Abstract
The ongoing obesity epidemic places about a third of American youth at risk for developing obesity-related insulin resistance. This is manifested by reduced multiorgan effects of insulin, leading to elevated insulin concentrations and, in some cases, inappropriately elevated glucose and lipid concentrations. Many risk factors for insulin resistance—including genetics, epigenetics, obesity, diet, and lifestyle—are in common between adolescents and adults. However, adolescents have a unique risk factor in the physiologic insulin resistance of puberty, which along with obesity can pose a “double hit” to the beta-cell, causing increased burden for insulin secretion.
The diagnosis of insulin resistance-related health conditions is more difficult in pediatric patients, due to physiological growth and change that makes the establishment of standards difficult. Such conditions include nonalcoholic fatty liver disease, polycystic ovary syndrome, metabolic syndrome, prediabetes, type 2 diabetes, and lipodystrophies, each of which would obligate additional screening or treatment. Research on treatment options generally addresses holistic changes in diet and lifestyle, with or without the addition of insulin sensitizers. Though these interventions require diligence and can be difficult to maintain, even small successes can create improvements to measured insulin resistance.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Insulin sensitivity
- Insulin resistance
- Obesity
- PCOS
- Lipodystrophy
- NAFLD
- NASH
- Type 2 diabetes
- Metabolic syndrome
Introduction
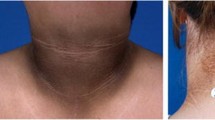
The obesity epidemic has led to a significant proportion of youth with comorbid conditions related to reduced insulin sensitivity, or “insulin resistance.” Insulin resistance manifests as a reduction in the metabolic effects of insulin on liver, muscle, and adipose tissue—the body tissues that store energy. Because insulin directly or indirectly affects nearly every tissue in the body, physical signs of insulin resistance or associated conditions may be evident. These include skin findings, such as acanthosis nigricans, and systemic conditions such as polycystic ovary syndrome (PCOS) and the cluster of metabolic abnormalities referred to as the metabolic syndrome. Prediabetes and type 2 diabetes are well-recognized diagnoses associated with insulin resistance in adults and are becoming more prevalent in children. The ongoing increase in obesity and insulin resistance makes a thorough understanding of this topic more salient to both pediatric endocrine and general pediatric practice. This chapter will provide an introduction to the understanding, the recognition of, and the care for insulin resistance in children and youth. Focusing on the core concept of insulin sensitivity and its contribution to related clinical conditions, fundamental aspects of development and assessment of insulin resistance will be briefly discussed. Additionally, recognition of various syndromes and health conditions associated with insulin resistance will be reviewed. Some are mild and a direct result of exogenously driven conditions, such as obesity-related insulin resistance. However, more severe forms of insulin resistance, such as the inherited lipodystrophies, can be the result of an endogenous genetic defect in insulin action. The current understanding of various medical and lifestyle interventions and reasonable expectations for their success are also examined.
Because this chapter is intended to be an introductory review, the topics covered here will be necessarily brief, and a great deal of complexity and interrelationships between the conditions covered will be left to later chapters. The overarching concept that should be clear throughout the following pages is that youth with insulin resistance should be assessed, evaluated, and treated commensurate with the risks and outcomes known for their age group, which is not necessarily comparable to those found in adults.
Metabolic Effects of Insulin
Insulin has metabolic effects in virtually every tissue of the body. Insulin acts in balance with glucagon and other hormones to maintain glucose homeostasis. Specifically, it acts as the anabolic end of the balance by encouraging cellular uptake of glucose and glycogen synthesis. Simultaneously, it decreases glycogenolysis and gluconeogenesis and limits provision of substrates (amino acids, fatty acids, and ketone bodies) for these processes, leading to a net effect of utilization of immediately available glucose [1, 2].
Once insulin binds to its target receptor, the exact pathway to the metabolic action of insulin depends on the tissue itself. In the liver, insulin inhibits glycogen phosphorylase, directly modulating glycogenolysis [1]. Insulin also decreases gluconeogenesis by decreasing free fatty acid and amino acid provision to the liver, as well as modulating glucagon secretion that would otherwise promote gluconeogenesis [2, 3]. In addition, insulin directly inhibits ketone production in the liver [1].
In skeletal muscle and, to a lesser degree, adipose tissue, insulin stimulates increased localization of cytoplasmic glucose transporter 4/GLUT-4 receptors to the cell membrane, resulting in greater intracellular glucose transport [4, 5]. Insulin binding also triggers a coordinated series of responses that favor the use of immediately available glucose over use of amino acids [2]. This is achieved by increasing utilization of amino acids for protein synthesis, inhibiting breakdown [2], and more indirectly through the inhibition of gluconeogenesis [2, 3].
Insulin additionally inhibits lipolysis in adipose tissue, thereby indirectly increasing glucose utilization as a fuel source in other tissues. Simultaneously, differential tissue effects of insulin favor the diversion of circulating lipids to adipose for storage, rather than for immediate use in muscle [6]. Decreased utilization of fatty acids minimizes available substrate for ketone production, which would be used as an alternative source of fuel in the absence of glucose. The decreased substrate exaggerates the direct inhibition of liver ketone production [1].
Lastly, insulin exaggerates its own effects by negating hormonal stimulation toward opposite goals. It does this by directly inhibiting glucagon secretion, while hyperglycemia can induce somatostatin, an additional inhibitor of glucagon secretion [3].
Insulin Resistance
Insulin resistance is a fundamental underlying mechanism in the development of type 2 diabetes. Whereas normal responsivity to insulin is integral for the multitude of tissue responses that contribute to glucose homeostasis, resistance to insulin action is characterized by diminishing metabolic response for a given degree of insulin secretion. Total body insulin resistance largely reflects reduced insulin action at the skeletal muscle tissue, resulting in lowered intracellular glucose uptake [5, 7], but tissue-specific effects are nevertheless important to understand overall effects of insulin resistance. Hepatic insulin resistance, for example, results in reduced inhibition of glycogen phosphorylase, increased hepatic glucose output, and reduced inhibition of ketone production [1, 5, 8]. Insulin resistance in adipose tissue results in increases in circulating lipids because of reduced insulin-mediated expression of genes vital for lipogenesis and, importantly, loss of suppression of hormone-sensitive lipase and lipoprotein lipase. Absent suppression of these enzymes controlling triglyceride mobilization leads to inappropriate fatty acid secretion from the adipocyte [8], contributing to the dyslipidemia often seen in association with insulin resistance.
In childhood, normoglycemia is predominantly maintained in the face of insulin resistance, where decreased tissue sensitivity is compensated for by higher insulin secretion to maintain normal glucose concentrations [9]. However, continued stress to the pancreatic β(beta)-cell, decreased total body insulin action, and increased demand for higher insulin concentrations can lead to progressive β(beta)-cell dysfunction and failure [9]. Further exacerbation of insulin resistance and β(beta)-cell dysfunction leads to an ever-greater mismatch between insulin needs and insulin secretion, ultimately resulting in type 2 diabetes [9, 10].
Insulin resistance is directly measured by hyperinsulinemic-euglycemic clamp studies, which are unreasonable to do in the clinical setting due to several practical barriers [11]. Fasting insulin, though easily obtainable in the clinical setting, is a poor correlate of clamp study results [10, 11]. Because of these issues, and because there are no widely accepted standards by which insulin resistance can be defined, assessment of insulin resistance in the clinical setting measuring fasting insulin is generally not recommended [10].
