Abstract
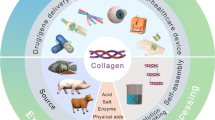
In the search for biomaterials that exhibit both versatility and compatibility with human-tissues, considerable interest has been shown in collagen-based biomaterial for the repair and replacement of the body tissues such as tendons, skin, vascular grafts, heart valves, dental and bones. Some of the general properties of collagen which makes it an interesting biomaterial are the high mechanical strength of the fibers, low antigenicity, its suitability as a substrate for cell growth, and its tunable stability by chemical or physical cross-linking. Collagen based composites are used in various biomedical applications as collagen shields in ophthalmology, sponges for burns and wounds, mini-pellets and tablets for protein delivery, gel formulation in combination with liposome for sustained drug delivery, as controlling material for transdermal delivery, basic matrices for cell culture systems, coating material of metal implant for bone replacement and 3-D printed matrix for various tissue engineering applications. For an adequate biomedical application of collagen, basic knowledge about collagen structure, hierarchical structural organisation and the processing technology in combination with understanding of the physico-chemical properties is of vital importance.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Collagen is the most abundant protein responsible for maintaining the structural integrity of vertebrates and many other multicellular organisms. Collagen represents the major structural protein accounting for approximately 30% of proteins found in the vertebrate organism. There are 28 types of collagen which have been identified (Gelse et al. 2003). Among these collagen types, type I collagen is the most abundant type found in the extracellular matrix (ECM) of most connective tissues such as tendon and bone (Gelse et al. 2003). Collagen type I is widely used in the fabrication of biomaterials for tissue engineering applications. In this review, we will discuss the structure of collagen, hierarchical structure of collagen, source of collagen, cross-linking of collagen, advantages of collagen over other biomaterials and the biomedical application of collagen based biomaterials. In addition, the challenges faced and solutions regarding collagen based biomedical research is also discussed in conclusion.
2 Structure of Collagen Type I
The mature collagen molecule contains three polypeptide helical α chains, two of which are identical, termed α1(I), and one α2(I) chain with a different amino acid composition (Fig. 1.1). Collagen molecules are synthesized as pro-collagen molecules which later converted into mature collagen molecules upon the removal of their pro-peptide domain present on either side, by pro-collagen peptidase. The pro-peptide released from the pro-collagen molecules serves as biomarkers of collagen synthesis. Further, Assembly and cross-linking of mature collagen molecules lead to the development of collagen fibrils and fibers. During physiological and pathological ECM remodelling, the telopeptides present on both amino and carboxyl terminal of mature collagen are cleaved resulting in the degradation of the collagen fibers. The cleaved telopeptides act as the biomarkers for collagen degradation.
Structure of collagen molecule (Fan et al. 2012)
In mature collagen, the three polypeptide chains are intertwined to form a right-handed super-helix (Fig. 1.2b) with a pitch of approximately 8.6 nm. The rod-shaped triple helical molecule has an average molecular weight of approximately 300 kDa, a length of 300 nm with a diameter of 1.5 nm. Each α-chain consists of more than 1000 amino acids. The amino acids are arranged in a unique triple-helix forming a sequence i.e. Gly-X-Y. Glycine with its smallest side group repeats at every third position on the sequence allowing the close packing of the chains into a helix leaving little space for residues in the core. About 35% of the non-glycine positions in the repeating units of Gly-X-Y sequence are occupied by proline in X-position and 4-hydroxyproline in the Y-position (Fig. 1.2a). Due to the steric repulsion between proline and hydroxyproline residues in the X- and Y‐positions, the central domain of the collagen α-chains is folded into a tight left-handed helix (Ramachandran and Kartha 1954). The occurrence of glycine in every third position in the triplet is essential for the formation of three left-handed helices which subsequently form a right-handed super helix. In the collagen molecule, all glycine residues are placed inside the coil while other amino acids project outside (Ramachandran and Kartha 1954).
Hierarchical structural organisation of collagen type I. a Primary amino acid sequence, b secondary left handed helix and tertiary right handed triple-helix structure and c staggered quaternary structure (Friess 1998)
2.1 Hierarchical Structural Organization of Collagen
Collagen fibrillogenesis is a sequence of self-assembling of the triple-helical collagen molecules which stagger longitudinally and laterally to form fibrils in the extracellular space with distinct periodicity (Fig. 1.2c). The collagen molecules aggregate into microfibrils consisting of four to eight collagen molecules and further into fibrils. These fibrils grow laterally from 10 to 500 nm in diameter depending on tissue type and stage of development (Nimni and Harkness 1988). In the collagen fibril, the triple-helices overlap by 0.4D and stagger by 0.6D where D equals 67 nm. The collagen fibrils organize into fibers, which on their part can form even larger fiber bundles.
3 Collagen Origin and Variability
Collagen is produced by fibroblast cells derived from the lineage of pluripotential advential cells. Some of the most common sources of collagen for biomedical applications include bovine skin and tendons, porcine skin and rat tail among others. Marine life forms also serve as a considerable source of collagen, which can be extracted from sponges, fish and jellyfish. Recently, collagen has been discovered in marine coral reefs and further research is going on to explore its biomedical application. These collagens find wide industrial applications, but comparatively used less in biomedical research and clinical usage. Further, the recombinant human collagen has been prepared by Fibrogen® with less immunogenic and identical in composition (Parenteau-Bareil et al. 2010). Collagen obtained from decellularized ECM serve as a scaffolding material for tissue engineering. Acellular ECM obtained from human/porcine dermis, swine intestine and bladder submucosa are also employed in biomedical applications (Badylak 2004).
