Abstract
Recently, pulse wave velocity (PWV), or its reciprocal pulse transit time (PTT), has been intensively investigated as a promising technique for continuous, cuffless, and noninvasive blood pressure (BP) monitoring. BP is mathematically derived through PTT, or the “time delay” in propagation of pressure waves in the vascular system, which can be easily derived from two pulse signals, including electrocardiography (ECG) and pulse plethysmography (PPG) signals, together with adequate calibration procedure. Practical steps in applying this method as well as mathematical models in estimating BP were reviewed; while limitations of this approach, such as the need for individual calibration and the need for a reasonably stable condition were discussed. The future of this technology can be potentially used in, but not limited to, continuous BP monitoring, BP change tracker, and trigger for absolute BP measurement. Furthermore, with machine learning, the initially extract surrogate cardiovascular indexes from physiological signals can be used to train and adapt to the model to further improve the accuracy of BP prediction.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Blood pressure
- Pulse wave velocity
- Pulse transit time
- Pulse arrival time
- Electrocardiogram
- Pulse plethysmography
- Wearable
- Cuffless
- Calibration
- Machine learning
History Overview
Inspired by the invasive intra-arterial continuous blood pressure measurement through clinical cannulation in 1949, researchers started exploring noninvasive alternative for continuous blood pressure monitoring. In 1963, arterial tonometry was used by Pressman et al. by applanating a superficial artery against a bone with an external transducer until the artery is flattened, where the tangential arterial wall tension no longer affects the vertical force measured [1]. Quickly, arterial tonometry was improved and widely used by the researchers in the measurement of pulse wave velocity (PWV) for evaluation of arterial stiffness and pulse wave analysis. The commonly applied sites include the radial artery, carotid artery, and femoral artery. The validity of measuring arterial BP depends on the applanation of the artery, and therefore encounters practical problems of sensor positioning, motion artifacts, calibration, etc. To enhance usability, tonometry sensor array was used to ensure at least one sensor among multiple ones is precisely positioned to capture good pulse wave signal. AtCor SphygmoCor (AtCor Medical, Sydney, Australia) and Form/VP-2000 and HEM-9000AI (Omron Healthcare Co., Ltd, Kyoto, Japan) are example of commercialized research tools that utilizing arterial tonometry. These tools were mainly for pulse wave analysis, arterial stiffness, and central BP measurement. Even though they are capable of providing continuous pulse waves for short period, they are not a true noninvasive continuous BP monitor.
Another development in noninvasive continuous BP monitoring field is the use of volume clamp on the finger technique proposed by Peñáz from Czech in 1973 [2]. The system has an inflatable finger cuff with a built-in photoplethysmograph (PPG) sensor, and a closed loop servo system to apply a pulsating cuff pressure to the finger arteries that is precisely opposite to the intra-arterial pressure. In equilibrium, when the cuff pressure equals the arterial pressure, the difference between the intra-arterial pressure and external applied cuff pressure will become zero, which is called the set point . The key to volume clamp method is to reach the set point. On the basis of the volume clamp method, some research tools were commercialized, including Finapres (Finapres Medical Systems, Enschede, The Netherlands) and CNSystems (CNSystems Medizintechnik GmbH, Graz, Austria).
Despite being noninvasive continuous blood pressure measurement techniques, both tonometry and volume clamp techniques are intrusive because both require the application of external pressure or force on cuff during the entire course of the monitoring, which leads to discomfort and motion artifacts. Another approach, which is based using PWV or its reciprocal, pulse transit time (PTT), can be an attractive alternative for measuring unobtrusive continuous BP. In principle, PWV depends on the property of the arterial wall, which varies with the arterial pressure. The usage of PTT can be dated back to 1959 when Weltman et al. designed the PWV computer by utilizing the ECG and a pulse signal to define pulse transit time over a known arterial length [3]. Recently, PTT became a popular way of achieving cuffless continuous BP measurement through using wearable objects including watches, rings, shirts, eyeglasses, smartphones, and cameras, as well as daily objects such as sleeping cushions, chairs, and weighing scales.
