Abstract
Total shoulder arthroplasty has gained remarkable popularity in the last few decades. Critical review of outcomes and large registries in different countries have led to newer designs and expanding indications, which have resulted in rapid growth around the world. Despite numerous advances in surgical techniques and implant designs, however, complications still occur, and with an active aging population and increasing popularity among upper extremity surgeons, we can expect those complications to increase both in volume and complexity. Recognition and timely diagnosis of adverse outcomes can help surgeons not only correct and salvage a poor outcome but understanding what leads to common complications may also help us to anticipate and avoid them altogether. Infection, component loosening, periprosthetic fracture, and instability are the major reasons for revision surgery. Though the diagnosis may be difficult, as many signs and symptoms may be subtle and nonspecific, a careful history and physical examination, critical review of radiographs, and a healthy level of suspicion may offer clues and guide the surgeon toward advanced imaging, laboratory studies, and other diagnostic measures. This chapter aims to provide the reader with the tools to successfully identify those patients who may need further surgical treatment in the setting of a total shoulder arthroplasty as well as to help avoid common pitfalls implicated in certain complications.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Total shoulder arthroplasty
- Reverse shoulder arthroplasty
- Infection
- Periprosthetic
- Fracture
- Instability
- Loosening
- Radiolucency
- Cutibacterium acnes
7.1 Introduction
The history of shoulder arthroplasty begins in the late nineteenth century with Themistocles Gluck and Jules Emile Péan [1], but its clinical contribution to shoulder surgery remained relatively anecdotal until the initial design created in 1952 by Dr. Charles Neer, which served as a solution for complex proximal humerus fractures in which avascular necrosis and ankylosis were common complications [2]. At that time, the design was a monobloc humeral stem with only three sizes, and there was no option to resurface the glenoid. In 1974, he published his results with total shoulder arthroplasty for primary osteoarthritis, with an early design of a cemented polyethylene glenoid component [3]. Since then, shoulder arthroplasty design has continued to evolve into a wide variety of anatomic and reverse systems, including stemmed, stemless, cemented, cementless, and modular implants.
7.2 Epidemiology
The incidence of shoulder replacement surgery is increasing worldwide. A 141.4% increase has been reported between 2008 and 2017 in Australia, with an annual incidence of 26/100,000 inhabitants in 2017 [4]. A similar trend has been reported in other countries, such as Norway, Sweden, New Zealand, Denmark, and Germany, with annual incidence rates anywhere from 8 to 34/100,000 [5].
Along with that dramatic increase, there has been a concomitant rise in the number of revision surgeries. With a reported revision rate of 2.8–10.9% [4, 6, 7], an increasing number of revision procedures can be expected as surgical indications are expanded.
The most common indications for total shoulder replacement include inflammatory arthritis, primary osteoarthritis, instability arthritis, post-capsulorrhaphy arthropathy, rotator cuff-deficient arthritis, advanced avascular necrosis, and intra-articular fractures. The underlying etiology seems to influence the clinical outcome and longevity of the implant.
In that regard, national registries have proven to be excellent tools to further analyze revision surgery. The Australia National Joint Registry of 2018, for example, is rich with information. In Australia, the revision rate for hemi-resurfacing arthroplasty is 12%, and the main reasons for revision are glenoid erosion and pain, which account for almost 50% of revision cases. This is followed by rotator cuff insufficiency and component loosening. Of the total revisions, 54% follow a reverse shoulder arthroplasty, and 45% follow an anatomic arthroplasty. Patients aged 65–74 have a 50% reduced hazard ratio for revision compared to patients <55 years. There appears to be no difference in revision rates when comparing the underlying diagnoses in stemmed hemiarthroplasty (8.4% for fracture vs. 9.3% for osteoarthritis).
The indication for revision, however, does seem to vary according to the underlying diagnosis. In those cases where hemiarthroplasty was performed for a fracture, the most common reasons for revision were rotator cuff insufficiency, followed by instability, or dislocation. For those whose primary diagnosis was osteoarthritis, glenoid erosion was the most frequent reason for revision, followed by instability. Cemented stems and patients older than 75 years had a lower revision rate in the fracture group.
Anatomic total shoulder arthroplasty in the Australian National Joint Registry has demonstrated a rapid decrease since 2010 with a simultaneous increase in reverse shoulder arthroplasty, which accounted for over 70% of all the total shoulder arthroplasties performed during 2017. The revision rate for anatomic versus reverse shoulder arthroplasty was 12.6% and 7% at 10 years, respectively. Anatomic shoulder arthroplasty did not show different revision rates when performed for fracture, osteonecrosis, or osteoarthritis.
Rotator cuff insufficiency, instability, and loosening account for almost two-thirds of the reasons for revision in anatomic arthroplasties. Half of the revisions were of the humeral component only, and 20% of them involved revision of both the humeral and glenoid components. There was an increased rate of revision with cementless glenoid components and in patients younger than 55 years.
In the case of reverse shoulder arthroplasty, there was an increased rate of revision at 3 months when performed for fracture, but the rate stabilized after that. Instability, infection, loosening, and fracture accounted for over 85% of the revision causes. Age was not a reason for revision when performed for osteoarthritis, but when performed for fracture or rotator cuff arthropathy, patients older than 75 years had a lower revision rate [4].