4 Cross-Linking of Collagen
Cross-linking is crucial to stabilize the collagen matrix. Various biocompatible cross-linkers are widely utilized to fabricate self-standing, stable collagen scaffolds. Cross-linking controls immunogenic properties, rapid degradation and calcification (Jorge-Herrero et al. 1999). Various cross-linkers widely employed for cross-linking the collagen based scaffolds are shown in the Fig. 1.3. The amine and carboxylic groups of collagen are mainly responsible for cross-linking. Glutaraldehyde is one of the cheap and highly reactive water soluble cross-linker and widely employed for stabilizing the collagen scaffolds. Amine groups of collagen favors the cross-linking through Schiff base formation. EDC and N-hydroxy succinimide forms a stable ester known as o-acylisourea, on reacting with carboxylic acid group of collagen and contributes to the cross-linking (Usha et al. 2012). Powell et al. fabricated collagen scaffolds which display prolonged degradation with collagenase enzyme and improved mechanical property without affecting the biocompatibility (Powell and Boyce 2006). It is also widely reported that amine groups of collagen can effectively crosslink with EDC in the absence of NHS. Genipin is extracted from plant extract and utilized for cross-linking collagen (Sundararaghavan et al. 2008). Transglutaminase enzyme was also successfully used for cross-linking collagen (Orban et al. 2004). Charulatha et al. provided a detailed review about various cross-linker and their influence on mechanical properties of collagen scaffolds. Briefly, GTA display enhanced degree of cross-linking and amino acid side chains are crucial for cross-linking, increasing degree of cross-linking significantly improves the mechanical properties (Charulatha and Rajaram 2003).
5 Collagen Over Other Biomaterials
Collagen is extracted from the wide range of various animal tissues by well studied isolation and purification process. The structural, physical, chemical and biological properties of the collagen are well studied (Stamov and Pompe 2012). Further collagen is biocompatible, biodegradable with less immunological property compared to other biomaterials. Collagen posses the cell binding RGD sequence is responsible for adhesion, growth and proliferation of cells which is almost absent in other biomaterials. Collagen can be casted into films, beads, hydrogels, sponges and 3-D matrices (Sinha and Trehan 2003). In addition, it can be easily functionalized with wide range of materials for its utilization in desired biomedical application. Hence, several clinical medicines are based on collagen for the wound dressing, hemostasis, hernia repair, nerve regeneration, drug delivery, heart valves, ocular device and as vascular grafts (Lee et al. 2001).
6 Collagen Based Scaffold in Tissue Engineering Applications
In recent years, the demand for the development of biomaterials aimed at the replacement of injured or damaged tissues in humans or animals has increased. Collagen can be easily made into different material forms such as particles, fibers, hydrogel, films/membranes, sponges, blends and composites. Collagen has then been employed in a wide diversity of applications in the field of medicine including: sutures, hemostatic agents, tissue replacement and regeneration materials (bone, cartilage, skin, blood vessels, trachea, oesophagus, etc.), cosmetic surgery (lips, skin), dental composites, skin regeneration templates, membrane oxygenators, contraceptives (barrier method), biodegradable matrices, protective wrapping of nerves, implants, corneal bandage, contact lens, drug delivery, etc. (Meena et al. 1999; Pannone 2007; Ratner et al. 2004). Here, we will discuss the tissue engineering applications of biomaterial fabricated from collagen, collagen blended with inorganic material, synthetic polymer and biopolymers, collagen composites with native structure and denatured collagen i.e. gelatin.
6.1 Collagen
Collagen is widely employed as biomaterial for the preparation of scaffold in tissue engineering. Collagen films with porous structure were developed by Liu et al. using NaCl ion leaching technique. Here the author had prepared cross-linked collagen films with different amount of NaCl. Further, the porosity of the film was created by leaching out of NaCl from the membrane. The developed porous membrane exhibit biocompatibility with corneal epithelial cells leading to the growth of 2–3 layers of corneal epithelial cells within a week. The author suggest that the developed porous collagen film can be used to solve the problems in corneal regeneration (Liu et al. 2012).
Collagen type II is widely used as a scaffold material for cartilage tissue engineering. Here the author has prepared lyophilized sponge of collagen type I and type II and studied its influence on chondrocytes. The in vitro studies revealed that the cells are viable and evenly distributed over both sponges. Further, Collagen type I and type II sponge exhibit similar gene expression of type I, type II and aggrecan. These results conclude that collagen type I sponge can be used for developing hyaline cartilage-like tissue at initial stage of tissue regeneration (Ohno et al. 2004).
Chen et al. prepared 20–30 nm diameter collagen I particles using high-voltage electrostatic field system. In vitro analysis done using rat Bone marrow stromal cells (BMSCs) revealed the promotion of cell proliferation, osteogenic differentiation and formation of bone nodules. From the observations the author suggested that collagen I nanospheres might have a greater potential for its application in bone tissue engineering (Chen et al. 2009).
Collagen hydrogel formed by cross-linking using four-armed polyethylene glycol succinimidyl glutarate (PEG-SG) (Fig. 1.4) resist fibroblast mediated contraction and thus act as a supporting dermal layer matrix for full thickness skin model. Cross-linked matrix are mechanically stable and resist the collagenase degradation can be used as skin graft equivalent after culturing with human epidermal keratinocytes (Lotz et al. 2017).
Schematic representation of development of skin graft equivalent using collagen and PEG-SG (Lotz et al. 2017)
6.2 Collagen/Inorganic Composite
Inorganic materials such as calcium phosphate (Cap), bioglass, clay, silica, zeolite are widely utilized to reinforce collagen. Inorganic materials are incorporated into collagen matrix in order to improve the bioactivity, mechanical stability and durability. Various methods are widely practiced to fabricate collagen/Cap scaffolds such as (I) In situ mineralization of inorganic particles in fibrous collagen scaffolds or interconnected non fibrous porous scaffolds, (II) Direct blending of inorganic particles along with the fibrous or non-fibrous collagen matrix.
Calcium phosphate has been utilized to fabricate the scaffolds for tissue engineering application owing to their osteoconductivity, osteoinductivity, biocompatibility, biointegration with the host and surrounding tissue and bioresorbability (Samavedi et al. 2013; Vallet-Regí and González-Calbet 2004). Calcium phosphate display various phases such as monocalcium phosphate monohydrate (MCPM), anhydrous monocalcium phosphate (MCPA), dicalcium phosphate dihydrate (DCPD), and anhydrous dicalcium phosphate (DCPA) which are known as acidic calcium phosphate. Other phases such as octacalcium phosphate (OCP), α- and β-tricalcium phosphate (α-TCP, β-TCP), amorphous calcium phosphate (ACP) and hydroxyapatite (HAp) are also observed (Dorozhkin 2012). The [Ca]/[P] ratio of various phase of calcium phosphates are specific which ranges from 0.5 to 2. Bone contains hydroxyapatite as one of the major inorganic constituent and collagen as an organic constituent. Hence mimicking the architecture of bone and fabricating scaffolds using collagen and calcium phosphate based materials can facilitate the healing of damaged bone tissues and considered as a suitable alternate for xenograft. Apart from chemical similarity of calcium phosphate with the chemical constituent of bone and teeth, it also displays enhanced osteoblast attachment, proliferation and differentiation which are indispensable properties of bioactive scaffolds (Villa et al. 2015).