Contributory Factors to Developing Insulin Resistance
Risk factors for developing insulin resistance can be grossly divided into nonmodifiable or modifiable categories. Non-modifiable risk factors, though unavoidable, do not consistently affect an individual over time and may result in fluctuating risk for dysglycemia throughout the lifespan.
Non-modifiable Factors
Puberty
Puberty is associated with rapid growth and development during which anabolic hormones, including insulin, are required in higher concentrations. The physiologic insulin resistance associated with the onset of puberty is thereby necessary to promote optimal growth and is naturally a self-limited process [12, 13]. Nonetheless, it is during this time that the risk for developing conditions associated with insulin resistance is heightened [12]. Typically, insulin sensitivity is decreased 30–50% even in lean and healthy children and is compensated for by a doubling of insulin secretion [12, 14]. In healthy children, this phenomenon is seen as early as the onset of puberty (Tanner 2), and insulin sensitivity returns to near prepuberty levels by the time puberty has completed [13].
Genetics
The heritable nature of susceptibility to insulin resistance is frequently seen in clinical practice, with a family history of type 2 diabetes considered a risk factor for developing type 2 diabetes in the individual [15]. Nevertheless, the development and progression of insulin resistance is multifactorial, and widespread genetic screening is not currently recommended. The list of genes associated with type 2 diabetes via increased propensity for insulin resistance or β(beta)-cell dysfunction is ever expanding, but the clinical utility of genetic studies is limited by expense and practicality, as well as overall usefulness. The many known polymorphisms explain only a small proportion of interindividual phenotypic variance [16], and broad assessments of the use of biomarkers have demonstrated only modest improvements in the ability to predict development of type 2 diabetes [15].
Epigenetics
More recently, research into the epigenetics of obesity has revealed potential implications for insulin resistance. Genome association studies demonstrating methylation alterations as a consequence of adiposity have identified influences on genes involved in lipid transport or dyslipidemia [17], as well as genes previously implicated in insulin resistance and type 2 diabetes risk or other metabolic defects [17]. Though causation of insulin resistance is unknown, changes such as hypomethylation of particular regions predispose the individual to later development of type 2 diabetes, rather than appearing after the development of insulin resistance or diabetes [16]. That the pattern of hypomethylation can be independent of the DNA sequence itself is suggestive of an entirely separate layer of control over metabolic processes affecting risk for insulin resistance [16]. Some of these methylation differences are present even in young adulthood, and it is postulated that resultant differences in expression, even if initially minor, may have substantial impact on risk for developing type 2 diabetes over the long term. In addition, specific epigenetic studies of high-risk ethnic populations have demonstrated an association of specific loci with high methylation scores and future development of type 2 diabetes relative to age- and gender-matched controls without diabetes, independent of more well-known risk factors.
Methylation is postulated to occur early in human development, including during fetal life. Infants who are born small for gestational age [18] are well known to be at risk for insulin resistance and metabolic sequelae in later life [19]. Animal models have provided early evidence of possible mechanisms for these sequelae, including methylation of genes that could result in decreased insulin sensitivity and altered response to lipid delivery to muscle cells, leading to metabolic inflexibility that furthers insulin resistance at the level of the muscle cell [20].
Ethnicity
Certain minority populations are well recognized to have a higher burden of type 2 diabetes, and this seems to be a reflection of their susceptibility to develop insulin resistance or to demonstrate inadequate compensatory insulin secretion. Physiologic differences have been revealed with ethnic minorities demonstrating greater insulin secretion compared to their non-Hispanic white peers, even at equal degrees of insulin sensitivity, causing subsequently higher β(beta)-cell stress [21]. In both healthy youth and youth with diabetes, for example, African Americans have been shown to have lower visceral adipose tissue and higher insulin secretion at a given degree of insulin sensitivity, compared to non-Hispanic white counterparts [22, 23]. Studies on racial differences in insulin sensitivity show that while different ethnic groups have similar patterns of adaptation to insulin resistance, African Americans have somewhat lower insulin sensitivity than Latinos. This would require more exaggerated hyperinsulinemia to maintain euglycemia, possibly creating greater demand on β(beta)-cells and encouraging decompensation with clinically evident dysglycemia [23,24,25]. Latinos, though they may have higher insulin sensitivity than African Americans as a whole, tend to have more dramatic declines in measured insulin sensitivity as they become more obese [25]. However, the more fundamental differences between ethnic groups contributing to differences in insulin sensitivity and β(beta)-cell response are as yet unknown [25]. These inequities are aggravated with higher treatment failure rates among African-American and Hispanic youth with diabetes in comparison to non-Hispanic white youth in large treatment-based studies such as the TODAY study (Treatment Options for type 2 Diabetes in Adolescents and Youth) [26].
The predilection for obesity and insulin resistance seems to be especially true when ethnic minorities are placed into an obesogenic environment, demonstrating a genetic vulnerability toward metabolic decompensation when exposed to the appropriate environmental stress on β(beta)-cell function. Native Americans, for example, have the highest prevalence of type 2 diabetes in the SEARCH for Diabetes in Youth study, followed by African Americans, Hispanics, and Asians [27]. These differences are likely to be multifactorial, incorporating influences from genetics and epigenetics, as well as environmental stressors and cultural differences in diet and activity. There is some evidence for differential epigenetic changes in certain ethnic groups [17], while others have demonstrated some physiologic basis for these differences at the level of the β(beta)-cell.
Modifiable Factors
Though characteristics such familial traits, genetics, ethnicity, and the onset of puberty are uniformly unavoidable, other contributory factors toward insulin resistance can be successfully modified, though many patients struggle to do so.
Obesity
Obesity, one of the most well-known contributors to insulin resistance, is a prevalent issue nationwide [28], with approximately 32% of children 2–19 years considered overweight or obese and 17% considered obese. This places approximately a third of the nation’s children at risk of complications associated with insulin resistance. Though alarming, this is only the beginning of a problem that increases exponentially by adulthood, with about 70% of the adult US population >20 years old considered overweight or obese, about 38% of the population considered obese, and an estimated 12% of the population estimated to have diabetes [29].
Consequently, the combination of obesity and puberty composes a “double hit” to adolescents in the development of pathologic insulin resistance. Physiologic insulin resistance in puberty is already characterized by decreased glucose oxidation and decreased insulin-mediated suppression of free fatty acid oxidation. Increased need for fat oxidation rising from obesity during puberty may exacerbate competition with glucose oxidation and amplify glycemic manifestations of insulin resistance [12]. Meanwhile, obesity alone is associated with a measured insulin response about half that seen in normal weight youth, after adjusting for individual insulin sensitivity [30]. This indicates poorer β(beta)-cell function in the face of insulin resistance occurring even among obese adolescent youth with normal glucose tolerance. Consequently, the insulin resistance of puberty in addition to excessive substrate for fatty acid oxidation may create enough demand to overwhelm β(beta)-cells resulting in prediabetes or diabetes, particularly in those patients who are predisposed to have underlying β(beta)-cell dysfunction.