7.3 Causes for Revision
7.3.1 Infection
Periprosthetic joint infection after total shoulder arthroplasty has been reported to have an incidence between 1% and 4% and accounts for 3–5% of all complications following anatomic total shoulder arthroplasty [8,9,10,11]. A higher infection rate has been reported following reverse shoulder arthroplasty, and it has been found to be up to 6.11 times greater than after anatomic shoulder arthroplasty [12]. It has been hypothesized that postsurgical hematoma formation in the subacromial space may contribute to its development [13, 14].
A systematic review by Zumstein et al. reported a 2.9% rate of deep infection rate after primary RTSA and 5.8% after revision RTSA [15]. Walch et al. compared their results after RTSA between the years 1995 to 2003 versus 2003 to 2007 and found a marked decrease in infection rates between those time periods (from 4% to 0.9%). The authors postulated that surgeon experience, perhaps through more refined indications and surgical technique, may be paramount to avoiding or minimizing this complication [16].
Some patient populations are at greater risk for deep infection, such as patients with rheumatoid arthritis. These patients reportedly have up to 2.6 times higher risk of infection after joint arthroplasty [17, 18]. A case-control study by Bala and colleagues found an increased risk of infection in patients with HIV infection (OR 1.36; 95% CI, 1.01–1.82) [19].
Smoking has also been correlated with increased infection rates with a hazard ratio of 7.27 for current smokers and 4.56 for former smokers (those who had not smoked within 1 month prior to surgery) [20]. In addition, an increased risk of infection after total shoulder arthroplasty has been found in male patients, those with a traumatic indication, prior local infection, prior non-arthroplasty shoulder surgery, revision arthroplasty, long-term corticosteroid use, and the need for perioperative allogeneic red blood cell transfusion [21, 22].
The most commonly cultured organisms in an infected shoulder arthroplasty are the Cutibacterium acnes (formerly Propionibacterium acnes) and coagulase-negative Staphylococcus spp. It is common to find these organisms in the setting of a subacute infection, where pain may be the only apparent manifestation and the classic signs of infection, such as fever, erythema, warmth, and purulence may be less prominent. A careful history can reveal details that help with the diagnosis, such as pain at rest and stiffness.
C. acnes is a well-known gram-positive rod found in the skin as a commensal, with younger male patients having a higher bacterial burden. It has been implicated in chronic skin diseases, such as acne vulgaris, and deep infections associated with prosthetic devices. Torrens et al. isolated positive cultures for C. acnes in the deep layers of 18.8% of their patients undergoing a primary reverse total shoulder arthroplasty. Of those cases, however, with a minimum follow-up of 1 year, only one patient (1.1%) developed an infection at 6 months after the procedure, suggesting that the presence of this organism does not guarantee an infection and may not be the only risk factor [23]. C. acnes infection is more frequently associated with male patients, cloudy synovial fluid, humeral osteolysis, humeral loosening, glenoid wear, and membrane formation (Fig. 7.1) [24].
More “classic” clinical findings suggesting infection may be encountered in the setting of a more aggressive organism, such as Staphylococcus or Streptococcus spp. In these instances, bone osteolysis and implant loosening, swelling, erythema, and increased blood infection markers may be present [12, 21, 25].
Diagnosis of infection can often be difficult, with pain and limited range of motion being the most common clinical complaints [26]. Good-quality radiographs can help rule out conditions that may mimic or coexist with an infected shoulder arthroplasty, such as post-arthroplasty rotator cuff failure. It is common practice to obtain a baseline laboratory analysis with white blood cells (WBC) (percentage polymorphonuclear cells), erythrocyte sedimentation rate (ESR), and C-reactive protein (CRP). Computed tomography (CT) scans can be useful to detect osteolysis and to assess remaining bone stock. Ultrasonography and magnetic resonance imaging (MRI) with metal subtraction protocols can determine the presence of local abscesses, effusion, or osteomyelitis. Scintigraphy can detect inflammation but may not be useful in low-grade infections [27, 28].
Synovial fluid analysis from an aspiration or at the time of revision surgery should include cell count, gram stain, cultures for aerobes, anaerobes, fungi, and mycobacteria and should be held for up to 4 weeks [24]. Unfortunately, a negative culture or gram stain does not always rule out infection. Intraoperatively, at least five biopsy samples should be sent for gram stain and frozen section [29]. Interestingly, increased body mass index, diabetes severity, and asymptomatic bacteriuria or abnormal urinalysis have not been associated with increased rates of infection [12, 30,31,32].
7.3.2 Instability
7.3.2.1 Instability After Anatomic Total Shoulder Arthroplasty (TSA)
Instability after anatomic total shoulder arthroplasty is a relatively common complication, with a reported prevalence ranging from 1% to 3% [11, 33]. It can occur secondary to insufficient bone stock, inadequate soft tissue balance, component malalignment, or loosening.