Porous collagen scaffolds display poor mechanical properties which can be significantly improved using nano HAp reinforcement (Sionkowska and Kozłowska 2013). HAp can be incorporated through mineralization or insitu precipitation methods. Kozlovska et al. fabricated interconnected porous scaffolds and successfully demonstrated the insitu precipitation of Cap in collagen matrix (Kozłowska and Sionkowska 2015). Simulated body fluid was also employed for mineralization of HAp on collagen scaffolds (Al-Munajjed et al. 2009). Incorporation of HAp into collagen maintains structural stability such as size, shape and pore architecture of the scaffolds compare to pristine collagen scaffolds as shown in the Fig. 1.5 (Kane et al. 2015). Collagen films incorporated with HAp also demonstrated as a composite material for tissue engineering. Yamauchi et al. synthesized multilayer sheets of collagen and HAP which are deposited alternatively through enzymatic mineralization. These scaffolds are transparent and display excellent mechanical stability (Tensile strength and modulus) (Yamauchi et al. 2004). 3D printing technique was also employed to fabricate collagen/HAp scaffolds. Ardelean et al. produced collagen-calcium ink and fabricated the 3D printed structures. 3D printed structures were cross-linked using GTA in phosphate buffer saline which allows the precipitation of HAp crystals in 3D printed matrix producing biocompatible scaffolds (Ardelean et al. 2018; Ogata et al. 2005). HAp displays poor osteoinductivity which can be improved through ionic substitution (carbonates, magnesium) (Samavedi et al. 2013). Collagen scaffolds are also fabricated using β-TCP and amorphous calcium phosphate. Baheiraei et al. fabricated β-TCP incorporated collagen scaffolds and cultured bone marrow derived mesenchymal stem cells (BMMSCs) which displayed improved differentiation of BMMSC into osteoblast (Baheiraei et al. 2018).
Histological sections (H&E stained) showing the infiltration of hASCs. a 0 vol.% and b 40 vol.% HA whiskers after 14 days in culture (Kane et al. 2015)
Bioglass (SiO2–CaO–P2O5) contains silica, calcium and phosphorous as a major chemical constituent. It is biocompatible, bioactive and facilitates the osteogenic differentiation (Jones et al. 2016; Li et al. 2016). Bioglass incorporated interconnected porous scaffolds are fabricated and utilized for bone tissue engineering application. Sharifi et al. fabricated bioglass incorporated collagen fibers and porous scaffolds which are highly biocompatible (Sharifi et al. 2016). Long et al. produced porous collagen/BG scaffolds which facilitate bone stromal cells attachment and proliferation (Long et al. 2015). Bioglass incorporated collagen scaffolds improve in vitro mineralization and accelerate the osteogenic differentiation of MC-3T3 cells (Marelli et al. 2011). Miri et al. synthesized injectable collagen/BG scaffolds and analysed their in vivo performance using adult rats which evidenced that the injectable hydrogels are highly osteoconductive (Miri et al. 2016). Collagen also used as a coating material to enhance cell attachment and proliferation. Hum et al. produced bioglass scaffolds and coated with collagen scaffolds which display improved cell attachment and proliferation (Hum and Boccaccini 2018). Incorporation of antimicrobial metal ion in bioglass imparts antimicrobial property which were utilized for the fabrication of collagen scaffolds for treating osteomyelitis (Ryan et al. 2019).
Clay is a layered silicate material, montmorillonite (MMT), saponite, smectite and hectorite are some of the clay materials which are widely employed for biomedical application. Clay has been employed for the fabrication of hydrogel, porous polymer scaffolds for tissue engineering applications (Dawson and Oreffo 2013). Reyna-Valencia et al. introduced laponite clay into collagen hydrogel which significantly improved its mechanical property (Reyna-Valencia et al. 2012). Zeolites which are aluminosilicate minerals were also tested for use in tissue engineering applications. Faraji et al. synthesized collagen/zeolite composite and evaluated their biological activity using rabbit in vivo model which evidenced that the composites are suitable for the reconstruction of bone defects (Faraji et al. 2017).
6.3 Collagen/Synthetic Polymer Composite
Various synthetic polymers have been employed to improve the mechanical property of collagen based matrix, such as synthetic polymers such as poly ethylene glycol (PEG), Poly caprolactone (PCL), polyacrylamide, poly(lactic-co-glycolic acid) (PLGA), polyvinyl alcohol (PVA), poly(glycolic acid) (PGA), poly(lactic acid) (PLA) etc. Collagen based synthetic polymeric based biomaterial has been prepared by coating, immobilization, coupling the collagen over the synthetic polymers.
PEG has been used in the preparation of scaffold because of its bio-inert property and its capability to be molded into variety of structures to form scaffold with different architectures. Sargeant et al. developed a collagen-PEG in situ hydrogel forming material for filling the defects with complex geometry and for adherence to adjacent tissue for supporting cell proliferation. The hydrogel exhibits tunable mechanical property, biodegradation and assisting cell adhesion and proliferation. These results suggest that the developed collagen-PEG hydrogel exhibit mechanical, physical and biological properties suitable for its application as an injectable tissue scaffold for the treatment of a variety of simple and complex tissue defects (Sargeant et al. 2012).
PCL having biocompatible, mechanical and biodegradable properties, has been widely used as a biomaterial in tissue engineering. PCL coated collagen matrix was developed by immobilizing collagen over acrylic acid modified 3-D printed PCL matrix. Sousa et al. developed square porous interconnected PCL filamentous network with filament diameter 350 µm using bioextruder. Collagen coated PCL matrix shows better biological properties such as adhesion, growth and proliferation of cells and could be used as a biomaterial for tissue engineering (Sousa et al. 2013).