Most of these methods are still in the research stage. The few commercially available devices include Sotera ViSi Mobile (Sotera Wireless, San Diego, USA) continuous, noninvasive BP (cNIBP) monitoring and SOMNOtouchTM-NIBP (SOMNOmedics GmbH, Randersacker, Germany), which are based on the PTT method. The ViSi’s cNIBP is determined on a beat-to-beat basis employing PTT and calibration with automatic noninvasive BP method.
Physiological Description
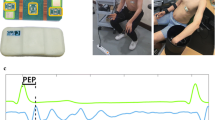
PWV is usually assessed using the arrival time of a pressure wave propagating through the arterial tree in a certain distance between the proximal and distal arterial sites, in the form of PWV = L/PTT, where L is the distance between the proximal and distal sites. Because of the complexity of distance measurement, PWV can be indirectly approximated using PTT, which can be easily derived from two pulse signals, including electrocardiography (ECG) and pulse plethysmography (PPG) signals (Fig. 6.1). By using a calibration procedure, the measured PTT can be translated into arterial pressure by using an appropriate model.
Pulse transit time refers to the time it takes a pulse wave to travel between two arterial sites. Like pulse wave velocity, PTT is a measure for arterial stiffness. When blood pressure increases, the vascular tone increases and the arterial wall becomes stiffer, causing the PTT to shorten. Conversely, when blood pressure falls, vascular tone decreases and the arterial wall becomes less stiffer, and PTT increases. Increased stiffness can be either structural—due to aging and atherosclerosis, or functional—due to higher sympathetic activity or elevated blood pressure [4]. But at the same time, the multiple factors besides blood pressure that influences vascular stiffness mechanisms itself make the technique based on PWV or PTT sensitive but not specific, particularly for peripheral/muscular arteries, and less prominent in central/elastic arteries. Such influence presents a limitation of PWV or PTT for blood pressure estimation in real life.
Another relevant concept and parameter is the pulse arrival time (PAT), as described in the previous chapter, which measures the time difference between the R-peak of ECG and a characteristic point of pulse plethysmography (PPG) waveform. PAT is the sum of PTT of the pressure wave and the pre-ejection period (PEP) delay:
PEP is the time needed to convert the electrical signal into a mechanical pumping force and isovolumetric contraction to open the aortic valve, and can be calculated by the delay between R-wave and impedance cardiography (ICG), as shown in Fig. 6.1. PEP is a delay that changes with various factors such as stress, physical activity, age and emotion [5]. The popularity of using PAT to estimate blood pressure is based on the simplicity of obtaining PAT by referencing ECG R wave, which is precise and easy to get. The disadvantage is the introducing the new variable of PEP, especially when PTT is small when the distal measurement point is close to the core body.
Studies showed that PEP accounts for 7% of the RR interval to approximately 20% of PTT measured at the finger tips at rest [6]. The impact of PEP decreases with distance from the heart, but becomes more significant when heart rate lowered, and therefore should be subtracted out to obtain PTT. It is generally thought that PTT has higher correlation with SBP, DBP, and MAP than PAT, but with some studies stating PAT is a better indicator of SBP due to its dependency on both ventricular contraction and vascular function.
Using PTT to effectively estimate blood pressure needs to be handled in a reasonably stable condition, where smooth muscle contraction is minimal and viscous effects are negligible. Such conditions are best met by measuring PTT through central arteries. It also needs to be individually calibrated to associate the PTT to the absolute blood pressure values. Furthermore, aging or diseases can potentially change the arterial elasticity characteristics; therefore, periodic recalibration is necessary to ensure the close correlation between PTT and blood pressure values during long-term BP monitoring.
Practical Approach
The practical PTT-based cuffless BP monitoring involves a three-step approach:
-
Step 1—obtain the proximal and distal arterial waveforms;
-
Step 2—calculate PTT from the waveforms, either from foot-to-foot, or peak-to-peak of the waveforms;
-
Step 3—calibration of PTT (in units of ms) to BP (in units of mmHg)
There are various challenges along the way during implementation of the above steps. First challenge is to reliably obtain good quality arterial waveforms in a reasonably convenient way. Generally speaking, the more convenient the waveform measurement is, the more noise contamination can be resulted. For example, when the proximal and distal sites are apart, the signal-to-noise ratio will be higher, but such design will lead to two different sensors, and compromise on the convenience. On the other hand, getting the two sites next to each other can make a great case on convenience, but the signal-to-noise ratio might be an issue given too small PTT between the two sites.
