Abstract
In this work, the effect of VUV radiation of the wavelength region Δλ = 166–180 nm on microscopic fungi propagules Cladosporium herbarum, Rhodotorula colostri, Saccharomyces cerevisiae was studied. In the course of the work, dependencies of the survival probability of propagules, which were at the exponential and stationary phases of development, on radiation doses were obtained. It was found that at the exponential stage of colonies development, the survival curves of propagules of different types of micromycetes coincide within the limits of error, but at the stationary phase they are different. IR spectroscopy and atomic force microscopy of irradiated propagules indicate a change in their cell wall. Electrophoresis of DNA molecules of irradiated propagules proves double-stranded breaks. Experiments with the use of an antioxidant show that the death of propagules during VUV irradiation occurs as a result of the direct and indirect effects of radiation, with the share of the latter being 10–15%. The results obtained allow us to conclude that the inactivation of propagules during irradiation with radiation of the long-wave region of the VUV range Δλ = 166–180 nm is the result of both direct and indirect effects with the destruction of both the cell wall and DNA.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
The presence of stress-tolerant species of microscopic fungi today has been established for communities of almost all known extreme habitats, ranging from polar deserts (Kirtsideli et al. 2010, 2018) to the Chernobyl nuclear power plant (Zhdanova et al. 2004). The first information about the resistance of microscopic fungi, including hyphal and yeast forms, to UV irradiation appeared in the first half of the last century (Luyet 1932; Smith 1936). The effect of UV on fungus activity is mainly associated with its mutagenic effect, as well as with its effect on the plasma membrane (Griffin 1996). UV has a significant effect on the biosynthesis of primary metabolites of fungi, in particular organic acids (Musilkova et al. 1983; Andersen et al. 2011; Prabu et al. 2012; Vasanthabharathi et al. 2013; Tembhurkar et al. 2012).
Studies of the effects of UV and radiation on microorganisms, including algae, bacteria and microscopic fungi, were more often carried out in the temperate and southern zones (Robson et al. 2004; Torres et al. 2004; Palffy and Voros 2006; Ozcelik 2007; Salcedo et al. 2007; Singaravelan et al. 2008; Selbmann et al. 2005, 2014; Siddiqui et al. 2011). Currently, changes are observed in the stratospheric layer of ozone and, consequently, in the intensity of ultraviolet (UV) radiation, which can lead to various effects in ecosystems. Ultraviolet radiation is involved in complex biochemical processes and leads to changes in the carbon cycle. The reactions of organisms to changes in UV radiation and interactions with climate change are considered in a number of papers (Zepp et al. 2007; Singh et al. 2010). Much less is known about the effect of radiation on microscopic fungi on the surface of the primary soils and soils of Antarctica. Antarctica is one of the most extreme habitats of our planet, characterized by geographic isolation, extremely low temperatures, low humidity, and ozone “holes” causing high levels of background ultraviolet (UV) radiation. This makes it possible to consider the microscopic fungi of Antarctica as a natural reservoir of microorganism strains adapted to extreme conditions, including a high dose of UV radiation (Salcedo et al. 2007; Selbmann et al. 2011; Vasilenko et al. 2010; Wynn-Williams and Edwards 2001). Microorganisms of Antarctica are affected by various doses of radiation, which fluctuate strongly due to seasonal changes in snow and ice cover and other factors (Walton 1984). Microorganisms of the Antarctic continent, develop, as a rule, on the surface or in an extremely thin layer of soil, because it is there that the substrate is heated. However, it is there that microscopic fungi are exposed to the strongest effects of UV irradiation.
The influence of Antarctic conditions on microscopic fungi is considered even as analogs of cosmic conditions (Wynn-Williams and Edwards 2001; Poulet et al. 2005; Onofri et al. 2004, 2008, 2012, 2015, 2018). When preparing experiments on the detection of life on Mars (Parnell et al. 2007), it is Antarctica that is considered to be the terrestrial analogue of expected habitats (Wynn-Williams and Edwards 2000). Therefore, it was the isolates of the Antarctic species that were used as test models for studying the mechanisms of the effects of vacuum ultraviolet (VUV) radiation on the survival of microorganisms.
