Abstract
Since the publication of the “The Ecology of Browsing and Grazing” (Gordon and Prins, The ecology of browsing and grazing. Springer, 2008), a number of researchers have taken the approach outlined in the book to assess the impacts of differences in food and nutrient supply on the ecology of other vertebrate taxa. In line with the slightly altered emphasis of the current book (The Ecology of Browsing and Grazing II), we also asked the authors of the Sections in this Chapter to provide insights into the impacts that these different vertebrate taxa have on the ecosystems in which they exist. As you will see, the depth of research on the ecology and impacts of the different herbivorous vertebrate taxa varies considerably and demonstrates the importance of further research endeavours, on herbivore/plant interactions, across the board.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Since the publication of the “The Ecology of Browsing and Grazing” (Gordon and Prins 2008), a number of researchers have taken the approach outlined in the book to assess the impacts of differences in food and nutrient supply on the ecology of other vertebrate taxa. The approach may not work in all vertebrate taxa but understanding the similarities and differences between herbivorous vertebrate taxa provides ecologists with a broader canvas upon which to develop and test hypotheses about herbivore/plant interactions. In line with the slightly altered emphasis of the current book (The Ecology of Browsing and Grazing II), we also asked the authors of the Sections in this Chapter to provide insights into the impacts that these different vertebrate taxa have on the ecosystems in which they exist. In this Chapter, the authors describe the findings from this research. As you will see, the depth of research on the ecology and impacts of the different herbivorous vertebrate taxa varies considerably and demonstrates the importance of further research endeavours, on herbivore/plant interactions, across the board. The taxa covered are:
-
Dinosaurs (Jordan Mallon)
-
Fish (Laura D. Puk)
-
Reptiles (Everton B. P. Miranda and Carolina Starling-Manne)
-
Birds (René van der Wal)
-
Marsupials (Ben Moore and William Foley)
-
Lagomorphs (Lucy Lush)
-
Rodents (Renan Maestri)
-
Primates (Ikki Matsuda and Marcus Clauss)
15.1 Dinosaurs
The browser-grazer continuum is not one that readily applies to the non-avian dinosaurs (hereafter, simply ‘dinosaurs’). Although grasses (Poaceae) had evolved by the Cretaceous (Prasad et al. 2005), they were not abundant and did not form widespread grasslands until the Miocene (Potts and Behrensmeyer 1992). It, therefore, makes little sense to speak of dinosaurian ‘grazers’ sensu stricto (but see ‘True Dinosaurian Grazers?’ below). Rather, lycopods, ferns, sphenopsids, cycadophytes, ginkgos, conifers, and (non-poaecean) angiosperms made up the bulk of the plant material available for dinosaurian consumption (Gee 2011; Tiffney 2012). Ferns, in particular, likely filled the role of low growing, herbaceous colonizers for most of the Mesozoic (Wing et al. 1993; Collinson 1996). For this reason, it makes more sense to speak of herbivorous dinosaurs in terms of concentrate, intermediate, and bulk feeders (sensu Hofmann and Stewart 1972, Mallon and Anderson 2014a).
15.1.1 Size and Shape
Whether particular herbivorous dinosaurs were concentrate, intermediate, or bulk feeders would have been primarily influenced by their respective body sizes (Peters 1983). Dinosaur body mass spanned seven orders of magnitude (Benson et al. 2014), so these animals undoubtedly adopted a variety of feeding strategies. Small (10–100 kg) herbivores—including heterodontosaurids, small ornithopods, early thyreophorans, most pachycephalosaurs, and basal ceratopsians, among others—almost certainly concentrated on nutritious shoots, fruits, and seeds to fuel a relatively high metabolism (Weishampel 1984). These dinosaurs were obligatory bipeds, and possessed narrow, pointed beaks with which to selectively crop their food (Coe et al. 1987). Their teeth, and associated jaw mechanics, varied from the simple to the complex, indicative of corresponding variability in dietary fibre intake (Norman and Weishampel 1985; Nabavizadeh 2016) (Fig. 15.1a). Some derived clades developed rudimentary tooth batteries having a single, continuous occlusal surface (e.g., Norman et al. 2011). The jaw joint was depressed below the plane of occlusion, increasing the lever arm of the external mandibular adductor musculature, enabling a more powerful bite. This system was functionally equal, but mechanically opposite, to that of ungulates , where the jaw joint is positioned above the occlusal plane to increase the lever arm of the masseter musculature (Greaves 1995). One lineage, the Ornithopoda, is traditionally thought to have developed a ‘pleurokinetic’ skull having multiple, mobile intracranial joints, allowing the upper jaw to flex laterally during tooth occlusion, to accommodate the lower cheek teeth . Given the isognathous nature of the jaws (where the teeth occlude on both sides when the jaws are closed), this would have resulted in a transverse power stroke, functionally analogous to that of ungulates (Norman and Weishampel 1985). This hypothesis has recently received some push-back—Rybczynski et al. (2008) and Cuthbertson et al. (2012) showed that the secondary intracranial movements imposed by the pleurokinetic model could not be accommodated by the corresponding joints. Rather, minimal rotation of the lower jaw rami, about their long axes, would have produced a similar power stroke, and is mechanically more feasible (Nabavizadeh and Weishampel 2016).
Herbivorous dinosaur ecomorphology . A, Herbivorous dinosaur craniodental adaptations, with beaks and teeth in dark grey (clockwise from upper left): heterodontosaurid, ankylosaur, stegosaur, sauropod, ceratopsian, hadrosaur (skulls not to scale). B, Herbivorous dinosaur feeding heights through time. Sauropodiform feeding heights (assuming vertical necks) were calculated following Upchurch and Barrett (2000). The methodology of Mallon et al. (2013) was used for calculating bipedal feeding heights for basal sauropodomorphs, basal ornithischians, heterodontosaurids, pachycephalosaurs, basal ceratopsians, and ornithopods, and quadrupedal feeding heights for stegosaurs, ankylosaurs, and derived ceratopsians (ceratopsids). Dashed lines indicate missing data. Herbivorous theropod groups (e.g., Ornithomimosauria, Therizinosauria) are not shown due to space restrictions. Taxonomic abbreviations: AL, Alamosaurus; AC, Acrotholus; AG, Agilisaurus; AN, Ankylosaurus; AU, Auroraceratops; BA, Bactrosaurus; BR, Brachiosaurus; CA, Camptosaurus; CE, Cetiosaurus; CH, Chaoyangsaurus; CN, Centrosaurus; ED, Edmontonia; EO, Eocursor; ER, Europelta; ET, Eotrachodon; EU, Euhelopus; FR, Fruitadens; FU, Futalongkosaurus; GA, Gargoyleosaurus; GB, Gobisaurus; GO, Gongxianosaurus; GR, Graciliceratops; GS, Gastonia; HA, Haya; HE, Heterodontosaurus; HN, Hungarosaurus; HU, Huayangosaurus; HY, Hypselospinus; IG, Iguanodon; IS, Isaberrysaura; JE, Jeholosaurus; JI, Jinzhousaurus; KO, Kotasaurus; LE, Leaellynasaura; LO, Loricatosaurus; LU, Lufengosaurus; MA, Mamenchisaurus; MC, Macrogryphosaurus; ME, Melanorosaurus; MG, Magnapaulia; MI, Miragaia; MN, Manidens; MO, Mosaiceratops; MY, Mymooropelta; NE, Neuquensaurus; OM, Omeiosaurus; OR, Orodromeus; OT, Othnielosaurus; OU, Ouranosaurus; PA, Panphagia; PC, Pachycephalosaurus; PK, Parksosaurus; PL, Plateosaurus; PR, Prenocephale; PS, Psittacosaurus; PT, Patagotitan; SA, Sauropelta; SC, Scutellosaurus; SE, Stenopelix; SH, Shantungosaurus; SP, Spinophorosaurus; ST, Stormbergia; TA, Talarurus; TI, Tianyulong; TL, Talenkauen; TR, Triceratops; WU, Wuerhosaurus; YI, Yinlong; YU, Yunnanosaurus; ZU, Zuniceratops. Other abbreviations: Ma, Mega-annum. Silhouette credits: R. Amos, A. Farke, S. Hartman, N. Tamura, M. Taylor, E. Willoughby
At the opposite end of the size spectrum, megaherbivorous dinosaurs (≥ 1 × 103 kg; sensu Owen-Smith 1988) included most sauropodomorphs, stegosaurs, ceratopsids, ankylosaurs, and iguanodontians. These forms were highly variable in morphology , and shared few ecomorphological features in common beyond large body size and minimally facultative quadrupedality. Stegosaurs and ankylosaurs retained primitively small, phylliform teeth, although the beaks of the latter were wider, and presumably predisposed to bulk feeding (Mallon and Anderson 2014a; Ősi et al. 2017). The exceptionally wide bellies of the ankylosaurs attest to lengthy gut-retention times and a preference for fibrous roughage (Bakker 1986).
Iguanodontians and ceratopsids both possessed complex dental batteries, but these functioned in quite different ways. Iguanodontian dental batteries, best exemplified by the hadrosaurids, consisted of hundreds of highly complex, tightly-spaced teeth, multiple rows of which contributed to the occlusal surface (Erickson et al. 2012; LeBlanc et al. 2016). This inclined surface was capable of both crushing and shearing functions. Tooth wear evidence further suggests some capacity for fore-aft grinding (Williams et al. 2009; Mallon and Anderson 2014b). This suite of functions would have allowed the hadrosaurids to rend all manner of plant types, substantiating their inferred role as herbivore generalists. By contrast, the tooth batteries of the ceratopsids were simpler, and the continuous occlusal surface of the teeth was limited to the vertical plane. Thus, ceratopsids were evidently more restricted in their diets, which is consistent with their narrow, selective beaks (Mallon and Anderson 2014a). In this sense, ceratopsids might be likened to the narrow-lipped black rhinoceros, Diceros bicornis, which selects for low-growing woody scrub (Owen-Smith 1988).
Largest of all were the sauropodomorphs, with some forms possibly approaching 90 tonnes (Benson et al. 2014). These were characterized by exceedingly long necks ending in proportionally tiny heads. The jaws were simple in construction, and the teeth were primitively small and leaf-shaped. Some derived forms (e.g., diplodocids) had simple, peg-like teeth restricted to the front of the jaws; these would have been mechanically ideal for stripping branches of twigs and leaves (Young et al. 2012). Other contemporaneous sauropods (i.e., camarasaurids) had mouths full of robust, chisel-like teeth capable of delivering higher, sustained bite forces, probably for ingestion of harder foodstuffs (Button et al. 2014). Nigersaurus taquietti is distinctive in having an unusually broad, flat muzzle lined with a battery of pencil-like teeth (Sereno et al. 2007). This configuration was almost certainly an adaptation for bulk feeding, low to the ground.
Unfortunately, the ecology of most other sauropods is not so easily discernible. Most problematic has been the use of their long necks, and debate has waged over whether they were held horizontally or more nearly vertically. Proponents of the first view maintain that the cervical articulations prevented the neck from being elevated very much (Stevens and Parrish 2005a, b), that the blood pressure necessary to perfuse the brain, with the neck held in a vertical position, would have been dangerously high (Seymour 2009), or that the neck was mechanically more efficient while in a horizontal posture (Woodruff 2017). Proponents of the second view retort that the cervical vertebrae alone are not good indicators of neck posture in modern taxa (Taylor et al. 2009), that stresses distributed through an upright neck would not have been prohibitive (Christian 2010), and that partitioning of the forest strata would have made ecological sense in sauropod-dominated communities (Bakker 1978) (Fig. 15.1b), as it does among ruminants today (du Toit 1990). The debate is, as yet, unsettled, and the arguments grow increasingly nuanced.
15.1.2 Energetics
Dinosaur energetics are among the most difficult aspects of their biology to glean from the fossil record , but palaeontologists are anything but faint of heart. Although a range of educated assumptions must invariably be made, these serve to constrain interpretations within the realm of possibility. Dinosaur energetics are typically considered from the perspectives of supply and demand. On the supply side, Hummel et al. (2008) investigated the suitability of various Mesozoic (non-angiosperm) plants as sauropod fodder by subjecting samples of related living tissue to in vitro fermentation experiments , using gas production as a measure of metabolizable energy, while also quantifying other nutrients. Horsetails, Equisetum spp., proved to be the most nutritious, having high levels of both metabolizable energy and crude protein (the high silica content was likely of little concern to those dinosaurs that relied on gut processing). Araucaria spp., Ginkgo spp. and Angiopteris spp. were also likely dietary staples, whereas cycads, tree ferns, and podocarps proved to be poor sources of energy.
On the demand side, the work of Farlow (1976) and colleagues (Farlow et al. 2010) is instructive. It is possible to bracket the possible energy requirements of dinosaurs via consideration of how those requirements scale with body size in both reptilian ectotherms and mammalian endotherms (birds , although descended from dinosaurs, are likely poor models for dinosaur energetics because their physiology is fine-tuned to the aerial realm). Thus, a single adult Brontosaurus excelsus, weighing 3 × 104 kg, might have needed anywhere from 2 × 104 to 50 × 104 kJ of energy per day, depending on the animal’s physiology (Farlow et al. 2010). By combining both supply and demand sides of the model, it is possible to further estimate the population density of B. excelsus on a Jurassic landscape, which varies from a few large adults.km−2 (endotherm model) to a few tens of large adults.km−2 (ectotherm model). Given that the most diverse sauropod communities of the Jurassic were situated in semi-arid climates at mid-latitudes (Noto and Grossman 2010; Mannion et al. 2011), primary productivity is unlikely to have been very high, and sauropods may have needed to seasonally migrate to find appropriate sustenance. Evidence for such migration, between lowland and upland environments, is preserved as oxygen isotope excursions in tooth enamel (Fricke et al. 2011).
15.1.3 True Dinosaurian Grazers?
In spite of the foregoing discussion, there do exist rare examples of grass-eating in the dinosaur fossil record . The first concerns a report of grass phytoliths found inside coprolites (fossil dung) attributed to Late Cretaceous titanosaurian sauropods from India (Prasad et al. 2005), based on their common association with diagnostic skeletal material. The variety of phytolith morphologies is suggestive of the presence of up to five different grass species, including relatives of modern rices, bamboos, and cool-season grasses. Other phytoliths, found within the same coprolites, are attributable to dicotyledons, conifers, and palms. Variation in phytolith preservation implies that some were purposefully ingested as forage, whereas others (particularly from the rarely preserved palms) were consumed incidentally.
A more recent example (Wu et al. 2017) concerns the discovery of grass phytoliths and silicified epidermis within the purported dental calculus (plaque) of the Early Cretaceous basal hadrosauroid Equijubus normani from China. These structures are assigned to Poaceae, based on the short-long cellular patterning and short-cell pairing of the epidermis, and on the equidimensional unlobed and slightly lobed morphologies of the phytoliths.
The presence of grass phytoliths in Cretaceous deposits is somewhat unexpected, as it was long held that these defensive structures evolved in response to later mammal grazing during the Miocene (Strömberg 2004). Could grass phytoliths have evolved in response to dinosaur herbivory instead? It seems unlikely; phytoliths are primarily thought to work by exacerbating tooth wear (Strömberg et al. 2016), which is hardly a concern for reptiles with unlimited tooth replacements, including dinosaurs. In the case of sauropods, these animals are unlikely to have done much oral processing, opting instead for hindgut fermentation (Hummel and Clauss 2011). The dental battery of Equijubus normani, and other iguanodontians, may have been well-suited for rending grass, but chance finds of gut and fecal contents in these animals indicates a quite varied diet, including conifer and angiosperm twigs and stems, bark, seeds, leaves, and even crustaceans (Currie et al. 1995; Tweet et al. 2008; Chin et al. 2017). There is, as yet, no solid evidence that dinosaurs co-evolved with any group of angiosperms (Barrett and Willis 2001; Butler et al. 2009). Rather, grass phytoliths may have evolved in response to predation from the hypsodont gondwanatherian mammals or insects (Prasad et al. 2005).
15.2 Fishes
Herbivorous fishes are found in both marine and freshwater ecosystems but are much more abundant in the marine environment. While in terrestrial ecosystems ‘grazing’ and ‘browsing’ refer to the feeding on monocotyl and dicotyl plants, in marine ecosystems, ‘grazing’ and ‘browsing’ refer to the removal of different kinds of algae (Green and Bellwood 2009). Marine grazing fishes remove small filamentous turf algae and marine browsing fishes remove larger macroalgae (Green and Bellwood 2009). In contrast to terrestrial ecosystems, ‘real’ grazing on angiosperms is rare and restricted to only one plant group: seagrasses (Beer 1989). In freshwater ecosystems the terminology is different yet again. The term ‘grazer’ is used for both plant—(‘macrophytes’; Fowler and Robson 1978) and algal—(‘periphyton’, Power 1983) removing fishes, whereas the term ‘browser’ is not used at all.
Where a distinction between grazing and browsing fishes is made, they are faced with different challenges in terms of intake and digestion of their respective food source. Grazers feeding on turf algae ingest a low energy but relatively easily digestible and abundant resource. Browsers feeding on macroalgae on the other hand are faced with a resource that provides more energy, but exhibits chemical and structural defences, is difficult to digest, and patchily distributed. Both groups exhibit adaptations to their respective resources, including differing home range sizes (Nash et al. 2013; Welsh and Bellwood 2014), bite rates (Randall 1967; Burkepile and Hay 2010), and digestive mechanisms (Clements et al. 2009). Browsers are often larger in body size than grazers, potentially enabling them to take bigger, stronger bites (Bonaldo and Bellwood 2008; Lokrantz et al. 2008). They also have larger home ranges (Nash et al. 2013), which helps them locate their food and they take less frequent bites (Cardoso et al. 2009; Burkepile and Hay 2010), because their resource is more energy-dense. After ingestion, browsing fishes utilise fermentation to break down the macroalgal cell walls to access the nutrients (Clements et al. 2009); a mechanism not required by grazers.
While the importance of herbivorous fishes in marine ecosystems has been recognized for a long time, research in freshwater systems mainly focused on insects (Lodge et al. 1998) but is now recognizing the impact of herbivorous fishes on macrophytes (Lodge 1991; Lodge et al. 1998). Herbivorous fishes hold crucial functions in both freshwater and in marine systems, albeit of a different nature. In freshwater systems, they contribute significantly to seed dispersal in seasonally flooded forest ecosystems (Anderson et al. 2011; Horn et al. 2011), exhibiting some of the longest dispersal distances discovered in animals, including African hornbills and Asian elephants (Anderson et al. 2011). However, due to their removal of macrophytes, they can increase phytoplankton biomass and shift lakes towards a turbid state if certain conditions are met (Van Donk and Otte 1996). Grazing and browsing fishes exhibit their highest diversity in marine ecosystems, especially on coral reefs (Gaines and Lubchenco 1982), which has led to a research focus on these ecosystems. Here, herbivorous fishes are considered to provide a key ecosystem function because they control algae, which compete with the primary reef builder—corals.
On coral reefs, herbivorous fishes can consume up to 90% of the primary production (Carpenter 1986; Polunin and Klumpp 1992) and, therefore, play a major role in controlling the reef state. Grazers are more abundant than browsers (Choat et al. 2004), and coupled with their higher feeding rates (Burkepile and Hay 2010), they are responsible for consuming most of the primary production (Burkepile and Hay 2010). Coral reefs dominated by corals are considered ‘healthy’, but if grazing pressure is reduced, they often become dominated by algae (Hughes et al. 2007; Webster et al. 2015). Grazing pressure can be reduced due to overfishing, which is simulated experimentally by excluding fishes with cages (Bellwood et al. 2006; Webster et al. 2015), or due to coral bleaching events which kill vast amounts of corals, freeing up space for algal settlement (Hoegh-Guldberg 1999). Sudden increased availability of space reduces the grazing pressure per unit area on a reef (Williams et al. 2001; Mumby et al. 2007; Mumby 2009), because the existing fish community now has to distribute its feeding activity over the additional free substrate. Once a reef is dominated by algae a return to a coral-dominated state is difficult as macroalgae inhibit coral recruitment (Kuffner et al. 2006; Webster et al. 2015) and survival (Box and Mumby 2007; Webster et al. 2015). Algal-dominated reefs provide less ecosystem services to people compared to their healthy coral-dominated counterparts (Moberg and Folke 1999).
Shifts to algal-dominated coral reefs are projected to increase in the future due to climate change (Hoegh-Guldberg 1999). Higher temperatures cause a higher frequency of coral bleaching events and subsequent mass coral mortality (Hoegh-Guldberg 1999; Hughes et al. 2018). Additionally, algae experience a net positive physiological effect with rising temperatures, whereas corals experience a net negative physiological effect (Elfwing and Tedengren 2000). This could shift the competitive interaction further in favour of algae. However, grazing intensity is greater at higher temperatures (Smith 2008) and may counteract the increased primary production (O’Connor 2009). Higher production and herbivory can strengthen the producer-consumer interaction (O’Connor 2009) which may become a feature of future ecosystems. A strengthening of this interaction could increase the importance of herbivorous fish for the health of consumer-dominated ecosystems such as coral reefs even further.
While the state of coral reefs is heavily controlled by herbivorous consumers, other ecosystems are less dependent on consumers and instead producer-dominated (Connell et al. 2011). One example are kelp forests, common temperate marine ecosystems, where native herbivores (mostly invertebrates such as sea urchins) exhibit only minor control over the ecosystem state (Connell et al. 2011). However, rising temperatures cause a range expansion of tropical herbivorous fishes, which could have detrimental effects on kelp forests (Vergés et al. 2014). After a kelp die-off in Western Australia, tropical browsing fish (kyphosids) that had moved into higher latitudes consumed kelp and created a new barren ecosystem state dominated by turf algae (Bennett et al. 2015). Herbivorous fishes may, therefore, shift the production-dominated temperate marine systems towards consumption-dominated alternative states (Bennett et al. 2015). The strengthening of the producer-consumer interaction (O’Connor 2009), and the switch from production-dominated to consumer-dominated temperate systems (Bennett et al. 2015), suggest a stronger influence of grazers and browsers on future marine ecosystems. Only time will tell.
Similarly strong changes may occur in freshwater ecosystems. Increased grazing, for example through higher metabolic rates associated with increased temperatures, could destabilize the clear-water macrophyte-rich state of lakes and shift them to a phytoplankton-dominated turbid state (Van Donk and Otte 1996). Additionally, increased frequencies of droughts may decrease the number or intensity of floods in forest ecosystems that experience substantial seed dispersal by fishes (Horn et al. 2011). This could decrease the dispersal of plants, especially upstream (Horn et al. 2011). Overfishing can further intensify the problem as bigger fish are often targeted first but they are also the most effective long-distance dispersers (Anderson et al. 2011). Whether the role of fish as a disperser is important enough to make their disappearance noticeable in the forest community structure is currently unknown.
The potentially significant future ecosystem changes connected to herbivorous fishes highlight the importance of understanding their ecosystem function in detail. The study of the role of browsing and grazing fishes is still in its infancy and there is a lot to be learnt from studies on terrestrial ecosystems. However, fish herbivory is likely to differ from terrestrial herbivory because producers and consumers are structurally and functionally distinct from their terrestrial counterparts. Research should focus on understanding the drivers of food choice in herbivorous fishes, how climate change and habitat degradation influence interactions between herbivores and their ecosystems , and identify potential feedbacks .
15.3 Reptiles
Among reptiles , lizards and chelonians are the only modern groups that strictly, or mostly, feed on plant-matter (King 1996). Snakes are obligate carnivores , and crocodiles have evolved herbivory habits a few times in their evolutionary history (Kley et al. 2010; Fiorelli et al. 2016), but there is no modern herbivorous crocodile species. However, a large diversity of chelonian and lizard species is adapted to feed on plants, with several insular radiations that represent most of this diversity.
Given the physiological demands of digesting cellulose and high-fibre plant material, reptile species specialized for grazing and browsing are typically large-sized tropical or sub-tropical species (Pough 1973; Cooper and Vitt 2002; but see Espinoza et al. 2004). The latitude constraint is caused by temperature-dependent limitations to fermentation rates by bacteria in the gut, which require high temperatures to perform their activities (Cooper and Vitt 2002). Being ectotherms, reptiles do not maintain their body temperature stable, and would likely lose their microbial symbionts over cold winters, so full herbivore reptiles are usually restricted to tropical and subtropical climates. In terms of size, large ectotherms become two to three times smaller per each 10 °C of decrease in ambient temperature (Makarieva et al. 2005), due to metabolic constraints. There is also the need of having enough space to allow the fermentation of large amounts of fibre and cellulose (Pough 1973), so herbivory in ectotherms usually requires a large body size.
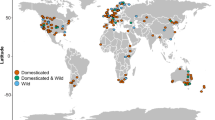
Consequently, many of the largest continental species of reptiles, and the megafauna in islands, are herbivorous lizards and chelonians (Hansen and Galetti 2009). Martin (1984) defined megafauna as animals above 44 kg, but throughout this section we will be using an operational concept of megafauna, which is animals whose evolution is constrained by the size of the land area each species inhabits (Burness et al. 2001; Hansen and Galetti 2009). Also, it is worth noting that reptiles are the vertebrates that achieve some of the highest densities and biomasses in terrestrial ecosystems, reaching up to around 600 kg.ha−1 (Arce-Nazario and Carlo 2012; Lovich et al. 2018; Fig. 15.2). Since the grazing and browsing pressure of an herbivore is magnified by its density and biomass, in some ecosystems the ecological role of reptiles is also magnified.