Second challenge is to calculate the PTT with suboptimal waveforms obtained by applying algorithm to enhance the signal-to-noise ratio. It is necessary to strike a balance between waveform quality and convenience, and rely on signal processing algorithm to optimize the suboptimal waveforms.
The third and the most difficult challenge is to choose the right calibration and appropriate calibration intervals. The calibration curve relating PTT to BP is dependent on quite a few factors, including individual specific characteristics such as body height, relevant artery cross-sectional area, and vascular tree compliance. Therefore, obtaining an individual specific calibration curve through multiple blood pressure readings which covers the target blood pressure range is optimal. However, constructing such a curve requires cuff BP measurements from the subject, and the calibration curve need to be updated at a rate faster than the arteriosclerotic process (e.g., up to a few years at a time).
A final major challenge is the independent determination of both systolic BP and diastolic BP at the same time. This challenge is important, as isolated systolic hypertension often occurs in the elderly. However, conventionally estimated PTT may only be a marker of diastolic BP.
Mathematical Modeling
PTT based BP estimation relies on two basic equations for arterial wave propagation. The first is the relationship between the Young’s modulus (E) and the arterial pressure (P):
where a is a parameter that is related to the vessel and Eo is the Young’s modulus for zero arterial pressure . The formula estimates the arterial pressure if a and Eo are adjusted for subject (age, gender, health condition, etc.) on the elasticity due to the change in the wall composition.
The second is the Moens–Korteweg equation in which the elasticity of arteries determines the propagation speed, which is the pressure’s pulse wave velocity (PWV).
where h is the thickness in an elastic artery, d is the diameter, and ρ is the blood density.
Combining Eqs. (6.2) and (6.3), the relationship between P and PWV can be derived by the Bramwell–Hill equation
where L is the length the pulse wave passed through, and ∆t is PTT, or the time delay [7]:
This equation indicates that an increase in pressure will lead to an increase in PWV and decrease in time delay.
Various mathematical models were used to approximate the relationships between BP and the PTT including the following:
-
1.
Logarithmic model—Rearranging the Bramwell–Hill equation, we will have the logarithmic relationship between BP and PTT in the form of [8]:
$$ P=\left(-\frac{2}{a}\right)\ln \left(\varDelta t\right)+\left(\frac{1}{a}\right)\ln \left(\frac{\rho d{L}^2}{h{E}_{\mathrm{o}}}\right) $$Or in a simplified version as:
$$ \mathrm{BP}=A\ast \ln \left(\varDelta t\right)+B $$(6.5)Here, A and B are subject-specific constants and they can be obtained through a regression analysis between the reference BP and the corresponding PTT, or ∆t—the time delay [9].
The above mathematical relationship is the theoretical basis between PTT and BP. The more generalized relationship can be summarized as
$$ \mathrm{BP}=A\ast f\left(\varDelta t\right)+B $$(6.6)where f(∆t) is a specific function of the PTT, or ∆t—the time delay. Among various approximation of BP from PTT, the following models were used besides the logarithmic model shown in (6.5):
-
2.
Linear model—Assuming there is a negligible change in the arterial thickness and diameter with pressure variation, BP and the time delay can be linearly related [10]:
$$ \mathrm{BP}=A\ast \varDelta t+B $$(6.7) -
3.
Inverse Square Model
$$ \mathrm{BP}=A/\varDelta {t}^2+B $$(6.8)where A and B are constants that are related to subject’s biometrics such as height and blood density [6]
-
4.
Inverse Model—an inverse relationship between BP and PTT [11]
$$ \mathrm{BP}=A/\varDelta t+B $$(6.9)
While PTT is the most important parameter for estimating BP, other factors, especially those that are related to cardiovascular activity can be added to enhance the robustness of the model. Specifically, heart rate (HR) represents the cardiac cycle and influences the heart’s preload and the cardiac output (CO), which positively impact BP as the pressure on the arterial walls. HR is proportional to the volume of blood ejected. HR and BP are regulated by the autonomic nervous system which has been found to be inversely related, depending on the baroreflex activity. HR is calculated from the RR interval in ECG signals and has been incorporated in several algorithms to estimate BP, contributing some improvement in accuracy.
where A, B, and C are constants, and ∆t and HR are two variables [12].