Yamamoto et al. fabricated collagen-polyacrylamide hydrogel by immobilizing the gradient of collagen type I into the polyacrylamide hydrogel. The authors prepared the biomaterial by coupling the amine groups of collagen with carboxyl group generated in the polyacrylamide hydrogel by sodium hydroxide treatment. The in vitro studies reveal that the number of L929 fibroblasts cells adhered to the hydrogel depend on the amount of collagen immobilized. These results suggest that the cell adhesion can be tuned by varying the concentration gradient of collagen. This diffusion controlled fabrication could be used to modify the functional group of the scaffold with chemicals for coupling with biomolecules for its desired biomedical applications (Yamamoto et al. 2010).
PLGA formed by copolymerization of PLA and PGA is used for the preparation of scaffold with designed shapes having high mechanical property. PLGA scaffold exhibit hydrophobic nature making it unsuitable for cell adhesion and cell growth. Hence, a hybrid 3D scaffolds combining the advantages of natural type I collagen and synthetic poly(lactic-co-glycolic acid) (PLGA) knitted mesh was developed by Dai et al. 2010. 3-D matrix was prepared by incorporating collagen in interstice, one side and both side of the skeletal PLGA mesh to give THIN, SEMI and SANDWICH composite respectively. SEM analysis clearly reveals the development of porous collagen sponge within the PLGA mesh (Fig. 1.6). When cultured with bovine chondrocytes all composites exhibit uniform distribution of cells with chondrocyte morphology and abundant cartilaginous ECM deposition. While SEMI and SANDWICH composites exhibit higher production of glycosaminoglycans (GAG) and expression of type II collagen and aggrecan mRNA. Hence, these scaffolds with the designed structure could be used with tunable thickness for tissue regeneration of articular cartilage. Also this method also provides a new route for the design and fabrication of 3D biodegradable porous scaffolds in tissue engineering (Dai et al. 2010).
SEM micrograph of composites. a, b, c top view of the THIN scaffolds; d, g top view of the SEMI and SANDWICH scaffolds, respectively; e, h bottom view of the SEMI and SANDWICH scaffolds, respectively; f, i cross-sectional view of the SEMI and SANDWICH scaffolds, respectively (Dai et al. 2010)
PVA is water soluble, biocompatible and biodegradable that is used for the preparation of biomaterials. Biomimetic 3-D matrix with collagen and PVA was prepared by coating collagen over the photo cross-linked electrospun fibers of methacrylated polyvinyl alcohol system. Morphological analysis showed uniform nanofibrous structure with fiber diameter of 220–250 µm. In vitro analysis of the 3-D matrix with 3T3 mouse fibroblasts and human umbilical vein endothelial cells (ECV304) cells revealed attachment and growth of cells throughout the matrix (Fig. 1.7). Oktay et al. concluded that the biocompatibility of the PVA fibres was enhanced by coating with the collagen molecules and thus fulfilling the requirements of the scaffold for tissue engineering application (Oktay et al. 2015).
Scanning electron microscopy images of attached cells on collagen-modified scaffold a after 2 h of cell culture, b 24 h, c 72 h (low-magnification), and d high-magnification (Oktay et al. 2015)
PGA is a synthetic polymer with biodegradability and mechanical resistance which could be combined with collagen to provide better mechanical and biological properties to the scaffolds. Hiraoka et al. found that incorporation of PGA fiber increased the compression modulus of collagen sponge by six fold with the weight ratio of collagen to PGA fiber at 0.2. Moreover, PGA incorporation lowers the shrinkage of collagen sponge thereby facilitating increased attachment of fibroblast L929 cells. It also favours deeper infiltration of cells when implanted subcutaneously on the back of the mice. The incorporation of PGA fiber reinforces collagen sponge without compromising the biocompatibility (Hiraoka et al. 2003).
PLA is the one such resorbable synthetic polymer with better mechanical strength than the most of the natural polymers which is used for the fabrication of tissue regeneration scaffolds. Collagen-PLA composite was developed by blending the PLA with the collagen fibres formed by extrusion and cross-linking techniques. The mechanical property of the developed collagen-PLA composites were found to be superior that the collagen-collagen composite, which is formed by grafting collagen with collagen fibers. The collagen-PLA composites support the formation of fibrous tissue in growth and exhibit delayed resorption when implanted into New Zealand white rabbit. Hence, Dunn et al. suggest that the developed composite can be used in the reconstruction of anterior cruciate ligament of knee (Dunn et al. 1997).
6.4 Collagen/Biopolymer Composites
Several biopolymers which are produced by the biological systems are utilized for the fabrication of the biomaterials. Biopolymers exhibit biocompatible and biodegradable property which is one of the major criteria of the scaffold. Here the collagen based scaffold with several biopolymers such as alginate, chitosan, silk fibroin, elastin, fibroin, hyaluronic acid and cellulose has been discussed below.
Alginate is a naturally occurring anionic and hydrophilic polysaccharide composed of (1→4) linked β-D mannouronate (M) and α-L guluronate (G) in different composition and sequence. Because of its properties in terms of biocompatibility, bioresorbability, non-antigenicity, and elasticity, alginate has been used as a biomaterial in tissue engineering. Here, Guillaume et al. had fabricated collagen-alginate composite by lyophilisation to obtain porous and shape memory property to fill the defects in degenerated intervertebral discs. In vitro and ex vivo organ defect model studies also reveal the compatibility of the composite towards bone marrow derived mesenchymal stem cells. Hence, it could be used as an effective biomaterial for intervertebral disc repair (Guillaume et al. 2015).
Chitosan is an amino cationic polysaccharide obtained by deacetylation of chitin. Chitosan is comprised of glucosamine and N-acetyl glucosamine connected through β (1–4) linkage. Chitosan mimics glycosaminoglycan (GAG) which is one of the components of extracellular matrix (ECM) involved in cell-cell and cell matrix interaction. Mighri et al. developed a material by coating chitosan over the collagen membrane and further it was stabilized by cross-linking with glutaraldehyde. This composite membrane support chondrocyte adhesion, proliferation, and IL-6 secretion which were confirmed by the in vitro studies using human chondrosarcoma cell line. This hybrid membrane could be employed as a potential biomaterial for cartilage regeneration (Mighri et al. 2015).