Densely packed green iguana (Iguana iguana) populations occur across a wide area of Neotropical forests. In this photo, several adult males display during the breeding season. http://www.thinkstockphotos.ca/image/stock-photo-iguanas-in-a-tree-in-mexico/464974882
15.3.1 Diversity
15.3.1.1 Insular
Some of the most conspicuous kinds of grazing and browsing reptiles are giant insular tortoises and iguanas (Gerlach 2014; Hastings et al. 2014). The colonization restrictions of islands normally results in a “reptile-only” megafauna (Hansen and Galetti 2009). In these ecosystems, tortoises have evolved, on multiple occasions, domed shells when grazing over grasslands and craning necks and saddleback shells when browsing over trees, shrubs, cacti, and palms. In the past, giant tortoises inhabited climates ranging from mediterranean to tropical climates, including archipelagos in the Mediterranean sea, and the Caribbean, Indic and Pacific oceans ( Turtle Extinctions Working Group 2015). Today, there are extant island giant tortoise populations of only two species complexes, in the Galápagos Islands (Chelonoidis niger spp.) and in the Seychelles Islands (Aldabracheys gigantea; Hansen et al. 2010).
Another group of browsing and grazing reptiles are iguanas (Cooper and Vitt 2002). They represent a diverse group (Uetz et al. 2016), with three insular radiative adaptations in the Caribbean (nine species in the Cyclura genus), the islands of Fiji and Tonga (six species in the Brachylophus and Lapitiguana genus, from which the two largest species are extinct), and the Galápagos archipelago (three terrestrial species from the Conolophus genus). As is the case with their continental counterparts, insular iguanas are mainly browsers, also being important for seed dispersal (Laurel et al. 2000; Meehan et al. 2002; Traveset et al. 2016). To complete the list, two other insular browsers are the monkey tailed lizard, Corucia zebrata, from the Solomon Islands, and the lacertid lizards, Galotia spp., from Mediterranean islands (Espinoza et al. 2004).
15.3.1.2 Continental
Continental browsing by lizards is restricted to iguanids in the Neotropics, such as the widespread green iguana, Iguana iguana, and by other species that are not so specialized on leaf or grass material, such as Ctenosaurus, Sauromalus, and Dipsosaurus (Vitt and Caldwell 2013). The only significant species outside the Neotropics are the mostly-grazing desert-specialists in the genus Uromastyx (Wilms et al. 2010), and the partial-browsers of the genus Hydrosaurus.
Large continental grazing chelonians (heavier than 44 kg) used to be found on all continents apart from Antarctica. As with most of the megafauna, they are now extinct in most of their historical range, except for the African continent (Martin 1984; Turtle Extinctions Working Group 2015). For herbivorous reptiles, the extinctions were caused mainly by direct and indirect effects of human action, including over-exploitation, and pressures exerted by invasive species (Turtle Extinctions Working Group 2015; Slavenko et al. 2016). Large continental grazing tortoises can only be found today in Africa (Turtle Extinctions Working Group 2015), likely due to their history of co-existence with hominids (Klein 1984). There are two widely known species of tortoises that fit this role, the leopard tortoise, Stygmochelis pardalis, and the spurred tortoise, Centrochelys sulcata. While the former may weigh up to 48 kg, and feeds almost exclusively on grasses (Branch et al. 1990), spurred tortoises are truly specialized grazers, that weight up to 96 kg (Lambert 1993), and are the last representatives of giant grazing tortoises on continental ecosystems. A few other smaller grazing tortoise species still inhabit continents, such as the Bolson tortoise, Gopherus flavomarginatus, from Mexico, the yellow-footed tortoise, Chelonoidis denticulatus, from South America and the Asian forest tortoise, Manouria emys, from Southeast Asia. Among the extinct giant species of continental tortoises are the Chelonoidis lutzae in South America, Hesperotestudo spp. in North America, Meiolanids (horned turtles) in Australia and the Megalochelys spp. in Asia (estimated weight: 1000 kg; Badam 1981)—being the latter the largest tortoises to have ever lived (Turtle Extinctions Working Group 2015).
15.3.1.3 Marine
Green sea turtles, Chelonia mydas, play a conspicuous role as a marine grazer, paralleling grazing ungulates in many aspects of their ecology (Christianen et al. 2014). They have some degree of endothermy which allows them to graze on water temperatures around 20 °C (Heath and Mcginnis 1980). Furthermore, two species of herbivorous reptiles are known as being associated with marine habitats. First, there is the marine iguana from the Galápagos Islands, which is a grazer, feeding on the rocky subtidal and intertidal zones of the archipelago (Shepherd and Hawkes 2005). Marine iguanas have tight schedules of basking and foraging—the second being usually restricted to only 1 h a day—which allows them to graze on the cold Galapagos waters. A second species, closely associated to marine ecosystems, is Ctenosaura bakeri (Köhler 2004), a browsing iguana, endemic to the mangroves of the Utila Island, in Honduras, in the Caribbean.
15.3.2 Effects on Vegetation Composition and Dynamics
Giant tortoises are highly influential on both insular and continental ecosystems, being best known as important seed dispersers (Falcon et al. 2018). They also play the, perhaps less appreciated, role of grazers and browsers on vegetation, affecting vegetation community composition and structure (Lovich et al. 2018).
Grazing herds of giant tortoises reportedly create and maintain a grassland ecosystem called tortoise turf on islands in the Indian Ocean (Gibson and Hamilton 1983; Cheke and Hume 2010). Composed of grasses, sedges and herbs, tortoise turf occurs in areas of high-tortoise concentration and, therefore, heavy grazing. Many of its characteristic plant species are dwarfs with highly specialized growth strategies (e.g., flowers and fruits produced at the base of the plants; Merton et al. 1976). This is a dominant ecosystem in the Seychelles and used to be widespread at the Mascarene Islands (Cheke and Hume 2010; Griffiths et al. 2010). Tortoise terraforming is, in its turn, important to other insular species of plants and animals (Griffiths et al. 2010, 2011, 2013). Many plants in the Mascarene archipelago have two kinds of foliage, from which their low-hanging leaves have adaptations to lower or no levels of herbivory by tortoises (Eskildsen et al. 2004). This phenomenon illustrates the high grazing pressure that can be exerted by large herds of tortoises. Tortoise-exclusion experiments are in operation on Aldabra, and are likely to show the importance of these keystone herbivores on the archipelago’s vegetation dynamics in the near future. On the other hand, on some islands, where native giant tortoises have been extirpated, similar-species tortoise introductions are being conducted, as ecological replacements to restore important lost ecosystem functions. For example, that approach is rebuilding trophic interactions (e.g., herbivory and seed dispersal networks) on some islands of the Mascarene archipelago (Griffiths et al. 2010, 2013).
On the Galápagos Islands, giant tortoises are known to maintain plant communities with an upper strata, formed by arborescent cacti (Gibbs et al. 2010). If tortoises have been extirpated, these plant communities become encroached by woody vegetation (Hunter and Gibbs 2014). The Anthropocene extinctions, translocations and population fluctuations of tortoises in the Galapagos archipelago provided many ecological quasi-experiments to test the effects of these ecosystem engineers (Gibbs et al. 2008; Froyd et al. 2014). Tortoise reintroductions, after decades of absence, cause a marked rebuilding of cactus population, reducing clustering and increasing juvenile cactus recruitment (Gibbs et al. 2010). Intense grazing, soil disturbance , browsing, seed dispersal , pool forming and direct vegetation damage are some of the means by which giant tortoises restore degraded ecosystems in the Galápagos (Hamann 1993; Blake et al. 2012, 2013; Froyd et al. 2014).
The effects of grazing and browsing on vegetation composition and dynamics, by continental tortoise species, are still largely uninvestigated (Falcon et al. 2018). The last representative of giant tortoises in continental ecosystems, the spurred tortoise, is threatened by extinction in the wild, mainly due to collection for the pet trade and the bush meat industry (Garrigues and Cadi 2011; Petrozzi et al. 2018). The ecological effects of the removal of a potential ecosystem engineer are poorly understood. The same problem of lack of vegetation impact studies occurs with other large-sized tortoise species, such as the yellow-footed tortoise (up to 54 kg), the leopard tortoise (up to 48 kg) and the Asian forest tortoise (up to 37 kg). Hopefully, the reintroductions planned for some of those species will mirror the ecological quasi-experiments in Galápagos and in the Mascarenes. Thereby, we would gain invaluable insight into the ecological roles of the last representatives of large tortoises on continental vegetation dynamics , other than as seed dispersers.
Whereas there are many excellent studies on the foraging behavior of iguanids, studies of their role on vegetation dynamics have mainly focused on seed dispersal (Valido and Olesen 2007). As with tortoises, many iguanas play an important role increasing the germination rate and decreasing the germination time of several angiosperm species, some of the plants also threatened (Traveset 1990; Laurel et al. 2000; Moura et al. 2015; Traveset et al. 2016). The smaller size of iguanas, and their low metabolism (averaging just ~70 kcal.day−1.kg−1 for Iguana iguana; Lichtenbelt 1992), suggest they may influence vegetation dynamics through browsing and grazing to a lesser degree than do tortoises. However, by attaining extremely high densities, in both continental and insular ecosystems (up to 364 ind.ha−1 in continental Iguana iguana; Rodda 1992), and by browsing or grazing over a small home range (Moura et al. 2015), they may be able to affect vegetation composition by: i.) reducing the leaf area of browsed species; ii.) increasing the amount of sunlight that reaches the undergrowth; and, iii.) affecting edaphic mosaics in the soil, by the repeated deposition of feces in latrines. It is hoped that these research gaps will be addressed in the future.
Marine , grazing reptiles, such as marine iguanas and green turtles, feed mostly on algae. Marine iguanas forage non-selectively on subtidal and intertidal zones, choosing abundant red and green algae species and avoiding brown algae (Shepherd and Hawkes 2005). While each adult consumes a fresh mass of just 35 g of algae per day, they can reach high densities, so populations of marine iguanas can crop a sizeable amount of fresh algal material (27–29 tons yearly for a single population in a 3 km2 peninsula on the Galápagos Islands; Nagy and Shoemaker 1984). Together with decreased water temperature, during El Niño years, population numbers of marine iguanas are often limited by bottom-up effects on the algae cover of rocky substrata (Wikelski and Thom 2000). Green turtles, the only other herbivorous marine reptile, also feed almost exclusively on algae and sea grass. They prefer low-fibre and high nitrogen species, mainly Thalassia spp. (turtle grasses), although all algae grazed by green turtles are low-quality forage (Bjorndal 1980). Sea turtles graze on algae and sea grasses, keeping them fertilized with their dung (Moran and Bjorndal 2005; Hearne et al. 2018). Sea turtles further parallel the terrestrial ungulates by having complex space use relationships with their predators. The food resource of turtles could be at risk under conditions that their natural predators, namely sharks, are rare or absent due to overfishing (Heithaus et al. 2014).
15.3.3 Direct Impact of Domestic Herbivores
Habitat destruction resulting from introduced ungulates is a classical threat to grazing and browsing reptiles (Fig. 15.3). The transformation of forest ecosystems into grasslands, or the poor management of ungulates in natural grasslands, continue to negatively affect grazing and browsing reptiles all over the world. Uromastyx lizards—important ecosystem engineers (Williams et al. 1999)—are negatively impacted by heavy grazing pressure imposed by livestock (Cunningham 2000). Other grazing species, such as the tortoises of the genus Gopherus and the spurred tortoise, are also negatively affected by competition for forage resources with cattle, Bos taurus, (Grandmaison et al. 2010; Ureña-Aranda et al. 2015; Becerra-López et al. 2017; Petrozzi et al. 2018). Also, habitat loss in the form of forest fires, set by cattle-ranchers in tropical ecosystems, are frequent all over the world (Gibbons 1984; Wiewandt and García 2000; García and Gerber 2016; Tershy et al. 2016). Direct ecosystem conversion for beef and dairy cattle production is bound to continue (Fearnside 2018), whereas the harvest of native species (including reptiles), for food, remains largely unmanaged, unsustainable and irresponsible in most tropical countries (Fa et al. 2002; Fernandez et al. 2012; Terborgh and Peres 2017).
A grassland grazed by introduced cattle on Santa Cruz Island, in Galápagos. In this photo, a cow and a giant tortoise, Chelonoidis porteri, share a field. https://www.alamy.com/stock-photo-wild-galapagos-giant-tortoise-geochelone-elephantopus-with-cow-on-164775731.html
15.4 Concluding Remarks and Further Developments
Rather than biological oddities, large-sized herbivorous reptiles are fundamental components of some vertebrate communities of tropical and subtropical environments, all over the world (Miranda 2017). The low metabolic rate of reptiles allows them to exist at extremely high biomasses, as testified by early naturalists (Von Humboldt 1877; Leguat 2017), or in ecosystems that have not yet been destroyed by humans (Bourn et al. 1999; Mourão et al. 2000). Many of these important species have disappeared, with a higher rate of extinction for giant lizards and tortoises (Turtle Extinctions Working Group 2015; Slavenko et al. 2016). Ecological networks have been impoverished by the loss of these large herbivores, likely resulting in simpler, shorter and less resilient trophic networks (Malhia et al. 2016). For the remaining herbivorous reptilian species, their direct impacts on vegetation dynamics are still being discovered. Results coming from natural and planned ecological quasi-experiments on islands show the importance of large herbivorous reptiles for ecosystem composition, structure and function (Gibbs et al. 2010; Griffiths et al. 2013). Another interesting research avenue, yet to be explored, is investigating the role reptilian aquatic herbivores play for the ecosystems in which they exist. Some freshwater turtles, such as the Amazon giant river turtle, Podocnemis expansa (up to 65 kg), and the Central American river turtle, Dermatemys mawii (up to 22 kg), feed mostly on leaves. These species are seasonal or year-round browsers, and hold the potential to exert pressure on the submerged arboreal vegetation, and act as links between terrestrial and aquatic ecosystems.
Finally, the last decade has seen a multitude of translocation programs targeting threatened tortoises, turtles, and herbivorous lizards (Jones 2002; Attum et al. 2010; Nussear et al. 2012; Gibbs et al. 2014; Grant and Hudson 2015; Falcón and Hansen 2018). These conservation initiatives have been restoring not only threatened species populations, but also lost or diminished ecological interactions. Knowledge about the effects of those translocations on the vegetation dynamics of continental floras will provide information on rebuilding reptile-driven ecological interactions (sensu Genes et al. 2017) such as trampling, seed dispersal , grazing and browsing. While considered by many as “primitive” and dull, reptiles are significant players in the ecosystems they inhabit, and perform important functions as predators, pollinators, burrowers and frugivores. Acquiring knowledge on their role as grazers and browsers is a promising ecological research venture.
Acknowledgements
EBPM greatly appreciates the generous financial support of the following donors: Rufford Small Grants Foundation (18743-1 and 23022-2), Rainforest Biodiversity Group, Idea Wild, The Mamont Scholars Program of the Explorer’s Club Exploration Fund, Cleveland Metroparks Zoo and the SouthWild.com Conservation Travel System. EBPM receives major logistical support from the Peugeot-ONF Brasil Carbon Sink Reforestation Project, based at Fazenda São Nicolau in the Municipality of Cotriguaçu, Mato Grosso, Brazil. This Project is a Peugeot initiative to fulfill some of the Kyoto Protocol directions and is run by the ONF Brasil enterprise. CSM’s research is supported by a scholarship from The National Council for Scientific and Technological Development (CNPq), within the Ministry of Science of Brazil, and by the Young Talents for Conservation Award, by the Luísa Pinho Sartori Institute. We thank Bernardo Araujo and our reviewers Iain Gordon and Herbert Prins for reviewing the manuscript.
15.5 Birds
15.5.1 Grazing and Browsing Birds
Foraging on plants is very widespread among birds. The most common form this takes is the selection of high energy and nutrient forage item such as seeds and fruits. Quite a number of species have specialised in this foraging strategy (see Whelan et al. 2015 for impacts of granivorous and frugivorous birds on ecosystems), but many more take these items as part of a much broader—often omnivorous—diet. Far fewer have adopted grazing or browsing as their main mode of foraging (see Lopes et al. 2016 for a classification of avian diet types), estimated to be the case for 101 species (80 grazers and 21 browsers—Table 15.1), and thus less than 1% of the Worlds’ extant bird species (9993—Jetz et al. 2012). The distribution of herbivorous birds is strongly skewed towards a single phylogenetic order and family therein, namely the Anatidae or ducks, geese and swans (Table 15.1). All swans and geese are obligate herbivores, although occasionally consuming other food items too (e.g., insects, bones or even soil—Abrahams 2013). The extent to which plant-eating ducks consume plant material (other than seeds) varies considerably among species and the time of the year (Olsen 2015). About 20 ducks (e.g., whistling ducks, some diving ducks including pochards, and several dabbling ducks including wigeons, gadwall, Anas strepera, and Australian wood duck, Chenonetta jubata, can be considered primarily herbivorous, for at least part of the year (Table 15.1), but all will shift towards a more omnivorous diet during spring and summer to meet protein requirements for breeding and growth (Sedinger 1997).
Whilst many of the above Anatidae also consume seeds when present (and thus are in part, and to a various extent, also granivorous), grazing of grasses, sedges and forbs is the predominant mode of foraging. Grazing typically concerns the consumption of aboveground tissue of non-woody plants (notably leaves but also stems and seed heads, the latter particularly in the case of agricultural crops), but this regularly spills over in the extraction of belowground plant organs such as tubers and rhizomes (Fox and Abraham 2017). For some species groups, shorter or longer periods of the year are dedicated to such foraging for energy -rich storage organs—captured by the term ‘grubbing’ (Anderson et al. 2012)—which requires considerable strength, and hence is typically conducted by larger species (e.g., ostrich, cranes, larger-bodied geese, swans; Zhiheng and Clarke 2016). Several other aquatic families, or members thereof, such as screamers, coots and some moorhens, are primarily grazers—or consumers of belowground plant parts—too, as are several flightless members of the phylogenetically oldest clade (Palaeognathae) of extant birds (emu, Dromaius novaehollandiae, rheas), the common wood pigeon, Columba palumbus, and the large, flightless, and critically-endangered kakapo, Strigops habroptilus, whose diet (e.g., clubmosses, ferns and bark, and thus leaning towards being a browser—Best 1984) is as unusual as its appearance. Many more bird species consume fresh green shoots, flowers and buds (e.g., pheasants, turkeys, rails, turacos), or extract rhizomes, roots and bulbs (e.g., bustards, sandgrouse, tinamous), but only in limited amounts, and at certain times of the year, and are thus not genuine grazers.
Grouse is the family of birds with most genuine browsers (all 17 species—Table 15.1), often almost exclusively living from shrubs, or tree leaves and needles, outside the breeding season. Many also consume seeds, with the exception of the two sage-grouse, which lack a muscular gizzard and, therefore, consume only soft-tissue foods (mainly leaves of sagebrush). Within the breeding season, grouse diets are generally enriched with invertebrates (Sedinger 1997). Other bird species that forage primarily on buds, leaves and twigs are the three plantcutter—the only folivorous passerines (Bucher et al. 2003)—and the Amazonian-dwelling hoatzin, Ophisthocomus hoazin. The latter has even developed a crop and oesophagus with fermentation functionality akin to rumen-digestion (Grajal et al. 1989; Godoy-Vitorino et al. 2011).
With the exception of the Rattites (large flightless birds including ostriches, rheas and emu) and the (poorly flying) hoatzin—both of which can digest cellulose (Swart et al. 1993; Grajal et al. 1989)—the requirement to fly has prevented birds from developing relatively heavy but efficient digestive systems to deal with e.g., plant material (Caviedes-Vidal et al. 2007; but see Hunt et al. 2019 for limited evidence between flying ability and caecal length—greatest for grazers—within birds). This has two important consequences: firstly, a limited ability to digest cellulose and other highly recalcitrant plant parts (Prop and Vulink 1992; Durant 2003), meaning that large amounts of plant material need to be consumed; secondly, there is an even greater premium on plant quality than is the case for most other herbivores, as selection of the most nutritious and/or energy rich (depending on requirements and time of the year) food items—in itself a time-consuming process—is the only way to maximise daily nutrient and/or energy gains. Combined, many herbivorous birds have to forage for most of the day (Prop and Vulink 1992). For grazers, having to use a bill as feeding device brings the additional constraint of reduced bite size as compared to mammalian grazers. This may, in part, be compensated for by employing a greater bite frequency in order to achieve comparable instantaneous food intake rates (Steuer et al. 2015), or require further adaptation, in the form of yet longer daily foraging times (Van Gils et al. 2007). This puts in sharp focus the trade-off between plant quantity and quality, and with it a set of key behavioural adaptions (see below) to try and achieve the highest possible intake rates of high quality food items. Whilst none of these adaptations is unique to birds, and indeed trade-offs between quantity and quality rule foraging decisions of all herbivores (and beyond), birds’ limited digestive ability of plant material tends to make conditions more extreme.
15.5.2 Ecosystem Impacts of Grazing and Browsing Birds
Many of the ecosystem impacts of birds observed today could be understood in the context of digestive constraints on herbivorous birds, whereby the ability to i) aggregate into groups to optimise foraging time and food finding (Stahl et al. 2001; Gyimes et al. 2010; Kułakowska et al. 2014); ii) undergo carefully scheduled movement and migration, to and from a limited number of sites, and thus requiring individuals, in a number of herbivorous bird species, to make decisions at larger spatial and temporal scales (Arzel et al. 2006; Van der Graaf et al. 2006; Shariatinajafabadi et al. 2014); and, iii) seek out, and subsequently utilise, new ‘high quality’ foraging opportunities (Van Eerden et al. 2005; Si et al. 2015; Fox and Madsen 2017; Buij et al. 2017), are key behavioural adaptations to those constraints.
For grazing or browsing birds, to exert large-scale impact on the vegetation, species need to be abundant and use a location for a reasonable length of time. As a consequence, herbivorous birds that are gregarious tend to have greatest impact on the ecosystems they inhabit (Wood et al. 2012; Kollars et al. 2017), though exceptions occur (e.g., ptarmigan controlling shrub architecture—Tape et al. 2010). Large aggregations concern mostly—but not exclusively (e.g., coots, cranes, wood pigeons)—“waterfowl”, a loosely defined label that typically concerns ducks, geese and swans, and sometimes also rails. As the label suggests, waterfowl use both terrestrial and aquatic ecosystem components, and impacts on land are often inextricable connected to those occurring in nearby freshwater , or marine , ecosystems and vice versa (Hessen et al. 2017; Kollars et al. 2017).
15.5.3 Bird Density and Existence of Plant Refuge Determine Marine Ecosystem Impact
Impacts on marine ecosystems are restricted to the intertidal or shallow subtidal, and those zones are typically used seasonally, coinciding with spring or autumn migrations. For example, beds of seagrass, Zostera spp., are in high demand by brent geese, Branta bernicla, black swan, Cygnus atratus, mute swan, Cygnus olor, and American wigeon, Anas americana, but Zostera also occurs frequent in the diet of a further 20 waterfowl species (Kollars et al. 2017). Consumption of both above- and belowground components may severely reduce seagrass abundance, though accumulation of seeds in feeding pits, in autumn, can facilitate seedling recruitment in spring, thereby contributing to the persistence of seagrass beds (Zipperle et al. 2010). In some areas waterfowl may control the abundance and distribution of seagrasses (Kollars et al. 2017), in other areas (e.g., Gulf of Mexico where redhead duck, Aythya americana, forages extensive on seagrass beds that represent economically important fish nursing habitat—Kennedy et al. 2018), impacts appear to be limited. Critical to the extent of bird herbivory impact, in shallow marine, as well as comparable freshwater , systems (e.g., swans foraging on pondweeds, Potamogeton spp—Klaasen and Nolet 2007), is not only the density of birds, and duration of resource use, but also the existence of spatial refuges for plants (e.g., belowground plant parts that are out of reach—Santamaria and Rodríguez-Gironés 2008), and safe sites for seedling establishment to ensure plant regrowth and recruitment. Although recovery of previously endangered waterfowl, due to successful conservation efforts, combined with ongoing expansion of already highly successful species, may exert further impact on seagrass beds (Kollars et al. 2017), the attraction of most Zostera-consuming species to agricultural land for foraging (Fox and Madsen 2017) is likely to mitigate against greater bird grazing impacts on marine ecosystems.
15.5.4 Expanding Waterfowl Populations Contribute to Top-Down Control of Freshwater Ecosystems
The impacts of ducks , geese, swans and rails on freshwater ecosystems are manifold, but key are the accumulation of nutrients, bio-turbidity and the consumption of macrophytes (Wood et al. 2012; Bakker et al. 2018). A further significant, although less directly observable, impact, is the contribution by waterfowl to wetland biodiversity around the world, through the transport of a wide variety of aquatic plants and animals between wetlands and water bodies (Van Leeuwen et al. 2012) Extensive consumption of water plants, in lakes, by birds, does occur, but in most cases this is limited to very shallow, and often heavily vegetated, systems (e.g., swamps), although ponds may also be subject to intense waterfowl herbivory (and indeed kept apparently free of vegetation—Van Onsem and Triest (2018)). Within deeper lakes, there may be brief episodes of heavy grazing, for example when large numbers of waterfowl gather to moult during which they are flightless, and hence prone to predation (e.g., moult migration by greylag geese, Anser anser—Zijlstra et al. 1991). Whereas 3 decades ago, recognition of waterfowl shaping freshwater ecosystems, beyond single sites, was limited, the rapid increase in waterfowl abundance in recent years has changed this. In a review, Bakker et al. (2018) concluded that, currently, herbivorous birds remove 40–48% of plant biomass in aquatic ecosystems, well in excess of exploitation levels found in most terrestrial ecosystems. Impacts are manifold with, for example, the dramatic expansions of greylag geese being held responsible for the decline in reed, Phragmites europaeus, beds across Europe (Bakker et al. 2018), with knock-on effects on numerous species of insects and breeding birds. Moreover, the accumulation of nutrients, deposited in the water as uric acid and faeces, in no small part originating from food plant consumed in the terrestrial realm, is now viewed as default, and increasingly the main eutrophicating agent in lakes and ponds (Chaichana et al. 2010; Hessen et al. 2017). Whilst there is strong evidence for birds causing eutrophication, bioturbation and the reduction of aquatic plant abundance, and for bird density to be related to the extent of plant biomass suppression, this is also the case for other species groups (e.g., mammals, crustaceans, molluscs, fish , echinoderms—Wood et al. 2016 and elsewhere in this Chapter and Book). Hence, the current, and likely further, increasing impacts of herbivorous birds on freshwater ecosystems may best be viewed as contributing to a wider top-down control of primary productivity. It is likely that only part of the complexity of herbivorous bird impact on freshwater ecosystems has been revealed because of the large number of species interactions and mechanisms involved (e.g., influence of carp on habitat choice of water birds—Haas et al. 2007). Whilst the impact of birds on macrophytes, in water bodies, is thus beyond doubt, its impact on rivers is still deemed limited, and mostly local (Franklin et al. 2008).
