Abstract
This study assessed the efficacy of adding metformin to a structured lifestyle intervention in reducing BMI in obese adolescents with insulin resistance. Obese adolescents (25) aged 10–16 years with a body mass index (BMI) > 95th percentile and insulin resistance (Homeostasis Model Assessment—HOMA) > 3.0 were assessed in a community clinic. A structured lifestyle intervention comprising nutritional and exercise education and motivational support in both individual and group sessions was delivered over 6 months. Subjects were randomized to lifestyle intervention alone or with metformin (1500 g daily). The primary outcome measures were a change in BMI and modification of metabolic risk factors, including insulin resistance, plasma lipids and adipocytokines. Eleven adolescents receiving lifestyle and metformin intervention and 14 receiving lifestyle alone completed the study. BMI decreased by 1.8 kg/m2 with lifestyle and metformin but did not change with lifestyle alone. HOMA was significantly decreased in the lifestyle intervention group, but not following metformin, while the adiponectin/leptin ratio improved significantly in both groups. Dyslipidemic profiles improved most significantly with metformin. We conclude that metformin in combination with a 6-month structured lifestyle intervention is effective in reducing BMI in obese adolescents but did not improve insulin resistance. Lifestyle intervention, with or without metformin, improved metabolic risk factors such as plasma lipids and adipocytokines.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Childhood obesity confers a high degree of risk for future development of type-2 diabetes (T2D) [1, 2] This risk increases in adolescence in association with increasing insulin resistance which plays a crucial role in the pathogenesis of T2D [3]. Rates of obesity and T2D have escalated in children and adolescents, most of whom are obese at diagnosis of T2D [4–6]. Although there are multiple risk factors for insulin resistance and T2D, increased body mass index (BMI) has been identified as one of the most significant [1, 7].
The abnormal metabolic profile of insulin resistance and dyslipidemia in obese adolescents extends to circulating adiponectin which is inversely correlated with BMI, plasma leptin and insulin resistance, and positively correlated with HDL-cholesterol in non-diabetic, healthy adolescents [8, 9]. In adults with T2D, resistin and leptin levels decline following weight loss or improvement of insulin sensitivity [10]. Low adiponectin levels are independently associated with metabolic syndrome in overweight and obese children and high leptin levels are associated with future weight gain in adolescent girls [11–13]. Therefore, changes in adiponectin levels combined with changes in leptin or resistin levels may represent an informative indicator of metabolic health.
Metformin has been shown to be effective in reducing BMI and fasting insulin levels in obese, insulin-resistant adolescents in randomized controlled trials [14–18]. Although these studies demonstrated variable improvement in anthropometry with metformin, none included an individually structured lifestyle component which is likely important to sustain improved BMI and insulin sensitivity. The effectiveness of addition of metformin to a community-based multidisciplinary team approach to structured lifestyle intervention was assessed within a randomized controlled trial of obese children with insulin resistance. The goal was to reduce BMI and obesity-associated metabolic risk. The specific primary outcome measures were change in BMI and modification of metabolic risk factors, including insulin resistance, plasma lipids and adipocytokines; and assessment of metformin on the attainment of a target metabolic profile.
Results
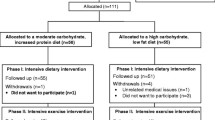
Sixty-five obese children aged between 10 and 16 years were screened of whom 31 met the inclusion criterion for insulin resistance and were enrolled. Six subjects dropped out prior to completion of this 6-month intervention, 3 at randomization and 3 due to non-adherence to the lifestyle intervention or social circumstances (Fig. 1). Twenty-five subjects (11 girls) with a mean age of 13.1 (range 10.1–16.1) years at entry completed the study, 11 (7 girls) received lifestyle intervention and metformin and 14 (5 girls) lifestyle intervention alone. All the subjects were Caucasian and had at least one second-degree relative with T2D while seven subjects had a first-degree relative with T2D. There was no history of maternal diabetes apart from gestational diabetes in 3 mothers. Fifteen subjects had acanthosis nigricans. Meformin was well tolerated at a maintenance dose of 1500 mg daily by all the subjects, and adherence was good as assessed by monthly pill counts. The average number of pills missed per month was 2 with a maximum of 5.