This radiation lies in a shorter wavelength region (10 nm < λ < 200 nm) compared to UV one (200 nm < λ < 400 nm). It has a higher quantum energy, which causes its large photochemical and photobiological properties. In particular, VUV irradiation of H2O and O2 molecules leads to the formation of highly reactive oxygen-containing products ·OH (Heit et al. 1998) and O (1D) (Atkinson et al. 2004):
VUV radiation also has high photobiological capabilities—it is effectively absorbed by such biologically important molecules as: DNA, proteins, sugars. VUV irradiation of DNA molecules leads to different types of destruction than UV exposure: to single- and double-stranded breaks (Michael et al. 1994). Absorption of VUV radiation by sugars (Dickinson and Johnson 1974) and proteins (Inagaki et al. 1975) can lead to the destruction of the chitin cell wall and phospholipid membrane.
Inactivation of microorganisms under the action of VUV radiation can be carried out either as a result of the direct effect of radiation quanta absorbed by the target molecules or by indirect effect of VUV photolysis products: reactive oxygen-containing species (ROS) and lipid oxidation products.
VUV radiation finds practical application in disinfection technologies using gas-discharge plasma. It occurs in gas discharges in mixtures containing rare gases and, together with plasma particles, participates in the inactivation of microorganisms (Weltmann et al. 2010). VUV radiation is present in space, which determines its importance in solving astrobiological problems associated with the survival of microorganisms in space, the formation of microflora in spacecraft, the origin of life on planets (Horneck et al. 2010).
A relatively small number of scientific papers are devoted to the effect of VUV radiation on microorganisms. The mechanisms of inactivation of microorganisms during VUV irradiation remain not fully understood. Among the articles in this area, one can mention a series of works (Ito et al. 1980, 1981, 1983; Hieda et al. 1984), where irradiation was performed at various wavelengths in the UV-VUV range of dry (in vacuum) and wet (in water vapor) Saccharomyces cerevisiae spores. Based on the absence of genetic changes of VUV irradiated spores and the proximity of the shape of the water absorption curve to the sensitivity curve to VUV irradiation of wet spores, the authors concluded that the main mechanism of VUV spore inactivation is destruction of the cell membrane by ROS produced by VUV water photolysis. In this series of works, it was noted that the depth of VUV radiation penetration, due to the sharp dependence of the absorption coefficient on the wavelength, will differ by two orders of magnitude in the long- and short-wavelength parts of the VUV range: in the wavelength range λ = 170 nm, most of the radiation passes through cell wall and membrane, and at a wavelength of λ = 150 nm they will be absorbed by them (Ito et al. 1983). Thus, when the VUV microorganisms are irradiated by the long- wavelength VUV emission, the effect on both the cell wall and DNA should be expected, while under irradiation with short-wavelength VUV radiation, the main role in inactivation will play cell wall destruction. The authors of the paper (Sarantopoulou et al. 2014) have point of view that the destruction of the cell wall of a spore is the main cause of death during VUV irradiation. In this work, VUV irradiation of spores of Cladosporium herbarum by laser VUV radiation (λ = 157 nm) and incoherent VUV radiation (Δλ = 110–180 nm) was carried out under normal conditions in a nitrogen atmosphere and also in vacuum at low temperature (T = 10 K). During the experiments, both the destruction of the cell wall and the breaks in the chains of DNA molecules were detected. The article (Nakonechnyj et al. 1996) investigated the destruction of algal cells Chlamydomonas reinhardtii with VUV irradiation at wavelengths of 120–130 nm, the authors, referring to the work (Ito et al. 1983), have concluded that cell death occurs as a result of cell walls destruction.
Most of the works devoted to the effects of VUV radiation on microorganisms have an astrobiological orientation. Thus, in the works (Horneck et al. 2010; Sarantopoulou et al. 2014) it is noted that the exposure to VUV radiation, along with the action of vacuum, is one of the factors determining the survival of microorganisms in space.