Silk fibroin is a protein extracted from silkworm cocoons mostly from Bombyx mori. Silk fibroin fibers possess excellent mechanical strength, toughness, biocompatibility, biodegradability and thermal stability. Hence, it represents one of the best biopolymers with superior properties to many synthetic and natural polymers. Tissue-engineered nerve conduit (TENC) was developed by blending silk fibroin-collagen nerve scaffold and co-cultured Schwann cells and adipose-derived stem cells. Morphology of the schwann cells exhibited elongated spindle shape and immunocytochemical analysis reveals the presence of S-100 protein which is the characteristic of positive Schwann cells (Fig. 1.8). It was developed with an intension to bridge a 1-cm gap in the rat sciatic nerve transection model. Electrophysiological examinations and morphological analyses of the developed TENC resulted in the nerve regenerative outcome similar to that of the autologous nerve grafts and superior to that of individual collagen and silk fibroin scaffold. Based on the results it is concluded that the developed TENC could be a promising material for peripheral nerve repair (Xu et al. 2016).
Morphology of Schwann cells. a Phase contrast microscopy. b An immunocytochemical assessment of Schwann cells. Scale bar, 100 (a) and 50 μm (b) (Xu et al. 2016)
Elastin is the major component of the elastic fibres present in the connective tissue of extra cellular matrix. It provides the mechanical strength and elasticity to the connective tissues. Minardi et al. developed a biomimetic mesh from the blend of collagen type 1 and elastin for the application in ventral hernia repair. They developed flat sheet and porous scaffolds from the blended composite to compare the morphological effect on the performance of the implant. Both the sheet and porous scaffolds were analysed for in vitro studies using human bone marrow-derived mesenchymal stem cells (h-BM-MSC) and then implanted in a rat ventral hernia model. Porous scaffold displayed mechanical property similar to that of native tissue and stimulated the gene expression of genes associated with de novo matrix deposition, angiogenesis, adipogenesis and skeletal muscles, when compared to that of sheet. Thus, the results conclude the capability of the collagen-elastin composite material for ventral hernia repair application (Minardi et al. 2017).
Fibrin is the insoluble network of fibrous protein derived from soluble fibrinogen after tissue injury to cease the bleeding of blood. Kim et al. developed a fibrin network on the collagen sponge by immersing the collagen sponge in the fibrin polymerisation solution having different concentration of fibrinogen and thrombin. The porosity and water uptake capacity of the collagen-fibrin matrix was reduced due to the cross-linking and fibrin network formation over the collagen sponge. The developed collagen-fibrin matrix seeded with MG-63 cells had shown enhanced cell attachment, proliferation and osteoblast differentiation. Suggesting the incorporation of fibrin network to the collagen scaffold support and improves the differentiation of osteoblast cells for stimulating bone repair (Kim et al. 2013).
Hyaluronic acid is an unsulfated glycosaminoglycan found in the extra cellular matrix of soft tissues. 3-D collagen–hyaluronic acid scaffolds with uniform, interconnected pore structure (total porosity of about 85%) were fabricated by freeze-drying technique and cross-linking with 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide hydrochloride. The addition of hyaluronic acid improved the thermal stability, compressive modulus and elastic collapse stress of the composite. In vitro studies of the composites with murine pre-adipocyte (3T3-L1) cell line has shown the increased expression of genes, adipsin and PPARγ (adipogenesis transcription factor). Thus collagen–hyaluronic acid scaffolds may exhibit porous and permeable 3-D matrices that support the growth and differentiation of mammary stromal tissue for its application in adipose tissue engineering (Davidenko et al. 2010).
Li et al. developed a porous collagen-cellulose matrix loaded gelatin microspheres having basic fibroblast growth factor (bFGF) for skin tissue regeneration. The material was fabricated for the long term release of the bFGF growth factor for enhancing angiogenesis, which is as revealed from ELIZA analysis. The composites also support the proliferation of human umbilical vein endothelial cells when cultured in vitro. Further, subcutaneous implantation of the composite into Sprague-Dawley rats also resulted in formation of new blood vessels as observed from hematoxylin and eosin and immunohistochemical staining. Thus the fabricated composite posses the capability to repair the damaged skin tissue and can be effectively used in skin tissue engineering (Li et al. 2015).
6.5 Collagen Composite with Native Structure
Biomaterial mimicking the native structures has been developed to improve the biomaterial interaction with surrounding tissues through biomolecular recognition (Sakiyama-Elbert and Hubbell 2001). Biomaterial mimicking the characteristics of extracellular matrix (ECM) will serve as an excellent biomaterial for tissue engineering application. To mimic the ECM, several researchers utilize collagen type I which is one of the component of the ECM and fabricate the biomaterial using self-assembling process to mimic the molecular structure of collagen fibril formation in natural tissue. Here, few of such collagen composites having native structures have been discussed below.
Biomimetic collagen–apatite scaffold with a multi-level lamellar structure was developed by combining a time-dependent self-compression process with controllable freeze casting of a mineralized collagen hydrogel. A mineralized collagen hydrogel composed of bone-like mineralized fiber bundles was prepared by a simple biomineralization technique using collagen containing m-SBF. The multilevel lamellar porous structure resulted in a 12-fold increase in Young’s modulus and a two-fold increase in the compression modulus along the aligned direction compared to the conventional scaffold with an equiaxed pore structure. Further, In vitro studies reveal that the lamellar scaffold supported adhesion and spreading of osteoblast over the scaffold. Therefore, Xia et al. concluded that the developed scaffold with hierarchical biomimetic structure showed improved anisotropic mechanical strength and has great potential for its application in bone tissue engineering (Xia et al. 2014).
Li et al. fabricated densified collagen films (up to 100 μm thick) by a plastic compression technique and cross-link them using carbodiimide chemistry. Initially the author developed mineralized collagen by using a polymer-induced liquid-precursor (PILP) mineralization process to mimic the in vivo mineralization process and used it for preparing densified film. The hardness and elastic modulus of the film in dry state was observed to be as comparable to that of woven bone. Hence the author suggest that the biomimetic mineralized collagen film with tunable mineral and mechanical stability can be used for the development of bone substitutes (Li et al. 2012).
Socrates et al. describes the preparation of a novel tAgNPs incorporated native fibrillar collagen-HAp composite having antibacterial property. The author prepared spherical silver nanoparticles of mean diameter 25 nm and incorporated on to collagen and initiated collagen fibril formation to yield composites exhibiting native collagen fibril with D-periodicity. Later the composites were biomimetically mineralized by using m-SBF for mimicking the bone mineral. The formed composites are biocompatible and antibacterial which was shown form in vitro studies with MG-63 cells and antibacterial activity with E. coli and Staphylococcus aureus respectively. Hence, the author concluded that their developed material can be used as a bone repair material for preventing infection and for faster calcified tissue repair (Socrates et al. 2015).