In order to apply a mathematical model, one needs to vary the BP over a considerable range to obtain the curve that can relate PTT or PAT closely to BP. Models under different conditions, generally exercising, hydrostatic posture, Valsalva maneuver, and medication, have been commonly used [13,14,15,16].
Technical Details on Measurement Methods
The most direct way is to measure the time delay between two arterial sites. In research, it is easy to handle such way, but in consumer oriented application, it is challenging, because the device needs to be simple and compact, which leads to short PTT due to the two testing sites being quite close to each other quite sensitive to error.
Alternative way is to calculate the difference between two PATs at two different sites: for example, one is at the finger tip, but the other is at the wrist along the same arm. The approach cancels the same pre-ejection period PEP. The disadvantage of this approach is that the user needs to wear sensors at two different sites.
The focus of the PPT measurement is on conveniently measuring waveforms, contact or noncontact, most of which are relevant waveforms instead of blood pressure waveforms.
PPG, bioimpedance, electrocardiography (ECG), ballistocardiography (BCG), and video plethysmography (VPG) are among the most popular methods. These are based on either optical or electrical principles.
PPG method
Uses optical transmittance or reflectance to measure waveforms of proximal and distal blood volumes—the same technique as used in pulse oximeters to measure arterial oxygen saturation [17]. Transmission-mode and reflectance-mode PPG offer different advantages and limitations.
The reflectance-mode PPG is less restrictive in measurement locations (including forehead, forearm, supraorbital artery, under the legs, and the wrist), greater signal amplitude and lower motion artifact [18]. The disadvantage includes lower signal-to-noise ratio, motion artifact, and variation due to relative distance between the light source and the sensor caused by the shorter green wavelength that is only able to penetrate the skin and reflects skin blood flow rather than deeper larger arteries.
The transmission-mode PPG measurements are limited in locations: such as the earlobe, fingertip, and toe for maximizing the signal-to-noise ratio. Infrared (IR) and red optical wavelengths are widely used for transmission-mode PPG due to higher tissue penetration depth. IR is less sensitive to the oxygen content of hemoglobin and thus yields waveforms that are more stable over time. Transmission-mode PPG at the fingertip has been the most widely used method for obtaining the distal waveform .
Bioimpedance method
Bioimpedance can be measured using skin surface electrode pairs placed on skin surface at both proximal and distal sites. Micro amp level electrical current with high frequency is applied onto the outer electrodes; and the resultant differential voltage is measured across the inner electrodes. The measured differential voltage has both an AC component for the pulsatile blood and the DC component for other stable tissue components (bone, fat, muscle, and interstitial fluid). For PTT measurements, bioimpedance plethysmography is used with four electrodes in two pairs, positioned locally along the same artery in the limb (such as the lower leg or forearm) to measure the differential voltage when blood is flowing from one set of electrodes to the other [19, 20].
ECG method
This method provides a time reference with ECG, an easy to measure and resistant to noise and artifact. The time delay between the ECG waveform and a distal arterial waveform is the pulse arrival time (PAT). The advantage is, ECG is easy to measure, but the disadvantage is, PAT does not have as tight relationship to BP as PTT, due to the pre-ejection period PEP [21].
Ballistocardiography (BCG) method
It measures the reaction forces of the body in response to cardiac ejection of blood into the aorta using daily common objects such as chairs, beds, weighing scales, and on-body accelerometers [22]. BCG provides a proximal waveform, but it can be applied at a distal location, such as at the feet with a weighing scale or at the wrist with an accelerometer.
Video plethysmography (VPG) method
A noncontact method uses camera such as that on a smartphone to measure arterial waveforms from the skin [23]. It uses ambient/external light serving as the excitation source. Both proximal and distal waveforms can be obtained from two sites, such as the face, finger, or hand. Infrared thermal imaging with a highly sensitive camera can be used to measure arterial waveforms from the skin, based on the principle that the measured skin temperature changes with pulsatile blood flow due to heat exchange between vessels and surrounding tissue.