Our paper is devoted to the study of the mechanisms of exposure to radiation of the long-wavelength region of the VUV range (Δλ = 166–180 nm) on microscopic fungi propagules and is a continuation of work (Zvereva et al. 2018).
1 Materials and Methods
Xenon excimer lamps developed at the S. I. Vavilov State Optical Institute were used in the experiments. The lamps produced a surface radiation intensity of I = 1.6 mJ/cm2, the emission had bandwidth Δλ = 166–180 nm with a maximum at λ = 172 nm. The radiation was excited by means of a barrier discharge in xenon at a pressure of P = 300 Torr, the amplitude of the supplied voltage was U = 4.4 kV, the frequency f = 1 kHz.
The absolute intensity of the VUV radiation was measured using a HAMAMATSU H8025 photodetector.
In the work were used cultures of microscopic fungi from the collection of the Komarov Botanical Institute (Cladosporium herbarum (Pers.) Link, Rhodotorula colostri T. Castelli). These cultures were isolated from Antarctic substrates. These species were isolated from the primary soils of Antarctica, the isolates of these species are known as biodestructors of natural and industrial materials, as well as pathogens. For experimental work, cultures of microfungi were grown on standard media (Chapek). We used also Saccharomyces cerevisiae (Desm.) Meyen culture from the St. Petersburg State University collection, which was grown on Saburo medium. Cultivation was carried out at a temperature of 20 °C, for 8–10 days. When studying the effect of the formation of the cell wall of microscopic fungi on their survival, cultures were grown for a longer period (up to 2 months). A suspension of propagules of these isolates was obtained using the method of flushing from the surface. For Cladosporium herbarum, the suspension was divided into spores and mycelium by filtration, conidium suspension was used for further studies.
A suspension of propagules was applied to the surface of the cover glass in an amount of 10 μl and dried on the surfaces of the glass in the form of a monolayer. Irradiation was carried out in air at a pressure of P = 1 atm, air temperature t = 20 °C. In order to avoid absorption of VUV radiation by air, the monolayer had direct contact with the lamp window. After irradiation, the propagules were washed off from the glass surface and cultured in Petri dishes in agar medium at t = 14 °C. After 10–15 days of cultivation, the number of colonies of fungi was counted and the probability of survival was determined.
An atomic force microscope (AFM) NTegra Aura (NT-MDT) was used for microscopic examination of the propagules surface. Surface analysis was carried out in semicontact mode.
The FTIR FSM spectrometer (Infraspek) was used to record the infrared transmission spectra of the spores.
DNA electrophoresis was performed using a Bio RAD Sub-cell Model 96 camera. Statistical processing of measurement results was carried out using software STADIA.
2 Experimental Results and Discussion
Determination of the survival probability of spores during VUV irradiation. In this part of the work propagules of micromycetes of Cladosporium herbarum, Rhodotorula colostri, Saccharomyces cerevisiae, isolated from colonies that were in the exponential phase of growth were exposed to radiation.
According to the results of 5 experiments, regression survival curves were obtained for each type of spores (Fig. 32.1). From the results presented in Fig. 32.1, it can be concluded, that despite the biological differences (the presence of a protective pigment in the cell wall of Cladosporium herbarum, the differences of the chemical composition and cell walls thickness), the survival curves of the different types propagules almost coincide (the difference is observed within error limits). This fact can be explained by the fact that in the exponential phase the propagules did not fully achieve their distinctive characteristics: they did not accumulate significant amount of pigment, the cell wall was not completely formed. It should be noted that the obtained values of the survival probabilities are in good agreement with the existing literature data. Thus, the obtained survival probabilities values of Saccharomyces cerevisiae spores within the 95% regression confidence interval coincide with the results of work (Ito et al. 1983), where the values of SP = 30% (λ = 175 nm) are achieved at a dose of I = 1.5 mJ/cm2. The survival probabilities of Cladosporium herbarum propagules obtained in (Sarantopoulou et al. 2014), where SP = 12% corresponds to the dose I = 8 mJ/cm2 (λ = 157 nm), also correlate with the results of Fig. 32.1.