Biomimetic fabrication of mineralized native fibrillar collagen-chitosan composites with incorporated silver nanoparticles exhibiting an antibacterial property was developed by Socrates et al. The surface and cross-sectional micrographs of the composite reveal the presence of fibrillar collagen similar to the native tissue. The composites exhibit mineralization of spherulitic calcium phosphate mineral onto the surface. The composites displayed collagen in the native fibrillar state, improved mechanical property, hemocompatibility, biocompatibility towards MG-63 cells and antibacterial property. These fabricated composites could be employed as a promising bone scaffold for preventing infection and assisting faster healing of the bone (Socrates et al. 2019).
7 Conclusion
Collagen based biomaterials are widely employed in several biomedical applications owing to their better biocompatibility, biodegradability and low immunogenicity. It was extracted and purified from various sources of animal tissues and functionalized with different inorganic and organic polymeric materials for improving its mechanical, biodegradable and other biological properties for specific biomedical application. Collagen scaffold faces several challenges such as stability and immunogenicity caused from utilization of collagen from xenogenic source. In order to reduce the immunogenicity caused from the xenogenic source non helical portion was cleaved during the extraction of collagen. In that course we are eliminating non helical portion, we are losing several cell binding sites and cross-linking sequences which may play a vital role in tissue engineering. To overcome this, recombinant collagen has been utilized for the preparation of composites for tissue engineering. At present several researches are being carried out on the fabrication of collagen based biomaterial cultured with cells to mimic the in vivo structures by biomimetic process to cut down the gap between the in vitro and in vivo studies.
References
Al‐Munajjed AA, Plunkett NA, Gleeson JP, Weber T, Jungreuthmayer C, Levingstone T, Hammer J, O’Brien FJ (2009) Development of a biomimetic collagen-hydroxyapatite scaffold for bone tissue engineering using a SBF immersion technique. J Biomed Mater Res B Appl Biomater 90B(2):584–591. https://doi.org/10.1002/jbm.b.31320
Ardelean IL, Gudovan D, Ficai D, Ficai A, Andronescu E, Albu-Kaya MG, Neacsu P, Ion RN, Cimpean A, Mitran V (2018) Collagen/hydroxyapatite bone grafts manufactured by homogeneous/heterogeneous 3D printing. Mater Lett 231:179–182. https://doi.org/10.1016/j.matlet.2018.08.042
Badylak Stephen F (2004) Xenogeneic extracellular matrix as a scaffold for tissue reconstruction. Transpl Immunol 12(3):367–377. https://doi.org/10.1016/j.trim.2003.12.016
Baheiraei N, Nourani MR, Mortazavi SM, Movahedin M, Eyni H, Bagheri F, Norahan MH (2018) Development of a bioactive porous collagen/β-tricalcium phosphate bone graft assisting rapid vascularization for bone tissue engineering applications. J Biomed Mater Res, Part A 106(1):73–85. https://doi.org/10.1002/jbm.a.36207
Charulatha V, Rajaram A (2003) Influence of different crosslinking treatments on the physical properties of collagen membranes. Biomaterials 24(5):759–767. https://doi.org/10.1016/S0142-9612(02)00412-X
Chen K-Y, Chung C-M, Kuo S-M, Chen Y-S, Yao C-H (2009) Influence of collagen I nanospheres on the growth and osteogenic difference of rat bone marrow stromal cells. J Med Biol Eng 29(6):284–289
Dai W, Kawazoe N, Lin X, Dong J, Chen G (2010) The influence of structural design of PLGA/collagen hybrid scaffolds in cartilage tissue engineering. Biomaterials 31(8):2141–2152. https://doi.org/10.1016/j.biomaterials.2009.11.070
Davidenko N, Campbell JJ, Thian ES, Watson CJ, Cameron RE (2010) Collagen–hyaluronic acid scaffolds for adipose tissue engineering. Acta Biomater 6(10):3957–3968. https://doi.org/10.1016/j.actbio.2010.05.005
Dawson JI, Oreffo ROC (2013) Clay: new opportunities for tissue regeneration and biomaterial design. Adv Mater 25(30):4069–4086. https://doi.org/10.1002/adma.201301034
Dorozhkin SV (2012) Biphasic, triphasic and multiphasic calcium orthophosphates. Acta Biomater 8(3):963–977. https://doi.org/10.1016/j.actbio.2011.09.003
Dunn MG, Bellincampi LD, Jr Tria, Alfred J, Zawadsky JP (1997) Preliminary development of a collagen-PLA composite for ACL reconstruction. J Appl Polym Sci 63(11):1423–1428. https://doi.org/10.1002/(SICI)1097-4628(19970314)63:11%3c1423:AID-APP4%3e3.0.CO;2-O
Fan D, Takawale A, Lee J, Kassiri Z (2012) Cardiac fibroblasts, fibrosis and extracellular matrix remodeling in heart disease. Fibrogenesis & Tissue Repair 5(1):15. https://doi.org/10.1186/1755-1536-5-15
Faraji D, Jahandideh A, Asghari A, Akbarzadeh A, Hesaraki S (2017) Effect of zeolite and zeolite/collagen nanocomposite scaffolds on healing of segmental femur bone defect in rabbits. Iran J Vet Surg 12(2):63–70. https://doi.org/10.22034/ivsa.2018.112807.1133
Friess W (1998) Collagen—biomaterial for drug delivery. Eur J Pharm Biopharm 45(2):113–136. https://doi.org/10.1016/S0939-6411(98)00017-4
Gelse K, Pöschl E, Aigner T (2003) Collagens—structure, function, and biosynthesis. Adv Drug Deliv Rev 55(12):1531–1546. https://doi.org/10.1016/j.addr.2003.08.002