The fundamental role of obesity in the development of insulin resistance becomes especially important when the epidemiology of the condition is considered. Unfortunately, those race/ethnic groups that already show a preexisting vulnerability toward insulin resistance often have higher rates of obesity, with almost 40% of Hispanic youth being overweight or obese and about 35% of African-American youth being overweight or obese [28]. This trend continues in the more severe categories, with 22% and 20% of Hispanic and African-American youth, respectively, categorized as obese, compared to 14% of non-Hispanic white youth [28]. This distribution of obesity essentially places the greatest physiological stress on those individuals who are intrinsically most likely to decompensate from that stress and places a disproportionate burden of morbidity from type 2 diabetes and metabolic syndrome within these populations.
Diet and Lifestyle
One important factor in constant interaction with ethnic or genetic predisposition toward insulin resistance is the surrounding environment [31]. The influence of an “obesogenic environment” common to a Westernized lifestyle, corresponding to higher fat, salt, sugar, refined grains, and high-calorie foods, and lacking in physical activity has been reported especially within countries with higher income levels [32, 33]. While the effects of an unhealthy diet can impact anyone, this influence is seen most strikingly in groups that shift toward higher rates of obesity upon encountering a truly obesogenic lifestyle for the first time. A number of studies, for example, have shown that adolescent and adult immigrants to the United States from lower-income countries have an increasing prevalence of obesity with increased years since immigration and, over the long term, the prevalence begins to approach rates similar to the country to which they have immigrated [33, 34]. This trend, on large national surveys, is at least present among Hispanics and Asians, two ethnic groups that are particularly susceptible to developing insulin resistance. Furthermore, these populations are less likely to discuss diet and exercise options with their clinicians, potentially leading to an education gap that can increase their participation in obesogenic habits [34].
Even in children, a similar association seems to hold true, with children of acculturated parents (i.e., having lived in a higher-income country for a longer period of time and implicitly having adapted more of the surrounding obesogenic behaviors and diet) much more likely to be overweight or obese than children in the originating country, children of new immigrants, or even children native to the high-income country [33, 35]. When examining dietary shifts, changes toward increased consumption of processed foods and decreases in high-fiber, high-nutrient foods are often reported particularly in younger generations, though it is by no means exclusive [32]. This shift is likely the result of multiple influences [32], including food availability (forced shift away from traditional foods), relative income (lower income possibly creating a reliance on cheaper and lower quality foods), education level, generation (younger generations being reported as more likely to change dietary habits), and others. These generalizations between time and risk for obesity, however, are often drawn from studies that fail to draw a distinction between acculturation (assimilation and exchange of cultural behaviors) and enculturation (a lesser degree of assimilation despite living within a different culture). Among studies that used scales to measure degree of acculturation rather than time from immigration in relationship with the development of obesity, there is generally a positive association between high degrees of acculturation and higher risk for obesity. Authors have theorized that migration forces a more rapid nutritional transition toward increased consumption of high-fat foods that are lower in nutrition, a transition that would normally occur over years along with the economic development of their country of origin [36].
Physical Activity
The other end of the equation in caloric balance affecting insulin resistance is the degree of physical activity. Longitudinally, a shift toward more sedentary activities is part of the obesogenic lifestyle. A lack of calorie expenditure leading to overall caloric excess can lead to increased weight gain, increasing fatty acid oxidation as competition for glucose oxidation as outlined above. However, even moderate physical activity increases muscle cell uptake of glucose by insulin-independent mechanisms. Exercise modulates translocation of the same GLUT4 transporters that are typically controlled by insulin secretion [37], increasing glucose uptake in an additive manner to insulin-mediated mechanisms and contributing to more efficient glucose homeostasis overall [38].
Conditions Associated with Insulin Resistance
Though the development of type 2 diabetes is the most obvious and direct consequence of insulin resistance and β(beta)-cell stress that proceeds unchecked, there are myriad other associations that healthcare providers should be aware of and screen for as indicated. Like insulin resistance, many of these conditions are more commonly seen in adulthood, but can certainly affect the pediatric population. Many of these disease associations and syndromes are not entirely surprising, given the multiorgan effects of insulin even beyond glucose homeostatic mechanisms. Resistance to insulin-mediated control of circulating fatty acids may predispose to worsening dyslipidemia, a well-known risk factor for cardiovascular disease. Insulin, in addition, increases androgen synthesis and simultaneously decreases liver production of sex hormone-binding globulin, thus providing a mechanism for the reproductive disruption seen in PCOS [39, 40]. Consequently, insulin resistance itself is associated with many well-known diseases and syndromes that incorporate other organ and system effects of insulin.
Metabolic Syndrome
In adults, the metabolic syndrome is comprised of a collection of cardiovascular risk factors associated with insulin resistance, including abdominal obesity (elevated waist circumference), hypertension, dyslipidemia, and dysglycemia. Approximately one-third of the adult population is estimated to have metabolic syndrome according to 2003–2012 National Health and Nutrition Examination Survey (NHANES) data [41]. Of note, criteria for metabolic syndrome in the adult population are known to underdiagnose the condition in certain ethnic populations, and thus the reported prevalence of the syndrome in African Americans may in fact be an underrepresentation [42].
With the increase in pediatric obesity, greater attention has been given to criteria that would be analogous in the pediatric population. As always in pediatrics, the growth and ever-changing physiology of children create a unique challenge in the recognition of important health conditions, and a single standard definition does not yet exist. Furthermore, the absence of long-term epidemiologic data linking any definition of metabolic syndrome to cardiovascular risk makes the designation of an evidence-based definition of metabolic syndrome impossible. However, based on extrapolation from adults, the International Diabetes Federation defines metabolic syndrome in children 10 to <16 years old as the presence of obesity >90th percentile by waist circumference, replacing the adult criterion for waist circumference alone, and the presence of at least two other criteria defined similarly to adults (hypertriglyceridemia, low high-density lipoprotein [HDL], hypertension, and dysglycemia or type 2 diabetes) [43]. Meanwhile, children 16 and older should be diagnosed according to adult criteria, and children <10 do not have particular criteria for diagnosis of metabolic syndrome, but should be screened for risk factors if abdominal obesity >90th percentile by waist circumference is present and if their family history of metabolic syndrome or cardiovascular risk is strong. Based on adolescent-adapted criteria, the overall prevalence of metabolic syndrome in patients 12–19 years old is thought to be about 10%, with an overall higher prevalence in males, Hispanics, and non-Hispanic whites [42]. In recent years, the prevalence of metabolic syndrome has been seen to decrease slightly in youth [42], while the prevalence has stabilized but not decreased among adults [41].
Like insulin resistance, the presence of metabolic syndrome has been shown to differ by ethnic group [21], and the prevalent components of metabolic syndrome also differ by ethnic background. African Americans, for example, have a somewhat lower prevalence of the diagnosis overall and are less likely than non-Hispanic whites to have dyslipidemia, but are more likely to demonstrate hypertension [21].
In the pediatric population, as in adults, the association between obesity and metabolic syndrome is strong, with higher prevalence in more severe degrees of obesity. In a population of obese children, almost 40% of moderately obese subjects and almost half of severely obese subjects met criteria for metabolic syndrome, while overweight or normal weight subjects are unlikely to meet criteria [44]. Severely obese white subjects have an especially high rate of metabolic syndrome even with an intermediate degree of insulin resistance. Other studies have shown the dyslipidemia associated with insulin resistance (low HDL, high triglycerides) is frequently found in adolescents [21].