The influence of the cell wall on propagules survival during VUV irradiation. To study the role of the cell wall in propagules inactivation by VUV irradiation, we compared the survival of propagules obtained from colonies at different (exponential and stationary) phases of development.
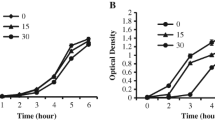
The survival probabilities curves of Cladosporium herbarum and Rhodotorula colostri propagules, formed in pure cultures of different ages, are presented in Fig. 32.2. These cultures had both exponential (8–18 days) and stationary (25–59 days) developmental stages.
Survival curves of the propagules isolated from colonies of different ages: Cladosporium herbarum (Zvereva et al. 2018) and Rhodotorula colostri
The increase of survival probabilities at stationary stage is observed in both types of spores, but to a greater degree for Cladosporium herbarum species, which may be due to the accumulation of melanin in the cell wall over the time.
Studies of the cell wall changes during VUV irradiation were carried out using AFM microscopy and IR spectrometry. Figure 32.3 shows the results of microscopic examination: the same area of the Rhodotorula colostri spore surface is presented before and after irradiation with a dose of I = 100 mJ/cm2. It can be seen that irradiation lead to the appearance of structures on the surface with a characteristic scale of the order of 100 nm.
In Fig. 32.4 shows the IR transmission spectra of irradiated propagules of Rhodotorula colostri at various doses of irradiation. The spectra demonstrate that with increasing of VUV irradiation dose, a decrease in the absorption of the glucan polysaccharide β(1–3) in the frequency region of Δf = 1000–1100 cm−1 and an increase in the absorption of proteins in the Δf = 1415–1650 cm−1 region.
A decrease of absorption at the frequency f = 1239 cm−1 by the PO2 anion, which is present in DNA and phospholipids, and an increase of the absorption of polysaccharide at the frequency f = 966 cm−1 with increasing of VUV radiation dose were observed in our experiments. As cell wall mainly consists of proteins and polysaccharides (Kamzolkina and Dunaevskiy 2015), absorbing in the VUV region (Inagaki et al. 1975; Dickinson and Johnson 1974), then changes in the IR spectra can be attributed to its destruction.
Studies have shown that the cell wall plays important role in the inactivation of spores during VUV irradiation. In the majority of studies on the effects of VUV radiation on microorganisms (Nakonechnyj et al. 1996; Ito et al. 1983; Sarantopoulou et al. 2014), as the main cause of inactivation were considered the destruction of the cell wall under the action of highly reactive photolysis products of water (·OH radicals). This is partly justified by the fact that VUV radiation is mainly absorbed by the water contained in the cell wall and does not pass inside a cell. However, when considering the depth of penetration, the wavelength of the VUV radiation should be taken into account. Thus, according to the data (Crapulli et al. 2014), the thickness of the water layer in which 90% of the radiation is absorbed is 10 μm for the wavelength λ = 172 nm and 0.1 μm for λ = 120–130 nm work. Considering that the characteristic size of micromycete spores is a few microns, in the case of the wavelength range used by us Δλ = 166–180 nm, we should expect the radiation to penetrate the internal parts of the spores and, in particular, to the DNA molecule.
DNA molecules change. DNA plays a key role in biological processes and is considered in radiation biology as the main target molecule. It is known that the effect of VUV radiation on DNA molecules leads to single- and, to a lesser extent, double-stranded breaks (Michael et al. 1994). Our research confirms these results. Electrophoresis of DNA molecules isolated from irradiated and non-irradiated (control) propagules shows the appearance of breaks in DNA molecules during VUV irradiation: the number of low molecular weight fragments increase in spore irradiated samples and a high molecular weight fragment, corresponding to a mass of 20,000 nucleotides appears as well (Fig. 32.5). The presence of DNA fragmentation indicates a double-stranded break of the strands of this molecule as a result of VUV irradiation.
