients, while they were absent in controls [18].
13.2.3 Clinical Studies Based on the Subjective Evaluation of Fundus Photographs
The RNFL consists of the RNFL axons of retinal ganglion cells, converging at the optic disc forms the origin of the optic nerve.
That retinal ganglion cell degeneration occurs in AD is further evident by means of the use of a boundary-tracking program and RNFL photographs. A higher proportion of AD patients showed signs of optic neuropathy manifesting as optic disc atrophy, pathologic optic disc cupping, and thinning of the neuroretinal rim and of the RNFL [64, 65]. Tsai et al. [65] observed an increased cup-to-disc ratio, cup volume, and decreased optic disc rim area in AD patients by optic nerve analyser.
Analyzing mild to moderate AD patients with RNFL fundus photographs, Lu and colleagues found that all the AD patients had different types of RNFL abnormalities, including diffuse and wedge shape nerve fiber layer drop-out [66]. These abnormalities in AD patients were associated with a higher cup–disc ratio of 39–43% when compared to that in the control group.
However, these studies required the subjective evaluation of fundus photographs with the inherent observer bias.
13.3 In-Vivo Morphological Analysis in AD and MCI Patients
One confounding factor in analysing MCI and AD-related late-onset progressive changes is that there are anatomical and physiological changes normally found in aging eyes. In animal [67] and human [67, 68] studies, a physiological age-dependent progressive loss of optic nerve fibers, with progressive decrease of mean axonal diameter, has been demonstrated [69,70,71].
Optical coherence tomography (OCT) is a technique that can also be used in ophthalmology for the measurement of the macular thickness and volume. OCT can determine RNFL thickness of the peripapillary region reflecting axons and would allow quantification of axonal loss, measurement of macular thickness and volume would reflect retinal neurons, allowing quantification of neuronal loss [30].
Several previous studies using OCT techniques have examined the relationship between age and RNFL measurements. These have consistently shown that optic nerve, RNFL, and macular measurements with OCT all varied with age [41, 72], with the greatest decrease in mean RNFL occurring in the superior [44] and inferior quadrants [42], especially after age 50 years [43]. There was less loss in RNFL thickness in the nasal and temporal quadrants [73, 74]. This age-related decline in normal RNFL measurements needs to be considered when considering or monitoring ocular disease.
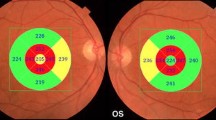
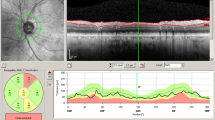
However, as described below, the reduction in RNFL thickness observed in most studies on AD patients was significantly greater than that observed in the age-matched controls and thus cannot be exclusively ascribed to aging (see some illustrative OCT imaging in Fig. 13.1 and a synopsis of published OCT studies in AD and MCI in Table 13.1).
Optical Coherence Tomography (OCT) imaging taken in cylindrical section of tissue surrounding the optic disc of a control eye and of 2 AD patients eyes. The images in the left panel are raw OCT data, while the maps in the right panel are quantitative data curve. In AD patients eyes OCT shows a decrease of RNFL reflection (reduced RNFL thickness) in each examined quadrant, with particular prevalence of the temporal ones
Parisi and colleagues were the first who used OCT to study a group of 17 AD patients affected from mild severity cognitive impairment and compared them with a group of 14 age-matched controls. In AD patients, OCT results showed thinning of the average peripapillary RNFL and in all quadrants examined, showing the involvement of the neuroretinal tissue in AD [75, 76].
The mean RNFL thickness was corroborated to be reduced in AD patients by several independent groups [18, 66, 77,78,79,80,81,82,83,84,85,86,87,88,89]. Patients affected with MCI have also shown some RNFL thinning [81, 86, 88,89,90]. Individual examination of the quadrants was even more revealing. Most studies observed a significant reduction of RNFL thickness in the superior quadrant [18, 66, 75, 76, 79, 80, 83,84,85,86,87,88, 90,91,92], but for some also in the inferior quadrant [66, 75, 76, 79, 80, 84,85,86,87,88, 90,91,92], and for a less extent also in the nasal [75, 76, 80, 84, 86] and temporal [75,76,77, 79, 80, 84,85,86, 88] quadrants. In contradistinction, the preferential involvement of each quadrant is more inconsistent in MCI patients [86, 88, 90]. For the sake of completeness, some researchers did not observe reduced RNFL thickness both in AD [93,94,95,96] and MCI [87, 92,93,94, 96] patients in comparison with controls.
When assessed, the mean ganglion cell layer plus inner plexiform layer thickness (GCL-IPL) thickness was found to be reduced in AD eyes by several independent groups [77, 81, 87], and even in some patients affected with MCI [28]. In one study GCL-IPL thickness was reduced in moderate AD and in frontotemporal dementia, but not in MCI and mild AD patients [81].
Some researchers studied optic nerves of AD patients by using confocal scanning laser ophthalmoscopy (cSLO). Ophthalmoscopy in combination with cSLO, revealed an enlargement in the mean vertical cup-to-disc ratio (clinician and instrument derived), rim volume, rim area, and reduced RNFL thickness in patients with AD with respect to controls [97]. Compared with individuals in the lowest quarter of values of cup-to-disc ratios, those with higher values generally had an increased risk of AD [97]. Lu et al. [66] also observed an enlarged cup-to-disc ratio and a thinner RNFL thickness in their 22 mild to moderate AD patients. In contrast, Kergoat et al. [98] found no differences in the regional distribution of RNFL thickness between patients with mild to moderate AD and healthy controls evaluated with scanning laser polarimetry. The same researchers did not observed optic nerve head structural abnormalities in AD by using real-time topographical images obtained with a Heidelberg retina tomograph (HRT) from individuals in the early stages of AD compared with age-matched controls [98]. The same negative results were obtained by Kurna and coworkers in a group of patients with mild to moderate AD [99]. Despite that, Csincsik et al. [100] used ultra-widefield (UWF) scanning laser ophthalmoscope of the retina to determine phenotypic variations in 59 patients with AD and 48 healthy controls and found significantly higher prevalence of a hard drusen phenotype in the periphery of AD patients compared to controls, as well as changes to the arterial and venular vasculature beyond the posterior pole. The number of optic nerve head drusen increased at the 2 years follow-up measurement in AD patients [100].