In addition, the prevalence of metabolic syndrome increases with increasing insulin resistance, even after adjusting for race or obesity [44]. When followed longitudinally without intervention, most youth persisted in having metabolic syndrome and even progressed at 2-year follow-up, with lower body mass index (BMI) being associated with a lower likelihood of persisting. This alarming persistence of cardiovascular risk factors early in life warrants close attention, follow-up, and intervention.
Prediabetes and Type 2 Diabetes
Insulin resistance is a major contributing factor to the development of prediabetes and type 2 diabetes. Within the general adolescent population, the prevalence of prediabetes (either impaired fasting glucose [IFG] or impaired glucose tolerance [IGT]) has been reported to be approximately 16.1% [45]. This is compared with 27.5% of US adults with prediabetes [46]. As with the prediabetic state, adults represent a far greater proportion of the burden of type 2 diabetes within the United States with a population prevalence of about 12.3 per 100 compared to 0.5 per 1000 in individuals <20 years of age [47]. Though adults have also seen greater increases in the prevalence of diabetes within the population, the recent 30–35% increase in type 2 diabetes in youth is nevertheless alarming [27, 48].
Polycystic Ovary Syndrome (PCOS)
Other hormonal alterations in association with insulin resistance together form the polycystic ovary syndrome (PCOS) in adolescent females—a collection of symptoms that classically encompasses chronic anovulation, polycystic ovaries, and hyperandrogenism [49]. Signs of insulin resistance are commonly present, and there is increased risk for the development of glucose intolerance and type 2 diabetes [50]. As with metabolic syndrome, PCOS was first defined in adults but is increasingly recognized within the pediatric population. And like metabolic syndrome, diagnostic criteria for PCOS are less clear in youth, owing to practical differences in clinical evaluation and assessment. Some elements of PCOS, such as irregular menses and anovulation, can be seen as components of normal puberty, making diagnosis more difficult [49, 51]. The core pathophysiological elements of hyperandrogenism, insulin resistance, and anovulation remain the same. However, thresholds for declaring these abnormalities differ by criteria, and currently obesity and measures of insulin resistance are not included in most proposed diagnostic criteria for adolescents.
Hyperandrogenism can be assessed as clinical symptoms or biochemical evidence. Cutoffs for laboratory tests when evaluating for biochemical evidence of hyperandrogenism differ by developmental stage and must be evaluated within the clinical context [51]. Currently, biochemical evaluation lacks standardization between assays and, especially in younger females, sensitivity to detect lower levels. Though some cutoff values have been proposed, these depend on the use of reliable assays and laboratories [49]. The assessment of hirsutism in a female who is not yet fully pubescent is difficult and highly subjective. The use of a Ferriman–Gallwey score has many limitations even in adults, among them subjectivity and ethnic variation, and data on use in adolescents is even more scarce. Acne vulgaris is common during puberty, though moderate or severe acne early in puberty is less common and should prompt further evaluation [49].
Though the presence of oligomenorrhea is recommended as a diagnostic feature in some criteria, the clinical assessment of menstrual irregularity in adolescents is confounded by the fact that anovulatory cycles may persist for years after menarche even in normal females. However, the overall pattern is to have progressively more regular cycles with time, causing some experts to recommend considering menstrual intervals of <20 days or >45 days 2 or more years after menarche, or consecutive menstrual intervals >90 days at any point, as evidence of persistent amenorrhea [49]. The presence of ovaries meeting the adult definition of “polycystic” is actually quite common in normal adolescent females, so is an unreliable predictor for PCOS. Consequently, most criteria do not require visualization of polycystic ovaries themselves for diagnosis in adolescents [52].
PCOS is well recognized to be associated with insulin resistance. Hyperinsulinemia is associated with increased androgen production and decreased sex hormone-binding globulin (SHBG) production. This creates functional hyperandrogenism that impairs normal GnRH regulation and creates or exacerbates the menstrual irregularities characteristic of PCOS [49, 52, 53]. Thus, the presence of insulin resistance should raise greater concern for PCOS, and the reverse is also true. However, currently, no adolescent criteria require obesity, insulin resistance, or hyperinsulinemia [49]. These and other commonly seen features in PCOS overlap with several features of metabolic syndrome, and the prevalence of metabolic syndrome is thought to be 37–47% within the adolescent PCOS population—higher than in adults with PCOS and much higher than in the general population [53]. Adolescents with PCOS have a similar fasting glucose and higher concurrent fasting insulin even compared to obese adolescents and a roughly 50% reduction in peripheral insulin sensitivity and subsequent higher insulin secretion during a hyperglycemic clamp [52]. Adolescents may have exaggerated compensatory insulin secretion in response to the insulin resistance of PCOS, in contrast to a dysfunctional secretory response, suggesting that adult PCOS could represent an extension of adolescent-onset PCOS. In this postulated scenario, a longer time of disease exposure results in gradual dysfunction and failure of β(beta)-cells to compensate for increased insulin resistance over the long term. In addition, recognition of these differences in adults and adolescents in PCOS could indicate that differential treatment by age and clinical stage may be warranted [52].
Current treatment of PCOS in the pediatric population, while materially similar to adults, should be approached differently in the clinic. Intervention has been shown to benefit hirsutism, quality of life, and cardiovascular risk profile at least in the short term [49]. While adolescents may consider issues of fertility to be a less immediate concern, lifestyle modifications, if successfully implemented, can still be beneficial to quality of life. Oral contraceptive pills, especially antiandrogenic forms such as drospirenone- or cyproterone-containing formulations, can help to combat hyperandrogenism and restore regular menses, but may worsen insulin resistance or other cardiovascular risk factors such as dyslipidemia [51]. In addition, considering the postulated fundamental role of insulin resistance in driving other features of PCOS, insulin sensitizers such as metformin have been shown to increase glucose tolerance and decrease androgen concentrations, in some studies promoting improved lipid profiles and restoring regular menses when in combination with lifestyle interventions [54, 55].
Nonalcoholic Fatty Liver Disease (NAFLD) and Nonalcoholic Steatohepatitis (NASH)
Nonalcoholic fatty liver disease (NAFLD) is the most common chronic liver disease in pediatric patients [56]; estimates of the prevalence of NAFLD are about 30–40% in obese youth [56]. The clinical spectrum can range from mild steatosis to cirrhosis. Hispanic individuals have the highest risk, and Asian and non-Hispanic white individuals have a higher risk of hepatic steatosis than African Americans. Studies consistently show higher prevalence of NAFLD among those with insulin resistance [57] and a higher likelihood of persistence of glucose dysregulation among patients with NAFLD [58]. Others have suggested that because fatty liver can be seen even before frank type 2 diabetes has developed, and because steatotic livers tend to be insulin resistant and produce excess glucose, NAFLD may be a contributor toward the progression of insulin resistance to type 2 diabetes [59]. However, few studies have truly addressed causality in the association between insulin resistance and NAFLD.
About a fourth of children in the Nonalcoholic Steatohepatitis Clinical Research network had prediabetes, and 6.5% had type 2 diabetes, rates far higher than within the general pediatric population. Children with evidence of dysglycemia tend to be older and more obese, with a higher waist circumference. In addition, females with NAFLD are more likely to have prediabetes and type 2 diabetes than males with NAFLD [57], despite a clear predominance of males within the overall population with NAFLD.