15.5.5 Agriculture Drives Grazing Bird Abundance and Terrestrial Ecosystem Impacts
Numerous studies have revealed the intricate nature of impacts exerted by grazing and browsing birds on terrestrial ecosystems. Key to large-scale herbivore impacts are changes in wider land use, government policy and climate which, collectively, have allowed, notably waterfowl, to greatly increase in abundance (e.g., Jefferies et al. 2004; Van Eerden et al. 2005). Long-term changes in the quality of the vegetation on offer in grasslands and arable lands have, together with reductions in hunting practice and the formation of nature reserves, driven down over-winter mortality of waterfowl (Fox and Madsen 2017). Particularly dramatic population expansions have occurred in arctic-breeding geese, raising the status of e.g., the lesser snow goose, Chen caerulescens, to one of the world’s most abundant grazing birds. The impact of this species on their breeding grounds has been evidenced particularly well in and around La Pérouse Bay, Hudson Bay area, subarctic Canada. Here, the relationship between snow geese and the subarctic tundra vegetation changed from one characterised by finely tuned herbivore-plant-soil feedbacks , which allowed geese to optimise food intake (Hik and Jefferies 1990), to a situation in which extensive loss in vegetation cover occurred, due to grubbing. The dramatic increase in the number of breeding and spring-staging lesser snow geese, together with a comparably low number of Ross’s geese, Chen rossii, set in motion a positive feedback loop, wherein grubbing for belowground plant parts caused vegetation cover decline and soil exposure, which led to the development of hypersaline topsoil, in turn killing off extant vegetation (Srivastava and Jefferies 1996), and reducing plant recovery potential (Handa et al. 2002). This resulted in—for avian herbivory—exceptionally large-scale denudation of, notably, saltmarsh habitat, and to a lesser extent also freshwater habitat, in and around snow goose colonies, all along the 2000 km-long Hudson Bay coastline (Jano et al. 1998; Jefferies et al. 2006). Because recovery is slow (freshwater marsh) or absent (saltmarsh; Peterson et al. 2013), lack of vegetation causes geese to move away and establish colonies elsewhere, resulting in further goose impacts across the region.
Similarly long-lasting, and large-scale, disruptions of the vegetation can now be observed across the Canadian Arctic. For example, between 1988 and 2011, well north of the Hudson Bay in Nunavut, a five-fold increase in area of exposed peat habitat, from 269 to 1373 km2 in a 36,370 km2 large study area, and a 48% loss of wet sedge meadow, were observed (Conkin and Alisauskas 2017). Although goose impacts in large parts of the Canadian Arctic remain limited, goose numbers are very high, colonies numerous, and local grazing impacts strong and replicated over large areas. Hence, region-wide ecosystem impacts are likely, including population declines of other bird species e.g., shorebirds and passerines (Flemming et al. 2016). Other Nearctic goose populations are less large, but otherwise following suit (e.g., Canada goose Branta canadensis, greater snow goose), and impact staging and breeding grounds elsewhere. Hunting pressure has been increased significantly in an attempt to curtail goose populations (e.g., Lefebvre et al. 2017), and reduce the pressure on arctic ecosystems as well as on agricultural land further south used by geese outside the breeding season, and which fuel the population expansions. Hunting has stabilised the population of greater snow geese (to around one million birds—Lefebvre et al. 2017), but the, more than an order of magnitude, more abundant population of lesser snow geese continues to grow despite huge hunting efforts (Alisauskas et al. 2011).
Whilst Palearctic goose populations expanded almost as spectacularly, impacts on their Arctic breeding grounds are far less pronounced. Pink-footed goose impacts, which like snow geese are sufficiently powerful to dig for belowground plant parts (i.e., grubbing) early on in the breeding season (Anderson et al. 2016), do now occur throughout the productive parts of Svalbard, but with the initial disruption of the vegetation overlying organic soils recovering swiftly (Speed et al. 2010). Colony-breeding geese that do not have the physical ability to grub extensively, such as the barnacle goose, can locally also be influential—suppressing e.g., vascular plant and moss biomass and the carbon sequestration ability of tundra—but again recovery, when grazing pressure reduces, is rapid (Sjögersten et al. 2008, 2011), and hence is best not labelled “habitat degradation” (Van der Wal 2006). A fundamental difference with the coastal zone of NE Canada, subject to isostatic uplift (Jefferies et al. 2006), is the absence of saline subsoil; when exposed through grubbing, soils in the Hudson Bay turn hypersaline which kills the vegetation, and puts vegetation succession on long-term hold. Thus, waterfowl impacts on terrestrial ecosystems may be strong and long-lasting, but particularly so when amplified by specific abiotic conditions.
Extensive use of agricultural land—often superior in foraging quality to semi-natural habitats (e.g., Fox and Madsen 2017; Dokter et al. 2018)—by a growing number of grazing birds (Van Eerden et al. 2005), has attracted widespread disapproval among, notably, the farming community, leading to loci of conflict around specific species. Evidence for such grazing impacts is mixed (see Fox et al. 2017 for a review), but the conflicts remain real. Whilst overwintering species (of notably geese) were initially seen as most problematic by some, there is a shift towards a broader discontent about extremely rapidly growing populations of year-round bird species. Within Europe this concerns is expressed most prominently towards greylag geese (a species which has increased particularly rapidly—Fox and Madsen 2017), but also towards other expanding species originating, at least in part, from collections (e.g., Canada goose, Egyptian goose, Alopochen aegyptiaca, mute swan, barnacle goose, white-fronted goose, Anser albifrons). Increasingly, questions are being asked about how such summer herbivory may impact various ecosystem functions, including food stocks of migratory bird species arriving in autumn (e.g., Gyimesi et al. 2011). In many countries, resident waterfowl species have also penetrated into urban environments, and established sizeable populations. Their omnipresence, and diversity of impacts on society, ranging from fouling of lawns and pavements to aggressive encounters, air traffic accidents, and concerns about zoonotic diseases (Buij et al. 2017), has rendered several species subject to sometimes dramatic population control. The rapid expansion of, notably, geese, but also ducks, cranes, coots, wood pigeons and other species benefitting from almost unlimited feeding opportunities provided by farmland landscapes, and the resulting diversification of impacts, is leading to significant shifts in the dynamics of different interest groups’ views on the future of herbivorous birds (Cusack et al. 2018). This is an area of growing academic, political and conservation interest, and is likely to spill over into other areas of society, across large areas of the northern hemisphere (Milton 2000; Fox and Madsen 2017).
15.6 Marsupials
Amongst marsupials, browsers and grazers are found almost exclusively among the Australasian order Diprotodontia (Hume 1999), with significant herbivory not reported, and certainly unstudied, in South American marsupial lineages. This assemblage evolved in the absence of eutherian browsers and grazers, to occupy a diversity of ecological niches , throughout the Australian continent (Tyndale-Biscoe 2005). However, the diversity, size range and impact of marsupial browsers and grazers is much reduced from that prior to the Pleistocene extinction of megafaunal browsers including the short-faced kangaroos, Simosthenurus spp., the chenopod specialist, Procoptodon goliah, the “marsupial tapir”, Palorchestes spp., and the diprotodontines, Euryzygoma spp., Diprotodon spp. and Zygomaturus spp., (Johnson 2006; Prideaux et al. 2009). The only native Australian non-marsupial mammalian browsers and grazers are rodents , including the highly-specialised, vole-like grass-eating broad-toothed rat, Mastacomys fuscus, the swamp rat Rattus lutreolus and the arid-zone sticknest rats, Leporillus spp., (Breed and Ford 2007; Aplin and Ford 2014). Other omnivorous and primarily seed- and fruit-eating rodents (e.g., Melmoys cervinipes), also include grass and leaves in their diets, to varying extents (Breed and Ford 2007). In consequence, marsupial browsers and grazers account for most Australian native vertebrate herbivory. The direct impacts of marsupial herbivory are rarely quantified, except where economic impacts occur, or are perceived, and where species are judged to be overabundant, and detrimentally affecting vegetation condition or biodiversity values (e.g., Barnes and Hill 1992; Coulson 2007; Di Stefano et al. 2007).
15.6.1 Marsupial Grazing
Grazing, as the main dietary habit amongst marsupials, is restricted to some species in the family Macropodidae (kangaroos and wallabies), and to all members of the family Vombatidae (wombats). Arman and Prideaux (2015) classified the diets of 37 extant macropods, and identified only 3 browsers (diet comprising >70% dicot foliage; these were two arboreal tree kangaroos and the small terrestrial quokka) and 9 grazers (>70% grass; large kangaroos and wallabies), while the greatest number (19) were mixed feeders. Previous classifications, with a greater emphasis on dental morphology, had identified many of these mixed feeders as browsers (e.g., Sanson 1989).
Because of their perceived economic impact, the impacts of large and abundant grazing marsupials have attracted the most attention from ecologists. Several species of large kangaroos, in particular red kangaroos (Osphranter rufus, formerly Macropus rufus—here we adopt the taxonomy of Jackson (2015)) together with the euro/wallaroo, Osphranter robustus, and eastern grey, Macropus giganteus, and western grey, M. fuliginosus, kangaroos, often graze the same pastures as domestic livestock, and macropods including grey kangaroos can aggregate in crops, causing cereal production losses (Coulson 2007). Agile wallabies, Notamacropus agilis, and black-striped wallabies, Notamacropus dorsalis, are sometimes, locally, identified as pests of pasture and/or crops in northern Australia and Central Queensland, respectively (Baxter et al. 2001; Bedoya-Pérez et al. 2017).
There has been a long-standing debate about the extent to which these macropods and livestock, particularly sheep, compete for the same pasture resources. Demonstrating intraspecific competition is difficult, and the evidence, for competion between livestock and macropods, can only be considered insufficient to weak (e.g., dietary overlap with a lack of evidence that resources are limiting; Spear and Chown 2009, Prins 2016). In arid New South Wales, Dawson and Ellis (1996) found little dietary overlap occurred between sympatric euros and sheep, but a large manipulative experiment, in the same region, showed that the diets of red kangaroos and sheep overlapped considerably. This was true whether they grazed together or separately, but the degree of overlap varied with rainfall and available pasture biomass (Dawson and Ellis 1994; Edwards et al. 1995, 1996). Although sheep diets were affected, during dry times, by the presence of kangaroos (they consumed more chenopod shrubs), kangaroo diet was unaffected by the presence of sheep. It appears possible then that competition may occur when red kangaroos deprive sympatric sheep of pasture when pasture biomass is low, but moderate or strong support for either competition or facilitation between macropods and livestock, such as evidence of detrimental effects on individuals or populations of putatively competing species, is lacking (Edwards et al. 1995; Dawson 2012).
Australian rangelands are characterized by non-equilibrium dynamics , and at times both kangaroos and sheep can reduce pasture to an ungrazeable level (Short 1985), at which times food must become, at least transiently, limiting. Whilst primary producers can re- or destock, to rapidly alter grazing pressure as circumstances demand, the numerical response of kangaroo species, although high (Bayliss and Choquenot 2002), is sometimes insufficient to take advantage of the transient availability of good pasture. Red kangaroos, and perhaps, euros are exceptions in that they can travel long distances to take advantage of local rainfall events (Croft 1991; Clancy and Croft 1992).
Only weak evidence can be found for competition or facilitation between macropods and cattle. Consistent with competition, black-striped wallabies, Notamacropus dorsalis, show very strong dietary overlap with cattle in Central Queensland and consume economically very significant quantities of pasture (Baxter et al. 2001). In the Simpson Desert, removal of cattle resulted in a progressive increase in kangaroo numbers over a period of approximately 2 years compared to areas where cattle remained (Frank et al. 2016), but over the short duration of that study, kangaroo feed intake (estimated from dung deposition) remained a tiny fraction of that of cattle. More consistent with facilitation, time-series data, from across a large area of the South Australian pastoral zone, revealed that the presence of sheep and cattle had a positive effect on the population growth rate of red kangaroos, although this result was interpreted by Jonzen et al. (2005) as indicating that livestock are a surrogate for resource availability beyond rainfall. At a smaller scale, Payne and Jarman (1999) observed immediate disturbance impacts of cattle on grazing kangaroos in eastern Australia and found these to be minimal, with kangaroo groups distributed more closely to cattle groups than expected by chance.
A second aspect of the debate about the impact of kangaroo grazing on domestic livestock focusses on the amount of food consumed by kangaroos relative to sheep. The safe stocking rate of rangelands for sheep is usually expressed in terms of “dry sheep equivalents” (DSE) which is the amount of food required to support a “typical” 2-year-old ~ 45 kg non-lactating Merino sheep (Turner and Alcock 2000). This unit is then used to compare different livestock management options. Dawson and Munn (2007) note that kangaroos are regarded, by some, as eating twice as much food as sheep (i.e., 2 DSE) but values of around 0.7 are typically assumed by Departments of Agriculture, because the basal metabolic rate of marsupials is generally about 70% of that of eutherian mammals (Munn and Dawson 2003). Dawson and Munn (2007) bring together data from many sources, including from heart-rate telemetry (Munn et al. 2009), and observations of bite rates (McLeod 1996) to estimate feeding rates of free-living kangaroos. They conclude that, on rangelands, kangaroos can be considered to be equivalent to 0.4 DSE in the event that competition is occurring.
Although much research has been directed towards the implications for domestic stock of grazing by kangaroos, attention is turning to the effects of grazing on ecosystems and biodiversity, particularly by high-density kangaroo populations. Grazing marsupials can engineer environments by maintaining lawns—bare-nosed wombats, Vombatus ursinus, can exclude woody plant establishment, and maintain lawns, in Tasmanian wetlands (Roberts et al. 2011), and both bare-nosed and hairy-nosed wombats, Lasiorhinus latifrons, can maintain lawns, in well-drained areas, elsewhere, by closely and repeatedly cropping their preferred tough native perennial grasses (Hume 1999; Tyndale-Biscoe 2005; Kirkpatrick 2016). Observations have been reported of up to 80 wombats grazing by day in some Tasmanian locations (Temby 1998). Red kangaroos can also maintain grazing lawns in Central Australia (and depend upon them during drought), but initial establishment of these lawns is facilitated by removal of senescent biomass by cattle (Newsome 1997).
Experimental studies have shown that high densities of kangaroos, at multiple locations in south-eastern Australia, have significant negative effects on ground layer plants (Sluiter et al. 1997; McIntyre et al. 2010, 2015), tree seedlings and saplings (Noble 2001; Meers and Adams 2003; Allcock and Hik 2004; Stapleton et al. 2017), beetles (Barton et al. 2011), grassland-dependent reptiles (earless dragons, Tympanocryptis pinguicolla, Howland et al. 2014, and striped legless lizards, Delma impar, Howland et al. 2016a), and reintroduced eastern barred bandicoots, Perameles gunnii, (Winnard and Coulson 2008). Browsing damage by kangaroos can be particularly significant during droughts, when grass biomass is depleted (Couslon and Norbury 1988). For several other groups, including web-spinning spiders (Foster et al. 2015) and birds (Howland et al. 2016b), the results were more complex with some species of birds being affected and others not. In semi-arid, low-productivity Australian rangelands and woodlands , where kangaroos exist at more moderate densities, the intensity of their grazing is substantially less than that of livestock or rabbits, Oryctolagus cuniculus, (Vandandorj et al. 2017). Structural equation and multiple linear regression models that have been designed to identify the relative impacts of different types of herbivory and abiotic factors, suggest that the impacts of kangaroos on plant species composition (Travers et al. 2018), plant species diversity (Eldridge et al. 2018), shrub and tree short-term recruitment and long-term regeneration (Tiver and Andrew 1997), soil health (Eldridge et al. 2017) and regulating and provisioning ecosystem services (Vandandorj et al. 2017), are correspondingly benign, again in contrast to past and present impacts of exotic herbivores, particularly sheep. Indeed, under low productivity, kangaroo grazing can increase native plant species richness (Eldridge et al. 2018). In contrast, Rees et al. (2017) experimentally excluded kangaroos from plots in the Strzelecki Desert, demonstrating that in the absence of dingoes (Canis dingo), which otherwise suppress their numbers, kangaroos can suppress pasture biomass and grass seed production. This in turn likely contributes to the decline of small graminivorous birds.
Solutions to the impacts of high kangaroo densities are varied and can involve restoring coarse woody debris into habitats to protect saplings and create micro-habitats for ground flora (Stapleton et al. 2017), direct reduction of kangaroo numbers through shooting (from densities of ~2–4 ha−1 to as low as 0.4 ha−1), and the possible re-introduction of apex predators such as the dingo (Letnic et al. 2009). Long-term studies indicate that grassland recovery , from heavy grazing pressure by kangaroos, can be a slow process, particularly if the grazing pressure from kangaroos is replaced by that of other herbivores, such as rabbits. Although direct reduction of kangaroo numbers is a simple long-term strategy, in some places there can be significant community and professional opposition to the process, and accompanying actions (Ben-Ami et al. 2014; McKinnon et al. 2018). Nonetheless, the four large kangaroo species are the focus of Kangaroo Management Plans, and permits for culling are assessed each year (Department of Parks and Wildlife, Western Australia 2013; Department of Environment, Water and Natural Resources, South Australia 2017; Department of Environment, Land, Water and Planning, Victoria 2017; Department of Environment and Heritage Protection, Queensland 2018). Southern hairy-nosed wombats and bare-nosed wombats are also occasionally culled under license, in response to perceived impacts on cereal crops and pasture (Marks 1998), although the impact of their digging and damage to fences is generally more problematic (Triggs 2009).
The potential also exists for competition between marsupial browsers and grazers and non-livestock herbivores species, most particularly rabbits. Strong, to very strong, experimental evidence has been presented to show that competition from rabbits negatively affects bare-nosed wombats (Cooke 1998) and red kangaroos (Cooke and Mutze 2018). Grazing by rabbits favours (often exotic) annual grasses and forbs over native perennial grasses, and these altered grasslands are less able to support the more protracted reproduction of marsupials (Tyndale-Biscoe 2005).
Both in Australia, and in its introduced range in New Zealand, the browsing common brushtail possum, Trichosurus vulpecula, sometimes feeds on pasture, particularly on clover, which can account for up to 30% of its diet (Harvie 1973). Possums have sometimes been considered to compete for pasture with livestock, with estimates of grazing equivalence as high as 0.072 stock units (SU; the New Zealand SU is for a 55 kg lactating ewe, so exceeds the Australian DSE) but a more convincing estimate is 0.01 SU (Cowan 2007). More strikingly, in Tasmania, the combined effects of possum and wallaby grazing have been suggested to reduce dry matter yields by up to 94% and 48% for improved and native pasture, respectively (Statham and Rayner 1995).
15.6.2 Marsupial Browsing
Marsupial browsing impacts can be attributed to browsing and mixed-feeding macropods, and a number of arboreal folivores. The arboreal folivores include several widespread and locally abundant species that specialize, to varying extents, on the foliage of eucalypts, and of these koalas, Phascolarctos cinereus, and common ringtail, Pseudochirus peregrinus, and common brushtail possums have all been linked to defoliation and mortality of trees, when they occur in high densities, although the impact of common brushtail possums has been most thoroughly investigated in its introduced range in New Zealand (Cowan and Waddington 1990; Pekelharing et al. 1998; Duncan et al. 2011).
In the absence of predation, or significant impacts of disease, koala populations can exhibit rapid population growth , resulting in population densities as high as 20 ha−1, reported from Cape Otway, Victoria (Whisson et al. 2016). At high koala population densities, browsing is unsustainable, and results in widespread tree and koala mortality (Fig. 15.4) (either directly from starvation or from euthanasia of malnourished captured koalas by management agencies; Martin 1985a, b, Department of Environment, Land, Water and Planning, Victoria 2016) and loss of koala habitat, as well as posing a threat to certain vegetation classes and their associated biodiversity (Department of Sustainability and Environment, Victoria 2004). Localised events, like these, have been recorded repeatedly since the early twentieth century and occur most commonly in isolated patches of habitat on islands or surrounded by cleared land (Kershaw 1934; Menkhorst 2008). Koala overabundance is usually associated with Eucalyptus viminalis across a variety of soil types in coastal regions of Victoria and South Australia, although it can also develop in E. ovata and other species (Martin 1985a), and has been reported in association with E. tereticornis from northern coastal NSW (Frith 1978).
Dense populations of arboreal browsing marsupials can cause severe overbrowsing of the forest canopy. In this forest at Cape Otway, Victoria, koalas have overbrowsed and killed all mature manna gum, Eucalyptus viminalis, trees, while the less preferred messmate stringybarks, E. obliqua, have survived. Photo Credit: Ben Moore
The population dynamics of koalas, in E. viminalis forest, have been well studied, and modelled (Todd et al. 2008; Whisson et al. 2016). Ramsey et al. (2016) estimated that the koala carrying capacity of the manna gum forest at Cape Otway is 5.3–8.3.ha−1, but cautioned that this estimate has low precision, and may be too high to allow recovery of previously overbrowsed trees; an appropriate management target for a “safe” population density is probably lower than this estimate. Most koalas show strong fidelity to their small home ranges (0.4–1.2 ha at Cape Otway; Whisson et al. 2016), even in the face of declining food resources, and despite adequate habitat connectivity making dispersal possible. A model of koala-manna gum dynamics, at Mt. Eccles in western Victoria, predicted the koala population increasing to peak abundance in 10–18 years before crashing—with the impact on tree mortality dependent upon the eventual maximum rate of decline of the koala population (Todd et al. 2008). Eucalypts have a remarkable capacity to replace foliage after defoliation or fire, but energy (starch) reserves can be depleted if repeated defoliation occurs over an extended period (Bamber and Humphreys 1965). A management strategy, suggested by Todd et al. (2008) on the basis of their modelling , requires the sterilization of a variable number of female koalas each year in order to maintain a fixed number of non-sterilised females. Chemical (and formerly, surgical) sterilization (Hynes et al. 2010) is a widely-implemented koala management strategy in South Australia and Victoria, as is translocation of koalas from overabundant populations.
Increasingly, koalas are now reaching high densities, in commercial plantations of E. globulus established in the 1990s in Victoria and South Australia (Natural Resources Kangaroo Island 2017, Department of Environment, Land, Water and Planning, Victoria 2018; Department of Environment, Water, and Natural Resources, Government of South Australia 2017). Although significant impacts on plantation productivity have not been claimed (nor measured), costs to the plantation industry are considerable, because harvesting operations must be modified, and significant reputational cost is incurred by forestry companies when koalas are killed or injured (e.g., ABC News 2013; HVP Plantations 2018). Marsupial browsing of eucalypt seedlings in Tasmanian hardwood plantations, is more obviously detrimental to productivity, because it reduces seedling growth and survival and affects tree form (Bulinski and McArthur 1999; Scott et al. 2002). Consequently, Tasmania has implemented intensive browser control measures and encouraged research into the problem (e.g., Miller et al. 2009; Close et al. 2010; Miller et al. 2011). While brushtail possums, pademelons and wallabies have all been implicated, Bulinski and McArthur (2003) suggested that the relative contribution of brushtail possums had previously been underestimated.
The second specialist folivore of eucalypts (in addition to the koala) is the greater glider, Petauroides volans, which does not appear to reach population densities that measurably affect tree canopy biomass. However, the two generalist browser species, that can feed to significant extents on eucalypt foliage, i.e., the common brushtail and ringtail possums, can both occur in very high densities, and can cause local canopy loss and eucalypt mortality (Loyn and Middleton 1980; Low 2002; Yugovic 2015).
Dramatic impacts of common brushtail possums, on native vegetation, have been reported when they have been introduced to islands, such as the Keppel Islands in Australia, and most famously, to New Zealand (Low 2002). Possums selectively browse on, and cause crown dieback of, numerous native forest trees and mistletoes in New Zealand (Sweetapple 2008; Sweetapple et al. 2016), and enormous efforts are expended to reduce and control possum populations. Holland et al. (2013) have produced a model to describe the impact of browsing by common brushtail possums on woody vegetation, and have parametrized, and run, this model for several NZ tree species (Holland et al. 2016). These models focus on tree mortality as an endpoint, and key input parameters are plant foliage cover, and an index of browse damage. These models are constructed such that the relationship of possum density to tree mortality is strongly non-linear, and high possum densities are required before browsing impacts become apparent. A strength of these models is the recognition, by the modellers, that browsing is selective at the level of browse species and individual plant (Windley et al. 2016). While many other modellers (e.g., Feng et al. 2009) average herbivore offtake evenly across plants in the landscape, the models of Holland and coworkers incorporate the recognition that herbivory is often concentrated on individual, preferred trees. Selective browsing, including that driven by strong differences in secondary chemistry among conspecific individual plants (e.g., Moore et al. 2010), explains why browsing impacts can become apparent even when overall herbivore densities appear to be too low to have an impact on foliar production at the ecosystem level. These models have not yet been applied to Australian eucalypts, although these trees might differ from the New Zealand examples used by Holland et al. (2016), in terms of leaf lifespan, carbohydrate storage and bud reserves to facilitate regrowth, and may show greater-between tree variability in herbivore preferences attributable to plant secondary metabolites.