Berisha et al. [101] tried to determine whether regional thinning of the RNFL occurred in the nine patients with mild to moderate AD and to determine whether the retinal circulation was abnormal in these patients. AD patients showed a marked narrowing of the retinal venous blood column diameter and a reduction in retinal blood flow rate compared with eight age-matched control subjects. OCT data confirmed a significant thinning of the peripapillary RNFL that was most pronounced in the superior quadrant, which did not correlate with the retinal blood flow [101]. They argued that the mechanisms producing reduced blood flow in the retina are related to those that produce cerebral blood flow abnormalities, which are known to occur in AD, such as increased venous wall thickness due to collagen and β-amyloid deposition [102].
In a cross-sectional study on 150 patients with low-to-moderate dementia and 61 controls, researchers compared the parameters provided by two commercially available spectral domain OCT devices, the Cirrus and the Spectralis OCT instruments [85]. With both devices, they confirmed retinal thinning in AD patients, and they found that the best parameter for distinguishing AD patients from healthy controls using OCT measurements was the Spectralis looking at RNFL [85]. Along these lines, Marziani et al. [84] used both Optovue RTVue-100 and Spectralis instruments to evaluate retinal thickness in 21 patients affected by AD in comparison with 21 healthy controls. Both instruments showed a significant difference in full retinal thickness between patients and controls in all the macular sectors except in the central sector and for the superior external sector [84].
In order to verify whether involvement of the retina is an early event in the course of AD, a few studies compared MCI versus AD patients and healthy aged people. Paquet and coworkers [82] enrolled 23 MCI patients, 14 mild AD, 12 moderate to severe AD patients, and 15 healthy subjects. Overall, mean RNFL thickness was reduced in all patient groups in respect to controls. The subgroup analyses revealed no difference between the results observed in MCI and in mild AD patients, whereas RNFL thickness assessed in moderate to severe AD patients was significantly reduced in respect with that assessed in MCI patients [82]. Kesler et al. [90] obtained similar results finding that the mean RNFL was significantly thinner in both AD and MCI patients groups compared to controls, and that the MCI group fell in between the other two groups. This difference was particularly prominent in the inferior quadrant, whereas the AD patients had significantly thinner retinal NFL values also in the superior quadrant [90].
Some investigators attempted to identify individuals who might be at a high risk of developing AD, and observed that, despite the reduction in peripapillary RNFL thickness, central 1-mm foveal thickness and macular volume were significantly increased in aMCI patients, but not in AD patients, when compared with age-matched controls. The authors argued that retinal swelling might be the first sign of macular retinal ganglion cell degeneration probably due to inflammation and/or gliosis in the early stages of AD [86]. Other independent research groups found decreased thickness of the RNFL in MCI patients, with values sometime equal to [81, 89] or in between [88] the control and AD groups, sometimes accompanied by a reduced macular volume [88]. However, we noticed that 5 studies were unable to find differences between MCI patients and controls [87, 92,93,94, 96] challenging the utility of OCT in differentiation MCI from mild-to-moderate AD.
Two studies, relying both on cross-sectional and longitudinal data, recruited individuals in the preclinical stage of the disease to perform a correlation analysis between retinal structural measurements and positron emission tomography (PET) Aβ binding in the neocortex. In adults with preclinical AD, the Aβ positive group of participants showed higher volume in the IPL in comparison with the Aβ negative group [103]. The same research group found that the mean RNFL volume reduction over 27 months was linearly related to neocortical Aβ accumulation, after controlling for normal aging [104].
Finally, it is worth noting that thinning of RNFL is also present in other types of dementia, such as frontotemporal dementia [81], dementia with Lewy bodies, dementia associated with Parkinson’s disease [105] and with cerebral autosomal dominant arteriopathy with subcortical infarcts and leuco-encephalopathy (CADASIL) [106] challenging the specificity of OCT findings in AD patients.
13.4 Structural-Functional Correlations
The functional activity of the visual system can be dissected by the use of electrophysiological techniques such as pattern electroretinogram (PERG) and visual evoked potentials (VEPs) that have the capability of assessing, respectively, the bioelectrical activity of retinal ganglion cells and their fibers, and the functional integrity of the entire visual pathways from retina to the visual cortex.
The implicit times were delayed and/or the amplitudes reduced [75, 76, 107,108,109,110] in most of the PERG studies performed in AD patients at various stages, reflecting retinal ganglion cell dysfunction. However, in some studies PERG parameters were within the range of normality [111, 112]. In one study luminance pattern ERGs were delayed and amplitudes reduced, whereas isoluminant chromatic ERGs were always within the normal ranges [110].
The implicit time of patterned VEP was normal in some studies [107, 110, 113, 114] and in others delayed [109, 112, 115, 116], with the amplitudes within the range of normality. A delayed P2 component of the flash VEP is a common finding across studies in AD patients [107, 113, 117, 118], with one exception [115].
Taken as a whole, these electrophysiological results are consistent with the early histological studies showing retinal ganglion cell dysfunction in AD [25, 118] and supports the notion that this damage in AD preferentially affects the larger, faster-conducting retinal ganglion cells and their retinocortical projections.
Parisi and coworkers [75, 76] used OCT and PERG in a group of 17 mild severity AD patients compared with a group of 14 healthy controls. They observed that the general reduction of peripapillary RNFL thickness was accompanied with delayed N35, P50 and N95 implicit times and reduced N35-P50 and P50-N95 amplitudes in their AD patients. More interestingly, in AD eyes, the RNFL overall values were positively correlated to the PERG P50 and N95 implicit times and PERG P50-N95 amplitude. This was not so in controls [75, 76]. However, Iseri et al. [91] did not find significant differences in VEP P100 implicit times and N75-P100 amplitudes recorded at high spatial frequency in a group of 14 mild to moderate AD patients when compared with a group of 15 controls. Moreover, they saw no correlation between any of the abnormally in RNFL and macular OCT thickness and VEP in AD [91]. This negative correlation, between VEPs parameters recorded at both high and low spatial frequency and OCT peripapillary scans, was later corroborated [119].
Liu et al. [28] for the first time observed that thinner macular GC-IPL is associated with lower integrity of the white matter microstructure, as verified using MRI diffusion tension imaging, in healthy controls in fronto-parieto-occipital and cingulate regions, as well as with lower grey matter volume in occipital pole and cerebellum. These results in controls were contrast with those obtained in AD and MCI patients where these relationships between macula and brain microstructure and volume were not detectable. The authors suggested that neurodegenerative processes related to the AD and MCI pathology can disrupt the physiological retina-brain coupling [28].