Severe primary osteoarthritis, as well as post-capsulorrhaphy arthritis, can lead to excessive acquired retroversion of the native glenoid. Anterior wear is more uncommon, but it can be found in patients with chronic anterior glenohumeral dislocations, glenoid fractures, or rheumatoid arthritis. Failure to identify and correct this deformity can result in glenoid component malalignment and either posterior or anterior instability. Humeral component malpositioning is usually less critical, but it can also play a role in instability.
Diagnosis can be difficult, and a careful physical examination is paramount. In some patients, dislocation of the glenohumeral joint can be obvious radiographically, but in the setting of subluxation, findings will be more subtle. Excessive translation of the humeral head or a positive load-and-shift test can help the examiner in the diagnosis of these cases [34, 35].
Anterior instability after anatomic total shoulder arthroplasty has been reported in 0.9% of the patients [11] and has been associated with subscapularis failure, retroversion of the humeral component of less than 20° [36], anterior glenoid deficiency, and anterior deltoid dysfunction [11]. Of these causes, it is thought that subscapularis dysfunction plays a major role. In these patients, a positive lift-off test and/or belly press test can be found [37].
Management of the subscapularis during the initial surgery remains controversial, as some authors report improved outcomes after a lesser tuberosity osteotomy (LTO) versus a tenotomy or peel technique [38]. This clinical finding has been supported by biomechanical analyses [39, 40]. However, the peel technique or tenotomy of the subscapularis avoids the potential complication of LTO nonunion [41]. To date, there is insufficient high-level clinical evidence to strongly support one technique over the others.
In addition to technique, overstuffing the joint with an excessively large humeral head and medialization of the tendon insertion may lead to failed subscapularis failure. Excessively early mobilization, aggressive physical therapy, or postoperative trauma can also disrupt the subscapularis tendon repair.
Posterior instability after TSA occurs with a similar frequency as anterior instability (1%) [11] and has been associated with soft tissue imbalance. While posterior rotator cuff dysfunction and capsular laxity have been most commonly implicated, component malalignment and posterior bone loss can also play a role [34, 42]. Glenoid retroversion over 20° and humeral component in more than 45° of retroversion have been described as potential causes of posterior instability [36]. Sanchez-Sotelo and colleagues recommended that surgeons pay close attention to the humeral neck cut angle and the subscapularis tendon repair and address any posterior glenoid bone loss to minimize the potential for this complication. In addition, posttraumatic osteoarthritis or preoperative humeral subluxation should be carefully evaluated [34].
Rotator cuff failure is one of the most common complications after anatomic total shoulder replacement. A recent analysis of complications reported to the US Food and Drug Administration (FDA) demonstrated that among all the complications found after 1673 anatomic total shoulder replacements, posterior-superior rotator cuff and subscapularis failure were second only to glenoid component failure, representing 15.4% of all the complications [9]. Rotator cuff failure allows the humeral head to migrate proximally, leading to superior instability (Fig. 7.2). Reported in up to 3% of cases [11], superior instability may be the single most common direction of instability following anatomic shoulder arthroplasty.
Rotator cuff failure can lead to superior instability. The radiograph on the left demonstrates proximal migration of the humerus, which led to glenoid component fixation failure through the so-called rocking horse mechanism. This patient eventually underwent revision surgery to a reverse shoulder arthroplasty (right)
The rotator cuff can be compromised during the index procedure, specifically if an aggressive humeral resection is performed or if the cut is placed in too much retroversion [43]. Postoperative rotator cuff failure can also occur, with reported rates from 1.3% to 5.8% [11, 37]. Several factors have been found to affect superior instability: fatty infiltration of the infraspinatus, rotator cuff tear size, coracoacromial arch insufficiency, anterior deltoid dysfunction, humeral head overstuffing and malpositioning, and tuberosity nonunion in the setting of fracture [44, 45].
Inferior instability often occurs when the humeral length is not restored and deltoid tensioning is therefore not achieved. This has been reported to be more common after four-part proximal humerus fractures, where the stem can be accidentally seated too low due to a loss of anatomic references. Warren recommends inferior distraction of the humerus to detect this issue intraoperatively. When this maneuver is performed, the head should ideally remain within the upper one-third of the glenoid. Inferior instability may also occur in a setting of an axillary nerve palsy or rotator interval insufficiency in which the dynamic stabilizers are inadequate to hold the glenohumeral joint reduced [42].
7.3.2.2 Instability After Revision Total Shoulder Arthroplasty (RTSA)
Trappey et al. reported an instability rate after RTSA of 5% following primary cases and 8% following revision arthroplasty [46]. The mechanism of dislocation is typically adduction and internal rotation and most commonly occurs within the first 3 months following surgery. Up to 50% of these will have good outcomes with conservative treatment after successful closed reduction. Late dislocations that occur over 3 months after the index procedure often require surgical treatment [47].
Abdelfattah et al. proposed a classification system for instability after reverse total shoulder arthroplasty. They described three main categories: loss of compression, loss of containment, and impingement.
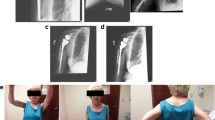
They further divided loss of compression into undersized implants, loss of deltoid contour, humeral height loss, subscapularis deficiency, acromial/scapular fracture, and deltoid dysfunction (Fig. 7.3).