As with grazing, browsing by marsupials can affect biodiversity, especially by limiting regeneration of tree species after disturbance (Allcock and Hik 2004). Overabundance of the mixed-feeding swamp wallaby, Wallabia bicolor, at Booderee National Park, in coastal NSW, suppresses regeneration of Eucalyptus pilularis seedlings, as well as other native and weedy trees, vines and shrubs, favouring the growth of bracken, Pteridium esculentum, (Dexter et al. 2013). Stutz et al. (2016) demonstrated the remarkable ability of these wallabies to locate and target small seedlings by olfaction, even when they are obscured by thick understorey. Exclosures were also used at Wilsons Promontory, Victoria, to demonstrate that secondary succession (from shrubland to forest) could only proceed in the absence of swamp wallabies, which were capable of pulling down and browsing saplings as tall as 2 m (Ashton and Chappill 1989). In different Victorian forests, swamp wallaby effects on forest regeneration, after harvesting, can range from minimal (Di Stefano et al. 2007) to substantial (Di Stefano 2005). Browsing can also, sometimes, threaten individual plant species such as the Tasmanian trees Eucalyptus gunnii (Calder and Kirkpatrick 2008) and the threatened Eucalyptus morrisbyi, in which one of two remaining populations is less able to resist brushtail possum herbivory, and suffers lower flowering as a consequence (Mann et al. 2012).
Both the burrowing, Bettongia lesueurii, and the rufous, Aepyprymnus rufescens, bettongs differ from the other, principally mycophagous, rat-kangaroos in consuming significant amounts of plant material. Although this is generally thought to comprise mostly roots and tubers (Claridge et al. 2007), foliage and branches can also be consumed, particularly in high-density populations (Bice and Moseby 2008; Linley et al. 2017). Woylies, Bettongia penicillata ogilbyi, too, include substantially higher amounts of plant food in their diet in a high-density, food-limited, fenced population than they do otherwise (Zosky et al. 2018). Anecdotally, bettong browsing has been suggested to have limited post-fire recruitment of eucalypt seedlings (Noble et al. 2007), and the decline of rufous bettongs was linked, again anecdotally, to increased recruitment of woody vegetation in western New South Wales (Rolls 1981). Noble et al. (2007) modelled the impact of browsing by burrowing bettongs, fire and rainfall on shrub population dynamics and suggested a potential for fire and browsing, either individually or in combination, to maintain low shrub densities.
As with grazing marsupials , the impacts of browsing marsupials may only become apparent in the absence of mechanisms that would otherwise suppress populations, or alter their foraging behavior (Pickett et al. 2005). This could include the cessation of hunting by humans, and a lack of predation by native owls (e.g., Kavanagh 1988), raptors and dingoes, due to human persecution, local extinction or exclusion from fenced reserves. The isolation of habitat patches, due to land clearing and the introduction of marsupial herbivores to islands, can also make dispersal impossible, thereby increasing population growth rates. For example, burrowing bettongs previously declined to extinction on mainland Australia, but now thrive in predator-exclusion reserves such as Arid Recovery in South Australia, where their densities are sufficient to reduce perennial plant species richness and reduce vegetation condition (Linley et al. 2017). These effects were not seen at lower bettong densities in the same habitat (Munro et al. 2009). Similarly, browsing by quokkas, Settonix brachyurus, acts, together with fire, to prevent tree seedling recruitment on Rottnest Island, Western Australia (Main 1992).
15.6.3 Conclusion
Australian mammalian herbivore communities have changed dramatically since the arrival of humans, which was followed by the loss of browsing and grazing megafauna. Not only the causes of these extinctions, but also their consequences continue to be debated, as researchers struggle to attribute evidence of changed vegetation to herbivory, fire and climate (Johnson 2006). Given the former diversity of large mammalian herbivores, one can imagine an impact on par with that of African herbivores today. A second wave of ecosystem change commenced with the invasion of Australia by Europeans 230 years ago. Since then, woody vegetation clearance, changes to fire regimes, provisioning of water, pasture improvement, and the introduction of exotic weedy and pasture species, have altered the environment in which marsupials forage. Australia’s apex predator, the dingo, has been rendered functionally extinct in large parts of the country, while exotic mesopredators such as the fox have had devasting impacts on many smaller mammalian herbivores. Grazing herbivores now coexist alongside, and possibly compete, with exotic sheep and cattle, rabbits, camels, goats and pigs, which themselves alter the foraging environment encountered by native marsupial herbivores (e.g., Cooke 1998; Tyndale-Biscoe 2005). As they have disappeared from across the landscape, or as the ecosystems around them have been altered, the prospect of understanding the former ecological role of many marsupial herbivores has slipped from our grasp, but much also remains to be learnt about their contemporary impacts.
15.7 Lagomorphs
Lagomorphs are small- to medium-sized mammals that inhabit a wide variety of habitats worldwide, both as native or introduced species (Hutchings and Harris 1996; Trout 2003). The lagomorph Order includes hares, Lepus spp., rabbits, Leporidae spp., and pikas, Ochotonidae spp., many of which are classed as endangered in their native ranges, with introduced species often becoming pests (Cowan and Hartley 2008; Jennings 2008). Despite their small size, lagomorphs can dramatically shape vegetation structure and composition, both positively and negatively (Boag et al. 1990; Crawley 1990; Van der Wal et al. 2000a, b; Stahl et al. 2006). The way land is managed can equally affect the distribution and behaviour of lagomorphs (Petrovan et al. 2013; Lush et al. 2014). Understanding the determinants of lagomorph distribution and behaviour, and interactions between these species and the ecosystems they inhabit (Sinclair et al. 2000), could provide management solutions for both conservation and biosecurity.
15.7.1 Feeding Ecology of Lagomorphs
Lagomorphs are selective feeders and can be both grazers and browsers, depending on the habitat, season and food availability (Chapuis 1990; Schai-Braun et al. 2015). In general, lagomorphs graze on grasses and herbs but may browse on saplings and woody plants, particularly when forage resources are limited during winter (Homolka 1982; Rao et al. 2003). Some species of hares, such as the Mountain hare, Lepus timidus, are specialist browsers, preferentially feeding on deciduous tree species (Hjältén et al. 2004).
Lagomorphs are hind gut fermenters and, unusually, they perform coprophagy (re-ingesting the soft faeces initially produced after a feeding bout) that enables them to more effectively digest lower quality forage (in comparison to other mammalian herbivore species their size) (Kuijper et al. 2004). This allows their diet to be incredibly varied and provides the flexibility to adapt to changes in plant availability, management of agricultural land or competition from other herbivores.
Rabbits and pikas are central placed foragers , which results in grazing gradients, with reduced grazing intensity and increased dietary selectivity in grasslands further away from their burrows or talus (Huntly 1987; McIntire and Hik 2005; Bakker et al. 2005). This can lead to higher standing biomass, vegetation height and decreased plant nutrient concentration at greater distances from burrows (Bakker et al. 2005). Unlike rabbits, pikas also carry out haying (caching plants) during periods of high vegetation biomass, enabling them to survive through periods when vegetation is dormant (Huntly et al. 1986). In contrast, hares often select fields with taller vegetation that provides cover from predation, and less intensively managed agricultural land, with higher levels of fat in plant material, whereas rabbits select for more intensively livestock grazed pastures with nitrogen rich shorter grass (Bakker et al. 2005; Lush et al. 2014, 2017).
15.7.2 Impacts of Lagomorph Grazing and Browsing
The flexible feeding ecology of lagomorphs can affect, both positively and negatively, the localised impact of their grazing behaviour on the landscape. For example, rabbits and pikas usually graze close to their burrows, which creates high intensity grazing areas (Huntly et al. 1986; Cowan et al. 1989). Hares can also affect vegetation structure through selectively browsing on plant species (Rose and Platt 1992), reducing woody biomass (Pease et al. 1979) and removing seedlings (Wong and Hickling 1999). This can benefit other species, for example, hare browsing restricted the growth of shrubs such as, Artemisia maritima and Atriplex portulacoides, on salt-marshes that created preferential foraging habitat for Brent geese (Van der Wal et al. 2000b). High population densities of lagomorphs can, therefore, lead to fundamental alterations to grassland structure and composition (Boag et al. 1990; Crawley 1990), and can cause damage to native grasses and forbs, resulting in reduced species richness, reduction in the area of grassland swards and increased weeds (Mutze et al. 2016). Studies, using exclusion experiments, revealed that preferential grazing of certain plants by lagomorphs reduced plant diversity and vegetation growth (Gibbens et al. 1993; McIntire and Hik 2005). However, these effects can be confounded by grazing by other types of herbivores, weather conditions and long-term effects of grazing pressure.
European rabbits, Oryctolagus cuniculus, have become a significant pest species in many countries (Cowan 1987). In the UK, damage to crops have been estimated at £115 million annually (Smith et al. 2007), and, in Australia are attributed to the loss of A$206 million in agriculture (Gong et al. 2009). However, rabbits can provide commercial revenue for example, A$36 million in Australia from the sale of products as a result of shooting (Gong et al. 2009).
Rabbit grazing can also benefit the environment, providing an important mechanism for maintaining certain habitats such as, calcareous grasslands, heathland and sand dune grasslands (Lees and Bell 2008; Trout 2003). When rabbits were removed from these habitats, plant biodiversity reduced and indirectly affected numbers of important invertebrate species (Barham and Stewart 2005). Similarly, the burrowing and grazing activity of pikas, Ochotona pallasi, has improved soil nutrients in arid habitats, and subsequently increased grass productivity, creating higher species diversity and vegetation abundance (Wesche et al. 2007; Yu et al. 2017). Although, in contrast, other studies found they had little impact on grassland plant species richness, and pikas were negatively affected by high intensity livestock grazing (Komonen et al. 2003).
Being prey species, lagomorphs can, to some extent, be naturally controlled by predators, such as the red fox, Vulpes vulpes, and lynx, Lynx lynx. There has been a 10 year cyclic relationship between snowshoe hare populations, Lepus americanus, and lynx, in North America, through a combination of predation and forage quantity and quality (Krebs et al. 2001). Similarly, rabbits, in their native ranges, are declining due to changes in land use, habitat loss and introduced viruses, resulting in declines in lynx populations (Virgós et al. 2003; Lees and Bell 2008). The changes in population density of lagomorphs can, in turn, affect grazing pressure, plant growth and composition.
15.7.3 Effect of Management on Lagomorphs
Agricultural intensification , and changes in management practices, have altered the habitat and food availability for lagomorphs. The winter planting of agricultural crops has provided an important food source for hares and rabbits, when other natural forage resources are limited; inadvertently resulting in an increase in rabbit populations (Tapper and Barnes 1986). Removal of hedgerows, woodlands and the planting of larger, monocultural fields, reduced habitat diversity and structure, which has negatively impacted some lagomorphs, such as the brown hare, Lepus europaeus, that require a diverse variety of plants and habitat structure (Tapper and Barnes 1986). Increased livestock grazing reduces the available forage and cover for lagomorphs, and has been shown to have a major impact on lagomorphs’ foraging distribution (jackrabbits, cottontails and brown hares), with higher numbers found on areas with moderate livestock grazing, as opposed to those areas that were heavily or lightly grazed (Milchunas et al. 1998; Karmiris and Nastis 2007). This could also create potential competition for resources between livestock and lagomorphs (Hulbert and Andersen 2001). Livestock grazing regimes, the application of fertiliser and planting different grass or crop species affects plant nutritional quality and, consequently, lagomorph diet (Bakker et al. 1983; Pavlů et al. 2006; Lush et al. 2017). In particular, the amount of nitrogen, crude fat and fibre available in plants have differing effects on lagomorph foraging distribution and body condition (Hackländer et al. 2002; Bakker et al. 2005; Lush et al. 2014).
15.7.4 Managing Lagomorph Grazing and Browsing
Biocontrol agents, such as, myxomatosis and rabbit haemorrhagic disease virus, were introduced in several countries to reduce the extremely high rabbit populations. Following the introduction of these agents, rabbit numbers in the UK reduced by 99.9% (Boag 1987), resulting in dramatic changes to the landscape, with increased grassland growth and regeneration of woodlands . The reduction in rabbit populations in the UK reduced plant diversity resulting in the extinction of some invertebrates , such as, the large blue butterfly, Maculinea arion, (Sumption and Flowerdew 1985). It also resulted in the decline of many predators that fed largely on rabbits such as, the stoat, Mustela ermineu, and buzzard, Buteo buteo, (Sumption and Flowerdew 1985); with knock on positive and negative effects on the environment and economy. Eradication, and control, of introduced rabbit populations, particularly on islands, has resulted in increased plant species richness and cover, although exotic plants often populate areas faster than native plants, highlighting the benefits of rabbit grazing in some situations (Schweizer et al. 2016). In Australia, the reduction of rabbits benefitted Australian agriculture by A$70 billion dollars (Cooke et al. 2013). Although, recently, increasing resistance to the virus, and different strains, have reduced the effectiveness of this control measure (Ross and Sanders 1984).
Less dramatic methods to control lagomorph populations and foraging behaviour can be mediated through changes in the structure and composition of available habitats (Boag 1987). Reduction of vegetation cover, removal of field boundaries and the use of set asides (removing areas of land from crop production creating strips of grassland at field edges), have reduced some crop losses from lagomorph grazing; however, at high lagomorph densities these become ineffective (Trout 2003). Studies found that planting of older, larger tree saplings reduced lagomorph browsing in plantations (McArthur and Appleton 2004); similarly, reducing tree planting density, and planting seedlings in tall vegetation to reduce their visibility, lowered browsing pressure by hares (Rao et al. 2003). Less intensive farming practices, such as reducing livestock grazing and other inputs to pastures e.g., fertilisers that alter the nutritional value of vegetation, could benefit some lagomorph species (Lush et al. 2014). However, each lagomorph species has differing nutritional requirements and therefore, changes could be positive or negative. Further research, and regular monitoring, is required to assess the impact of different management practices on lagomorph species and measure the effectiveness of management interventions at a landscape scale.
15.8 Rodents
Rodents outnumber all other orders of mammals, in term of number of species (Wilson and Reeder 2005); approximately 42% of mammals are rodents, which corresponds to around 2400 species. Rodent radiations have occurred repeatedly across the globe, and they occupy all continents with the exception of Antarctica (Lacher et al. 2016), showing a parallelism in morphological and ecological features in each radiation (Wood 1947; Aplin and Ford 2014). The success of rodents in colonizing different environments is directly related to rodent’s ability to exploit diverse food items, such as grasses, roots, leaves, fruits, seeds and insects. However, most rodents are herbivorous, or include some plant material in their diet, and have many adaptations for herbivory (Samuels 2009). This is apparent from the smallest rodents such as the pygmy jerboa, Salpingotulus michaelis (~4 g), that feed on desert-adapted leaves, to the largest living rodent, Hydrochoerus hydrochaeris (~60 kg), that feed on grasses, and even the largest extinct rodent, Josephoartigasia monesi (~1000 kg), is thought to have fed on soft vegetation and fruits (Rinderknecht and Blanco 2008).
A classification of rodents into browsing vs. grazing categories is uncommon compared to more traditional categories that divide rodents into chewing vs. gnawing for feeding habits, and into folivory (leaf eaters) vs. gramnivory (grass eaters), among others, for food preferences. A comparative assessment of adaptations for herbivory, considering these categories, reveals many morphological and physiological differences among living rodents, which are reflected in their impact on the ecosystems through feeding activities, reviewed below.
The herbivorous habit of rodents is related to their characteristic skull shape, with enlarged and ever-growing incisors that are adapted for biting (also gnawing ) (Lacher et al. 2016). The check teeth, which are found behind the incisors and the diastema, are effective at chewing and grinding of plant material. Gnawing with the incisors and grinding with the check teeth are the main activities performed by rodents whilst feeding. Both functions take place in alternation: when the incisors are engaging, the jaw is positioned forward in a position that means that the check teeth do not meet each other; when the check teeth are positioned for grinding and chewing, the incisors are not positioned to allow gnawing (Vaughan et al. 2015). This difference in position relates to a trade-off between specialization for gnawing or chewing , and rodents can be roughly classified depending on their jaw musculature and skull adaptations for increased gnawing over chewing or vice versa (Cox et al. 2012).
Morphological adaptations for feeding, mainly related to the position and form of masseter muscles, can be used to segregate rodents in three non-monophyletic groups: sciuromorph , hystricomorph and myomorph (Simpson 1945). In the sciuromorph rodents the masseter lateralis and temporalis muscles are relatively large and the masseter lateralis extends onto the rostrum (Korth 1994). This leads to changes in feeding processes that, together, increase the gnawing abilities of these rodents. Greater gnawing abilities are associated with feeding on large seeds, nuts and roots. Examples of sciuromorph rodents are squirrels, beavers and pocket gophers. In the hystricomorph rodents it is the masseter medialis that is greatly developed and extended, passing through an enlarged infraorbital foramen before attaching to the rostrum (Wood 1965; Korth 1994). Rodents with this adaptation have an increased ability to perform varied movements with the jaw, resulting in an improved capacity for chewing and grinding at the same time (Cox et al. 2012). These adaptations allow increased processing efficiency when feeding on plant material, and most strictly grazing rodents have this morphotype. Examples of hystricomorph rodents are jerboas plus some Old-World rodents, the South American porcupines and many other caviomorph species; the largest rodent in the world, the South American capybara, Hydrochoerus hydrochaeris, is a grazing rodent with these features (Herrera 2013). The myomorph rodents have a combination of features from both the sciuromorph and the hystricomorph morphotypes, where the masseter lateralis extends onto the rostrum, as in sciuromorphs, and the masseter medialis passes inside the infraorbital foramen, as in hystricomorphs (Korth 1994). This combination of features produces a phenotype capable of performing both gnawing and chewing functions that leads to effective feeding on seeds, fruits, grains and plant material in general (Cox et al. 2012; Maestri et al. 2016).
Dietary shifts also trigger corresponding changes in the shape of the jawbone (e.g., Hautier et al. 2012; Maestri et al. 2016). A narrow angular process of the mandible is usually associated with the hystricomorph rodents, while a more robust angular process characterizes sciuromorphy. Even among hystricomorphs, those families feeding exclusively on grasses have a narrower and thinner angular process than those feeding on fruits and seeds (Hautier et al. 2011). Furthermore, a shorter, and curved, diastema, and a deeper ramus, characterize families that feed on fruits and seeds, as opposed to those that feed on grasses (Hautier et al. 2011). Other general differences in skull morphology occur according to whether a species feeds on grasses versus fruits and seeds (see Hautier et al. 2012), and still another set of morphological characteristics are found among rodents that feed on meat (e.g., Woollard et al. 1978; Rowe et al. 2016).
Herbivory in rodents can also be augmented by adaptations of the teeth (e.g., Willians and Kay 2001; Ma et al. 2016). Hypsodonty , one of the hallmarks of herbivory for mammals in general (Saarinen Chap. 2), is also a feature of herbivorous rodents. High crowned check teeth can increase the capacity for processing plant material consumed during grazing, and these type of teeth are found, for instance, among caviomorphs and several species of small rats and mice. A few studies of South American sigmodontines rodents have shown that the proportion of high-crowned teeth is greatest in species found in cold and dry and semiarid climates, while few high-crowned species are present in wet and hot climates (see Madden 2015). In addition , high teeth crowns, and larger check teeth, are positively correlated with the more seasonal and open environments (Madden 2015; Maestri et al. 2017).
Another related adaptation is the development of ever-growing check teeth in some caviomorph rodents such as the caviids and chinchillids. Nevertheless, relating hypsodonty to diet in rodents is limited by the scarcity of work on dietary composition for rodents in general (Madden 2015; Arregoitia 2016). Analyses of stomach contents using, for example, metabarcoding (Lopes et al. 2015), have revealed a great overlap in plant families consumed by rodent species. Intestinal physiology studies can contribute by comparing the concentration of types of bacteria between rodents and grazing ungulates, revealing, for example, a similarity in intestinal physiology between the capybara and bovids (Borges et al. 1996, see Herrera 2013 for a distinction between foregut and hindgut fermenters ). Enamel microwear investigations are also a useful tool that helped to classify grazing and browsing mammals (Townsend and Croft 2008), and are applicable to distinguish food preferences (e.g., omnivore, frugivore, gramnivore) among rodents (Caporale and Ungar 2016). Multiple approaches are urgently needed to analyse rodent diet composition, so as to move away from the broad description of rodents as “opportunistic” and “omnivorous”.
Given the diversity and wide geographical distribution of rodent species, and the propensity of its members to feed on many types of plant material, it is important to understand how the foraging behavior of rodents impacts ecosystems through grazing and browsing activities. A few studies have investigated the impacts of rodents on ecosystems through grazing (e.g., Howe et al. 2002; Bilodeau et al. 2014) and browsing (Ravolainen et al. 2014), yet most of the literature has focused on large mammals or rabbits (previous section Katona and Coetsee Chap. 12). Nevertheless, the impact of grazing rodents could be, at least, as great as that of rabbits at some regions, as in South America: Madden (2015) suggests that the invasion of South America by herbivorous caviomorph rodents (see also Saarinen Chap. 2) could have generated increased soil erosion resulting from their grazing activities. For example, there is evidence that the feeding behavior and burrowing activities of tuco-tucos (genus Ctenomys) reduces forage and leads to vegetation changes and habitat degradation (Jackson 1988). When these rodents cover large areas, they can alter the soil dynamics and the vegetation growth (Massoia 1970; Galiano et al. 2014). Similarly, the feeding behavior of vizcacha (genus Lagostomus), another caviomorph, can lead to reductions in vegetation cover and abundance and they even compete with livestock for food; they are considered a pest in some areas of Argentina (Jackson 1988).
The effects of grazing and browsing of small rats and mice might also impact ecosystems, but may go unnoticed due to the difficulty of measuring their impacts. Limited direct evidence exists on the role of small cricetid rodents in damaging pastures and being pests of cultivated plants (Jackson 1988). Nevertheless, damage of human cultivated crops, such as rice and cereal grains, by small rodents, has been estimated to cause a billions of dollars per year of damage worldwide (Lacher et al. 2016). Using experimental plots in England, Hulme (1994, 1996) compared the impact of small rodents and invertebrates on seedlings predation and the growth of grasslands. He showed that rodents and molluscs had similar negative effects on herbaceous seedlings, consuming about 30% within a given plot. Nevertheless, rodents had their greatest effect on the growth of grassland plants, being responsible for a 50% reduction in mean plant biomass, greater than the impact of molluscs or arthropods. Hagenah et al. (2009) found a proportionally higher effect of murid rodents on plant biomass in a South African savanna, after excluding large herbivores from experimental plots; the exclusion of large herbivores had the effect of increasing small rodent abundance (Hagenah et al. 2009; Luza et al. 2018). Howe and Brown (1999) compared the effects of herbivory between small browsing rodents and birds in a tallgrass praire, and found that the voles had negative effects on grass biomass and altered plant community structure, especially in low-density communities; while the effects of bird were more pronounced in high-density plantings. Browsing voles, such as Microtus pennsylvanicus, can even be considered a plague in prairie grasslands in North America, because they can cause drops in plant diversity (37% drop in Simpson’s diversity index), greatly altering community composition (Howe et al. 2002).
Other studies have found contrasting results, showing that rodents have negligible top-down control on plant assemblages, which were mostly regulated by bottom-up processes, such as in native grasslands of North America (Báez et al. 2006). Similarly, grazing by lemmings had very weak effects on plant biomass at the Canadian arctic (Bilodeau et al. 2014). Therefore, while rodents have a pronounced effect on growth of grasses in some cases, the literature on the effects of browsing and grazing by rodents shows varying overall impacts on ecosystems. Furthermore work is needed, not just focusing specifically on rodents but also comparing the effects of rodents with those of large mammals, to reach a consensus about how great are the ecosystem impacts of grazing and browsing by rodents.
15.9 Primates
Non-human primates (hereafter referred to as ‘primates’) cover various trophic niches, from nearly exclusive folivory to frugivory, gummivory, insectivory and omnivory; although the majority of species are folivorous, frugivorous, or both (Campbell et al. 2011). One exceptional species, the gelada baboon, Theropithecus gelada, was thought to be graminivorous (a ‘grazer’); however, a recent study showed that geladas also consume a substantial amount of non-grass (non-monocot) foods, implying that they are not strict grazers as previously thought (Fashing et al. 2014). Folivorous primates that exhibit hindgut and foregut fermentation such as colobines (foregut fermenters : Asia and Africa), lemurs (hindgut fermenters : Africa) and gorillas (hindgut fermenters: Africa) are widely distributed in the Old World, and hindgut fermenting howlers are found in the New World (Mittermeier et al. 2013). Recent studies on the feeding ecology of some primates, technically classified as folivorous, have reported high levels of fruit and/or seed consumption in response to local habitat conditions (Campbell et al. 2011). Thus, classifying primates as strictly folivorous is not a simple matter.
15.9.1 Anatomical Adaptations to Diet
In parallel to the dichotomy of hindgut and foregut fermenters seen in mammals, primates generally have two contrasting digestive strategies: high intake with fast throughput for low digestive efficiency or low intake with slow throughput for high digestive efficiency (Clauss et al. 2008). Folivorous primates that exhibit foregut fermentation are mostly limited to the high digestive efficiency strategy (Clauss et al. 2008). Another key digestive strategy in folivorous primates entails fine-tuning of salivary protein composition. Howler monkeys, the most folivorous New World primates, show continuous expression of tannin-binding salivary proline-rich proteins; this allows them to consume a diet with variable tannin content (Espinosa Gómez et al. 2015). Such salivary tannin defences have been demonstrated in a few primate species, including omnivorous baboons and macaques (Espinosa-Gómez et al. 2018). On the other hand, as do a great variety of other grazing mammals, graminivorous geladas completely lack proline-rich proteins, and a capacity to bind tannins, demonstrating their narrower dietary niche compared to that of other baboon species (Mau et al. 2009). Given that colobines generally have large salivary glands, they may use a similar strategy to howlers (Kay et al. 1976; Matsuda et al. 2017b); however, this hypothesis awaits further testing.