There was no difference in BMI at baseline for the 2 treatment groups (Table 1). BMI, both as a direct measure and expressed as a z-score, decreased with lifestyle and metformin but did not change significantly with lifestyle alone (Table 1). The change in BMI or BMI z-score was significantly greater with lifestyle and metformin than with lifestyle alone. No significant change in waist circumference occurred. There was no difference in systolic or diastolic blood pressure between the groups at study entry and subjects were normo-tensive (combined mean systolic 119 ± 5, diastolic 69 ± 2). There was no significant change in blood pressure following treatment in either group. Fasting insulin levels and HOMA both decreased in the lifestyle alone intervention group from baseline to 6 months, but did not significantly change with lifestyle and metformin (Table 2). However, the ∆ changes did not significantly differ between the two treatments. Dyslipidemic lipid profiles at baseline improved most significantly in the lifestyle with metformin group, with a significant decline in triglycerides and LDL cholesterol and for both groups an improvement in HDL cholesterol (Table 2). The decrease in triglycerides and LDL cholesterol with lifestyle and metformin was significantly greater than that seen with lifestyle alone. Serum levels of adiponectin increased (7.05 ± 0.93 ug/ml vs. 7.70 ± 0.97, NS lifestyle alone, and 6.24 ± 0.57 ug/ml vs. 7.31 ± 0.67, P < 0.05 lifestyle and metformin) while levels of leptin decreased (41.3 ± 3.97 ng/ml vs. 38.46 ± 5.40, NS lifestyle alone and 46.50 ± 3.36 ng/ml vs. 36.12 ± 6.62, P < 0.05 lifestyle and metformin), such that the adiponectin/leptin ratio increased significantly in both groups (0.19 ± 0.03 vs. 0.24 ± 0.04, P < 0.05 lifestyle alone and 0.15 ± 0.01 vs. 0.33 ± 0.08, P < 0.05 lifestyle and metformin), and significantly more so for lifestyle with metformin (Fig. 2). Linear regression analysis showed a significant inverse relationship between the net change in adiponectin/leptin ratio and the net change in BMI in both groups (r 2 = 0.19, P < 0.05). Resistin levels in serum were unaltered following intervention, and did not differ between groups. Neither the adiponectin/resistin ratio nor the resistin/leptin ratio differed before and after intervention in either group.
A parallel group of 25 pubertal adolescents (12 girls) matched at entry for age, BMI (34.9 ± 1.4), and BMI z-score (2.4 ± 0.1) and given only the usual standard of care (a diet and exercise counselling session) demonstrated a significant increase over 6 months in BMI (+1.1 ± 0.31, P < 0.005) and BMI z-score (+0.06 ± 0.02, P < 0.05).
Discussion
In the absence of an intervention, obese children and adolescents tend to continue to gain weight more rapidly than normal, as seen in the parallel group to this study cohort, and become obese adults. This study demonstrates that metformin therapy in conjunction with a structured 6-month individualized lifestyle intervention in obese adolescents can lead to a significant reduction in BMI that was not seen with lifestyle change alone. The addition of metformin also significantly improved the plasma lipid profile with respect to LDL cholesterol and triglycerides.
The changes seen here in BMI are more definitive than in previous studies assessing the impact of metformin on obesity in small cohorts of children and adolescents, where decreases in BMI ranged from 0.4 to 1.3 kg/m2 during a 4–6-month intervention [15–18]. Gender and ethnicity were both reported to influence the response to metformin with the reduction in BMI limited to girls in one study and to Caucasians in another which included African-Americans [17, 18]. Where insulin sensitivity was evaluated it showed improvement [14, 16]. In this study, the adherence rate for metformin was high, attributed in part to the weekly study co-ordinator contact. Therefore the failure of metformin to significantly impact on insulin sensitivity does not appear to be due to non-adherence, but could be due to variance in insulin sensitivity during puberty. Although insulin sensitivity did not improve significantly with metformin, the reduction in BMI was significant. Since obesity is an independent risk factor for T2D and usually precedes the development of insulin resistance, an initial reduction in BMI may be of greater value in reducing risk for T2D. A prospective study of over 1600 Pima Indian children and adolescents analysed predictive risk factors for T2D and identified fasting glucose, 2 h glucose, HDL cholesterol and BMI as independent modifiable diabetes predictors [1]. Although fasting insulin, which correlates with HOMA, was individually predictive of diabetes, this did not provide additional information to other predictors [1, 19]. Obesity was also shown to have a greater contribution to clustering of risk factors for diabetes and cardiovascular disease than insulin resistance, and the effect of insulin resistance was accounted for by obesity [20].