13.5 Correlations with Psychometric Measures
Along with personal history, physical examination and laboratory tests, questionnaires are often used to help test a range of everyday mental skills of persons with symptoms of dementias. Widely used tests include the mini mental state examination (MMSE), the Alzheimer’s disease assessment scale- (ADAS-Cog), clinical dementia rating (CDR) score, and the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS).
The previously mentioned parameters of increased cup-to-disc ratio, cup volume, and decreased optic disc rim area, all found in AD patients by optic nerve analyser, positively correlated with ADAS-Cog scores and disease’s duration [65]. In AD patients, a significant relationship between inferior RNFL thickness change and ADAS-Cog scores change as well as CDR scores at 12 months follow-up was observed. Interestingly, the linear regression model revealed that for every 10 μm decrease of RNFL thickness, there was an accompanying ADAS-Cog increase of 5 units [95].
Moreover, the reduced total macular volume in patients with AD was positively correlated with the severity of the disease as assessed by the MMSE [79, 89, 91, 95, 105]. Other studies failed to find the same associations [82, 83, 90, 119, 120]. However, some authors observed that the thinner the RNFL, ganglion cell and inner plexiform layers the longer the AD disease duration, even if the latter were not significantly related to severity of the disease (assessed with MMSE score) [77]. Nonetheless, RNFL thickness of AD or MCI patients was not associated with other patient demographic features, such as age [82, 85], gender, and AD stage [120]. In another study assessing the association between macular GC-IPL and peripapillary RNFL thicknesses with disease progression in MCI and AD, the CDR scale score exhibited significant negative relationships with the average GC-IPL thickness and GC-IPL thickness in the supero-temporal, supero-nasal, and infero-nasal sectors [121]. Furthermore, the composite memory score exhibited significant positive association with the average GC-IPL thickness and the GCIPL thickness in the supero-temporal, inferonasal, and inferotemporal sectors. A positive association between the central macular thickness and the MMSE score was also observed [121].
More recently, La Morgia et al. [18] with the scope to verify the integrity of a subpopulation of retinal ganglion cells expressing the photopigment melanopsin, observed that clinical parameters (age, age at onset, disease duration, and MMSE score) and OCT data were not correlated to AD patients actigraphic data assessing rest–activity circadian rhythms. There may have been a minimum threshold such that relatively few melanopsin retinal ganglion cells sufficed to maintain circadian rhythms.
In AD patients, but not in aMCI, multiple linear regression models showed a strong association between overall RNFL thickness and MMSE score. The same association was found between MMSE and RNFL thickness in superior and nasal quadrants. Macular thickness and volume were not associated with MMSE score either in aMCI or in AD patients [86].
A prospective study attempted to determine whether deterioration of patients’ cognitive functions, as verified with the RBANS test, correlated to specific OCT findings [122,123,124]. These investigators followed, for a period of 25 months, two groups of subjects: 82 participants with a normal cognitive status and 22 MCI patients. The participants who completed the observational period were further categorized as 60 participants experiencing a stable cognitive status and 18 participants who experienced a worsening of their cognitive status. The investigators found that in those participants who remained stable, greater attenuation of RNFL in the superior quadrant could predict specific cognitive deteriorations. In contradistinction, in participants who worsened, less attenuation of RNFL in the inferior quadrant could predict greater cognitive deterioration [122,123,124]. These findings suggest that patients who have less reduction in the thickness of the inferior quadrant of RNFL over time may have a higher risk of developing MCI and dementia, leading the authors to put forward RNFL thickness as a potential biomarker of dementia.
13.6 Conclusions
The diagnosis of AD is often based on psychometric assessments by a multidisciplinary team (neurologists, psychiatrists, and psychologist) and a neurological examination. Because of inter- and intra-individual variability, neurophysiological and neuroimaging tests have not yet been considered as criteria on which to base a diagnosis. In fact, neurophysiological as well as other paraclinical tests are recommended only on suspicion of secondary causes of dementia as part of the differential diagnosis of AD. The AD diagnosis is confirmed only with post mortem histopathology. Nonetheless, the last few decades have seen the use of structural and functional techniques as potential biomarkers that might also identify factors that may predispose individuals to AD. The optical coherence tomography (OCT) technique for the measurement of the peripapillary RNFL, the macular thickness and volume, has been demonstrated as useful for the demonstration of significant retinal changes in patients that roughly correlate with the severity of the disease (Fig. 13.2).
Particularly intriguing results of recent experiments include:
-
In animal models, expressing mutant forms of amyloid precursors, and histological studies on post mortem AD eyes, researchers have observed various retinal pathological changes, such as accumulation of Aβ aggregates within the retina and its microvasculature, retina ganglion cell death and signs of neuroinflammation.
-
In evaluations with the optic nerve analyser, a higher proportion of AD patients show signs of optic neuropathy manifesting as optic disc atrophy, pathologic optic disc cupping, and thinning of the neuroretinal rim and of the RNFL.
-
In agreement with post mortem studies, OCT data studies indicate a significant decline in peripapillary RNFL and also changes in macular thickness and volume that is progressive from MCI to AD eyes.
-
Electrophysiological and OCT studies in AD patients show RNFL thickness reduction that is related to neuronal degeneration in the retinal ganglion cell layer that is not a consequence of retrograde degeneration from the post-chiasmatic visual pathway.
-
The acquisition in the same patients of OCT and objective measurements of brain microstructure and volume shows that RNFL cannot be used as a surrogate for magnetic resonance imaging measurements of the brain.
-
The correlations between morphological and clinical features tend to suggest a potential role for optic nerve head analysis in monitoring the progression of AD and as outcome measures in the assessment of any purported AD treatments.
Overall, these results suggest that degeneration of the retinal ganglion cells should be added to the constellation of neuropathologic changes found in patients with Alzheimer’s disease.
Other neurodegenerative disorders, like Parkinson’s disease and glaucoma, may enter in the differential diagnosis of RNFL thinning [125]. A particular mention merits the controversial association between primary open angle glaucoma (POAG) and AD. This is based on various indirect evidence of some shared genetic, histopathological and functional features [126, 127]. The association of AD and POAG is poorly supported by epidemiological studies [128, 129]. Nonetheless, for some authors glaucoma could be, in a subgroup of patients, the expression of a neurodegenerative process of the CNS that may be partially influenced by the risk factor of intraocular pressure (IOP) [130, 131]. However, the link between AD and the risk of developing glaucoma persists even without elevated IOP levels [78].