The microbial community in the gastrointestinal tract is believed to play an important role in facilitating the consumption of hard-to-digest foods, such as leaves of trees and grasses. As a result of recent developments in sequencing technology, gut microbiota analysis, based on large amplicon libraries of 16S ribosomal RNA (rRNA) genes and mostly faecal DNA, has increasingly been conducted on folivorous primates (Ley et al. 2008). In folivorous primates, the distal gut microbiome varies even within a species according to diet and/or living conditions (Amato et al. 2013; Clayton et al. 2017); gut microbial diversity in captive primates is generally reduced compared with that in their wild counterparts. A recent study suggested that gastrointestinal distress in folivorous primates, such as infestation with or disease caused by parasites, may be associated with an imbalance between the types of organism present in their natural microflora, especially that of the gut (Amato et al. 2016).
Nutritional studies have revealed that both hindgut and foregut fermenters generally prefer leaves rich in protein and lower in fibre (Ganzhorn et al. 2016). However, there are some folivorous primates that do not display a strong preference, indicating that a preference for protein depends on the overall protein availability in the environment; the preference for protein is only clearly demonstrated in environments with a low average protein content (Ganzhorn et al. 2016). Other factors that may affect dietary selection in folivorous primates are mechanical toughness and leaf digestibility . However, little information is available regarding primates’ dietary choices, and studies evaluating a variety of nutritional and mechanical factors with diet digestibility are particularly lacking. One study providing new insights into in vitro digestibility, toughness and nutrients of leaves shows that the preferred leaves of foregut fermenting proboscis monkeys not only contain more protein and less fibre but are also less tough and more digestible than the alternatives (Matsuda et al. 2017b).
15.9.2 Behavioural Adaptations to Diet
The way a primate rests could be related to their digestive physiology . Among folivorous primates, this is most evident in colobines , that have a characteristic long resting period (over 70% of daylight hours) sitting with a vertical posture (Matsuda et al. 2017a). This might be because the position of the digestive chamber, and the need to frequently eructate digestive gases, force colobines to assume a posture that reduces pressure on the thorax and respiratory organs. In contrast to ruminants that are characterised by a sorting mechanism in their forestomach that operates based on the density of different-sized food particles (Lechner-Doll et al. 1991), with smaller particles generally having a higher density than larger ones (Clauss et al. 2009), experiments with captive colobines have shown no evidence for a forestomach sorting mechanism (Schwarm et al. 2009; Matsuda et al. 2015, 2019). Passage studies in general suggest that, as a group, primates do not pass solutes faster than particles, i.e., lack the physiological or mechanical ability to wash particulate digesta with fluids in the digestive tract that has evolved in all other mammalian herbivore clades (Müller et al. 2011). An interesting peculiarity is that a non-obligatory rumination-like behaviour has been demonstrated in one colobine species, the proboscis monkey (Matsuda et al. 2011; Matsuda et al. 2014).
As with most primates, folivorous primates live in groups varying in their sex-composition and -disperal, e.g., one-male-multi-female or multi-female-multi-male groups with both-sex-dispersal or female-philopatric/male-dispersal. As leaves are generally abundant and evenly distributed in their habitats, socioecological models indicate that food competition within folivorous primate groups can be assumed to be weak/absent and that populations and groups are not constrained by the availability of food (Wrangham 1980; Janson and Goldsmith 1995; Sterck et al. 1997). Increasing day range with increasing group size has been used as a behavioural indicator of food competition within primate groups, and this hypothesis on weak/absent of food competition is supported by the fact that a flat relationship exists between group size and day range across various folivorous primates (e.g., Yeager and Kirkpatrick 1998). However, many folivorous primates, despite this assumed lack of feeding competition within their groups, often live in relatively small groups, though larger groups are expected to have lower costs of predation due to better detection of predators. This inconsistency is referred to as the ‘folivore paradox ’ (Steenbeek and van Schaik 2001). Indeed, the results contradict the assumption of leaves as ubiquitous or non-patchy resources in some colobines , e.g., red colobus, Procolobus rufomitratus, (Snaith and Chapman 2005) and gray langurs, Semnopithecus entellus, (Sayers and Norconk 2008). Infanticide has been suggested as a mechanism that could regulate group sizes in groups with a high proportion of males, although this theory alone cannot explain the folivore paradox in all cases (reviewed by Snaith and Chapman 2007).
15.9.3 Conservation of Primates
Nearly half of all primate species are threatened with extinction as a result of habitat destruction and poaching , and folivorous primates are no exception (Estrada et al. 2017). A fundamental challenge facing primate conservation today is a lack of knowledge regarding the status of endangered populations (Wich and Marshall 2016). Such information is essential in order to develop effective, long-term management plans for conservation. Therefore, understanding the determinants of abundance in folivorous primates is of utmost importance (Chapman and Peres 2001). It has been suggested that the biomass of folivorous primates, especially colobines , is related to the protein-to-fibre ratio of the leaves in their habitats (Chapman et al. 2002). In line with this protein-to-fibre model, the effect of global change on folivorous populations has been examined; a decline in colobine abundance may be explained by the fact that the fibre concentration in their consumed leaves has increased and protein content has decreased over the past 30 years (Rothman et al. 2015).
Contrary to the earlier reports that colobine monkeys are primarily seed-eaters (e.g., Sun et al. 2007), an impact of colobines on their ecosytems as seed dispersers has recently been reported in several taxa such as Nasalis, Presbytis, Rhinopithecus and Trachypithecus (Tsuji et al. 2017; Chen et al. 2018; McConkey 2018; Thiry et al. 2019). Even if they are not comparatively efficient endozoochorous and epizoochorous seed dispersers in forest ecosystems, their high abundance and biomass could make them quantitatively significant in seed dispersal (Matsuda et al. 2013).
Available data showing the impact of climate change on food quality and colobine abundance are very limited, and as colobines have been considered as seed dispersers only very recently, the available data testing its impact on their living-ecosystem are also scarce. Thus, this remains a highly relevant topic that can aid, not only in a basic understanding of colobine population and behavioural ecology, but also in the development of conservation strategies for colobine species. Further work on the importance of colobines in ecological processes (e.g., seed dispersal , nutrient cycling) and their reduced populations on the dynamics of ecosystems is clearly needed.
References
ABC News (2013) Woodchip giant Australian Bluegum plantations stripped of environmental certification over koala deaths
Abrahams PW (2013) Geophagy and the involuntary ingestion of soil. In: Selinus O (ed) Essentials of medical geology. Springer, Dordrecht
Alisauskas RT, Rockwell RF, Dufour KW, Cooch EG, Zimmerman G, Drake KL, Leafloor JO, Moser TJ et al (2011) Harvest, survival, and abundance of midcontinent lesser snow geese relative to population reduction efforts. Wildl Monogr 179:1–42
Allcock KG, Hik DS (2004) Survival, growth, and escape from herbivory are determined by habitat and herbivore species for three Australian woodland plants. Oecologia 138:231–241
Amato KR et al (2013) Habitat degradation impacts black howler monkey (Alouatta pigra) gastrointestinal microbiomes. ISME J 7:1344–1353
Amato KR et al (2016) Using the gut microbiota as a novel tool for examining colobine primate GI health. Glob Ecol Conserv 7:225–237
Anderson JT, Nuttle T, Rojas JSS et al (2011) Extremely long-distance seed dispersal by an overfished amazonian frugivore. Proc R Soc B Biol Sci 278:3329–3335. https://doi.org/10.1098/rspb.2011.0155
Anderson HB, Godfrey TG, Woodin SJ, Van der Wal R (2012) Finding food in a highly seasonal landscape: where and how pink footed geese (Anser brachyrhynchus) forage during the Arctic spring. J Avian Biol 43:415–422
Anderson HB, Speed JDM, Madsen J, Pedersen ÅØ, Tombre I, Van der Wal R (2016) Late snow melt moderates herbivore disturbance of the Arctic tundra. Ecoscience 23:29–39
Aplin K, Ford F (2014) Murine rodents: late but highly successful invaders. In: Prins HHT, Gordon IJ (eds) Invasion biology and ecological theory: insights from a continent in transformation. Cambridge University Press, Cambridge, pp 196–240
Arce-Nazario JA, Carlo TA (2012) Iguana iguana invasion in Puerto Rico: facing the evidence. Biol Invasions 14(9):1981–1984
Arman SD, Prideaux GJ (2015) Dietary classification of extant kangaroos and their relatives (Marsupialia: Macropodoidea). Austral Ecol 40:909–922
Arregoitia LDV (2016) Rethinking omnivory in rodents. Preprint https://doi.org/10.20944/preprints201609.0017.v1
Arzel C, Elmberg J, Guillemain M (2006) Ecology of spring-migrating Anatidae: a review. J Ornithol 147:167–184
Ashton DH, Chappill JA (1989) Secondary succession in post-fire scrub dominated by Acacia verticillata (L’Herit) Willd at Wilsons promontory, Victoria. Aust J Bot 37:1–18
Attum O et al (2010) Retention rate of hard-released translocated Egyptian tortoises Testudo kleinmanni. Endanger Species Res 12(1):11–15. https://doi.org/10.3354/esr00271
Badam GL (1981) Colossochelys atlas, a Giant tortoise from the upper Siwaliks of North India. Bull Deccan Coll Res Inst 40:149–153
Báez S, Collins SL, Lightfoot D, Koontz TL (2006) Bottom-up regulation of plant community structure in an aridland ecosystem. Ecology 87:2746–2754
Bakker RT (1978) Dinosaur feeding behaviour and the origin of flowering plants. Nature 274:661–663
Bakker RJ (1986) The dinosaur heresies. Kensington Publishing, New York
Bakker JP, de Leeuw J, Van Wieren SE (1983) Micro-patterns in grassland vegetation created and sustained by sheep-grazing. Vegetatio 55:153–161
Bakker ES, Reiffers RC, Olff H, Gleichman JM (2005) Experimental manipulation of predation risk and food quality: effect on grazing behaviour in a central-place foraging herbivore. Oecologia 146:157–167. https://doi.org/10.1007/s00442-005-0180-7
Bakker ES, Veen C, Ter Heerdt G, Huig N, Sameel J (2018) High grazing pressure of geese threatens conservation and restoration of reed belts. Front Plant Sci:12. https://doi.org/10.3389/fpls.2018.01649
Bamber RK, Humphreys FR (1965) Variations in sapwood starch levels in some Australian forest species. Aust For 29:15–23
Barham DF, Stewart AJA (2005) Differential indirect effects of excluding livestock and rabbits from chalk Heath on the associated leafhopper (Hemiptera: Auchenorrhyncha) Fauna. J Insect Conserv 9:351–361. https://doi.org/10.1007/s10841-005-0517-x
Barnes A, Hill GJE (1992) Estimating kangaroo damage to winter wheat crops in the Bungunya district of southern Queensland. Wildl Res 19:417–427
Barrett PM, Willis KJ (2001) Did dinosaurs invent flowers? Dinosaur–angiosperm coevolution revisited. Biol Rev 76:411–447
Barton PS, Manning AD, Gibb H, Wood JT, Lindenmayer DB, Cunningham SA (2011) Experimental reduction of native vertebrate grazing and addition of logs benefit beetle diversity at multiple scales. J Appl Ecol 48:943–951
Baxter GS, Moll EJ, Lisle AT (2001) Pasture grazing by black-striped wallabies (Macropus dorsalis) in Central Queensland. Wildl Res 28:269–276
Bayliss P, Choquenot D (2002) The numerical response: rate of increase and food limitation in herbivores and predators. Philos Trans R Soc Lond B Biol Sci 357:1233–1248
Becerra-López JL et al (2017) Effect of climate change on halophytic grasslands loss and its impact in the viability of Gopherus flavomarginatus. Nat Conserv 21:39–55
Bedoya-Pérez M, Lawes M, McMahon C (2017) Final report: protecting vulnerable land from high wallaby densities. Charles Darwin University and Meat and Livestock Australia, North Sydney
Beer S (1989) Photosynthesis and photorespiration in marine phytoplankton. Aquat Bot 34:105–130. https://doi.org/10.1016/0304-3770(89)90052-1
Bellwood DR, Hughes TP, Hoey AS (2006) Sleeping functional group drives coral-reef recovery. Curr Biol 16:2434–2439. https://doi.org/10.1016/j.cub.2006.10.030
Ben-Ami D, Boom K, Boronyak L, Townend C, Ramp D, Croft DB, Bekoff M (2014) The welfare ethics of the commercial killing of free-ranging kangaroos: an evaluation of the benefits and costs of the industry. Anim Welf 23:1–10
Bennett S, Wernberg T, Harvey ES et al (2015) Tropical herbivores provide resilience to a climate-mediated phase shift on temperate reefs. Ecol Lett 18:714–723. https://doi.org/10.1111/ele.12450
Benson RB, Campione NE, Carrano MT et al (2014) Rates of dinosaur body mass evolution indicate 170 million years of sustained ecological innovation on the avian stem lineage. PLoS Biol 12(5):e1001853. https://doi.org/10.1371/journal.pbio.1001853
Best HA (1984) The foods of kakapo on Stewart Island as determined from their feeding sign. N Z J Ecol 7:71–83
Bice JK, Moseby KE (2008) Diet of the re-introduced greater bilby (Macrotis lagotis) and burrowing bettong (Bettongia lesueur) in the arid recovery reserve, northern South Australia. Aust Mammal 30:1–12
Bilodeau F, Gauthier G, Fauteux D, Berteaux D (2014) Does lemming winter grazing impact vegetation in the Canadian Arctic? Polar Biol 37:845–857
Bjorndal KA (1980) Nutrition and grazing behavior of the green turtle Chelonia mydas. Mar Biol 56(2):147–154
Blake S et al (2012) Seed dispersal by Galápagos tortoises. J Biogeogr 39(11):1961–1972. https://doi.org/10.1111/j.1365-2699.2011.02672.x
Blake S et al (2013) Vegetation dynamics drive segregation by body size in Galapagos tortoises migrating across altitudinal gradients. J Anim Ecol 82(2):310–321
Boag B (1987) Reduction in numbers of the wild rabbit (Oryctolagus cuniculus) due to changes in agricultural practices and land use. Crop Prot 6:347–351
Boag B, Macfarlane Smith WH, Griffiths DW (1990) Effects of grazing by wild rabbits (Oryctolagus cuniculus) on the growth and yield of oilseed and fodder rape (Brasslca napus sub. Sp. oleifera). Crop Prot 9:155–159
Bonaldo RM, Bellwood DR (2008) Size-dependent variation in the functional role of the parrotfish Scarus rivulatus on the great barrier reef, Australia. Mar Ecol Prog Ser 360:237–244. https://doi.org/10.3354/meps07413
Borges PA, Dominguez-Bello MG, Herrera EA (1996) Digestive physiology of wild capybara. J Comp Physiol B 166:55–60
Bourn D et al (1999) The rise and fall of the Aldabran giant tortoise population. Proc R Soc Lond Ser B Biol Sci 266(1424):1091–1100. https://doi.org/10.1098/rspb.1999.0748
Box SJ, Mumby PJ (2007) Effect of macroalgal competition on growth and survival of juvenile Caribbean corals. Mar Ecol Prog Ser 342:139–149. https://doi.org/10.3354/meps342139
Branch WR, Baard E, De Villiers A (1990) Some exceptionally large southern African chelonians. Afr J Herpetol 37(1):53–54
Breed B, Ford F (2007) Native mice and rats. CSIRO Publishing, Collingwood, Vic
Bucher EH, Tamburini D, Abril A, Torres P (2003) Folivory in the white-tipped plantcutter Phytotoma rutila: seasonal variations in diet composition and quality. J Avian Biol 34:211–216
Buij R, Melman TCP, Loonen MJJE, Fox AD (2017) Balancing ecosystem function, services and disservices resulting from expanding goose populations. Ambio 46:301–318
Bulinski J, McArthur C (1999) An experimental field study of the effects of mammalian herbivore damage on Eucalyptus nitens seedlings. For Ecol Manag 113:241–249
Bulinski J, McArthur C (2003) Identifying factors related to the severity of mammalian browsing damage in eucalypt plantations. For Ecol Manag 183:239–247
Burkepile DE, Hay ME (2010) Impact of herbivore identity on algal succession and coral growth on a Caribbean reef. PLoS One. https://doi.org/10.1371/journal.pone.0008963
Burness GP, Diamond J, Flannery T (2001) Dinosaurs, dragons, and dwarfs: the evolution of maximal body size. Proc Natl Acad Sci 98(5):14518–14523
Butler RJ, Barrett PM, Kenrick P et al (2009) Diversity patterns amongst herbivorous dinosaurs and plants during the cretaceous: implications for hypotheses of dinosaur/angiosperm co-evolution. J Evol Biol 22:446–459
Button DJ, Rayfield EJ, Barrett PM (2014) Cranial biomechanics underpins high sauropod diversity in resource-poor environments. Proc R Soc B 281:20142114. https://doi.org/10.1098/rspb.2014.2114
Calder JA, Kirkpatrick JB (2008) Climate change and other factors influencing the decline of the Tasmanian cider gum (Eucalyptus gunnii). Aust J Bot 56:684–692
Campbell CJ, Fuentes A, MacKinnon KC, Bearder SK, Stumpf RM (2011) Primates in perspective. Oxford University Press, New York
Caporale SS, Ungar PS (2016) Rodent incisor microwear as a proxy for ecological reconstruction. Palaeogeogr Palaeoclimatol Palaeoecol 446:225–233
Cardoso SC, Soares MC, Oxenford HA, Côté IM (2009) Interspecific differences in foraging behaviour and functional role of Caribbean parrotfish. Mar Biodivers Rec 2:e148. https://doi.org/10.1017/S1755267209990662
Carpenter RC (1986) Partitioning Herbivory and its effects on coral reef algal communities. Ecol Monogr 56:345–363. https://doi.org/10.2307/1942551
Caviedes-Vidal E, McWhorter TJ, Lavin SR, Chediack JG, Tracy CR, Karasov WH (2007) The digestive adaptation of flying vertebrates: high intestinal paracellular absorption compensates for smaller guts. PNAS 104:19132–19137
Chaichana R, Leah R, Moss B (2010) Birds as eutrophicating agents: a nutrient budget for a small lake in a protected area. Hydrobiologia 646:111–121
Chapman CA, Peres CA (2001) Primate conservation in the new millennium: the role of scientists. Evol Anthropol: Issues News Rev 10:16–33
Chapman CA, Chapman LJ, Bjorndal KA, Onderdonk DA (2002) Application of protein-to-fiber ratios to predict colobine abundance on different spatial scales. Int J Primatol 23:283–310
Chapuis JL (1990) Comparison of the diets of two sympatric lagomorphs, Lepus europaeus (Pallas) and Oryctolagus cuniculus (L.) in an agroecosystem of the Ile-de-France. Zeitschrift fur Saugetierkd 55:176–185
Cheke A, Hume JP (2010) Lost land of the dodo: the ecological history of Mauritius, Réunion and Rodrigues. Yale University Press, London
Chen Y, Chen H, Zhang Y, Yao H, Yang W, Zhao Y, Ruan X, Xiang Z (2018) First evidence of epizoochorous seed dispersal by golden snub-nosed monkeys (Rhinopithecus roxellana) in temperate forest. Plant Ecol 219:417–427
Chin K, Feldmann RM, Tashman JN (2017) Consumption of crustaceans by megaherbivorous dinosaurs: dietary flexibility and dinosaur life history strategies. Sci Rep 7(1):11163. https://doi.org/10.1038/s41598-017-11538-w
Choat JH, Robbins WD, Clements KD (2004) The trophic status of herbivorous fishes on coral reefs. Mar Biol 145:445–454. https://doi.org/10.1007/s00227-004-1341-7
Christian A (2010) Some sauropods raised their necks—evidence for high browsing in Euhelopus zdanskyi. Biol Lett 6:823–825
Christianen MJA et al (2014) Habitat collapse due to overgrazing threatens turtle conservation in marine protected areas. Proc R Soc Lond B Biol Sci 281(1777):20132890
Clancy TF, Croft DB (1992) Population dynamics of the common wallaroo (Macropus robustus erubescens) in arid New South Wales. Wildl Res 19:1–16
Claridge AW, Seebeck JH, Rozse R (2007) Bettongs, potoroos and the musky rat-kangaroo. CSIRO Publishing, Collingwood, Victoria
Clauss M, Streich WJ, Nunn CL, Ortmann S, Hohmann G, Schwarm A, Hummel J (2008) The influence of natural diet composition, food intake level, and body size on ingesta passage in primates. Comp Biochem Physiol A Mol Integr Physiol 150:274–281
Clauss M, Fritz J, Bayer D, Nygren K, Hammer S, Hatt JM, Südekum KH, Hummel J (2009) Physical characteristics of rumen contents in four large ruminants of different feeding type, the addax (Addax nasomaculatus), bison (Bison bison), red deer (Cervus elaphus) and moose (Alces alces). Comp Biochem Physiol A Mol Integr Physiol 152:398–406
Clayton JB et al (2017) Associations between nutrition, gut microbiome, and health in a novel nonhuman primate model. bioRxiv:177295
Clements KD, Raubenheimer D, Choat JH (2009) Nutritional ecology of marine herbivorous fishes: ten years on. Funct Ecol 23:79–92. https://doi.org/10.1111/j.1365-2435.2008.01524.x
Close DC, Paterson S, Corkrey R, McArthur C (2010) Influences of seedling size, container type and mammal browsing on the establishment of Eucalyptus globulus in plantation forestry. New For 39:105–115
Coe MJ, Dilcher DL, Farlow JO, Jarzen DM, Russell DA (1987) Dinosaurs and land plants. In: Friis EM, Chaloner WG, Crane PR (eds) Origins of angiosperms and their biological consequences. Cambridge University Press, Cambridge, pp 225–258
Collinson ME (1996) ‘What use are fossil ferns?’ ± 20 years on: with a review of the fossil history of extant pteridophyte families and genera. In: Camus JM, Gibby M, Johns RJ (eds) Pteridology in perspective. Kew, Royal Botanic Gardens, London, pp 349–394
Conkin J, Alisauskas RT (2017) Conversion of tundra to exposed peat habitat by snow geese (Chen caerulescens caerulescens) and Ross’s geese (C. rossii) in the Central Canadian Arctic. Polar Biol 40:563–576
Connell SD, Russell BD, Irving AD (2011) Can strong consumer and producer effects be reconciled to better forecast “catastrophic” phase-shifts in marine ecosystems? J Exp Mar Bio Ecol 400:296–301. https://doi.org/10.1016/j.jembe.2011.02.031
Cooke BD (1998) Did introduced rabbits Oryctolagus cuniculus (L.) displace common wombats Vombatus ursinus (Shaw) from paert of their range in South Australia? In: Wells RT, Pridmore PA (eds) Wombats. Surrey Beatty, Chipping Norton, pp 262–270
Cooke BD, Mutze GJ (2018) How introduced rabbits Oryctolagus cuniculus limit the abundance of red kangaroos Macropus Rufus and other native grazers in Australia. Food Webs 15:e00079
Cooke B, Chudleigh P, Simpson S, Saunders G (2013) The economic benefits of the biological control of rabbits in Australia, 1950-2011. Aust Econ Hist Rev 53:91–107. https://doi.org/10.1111/aehr.12000
Cooper WE, Vitt LJ (2002) Distribution, extent, and evolution of plant consumption by lizards. J Zool 257(4):487–517
Coulson GM (2007) Exploding kangaroos: assessing problems and setting targets. In: Lunney D, Eby P, Hutchings P, Burgin S (eds) Pest or guest: the zoology of overabundance. Royal Zoological Society of New South Wales, Sydney
Couslon GM, Norbury GL (1988) Ecology and management of western grey kangaroos (Macropus fuliginosus) at Hattah-Kulkyne National Park. Arthur Rylah Institute for Environmental Research, Conservation, Forests & Lands, National Parks and Wildlife Division, Melbourne
Cowan DP (1987) Group living in the European rabbit (Oryctolagus cuniculus): mutual benefit or resource localization? J Anim Ecol 56:779–795