We found an increase in HDL cholesterol in both groups, presumably due to the increased physical activity levels promoted by the lifestyle intervention, while the addition of metformin was associated with a significant reduction in both LDL cholesterol and triglycerides that was not seen with lifestyle change alone. There are limited data on lipid profiles in response to metformin in obese, insulin-resistant adolescents. One study found no change in plasma lipids while another reported improvement in total cholesterol and triglycerides but did not assess LDL and HDL cholesterol [14, 15]. Those studies did not include a structured individualized dietary intervention as this study has done. It is likely this dietary component resulted in the observed improvement in all the lipid parameters augmented by the addition of metformin.
We found that changes in the adiponectin/leptin ratio showed a negative relationship with the corresponding change in BMI, suggesting that this could represent a useful indicator of change in BMI, with possible prognostic value. An increase in adiponectin levels was demonstrated in response to a 6-week activity intervention whilst a reduction in leptin levels correlated with a decrease in fat mass, in obese boys but not girls [21–23]. A more prolonged 1-year lifestyle intervention in overweight and obese children was associated with a significant change in adiponectin level irrespective of weight change, and was associated with loss of fat mass [23]. The adiponectin-to-leptin ratio as shown in this study appears to provide a better index of improvement in BMI than either cytokine level alone.
Key elements of the lifestyle intervention were collaboration with the child to develop individual goals, commitment of a parent ‘coach’ and regular contact with a multidisciplinary team including weekly telephone calls, monthly clinic visits and family education sessions. A recent meta-analysis of weight-loss treatments for children identified that a family behavioural component produced larger effect sizes than the alternative treatment groups [24]. Three subjects discontinued this study due to non-adherence to the lifestyle intervention or social circumstances, highlighting the importance of screening to address psychosocial issues that may become barriers to success.
Limitations to this study include the relatively small sample size and the absence of a placebo control group. Over half of the obese adolescents screened did not meet the criterion for insulin resistance, and 23% did not complete the 6-month intervention, consistent with dropout rates reported in other studies in this population [14, 16, 25]. Review of barriers to study entry identified commitment to monthly visits as the most substantial. Reasons for withdrawal from the study included dissatisfaction with the allotted group at randomization or lack of commitment to the lifestyle intervention, underscoring the importance of including a placebo control in future studies.
The key motivator for participation in this study was weight loss, not improved health or diabetes prevention. We conclude that in obese, insulin-resistant adolescents, metformin in conjunction with nutrition and activity intervention is effective in reducing BMI and improving metabolic profile. Contrary to lifestyle intervention alone, it did not lead to an improvement in insulin resistance. It is likely that those children who lose weight in response to metformin will be encouraged by this positive response and become more motivated to maintain the promoted lifestyle changes which are essential to prevent continuing weight gain and maintain improvement in anthropometric and metabolic parameters. Key questions remain including the long-term efficacy and sustainability of intervention programs for pediatric obesity, particularly strategies to optimize adherence to lifestyle interventions.
Materials and methods
Obese subjects aged 10–16 years, defined as BMI greater than the 95th percentile for age and gender, and who were also insulin resistant (defined by HOMA greater than 3.0, calculated as fasting plasma insulin (mU/l) × fasting serum blood glucose (mmol/l)/22.5) were enrolled over a 15 month period between 2005 and 2007. All the subjects were assessed to be in puberty throughout the study. HOMA values greater than 3 in adolescents are indicative of insulin resistance [26–28]. Exclusion criteria included fasting blood glucose ≥6.0 mmol/l and contraindications to metformin therapy. Subjects were randomized using computer random number generation to lifestyle intervention alone or lifestyle in combination with metformin. Those subjects receiving metformin started therapy at 500 mg/day, increasing by 500 mg/day every 7 days to a maximum tolerated dose of 500 mg × 3 per day. Metformin pills were distributed in monthly supplies, and pill counts conducted each month. In parallel, a comparison was made with a group of 25 age-matched obese adolescents who were managed with standard care comprising dietary advice and recommendations aimed at increasing physical activity with a follow-up visit at 6 months. This study was approved by the University of Western Ontario Research Ethics Board and Health Canada and informed consent was obtained from all the study subjects.
At study entry potential risk factors for development of T2D, in addition to obesity and insulin resistance, were assessed, including family history of T2D, gestational diabetes, ethnicity and presence of acanthosis nigricans. BMI z-scores were calculated from the U.S. Centers for Disease Control and Prevention reference data [29]. Waist circumference was measured in the standing position at the level of the umbilicus to the nearest 0.1 cm using a constant tension tape.