This patient sustained an early dislocation after a reverse total shoulder arthroplasty (left). He underwent a closed reduction, but examination under anesthesia revealed instability of the implant (center). Therefore, revision to a larger glenosphere and a retentive polyethylene was warranted (right)
Loss of containment can be subclassified into alteration of depth/radius ratio of the humerosocket and mechanical failure (such as glenosphere-baseplate dissociation, stem fracture, or humerosocket dissociation at the trunnion).
Impingement can occur in a setting of a large body habitus, with the axillary soft tissue creating a levering-out effect with traction from the weight of the arm. Furthermore, soft tissue or bony impingement can occur in a fracture setting with unreduced retained tuberosities, malunion, or heterotopic ossification; prosthetic malalignment may play a role if the humeral component prematurely contacts the glenoid neck in adduction [48]. This can be modified by changing the glenosphere size, the baseplate placement, offset or tilt or the neck-shaft angle, and version of the humeral component [49].
Trappey and colleagues also found that patients with an irreparable subscapularis had a higher rate of instability [46]. A meta-analysis by Matthewson et al. concluded that subscapularis repair decreases the rate of instability, and in those cases when it cannot be repaired, a lateralized center of rotation results in significantly lower dislocation [50]. Owing to the preservation of the subscapularis tendon insertion, a superior subscapularis-sparing approach may lower the risk of dislocation, with reported rates of instability as low as 0%. However, glenoid exposure and baseplate placement using this approach may be significantly more challenging [51]. Subscapularis involvement in RTSA instability remains controversial in the existing literature, as similar clinical results with or without subscapularis repair have been reported [52].
7.3.3 Component Loosening
7.3.3.1 Anatomic Total Shoulder Arthroplasty Loosening
Prosthetic loosening has been reported to represent 12.4–39% of the complications after anatomic total shoulder arthroplasty [11]. Radiolucencies, calcar resorption, or scapular notchings are common findings after anatomic and reverse shoulder replacement, but not all of them may be clinically relevant. In the presence of pain or gross implant migration, however, further investigation is warranted.
Glenoid component loosening occurs more frequently than aseptic humeral component loosening, representing over 80% of fixation failures [11]. Positive radiographic findings of lucencies about the component vary from 12% to 94% in the literature, but these do not necessarily correlate with clinical findings. In this regard, surgical technique must be meticulous, as it has been suggested that the presence of lucent lines and further frank loosening may be related to the presence of cement on the backside of the glenoid component. This may indicate suboptimal bone preparation of the native glenoid and/or suboptimal seating of the component [53, 54].
Loosening can occur due to uneven force distribution in the setting of glenohumeral instability (the so-called rocking horse mechanism) [55] due to proximal migration of the humeral head in the setting of rotator cuff failure or due to infection, lack of bone stock, or poor bone fixation. Shoulder biomechanics may also play a role. Compared to other joints, the humeral head appears to have larger “play in the socket,” which may explain the faster polyethylene wear that has been found in explanted shoulder liners when compared to equivalent hip inserts [56].
Papadonikolakis found an asymptomatic radiolucency rate of 7.3% per year and symptomatic loosening of 1.2% per year, with more asymptomatic lucencies found in keeled versus pegged implants [57]. Biconcavity of the native glenoid and increased glenoid retroversion may also lead to increased component loosening. Walch et al. found a 21% loosening rate in biconcave glenoids and a 44% complication rate associated to retroversion greater than 27° [58].
Others have found that metal-backed glenoid implants have a revision rate up to three times higher than all-polyethylene components [57]. The Australian registry demonstrated an increased revision rate in both fixed and modular metal-backed glenoid components. They reported a significantly higher revision rate of non-cross-linked vs. cross-linked glenoid components with a hazard ratio of 2.38, but they found no differences in the revision rate between cemented versus hybrid glenoid components in total shoulder arthroplasties [4].
Stem aseptic loosening is much less common than glenoid failure, accounting for 7% of the complications after TSA [11]. In defining stem loosening in non-cemented stems, Sperling described eight radiographic zones around the humeral stem and concluded that a humeral component was “at risk” if a lucent line 2 mm or greater was found in at least three zones [59]. Sanchez-Sotelo used the same parameters to successfully evaluate radiographic loosening in cemented stems [60]. Changes at the bone-implant interface on the humeral side in the presence of a glenoid component have raised concerns about osteolysis and symptomatic loosening in the setting of polyethylene particle debris [45, 60].
7.3.3.2 Reverse Total Shoulder Arthroplasty Loosening
Boileau reported that among all the causes that led to revision surgery after a failed RTSA, 21% were due to humeral side complications. It was the second most common cause of revision after instability. He found that humeral loosening was often related to biological causes (polyethylene wear and metallic debris), in addition to mechanical causes (rotational forces) [47]. Radiographic loosening is rare, with a reported prevalence of less than 1% [61], but proximal humerus bone loss in a proximal humerus fracture setting, for instance, can decrease mechanical strength of the humeral stem leading to an increased risk of humeral-sided failure [62].
Glenoid component loosening is uncommon in the setting of RTSA and can be minimized by careful surgical technique [63]. Avoidance of superior tilt, placement of the baseplate at the most inferior aspect of the glenoid, and achievement of adequate primary stability that allows bone ingrowth are paramount [47, 63].