The actual measurement involves following measurement steps:
-
(a)
Sampling and filtering the proximal and distal waveforms.
-
(b)
Detecting the beats in the waveforms.
-
(c)
Detecting the feet or other features within the beats.
-
(d)
Calculating PTT as the time delay between the features. Handling artifacts in the waveforms (e.g., due to motion) is also crucial in practice yet is often not mentioned.
Calibration of PTT to BP
PTT is a relative parameter that correlates with blood pressure. It needs calibration to have absolute value of blood pressure. Calibration can be done with automatic blood pressure monitors. Calibration process is to set up the necessary parameters for the model to establish one to one mapping relationship between the actual BP readings and the PTT measurements.
There are several aspects of calibration: the first is how to calibrate, the second one is how frequent to calibrate, and lastly is if the calibration can be done by population instead of by individuals.
The goal is to construct a calibration curve to map PTT measurement to absolute value of BP readings. The actual steps of calibration involve the following steps:
-
1.
Define a mathematical model to relate PTT to BP, either using physical models or empirical regression models. Most of the physical models are based on the Moens–Korteweg and Bramwell–Hill equations with an assumed function to relate the elastic modulus or compliance to BP.
-
2.
Measure multiple pairs of PTT and BP values from a subject during interventions that change the BP in a wide range. Commonly employed interventions to change BP include exercise (e.g., climbing steps, cycling on an ergometer), postural changes (i.e., seated, supine, standing), sustained handgrip, and the Valsalva maneuver. Large BP changes can be achieved using anesthesia induction, surgery, and ICU therapies but are limited to hospitalized subjects and are therefore not valid for chronic hypertension management.
-
3.
Estimate the parameters by fitting the model to the PTT-BP multiple measurements. The accuracy of the parameter estimation generally improves with the ratio of the number of data pairs to the number of parameters. Least squares regression has been commonly used for parameter estimation.
Calibration Frequency
The calibration curve can be constructed either one-time at the beginning, or periodically throughout a period [24, 25]. For the studies require periodic calibration, the period between calibrations was within 2 h. Such frequent calibration is to account for body system changes such as vascular tone change or smooth muscle contraction.
Shorter PTT is directly linked to the increased arterial stiffness, which is driven by either higher sympathetic nerve activity or elevated blood pressure, so the sympathetic nerve activity can also change the individual’s arterial stiffness “black box,” and therefore such change will require the calibration again.
Besides individualized calibration, generalized calibration approach can also be utilized. The process is to use a population average value for one model parameter while estimating the other parameter from cuff BP measurement [26]. The advantage of this approach is to avoid BP perturbation. The disadvantage of using population averages is the less accurate BP values. To implement this method, the following steps would have to be performed: (a) collection of training data comprising pairs of PTT estimates and BP values during a set of BP varying interventions per subject from a vast number of diverse subjects; (b) estimation of the parameters of a calibration model for each subject; and (c) regression of these parameters on simple subject information. Collecting the necessary training data is a serious endeavor but may be the best way to popularize the PTT-based BP monitoring approach .
There are challenges to have independent determination of systolic and diastolic BP, if these two BP values do not vary in the same direction (e.g., isolated systolic hypertension). This problem could be addressed by including additional simple covariates, such as heart rate, in the calibration model or by estimating multiple PTT values per beat via arterial modeling. Note that PTT estimates should correlate better with BP values than these covariates do to offer any real value . Limited by length and depth, this chapter is just aimed to serve as a general overview of key elements in PWV or PTT related cuffless continuous BP monitoring. Interested readers who want to study on mathematical models, calibration, and practical approaches should further reference to relevant literature in this field [27,28,29].
The Future of Technology
With the clear advantage of this PWV or PTT based technology being less obstructive and therefore continuous, there are three potential applications for such technology to play meaningful roles in the real world:
-
1.