At screening and at study completion at 6 months, blood was sampled under fasting conditions for measurement of glucose, insulin, LDL and HDL cholesterol, triglycerides, AST, ALT, BUN, creatinine and adipocytokines. Serum glucose was measured by the glucose oxidase technique, and insulin levels determined by a double antibody radioimmunoassay using a recombinant human insulin standard and the minimum detection level of <2 μU/ml. Within- and between-assay coefficients of variation were 5.8% and 6.5%, respectively. Resistin, leptin and total adiponectin levels in serum were each measured by specific, commercial ELISAs (Quantikine, R&D Systems, Minneapolis, MN). The minimum level of detection and intra- and inter-assay precision for resistin was 0.03 ng/ml, 5% and 8%, respectively, for leptin 7 pg/ml, 3% and 4%, and for adiponectin 0.25 ng/ml, 4% and 6%.
The 6 month lifestyle intervention was multidisciplinary with monthly individual visits and 2 group sessions. Dietary advice was provided in individual sessions every 2 months. Following an initial fitness assessment subjects were asked to choose a ‘fun’ activity(ies) to do for at least 30-min per day and an action plan developed to facilitate achievement of this goal. The fitness specialist supervised subjects in an individual 30 min exercise session every 2 months and provided recommendations, handouts and equipment for home use (dynabands and fit balls). Subjects were screened by a social worker to identify potential psychosocial barriers to study participation and provide support in goal achievement. A caregiver/parent committed to support the subject and was counselled on ‘coaching strategies’. The study co-ordinator reviewed and promoted progress in weekly telephone calls and at monthly visits. The lifestyle intervention was identical for the lifestyle alone and lifestyle with metformin groups.
Statistical analysis
Values are expressed as mean ± SEM. The normality of variables was evaluated by the Shapiro–Wilk W test, and all the values were found to be normally distributed. Statistical differences between groups were analysed by analysis of variance using Tukey/Kramer and Games/Howell post-hoc tests, and by paired t test. Linear regression analysis was performed to compare changes in adipocytokine levels with changes in BMI or HOMA.
References
P.W. Franks, R.L. Hanson, W.C. Knowler et al., Childhood predictors of young—onset type 2 diabetes. Diabetes 56, 2964–2972 (2006)
S.E. Kahn, R.L. Hull, K.M. Utzschneider, Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 444, 840–846 (2006)
S. Arslanian, Type 2 diabetes mellitus in children: pathophysiology and risk factors. J. Pediatr. Endocrinol. Metab. 13, 1385–1394 (2000)
M.S. Tremblay, J.D. Willms, Secular trends in the body mass index of Canadian children. Can. Med. Assoc. J. 163, 1429–1433 (2000)
A. Fagot-Campagna, D.J. Pettitt, M.M. Engelgau et al., Type 2 diabetes among North American children and adolescents; an epidemiologic review and a public health perspective. J. Pediatr. 136, 664–672 (2000)
H.J. Dean, R.L. Mundy, M. Moffatt, Non-insulin dependent diabetes mellitus in Indian Children in Manitoba. Can. Med. Assoc. J. 147, 52–57 (1992)
R.Y. Sung, P.C. Tong, C.W. Yu et al., High prevalence of insulin resistance and metabolic syndrome in overweight/obese preadolescent Hong Kong Chinese children aged 9–12 years. Diabetes Care 26, 250–251 (2003)
N. Stefan, J.C. Bunt, A.D. Salbe et al., Plasma adiponectin concentrations in children: relationships with obesity and insulinemia. J. Clin. Endocrinol. Metab. 87, 4652–4656 (2002)
F. Bacha, R. Saad, N. Gungor et al., Adiponectin in youth: relationship to visceral obesity, insulin sensitivity, and beta cell function. Diabetes Care 27, 547–552 (2007)
M. Bajaj, S. Suraamornkul, L.J. Hardies et al., Plasma resistin concentration, hepatic fat content, and hepatic and peripheral insulin resistance in pioglitazone-treated type II diabetic patients. Int. J. Obes. Relat. Metab. Disord. 28, 783–789 (2004)
L. Gilardini, P.G. McTernan, A. Girola et al., Adiponectin is a candidate marker of metabolic syndrome in obese children and adolescents. Atherosclerosis 189, 401–407 (2006)
G.Q. Shaibi, M.L. Cruz, M.J. Weigensberg et al., Adiponectin independently predicts metabolic syndrome in overweight Latino youth. J. Clin. Edocrinol. Metab. 92, 1809–1813 (2007)