The influence of scapular notching on glenoid component loosening after reverse shoulder arthroplasty remains controversial, as some series report increased loosening rates related to scapular notching (Fig. 7.4) [14, 64, 65], while others report no association [15, 66, 67]. The use of a superior approach has been reported to increase prevalence of scapular notching [67], which suggests that this approach may indirectly increase the risk of loosening. Lateral and inferior offset of the glenosphere, on the other hand, may minimize radiographic loosening, though some lateralized designs have been reported to potentially lead to a higher rate of component dissociation [68].
7.3.4 Periprosthetic Fractures
Periprosthetic fractures can occur both intraoperatively and postoperatively. The rate of intraoperative periprosthetic fractures has been reported to be between 1.3% and 5.1% [9, 11, 69], with a similar distribution between humeral and glenoid fractures [25]. Female sex, greater number of comorbidities, and a primary diagnosis of posttraumatic osteoarthritis have all been associated to higher rates of periprosthetic fracture [25].
The increased risk in women may be explained by the fact that rheumatoid arthritis and osteoporosis are more common in this population [70]. The relationship between posttraumatic osteoarthritis and intraoperative fracture may be related to the increased joint stiffness in these patients, placing greater torque forces during retraction, which eventually may lead to an intraoperative fracture. Implant stability and fracture pattern may ultimately determine if further intervention is required, such as exchange to a longer stem or open treatment with internal fixation [71].
Postoperative periprosthetic fractures have been reported to occur in 1–3% of cases [72]. Wright and Cofield described the most widely used classification of periprosthetic fractures. According to their classification, type A fractures do not extend beyond the tip of the stem, type B fractures start around the stem and end distal to the tip of it, and type C fractures are distal to the tip of the stem [73].
When evaluating these fractures, implant stability and remaining bone stock will determine further treatment (Fig. 7.5). Campbell described a system to classify bone quality, in which the bone is considered normal if the ratio between the mid-shaft cortices and the shaft diameter is greater than 50%, mild osteopenia if it is between 25% and 50%, and severe osteopenia if it is below 25%. He found that 75% of the patients in his series of periprosthetic fractures met the definition for osteopenia.
While implant stability is ultimately determined intraoperatively, preoperative radiographs can help the surgeon plan and predict fixation stability. As described earlier, when lucent lines greater than 2 mm are found in at least three of the eight zones described by Sperling, the surgeon may anticipate stem loosening [59]. Implant subsidence or tilt can also help determine the quality of stem fixation before the procedure and allow the surgeon to prepare accordingly.
7.4 Conclusions
While total shoulder replacement has added to our ability to salvage painful shoulders following severe trauma or late-stage arthrosis with and without rotator cuff deficiency, we have also learned that there are limitations to the expectation for a functional, pain-free shoulder. While complications following shoulder arthroplasty can be frustrating for both the patient and surgeon alike, it is unfortunately a reality that all arthroplasty surgeons will encounter at some point in their career.
Recognizing complications and potential failure may be difficult, since many of the signs and symptoms can be nonspecific, such as pain, weakness, and stiffness. However, timely recognition and accurate diagnosis are critical to avoiding a suboptimal outcome. Careful history, physical examination, and good quality imaging studies are essential, but further testing is often necessary and may include blood work, aspiration, CT, MRI or ultrasonography.
Perhaps as important as early diagnosis, however, may be striving to avoid complications altogether. By understanding the common modes of failure, learning to avoid them, and careful patient selection, surgeons may ensure better outcomes for their patients. As we continue to care for ever increasing numbers of patients with end-stage shoulder degeneration and severe trauma, we must continue to exercise judicious indications and meticulous technique and undertake thoughtful review of our outcomes.
References
Flatow EL, Harrison AK. A history of reverse total shoulder arthroplasty. Clin Orthop Relat Res. 2011;469:2432–9.
Neer CS II. Articular replacement for the humeral head. J Bone Joint Surg Am. 1955;37-A:215–28.
Neer CS. Replacement arthroplasty for glenohumeral osteoarthritis. J Bone Joint Surg Am. 1974;56-A:1–13.
Australian Orthopaedic Association National Joint Replacement Registry. Hip, knee & shoulder arthroplasty. Annual reports. Adelaide, SA: AOANJRR; 2018.
Lübbeke A, Rees JL, Barea C, Combescure C, Carr AJ, Silman AJ. International variation in shoulder arthroplasty: incidence, indication, type of procedure, and outcomes evaluation in 9 countries. Acta Orthop. 2017;88:592–9.
NJR. 15th Annual report 2018. Hemel Hempstead: NJR; 2018. National Joint Registry for England, Wales, Northern Ireland and the Isle of Man.
Bergen H. Norwegian National Advisory Unit on Arthroplasty and Hip Fractures Norwegian Arthroplasty Register Norwegian Cruciate Ligament Register Norwegian Hip Fracture Register Norwegian Paediatric Hip Register. Trondheim: Norwegian National Advisory Unit; 2018.