It should be noted that a change in the structure of DNA spores during VUV irradiation was also recorded in the work (Sarantopoulou et al. 2014): using mass spectroscopy, where DNA photo-fragments were detected, and during electrophoresis, where a decrease in the intensity of the upper band of the pattern was observed with increasing VUV dose.
It should be noted, that in a series of works (Ito et al. 1980, 1981, 1983; Hieda et al. 1984), electrophoresis of DNA molecules was not carried out and the conclusion that DNA was not involved in the inactivation of microorganisms during VUV irradiation based on the absence of genetic changes.
The role of indirect effects. VUV irradiation of H2O and O2 molecules leads to the formation of such ROS as ·OH (Heit et al. 1998) and O(1D) (Atkinson et al. 2004), as well as lipid oxidation products (Kudryashov 2004), which may play a role in the death of microorganisms.
The experiments with the use of antioxidants were made to study the role of reactive radicals. For these purposes iodine I2 was added to cultivation media. In alkaline environment I2 molecules is known quickly to dissociate with negative ions I− (iodide) formation, the letter are antioxidants and scavengers of free radicals (Winkler 2015) including ROS (Reactions 32.3 and 32.4), and phospholipid radicals LOOH (Reaction 32.5):
Survival probability curves for propagules grown in iodine containing medium and in free of iodine one are shown in the Fig. 32.6. In accordance with these curves iodine indirect effects provide 10–15% of the survival probability.
Since the irradiation was performed in the air, it is necessary to evaluate the effect of ozone, formed by VUV photolysis of oxygen, on spores survival probability. Evaluation shows that the concentration of ozone in our conditions will be not more than 1013 cm−3. In the work (Ali 2013) it was found that for 99.5% inactivation of spores of microscopic fungi C. albicans at ozone concentrations of 3 1013 cm−3, a time equal to 180 min were required. As irradiation time in our experiments was less than 30 s, the effect of ozone on spores SP values can be ignored.
3 Conclusion
Thus, as a result of studying of the effects of VUV radiation in the wavelength region Δλ = 166–180 nm on propagules of microscopic fungi Cladosporium herbarum, Rhodotorula colostri, Saccharomyces cerevisiae, dependences of the survival probability of propagules on the exponential and stationary development phases were obtained. It was found that at the exponential stage of colonies development, the SP curves of various types of micromycetes propagules coincide within the limits of error, but at the stationary phase they differ. IR spectroscopy and atomic force microscopy of irradiated propagules indicate a change in their cell wall. Electrophoresis of DNA molecules irradiated by propagules proves double-stranded breaks. Experiments with the use of an antioxidant show that the death of propagules during VUV irradiation occurs as a result of the direct and indirect effects of radiation, with the share of the latter being not less than 10–15%. The results obtained allow us to conclude that the inactivation of propagules during irradiation in long-wavelength VUV region (λ = 166–180 nm) results from both direct and indirect effects with the destruction of both the cell wall and DNA.
References
Ali EM (2013) Ozone application for preventing fungal infection in diabetic foot ulcers. Diabetol Croat 42(1):3–22