Guillaume O, Naqvi SM, Lennon K, Buckley CT (2015) Enhancing cell migration in shape-memory alginate–collagen composite scaffolds: in vitro and ex vivo assessment for intervertebral disc repair. J Biomater Appl 29(9):1230–1246. https://doi.org/10.1177/0885328214557905
Hiraoka Y, Kimura Y, Ueda H, Tabata Y (2003) Fabrication and biocompatibility of collagen sponge reinforced with poly(glycolic acid) fiber. Tissue Eng 9(6):1101–1112. https://doi.org/10.1089/10763270360728017
Hum J, Boccaccini AR (2018) Collagen as coating material for 45S5 bioactive glass-based scaffolds for bone tissue engineering. Int J Mol Sci 19(6):1807
Jones JR, Brauer DS, Hupa L, Greenspan DC (2016) Bioglass and bioactive glasses and their impact on healthcare. Int J Appl Glass Sci 7(4):423–434. https://doi.org/10.1111/ijag.12252
Jorge-Herrero E, Fernandez P, Turnay J, Olmo N, Calero P, Garcı́a R, Freile I, Castillo-Olivares JL (1999) Influence of different chemical cross-linking treatments on the properties of bovine pericardium and collagen. Biomaterials 20(6):539–545. https://doi.org/10.1016/S0142-9612(98)90205-8
Kane RJ, Weiss-Bilka HE, Meagher MJ, Liu Y, Gargac JA, Niebur GL, Wagner DR, Roeder RK (2015) Hydroxyapatite reinforced collagen scaffolds with improved architecture and mechanical properties. Acta Biomater 17:16–25. https://doi.org/10.1016/j.actbio.2015.01.031
Kim B-S, Kim JS, Lee J (2013) Improvements of osteoblast adhesion, proliferation, and differentiation in vitro via fibrin network formation in collagen sponge scaffold. J Biomed Mater Res, Part A 101A(9):2661–2666. https://doi.org/10.1002/jbm.a.34567
Kozłowska J, Sionkowska A (2015) Effects of different crosslinking methods on the properties of collagen–calcium phosphate composite materials. Int J Biol Macromol 74:397–403. https://doi.org/10.1016/j.ijbiomac.2014.12.023
Lee CH, Singla A, Lee Y (2001) Biomedical applications of collagen. Int J Pharm 221(1):1–22. https://doi.org/10.1016/S0378-5173(01)00691-3
Li H, He J, Yu H, Green CR, Chang J (2016) Bioglass promotes wound healing by affecting gap junction connexin 43 mediated endothelial cell behavior. Biomaterials 84:64–75. https://doi.org/10.1016/j.biomaterials.2016.01.033
Li W, Lan Y, Guo R, Zhang Y, Xue W, Zhang Y (2015) In vitro and in vivo evaluation of a novel collagen/cellulose nanocrystals scaffold for achieving the sustained release of basic fibroblast growth factor. J Biomater Appl 29(6):882–893. https://doi.org/10.1177/0885328214547091
Li Y, Thula TT, Jee S, Perkins SL, Aparicio C, Douglas EP, Gower LB (2012) Biomimetic mineralization of woven bone-like nanocomposites: role of collagen cross-links. Biomacromol 13(1):49–59. https://doi.org/10.1021/bm201070g
Liu Y, Ren L, Yao H, Wang Y (2012) Collagen films with suitable physical properties and biocompatibility for corneal tissue engineering prepared by ion leaching technique. Mater Lett 87:1–4. https://doi.org/10.1016/j.matlet.2012.07.091
Long T, Yang J, Shi S-S, Guo Y-P, Ke Q-F, Zhu Z-A (2015) Fabrication of three-dimensional porous scaffold based on collagen fiber and bioglass for bone tissue engineering. J Biomed Mater Res B Appl Biomater 103(7):1455–1464. https://doi.org/10.1002/jbm.b.33328
Lotz C, Schmid FF, Oechsle E, Monaghan MG, Walles H, Groeber-Becker F (2017) Cross-linked collagen hydrogel matrix resisting contraction to facilitate full-thickness skin equivalents. ACS Appl Mater Interfaces 9(24):20417–20425. https://doi.org/10.1021/acsami.7b04017
Marelli B, Ghezzi CE, Mohn D, Stark WJ, Barralet JE, Boccaccini AR, Nazhat SN (2011) Accelerated mineralization of dense collagen-nano bioactive glass hybrid gels increases scaffold stiffness and regulates osteoblastic function. Biomaterials 32(34):8915–8926. https://doi.org/10.1016/j.biomaterials.2011.08.016
Meena C, Mengi SA, Deshpande SG (1999) Biomedical and industrial applications of collagen. Proc Indian Acad Sci—Chem Sci 111(2):319–329. https://doi.org/10.1007/BF02871912
Mighri N, Mao J, Mighri F, Ajji A, Rouabhia M (2015) Chitosan-coated collagen membranes promote chondrocyte adhesion, growth, and interleukin-6 secretion. Materials 8(11):5413
Minardi S, Taraballi F, Wang X, Cabrera FJ, Van Eps JL, Robbins AB, Sandri M, Moreno MR, Weiner BK, Tasciotti E (2017) Biomimetic collagen/elastin meshes for ventral hernia repair in a rat model. Acta Biomater 50:165–177. https://doi.org/10.1016/j.actbio.2016.11.032
Miri AK, Muja N, Kamranpour NO, Lepry WC, Boccaccini AR, Clarke SA, Nazhat SN (2016) Ectopic bone formation in rapidly fabricated acellular injectable dense collagen-bioglass hybrid scaffolds via gel aspiration-ejection. Biomaterials 85:128–141. https://doi.org/10.1016/j.biomaterials.2016.01.047
Nimni ME, Harkness RD (1988) Molecular structures and functions of collagen. In: Nimni ME (ed) Collagen–biochemistry (pp 1–79). CRC Press, Boca Raton. https://doi.org/10.1201/9781351070799
Ogata K, Imazato S, Ehara A, Ebisu S, Kinomoto Y, Nakano T, Umakoshi Y (2005) Comparison of osteoblast responses to hydroxyapatite and hydroxyapatite/soluble calcium phosphate composites. J Biomed Mater Res, Part A 72A(2):127–135. https://doi.org/10.1002/jbm.a.30146
Ohno T, Tanisaka K, Hiraoka Y, Ushida T, Tamaki T, Tateishi T (2004) Effect of type I and type II collagen sponges as 3D scaffolds for hyaline cartilage-like tissue regeneration on phenotypic control of seeded chondrocytes in vitro. Mater Sci Eng, C 24(3):407–411. https://doi.org/10.1016/j.msec.2003.11.011