The frequent coexistence of type 2 diabetes and NAFLD in adults is even more clinically relevant because type 2 diabetes is associated with nonalcoholic steatohepatitis (NASH), the more progressive form that is more often associated with end-stage liver disease. As in adults, biochemical or clinical evidence of insulin resistance is associated with NASH, and individuals with type 2 diabetes and NAFLD have approximately double the risk of NASH compared to those with normal glucose tolerance.
Lipodystrophies: Inherited and Acquired
Four broad categories of genetic lipodystrophies exist, each with multiple genetic causes or multiple subtypes: (1) congenital generalized lipodystrophy, (2) acquired generalized lipodystrophy, (3) familial partial lipodystrophy, and (4) acquired partial lipodystrophy—though lipodystrophy can also be a feature of other syndromes. In particular, lipodystrophy secondary to highly active antiretroviral therapy (HAART) is not genetically inherited but is now the most common form of lipodystrophy, present in about half of patients receiving HAART.
Lipodystrophy is rare in children, with an overall prevalence of less than one in a million individuals. The disorder consists of the absence of adipose, either generalized or partial. Consequently, individuals can appear unusually muscular; but despite this clinical feature, insulin resistance is frequently found in association with lipodystrophies. When adipocytes are not present, lipid molecules normally stored within the adipocyte are stored in other organs, including the muscle, myocardium, pancreas, and liver, causing metabolic instability [60]. Metabolic derangements can occur when the limited ability to store triglycerides causes increased fatty acid circulation. Increased substrate availability promotes utilization by skeletal muscle, competing with glucose utilization, thus resulting in clinical manifestations of insulin resistance [61]. Specifically, diabetes, hypertriglyceridemia, and acanthosis nigricans are frequently reported in association with lipodystrophy, and PCOS is common in women with lipodystrophy [62] Women with partial lipodystrophy may have more severe manifestations of metabolic complications in general [63]. The insulin resistance can be worsened by reduced levels of leptin in patients with severe loss of adipose tissue, promoting increased appetite that can make insulin resistance harder to treat clinically [64].
The prevalence of diabetes mellitus in lipodystrophy patients varies by type from 35% to 70%. Patients with acquired generalized lipodystrophy have the highest rate of diabetes mellitus, though patients with congenital generalized lipodystrophy have the youngest age of onset. In these patients, diabetes can present as young as school age. However, the absence of ketosis and presence of acanthosis nigricans and elevated serum insulin suggests dysglycemia secondary to insulin resistance rather than an autoimmune process [62].
The etiology of lipodystrophies involves mutations directly related to lipid storage or adipocyte differentiation and function. As adipocytes are an important target of insulin action and vital for decreasing fatty acid circulation and competition for glucose oxidation, any fundamental disruption in this process would directly decrease tissue responsiveness to insulin. Of the known genetic mutations resulting in autosomal recessive congenital generalized lipodystrophy, many—including AGPAT2, BSCL2, CAV1, and PTRF—are related to appropriate adipocyte development and function [64]. In (mostly) autosomal dominantly inherited familial partial lipodystrophy, the most commonly mutated gene is LMNA, encoding lamin A and lamin C, which are thought to result in abnormal nuclear interaction with transcription factors involved in adipogenesis [65], or premature apoptosis in adipocytes. Mutations in PPARG, a regulator of adipogenesis and target for thiazolidinediones [66], are present in many cases of lipodystrophy, while PLIN1 mutations affect a protein that is necessary for normal lipid storage [60].
Acquired lipodystrophy seen with HAART therapy may be related to protease inhibitor inhibition of zinc metalloprotease, which is involved in processing of lamin A, thus possibly resulting in lipodystrophy in a manner similar to LMNA mutations [67].
Management of lipodystrophies primarily centers on management of metabolic abnormalities. As with other patients with insulin resistance, diet and exercise are important. A recommended diet for lipodystrophy patients contains a somewhat lower proportion of carbohydrate compared to other patients, at about 50–60% of daily calories [64]. In addition, many patients require metformin, or a combination of metformin and insulin; insulin needs may be substantial due to the severe insulin resistance. Though metreleptin, or recombinant human leptin, has not been effective in treatment of insulin resistance in obese subjects, who presumably have high endogenous levels of leptin, the administration of metreleptin seems to be beneficial for lipodystrophy patients who have low baseline levels of leptin [68]. In very limited data available on metreleptin use in children, it reduces serum insulin and hemoglobin A1c when used in combination with lifestyle changes [62]. Metreleptin continues to be experimental for partial lipodystrophy.
Interventional Options
In both adults and children with insulin resistance, lifestyle modifications including diet and exercise have long been mainstays of treatment. Many intervention studies are multifaceted, aiming to alter both, and seem to show overall improvement in insulin resistance when there is weight loss and supervised physical activity [69, 70]. Studies that examine exercise or diet modifications alone are few, and results have been variable. Aerobic exercise specifically has been associated with improvement in insulin resistance in some studies [71], whereas resistance exercise has been shown to improve insulin resistance in male adolescents but not females [72, 73]. Studies assessing exercise independent of weight loss or attempted calorie restriction show transiently improved insulin sensitivity. While physical activity alone does not completely reverse the metabolic consequences of obesity alone, studies specifically examining physical activity suggest independent contributions from obesity and physical inactivity toward cardiometabolic risk [74]. Some have noted correlations between insulin sensitivity and changes in body composition, suggesting that improvements can come from changes in adiposity and muscle mass, even without weight change. Other studies show inverse associations between the time spent on moderate and vigorous physical activity and fasting insulin in adolescents, a relationship that remains relevant regardless of the time spent sedentary [75]. Perhaps most encouraging for the sedentary obese individual, greater improvements come from the transition from being sedentary to being moderately active, though additional benefits accrue from greater degrees of exercise [73].
Though significant periods of exercise may be difficult to achieve as a consistent lifestyle change, adult data suggest that even small improvements in daily physical activity can lead to positive gains. Studies examining various degrees of interrupted sedentary activity show that at least moderate activity is required to induce a change in ATP production that would likely lead to weight change, but even light activity could result in modulated glucose uptake and improved postprandial glycemic indices within days of intervention, possibly through both activity- and insulin-mediated pathways [37]. Adults with type 2 diabetes who replaced several hours a day of sitting with standing or light walking have been shown to have significantly lower glycemia over a 24-hour period compared with adults who did not replace periods of sitting with activity. Over the course of 4–5 days, insulin sensitivity also improved in groups that replaced periods of sitting with exercise. These changes appear to occur through mediation of contraction-induced glucose uptake rather than insulin-mediated glucose uptake. Thus, improvement in insulin sensitivity can be seen even over a short period of dedicated increase in non-strenuous activity [76, 77].
Reversal of obesity may also reverse its deleterious effects in the insulin-resistant individual. In cohort studies that demonstrate successful weight control through intensive lifestyle intervention, an improvement in insulin sensitivity can be seen with a BMI-SDS (standardized BMI) reduction as small as 0.25–0.5 mg/m2—a difference that was most apparent among pubertal and extremely obese children [69]. Clinically, this is manifested as improvement in rates of successful medical treatment with the addition of lifestyle intervention in youth with type 2 diabetes, though the overall treatment success rates were still discouraging [78].