Andersen MR, Salazar MP, Schaap PJ, van de Vondervoort PJ, Culley D, Thykaer J, Frisvad JC, Nielsen KF, Albang R, Albermann K, Berka RM, Braus GH, Braus-Stromeyer SA, Corrochano LM, Dai Z, van Dijck PW, Hofmann G, Lasure LL, Magnuson JK, Menke H, Meijer M, Meijer SL, Nielsen JB, Nielsen ML, van Ooyen AJ, Pel HJ, Poulsen L, Samson RA, Stam H, Tsang A, van den Brink JM, Atkins A, Aerts A, Shapiro H, Pangilinan J, Salamov A, Lou Y, Lindquist E, Lucas S, Grimwood J, Grigoriev IV, Kubicek CP, Martinez D, van Peij NN, Roubos JA, Nielsen J, Baker SE (2011) Comparative genomics of citric-acid-producing Aspergillus niger ATCC 1015 versus enzyme-producing CBS 513.88. Genome Res 21(6):885–897
Atkinson R, Baulch DL, Cox RA, Crowley JN, Hampson RH, Hynes RG, Jenkin ME, Rossi MJ, Troe J (2004) Evaluated kinetic and photochemical data for atmospheric chemistry: volume I—gas phase reactions of Ox, HOx, NOx and SOx species. Atmos Chem Phys 4:1461–1738
Crapulli F, Santoro D, Sasges MR, Ray AK (2014) Mechanistic modeling of VUV advanced oxidation process in an annular photoreactor. Water Res 64:209–225
Dickinson HR, Johnson WC (1974) Optical properties of sugars. II. Vacuum-ultraviolet absorption of model compounds. J Am Chem Soc 96:5050–5054
Griffin DH (1996) Fungal physiology. Wiley, New York
Heit G, Neuner A, Saugy PY, Braun AM (1998) Vacuum-UV actinometry. The quantum yield of the photolysis of water. J Chem Phys A 102:5551–5561
Hieda K, Kobayashi K, Ito A, Ito T (1984) Comparisions of the effect of vacuum-UV and Far-UV synchrotron radiation on dry yeast cells of different UV sensitivities. Rad Res 98:74–81
Horneck G, Klaus DM, Mancinelli RL (2010) Space microbiology. Microbiol Mol Biol Rev 74:121–156
Inagaki T, Hamm RN, Arakawa ET, Birkhoff RD (1975) Optical property of bovine plasma albumin between 2 and 82 eV. Biopolymers 14:839–847
Ito T, Kobayashi K, Ito A (1980) Effect of broad-band vacuum-UV synchrotron radiationon wet yeast cells. Rad Res 82:364–373
Ito T, Ito A, Kobayashi K (1981) Effect of 120- to 165-nm vacuum-UV light on wet yeast cells. Rad Res 85(1):161–172
Ito T, Ito A, Hieda K, Kobayashi K (1983) Wavelength dependence of inactivation and membrane damage to Saccharomyces cerevisiae cells by monochromatic synchrotron vacuum-UV radiation (145–190 nm). Rad Res 96:532–548
Kamzolkina OV, Dunaevskiy YE (2015) Biology of the microfungi cell. KMK Scientific Publications Partnership, Moscow (in Russian)
Kirtsideli IYu, Vlasov DYu, Abakumov EV, Gilichinsky DA (2010) Diversity and enzyme activity of microfungi from antarctic soils. Mikologiya I Fitopatologiya 44(5):387–397 (in Russian)
Kirtsideli IYu, Vlasov DYu, Novozhilov YuK, Abakumov EV, Barantsevich EP (2018) Assessment of anthropogenic influence on Antarctic mycobiota in areas of Russian Polar Stations. Contemp Probl Ecol 11(5): 449–457
Kudryashov YuB (2004) Radiation biophysics (ionizing radiation). Fizmatlit, Moscow (in Russian)
Luyet BJ (1932) The effect of UV, X-and cathode rays on the spores of Mucoraceae. Radiology 18:1019–1023
Michael BD, Prise KM, Folkard M, Vojnovic B, Brocklehurst B, Munro IH, Hopkirk A (1994) Action spectra for single- and double-strand break induction in plasmid DNA: studies using synchrotron radiation. Int J Radiat Biol 66:569–572
Musilkova M, Ujcova E, Seichert L, Fencl Z (1983) Effect of changed cultivation conditions on themorphology of Aspergillus niger and citric acid biosynthesis in laboratory cultivation. Folia Microbiol 27:328–332
Nakonechnyj YuV, Pakhatova OV, Dodonova NYa (1996) The vaccum ultraviolet irradiation of green inicellular alga chlamydomonas reinhardtii. Biofizika 41(2):421–427 (in Russian)