M. Savoye, J. Dziura, J. Castle et al., Importance of plasma leptin in predicting future weight gain in obese children: a two-and-a-half-year longitudinal study. Int. J. Obes. Relat. Metab. Disord. 26, 942–946 (2002)
J.P. Kay, R. Alemzadeh, G. Langley et al., Beneficial effects of metformin in normoglycemic morbidly obese adolescents. Metabolism 50, 1457–1461 (2001)
M. Freemark, D. Bursey, The effects of metformin on body mass index and glucose tolerance in obese adolescents with fasting hyperinsulinemia and a family history of type 2 diabetes. Pediatrics 107, E55 (2001)
S. Srinivasan, G.R. Ambler, L.A. Baur et al., Randomized controlled trial of metformin for obesity and insulin resistance in children and adolescents: improvement in body composition and fasting insulin. J. Clin. Endocrinol. Metab. 91, 2074–2080 (2006)
K. Love-Osborne, J. Sheeder, P. Zeitler, Addition of metformin to a lifestyle modification program in adolescents with insulin resistance. J. Pediatr. 152, 817–822 (2008)
R.H. Lustig, M.L. Mietus-Snyder, P. Bacchetti et al., Insulin dynamics predict body mass index and z-score response to insulin suppression or sensitization pharmacotherapy in obese children. Pediatrics 148, 23–29 (2006)
B. Schwartz, D.R. Jacobs, A. Moran et al., Measurement of insulin sensitivity in children: comparison between the euglycemic hyperinsulinemic clamp and surrogate measures. Diabetes Care 31, 783–788 (2008)
S.D. Ferranti, S.K. Osganian, Epidemiology of paediatric metabolic syndrome and type 2 diabetes mellitus. Diabetes Vasc. Dis. Res. 4, 285–296 (2007)
E.S. Kim, J.A. Im, K.C. Kim et al., Improved insulin sensitivity and adiponectin level after exercise training in obese Korean youth. Obesity 15, 3023–3030 (2007)
E.E. Reiterer, K.M. Sudi, A. Mayer et al., Changes in leptin, insulin and body composition in obese children during a weight reduction program. J. Pediatr. Endocrinol. Metab. 12, 853–862 (1999)
V.M. Cambuli, M.C. Musiu, M. Incani et al., Assessment of adiponectin and leptin as biomarkers of positive metabolic outcomes after lifestyle intervention in overweight and obese children. J. Clin. Edocrinol. Metab. 93, 3051–3057 (2008)
M. Zeller, S. Kirk, R. Claytor et al., Predictors of attrition from a pediatric weight management program. J. Pediatr. 144, 466–470 (2004)
K.M. Young, J.J. Northern, K.M. Lister, J.A. Drummond, W.H. O’Brien et al., A meta-analysis of family-behavioural weight-loss treatments for children. Clin. Psychol. Rev. 27, 240–249 (2007)
N. Gungor, R. Saad, J. Janosky et al., Validation of surrogate estimates of insulin sensitivity and insulin secretion in children and adolescents. J. Pediatr. 144, 47–55 (2004)
M. Keskin, S. Kurtoglu, M. Kendirci et al., Homeostasis model assessment is more reliable than the fasting glucose/insulin ratio and quantitative insulin sensitivity check index for assessing insulin resistance among obese children and adolescents. Pediatrics 115, 500–503 (2005)
P. Allard, E.E. Delvin, G. Paradis et al., Distribution of fasting plasma insulin, free fatty acids, and glucose concentrations and of homeostasis model assessment of insulin resistance in a representative sample of Quebec children and adolescents. Clin. Chem. 49, 644–649 (2003)
2005 CDC growth charts for the United States, Methods and development. (2005), www.cdc.gov/nchs/data/series/sr_11/sr11_246.pdf
Acknowledgements
We are grateful to Lynda Bere, Bonnie Hill, Johanna Kaipainen and Ruth Aitchison for clinical and administrative support and Brenda Strutt who provided expert technical assistance. We acknowledge the financial support for this study provided by the Lawson Foundation, the Canadian Institutes of Health Research and the Lawson Health Research Institute.
Author information
Authors and Affiliations
Corresponding author
Additional information
The sponsors had no involvement in the study design, collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Rights and permissions
About this article
Cite this article
Clarson, C.L., Mahmud, F.H., Baker, J.E. et al. Metformin in combination with structured lifestyle intervention improved body mass index in obese adolescents, but did not improve insulin resistance. Endocr 36, 141–146 (2009). https://doi.org/10.1007/s12020-009-9196-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-009-9196-9