Lehtimäki K, Rasmussen JV, Mokka J, Salomonsson B, Hole R, Jensen SL, Äärimaa V. Risk and risk factors for revision after primary reverse shoulder arthroplasty for cuff tear arthropathy and osteoarthritis: a Nordic Arthroplasty Register Association study. J Shoulder Elbow Surg. 2018;27:1596–601.
Somerson JS, Hsu JE, Neradilek MB, Matsen FA. Analysis of 4063 complications of shoulder arthroplasty reported to the US Food and Drug Administration from 2012 to 2016. J Shoulder Elbow Surg. 2018;27:1978–86.
Gonzalez J-F, Alami GB, Baque F, Walch G, Boileau P. Complications of unconstrained shoulder prostheses. J Shoulder Elbow Surg. 2011;20:666–82.
Bohsali KI, Wirth MA, Rockwood CA. Complications of total shoulder arthroplasty. J Bone Joint Surg Am. 2006;88-A:2279–92.
Richards J, Inacio MCS, Beckett M, Navarro RA, Singh A, Dillon MT, Sodl JF, Yian EH. Patient and procedure-specific risk factors for deep infection after primary shoulder arthroplasty. Clin Orthop Relat Res. 2014;472:2809–15.
Gerber C, Pennington SD, Nyffeler RW. Reverse total shoulder arthroplasty. J Am Acad Orthop Surg. 2009;17:284–95.
Werner CML, Steinmann PA, Gilbart M, Gerber C. Treatment of painful pseudoparesis due to irreparable rotator cuff dysfunction with the Delta III reverse-ball-and-socket total shoulder prosthesis. J Bone Joint Surg Am. 2005;87:1476–86.
Zumstein MA, Pinedo M, Old J, Boileau P. Problems, complications, reoperations, and revisions in reverse total shoulder arthroplasty: a systematic review. J Shoulder Elbow Surg. 2011;20:146–57.
Walch G, Bacle G, Lädermann A, Nové-Josserand L, Smithers CJ. Do the indications, results, and complications of reverse shoulder arthroplasty change with surgeon’s experience? J Shoulder Elbow Surg. 2012;21:1470–7.
Howe CR, Gardner GC, Kadel NJ. Perioperative medication management for the patient with rheumatoid arthritis. J Am Acad Orthop Surg. 2006;14:544–51.
Luessenhop CP, Higgins LD, Brause BD, Ranawat CS. Multiple prosthetic infections after total joint arthroplasty: risk factor analysis. J Arthroplasty. 1996;11:862–8.
Bala A, Penrose CT, Visgauss JD, Seyler TM, Randell TR, Bolognesi MP, Garrigues GE. Total shoulder arthroplasty in patients with HIV infection: complications, comorbidities, and trends. J Shoulder Elbow Surg. 2016;25:1971–9.
Hatta T, Werthel JD, Wagner ER, Itoi E, Steinmann SP, Cofield RH, Sperling JW. Effect of smoking on complications following primary shoulder arthroplasty. J Shoulder Elbow Surg. 2017;26:1–6.
Everhart JS, Bishop JY, Barlow JD. Medical comorbidities and perioperative allogeneic red blood cell transfusion are risk factors for surgical site infection after shoulder arthroplasty. J Shoulder Elbow Surg. 2017;26:1922–30.
Werthel JD, Hatta T, Schoch B, Cofield R, Sperling JW, Elhassan BT. Is previous nonarthroplasty surgery a risk factor for periprosthetic infection in primary shoulder arthroplasty? J Shoulder Elbow Surg. 2017;26:635–40.
Torrens C, Marí R, Alier A, Puig L, Santana F, Corvec S. Cutibacterium acnes in primary reverse shoulder arthroplasty: from skin to deep layers. J Shoulder Elbow Surg. 2019;28:839–46.
Pottinger P, Butler-Wu S, Neradilek MB, Merritt A, Bertelsen A, Jette JL, Warme WJ, Matsen FA. Prognostic factors for bacterial cultures positive for Propionibacterium acnes and other organisms in a large series of revision shoulder arthroplasties performed for stiffness, pain, or loosening. J Bone Joint Surg Am. 2012;94:2075–83.
Singh JA, Sperling JW, Schleck C, Harmsen WS, Cofield RH. Peri-prosthetic infections after shoulder hemiarthroplasty. J Shoulder Elbow Surg. 2012;21:1304–9.
Weber P, Utzschneider S, Sadoghi P, Andress HJ, Jansson V, Müller PE. Management of the infected shoulder prosthesis: a retrospective analysis and review of the literature. Int Orthop. 2011;35:365–73.
Topolski MS, Chin PYK, Sperling JW, Cofield RH. Revision shoulder arthroplasty with positive intraoperative cultures: the value of preoperative studies and intraoperative histology. J Shoulder Elbow Surg. 2006;15:402–6.
Alesi D, Roberti di Sarsina T, Zaffagnini S, Marcheggiani Muccioli G, Lullini G, Rotini R, Cammisa E, Fratini S, Rinaldi V, Guerra E. Diagnosis and treatment of infected shoulder arthroplasty: current concepts review. Joints. 2018;6:173–6.