Onofri S, Selbmann L, Zucconi L, Pagano S (2004) Antarctic microfungi as models for exobiology. Planet Space Sci 52:229–237
Onofri S, Barreca D, Selbmann L, Isola D, Rabbow E, Horneck G, de Vera JP, Hatton J, Zucconi L (2008) Resistance of Antarctic black fungi and cryptoendolithic communities to simulated space and martian conditions. Stud Mycol 61:99–109
Onofri S, de la Torre R, de Vera JP, Ott S, Zucconi L, Selbmann L, Scalzi G, Venkateswaran KJ, Rabbow E, Sa´nchez Inigo FJ, Horneck G (2012) Survival of rockcolonizing organisms after 1.5 years in outer space. Astrobiology 12:508–516
Onofri S, de Vera JP, Zucconi L, Selbmann L, Scalzi G, Venkateswaran KJ, Rabbow E, de la Torre R, Horneck G (2015) Survival of Antarctic cryptoendolithic fungi in simulated martian conditions on board the International Space Station. Astrobiology 12:1–9
Onofri S, Selbmann L, Pacelli C, Hallsworth JE, Zucconi L (2018) Integrity of the DNA and cellular ultrastructure of cryptoendolithic fungi in space or mars conditions: a 1.5-year study at the international space station. Life 8(2):23
Ozcelik B (2007) Fungi/bactericidal and static effects of ultraviolet light in 254 and 354 nm wavelengths. Res J Microbiol 2:42–49
Palffy K, Voros L (2006) Effects of UV-A radiation on desmodesmus armatus: changes in growth rate, pigment content and morphological appearance. Int Rev Hydrobiol 91(5):451–465
Parnell J, Cullen D, Sims MR, Bowden S, Cockell CS, Court R, Ehrenfreund P, Gaubert F, Grant W, Parro V, Rohmer M, Sephton M, Stan-Lotter H, Steele A, Toporski J, Vago J (2007) Searching for life on Mars: selection of molecular targets for ESA’s aurora ExoMars mission. Astrobiology 7:578–604
Poulet F, Bibring JP, Mustard JF, Gendrin A, Mangold N, Langevin Y, Arvidson RE, Gondez B, Gomez D (2005) Phyllosilicates on Mars and implications for early Martian climate. Nature 438:623–627
Prabu R, Chand T, Raksha S (2012) Improvement of Aspergillus niger for sodium gluconate synthesis by UV mutation method. E-J Chem 9(4):2052–2057
Robson TM, Pancotto VA, Ballaré CL, Sala OE, Scopel AL, Caldwell MM (2004) Reduction of solar UV-B mediates changes in the Sphagnum capitulum microenvironment and the peatland microfungal community. Oecologia 140(3):480–490
Salcedo I, Andrade JA, Quiroga JM, Nebot E (2007) Photoreactivation and dark repair in UV-treated microorganisms: effect of temperature. Appl Environ Microbiol 73(5):1594–1600
Sarantopoulou E, Stefi A, Kollia Z, Palles D, Petrou PS, Bourkoula A, Koukouvinos G, Velentzas AD, Kakabakos S, Cefalas AC (2014) Viability of Cladosporium herbarum spores under 157 nm laser and vacuum ultraviolet irradiation, low temperature (10 K) and vacuum. J Appl Phys 116:104701-1-15
Selbmann L, de Hoog GS, Mazzaglia A, Friedmann EI, Onofri S (2005) Fungi at the edge of life: cryptoendolithic black fungi from Antarctic desert. Stud Mycol 51:1–32
Selbmann L, Isola D, Zucconi L, Onofri S (2011) Resistance to UV-B induced DNA damage in extremetolerant cryptoendolithic Antarctic fungi: detection by PCR assays. Fungal Biol 115:937–944
Selbmann L, de Hoog GS, Zucconi L, Isola D, Onofri S (2014) Black yeasts from cold habitats. In: Buzzini P (ed) Yeasts from cold habitats
Siddiqui A, Dawar S, Zaki M., Hamid (2011) Role of ultra violet (UV-C) radiation in the control of root infecting fungi on groundnut and mung bean. Pak J Bot 43(4): 2221–2224
Singaravelan N, Grishkan I, Beharav A, Wakamatsu K, Ito S, Nevo E (2008) Adaptive melanin response of the soil fungus Aspergillus niger to UV radiation stress at “Evolution Canyon”, Mount Carmel, Israel. Melanic Adapt Fungus 3(8):1–5