Future research in OCT as a biomarker of AD requires establishing a better specificity of OCT findings in AD. These and similar studies using OCT parameters of retinal changes will be important as objective in vivo outcome measures against which to evaluate new molecules purported to help in the treatment of AD.
Abbreviations
- AD:
-
Alzheimer’s disease
- ADAS-Cog:
-
Alzheimer’s disease assessment scale
- aMCI:
-
Amnestic type of mild cognitive impairment
- APP:
-
Beta-amyloid precursor protein
- ERG:
-
Electroretinogram
- GCL:
-
Ganglion cell layer
- HRT:
-
Heidelberg retina tomograph
- IPL:
-
Inner plexiform layer
- OCT:
-
Optical coherence tomography
- ON:
-
Optic nerve
- PERG:
-
Pattern electroretinogram
- PS1:
-
Presenilin 1
- RBANS:
-
Repeatable Battery for the Assessment of Neuropsychological Status
- RNFL:
-
Retina nerve fiber layer
- VEP:
-
Visual evoked potential
References
Stefanacci R. The costs of Alzheimer’s disease and the value of effective therapies. Am J Manag Care. 2011;13:S356–62.
Alzheimer’s Association. 2016 Alzheimer’s disease facts and figures. Alzheimers Dement. 2016;12:459–509.
Nichols E, Szoeke CEI, Vollset SE, et al. Global, regional, and national burden of Alzheimer’s disease and other dementias, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18:88–106.
Blennow K, de Leon MJ, Zetterberg H. Alzheimer’s disease. Lancet (London, England). 2006;368:387–403.
Waring S, Rosenberg RN. Genome-wide association studies in Alzheimer disease. Arch Neurol. 2008;65:329–34.
Morris J, Storandt M, Miller JP, McKeel DW, Price JL, Rubin EH, Berg L. Mild cognitive impairment represents early-stage Alzheimer disease. Arch Neurol. 2001;58:397–405.
Winblad B, Palmer K, Kivipelto M, et al. Mild cognitive impairment--beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med. 2004;256:240–6.
Litvan I, Goldman JG, Tröster AI, et al. Diagnostic criteria for mild cognitive impairment in Parkinson’s disease: Movement Disorder Society Task Force guidelines. Mov Disord. 2012;27:349–56.
Whitehouse PJ, Price DL, Clark AW, Coyle JT, DeLong MR. Alzheimer disease: evidence for selective loss of cholinergic neurons in the nucleus basalis. Ann Neurol. 1981;10:122–6.
van de Nes JAP, Nafe R, Schlote W. Non-tau based neuronal degeneration in Alzheimer’s disease—an immunocytochemical and quantitative study in the supragranular layers of the middle temporal neocortex. Brain Res. 2008;1213:152–65.
Leuba G, Kraftsik R. Visual cortex in Alzheimer’s disease: occurrence of neuronal death and glial proliferation, and correlation with pathological hallmarks. Neurobiol Aging. 1994;15:29–43.
Armstrong RA. Is there a spatial association between senile plaques and neurofibrillary tangles in Alzheimer’s disease? Folia Neuropathol. 2005;43:133–8.
Leuba G, Saini K. Pathology of subcortical visual centres in relation to cortical degeneration in Alzheimer’s disease. Neuropathol Appl Neurobiol. 1995;21:410–22.
Baloyannis S, Mauroudis I, Manolides SL, Manolides LS. Synaptic alterations in the medial geniculate bodies and the inferior colliculi in Alzheimer’s disease: a Golgi and electron microscope study. Acta Otolaryngol. 2009;129:416–8.
Dugger B, Tu M, Murray ME, Dickson DW. Disease specificity and pathologic progression of tau pathology in brainstem nuclei of Alzheimer’s disease and progressive supranuclear palsy. Neurosci Lett. 2011;491:122–6.
Hinton DR, Sadun AA, Blanks JC, Miller CA. Optic-nerve degeneration in Alzheimer’s disease. N Engl J Med. 1986;315:485–7.
Löffler K, Edward DP, Tso MO. Immunoreactivity against tau, amyloid precursor protein, and beta-amyloid in the human retina. Invest Ophthalmol Vis Sci. 1995;36:24–31.
La Morgia C, Ross-Cisneros FN, Koronyo Y, et al. Melanopsin retinal ganglion cell loss in Alzheimer disease. Ann Neurol. 2016;79:90–109.
Hutton J, Morris JL, Elias JW, Poston JN. Contrast sensitivity dysfunction in Alzheimer’s disease. Neurology. 1993;43:2328–30.
Gilmore G, Whitehouse PJ. Contrast sensitivity in Alzheimer’s disease: a 1-year longitudinal analysis. Optom Vis Sci. 1995;72:83–91.
Crow R, Levin LB, LaBree L, Rubin R, Feldon SE. Sweep visual evoked potential evaluation of contrast sensitivity in Alzheimer’s dementia. Invest Ophthalmol Vis Sci. 2003;44:875–8.
Gilmore G, Wenk HE, Naylor LA, Koss E. Motion perception and Alzheimer’s disease. J Gerontol. 1994;49:P52–7.
Rizzo M, Nawrot M. Perception of movement and shape in Alzheimer’s disease. Brain. 1998;121:2259–70.
Wijk H, Berg S, Sivik L, Steen B. Colour discrimination, colour naming and colour preferences among individuals with Alzheimer’s disease. Int J Geriatr Psychiatry. 1999;14:1000–5.
Wijk H, Berg S, Bergman B, Hanson AB, Sivik L, Steen B. Colour perception among the very elderly related to visual and cognitive function. Scand J Caring Sci. 2002;16:91–102.
Salamone G, Di L, Mosti S, Lupo F, Cravello L, Palmer K, Musicco M, Caltagirone C. Color discrimination performance in patients with Alzheimer’s disease. Dement Geriatr Cogn Disord. 2009;27:501–7.
Sadun A, Bassi CJ. Optic nerve damage in Alzheimer’s disease. Ophthalmology. 1990;97:9–17.