More holistic lifestyle intervention programs have been shown to prevent the progression of prediabetes to diabetes in adults, but similar programs have not been as frequently studied in the pediatric population. In an intensive diet, exercise, and behavioral modification program for children with clinically measurable insulin resistance, children showed greater improvements in insulin sensitivity compared to those receiving counseling alone. In addition, a greater proportion of children in the intensive program converted to normal glucose tolerance as shown by oral glucose tolerance test (OGTT) on follow-up [79].
While insulin sensitizers such as metformin have been studied in adults and shown to decrease risk for type 2 diabetes, equivalent studies in children are scarce. Several studies have shown modest benefit of metformin in reducing BMI in obese children in the short term only [18, 80,81,82,83]. These studies have been limited by small size, and differences in measures of insulin resistance have been modest [80]. At this time, metformin is only approved for use in youth with type 2 diabetes. Metformin is an accepted part of treatment of PCOS, but few studies have closely examined the effect of the medication alone on long-term risk for progression in the obese, insulin-resistant child.
Conclusions
The phenomenon of insulin resistance is widespread among both adults and youth. Insulin resistance can exist without further clinical symptoms or in the context of several syndromes that can further negatively impact an individual’s health; most importantly, insulin resistance can be present even with normal glucose tolerance. The risk for insulin resistance is greatly increased in the setting of obesity, and youth have unique risk factors, such as puberty, that create added stress to the β(beta)-cell.
Syndromes that were previously associated with adulthood—such as fatty liver, metabolic syndrome, and PCOS—are now frequently recognized in association with insulin resistance in youth. As in adults, these syndromes have a great deal of overlap with each other and convey additional cardiovascular risk and potential long-term health consequences. The early onset of these syndromes is alarming, as the lengthened time of exposure likely places adolescents at higher risk of developing end-organ damage and long-term consequences, which will then cause personal health and healthcare system burden on a massive scale.
Many, if not all, interventions recommended as effective treatment for insulin resistance, prediabetes, or type 2 diabetes require diligence on the part of both patient and provider. Poor adherence observed in clinical practice is a testament to the difficulty of building new and healthier habits. However, patients and providers may be encouraged that improvement in insulin resistance and general health does not always require herculean transformation and that small victories and simpler interventions are not wasted. Small changes to BMI or moderate interruptions to an otherwise sedentary lifestyle can create improvements to cardiovascular risk factors that, though modest, could have impressive impact on the population scale. Therefore, prompt attention and intervention to obesity and insulin resistance to prevent adverse long-term health consequences are both vital and urgent, in order to provide the greatest benefit to patients.
References
Keller U, Gerber PP, Stauffacher W. Fatty acid-independent inhibition of hepatic ketone body production by insulin in humans. Am J Physiol. 1988;254(6 Pt 1):E694–9.
Jefferson LS. Role of insulin in the regulation of protein synthesis. Diabetes. 1980;29(6):487–96.
Philippe J. Insulin regulation of the glucagon gene is mediated by an insulin-responsive DNA element. Proc Natl Acad Sci U S A. 1991;88(16):7224–7.
Kahn BB. Glucose transport: pivotal step in insulin action. Diabetes. 1996;45(11):1644–54.
DeFronzo RA. The triumvirate: cell, muscle, liver: a collusion responsible for NIDDM. Diabetes. 1988;37(6):667–87.
Fielding BA, Frayn KN. Lipoprotein lipase and the disposition of dietary fatty acids. Br J Nutr. 1998;80(6):495–502.
Carnagarin R, Dharmarajan AM, Dass CR. Molecular aspects of glucose homeostasis in skeletal muscle–a focus on the molecular mechanisms of insulin resistance. Mol Cell Endocrinol. 2015;417:52–62.
Meshkani R, Adeli K. Hepatic insulin resistance, metabolic syndrome and cardiovascular disease. Clin Biochem. 2009;42(13–14):1331–46.
Hannon TS, Rao G, Arslanian SA. Childhood obesity and type 2 diabetes mellitus. Pediatrics. 2005;116(2):473–80.
Levy-Marchal C, Arslanian S, Cutfield W, Sinaiko A, Druet C, Marcovecchio ML, et al. Insulin resistance in children: consensus, perspective, and future directions. J Clin Endocrinol Metab. 2010;95(12):5189–98.
Brown RJ, Yanovski JA. Estimation of insulin sensitivity in children: methods, measures and controversies. Pediatr Diabetes. 2014;15(3):151–61.
Hannon TS, Janosky J, Arslanian SA. Longitudinal study of physiologic insulin resistance and metabolic changes of puberty. Pediatr Res. 2006;60(6):759–63.
Moran A, Jacobs DR, Steinberger J, Hong CP, Prineas R, Luepker R, et al. Insulin resistance during puberty: results from clamp studies in 357 children. Diabetes. 1999;48(10):2039–44.
Caprio S, Plewe G, Diamond MP, Simonson DC, Boulware SD, Sherwin RS, et al. Increased insulin secretion in puberty: a compensatory response to reductions in insulin sensitivity. J Pediatr. 1989;114(6):963–7.
Lyssenko V, Jonsson A, Almgren P, Pulizzi N, Isomaa B, Tuomi T, et al. Clinical risk factors, DNA variants, and the development of type 2 diabetes. N Engl J Med. 2008;359(21):2220–32.
Toperoff G, Aran D, Kark JD, Rosenberg M, Dubnikov T, Nissan B, et al. Genome-wide survey reveals predisposing diabetes type 2-related DNA methylation variations in human peripheral blood. Hum Mol Genet. 2012;21(2):371–83.
Chambers JC, Loh M, Lehne B, Drong A, Kriebel J, Motta V, Wahl S, Elliot HR, Rota F, Scott WR, Zhang W, Tan ST, Campanella G, Chadeau-Hyam M, Yengo L, Richmond RC, Adamowicz-Brice M, Afzal U, Bozaoglu K, Kooner JS. Epigenome-wide association of DNA methylation markers in peripheral blood from Indian Asians and Europeans with incident type 2 diabetes: a nested case-control study. Lancet Diabetes Endocrinol. 2015;3(7):526–34.
Wilson DM, Abrams SH, Aye T, Lee PD, Lenders C, Lustig RH, et al. Metformin extended release treatment of adolescent obesity: a 48-week randomized, double-blind, placebo-controlled trial with 48-week follow-up. Arch Pediatr Adolesc Med. 2010;164(2):116–23.
Vaag A, Jensen CB, Poulsen P, Brons C, Pilgaard K, Grunnet L, et al. Metabolic aspects of insulin resistance in individuals born small for gestational age. Horm Res. 2006;65(Suppl 3):137–43.
Holness MJ, Sugden MC. Epigenetic regulation of metabolism in children born small for gestational age. Curr Opin Clin Nutr Metab Care. 2006;9(4):482–8.
Weiss R, Kaufman FR. Metabolic complications of childhood obesity: identifying and mitigating the risk. Diabetes Care. 2008;31(Suppl 2):S310–6.
Arslanian SA, Saad R, Lewy V, Danadian K, Janosky J. Hyperinsulinemia in African-American children: decreased insulin clearance and increased insulin secretion and its relationship to insulin sensitivity. Diabetes. 2002;51(10):3014–9.