Cowan P (2007) How many possums make a cow? N Z J Ecol 31:261–262
Cowan DP, Hartley FG (2008) Order lagomorpha: rabbit. In: Harris S, Yalden D (eds) Mammals of the British Isles handbook, 4th edn. The Mammal Society, London, pp 201–210
Cowan PE, Waddington DC (1990) Suppression of fruit production of the endemic forest tree, Elaeocarpus dentatus, by introduced marsupial brushtail possums, Trichosurus vulpecula. N Z J Bot 28:217–224
Cowan DP, Hardy AR, Vaughan JP, Christie WG (1989) Rabbit ranging behaviour and its implications for the management of rabbit populations. In: Putman RJ (ed) Mammals as pests. The Mammal Society, London, pp 178–185
Cox PG, Rayfield EJ, Fagan MJ, Herrel A, Pataky TC, Jeffery N (2012) Functional evolution of the feeding system in rodents. PLoS One 7:e36299
Crawley MJ (1990) Rabbit grazing, plant competition and seedling recruitment in acid grassland. J Appl Ecol 27(3):803–820
Croft DB (1991) Home range of the red kangaroo Macropus rufus. J Arid Environ 20:83–98
Cunningham P (2000) Daily activity pattern and diet of a population of the Spinytailed lizard, Uromastyx aegyptius microlepis, during summer in the United Arab Emirates. Zool Middle East 21(1):37–46
Currie J, Koppelhus EB, Muhammad AF (1995) “Stomach” contents of a hadrosaur from the Dinosaur Park Formation (Campanian, Upper Cretaceous) of Alberta, Canada. In: Sun AY, Wang Y (eds) Sixth symposium of Mesozoic terrestrial ecosystems and biota. China Ocean, Beijing, pp 111–114
Cusack JJ, Duthie AD, Sarobidy O, Rocío R, Pozo A, Mason THE, Månsson J, Nilsson L, Tombre IM, Eythórsson E, Madsen J, Tulloch A, Hearn RD, Redpath S, Bunnefeld N (2018) Time series analysis reveals synchrony and asynchrony between conflict management effort and increasing large grazing bird populations in northern Europe. Conserv Lett 12:e12450
Cuthbertson RS, Tirabasso A, Rybczynski N et al (2012) Kinetic limitations of intracranial joints in Brachylophosaurus canadensis and Edmontosaurus regalis (Dinosauria: Hadrosauridae), and their implications for the chewing mechanics of hadrosaurids. Anat Rec 295:968–979
Dawson TJ (2012) Kangaroos. CSIRO Publishing, Collingwood, VIC
Dawson TJ, Ellis BA (1994) Diets of mammalian herbivores in Australian arid shrublands - seasonal effects on overlap between red kangaroos, sheep and rabbits on dietary niche breadths and selectivities. J Arid Environ 26:257–271
Dawson TJ, Ellis BA (1996) Diets of mammalian herbivores in Australian arid, hilly shrublands: seasonal effects on overlap between euros (hill kangaroos), sheep and feral goats, and on dietary niche breadths and electivities. J Arid Environ 34:491–506
Dawson TJ, Munn AJ (2007) How much do kangaroos of differing age and size eat relative to domestic stock?: implications for the arid rangelands. In: Dickman C, Lunney D, Burgin S (eds) Animals of arid Australia: out on their own. Royal Zoological Society of New South Wales, Mosman, NSW, pp 96–101
Department of Environment and Heritage Protection, Queensland (2018) Overview of the Queensland macropod industry. Version 1.5
Department of Environment, Land, Water and Planning, Victoria (2016) Koalas at Cape Otway: Managing Koalas at Cape Otway (Factsheet)
Department of Environment, Land, Water and Planning, Victoria (2017) Kangaroo Population Management. Melbourne
Department of Environment, Land, Water and Planning, Victoria (2018) Koalas in blue gum plantations
Department of Environment, Water, and Natural Resources, Government of South Australia (2017) South Australian Commercial Kangaroo Management Plan 2018–2022. Adelaide
Department of Parks and Wildlife, Western Australia (2013) Management Plan for the Commercial Harvest of Kangaroos in Western Australia 2014–2018. Perth
Department of Sustainability and Environment, Victoria (2004) Victoria’s Koala Management Strategy. Melbourne
Dexter N, Hudson M, James S, MacGregor C, Lindenmayer DB (2013) Unintended consequences of invasive predator control in an Australian Forest: overabundant wallabies and vegetation change. PLoS One 8:e69087
Di Stefano J (2005) Mammalian browsing damage in the Mt. Cole state Forest, southeastern Australia: analysis of browsing patterns, spatial relationships and browse selection. New For 29:43–61
Di Stefano J, Anson JA, York A, Greenfield A, Coulson G, Berman A, Bladen M (2007) Interactions between timber harvesting and swamp wallabies (Wallabia bicolor): space use, density and browsing impact. For Ecol Manag 253:128–137
Dokter AM, Fokkema W, Ebbinge BS, Olff H, Van der Jeugd H, Nolet BA (2018) Agricultural pastures challenge the attractiveness of natural saltmarsh for a migratory goose. J Appl Ecol 55:2707–2718
du Toit JT (1990) Feeding-height stratification among African browsing ruminants. Afr J Ecol 28:55–61
Duncan RP, Holland EP, Pech RP, Barron M, Nugent G, Parkes JP (2011) The relationship between possum density and browse damage on kamahi in New Zealand forests. Austral Ecol 36:858–869
Durant D (2003) The digestion of fibre in herbivorous Anatidae – a review. Wild 54:7–24
Edwards G, Dawson T, Croft D (1995) The dietary overlap between red kangaroos (Macropus rufus) and sheep (Ovis aries) in the arid rangelands of Australia. Austral Ecol 20:324–334
Edwards GP, Croft DB, Dawson TJ (1996) Competition between red kangaroos (Macropus rufus) and sheep (Ovis aries) in the arid rangelands of Australia. Aust J Ecol 21:165–172
Eldridge DJ, Delgado-Baquerizo M, Travers SK, Val J, Oliver I (2017) Do grazing intensity and herbivore type affect soil health? Insights from a semi-arid productivity gradient. J Appl Ecol 54:976–985
Eldridge DJ, Delgado-Baquerizo M, Travers SK, Val J, Oliver I, Dorrough JW, Soliveres S (2018) Livestock activity increases exotic plant richness, but wildlife increases native richness, with stronger effects under low productivity. J Appl Ecol 55:766–776
Elfwing T, Tedengren M (2000) A comparison of production effects between corals and macroalgae at increased seawater temperature. Proc Ninth Int Coral Reef Symp 2:1139–1142
Erickson GM, Krick BA, Hamilton M, Bourne GR, Norell MA, Lilleodden E, Sawyer WG (2012) Complex dental structure and wear biomechanics in hadrosaurid dinosaurs. Science 338:98–101
Eskildsen LI, Olesen JM, Jones CG (2004) Feeding response of the Aldabra giant tortoise (Geochelone gigantea) to island plants showing heterophylly. J Biogeogr 31(11):1785–1790
Espinosa Gómez F, Santiago García J, Gómez Rosales S, Wallis IR, Chapman CA, Morales Mávil J, Canales Espinosa D, Hernández SL (2015) Howler monkeys (Alouatta palliata mexicana) produce tannin-binding salivary proteins. Int J Primatol 36:1086–1100
Espinosa-Gómez FC, Serio-Silva JC, Santiago-García JD, Sandoval-Castro CA, Hernández-Salazar LT, Mejía-Varas F, Ojeda-Chávez J, Chapman CA (2018) Salivary tannin-binding proteins are a pervasive strategy used by the folivorous/frugivorous black howler monkey. Am J Primatol 80:e22737. https://doi.org/10.1002/ajp.22737
Espinoza RE, Wiens JJ, Tracy CR (2004) Recurrent evolution of herbivory in small, cold-climate lizards: breaking the ecophysiological rules of reptilian herbivory. Proc Natl Acad Sci 101(48):16819–16824
Estrada A et al (2017) Impending extinction crisis of the world's primates: why primates matter. Sci Adv 3:e1600946
Fa JE, Peres CA, Meeuwig J (2002) Bushmeat exploitation in tropical forests: an intercontinental comparison. Conserv Biol 16(1):232–237
Falcón W, Hansen DM (2018) Island rewilding with giant tortoises in an era of climate change. Philos Trans R Soc Lond B Biol Sci 373(1761):20170442
Falcon, W., Hansen, D. M. and Moll, D. (2018) Frugivory and seed dispersal by chelonians: a review and synthesis, bioRxiv, p. 379933
Farlow JO (1976) A consideration of the trophic dynamics of a Late Cretaceous large-dinosaur community (Oldman formation). Ecology 57:841–857
Farlow JO, Coroian ID, Foster JR (2010) Giants on the landscape: modelling the abundance of megaherbivorous dinosaurs of the Morrison formation (late Jurassic, western USA). Hist Biol 22:403–429
Fashing PJ, Nguyen N, Venkataraman VV, Kerby JT (2014) Gelada feeding ecology in an intact ecosystem at Guassa, Ethiopia: variability over time and implications for theropith and hominin dietary evolution. Am J Phys Anthropol 155:1–16
Fearnside PM (2018) Challenges for sustainable development in Brazilian Amazonia. Sustain Dev 26(2):141–149
Feng ZL, Liu RS, DeAngelis DL, Bryant JP, Kielland K, Stuart Chapin F, Swihart R (2009) Plant toxicity, adaptive herbivory, and plant community dynamics. Ecosystems 12:534–547
Fernandez FA et al (2012) How sustainable is the use of natural resources in Brazil? Natureza & Conservação 10(1):77–82
Fiorelli LE et al (2016) A new late cretaceous crocodyliform from the western margin of Gondwana (La Rioja Province, Argentina). Cretac Res 60:194–209
Flemming SA, Calvert A, Nol E, Smith PA (2016) Do hyperabundant Arctic-nesting geese pose a problem for sympatric species? Environ Rev 24:1–10
Foster C, Barton P, Wood J, Lindenmayer D (2015) Interactive effects of fire and large herbivores on web-building spiders. Oecologia 179:237–248
Fowler MC, Robson TO (1978) The effects of food preference and stocking rates of grass carp (Ctenopharyngodon idella VAL.) on mixed plant communities. Aquat Bot 5:261–276
Fox AD, Abraham KF (2017) Why geese benefit from the transition from natural vegetation to agriculture. Ambio 46:S188–S197
Fox AD, Madsen J (2017) Threatened species to super-abundance: the unexpected international implications of successful goose conservation. Ambio 46:179–187
Fox AD, Elmberg J, Tombre IM, Hessel R (2017) Agriculture and herbivorous waterfowl: a review of the scientific basis for improved management. Biol Rev 92:854–877. https://doi.org/10.1111/brv.12258
Frank ASK, Wardle GM, Greenville AC, Dickman CR (2016) Cattle removal in arid Australia benefits kangaroos in high quality habitat but does not affect camels. Rangel J 38:73–84
Franklin P, Dunbar M, Whitehead P (2008) Flow controls on lowland river macrophytes: a review. Sci Total Environ 400:369–378. https://doi.org/10.1016/j.scitotenv.2008.06.018
Fricke HC, Hencecroth J, Hoerner ME (2011) Lowland–upland migration of sauropod dinosaurs during the late Jurassic epoch. Nature 480:513–515
Frith HJ (1978) Wildlife conservation. Angus and Robertson, Sydney
Froyd CA et al (2014) The ecological consequences of megafaunal loss: Giant tortoises and wetland biodiversity. Ecol Lett 17(2):144–154. https://doi.org/10.1111/ele.12203
Gaines SD, Lubchenco J (1982) A unified approach to marine plant-herbivore interactions. II. Biogeography. Annu Rev Ecol Syst 13:111–138
Galiano D, Kubiak BB, Overbeck GE, de Freitas TRO (2014) Effects of rodents on plant cover, soil hardness, and soil nutrient content: a case study on tuco-tucos (Ctenomys minutus). Acta Theriol 59:583–587
Ganzhorn JU et al (2016) The importance of protein in leaf selection of folivorous primates. Am J Primatol 79:1–13
García M, Gerber GP (2016) Conservation and management of Cyclura iguanas in Puerto Rico. Herpetol Conserv Biol 11:61–67
Garrigues L, Cadi A (2011) Re-introduction of African spurred tortoise in north Ferlo, Senegal. In: Soorae P (ed) Global re-introduction perspectives: more case studies from around the globe. IUCN/SSC Re-introduction Specialist Group & Environment Agency-ABU DHABI, Abu Dhabi, pp 94–97
Gee CT (2011) Dietary options for the sauropod dinosaurs from an integrated botanical and paleobotanical perspective. In: Klein N, Remes K, Gee CT, Sander PM (eds) Biology of the sauropod dinosaurs: understanding the life of giants. Indiana University Press, Bloomington, pp 34–56
Genes L et al (2017) Credit of ecological interactions: a new conceptual framework to support conservation in a defaunated world. Ecol Evol 7(6):1892–1897. https://doi.org/10.1002/ece3.2746
Gerlach J (2014) Western Indian Ocean tortoises: ecology, diversity, evolution, conservation, Palaeontology. Siri Scientific Press, Rocjdale
Gibbens RP, Havstad KM, Billheimer DD, Herbel CH (1993) Creosotebush vegetation after 50 years of lagomorph exclusion. Oecologia 94:210–217. https://doi.org/10.1007/BF00341319
Gibbons J (1984) Iguanas of the South Pacific. Oryx 18(2):82–91
Gibbs JP, Marquez C, Sterling EJ (2008) The role of endangered species reintroduction in ecosystem restoration: tortoise–Cactus interactions on Española Island, Galápagos. Restor Ecol 16(1):88–93. https://doi.org/10.1111/j.1526-100X.2007.00265.x
Gibbs JP, Sterling EJ, Zabala FJ (2010) Giant tortoises as ecological engineers: a long-term quasi-experiment in the Galápagos Islands. Biotropica 42(2):208–214. https://doi.org/10.1111/j.1744-7429.2009.00552.x
Gibbs JP et al (2014) Demographic outcomes and ecosystem implications of giant tortoise reintroduction to Española Island, Galapagos. PLoS One 9(10):e110742
Gibson CWD, Hamilton J (1983) Feeding ecology and seasonal movements of giant tortoises on Aldabra atoll. Oecologia 56(1):84–92
Godoy-Vitorino F, Goldfarb KC, Karaoz U, Leal S, Garcia-Amado MA, Hugenholtz P, Tringe SG, Brodie EL, Dominguez-Bello MG (2011) Comparative analyses of foregut and hindgut bacterial communities in hoatzins and cows. ISME J 6:531–541
Gong W, Sinden J, Braysher M, Jones R (2009) The economic impacts of vertebrate pests in Australia. Invasive Animals Cooperative Research Centre, Canberra
Gordon IJ, Prins HHT (2008) The ecology of browsing and grazing. Springer, Berlin
Grajal A, Strahl SD, Parra R, Dominguez MG, Neher A (1989) Foregut fermentation in the hoatzin, a neotropical leaf-eating bird. Science 245:1236–1238
Grandmaison DD, Ingraldi MF, Peck FR (2010) Desert tortoise microhabitat selection on the Florence military reservation, south-Central Arizona. J Herpetol 44(4):581–590
Grant TD, Hudson RD (2015) West Indian iguana Cyclura spp reintroduction and recovery programmes: zoo support and involvement. Int Zoo Yearb 49(1):49–55
Greaves WS (1995) Functional predictions from theoretical models of the skull and jaws of reptiles and mammals. In: Thomason JJ (ed) Functional morphology in vertebrate paleontology. Cambridge University Press, Cambridge, pp 99–115
Green AL, Bellwood DR (2009) Monitoring functional groups of herbivorous reef fishes as indicators of coral reef resilience – a practical guide for coral reef managers in the Asia Pacific region. Switzerland, IUCN working group on Climate Change and Coral Reefs, Gland, p 70
Griffiths CJ et al (2010) The use of extant non-indigenous tortoises as a restoration tool to replace extinct ecosystem engineers. Restor Ecol 18(1):1–7. https://doi.org/10.1111/j.1526-100X.2009.00612.x
Griffiths CJ et al (2011) Resurrecting extinct interactions with extant substitutes. Curr Biol 21:762–765. https://doi.org/10.1016/j.cub.2011.03.042
Griffiths CJ et al (2013) Assessing the potential to restore historic grazing ecosystems with tortoise ecological replacements. Conserv Biol 27(4):690–700. https://doi.org/10.1111/cobi.12087
Gyimes A, Van Rooij AP, Nolet BA (2010) Nonlinear effects of food aggregation on interference competition in mallards. Behav Ecol Sociobiol 64:1897–1904
Gyimesi A, De Vries PP, De Boer T, Nolet BA (2011) Reduced tuber banks of fennel pondweed due to summer grazing by waterfowl. Aquat Bot 36:24–28
Haas K, Köhler U, Diehl S, Köhler P, Dietrich S, Holler S, Jaensch A, Niedermaier M, Vilsmeier J (2007) Influence of fish on habitat choice of water birds: a whole system experiment. Ecology 88:2915–2925
Hackländer K, Tataruch F, Ruf T (2002) The effect of dietary fat content on lactation energetics in the European hare (Lepus europaeus). Physiol Biochem Zool 75:19–28. https://doi.org/10.1086/324770
Hagenah N, Prins HHT, Olff H (2009) Effects of large herbivores on murid rodents in a south African savanna. J Trop Ecol 25:483–492
Hamann O (1993) On vegetation recovery, goats and giant tortoises on Pinta Island, Galápagos, Ecuador. Biodivers Conserv 2(2):138–151. https://doi.org/10.1007/BF00056130
Handa IT, Harmsen R, Jefferies RL (2002) Patterns of vegetation change and the recovery potential of degraded areas in a coastal marsh system of the Hudson Bay lowlands. J Ecol 90:86–99
Hansen DM, Galetti M (2009) The forgotten megafauna. Science (New York, NY) 324(5923):42–43. https://doi.org/10.1126/science.1172393
Hansen DM et al (2010) Ecological history and latent conservation potential: large and giant tortoises as a model for taxon substitutions. Ecography 33(2):272–284. https://doi.org/10.1111/j.1600-0587.2010.06305.x
Harvie AE (1973) Diet of the opossum (Trichosurus vulpecula Kerr) on farmland northeast of Waverley, New Zealand. Proc N Z Ecol Soc 20:48–52
Hastings AK et al (2014) Domination by reptiles in a terrestrial food web of the Bahamas prior to human occupation. J Herpetol 48(3):380–388. https://doi.org/10.1670/13-091R1
Hautier L, Lebrun R, Saksiri S, Michaux J, Vianey-Liaud M, Marivaux L (2011) Hystricognathy vs sciurognathy in the rodent jaw: a new morphometric assessment of hystricognathy applied to the living fossil Laonastes (Diatomyidae). PLoS One 6:e18698
Hautier L, Lebrun R, Cox PG (2012) Patterns of covariation in the masticatory apparatus of hystricognathous rodents: implications for evolution and diversification. J Morphol 273:1319–1337
Hearne EL et al (2018) Effects of green turtle grazing on seagrass and macroalgae diversity vary spatially among seagrass meadows. Aquat Bot 152:10–15
Heath ME, Mcginnis SM (1980) Body temperature and heat transfer in the Green Sea turtle, Chelonia mydas. Coastal Mar Sci 1980(4):767–773
Heithaus MR et al (2014) Seagrasses in the age of sea turtle conservation and shark overfishing. Front Mar Sci 1:28. https://doi.org/10.3389/fmars.2014.00028
Herrera EA (2013) Capybara digestive adaptations. In: Capybara. Springer, New York, NY, pp 97–106
Hessen DO, Tombre IM, Van Geest G, Alfsnes K (2017) Global change and ecosystem connectivity: how geese link fields of central Europe to eutrophication of Arctic freshwaters. Ambio 46:40–47
Hik DS, Jefferies RL (1990) Increases in the net above-ground primary production of a salt-marsh forage grass: a test of the predictions of the herbivore optimization model. J Ecol 78:180–195
Hjältén J, Danell K, Ericson L (2004) Hare and vole browsing preferences during winter. Acta Theriol (Warsz) 49:53–62
Hoegh-Guldberg O (1999) Climate change, coral bleaching and the future of the world’s coral reefs. Mar Freshw Res 50:839–866. https://doi.org/10.1071/MF99078
Hofmann RR, Stewart DRM (1972) Grazer or browser: a classification based on the stomach-structure and feeding habits of east African ruminants. Mammalia 36:226–240
Holland EP, Pech RP, Ruscoe WA, Parkes JP, Nugent G, Duncan RP (2013) Thresholds in plant-herbivore interactions: predicting plant mortality due to herbivore browse damage. Oecologia 172:751–766
Holland EP, Gormley AM, Pech RP (2016) Species- and site-specific impacts of an invasive herbivore on tree survival in mixed forests. Ecol Evol 6:1954–1966
Homolka M (1982) The food of Lepus europaeus in a meadow and woodland complex. Folia Zool 31:243–253
Horn MH, Correa SB, Parolin P et al (2011) Seed dispersal by fishes in tropical and temperate fresh waters: the growing evidence. Acta Oecol 37:561–577. https://doi.org/10.1016/j.actao.2011.06.004
Howe HF, Brown JS (1999) Effects of birds and rodents on synthetic tallgrass communities. Ecology 80:1776–1781
Howe HF, Brown JS, Zorn-Arnold B (2002) A rodent plague on prairie diversity. Ecol Lett 5:30–36
Howland B, Stojanovic D, Gordon IJ, Manning AD, Fletcher D, Lindenmayer DB (2014) Eaten out of house and home: impacts of grazing on ground-dwelling reptiles in Australian grasslands and grassy woodlands. PLoS One 9:e105966
Howland BW, Stojanovic D, Gordon IJ, Fletcher D, Snape M, Stirnemann IA, Lindenmayer DB (2016a) Habitat preference of the striped legless lizard: implications of grazing by native herbivores and livestock for conservation of grassland biota. Austral Ecol 41:455–464
Howland BW, Stojanovic D, Gordon IJ, Radford J, Manning AD, Lindenmayer DB (2016b) Birds of a feather flock together: using trait-groups to understand the effect of macropod grazing on birds in grassy habitats. Biol Conserv 194:89–99
Hughes TP, Rodrigues MJ, Bellwood DR et al (2007) Phase shifts, herbivory, and the resilience of coral reefs to climate change. Curr Biol 17:360–365. https://doi.org/10.1016/j.cub.2006.12.049
Hughes TP, Anderson KD, Connolly SR, Heron SF, Kerry JT, Lough JM, Baird AH, Baum JK, Berumen ML, Bridge TC, Claar DC, Eakin CM, Gilmour JP, Graham NAJ, Harrison H, Hobbs J-PA, Hoey AS, Hoogenboom M, Lowe RJ, McCulloch MT, Pandolfi JM, Pratchett M, Schoepf V, Torda G, Wilson SK (2018) Spatial and temporal patterns of mass bleaching of corals in the Anthropocene. Science 359(6371):80–83
Hulbert IA, Andersen R (2001) Food competition between a large ruminant and a small hindgut fermentor: the case of the roe deer and mountain hare. Oecologia 128:499–508. https://doi.org/10.1007/s004420100683
Hulme PE (1994) Seedling herbivory in grassland: relative impact of vertebrate and invertebrate herbivores. J Ecol 82:873–880
Hulme PE (1996) Herbivores and the performance of grassland plants: a comparison of arthropod, mollusk and rodent herbivory. J Ecol 84:43–51
Hume ID (1999) Marsupial Nutrition. Cambridge University Press, Cambridge
Hummel J, Clauss M (2011) Sauropod feeding and digestive physiology. In: Remes K, Gee CT, Sander PM (eds) Biology of the sauropod dinosaurs: understanding the life of giants. Indiana University Press, Bloomington, pp 11–33
Hummel J, Gee CT, Südekum KH et al (2008) In vitro digestibility of fern and gymnosperm foliage: implications for sauropod feeding ecology and diet selection. Proc R Soc B 275:1015–1021
Hunt A, Al-Nakkash L, Lee AH, Smith HF (2019) Phylogeny and herbivory are related to avian cecal size. Sci Rep 9:4243
Hunter EA, Gibbs JP (2014) Densities of ecological replacement herbivores required to restore plant communities: a case study of giant tortoises on Pinta Island, Galápagos. Restor Ecol 22(2):248–256
Huntly NJ (1987) Influence of refuging consumers (pikas: Ochotona princeps) on subalpine meadow vegetation. Ecology 68:274–283. https://doi.org/10.2307/1939258
Huntly NJ, Smith AT, Ivins BL (1986) Foraging behavior of the Pika (Ochotona princeps), with comparisons of grazing versus haying. J Mammal 67:139–148
Hutchings MR, Harris S (1996) The current status of the brown hare (Lepus europaeus) in Britain. 1–76. https://doi.org/10.1007/s13398-014-0173-7.2
HVP Plantations (2018) Koalas. https://www.hvp.com.au/hvp-environment-conservation/koalas/. Accessed 9 July 2018
Hynes EF, Handasyde KA, Shaw G, Renfree MB (2010) Levonorgestrel, not etonogestrel, provides contraception in free-ranging koalas. Reprod Fertil Dev 22:913–919
Jackson JE (1988) Terrestrial mammalian pests in Argentina—an overview. Proc Vertebr Pest Conf 13:196–198
Jackson SM (2015) Taxonomy of Australian mammals. CSIRO Publishing, Collingwood
Jano AP, Jefferies RL, Rockwell RF (1998) The detection of vegetational change by multitemporal analysis of LANDSAT data: the effects of goose foraging. J Ecol 86:93–99
Janson CH, Goldsmith ML (1995) Predicting group size in primates: foraging costs and predation risks. Behav Ecol 6:326–336
Jarvis ED et al (2014) Whole-genome analyses resolve early branches in the tree of life of modern birds. Science 346:1320–1331
Jefferies RL, Rockwell RF, Abraham KE (2004) Agricultural food subsidies, migratory connectivity and large-scale disturbance in arctic coastal systems: a case study. Integr Comp Biol 44:130–139