Liu S, Ong Y-T, Hilal S, Loke YM, Wong TY, Chen CL-H, Cheung CY, Zhou J. The association between retinal neuronal layer and brain structure is disrupted in patients with cognitive impairment and Alzheimer’s disease. J Alzheimers Dis. 2016;54:585–95.
Coppola G, Di Renzo A, Ziccardi L, Martelli F, Fadda A, Manni G, Barboni P, Pierelli F, Sadun AA, Parisi V. Optical coherence tomography in Alzheimer’s disease: a meta-analysis. PLoS One. https://doi.org/10.1371/journal.pone.0134750.
Huang D, Swanson EA, Lin CP, et al. Optical coherence tomography. Science (New York, NY). 1991;254:1178–81.
Puliafito C, Hee MR, Lin CP, Reichel E, Schuman JS, Duker JS, Izatt JA, Swanson EA, Fujimoto JG. Imaging of macular diseases with optical coherence tomography. Ophthalmology. 1995;102:217–29.
Yaqoob Z, Wu J, Yang C. Spectral domain optical coherence tomography: a better OCT imaging strategy. BioTechniques. 2005;39:S6–13.
Kiernan D, Mieler WF, Hariprasad SM. Spectral-domain optical coherence tomography: a comparison of modern high-resolution retinal imaging systems. Am J Ophthalmol. 2010;149:18–31.
Schuman JS, Hee MR, Puliafito CA, Wong C, Pedut-Kloizman T, Lin CP, Hertzmark E, Izatt JA, Swanson EA, Fujimoto JG. Quantification of nerve fiber layer thickness in normal and glaucomatous eyes using optical coherence tomography. Arch Ophthalmol. 1995;113:586–96.
Parisi V, Manni G, Gandolfi SA, Centofanti M, Colacino G, Bucci MG. Visual function correlates with nerve fiber layer thickness in eyes affected by ocular hypertension. Invest Ophthalmol Vis Sci. 1999;40:1828–33.
Parisi V, Manni G, Spadaro M, Colacino G, Restuccia R, Marchi S, Bucci MG, Pierelli F. Correlation between morphological and functional retinal impairment in multiple sclerosis patients. Invest Ophthalmol Vis Sci. 1999;40:2520–7.
Parisi V, Manni G, Centofanti M, Gandolfi SA, Olzi D, Bucci MG. Correlation between optical coherence tomography, pattern electroretinogram, and visual evoked potentials in open-angle glaucoma patients. Ophthalmology. 2001;108:905–12.
Kardon RH. Role of the macular optical coherence tomography scan in neuro-ophthalmology. J Neuroophthalmol. 2011;31:353–61.
Sung K, Wollstein G, Bilonick RA, Townsend KA, Ishikawa H, Kagemann L, Noecker RJ, Fujimoto JG, Schuman JS. Effects of age on optical coherence tomography measurements of healthy retinal nerve fiber layer, macula, and optic nerve head. Ophthalmology. 2009;116:1119–24.
Mwanza J, Durbin MK, Budenz DL, Girkin CA, Leung CK, Liebmann JM, Peace JH, Werner JS, Wollstein G. Profile and predictors of normal ganglion cell-inner plexiform layer thickness measured with frequency-domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2011;52:7872–9.
Girkin C, McGwin G, Sinai MJ, et al. Variation in optic nerve and macular structure with age and race with spectral-domain optical coherence tomography. Ophthalmology. 2011;118:2403–8.
Alasil T, Wang K, Keane PA, Lee H, Baniasadi N, de Boer JF, Chen TC. Analysis of normal retinal nerve fiber layer thickness by age, sex, and race using spectral domain optical coherence tomography. J Glaucoma. 2013;22:532–41.
Parikh R, Parikh SR, Sekhar GC, Prabakaran S, Babu JG, Thomas R. Normal age-related decay of retinal nerve fiber layer thickness. Ophthalmology. 2007;114:921–6.
Feuer W, Budenz DL, Anderson DR, Cantor L, Greenfield DS, Savell J, Schuman JS, Varma R. Topographic differences in the age-related changes in the retinal nerve fiber layer of normal eyes measured by Stratus optical coherence tomography. J Glaucoma. 2011;20:133–8.
Lee J, Hwang YH, Lee SM, Kim YY. Age and retinal nerve fiber layer thickness measured by spectral domain optical coherence tomography. Korean J Ophthalmol. 2012;26:163–8.
Hardy J, Allsop D. Amyloid deposition as the central event in the aetiology of Alzheimer’s disease. Trends Pharmacol Sci. 1991;12:383–8.
Braidy N, Muñoz P, Palacios AG, Castellano-Gonzalez G, Inestrosa NC, Chung RS, Sachdev P, Guillemin GJ. Recent rodent models for Alzheimer’s disease: clinical implications and basic research. J Neural Transm. 2012;119:173–95.
Ning A, Cui J, To E, Ashe KH, Matsubara J. Amyloid-beta deposits lead to retinal degeneration in a mouse model of Alzheimer disease. Invest Ophthalmol Vis Sci. 2008;49:5136–43.
Perez S, Lumayag S, Kovacs B, Mufson EJ, Xu S. Beta-amyloid deposition and functional impairment in the retina of the APPswe/PS1DeltaE9 transgenic mouse model of Alzheimer’s disease. Invest Ophthalmol Vis Sci. 2009;50:793–800.
Koronyo-Hamaoui M, Koronyo Y, Ljubimov AV, Miller CA, Ko MK, Black KL, Schwartz M, Farkas DL. Identification of amyloid plaques in retinas from Alzheimer’s patients and noninvasive in vivo optical imaging of retinal plaques in a mouse model. NeuroImage. 2011;1:S204–17.
Koronyo Y, Salumbides BC, Black KL, Koronyo-Hamaoui M. Alzheimer’s disease in the retina: imaging retinal Aß plaques for early diagnosis and therapy assessment. Neurodegener Dis. 2012;10:285–93.
Liu B, Rasool S, Yang Z, Glabe CG, Schreiber SS, Ge J, Tan Z. Amyloid-peptide vaccinations reduce {beta}-amyloid plaques but exacerbate vascular deposition and inflammation in the retina of Alzheimer’s transgenic mice. Am J Pathol. 2009;175:2099–110.
Cordeiro M, Guo L, Coxon KM, et al. Imaging multiple phases of neurodegeneration: a novel approach to assessing cell death in vivo. Cell Death Dis. 2010;1:10.