Singh J, Anand K, Rudra D, Singh P (2010) Antarctic terrestrial ecosystem and role of pigments in enhanced UV-B radiations. Rev Environ Sci Biotechnol 63(1):63–77
Smith EC (1936) The effects of radiation on fungi. In: Duggar BM (ed) Biological effect of radiations, vol 2, pp 889–918
Tembhurkar VR, Joshi SV, Dama LB, Singh PP, Pawase SR, Nighute SB (2012) Random mutageneisis stimulated overproduction of citric acid by Aspergillus niger. DAV Int J Sci 1(2):53–55
Torres A, Hochberg M, Pergament I, Smoum R, Niddam V, Dembitsky VM, Temina M, Dor I, Lev O, Srebnik M, Enk CD (2004) A new UV-B absorbing mycosporine with photo protective activity from the lichenized ascomycete Collema cristatum. Eur J Biochem 271(4):780–784
Vasanthabharathi V, Sajitha N, Jayalakshmi S (2013) Citric acid production from U-V mutated estuarine Aspergillus niger. Adv Biol Res 7(3):89–94
Vasilenko T, Slezak M, Kovac I, Bottkova Z, Jakubco J, Kostelnicova M, Tomori Z, Gal P (2010) The effect of equal daily dose achieved by different power densities of low-level laser therapy at 635 and 670 nm on wound tensile strength in rats: a short report. Photomed Laser Surg 28(2):281–283
Walton DWH (1984) The terrestrial environment. In: Laws RM (ed) Antarctic ecology, vol 1. Academic Press, London, pp 1–60
Weltmann KD, Kindel E, Woedtke T, Hähnel M, Stieber M, Brandenburg R (2010) Atmospheric-pressure plasma sources: prospective tools for plasma medicine. Pure Appl Chem 82:1223–1237
Winkler R (2015) Iodine—a potential antioxidant and the role of Iodine/Iodide in health and disease. Nat Sci 7:548–557
Wynn-Williams DD, Edwards HGM (2000) Antarctic ecosystems are models for extraterrestrial surface habitats. Planet Space Sci 48:1065–1075
Wynn-Williams DD, Edwards HGM (2001) Environmental UV radiation: biological strategies for protection and avoidance, In: Horneck G, Baumstark-Khan C (eds) Astrobiology: the quest for the conditions of life. Springer, Berlin, pp 244–259
Zepp RG, Erickson DJ, Paulc ND, Sulzbergerd B (2007) Interactive effects of solar UV radiation and climate change on biogeochemical cycling. Environ Eff Assess Panel Rep 6(3):135–164
Zhdanova NN, Tugay T, Dighton J, Zheltnozhsky V, MCDermott P (2004) Ionizing radiation attracts soil fungi. Mycol Res 108:1089–1096
Zvereva G, Kirtsideli I, Machs E, Vangonen A (2018) Mechanisms of the effect of VUV radiation on the microfungi. Proc SPIE 10614:106141S
Acknowledgements
The authors want to express their gratitude to the E. Machs (Komarov Botanical Institute) for his help in carrying out DNA electrophoresis and to A. Vangonen (Vavilov State optical institute) for his help in recording the infrared transmission spectra of propagules.
This study was carried out as part of the state assignment according to the thematic plan of the Botanical Institute of the Russian Academy of Sciences (theme no. 01201255604) and the Basic Research Program of the Presidium of the Russian Academy of Sciences.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this paper
Cite this paper
Zvereva, G.N., Kirtsideli, I.Y. (2020). Features of the Effect of VUV Radiation on Microfungi from Polar Regions. In: Frank-Kamenetskaya, O., Vlasov, D., Panova, E., Lessovaia, S. (eds) Processes and Phenomena on the Boundary Between Biogenic and Abiogenic Nature. Lecture Notes in Earth System Sciences. Springer, Cham. https://doi.org/10.1007/978-3-030-21614-6_32
Download citation
DOI: https://doi.org/10.1007/978-3-030-21614-6_32
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-21613-9
Online ISBN: 978-3-030-21614-6
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)