Continuous BP monitoring —This is the most active field in which lots of startup companies or research interests reside. In order to provide BP readings on a continuous basis, the challenge is all around the calibration—specifically, how to improve calibration of PTT to BP accuracy, how to make calibration simple and easy, and how to make calibration less frequently are among the bottlenecks that could present as the biggest hurdle for PWV/PTT based technology become reality. One potential method is the universal calibration wherein the parameters of the model relating PTT to BP are determined simply from the subject’s age, gender, and other such information including cardiovascular risk factors.
Another approach is to involve more of an individual’s biometrics parameters and use machine learning to improve the predictive power of future BP.
-
2.
BP change tracker —This application will reflect the BP changes, without giving out the absolute BP value. Such BP changes are in mmHg unit, and such changes could be used to guide individuals to take appropriate measures.
-
3.
Trigger for absolute BP measurement—In this application, the PTT device serves as a trigger point based on individualized setup. Once BP changes monitored by the PTT technology reach a preset value, the trigger will alert the individuals to take a BP measurement using traditional cuff-based devices. Even though the PWV or PTT based technology is not the main unit for BP measurement, such technology provide a quite user friendly setting with continuous protection against elevated BP level.
Machine learning
Nowadays artificial intelligence through machine learning has been used in research for BP prediction and cuffless BP measurement . The general approach is to initially extract surrogate cardiovascular indexes from physiological signals, and then to use machine learning to train and adapt to the model, and finally to predict BP using the trained model.
Key features could be extracted from physiological signals such as ECG, PPG, activity, or sleep using either time domain or frequency domain. A feature selection method was used to remove irrelevant or redundant features to avoid over fitting. Machine learning methods such as linear regression, neural network, and Bayesian network can be used to establish the BP prediction model.
Chiang et al. used Random Forest with Feature Selection to enhance the performance of the BP prediction by filtering out unnecessary features from wearable sensors signals and past BP readings [30]. Xing et al. used fast Fourier transform on the PPG signal to extract the amplitude and frequency features to train an artificial neural network to estimate BP [31]. Kachuee et al. extracted multiple physiological parameters from ECG and PPG signals using multiple regression [32].
Future machine learning and math modeling—select optimal features that can best contribute to dynamic BP changes and then combine the physiological and mathematical modeling to predict continuous BP noninvasively and continuously. Considering the complexity of the cardiovascular system, selection of multiple indicators and an appropriate model is critical and requires full-system integration to ensure the accuracy of indirect measurements .
References
Pressman G, et al. A transducer for the continuous external measurement of arterial blood pressure. IEEE Trans Biomed Electron. 1963;10:73–81.
Peñáz J. Photoelectric measurement of blood pressure, volume and flow in the finger. In: Digest of the 10th International Conference on Medical and Biological Engineering. 1973.
Weltman G, et al. The continuous measurement of arterial pulse wave velocity. Med Electron Biol Eng. 1964;2:145–54.
Kortekaas MC, et al. Small intra-individual variability of the preejection period justifies the use of pulse transit time as approximation of the vascular transit. PLoS One. 2018;13(10):e0204105.
Peter L, et al. A review of methods for non-invasive and continuous blood pressure monitoring: pulse transit time method is promising? IRBM. 2014;35:271–82.
Fung P, et al. Continuous noninvasive blood pressure measurement by pulse transit time. Conf Proc IEEE Eng Med Biol Soc. 2004;1:738–41.
Nichols WW, et al. McDonald’s blood flow in arteries: theoretical, experimental and clinical principles, 3rd ed. J Cardiopulm Rehabil Prev. 1991;11:407.
Proença J, et al. Is pulse transit time a good indicator of blood pressure changes during short physical exercise in a young population? In: Proceedings of the 2010 Annual International Conference of the IEEE Engineering in Medicine and Biology (EMBC), Buenos Aires, Argentina. 31 Aug–4 Sept 2010. p. 598–601.
Geddes L, et al. Pulse transit time as an indicator of arterial blood pressure. Psychophysiology. 1981;18:71–4.
Wong MY, et al. An evaluation of the cuffless blood pressure estimation based on pulse transit time technique: a half year study on normotensive subjects. Cardiovasc Eng. 2009;9:32–8.
Masè M, et al. Feasibility of cuff-free measurement of systolic and diastolic arterial blood pressure. J Electrocardiol. 2011;44:201–7.