Jefferies RL, Jano AP, Abraham KF (2006) A biotic agent promotes large-scale catastrophic change in the coastal marshes of Hudson Bay. J Ecol 94:234–242
Jennings NV (2008) Order lagomorpha: Brown hare. In: Harris S, Yalden D (eds) Mammals of the British Isles handbook, 4th edn. The Mammal Society, London, pp 210–220
Jetz W, Thomas GH, Joy JB, Hartmann K, Mooers AO (2012) The global diversity of birds in space and time. Nature 491:444–448
Johnson CN (2006) Australia's mammal extinctions: a 50,000 year history. Cambridge University Press, Cambridge
Jones CG (2002) Reptiles and amphibians. In: Perrow M, David A (eds) Handbook of ecological restoration. Cambridge University Press, New York, pp 355–375
Jonzen N, Pople AR, Grigg GC, Possingham HP (2005) Of sheep and rain: large-scale population dynamics of the red kangaroo. J Anim Ecol 74:22–30
Karmiris IE, Nastis AS (2007) Intensity of livestock grazing in relation to habitat use by brown hares (Lepus europaeus). J Zool 271:193–197. https://doi.org/10.1111/j.1469-7998.2006.00199.x
Kavanagh RP (1988) The impact of predation by the powerful owl, Ninox strenua, on a population of the greater glider, Petauroides volans. Aust J Ecol 13:445–450
Kay RNB, Hoppe P, Maloiy GMO (1976) Fermentative digestion of food in the colobus monkey Colobus polykomos. Experientia 32:485–487
Kennedy MA, Heck KL, Michot TC (2018) Impacts of wintering redhead ducks (Aythya americana) on seagrasses in the northern Gulf of Mexico. J Exp Mar Biol Ecol 506:42–48
Kershaw JA (1934) The koala on Wilsons promontory. Victorian Nat 51:76–77
King GM (1996) Reptiles and herbivory. Springer Science & Business Media, New York
Kirkpatrick J (2016) Pattern and process in riparian vegetation of the treeless high country of Australia. In: Capon S, James C, Reid M (eds) Vegetation of Australian riverine landscapes: biology, ecology and management. CSIRO Publishing, Melbourne, pp 201–220
Klaasen M, Nolet BA (2007) The role of herbivorous water birds in aquatic systems through interactions with aquatic macrophytes, with special reference to the Bewick’s swan – fennel pondweed system. Hydrobiologia 584:205–213
Klein RG (1984) Mammalian extinctions and stone age people in Africa. In: Martin PS, Klein RG (eds) Quaternary extinctions: a prehistoric revolution. University of Arizona Press, Tucson, pp 553–573
Kley NJ et al (2010) Craniofacial morphology of Simosuchus clarki (Crocodyliformes: Notosuchia) from the late cretaceous of Madagascar. J Vertebr Paleontol 30(1):13–98
Kollars NM, Henry AK, Whalen MA, Boyer KE, Cusson M, Eklöf JS, Hereu CM, Jorgensen P, Kiriakopolos SL, Reynolds PL, Tomas F, Turner MS, Ruesink JL (2017) Meta-analysis of reciprocal linkages between temperate seagrasses and waterfowl with implications for conservation. Front Plant Sci 8:2119
Köhler G (2004) Conservation status of spiny-tailed iguanas (genus Ctenosaura), with special emphasis on the Utila Iguana (C. bakeri). Iguana 11:207–211
Komonen M, Komonen A, Otgonsuren A (2003) Daurian pikas (Ochotona daurica) and grassland condition in eastern Mongolia. J Zool 259:281–288
Korth WW (1994) The tertiary record of rodents in North America. Springer Science, New York
Krebs CJ, Boonstra R, Boutin S, Sinclair ARE (2001) What drives the 10-year cycle of snowshoe hares? Bioscience 51:25. https://doi.org/10.1641/0006-3568(2001)051[0025,WDTYCO]2.0.CO;2
Kułakowska KA, Kułakowski TM, Inglis R, Smith GC, Haynes PJ, Prosser P, Thorbek P, Sibly RM (2014) Using an individual-based model to select among alternative foraging strategies of woodpigeons: data support a memory-based model with a flocking mechanism. Ecol Model 280:89–101
Kuffner IB, Walters LJ, Becerro MA et al (2006) Inhibition of coral recruitment by macroalgae and cyanobacteria. Mar Ecol Prog Ser 323:107–117. https://doi.org/10.3354/meps323107
Kuijper DJP, van Wieren SE, Bakker JP (2004) Digestive strategies in two sympatrically occurring lagomorphs. J Zool 264:171–178. https://doi.org/10.1017/S0952836904005722
Lacher TE, Murphy WJ, Rogan J, Smith AT, Upham NS (2016) Evolution, phylogeny, ecology, and conservation of the clade Glires: Lagomorpha and Rodentia. In: Wilson DE, Lacher JTE, Mittermeier RA (eds) Handbook of mammals of the world, volume 6: lagomorphs and rodents. Lynx Ediciones, Barcelona, pp 15–26
Lambert MRK (1993) On growth, sexual dimorphism, and the general ecology of the African spurred tortoise, Geochelone sulcata. Chelonian Conserv Biol 4:37–46
Laurel MH, Richard E, Amy L (2000) Germination rates of seeds consumed by two species of rock iguanas (Cyclura spp.) in the Dominican Republic. Caribb J Sci 36(1–2):149–151
LeBlanc AR, Reisz RR, Evans DC, Bailleul AM (2016) Ontogeny reveals function and evolution of the hadrosaurid dinosaur dental battery. BMC Evol Biol 16:152. https://doi.org/10.1186/s12862-016-0721-1
Lechner-Doll M, Kaske M, Engelhardt WV (1991) Factors affecting the mean retention time of particles in the forestomach of ruminants and camelids. In: Tsuda T, Sasaki Y, Kawashima R (eds) Physiological aspects of digestion and metabolism in ruminants. Academic, San Diego, pp 455–482
Lees AC, Bell DJ (2008) A conservation paradox for the 21st century: the European wild rabbit Oryctolagus cuniculus, an invasive alien and an endangered native species. Mamm Rev 38:304–320. https://doi.org/10.1111/j.1365-2907.2008.00116.x
Lefebvre J, Gauthier G, Giroux J-F, Reed A, Reed ET, Bélanger L (2017) The greater snow goose Anser caerulescens atlanticus: managing an overabundant population. Ambio 46:262–274
Leguat F (2017) The voyage of François Leguat of Bresse to Rodriguez, Mauritius, Java, and the Cape of Good Hope: volume I. Hakluyt Society, London
Letnic M, Koch F, Gordon C, Crowther MS, Dickman CR (2009) Keystone effects of an alien top-predator stem extinctions of native mammals. Proc Biol Sci 276:3249–3256
Ley RE et al (2008) Evolution of mammals and their gut microbes. Science 320:1647–1651
Lichtenbelt WDM (1992) Digestion in an ectothermic herbivore, the green iguana (Iguana iguana): effect of food composition and body temperature. Physiol Zool 65(3):649–673
Linley GD, Moseby KE, Paton DC (2017) Vegetation damage caused by high densities of burrowing bettongs (Bettongia lesueur) at arid recovery. Aust Mammal 39:33–41
Lodge DM (1991) Herbivory on freshwater macrophytes. Aquat Bot 41:195–224. https://doi.org/10.1016/0304-3770(91)90044-6
Lodge DM, Cronin G, van Donk E, Froelich AJ (1998) Impact of Herbivory on plant standing crop: comparisons among biomes, between vascular and nonvascular plants, and among freshwater herbivore taxa. In: Jeppesen E et al (eds) The structuring role of submerged Marcophytes in lakes. Springer, New York, pp 149–174
Lokrantz J, Nyström M, Thyresson M, Johansson C (2008) The non-linear relationship between body size and function in parrotfishes. Coral Reefs 27:967–974. https://doi.org/10.1007/s00338-008-0394-3
Lopes CM, Barba M, Boyer F, Mercier C, Filho PJSS, Heidtmann LM, Galiano D, Kubiak BB, Langone PQ, Garcias FM, Giely L, Coissac E, Freitas TRO, Taberlet P (2015) DNA metabarcoding diet analysis for species with parapatric vs sympatric distribution: a case study on subterranean rodents. Heredity 114:525–536
Lopes LE, Fernandes AM, Medeiros MCI, Marini MA (2016) A classification scheme for avian diet types. J Field Ornithol 87:309–322
Lovich JE et al (2018) Where have all the turtles gone, and why does it matter? Bioscience XX(X):1–11. https://doi.org/10.1093/biosci/biy095
Low T (2002) The new nature: winners and losers in wild Australia. Penguin, Melbourne
Loyn RH, Middleton WGD (1980) Eucalypt decline and wildlife in rural areas. In: Old KM, Kile GA, Ohmart CP (eds) Eucalypt dieback in forests and woodlands. Forest Research CSIRO, Melbourne
Lush L, Ward AI, Wheeler P (2014) Opposing effects of agricultural intensification on two ecologically similar species. Agric Ecosyst Environ 192:61–66. https://doi.org/10.1016/j.agee.2014.03.048
Lush L, Ward AI, Wheeler P (2017) Dietary niche partitioning between sympatric brown hares and rabbits. J Zool 303:36–45. https://doi.org/10.1111/jzo.12461
Luza AL, Trindade JPP, Maestri R, Duarte LDS, Hartz SM (2018) Rodent occupancy in grassland paddocks subjected to different grazing intensities in South Brazil. Perspect Ecol Conserv. https://doi.org/10.1016/j.pecon.2018.06.006
Ma H, Ge D, Shenbrot G, Pisano J, Yang Q, Zhang Z (2016) Hypsodonty of Dipodidae (Rodentia) in correlation with diet preferences and habitats. J Mamm Evol. https://doi.org/10.1007/s10914-016-9352-y
Madden RH (2015) Hypsodonty in mammals: evolution, geomorphology and the role of earth surface processes. Cambridge University Press, Cambridge
Maestri R, Patterson BD, Fornel R, Monteiro LR, Freitas TRO (2016) Diet, bite force and skull morphology in the generalista rodent morphotype. J Evol Biol 29:2191–2204
Maestri R, Monteiro LR, Fornel R, Freitas TRO, Patterson BD (2017) Geometric morphometrics meets metacommunity ecology: environment and lineage distribution affects spatial variation in shape. Ecography 41:90–100
Main AR (1992) Management to retain biodiversity in the face of uncertainty. In: Hobbs RJ (ed) Biodiversity in Mediterranean ecosystems in Australia. Surrey Beatty & Sons, Chipping Norton, NSW
Makarieva AM, Gorshkov VG, Li B-L (2005) Gigantism, temperature and metabolic rate in terrestrial poikilotherms. Proc R Soc B Biol Sci 272(1578):2325–2328. https://doi.org/10.1098/rspb.2005.3223
Malhia Y et al (2016) Megafauna and ecosystem function from the Pleistocene to the Anthropocene. Proc Natl Acad Sci 113(4):838–846
Mallon JC, Anderson JS (2014a) Implications of beak morphology for the evolutionary paleoecology of the megaherbivorous dinosaurs from the Dinosaur Park formation (upper Campanian) of Alberta, Canada. Palaeogeogr Palaeoclimatol Palaeoecol 394:29–41
Mallon JC, Anderson JS (2014b) The functional and palaeoecological implications of tooth morphology and wear for the megaherbivorous dinosaurs from the Dinosaur Park formation (upper Campanian) of Alberta, Canada. PLoS One 9(6):e98605. https://doi.org/10.1371/journal.pone.0098605
Mallon JC, Evans DC, Ryan MJ, Anderson JS (2013) Feeding height stratification among the herbivorous dinosaurs from the Dinosaur Park formation (upper Campanian) of Alberta, Canada. BMC Ecol 13(1):14. https://doi.org/10.1186/1472-6785-13-14
Mann AN, O'Reilly-Wapstra JM, Iason GR, Sanson G, Davies NW, Tilyard P, Williams D, Potts BM (2012) Mammalian herbivores reveal marked genetic divergence among populations of an endangered plant species. Oikos 121:268–276
Mannion PD, Upchurch P, Carrano MT et al (2011) Testing the effect of the rock record on diversity: a multidisciplinary approach to elucidating the generic richness of sauropodomorph dinosaurs through time. Biol Rev 86:157–181
Marks CA (1998) Review of the humaneness of destruction techniques used on the common wombat Vombatus ursinus in Victoria. In: Wells RT, Pridmore PA (eds) Wombats. Surrey Beatty and Sons, Sydney, pp 287–297
Martin PS (1984) Prehistoric overkill: the global model. In: Martin PS, Klein RG (eds) Quaternary extinctions: a prehistoric revolution. University of Arizona Press, Tucson, pp 354–403
Martin RW (1985a) Overbrowsing, and decline of a population of the koala, Phascolarctos cinereus, in Victoria. 1. Food preference and food tree defoliation. Aust Wildl Res 12:355–365
Martin RW (1985b) Overbrowsing, and decline of a population of the koala, Phascolarctos cinereus, in Victoria. 2. Population condition. Aust Wildl Res 12:367–375
Massoia E (1970) Mamiferos que contribuyen a deteriorar suelos y pasturas en la Republica Argentina. IDIA 276:14–17
Matsuda I, Murai T, Clauss M, Yamada T, Tuuga A, Bernard H, Higashi S (2011) Regurgitation and remastication in the foregut-fermenting proboscis monkey (Nasalis larvatus). Biol Lett 7:786–789
Matsuda I, Higashi S, Otani Y, Tuuga A, Bernard H, Corlett RT (2013) A short note on seed dispersal by colobines: the case of the proboscis monkey. Integr Zool 8:395–399
Matsuda I et al (2014) Faecal particle size in free-ranging primates supports ‘rumination’ strategy in the proboscis monkey (Nasalis larvatus). Oecologia 174:1127–1137
Matsuda I et al (2015) Excretion patterns of solute and different-sized particle passage markers in foregut-fermenting proboscis monkey (Nasalis larvatus) do not indicate an adaptation for rumination. Physiol Behav 149:45–52
Matsuda I, Chapman CA, Shi Physilia CY, Mun Sha JC, Clauss M (2017a) Primate resting postures: constraints by foregut fermentation? Physiol Biochem Zool 90:383–391
Matsuda I, Clauss M, Tuuga A, Sugau J, Hanya G, Yumoto T, Bernard H, Hummel J (2017b) Factors affecting leaf selection by foregut-fermenting proboscis monkeys: new insight from in vitro digestibility and toughness of leaves. Sci Rep 7:42774
Matsuda I, Espinosa-Gómez FC, Ortmann S, Sha JCM, Osman I, Nijboer J, Schwarm A, Ikeda T, Clauss M (2019) Retention marker excretion suggests incomplete digesta mixing across the order primates. Physiol Behav 208:112558. https://doi.org/10.1016/j.physbeh.2019.112558
Mau M, Südekum KH, Johann A, Sliwa A, Kaiser TM (2009) Saliva of the graminivorous Theropithecus gelada lacks proline-rich proteins and tannin-binding capacity. Am J Primatol 71:663–669
McArthur C, Appleton R (2004) Effect of seedling characteristics at planting on browsing of Eucalyptus globulus by rabbits. Aust For 67:25–29. https://doi.org/10.1080/00049158.2004.10676202
McConkey KR (2018) Seed dispersal by Primates in Asian habitats: from species, to communities, to conservation. Int J Primatol 39:466–492
McIntire EJB, Hik DS (2005) Influences of chronic and current season grazing by collared pikas on above-ground biomass and species richness in subarctic alpine meadows. Oecologia 145:288–297. https://doi.org/10.1007/s00442-005-0127-z
McIntyre S, Stol J, Harvey J, Nicholls A, Campbell M, Reid A, Manning AD, Lindenmayer D (2010) Biomass and floristic patterns in the ground layer vegetation of box-gum grassy eucalypt woodland in Goorooyarroo and mulligans flat nature reserves, Australian Capital Territory. Cunninghamia 11:9–357
McIntyre S, Cunningham R, Donnelly C, Manning A (2015) Restoration of eucalypt grassy woodland: effects of experimental interventions on ground-layer vegetation. Aust J Bot 62:570–579
McKinnon M, Ahmad M, Bongers M, Chevalier R, Telfer I, Van Dorssen C (2018) Media coverage of lethal control: a case study of kangaroo culling in the Australian Capital Territory. Hum Dimens Wildl 23:90–99
McLeod SR (1996) The foraging behaviour of the arid zone herbivores, the red kangaroo (Macropus rufus) and the sheep (Ovis aries) and its role in their competitive interactions, population dynamics and life-history strategies. University of New South Wales, Sydney
Meehan HJ, McConkey KR, Drake DR (2002) Potential disruptions to seed dispersal mutualisms in Tonga, Western Polynesia. J Biogeogr 29(5–6):695–712. https://doi.org/10.1046/j.1365-2699.2002.00718.x
Meers T, Adams R (2003) The impact of grazing by eastern grey kangaroos (Macropus giganteus) on vegetation recovery after fire at Reef Hill Regional Park, Victoria. Ecol Manag Restor 4:126–132
Menkhorst P (2008) Hunted, marooned, re-introduced, contracepted: a history of koala management in Victoria. In: Lunney D, Munn A, Meikle W (eds) Too Close for comfort: contentious issues in human-wildlife encounters. Royal Zoological Society of New South Wales, Mosman, pp 73–92
Merton LFH, Bourn DM, Hnatiuk RJ (1976) Giant tortoise and vegetation interactions on Aldabra atoll—part 1: inland. Biol Conserv 9(4):293–304
Milchunas DG, Lauenroth WK, Burke IC (1998) Livestock grazing: animal and plant biodiversity of Shortgrass steppe and the relationship to ecosystem function. Oikos 83:65. https://doi.org/10.2307/3546547
Miller AM, O'Reilly-Wapstra JM, Potts BM, McArthur C (2009) Non-lethal strategies to reduce browse damage in eucalypt plantations. For Ecol Manag 259:45–55
Miller AM, O'Reilly-Wapstra JM, Potts BM, McArthur C (2011) Repellent and stocking guards reduce mammal browsing in eucalypt plantations. New For 42:301–316
Milton K (2000) Ducks out of water: nature conservation as boundary maintenance. In: Knight J (ed) Natural enemies: people-wildlife conflicts in anthropological perspective. Berg, Oxford, pp 229–246
Miranda EBP (2017) The plight of reptiles as ecological actors in the tropics. Front Ecol Evol 5:159
Mittermeier RA, Rylands AB, Wilson DE (2013) Handbook of the mammals of the world, vol 3. Primates, Lynx Edicions, Barcelona
Moberg F, Folke C (1999) Ecological goods and services of coral reef ecosystems. Ecol Econ 29:215–233. https://doi.org/10.1016/S0921-8009(99)00009-9
Moore BD, Lawler IR, Wallis IR, Beale CM, Foley WJ (2010) Palatability mapping: a koala's eye view of spatial variation in habitat quality. Ecology 91:3165–3176
Moran KL, Bjorndal KA (2005) Simulated green turtle grazing affects structure and productivity of seagrass pastures. Mar Ecol Prog Ser 305:235–247
Moura ADA et al (2015) Can green iguanas compensate for vanishing seed dispersers in the Atlantic forest fragments of north-East Brazil? J Zool 295(3):189–196
Mourão G et al (2000) Aerial surveys of caiman, marsh deer and pampas deer in the Pantanal wetland of Brazil. Biol Conserv 92(2):175–183
Müller DWH, Caton J, Codron D, Schwarm A, Lentle R, Streich WJ, Hummel J, Clauss M (2011) Phylogenetic constraints on digesta separation: variation in fluid throughput in the digestive tract in mammalian herbivores. Comp Biochem Physiol A 160:207–220
Mumby PJ (2009) Phase shifts and the stability of macroalgal communities on Caribbean coral reefs. Coral Reefs 28:761–773. https://doi.org/10.1007/s00338-009-0506-8
Mumby PJ, Hastings A, Edwards HJ (2007) Thresholds and the resilience of Caribbean coral reefs. Nature 450:98–101. https://doi.org/10.1038/nature06252
Munn A, Dawson T (2003) Energy requirements of the red kangaroo (Macropus rufus): impacts of age, growth and body size in a large desert-dwelling herbivore. J Comp Physiol B 173:575–582
Munn A, Dawson T, McLeod S, Croft D, Thompson M, Dickman C (2009) Field metabolic rate and water turnover of red kangaroos and sheep in an arid rangeland: an empirically derived dry-sheep-equivalent for kangaroos. Aust J Zool 57:23–28
Munro NT, Moseby KE, Read JL (2009) The effects of browsing by feral and re-introduced native herbivores on seedling survivorship in the Australian rangelands. Rangel J 31:417–426
Mutze G, Cooke B, Jennings S (2016) Density-dependent grazing impacts of introduced European rabbits and sympatric kangaroos on Australian native pastures. Biol Invasions 18:2365–2376. https://doi.org/10.1007/s10530-016-1168-4
Nabavizadeh A (2016) Evolutionary trends in the jaw adductor mechanics of ornithischian dinosaurs. Anat Rec 299:271–294
Nabavizadeh A, Weishampel DB (2016) The predentary bone and its significance in the evolution of feeding mechanisms in ornithischian dinosaurs. Anat Rec 299:1358–1388
Nagy KA, Shoemaker VH (1984) Field energetics and food consumption of the Galápagos marine Iguana, Amblyrhynchus cristatus. Physiol Zool 57(3):281–290
Nash KL, Graham NAJ, Bellwood DR (2013) Fish foraging patterns, vulnerability to fishing, and implications for the management of ecosystem function across scales. Ecol Appl 23:1632–1644
Natural Resources Kangaroo Island, Department for Environment and Water, South Australia (2017) Unexpected rise in koala numbers on KI. http://www.naturalresources.sa.gov.au/kangarooisland/news/170829-unexpected-rise-in-koala-numbers Accessed 9 July 2018
Newsome AE (1997) Reproductive anomalies in the red kangaroo in Central Australia explained by aboriginal traditional knowledge and ecology. In: Saunders NR, Hinds LA (eds) Marsupial biology: recent research, new perspectives. University of New South Wales Press, Sydney, pp 229–236
Noble JC (2001) Regulating Callitris populations: a tale of two pineries. In: Dargavel J, Hart D, Libbis B (eds) Perfumed pineries: environmental histories of Australia’s cypress pines. Centre for Resource and Environmental Studies, Australian National University, Canberra
Noble JC, Hik DS, Sinclair ARE (2007) Landscape ecology of the burrowing bettong: fire and marsupial biocontrol of shrubs in semi-arid Australia. Rangel J 29:107–119
Norman DB, Weishampel DB (1985) Ornithopod feeding mechanisms: their bearing on the evolution of herbivory. Am Nat 126:151–164
Norman DB, Crompton AW, Butler RJ et al (2011) The lower Jurassic ornithischian dinosaur Heterodontosaurus tucki Crompton & Charig, 1962: cranial anatomy, functional morphology, taxonomy, and relationships. Linnean Soc 163:182–276
Noto CR, Grossman A (2010) Broad-scale patterns of late Jurassic dinosaur paleoecology. PLoS One 5(9):e12553. https://doi.org/10.1371/journal.pone.0012553
Nussear KE et al (2012) Translocation as a conservation tool for Agassiz’s desert tortoises: survivorship, reproduction, and movements. J Wildl Manag 76(7):1341–1353. https://doi.org/10.1002/jwmg.390
O’Connor MI (2009) Warming strengthens an herbivore-plant interaction. Ecology 90:388–398. https://doi.org/10.1890/08-0034.1
Olsen AM (2015) Exceptional avian herbivores: multiple transitions toward herbivory in the bird order Anseriformes and its correlation with body mass. Ecol Evol 5:5016–5032
Ősi A, Prondvai E, Mallon J et al (2017) Diversity and convergences in the evolution of feeding adaptations in ankylosaurs (Dinosauria: Ornithischia). Hist Biol 29:539–570
Owen-Smith RN (1988) Megaherbivores. Cambridge University Press, Cambridge
Pavlů V, Hejcman M, Pavlů L et al (2006) Effect of continuous grazing on forage quality, quantity and animal performance. Agric Ecosyst Environ 113:349–355. https://doi.org/10.1016/j.agee.2005.10.010
Payne AL, Jarman PJ (1999) Macropod studies at Wallaby Creek. X. Responses of eastern grey kangaroos to cattle. Wildl Res 26:215–225
Pease JL, Vowles RH, Keith LB (1979) Interaction of snowshoe hares and Woody vegetation. J Wildl Manag 43:43–60
Pekelharing CJ, Frampton CM, Suisted PA (1998) Seasonal variation in the impacts of brushtailed possums (Trichosurus vulpecula) on five palatable plant species in New Zealand beech (Nothofagus) forest. N Z J Ecol 22:141–148
Peters RH (1983) The ecological implications of body size. Cambridge University Press, Cambridge
Peterson SL, Rockwell RF, Witte CR, Koons DN (2013) The legacy of destructive snow goose foraging on supratidal marsh habitat in the Hudson Bay lowlands. Arct Antarct Alp Res 45:575–583
Petrovan SO, Ward AI, Wheeler PM (2013) Habitat selection guiding Agri-environment schemes for a farmland specialist, the brown hare. Anim Conserv 16:344–352. https://doi.org/10.1111/acv.12002
Petrozzi F et al (2018) Exploring the main threats to the threatened African spurred tortoise Centrochelys sulcata in the west African Sahel. Oryx 52(3):544–551
Pickett KN, Hik DS, Newsome AE, Pech RP (2005) The influence of predation risk on foraging behaviour of brushtail possums in Australian woodlands. Wildl Res 32:121–130
Polunin NV, Klumpp D (1992) Algal food supply and grazer demand in a very productive coral-reef zone. J Exp Mar Bio Ecol 164:1–15. https://doi.org/10.1016/0022-0981(92)90132-T
Potts R, Behrensmeyer AK (1992) Late Cenozoic terrestrial ecosystems. In: Behrensmeyer AK, Damuth JD, DiMichele WA et al (eds) Terrestrial ecosystems through time. Chicago University Press, Chicago, pp 419–541
Pough FH (1973) Lizard energetics and diet. Ecology 54(4):837–844
Power ME (1983) Grazing responses of tropical freshwater fishes to different scales of variation in their food. Environ Biol Fish 9:103–115. https://doi.org/10.1007/bf00690856