Fink B, Sevelda F. Periprosthetic joint infection of shoulder arthroplasties: diagnostic and treatment options. Biomed Res Int. 2017;2017:1–10.
Gómez-Ochoa SA, Espín-Chico BB, García-Rueda NA, Vega-Vera A, Osma-Rueda JL. Risk of surgical site infection in patients with asymptomatic bacteriuria or abnormal urinalysis before joint arthroplasty: systematic review and meta-analysis. Surg Infect (Larchmt). 2019;20:159–66.
McElvany MD, Chan PH, Prentice HA, Paxton EW, Dillon MT, Navarro RA. Diabetes disease severity was not associated with risk of deep infection or revision after shoulder arthroplasty. Clin Orthop Relat Res. 2019;477:1358. https://doi.org/10.1097/CORR.0000000000000642.
Wagner ER, Houdek MT, Schleck C, Harmsen WS, Sanchez-Sotelo J, Cofield R, Sperling JW, Elhassan BT. Increasing body mass index is associated with worse outcomes after shoulder arthroplasty. J Bone Joint Surg Am. 2017;99:929–37.
Bohsali KI, Bois AJ, Wirth MA. Complications of shoulder arthroplasty. J Bone Joint Surg Am. 2017;99:256–69.
Sanchez-Sotelo J, Sperling JW, Rowland CM, Cofield RH. Instability after shoulder arthroplasty: results of surgical treatment. J Bone Joint Surg Am. 2003;85:622–31.
Moeckel B, Altchek DW, Warren RF, Wickiewicz TL, Dines DM. Instability of the shoulder after arthroplasty. J Bone Joint Surg Am. 1993;75:492–7.
Wirth MA, Rockwood CA. Current concepts review - complications of total shoulder-replacement arthroplasty. J Bone Joint Surg Am. 2016;78:603–16.
Miller BS, Joseph TA, Noonan TJ, Horan MP, Hawkins RJ. Rupture of the subscapularis tendon after shoulder arthroplasty: diagnosis, treatment, and outcome. J Shoulder Elbow Surg. 2005;14:492–6.
Jackson JD, Cil A, Smith J, Steinmann SP. Integrity and function of the subscapularis after total shoulder arthroplasty. J Shoulder Elbow Surg. 2010;19:1085–90.
Van den Berghe GR, Nguyen B, Patil S, D’Lima DD, Mahar A, Pedowitz R, Hoenecke HR. A biomechanical evaluation of three surgical techniques for subscapularis repair. J Shoulder Elbow Surg. 2008;17:156–61.
Ponce BA, Ahluwalia RS, Mazzocca AD, Gobezie RG, Warner JJP, Millett PJ. Biomechanical and clinical evaluation of a novel lesser tuberosity repair technique in total shoulder arthroplasty. J Bone Joint Surg Am. 2005;87:1–8.
Caplan JL, Whitfield B, Neviaser RJ. Subscapularis function after primary tendon to tendon repair in patients after replacement arthroplasty of the shoulder. J Shoulder Elbow Surg. 2009;18:193–6.
Warren RF, Coleman SH, Dines JS. Instability after arthroplasty: the shoulder. J Arthroplasty. 2002;17:28–31.
Pearl ML, Volk AG. Retroversion of the proximal humerus in relationship to prosthetic replacement arthroplasty. J Shoulder Elbow Surg. 1995;4:286–9.
Young AA, Walch G, Pape G, Gohlke F, Favard L. Secondary rotator cuff dysfunction following total shoulder arthroplasty for primary glenohumeral osteoarthritis: results of a multicenter study with more than five years of follow-up. J Bone Joint Surg Am. 2012;94:685–93.
Haines JF, Trail IA, Nuttall D, Birch A, Barrow A. The results of arthroplasty in osteoarthritis of the shoulder. J Bone Joint Surg Br. 2006;88-B:496–501.
Trappey GJ, O’Connor DP, Edwards TB. What are the instability and infection rates after reverse shoulder arthroplasty? Clin Orthop Relat Res. 2011;469:2505–11.
Boileau P. Complications and revision of reverse total shoulder arthroplasty. Orthop Traumatol Surg Res. 2016;102:S33–43.
Abdelfattah A, Otto RJ, Simon P, Christmas KN, Tanner G, LaMartina J, Levy JC, Cuff DJ, Mighell MA, Frankle MA. Classification of instability after reverse shoulder arthroplasty guides surgical management and outcomes. J Shoulder Elbow Surg. 2018;27:e107–18.
Barco R, Savvidou OD, Sperling JW, Sanchez-Sotelo J, Cofield RH. Complications in reverse shoulder arthroplasty. EFORT Open Rev. 2016;1:72–80.
Matthewson G, Kooner S, Kwapisz A, Leiter J, Old J, MacDonald P. The effect of subscapularis repair on dislocation rates in reverse shoulder arthroplasty: a meta-analysis and systematic review. J Shoulder Elbow Surg. 2019;28:989–97.
Mole D, Favard L. Excentered scapulohumeral osteoarthritis [article in French]. Rev Chir Orthop Reparatrice Appar Mot. 2007;93:37–94.