Cohen R, Rezai-Zadeh K, Weitz TM, et al. A transgenic Alzheimer rat with plaques, tau pathology, behavioral impairment, oligomeric aβ, and frank neuronal loss. J Neurosci. 2013;33:6245–56.
Tsai Y, Lu B, Ljubimov AV, Girman S, Ross-Cisneros FN, Sadun AA, Svendsen CN, Cohen RM, Wang S. Ocular changes in TgF344-AD rat model of Alzheimer’s disease. Invest Ophthalmol Vis Sci. 2014;55:523–34.
Andorfer C, Acker CM, Kress Y, Hof PR, Duff K, Davies P. Cell-cycle reentry and cell death in transgenic mice expressing nonmutant human tau isoforms. J Neurosci. 2005;25:5446–54.
Rodriguez L, Mdzomba JB, Joly S, Boudreau-Laprise M, Planel E, Pernet V. Human tau expression does not induce mouse retina neurodegeneration, suggesting differential toxicity of tau in brain vs. retinal neurons. Front Mol Neurosci. 2018;11:293.
Curcio C, Drucker DN. Retinal ganglion cells in Alzheimer’s disease and aging. Ann Neurol. 1993;33:248–57.
Davies D, McCoubrie P, McDonald B, Jobst KA. Myelinated axon number in the optic nerve is unaffected by Alzheimer’s disease. Br J Ophthalmol. 1995;79:596–600.
Blanks J, Torigoe Y, Hinton DR. Blanks RH retinal pathology in Alzheimer’s disease. I. Ganglion cell loss in foveal/parafoveal retina. Neurobiol Aging. 1996;17:377–84.
Blanks JC, Schmidt SY, Torigoe Y, Porrello KV, Hinton DR. Blanks RHI retinal pathology in Alzheimer’s disease. II. Regional neuron loss and glial changes in GCL. Neurobiol Aging. 1996;17:385–95.
Blanks J, Hinton DR, Sadun AA, Miller CA. Retinal ganglion cell degeneration in Alzheimer’s disease. Brain Res. 1989;501:364–72.
Schön C, Hoffmann NA, Ochs SM, et al. Long-term in vivo imaging of fibrillar tau in the retina of P301S transgenic mice. PLoS One. 2012;7:10.
Hedges TR, Perez G, Speigelman D, Barbas NR, Peli E, Yardley CJ. Retinal nerve fiber layer abnormalities in Alzheimer’s disease. Acta Ophthalmol Scand. 1996;74:271–5.
Tsai C, Ritch R, Schwartz B, Lee SS, Miller NR, Chi T, Hsieh FY. Optic nerve head and nerve fiber layer in Alzheimer’s disease. Arch Ophthalmol. 1991;109:199–204.
Lu Y, Li Z, Zhang X, Ming B, Jia J, Wang R, Ma D. Retinal nerve fiber layer structure abnormalities in early Alzheimer’s disease: evidence in optical coherence tomography. Neurosci Lett. 2010;480:69–72.
Balazsi AG, Rootman J, Drance SM, Schulzer M, Douglas GR. The effect of age on the nerve fiber population of the human optic nerve. Am J Ophthalmol. 1984;97:760–6.
Fortune B, Reynaud J, Cull G, Burgoyne CF, Wang L. The effect of age on optic nerve axon counts, SDOCT scan quality, and peripapillary retinal nerve fiber layer thickness measurements in rhesus monkeys. Transl Vis Sci Technol. 2014;3:2.
Johnson BM, Miao M, Sadun AA. Age-related decline of human optic nerve axon populations. Age (Omaha). 1987;10:5–9.
Repka M, Quigley HA. The effect of age on normal human optic nerve fiber number and diameter. Ophthalmology. 1989;96:26–32.
Sadun AA. Discussion of the effect of age on normal human optic nerve fiber number and diameter. Ophthalmology. 1989;96:31–2.
Bowd C, Zangwill LM, Blumenthal EZ, Vasile C, Boehm AG, Gokhale PA, Mohammadi K, Amini P, Sankary TM, Weinreb RN. Imaging of the optic disc and retinal nerve fiber layer: the effects of age, optic disc area, refractive error, and gender. J Opt Soc Am Opt Image Sci Vision. 2002;19:197–207.
Leung C, Yu M, Weinreb RN, Ye C, Liu S, Lai G, Lam DS. Retinal nerve fiber layer imaging with spectral-domain optical coherence tomography: a prospective analysis of age-related loss. Ophthalmology. 2012;119:731–7.
Celebi A, Mirza GE. Age-related change in retinal nerve fiber layer thickness measured with spectral domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2013;54:8095–103.
Parisi V, Restuccia R, Fattapposta F, Mina C, Bucci MG, Pierelli F. Morphological and functional retinal impairment in Alzheimer’s disease patients. Clin Neurophysiol. 2001;112:1860–7.
Parisi V. Correlation between morphological and functional retinal impairment in patients affected by ocular hypertension, glaucoma, demyelinating optic neuritis and Alzheimer’s disease. Semin Ophthalmol. 2003;18:50–7.
Garcia-Martin E, Bambo MP, Marques ML, Satue M, Otin S, Larrosa JM, Polo V, Pablo LE. Ganglion cell layer measurements correlate with disease severity in patients with Alzheimer’s disease. Acta Ophthalmol. 2016;94:e454–9.
Cesareo M, Martucci A, Ciuffoletti E, Mancino R, Cerulli A, Sorge RP, Martorana A, Sancesario G, Nucci C. Association between Alzheimer’s disease and glaucoma: a study based on Heidelberg retinal tomography and frequency doubling technology perimetry. Front Neurosci. 2015;9:479.
Cunha LP, Lopes LC, Costa-Cunha LVF, Costa CF, Pires LA, Almeida ALM, MLR M. Macular thickness measurements with frequency domain-OCT for quantification of retinal neural loss and its correlation with cognitive impairment in Alzheimerʼs disease. PLoS One. 2016;11:e0153830.
Cunha JP, Proença R, Dias-Santos A, Almeida R, Águas H, Alves M, Papoila AL, Louro C, Castanheira-Dinis A. OCT in Alzheimer’s disease: thinning of the RNFL and superior hemiretina. Graefes Arch Clin Exp Ophthalmol. 2017;255:1827–35.
Ferrari L, Huang S-C, Magnani G, Ambrosi A, Comi G, Leocani L. Optical coherence tomography reveals retinal neuroaxonal thinning in frontotemporal dementia as in Alzheimer’s disease. J Alzheimers Dis. 2017;56:1101–7.