Baek HJ, et al. Enhancing the estimation of blood pressure using pulse arrival time and two confounding factors. Physiol Meas. 2009;31:145–57.
Marcinkevics Z, et al. Relationship between arterial pressure and pulse wave velocity using photoplethysmography during the post-exercise recovery period. Acta Univ Latv Biol. 2009;753:59–68.
Longo A, et al. Posture changes and subfoveal choroidal blood flow. Investig Ophthalmol Vis Sci. 2004;45:546–51.
Parati G, et al. Comparison of finger and intra-arterial blood pressure monitoring at rest and during laboratory testing. Hypertension. 1989;13:647–55.
Steptoe A, et al. Pulse wave velocity and blood pressure change: calibration and applications. Psychophysiology. 1976;13:488–93.
Shelley KH. Photoplethysmography: beyond the calculation of arterial oxygen saturation and heart rate. Anesth Analg. 2007;105:S31–6.
Maeda Y, et al. Relationship between measurement site and motion artifacts in wearable reflected photoplethysmography. J Med Syst. 2011;35:969–76.
Risacher F, et al. Impedance plethysmography for the evaluation of pulse-wave velocity in limbs. Med Biol Eng Comput. 1993;31:318–22.
Critchley LA. Impedance cardiography. The impact of new technology. Anaesthesia. 1998;53:677–84.
Geddes LA, et al. Pulse arrival time as a method of obtaining systolic and diastolic blood pressure indirectly. Med Biol Eng Comput. 1981;19:671–2.
Inan OT, et al. Ballistocardiography and seismocardiography: a review of recent advances. IEEE J Biomed Health Inform. 2015;19(4):1414–27.
Yoshizawa M, et al. A great impact of green video signals on tele-healthcare in daily life, especially for rural or disaster areas. Trans Jpn Soc Med Biol Eng. 2013;51:M-55.
Chen W, et al. Continuous estimation of systolic blood pressure using the pulse arrival time and intermittent calibration. Med Biol Eng Comput. 2000;38:569–74.
McCarthy BM, et al. An investigation of pulse transit time as a non-invasive blood pressure measurement method. J Phys Conf Ser. 2011;307:012060.
McCarthy BM, et al. An examination of calibration intervals required for accurately tracking blood pressure using pulse transit time algorithms. J Hum Hypertens. 2013;27:744–50.
Mukkamala R, Hahn JO, Inan OT, Mestha LK, Kim CS, Töreyin H, Kyal S. Toward ubiquitous blood pressure monitoring via pulse transit time: theory and practice. IEEE Trans Biomed Eng. 2015;62(8):1879–901.
Sharma M, Barbosa K, Ho V, Griggs D, Ghirmai T, Krishnan SK, Hsiai TK, Chiao JC, Cao H. Cuff-less and continuous blood pressure monitoring: a methodological review. Technologies. 2017;5:21.
Ding XR, et al. Continuous blood pressure measurement from invasive to unobtrusive: Celebration of 200th birth anniversary of Carl Ludwig. IEEE J Biomed Health Inform. 2016;20(6):1455–65.
Chiang PH, et al. Personalized effect of health behavior on blood pressure: machine learning based prediction and recommendation. In: IEEE 20th International Conference on e-Health Networking, Applications and Services (Healthcom). 2018. https://doi.org/10.1109/HealthCom.2018.8531109.
Xing X, et al. Optical blood pressure estimation with photoplethysmography and FFT-based neural networks. Biomed Opt Express. 2016;7:3007–20.
Kachuee M, et al. Cuff-less high-accuracy calibration-free blood pressure estimation using pulse transit time. In: 2015 IEEE International Symposium on Circuits and Systems (ISCAS). 2015. p. 1006–9.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Li, J. (2019). Pulse Wave Velocity Techniques. In: Solà, J., Delgado-Gonzalo, R. (eds) The Handbook of Cuffless Blood Pressure Monitoring. Springer, Cham. https://doi.org/10.1007/978-3-030-24701-0_6
Download citation
DOI: https://doi.org/10.1007/978-3-030-24701-0_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-24700-3
Online ISBN: 978-3-030-24701-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)