Prasad V, Strömberg CA, Alimohammadian H et al (2005) Dinosaur coprolites and the early evolution of grasses and grazers. Science 310:1177–1180
Prideaux GJ, Ayliffe LK, DeSantis LRG, Schubert BW, Murray PF, Gagan MK, Cerling TE (2009) Extinction implications of a chenopod browse diet for a giant Pleistocene kangaroo. Proc Natl Acad Sci U S A 106:11646–11650
Prins HHT (2016) Interspecific resource competition in antelopes: search for evidence. In: Bro-Jorgensen J, Mallon DP (eds) Antelope conservation: from diagnosis to action. Wiley, Hoboken, NJ, pp 51–77
Prop J, Vulink T (1992) Digestion by barnacle geese in the annual cycle: the interplay between retention time and food quality. Funct Ecol 6:180–189
Ramsey DSL, Tolsma AD, Brown GW (2016) Towards a habitat condition assessment method for guiding the management of overabundant koala populations. Victoria Arthur Rylah Institute for Environmental Research, Department of Environment, Land, Water and Planning, Heidelberg
Randall JE (1967) Food habits of reef fishes of the West Indies. Stud Trop Oceanogr 5:665–847
Rao SJ, Iason GR, Hulbert IAR et al (2003) The effect of sapling density, heather height and season on browsing by mountain hares on birch. J Appl Ecol 40:626–638. https://doi.org/10.1046/j.1365-2664.2003.00838.x
Ravolainen VT, Brathen KA, Yoccoz NG, Nguyen JK, Ims RA (2014) Complementary impacts of small rodents and semi-domesticated ungulates limit tall shrub expansion in the tundra. J Appl Ecol 51:234–241
Rees JD, Kingsford RT, Letnic M (2017) In the absence of an apex predator, irruptive herbivores suppress grass seed production: implications for small granivores. Biol Conserv 213:13–18
Rinderknecht A, Blanco RE (2008) The largest fossil rodent. Proc R Soc B 275:1637
Roberts C, Kirkpatrick JB, McQuillan PB (2011) Tasmanian lentic wetland lawns are maintained by grazing rather than inundation. Austral Ecol 36:303–309
Rodda GH (1992) The mating behavior of Iguana iguana. Smithson Contrib Zool 534:1–38
Rolls EC (1981) A million wild acres: 200 years of man and an Australian forest. GHR Press, Sydney
Rose AB, Platt KH (1992) Snow tussock (Chionochloa) population responses to removal of sheep and European hares, Canterbury, New Zealand. N Z J Bot 30:373–382. https://doi.org/10.1080/0028825X.1992.10412917
Ross J, Sanders MF (1984) The development of genetic resistance to myxomatosis in wild rabbits in Britain. J Hyg (Lond) 92:255–261. https://doi.org/10.1017/S0022172400064494
Rothman JM, Chapman CA, Struhsaker TT, Raubenheimer D, Twinomugisha D, Waterman PG (2015) Long-term declines in nutritional quality of tropical leaves. Ecology 96:873–878
Rowe KC, Achmadi AS, Esselstyn JA (2016) Repeated evolution of carnivory among indo-Australian rodents. Evolution 70:653–665
Rybczynski N, Tirabasso A, Bloskie P et al (2008) A three-dimensional animation model of Edmontosaurus (Hadrosauridae) for testing chewing hypotheses. Palaeontol Electr 11(2):9A. http://palaeo-electronica.org/2008_2/132/index.html
Samuels JX (2009) Cranial morphology and dietary habits of rodents. Zool J Linnean Soc 156:864–888
Sanson GD (1989) Morphological adaptations of teeth to diets and feeding in the Macropodoidea. In: Grigg G, Jarman PJ, Hume ID (eds) Kangaroos, Wallabies and Rat-Kangaroos. Surrey Beatty and Sons, Sydney, pp 151–168
Santamaria L, Rodríguez-Gironés MA (2008) Hiding from swans: optimal burial depth of sago pondweed tubers foraged by Bewick’s swans. J Ecol 90:303–315
Sayers K, Norconk MA (2008) Himalayan Semnopithecus entellus at Langtang National Park, Nepal: diet, activity patterns, and resources. Int J Primatol 29:509–530
Schai-Braun SC, Reichlin TS, Ruf T et al (2015) The European hare (Lepus europaeus): a picky herbivore searching for plant parts rich in fat. PLoS One 10:1–16. https://doi.org/10.1371/journal.pone.0134278
Schwarm A, Ortmann S, Wolf C, Streich WJ, Clauss M (2009) Passage marker excretion in red kangaroo (Macropus rufus), collared peccary (Pecari tajacu) and colobine monkeys (Colobus angolensis, C. polykomos, Trachypithecus johnii). J Exp Zool A Ecol Genet Physiol 311:647–661
Schweizer D, Jones HP, Holmes ND (2016) Literature review and meta-analysis of vegetation responses to goat and European rabbit eradications on islands. Pac Sci 70:55–71. https://doi.org/10.2984/70.1.5
Scott SL, McArthur C, Potts BM, Joyce K (2002) Possum browsing - the downside to a eucalypt hybrid developed for frost tolerance in plantation forestry. For Ecol Manag 157:231–245
Sedinger JS (1997) Adaptations to and consequences of an herbivorous diet in grouse and waterfowl. Condor 99:314–326
Sereno PC, Wilson JA, Witmer LM et al (2007) Structural extremes in a Cretaceous dinosaur. PLoS One 2(11):e1230. https://doi.org/10.1371/journal.pone.0001230
Seymour RS (2009) Raising the sauropod neck: it costs more to get less. Biol Lett 5:317–319
Shariatinajafabadi M, Wang T, Skidmore AK, Toxopeus AG, Kölzsch A, Nolet BA, Exo K-M, Griffin L, Stahl J, Cabot D (2014) Migratory herbivorous waterfowl track satellite-derived green wave index. PLoSOne 9:e108331
Shepherd SA, Hawkes MW (2005) Algal food preferences and seasonal foraging strategy of the marine iguana, Amblyrhynchus cristatus, on Santa Cruz, Galapagos. Bull Mar Sci 77(1):51–72
Short J (1985) The functional-response of kangaroos, sheep and rabbits in an arid grazing system. J Appl Ecol 22:435–447
Si Y, Xin Q, Prins HHT, De Boer WF, Gong P (2015) Improving the quantification of waterfowl migration with remote sensing and bird tracking. Sci Bull 60:1984–1993
Simpson GG (1945) The principles of classification and a classification of mammals. Bull Am Mus Nat Hist 85:1–350
Sinclair ARE, Krebs CJ, Fryxell JM et al (2000) Testing hypotheses of trophic level interactions: a boreal forest ecosystem. Oikos 89:313–328. https://doi.org/10.1034/j.1600-0706.2000.890213.x
Sjögersten S, Van der Wal R, Woodin SJ (2008) Habitat sensitivity determines herbivory controls over CO2 fluxes in a warmer arctic. Ecology 89:2103–2116
Sjögersten S, Van der Wal R, Loonen MJJE, Woodin SJ (2011) Recovery of ecosystem carbon fluxes and storage from herbivory. Biogeochemistry 106:357–370
Slavenko A et al (2016) Late quaternary reptile extinctions: size matters, insularity dominates. Glob Ecol Biogeogr 25(11):1308–1320. https://doi.org/10.1111/geb.12491
Sluiter IRK, Allen GG, Morgan DG, Walker IS (1997) Vegetation responses to stratified kangaroo grazing pressure at Hattah-Kulkyne National Park, 1992–96. Flora and Fauna technical report no. 149. Department of Natural Resources and Environment, East Melbourne
Smith TB (2008) Temperature effects on herbivory for an indo-Pacific parrotfish in Panamá: implications for coral–algal competition. Coral Reefs 27:397–405. https://doi.org/10.1007/s00338-007-0343-6
Smith GC, Prickett AJ, Cowan DP (2007) Costs and benefits of rabbit control options at the local level. Int J Pest Manag 53:317–321
Snaith TV, Chapman CA (2005) Towards an ecological solution to the folivore paradox: patch depletion as an indicator of within-group scramble competition in red colobus monkeys (Piliocolobus tephrosceles). Behav Ecol Sociobiol 59:185–190
Snaith TV, Chapman CA (2007) Primate group size and interpreting socioecological models: do folivores really play by different rules? Evol Anthropol: Issues News Rev 16:94–106
Spear D, Chown SL (2009) Non-indigenous ungulates as a threat to biodiversity. J Zool 279:1–17
Speed JDM, Cooper EJ, Jónsdóttir IS, Van der Wal R, Woodin SJ (2010) Plant community properties predict vegetation resilience to herbivore disturbance in the Arctic. J Ecol 98:1002–1013
Srivastava DS, Jefferies RL (1996) A positive feedback: herbivory, plant growth, salinity, and the desertification of an Arctic salt-marsh. J Ecol 84:31–42
Stahl J, Tolsma PH, Loonen MJJE, Drent RH (2001) Subordinates explore but dominants profit: resource competition in high Arctic barnacle goose flocks. Anim Behav 61:257–264
Stahl J, Van Der Graaf AJ, Drent RH, Bakker JP (2006) Subtle interplay of competition and facilitation among small herbivores in coastal grasslands. Funct Ecol 20:908–915. https://doi.org/10.1111/j.1365-2435.2006.01169.x
Stapleton JP, Ikin K, Freudenberger D (2017) Coarse woody debris can reduce mammalian browsing damage of woody plant saplings in box-gum grassy woodlands. Ecol Manage Restor 18:223–230
Statham M, Rayner RJ (1995) Loss of pasture and crop to native animals in Tasmania. In: Proceedings of 10th annual vertebrate Pest conference. Hobart, Department of Primary Industries and Fisheries, pp 171–176
Steenbeek R, van Schaik CP (2001) Competition and group size in Thomas's langurs (Presbytis thomasi): the folivore paradox revisited. Behav Ecol Sociobiol 49:100–110
Sterck EHM, Watts DP, van Schaik CP (1997) The evolution of female social relationships in nonhuman primates. Behav Ecol Sociobiol 41:291–309
Steuer P, Hummel J, Grosse-Brinkhaus C, Südekum K-H (2015) Food intake rates of herbivorous mammals and birds and the influence of body mass. Eur J Wildl Res 61:91–102
Stevens KA, Parrish JM (2005a) Neck posture, dentition, and feeding strategies in Jurassic sauropod dinosaurs. In: Tidwell V, Carpenter K (eds) Thunder-lizards: the sauropodomorph dinosaurs. Indiana University Press, Bloomington, pp 212–232
Stevens KA, Parrish JM (2005b) Digital reconstructions of sauropod dinosaurs and implications for feeding. In: Curry Rogers KA, Wilson JA (eds) The sauropods: evolution and paleobiology. University of California Press, Berkeley, pp 178–200
Strömberg CA (2004) Using phytolith assemblages to reconstruct the origin and spread of grass-dominated habitats in the great plains of North America during the late Eocene to early Miocene. Palaeogeogr Palaeoclimatol Palaeoecol 207:239–275
Strömberg CA, Di Stilio VS, Song Z (2016) Functions of phytoliths in vascular plants: an evolutionary perspective. Funct Ecol 30:1286–1297
Stutz RS, Banks PB, Proschogo N, McArthur C (2016) Follow your nose: leaf odour as an important foraging cue for mammalian herbivores. Oecologia 182:643–651
Sumption KJ, Flowerdew JR (1985) The ecological effects of the decline in rabbits Oryctolagus cuniculus L. due to myxomatosis. Mamm Rev 15:151–186. https://doi.org/10.1111/j.1365-2907.1985.tb00396.x
Sun IF, Chen Y-Y, Hubbell SP, Wright SJ, Noor NSM (2007) Seed predation during general flowering events of varying magnitude in a Malaysian rain forest. J Ecol 95:818–827
Swart D, Mackie RI, Hayes JP (1993) Fermentative digestion in the ostrich (Struthio camelus var. domesticus), a large avian species that utilizes cellulose 1. S Afr J Anim Sci 23:127–135
Sweetapple PJ (2008) Spatial variation in impacts of brushtail possums on two Loranthaceous mistletoe species. N Z J Ecol 32:177–185
Sweetapple PJ, Nugent G, Whitford J, Latham MC, Pekelharing K (2016) Long-term response of temperate canopy trees to removal of browsing from an invasive arboreal herbivore in New Zealand. Austral Ecol 41:538–548
Tape KD, Lord R, Marshall H-P, Ruess RW (2010) Snow-mediated ptarmigan browsing and shrub expansion in arctic Alaska. Ecoscience 17:186–193
Tapper SC, Barnes RFW (1986) Influence of farming practice on the ecology of the brown hare (Lepus europaeus). J Appl Ecol 23:39–52
Taylor MP, Wedel MJ, Naish D (2009) Head and neck posture in sauropod dinosaurs inferred from extant animals. Acta Palaeontol Pol 54:213–220
Temby ID (1998) The law and wombats in Australia. In: Wells RT, Pridmore PA (eds) Wombats. Surrey Beatty and Sons, Sydney, pp 305–311
Terborgh J, Peres CA (2017) Do Community-managed forests work? A biodiversity perspective. Land 6(22):1–7
Tershy B et al (2016) The biogeography of threatened insular iguanas and opportunities for invasive vertebrate management. Herpetol Conserv Biol 11:222–236
Thiry V, Bhasin O, Stark DJ, Beudels-Jamar RC, Drubbel RV, Nathan SKSS, Goossens B, Vercauteren M (2019) Seed dispersal by proboscis monkeys: the case of Nauclea spp. Primates. https://doi.org/10.1007/s10329-019-00736-x
Tiffney BH (2012) Land plants as a source of food and environment in the age of dinosaurs. In: Brett-Surman MK, Holts TR Jr, Farlow JO (eds) The complete dinosaur, 2nd edn. Indiana University Press, Bloomington, pp 568–587
Tiver F, Andrew MH (1997) Relative effects of herbivory by sheep, rabbits, goats and kangaroos on recruitment and regeneration of shrubs and trees in eastern South Australia. J Appl Ecol 34:903–914
Todd CR, Forsyth DM, Choquenot D (2008) Modelling the effects of fertility control on koala-forest dynamics. J Appl Ecol 45:568–578
Townsend KEB, Croft DA (2008) Diets of notoungulates from the Santa Cruz formation, Argentina: new evidence from enamel microwear. J Vertebr Paleontol 28:217–230
Travers SK, Eldridge DJ, Dorrough J, Val J, Oliver I (2018) Introduced and native herbivores have different effects on plant composition in low productivity ecosystems. Appl Veg Sci 21:45–54
Traveset A (1990) Ctenosaura similis gray (Iguanidae) as a seed disperser in a central American deciduous forest. Am Midl Nat 123(2):402–404
Traveset A et al (2016) Galápagos land iguana (Conolophus subcristatus) as a seed dispersers. Integr Zool 11(3):207–213
Triggs B (2009) Wombats, 2nd edn. CSIRO Publishing, Collingwood
Trout RC (2003) Rabbits in the farmland ecosystem. In: Tattersall F, Manly WJ (eds) Conservation and conflict. Westbury Publishing, Otley, West Yorkshire, pp 198–210
Tsuji Y, Ningsih JIDP, Kitamura S, Widayati KA, Suryobroto B (2017) Neglected seed dispersers: endozoochory by Javan lutungs (Trachypithecus auratus) in Indonesia. Biotropica 49:539–545
Turner BW, Alcock D (2000) The dry sheep equivalent - redefining a 'standard'. Asian Australas J Anim Sci 13:215–215
Turtle Extinctions Working Group et al (2015) Turtles and tortoises of the world during the rise and global spread of humanity: first checklist and review of extinct Pleistocene and Holocene chelonians. 69(5). https://doi.org/10.3854/crm.5.000e.fossil.checklist.v1.2015
Tweet JS, Chin K, Braman DR et al (2008) Probable gut contents within a specimen of Brachylophosaurus canadensis (Dinosauria: Hadrosauridae) from the Upper Cretaceous Judith River Formation of Montana. PALAIOS 23:624–635
Tyndale-Biscoe H (2005) Life of marsupials. CSIRO Publishing, Collingwood
Uetz P, Freed P Hošek J (2016) The reptile database, Species Number. http://www.reptile-database.org/db-info/SpeciesStat.html. Accessed 19 Oct 2017
Upchurch P, Barrett PM (2000) The evolution of sauropod feeding mechanisms. In: Sues H-D (ed) Evolution of herbivorous in terrestrial vertebrates. Cambridge University Press, Cambridge, pp 79–122
Ureña-Aranda CA et al (2015) Using range-wide abundance modeling to identify key conservation areas for the micro-endemic bolson tortoise (Gopherus flavomarginatus). PLoS One 10(6):e0131452
Valido A, Olesen JM (2007) The importance of lizards as frugivores and seed dispersers. In: Dennis AJ et al (eds) Seed dispersal: theory and its application in a changing world. CAB International, Wallingford, pp 124–147
Van der Graaf AJ, Stahl J, Klimkowska A, Bakker JP, Drent RH (2006) Surfing on a green wave – how plant growth drives spring migration in the Barnacle Goose Branta leucopsis. Ardea 94:567–577
Van der Wal R (2006) Do herbivores cause habitat degradation or vegetation state transition? Evidence from the tundra. Oikos 114:177–186
Van der Wal R, Egas M, Van Der Veen A, Bakker J (2000a) Effects of resource competition and herbivory on plant performance along a natural productivity gradient. J Ecol 88:317–330
Van der Wal R, Van Wijnen H, Van Wieren S et al (2000b) On facilitation between herbivores: how Brent geese profit from brown hares. Ecology 81:969–980
Van Donk E, Otte A (1996) Effects of grazing by fish and waterfowl on the biomass and species composition of submerged macrophytes. Hydrobiologia 340:285–290. https://doi.org/10.1007/BF00012769
Van Eerden MR, Drent RH, Stahl J, Bakker JP (2005) Connecting seas: western Palaearctic continental flyway for water birds in the perspective of changing land use and climate. Glob Chang Biol 11:894–908
Van Gils JA, Gymesi A, Van Lith B (2007) Avian herbivory: an experiment, a field test, and an allometric comparison with mammals. Ecology 88:2926–2935
Van Leeuwen CHA, Van der Velde G, Van Groenendael JM, Klaassen M (2012) Gut travellers: internal dispersal of aquatic organisms by waterfowl. J Biogeogr 39:2031–2040
Van Onsem S, Triest L (2018) Turbidity, waterfowl herbivory, and propagule banks shape submerged aquatic vegetation in ponds. Front Plant Sci 9:1514
Vandandorj S, Eldridge DJ, Travers SK, Delgado-Baquerizo M (2017) Contrasting effects of aridity and grazing intensity on multiple ecosystem functions and services in Australian woodlands. Land Degrad Dev 28:2098–2108
Vaughan TA, Ryan JN, Czaplewski NJ (2015) Mammalogy, 6th edn. Jones & Bartlett, Burlington
Vergés A, Steinberg PD, Hay ME et al (2014) The tropicalization of temperate marine ecosystems: climate-mediated changes in herbivory and community phase shifts. Proc Biol Sci 281:1–10. https://doi.org/10.1098/rspb.2014.0846
Virgós E, Cabezas-díaz S, Malo A et al (2003) Factors shaping European rabbit abundance in continuous and fragmented populations of Central Spain. Acta Theriol (Warsz) 48:113–122
Vitt LJ, Caldwell JP (2013) Herpetology: an introductory biology of amphibians and reptiles. Academic, Cambridge
Von Humboldt A (1877) Personal narrative of travels to the equinoctial regions of the new continent during the years 1799–1804. AMS Press, New York
Webster FJ, Babcock RC, Van Keulen M, Loneragan NR (2015) Macroalgae inhibits larval settlement and increases recruit mortality at Ningaloo reef, Western Australia. PLoS One. https://doi.org/10.1371/journal.pone.0124162
Weishampel DB (1984) Interactions between Mesozoic plants and vertebrates: fructifications and seed predation. N Jb Geol Paläont Abh 167:224–250
Welsh JQ, Bellwood DR (2014) Herbivorous fishes, ecosystem function and mobile links on coral reefs. Coral Reefs 33:303–311. https://doi.org/10.1007/s00338-014-1124-7
Wesche K, Nadrowski K, Retzer V (2007) Habitat engineering under dry conditions: the impact of pikas (Ochotona pallasi) on vegetation and site conditions in southern Mongolian steppes. J Veg Sci 18:665–674. https://doi.org/10.1111/j.1654-1103.2007.tb02580.x
Whelan CJ, Şekercioğ ÇH, Wenny DG (2015) Why birds matter: from economic ornithology to ecosystem services. J Ornithol 156:227–238
Whisson DA, Dixon V, Taylor ML, Melzer A (2016) Failure to respond to food resource decline has catastrophic consequences for koalas in a high-density population in southern Australia. PLoS One 11:12
Wich SA, Marshall AJ (2016) An introduction to Primate Conservation. Oxford University Press, New York
Wiewandt TA, García M (2000) Mona Island iguana Cyclura cornuta stejnegeri. In: Alberts A (ed) West Indian iguanas: status survey and conservation action plan. IUCN SSC West Indian Iguana Specialist Group, Gland, pp 27–31
Wikelski M, Thom C (2000) Marine iguanas shrink to survive El Niño. Nature 403(6765):37
Williams J, Tieleman B, Shobrak M (1999) Lizard burrows provide thermal refugia for larks in the Arabian Desert. Condor 101(3):714–717
Williams ID, Polunin NVC, Hendrick VJ (2001) Limits to grazing by herbivorous fishes and the impact of low coral cover on macroalgal abundance on a coral reef in Belize. Mar Ecol Prog Ser 222:187–196. https://doi.org/10.3354/meps222187
Williams VS, Barrett PM, Purnell MA (2009) Quantitative analysis of dental microwear in hadrosaurid dinosaurs, and the implications for hypotheses of jaw mechanics and feeding. Proc Natl Acad Sci 106:11194–11199
Willians SH, Kay RF (2001) A comparative test of adaptive explanations for hypsodonty in ungulates and rodents. J Mamm Evol 8:207–229
Wilms T, Wagner P, Shobrak M (2010) Aspects of the ecology of the Arabian spiny-tailed lizard (Uromastyx aegyptia microlepis Blanford, 1875) at Mahazat as-Sayd protected area, Saudi Arabia. Salamandra 46(3):131–140
Wilson DE, Reeder DM (eds) (2005) Mammal species of the world: a taxonomic and geographic reference, 3rd edn. Johns Hopkins University Press, Baltimore, MD
Windley HR, Barron MC, Holland EP, Starrs D, Ruscoe WA, Foley WJ (2016) Foliar nutritional quality explains patchy browsing damage caused by an invasive mammal. PLoS One 11:e0155216
Wing SL, Hickey LJ, Swisher CC (1993) Implications of an exceptional fossil flora for late cretaceous vegetation. Nature 363:342–344
Winnard AL, Coulson G (2008) Sixteen years of eastern barred bandicoot Perameles gunnii reintroductions in Victoria: a review. Pac Conserv Biol 14:34–53
Wong V, Hickling GJ (1999) Assessment and management of hare impact on high-altitude vegetation. Sci Conserv 116:2–40
Wood AE (1947) Rodents – a study in evolution. Evolution 1:154–162
Wood AE (1965) Grades and clades among rodents. Evolution 19:115–130
Wood KA, Stillman RA, Clarke RT, Daunt F, O’Hare MT (2012) The impact of waterfowl herbivory on plant standing crop: a meta-analysis. Hydrobiologia 686:157–167
Wood KA, O’Hare MT, McDonald C, Searle KR, Daunt F, Stillman RA (2016) Herbivore regulation of plant abundance in aquatic ecosystems. Biol Rev 92:1128–1141
Woodruff DC (2017) Nuchal ligament reconstructions in diplodocid sauropods support horizontal neck feeding postures. Hist Biol 29:308–319
Woollard P, Vestjens WJM, Maclean L (1978) The ecology of the eastern water rat Hydromys chrysogaster at Griffith, Nsw: food and feeding habits. Wildl Res 5:59–73
Wrangham RW (1980) An ecological model of female-bonded primate groups. Behaviour 75:262–300
Wu Y, You HL, Li XQ (2017) Dinosaur-associated Poaceae epidermis and phytoliths from the early cretaceous of China. Natl Sci Rev. https://doi.org/10.1093/nsr/nwx145
Yeager CP, Kirkpatrick RC (1998) Asian colobine social structure: ecological and evolutionary constraints. Primates 39:147–155
Young MT, Rayfield EJ, Holliday CM et al (2012) Cranial biomechanics of Diplodocus (Dinosauria, Sauropoda): testing hypotheses of feeding behaviour in an extinct megaherbivore. Naturwissenschaften 99:637–643
Yu C, Pang XP, Wang Q et al (2017) Soil nutrient changes induced by the presence and intensity of plateau pika (Ochotona curzoniae) disturbances in the Qinghai-Tibet plateau, China. Ecol Eng 106:1–9. https://doi.org/10.1016/j.ecoleng.2017.05.029
Yugovic J (2015) Do ecosystems need top predators? A review of native predator-prey imbalances in south-East Australia. Vict Nat 132:4–11
Zhiheng L, Clarke JA (2016) The craniolingual morphology of waterfowl (Aves, Anseriformes) and its relationship with feeding mode revealed through contrastenhanced x-ray computed tomography and 2D morphometrics. Evol Biol 43:12–25
Zijlstra M, Loonen MJJE, Van Eerden M, Dubbeldam W (1991) The Oostvaardersplassen as a key moulting site for Greylag Geese Anser anser in western Europe. Wild 42:45–52
Zipperle AM, Coyer JA, Reise K, Stam W, Olsena JL (2010) Waterfowl grazing in autumn enhances spring seedling recruitment of intertidal Zostera noltii. Aquat Bot 93:202–205
Zosky KL, Wayne AF, Bryant KA, Calver MC, Scarff FR (2018) Diet of the critically endangered woylie (Bettongia penicillata ogilbyi) in South-Western Australia. Aust J Zool 65:302–312
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Gordon, I.J. et al. (2019). The Ecology of Browsing and Grazing in Other Vertebrate Taxa. In: Gordon, I., Prins, H. (eds) The Ecology of Browsing and Grazing II. Ecological Studies, vol 239. Springer, Cham. https://doi.org/10.1007/978-3-030-25865-8_15
Download citation
DOI: https://doi.org/10.1007/978-3-030-25865-8_15
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-25864-1
Online ISBN: 978-3-030-25865-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)