Bacha F, Gungor N, Lee S, Arslanian SA. Type 2 diabetes in youth: are there racial differences in beta-cell responsiveness relative to insulin sensitivity? Pediatr Diabetes. 2012;13(3):259–65.
Weiss R, Dziura JD, Burgert TS, Taksali SE, Tamborlane WV, Caprio S. Ethnic differences in beta cell adaptation to insulin resistance in obese children and adolescents. Diabetologia. 2006;49(3):571–9.
Hasson RE, Adam TC, Davis JN, Weigensberg MJ, Ventura EE, Lane CJ, et al. Ethnic differences in insulin action in obese African-American and Latino adolescents. J Clin Endocrinol Metab. 2010;95(8):4048–51.
TODAY Study Group, Zeitler P, Hirst K, Pyle L, Linder B, Copeland K, et al. A clinical trial to maintain glycemic control in youth with type 2 diabetes. N Engl J Med. 2012;366(24):2247–56.
Dabelea D, Mayer-Davis EJ, Saydah S, Imperatore G, Linder B, Divers J, et al. Prevalence of type 1 and type 2 diabetes among children and adolescents from 2001 to 2009. JAMA. 2014;311(17):1778–86.
Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA. 2014;311(8):806–14.
National Center for Health Statistics. Health, United States, 2015: with special feature on racial and ethnic health dsiparities. Hyattsville: National Center for Health Statistics; 2016.
Sjaarda L, Lee S, Tfayli H, Bacha F, Bertolet M, Arslanian S. Measuring beta-cell function relative to insulin sensitivity in youth: does the hyperglycemic clamp suffice? Diabetes Care. 2013;36(6):1607–12.
Wagner KA, Armah SM, Smith LG, Pike J, Tu W, Campbell WW, et al. Associations between diet behaviors and measures of glycemia, in clinical setting, in obese adolescents. Child Obes. 2016;12(5):341–7.
Gilbert PA, Khokhar S. Changing dietary habits of ethnic groups in Europe and implications for health. Nutr Rev. 2008;66(4):203–15.
Kaufman-Shriqui V, Fraser D, Friger M, Bilenko N, Vardi H, Abu-Saad K, et al. Factors associated with childhood overweight and obesity among acculturated and new immigrants. Ethn Dis. 2013;23(3):329–35.
Goel MS, McCarthy EP, Phillips RS, Wee CC. Obesity among US immigrant subgroups by duration of residence. JAMA. 2004;292(23):2860–7.
Gualdi-Russo E, Zaccagni L, Manzon VS, Masotti S, Rinaldo N, Khyatti M. Obesity and physical activity in children of immigrants. Eur J Pub Health. 2014;24(Suppl 1):40–6.
Delavari M, Sonderlund AL, Swinburn B, Mellor D, Renzaho A. Acculturation and obesity among migrant populations in high income countries–a systematic review. BMC Public Health. 2013;13:458.
Bergouignan A, Latouche C, Heywood S, Grace MS, Reddy-Luthmoodoo M, Natoli AK, et al. Frequent interruptions of sedentary time modulates contraction- and insulin-stimulated glucose uptake pathways in muscle: ancillary analysis from randomized clinical trials. Sci Rep. 2016;6:32044.
McGarrah RW, Slentz CA, Kraus WE. The effect of vigorous-versus moderate-intensity aerobic exercise on insulin action. Curr Cardiol Rep. 2016;18(12):117.
Nestler JE, Powers LP, Matt DW, Steingold KA, Plymate SR, Rittmaster RS, et al. A direct effect of hyperinsulinemia on serum sex hormone-binding globulin levels in obese women with the polycystic ovary syndrome. J Clin Endocrinol Metab. 1991;72(1):83–9.
Engmann L, Jin S, Sun F, Legro RS, Polotsky AJ, Hansen KR, et al. Racial and ethnic differences in the polycystic ovary syndrome (PCOS) metabolic phenotype. Am J Obstet Gynecol. 2017;216(5):493.e1–493.e13.
Aguilar M, Bhuket T, Torres S, Liu B, Wong RJ. Prevalence of the metabolic syndrome in the United States, 2003–2012. JAMA. 2015;313(19):1973–4.
Lee AM, Gurka MJ, DeBoer MD. Trends in metabolic syndrome severity and lifestyle factors among adolescents. Pediatrics. 2016;137(3):e20153177.
Zimmet P, Alberti G, Kaufman F, Tajima N, Silink M, Arslanian S, et al. The metabolic syndrome in children and adolescents. Lancet. 2007;369(9579):2059–61.
Weiss R, Dziura J, Burgert TS, Tamborlane WV, Taksali SE, Yeckel CW, et al. Obesity and the metabolic syndrome in children and adolescents. N Engl J Med. 2004;350(23):2362–74.
Li C, Ford ES, Zhao G, Mokdad AH. Prevalence of pre-diabetes and its association with clustering of cardiometabolic risk factors and hyperinsulinemia among U.S. adolescents: National Health and Nutrition Examination Survey 2005–2006. Diabetes Care. 2009;32(2):342–7.
American Diabetes Association. Statistics about diabetes. Available from: http://www.diabetes.org/diabetes-basics/statistics/.
Centers for Disease Control and Prevention. National Diabetes Statistics Report: estimates of diabetes and its burden in the United States. Atlanta: U.S. Department of Health and Human Services; 2014.
Hamman RF, Bell RA, Dabelea D, D’Agostino RB Jr, Dolan L, Imperatore G, et al. The SEARCH for Diabetes in Youth study: rationale, findings, and future directions. Diabetes Care. 2014;37(12):3336–44.
Witchel SF, Oberfield S, Rosenfield RL, Codner E, Bonny A, Ibanez L, et al. The diagnosis of polycystic ovary syndrome during adolescence. Horm Res Paediatr. 2015;83(6):376–89.
Carreau AM, Baillargeon JP. PCOS in adolescence and type 2 diabetes. Curr Diab Rep. 2015;15(1):564.
Williams RM, Ong KK, Dunger DB. Polycystic ovarian syndrome during puberty and adolescence. Mol Cell Endocrinol. 2013;373(1–2):61–7.
Lewy VD, Danadian K, Witchel SF, Arslanian S. Early metabolic abnormalities in adolescent girls with polycystic ovarian syndrome. J Pediatr. 2001;138(1):38–44.
Tfayli H, Arslanian S. Menstrual health and the metabolic syndrome in adolescents. Ann N Y Acad Sci. 2008;1135:85–94.
Glueck CJ, Aregawi D, Winiarska M, Agloria M, Luo G, Sieve L, et al. Metformin-diet ameliorates coronary heart disease risk factors and facilitates resumption of regular menses in adolescents with polycystic ovary syndrome. J Pediatr Endocrinol Metab. 2006;19(6):831–42.
Arslanian SA, Lewy V, Danadian K, Saad R. Metformin therapy in obese adolescents with polycystic ovary syndrome and impaired glucose tolerance: amelioration of exaggerated adrenal response to adrenocorticotropin with reduction of insulinemia/insulin resistance. J Clin Endocrinol Metab. 2002;87(4):1555–9.