Clark JC, Ritchie J, Song FS, Kissenberth MJ, Tolan SJ, Hart ND, Hawkins RJ. Complication rates, dislocation, pain, and postoperative range of motion after reverse shoulder arthroplasty in patients with and without repair of the subscapularis. J Shoulder Elbow Surg. 2012;21:36–41.
Lazarus MD, Jensen KL, Southworth C, Matsen FA 3rd. The radiographic evaluation of keeled and pegged glenoid component insertion. J Bone Joint Surg Am. 2002;84:1174–82.
Hsu JE, Hackett DJJ, Vo KV, Matsen FA 3rd. What can be learned from an analysis of 215 glenoid component failures? J Shoulder Elbow Surg. 2018;27:478–86.
Franklin JL, Barrett WP, Jackins SE, Matsen FA. Glenoid loosening in total shoulder arthroplasty. J Arthroplasty. 1988;3:39–46.
Kocsis G, Payne CJ, Wallace A, McNally D. Wear analysis of explanted conventional metal back polyethylene glenoid liners. Med Eng Phys. 2018;59:1–7.
Papadonikolakis A, Neradilek MB, Matsen FA. Failure of the glenoid component in anatomic total shoulder arthroplasty. J Bone Joint Surg Am. 2013;95:2205–12.
Walch G, Moraga C, Young A, Castellanos-Rosas J. Results of anatomic nonconstrained prosthesis in primary osteoarthritis with biconcave glenoid. J Shoulder Elbow Surg. 2012;21:1526–33.
Sperling JW, Cofield RH, O’Driscoll SW, Torchia ME, Rowland CM. Radiographic assessment of ingrowth total shoulder arthroplasty. J Shoulder Elbow Surg. 2000;9:507–13.
Sanchez-Sotelo J, O’Driscoll SW, Torchia ME, Cofield RH, Rowland CM. Radiographic assessment of cemented humeral components in shoulder arthroplasty. J Shoulder Elbow Surg. 2001;10:526–31.
Gilot G, Alvarez-Pinzon AM, Wright TW, Flurin PH, Krill M, Routman HD, Zuckerman JD. The incidence of radiographic aseptic loosening of the humeral component in reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2015;24:1555–9.
Cuff D, Levy JC, Gutiérrez S, Frankle MA. Torsional stability of modular and non-modular reverse shoulder humeral components in a proximal humeral bone loss model. J Shoulder Elbow Surg. 2011;20:646–51.
Boileau P, Melis B, Duperron D, Moineau G, Rumian AP, Han Y. Revision surgery of reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22:1359–70.
Lévigne C, Boileau P, Favard L, Garaud P, Molé D, Sirveaux F, Walch G. Scapular notching in reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2008;17:925–35.
Vanhove B, Beugnies A. Grammont’s reverse shoulder prosthesis for rotator cuff arthropathy. A retrospective study of 32 cases. Acta Orthop Belg. 2004;70:219–25.
Wall B, Nové-Josserand L, O’Connor DP, Edwards TB, Walch G. Reverse total shoulder arthroplasty: a review of results according to etiology. J Bone Joint Surg Am. 2007;89:1476–85.
Melis B, DeFranco M, Lädermann A, Molé D, Favard L, Nérot C, Maynou C, Walch G. An evaluation of the radiological changes around the Grammont reverse geometry shoulder arthroplasty after eight to 12 years. J Bone Joint Surg Br. 2011;93-B:1240–6.
Cusick MC, Hussey MM, Steen BM, Hartzler RU, Clark RE, Cuff DJ, Cabezas AF, Santoni BG, Frankle MA. Glenosphere dissociation after reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2015;24:1061–8.
Wagner ER, Houdek MT, Elhassan BT, Sanchez-Sotelo J, Cofield RH. Sperling JW (2015) What are risk factors for intraoperative humerus fractures during revision reverse shoulder arthroplasty and do they influence outcomes? Clin Orthop Relat Res. 2015;473:3228–34.
Campbell JT, Moore RS, Iannotti JP, Norris TR, Williams GR. Periprosthetic humeral fractures: mechanisms of fracture and treatment options. J Shoulder Elbow Surg. 1998;7:406–13.
García-Fernández C, Lópiz-Morales Y, Rodríguez A, López-Durán L, Martínez FM. Periprosthetic humeral fractures associated with reverse total shoulder arthroplasty: incidence and management. Int Orthop. 2015;39:1965–9.
Williams GRJ, Iannotti JP. Management of periprosthetic fractures: the shoulder. J Arthroplasty. 2002;17:14–6.
Wright TW, Cofield RH. Humeral fractures after shoulder arthroplasty. J Bone Joint Surg Am. 1995;77:1340–6.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Tabeayo, E., Gruson, K.I., Saucedo, J.M. (2020). Revision Total Shoulder Arthroplasty: Epidemiology and Causes. In: Rodríguez-Merchán, E. (eds) Revision Total Joint Arthroplasty. Springer, Cham. https://doi.org/10.1007/978-3-030-24773-7_7
Download citation
DOI: https://doi.org/10.1007/978-3-030-24773-7_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-24772-0
Online ISBN: 978-3-030-24773-7
eBook Packages: MedicineMedicine (R0)