Paquet C, Boissonnot M, Roger F, Dighiero P, Gil R, Hugon J. Abnormal retinal thickness in patients with mild cognitive impairment and Alzheimer’s disease. Neurosci Lett. 2007;420:97–9.
Kirbas S, Turkyilmaz K, Anlar O, Tufekci A, Durmus M. Retinal nerve fiber layer thickness in patients with Alzheimer disease. J Neuroophthalmol. 2013;33:58–61.
Marziani E, Pomati S, Ramolfo P, Cigada M, Giani A, Mariani C, Staurenghi G. Evaluation of retinal nerve fiber layer and ganglion cell layer thickness in Alzheimer’s disease using spectral-domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2013;54:5953–8.
Larrosa J, Garcia-Martin E, Bambo MP, Pinilla J, Polo V, Otin S, Satue M, Herrero R, Pablo LE. Potential new diagnostic tool for Alzheimer’s disease using a linear discriminant function for Fourier domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2014;55:3043–51.
Ascaso F, Cruz N, Modrego PJ, Lopez-Anton R, Santabárbara J, Pascual LF, Lobo A, Cristóbal JA. Retinal alterations in mild cognitive impairment and Alzheimer’s disease: an optical coherence tomography study. J Neurol. 2014;261:1522–30.
Cheung C, Ong Y, Hilal S, et al. Retinal ganglion cell analysis using high-definition optical coherence tomography in patients with mild cognitive impairment and Alzheimer’s disease. J Alzheimers Dis. 2015;45:45–56.
Gao L, Liu Y, Li X, Bai Q, Liu P. Abnormal retinal nerve fiber layer thickness and macula lutea in patients with mild cognitive impairment and Alzheimer’s disease. Arch Gerontol Geriatr. 2015;60:162–7.
Oktem EO, Derle E, Kibaroglu S, Oktem C, Akkoyun I, Can U. The relationship between the degree of cognitive impairment and retinal nerve fiber layer thickness. Neurol Sci. 2015;36:1141–6.
Kesler A, Vakhapova V, Korczyn AD, Naftaliev E, Neudorfer M. Retinal thickness in patients with mild cognitive impairment and Alzheimer’s disease. Clin Neurol Neurosurg. 2011;113:523–6.
Iseri P, Altinaş O, Tokay T, Yüksel N. Relationship between cognitive impairment and retinal morphological and visual functional abnormalities in Alzheimer disease. J Neuroophthalmol. 2006;26:18–24.
Kwon JY, Yang JH, Han JS, Kim DG. Analysis of the retinal nerve fiber layer thickness in Alzheimer disease and mild cognitive impairment. Korean J Ophthalmol. 2017;31:548.
Feke GT, Hyman BT, Stern RA, Pasquale LR. Retinal blood flow in mild cognitive impairment and Alzheimer’s disease. Alzheimer’s Dement (Amsterdam, Netherlands). 2015;1:144–51.
Pillai JA, Bermel R, Bonner-Jackson A, Rae-Grant A, Fernandez H, Bena J, Jones SE, Ehlers JP, Leverenz JB. Retinal nerve fiber layer thinning in Alzheimer’s disease. Am J Alzheimers Dis Other Dement. 2016;31:430–6.
Trebbastoni A, D’Antonio F, Bruscolini A, Marcelli M, Cecere M, Campanelli A, Imbriano L, de Lena C, Gharbiya M. Retinal nerve fibre layer thickness changes in Alzheimer’s disease: results from a 12-month prospective case series. Neurosci Lett. 2016;629:165–70.
Golzan SM, Goozee K, Georgevsky D, et al. Retinal vascular and structural changes are associated with amyloid burden in the elderly: ophthalmic biomarkers of preclinical Alzheimer’s disease. Alzheimers Res Ther. 2017;9:13.
Danesh-Meyer H, Birch H, Ku JY, Carroll S, Gamble G. Reduction of optic nerve fibers in patients with Alzheimer disease identified by laser imaging. Neurology. 2006;67:1852–4.
Kergoat H, Kergoat MJ, Justino L, Chertkow H, Robillard A, Bergman H. An evaluation of the retinal nerve fiber layer thickness by scanning laser polarimetry in individuals with dementia of the Alzheimer type. Acta Ophthalmol Scand. 2001;79:187–91.
Kurna SA, Akar G, Altun A, Agirman Y, Gozke E, Sengor T. Confocal scanning laser tomography of the optic nerve head on the patients with Alzheimer’s disease compared to glaucoma and control. Int Ophthalmol. 2014;34:1203–11.
Csincsik L, MacGillivray TJ, Flynn E, et al. Peripheral retinal imaging biomarkers for Alzheimer’s disease: a pilot study. Ophthalmic Res. 2018;59:182–92.
Berisha F, Feke GT, Trempe CL, McMeel JW, Schepens CL. Retinal abnormalities in early Alzheimer’s disease. Invest Ophthalmol Vis Sci. 2007;48:2285–9.
Jellinger KA. Alzheimer disease and cerebrovascular pathology: an update. J Neural Transm. 2002;109:813–36.
Snyder PJ, Johnson LN, Lim YY, Santos CY, Alber J, Maruff P, Fernández B. Nonvascular retinal imaging markers of preclinical Alzheimer’s disease. Alzheimers Dement. 2016;4:169–78.
Santos CY, Johnson LN, Sinoff SE, Festa EK, Heindel WC, Snyder PJ. Change in retinal structural anatomy during the preclinical stage of Alzheimer’s disease. Alzheimers Dement. 2018;10:196–209.
Moreno-Ramos T, Benito-León J, Villarejo A, Bermejo-Pareja F. Retinal nerve fiber layer thinning in dementia associated with Parkinson’s disease, dementia with Lewy bodies, and Alzheimer’s disease. J Alzheimers Dis. 2013;34:659–64.
Parisi V, Pierelli F, Coppola G, Restuccia R, Ferrazzoli D, Scassa C, Bianco F, Parisi L, Fattapposta F. Reduction of optic nerve fiber layer thickness in CADASIL. Eur J Neurol. 2007;14:627–31.
Katz B, Rimmer S, Iragui V, Katzman R. Abnormal pattern electroretinogram in Alzheimer’s disease: evidence for retinal ganglion cell degeneration? Ann Neurol. 1989;26:221–5.