Vos MB, Abrams SH, Barlow SE, Caprio S, Daniels SR, Kohli R, et al. NASPGHAN clinical practice guideline for the diagnosis and treatment of nonalcoholic fatty liver disease in children. J Pediatr Gastroenterol Nutr. 2017;64(2):319–34.
Newton KP, Hou J, Crimmins NA, Lavine JE, Barlow SE, Xanthakos SA, et al. Prevalence of prediabetes and type 2 diabetes in children with nonalcoholic fatty liver disease. JAMA Pediatr. 2016;170(10):e161971.
Kim G, Giannini C, Pierpont B, Feldstein AE, Santoro N, Kursawe R, et al. Longitudinal effects of MRI-measured hepatic steatosis on biomarkers of glucose homeostasis and hepatic apoptosis in obese youth. Diabetes Care. 2013;36(1):130–6.
Cali AM, De Oliveira AM, Kim H, Chen S, Reyes-Mugica M, Escalera S, et al. Glucose dysregulation and hepatic steatosis in obese adolescents: is there a link? Hepatology. 2009;49(6):1896–903.
Nolis T. Exploring the pathophysiology behind the more common genetic and acquired lipodystrophies. J Hum Genet. 2014;59(1):16–23.
Abate N. Adipocyte maturation arrest: a determinant of systemic insulin resistance to glucose disposal. J Clin Endocrinol Metab. 2012;97(3):760–3.
Gupta N, Asi N, Farah W, Almasri J, Moreno Barrionuevo P, Alsawas M, et al. Clinical features and management of non-HIV related lipodystrophy in children: a systematic review. J Clin Endocrinol Metab. 2016;102(2):363–74. https://doi.org/10.1210/jc.2016-2271.
Garg A. Gender differences in the prevalence of metabolic complications in familial partial lipodystrophy (Dunnigan variety). J Clin Endocrinol Metab. 2000;85(5):1776–82.
Hussain I, Garg A. Lipodystrophy syndromes. Endocrinol Metab Clin N Am. 2016;45(4):783–97.
Gambineri A, Semple RK, Forlani G, Genghini S, Grassi I, Hyden CS, et al. Monogenic polycystic ovary syndrome due to a mutation in the lamin A/C gene is sensitive to thiazolidinediones but not to metformin. Eur J Endocrinol. 2008;159(3):347–53.
Altshuler D, Hirschhorn JN, Klannemark M, Lindgren CM, Vohl MC, Nemesh J, et al. The common PPARgamma Pro12Ala polymorphism is associated with decreased risk of type 2 diabetes. Nat Genet. 2000;26(1):76–80.
Hudon SE, Coffinier C, Michaelis S, Fong LG, Young SG, Hrycyna CA. HIV-protease inhibitors block the enzymatic activity of purified Ste24p. Biochem Biophys Res Commun. 2008;374(2):365–8.
Diker-Cohen T, Cochran E, Gorden P, Brown RJ. Partial and generalized lipodystrophy: comparison of baseline characteristics and response to metreleptin. J Clin Endocrinol Metab. 2015;100(5):1802–10.
Reinehr T, Lass N, Toschke C, Rothermel J, Lanzinger S, Holl RW. Which amount of BMI-SDS reduction is necessary to improve cardiovascular risk factors in overweight children? J Clin Endocrinol Metab. 2016;101(8):3171–9.
Garnett SP, Gow M, Ho M, Baur LA, Noakes M, Woodhead HJ, et al. Improved insulin sensitivity and body composition, irrespective of macronutrient intake, after a 12 month intervention in adolescents with pre-diabetes; RESIST a randomised control trial. BMC Pediatr. 2014;14:289.
Marson EC, Delevatti RS, Prado AK, Netto N, Kruel LF. Effects of aerobic, resistance, and combined exercise training on insulin resistance markers in overweight or obese children and adolescents: a systematic review and meta-analysis. Prev Med. 2016;93:211–8.
Lee S, Deldin AR, White D, Kim Y, Libman I, Rivera-Vega M, et al. Aerobic exercise but not resistance exercise reduces intrahepatic lipid content and visceral fat and improves insulin sensitivity in obese adolescent girls: a randomized controlled trial. Am J Physiol Endocrinol Metab. 2013;305(10):E1222–9.
Lee S, Kim Y. Effects of exercise alone on insulin sensitivity and glucose tolerance in obese youth. Diabetes Metab J. 2013;37(4):225–32.
Loprinzi PD, Tudor-Locke C. Weight-activity associations with cardiometabolic risk factors among U.S. youth. Physiol Behav. 2015;149:165–8.
Ekelund U, Luan J, Sherar LB, Esliger DW, Griew P, Cooper A, et al. Moderate to vigorous physical activity and sedentary time and cardiometabolic risk factors in children and adolescents. JAMA. 2012;307(7):704–12.
Duvivier BM, Schaper NC, Hesselink MK, van Kan L, Stienen N, Winkens B, et al. Breaking sitting with light activities vs structured exercise: a randomised crossover study demonstrating benefits for glycaemic control and insulin sensitivity in type 2 diabetes. Diabetologia. 2016;60(3):490–8.
Dunstan DW, Kingwell BA, Larsen R, Healy GN, Cerin E, Hamilton MT, et al. Breaking up prolonged sitting reduces postprandial glucose and insulin responses. Diabetes Care. 2012;35(5):976–83.
TODAY Study Group. Effects of metformin, metformin plus rosiglitazone, and metformin plus lifestyle on insulin sensitivity and beta-cell function in TODAY. Diabetes Care. 2013;36(6):1749–57.
Savoye M, Caprio S, Dziura J, Camp A, Germain G, Summers C, et al. Reversal of early abnormalities in glucose metabolism in obese youth: results of an intensive lifestyle randomized controlled trial. Diabetes Care. 2014;37(2):317–24.
Kendall D, Vail A, Amin R, Barrett T, Dimitri P, Ivison F, et al. Metformin in obese children and adolescents: the MOCA trial. J Clin Endocrinol Metab. 2013;98(1):322–9.
Burgert TS, Duran EJ, Goldberg-Gell R, Dziura J, Yeckel CW, Katz S, et al. Short-term metabolic and cardiovascular effects of metformin in markedly obese adolescents with normal glucose tolerance. Pediatr Diabetes. 2008;9(6):567–76.
Yanovski JA, Krakoff J, Salaita CG, McDuffie JR, Kozlosky M, Sebring NG, et al. Effects of metformin on body weight and body composition in obese insulin-resistant children: a randomized clinical trial. Diabetes. 2011;60(2):477–85.
Clarson CL, Mahmud FH, Baker JE, Clark HE, McKay WM, Schauteet VD, et al. Metformin in combination with structured lifestyle intervention improved body mass index in obese adolescents, but did not improve insulin resistance. Endocrine. 2009;36(1):141–6.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Chen, M.E., Hannon, T.S. (2020). Clinical Manifestations of Insulin Resistance in Youth. In: Zeitler, P., Nadeau, K. (eds) Insulin Resistance. Contemporary Endocrinology. Humana, Cham. https://doi.org/10.1007/978-3-030-25057-7_1
Download citation
DOI: https://doi.org/10.1007/978-3-030-25057-7_1
Published:
Publisher Name: Humana, Cham
Print ISBN: 978-3-030-25055-3
Online ISBN: 978-3-030-25057-7
eBook Packages: MedicineMedicine (R0)