Trick G, Barris MC, Bickler-Bluth M. Abnormal pattern electroretinograms in patients with senile dementia of the Alzheimer type. Ann Neurol. 1989;26:226–31.
Krasodomska K, Lubiński W, Potemkowski A, Honczarenko K. Pattern electroretinogram (PERG) and pattern visual evoked potential (PVEP) in the early stages of Alzheimer’s disease. Doc Ophthalmol. 2010;121:111–21.
Sartucci F, Borghetti D, Bocci T, Murri L, Orsini P, Porciatti V, Origlia N, Domenici L. Dysfunction of the magnocellular stream in Alzheimer’s disease evaluated by pattern electroretinograms and visual evoked potentials. Brain Res Bull. 2010;82:169–76.
Strenn K, Dal-Bianco P, Weghaupt H, Koch G, Vass C, Gottlob I. Pattern electroretinogram and luminance electroretinogram in Alzheimer’s disease. J Neural Transm. 1991;33:73–80.
Kergoat H, Kergoat MJ, Justino L, Chertkow H, Robillard A, Bergman H. Visual retinocortical function in dementia of the Alzheimer type. Gerontology. 2002;48:197–203.
Philpot M, Amin D, Levy R. Visual evoked potentials in Alzheimer’s disease: correlations with age and severity. Electroencephalogr Clin Neurophysiol. 1990;77:323–9.
Justino L, Kergoat M, Bergman H, Chertkow H, Robillard A, Kergoat H. Neuroretinal function is normal in early dementia of the Alzheimer type. Neurobiol Aging. 2001;22:691–5.
Pollock V, Schneider LS, Chui HC, Henderson V, Zemansky M, Sloane RB. Visual evoked potentials in dementia: a meta-analysis and empirical study of Alzheimer’s disease patients. Biol Psychiatry. 1989;25:1003–13.
Partanen J, Hartikainen P, Könönen M, Jousmäki V, Soininen H, Riekkinen P. Prolonged latencies of pattern reversal visual evoked early potentials in Alzheimer disease. Alzheimer Dis Assoc Disord. 1994;8:250–8.
Coburn K, Ashford JW, Moreno MA. Visual evoked potentials in dementia: selective delay of flash P2 in probable Alzheimer’s disease. J Neuropsychiatry Clin Neurosci. 1991;3:431–5.
Moore N, Tucker KA, Jann MW, Hostetler RM, Coburn KL. Flash P2 delay in primary degenerative dementia of the Alzheimer type. Prog Neuro-Psychopharmacol Biol Psychiatry. 1995;19:403–10.
Kromer R, Serbecic N, Hausner L, Froelich L, Beutelspacher S. Comparison of visual evoked potentials and retinal nerve fiber layer thickness in Alzheimer’s disease. Front Neurol. 2013;4:203.
Kromer R, Serbecic N, Hausner L, Froelich L, Aboul-Enein F, Beutelspacher S. Detection of retinal nerve fiber layer defects in Alzheimer’s disease using SD-OCT. Front Psych. 2014;5:22.
Choi SH, Park SJ, Kim NR. Macular ganglion cell-inner plexiform layer thickness is associated with clinical progression in mild cognitive impairment and Alzheimers disease. PLoS One. 2016;11:e0162202.
Shen Y, Shi Z, Jia R, Zhu Y, Cheng Y, Feng W, Li C. The attenuation of retinal nerve fiber layer thickness and cognitive deterioration. Front Cell Neurosci. 2013;7:142.
Shen Y, Liu L, Cheng Y, Feng W, Shi Z, Zhu Y, Wu W, Li C. Retinal nerve fiber layer thickness is associated with episodic memory deficit in mild cognitive impairment patients. Curr Alzheimer Res. 2014;11:259–66.
Shi Z, Wu Y, Wang M, Cao J, Feng W, Cheng Y, Li C, Shen Y. Greater attenuation of retinal nerve fiber layer thickness in Alzheimer’s disease patients. J Alzheimers Dis. 2014;40:277–83.
Yu J, Feng Y, Xiang Y, Huang J, Savini G, Parisi V, Yang W, Fu X. Retinal nerve fiber layer thickness changes in Parkinson disease: a meta-analysis. PLoS One. 2014;9:10.
Janssen S, Gorgels TG, Ramdas WD, Klaver CC, van Duijn CM, Jansonius NM, Bergen AA. The vast complexity of primary open angle glaucoma: disease genes, risks, molecular mechanisms and pathobiology. Prog Retin Eye Res. 2013;37:31–67.
Ghiso JA, Doudevski I, Ritch R, Rostagno AA. Alzheimer’s disease and glaucoma: mechanistic similarities and differences. J Glaucoma. 2013;22(Suppl 5):S36–8.
Tamura H, Kawakami H, Kanamoto T, Kato T, Yokoyama T, Sasaki K, Izumi Y, Matsumoto M, Mishima HK. High frequency of open-angle glaucoma in Japanese patients with Alzheimer’s disease. J Neurol Sci. 2006;246:79–83.
Ou Y, Grossman DS, Lee PP, Sloan FA. Glaucoma, Alzheimer disease and other dementia: a longitudinal analysis. Ophthalmic Epidemiol. 2012;19:285–92.
Nucci C, Martucci A, Cesareo M, Mancino R, Russo R, Bagetta G, Cerulli L, Garaci FG. Brain involvement in glaucoma: advanced neuroimaging for understanding and monitoring a new target for therapy. Curr Opin Pharmacol. 2013;13:128–33.
Nucci C, Martucci A, Cesareo M, Garaci F, Morrone LA, Russo R, Corasaniti MT, Bagetta G, Mancino R. Links among glaucoma, neurodegenerative, and vascular diseases of the central nervous system. Prog Brain Res. 2015;221:49–65.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Coppola, G., Parisi, V., Manni, G., Pierelli, F., Sadun, A.A. (2020). Optical Coherence Tomography in Alzheimer’s Disease. In: Grzybowski, A., Barboni, P. (eds) OCT and Imaging in Central Nervous System Diseases. Springer, Cham. https://doi.org/10.1007/978-3-030-26269-3_13
Download citation
DOI: https://doi.org/10.1007/978-3-030-26269-3_13
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-26268-6
Online ISBN: 978-3-030-26269-3
eBook Packages: MedicineMedicine (R0)