Oktay B, Kayaman-Apohan N, Erdem-Kuruca S, Süleymanoğlu M (2015) Fabrication of collagen immobilized electrospun poly(vinyl alcohol) scaffolds. Polym Adv Technol 26(8):978–987. https://doi.org/10.1002/pat.3512
Orban JM, Wilson LB, Kofroth JA, El-Kurdi MS, Maul TM, Vorp DA (2004) Crosslinking of collagen gels by transglutaminase. J Biomed Mater Res, Part A 68A(4):756–762. https://doi.org/10.1002/jbm.a.20110
Pannone PJ (2007) Trends in biomaterials research. Nova Publishers
Parenteau-Bareil R, Gauvin R, Berthod F (2010) Collagen-based biomaterials for tissue engineering applications. Materials 3(3):1863–1887. https://doi.org/10.3390/ma3031863
Powell HM, Boyce ST (2006) EDC cross-linking improves skin substitute strength and stability. Biomaterials 27(34):5821–5827. https://doi.org/10.1016/j.biomaterials.2006.07.030
Ramachandran GN, Kartha G (1954) Structure of collagen. Nature 174(4423):269–270. https://doi.org/10.1038/174269c0
Ratner BD, Hoffman AS, Schoen FJ, Lemons JE (2004) Biomaterials science: an introduction to materials in medicine. Elsevier
Reyna-Valencia A, Chevallier P, Mantovani D (2012) Development of a collagen/clay nanocomposite biomaterial. Mater Sci Forum 706–709:461–466
Ryan EJ, Ryan AJ, González-Vázquez A, Philippart A, Ciraldo FE, Hobbs C, Nicolosi V, Boccaccini AR, Kearney CJ, O’Brien FJ (2019) Collagen scaffolds functionalised with copper-eluting bioactive glass reduce infection and enhance osteogenesis and angiogenesis both in vitro and in vivo. Biomaterials 197:405–416. https://doi.org/10.1016/j.biomaterials.2019.01.031
Sakiyama-Elbert SE, Hubbell JA (2001) Functional biomaterials: design of novel biomaterials. Annu Rev Mater Res 31(1):183–201. https://doi.org/10.1146/annurev.matsci.31.1.183
Samavedi S, Whittington AR, Goldstein AS (2013) Calcium phosphate ceramics in bone tissue engineering: a review of properties and their influence on cell behavior. Acta Biomater 9(9):8037–8045. https://doi.org/10.1016/j.actbio.2013.06.014
Sargeant TD, Desai AP, Banerjee S, Agawu A, Stopek JB (2012) An in situ forming collagen–PEG hydrogel for tissue regeneration. Acta Biomater 8(1):124–132. https://doi.org/10.1016/j.actbio.2011.07.028
Sharifi E, Ebrahimi‐Barough S, Panahi M, Azami M, Ai A, Barabadi Z, Kajbafzadeh AM, Ai J (2016) In vitro evaluation of human endometrial stem cell-derived osteoblast-like cells’ behavior on gelatin/collagen/bioglass nanofibers’ scaffolds. J Biomed Mater Res, Part A 104(9):2210–2219. https://doi.org/10.1002/jbm.a.35748
Sinha VR, Trehan Aman (2003) Biodegradable microspheres for protein delivery. J Controlled Release 90(3):261–280. https://doi.org/10.1016/S0168-3659(03)00194-9
Sionkowska A, Kozłowska J (2013) Properties and modification of porous 3-D collagen/hydroxyapatite composites. Int J Biol Macromol 52:250–259. https://doi.org/10.1016/j.ijbiomac.2012.10.002
Socrates R, Prymak O, Loza K, Sakthivel N, Rajaram A, Epple M, Narayana Kalkura S (2019) Biomimetic fabrication of mineralized composite films of nanosilver loaded native fibrillar collagen and chitosan. Mater Sci Eng, C 99:357–366. https://doi.org/10.1016/j.msec.2019.01.101
Socrates R, Sakthivel N, Rajaram A, Ramamoorthy Usha, Narayana Kalkura S (2015) Novel fibrillar collagen–hydroxyapatite matrices loaded with silver nanoparticles for orthopedic application. Mater Lett 161:759–762. https://doi.org/10.1016/j.matlet.2015.09.089
Sousa I, Mendes A, Bártolo PJ (2013) PCL scaffolds with collagen bioactivator for applications in tissue engineering. Procedia Eng 59:279–284. https://doi.org/10.1016/j.proeng.2013.05.122
Stamov DR, Pompe T (2012) Structure and function of ECM-inspired composite collagen type I scaffolds. Soft Matter 8(40):10200–10212. https://doi.org/10.1039/C2SM26134K
Sundararaghavan HG, Monteiro GA, Lapin NA, Chabal YJ, Miksan JR, Shreiber DI (2008) Genipin-induced changes in collagen gels: correlation of mechanical properties to fluorescence. J Biomed Mater Res, Part A 87A(2):308–320. https://doi.org/10.1002/jbm.a.31715
Usha R, Sreeram KJ, Rajaram A (2012) Stabilization of collagen with EDC/NHS in the presence of l-lysine: a comprehensive study. Colloids Surf, B 90:83–90. https://doi.org/10.1016/j.colsurfb.2011.10.002
Vallet-Regí M, González-Calbet JM (2004) Calcium phosphates as substitution of bone tissues. Prog Solid State Chem 32(1):1–31. https://doi.org/10.1016/j.progsolidstchem.2004.07.001
Villa MM, Wang L, Huang J, Rowe DW, Wei M (2015) Bone tissue engineering with a collagen–hydroxyapatite scaffold and culture expanded bone marrow stromal cells. J Biomed Mater Res B Appl Biomater 103(2):243–253. https://doi.org/10.1002/jbm.b.33225
Xia Z, Villa MM, Wei M (2014) A biomimetic collagen–apatite scaffold with a multi-level lamellar structure for bone tissue engineering. J Mater Chem B 2(14):1998–2007. https://doi.org/10.1039/C3TB21595D
Xu Y, Zhang Z, Chen X, Li R, Li D, Feng S (2016) A silk fibroin/collagen nerve scaffold seeded with a co-culture of Schwann cells and adipose-derived stem cells for sciatic nerve regeneration. PLoS ONE 11(1):e0147184. https://doi.org/10.1371/journal.pone.0147184
Yamamoto M, Yanase K, Tabata Y (2010) Generation of type I collagen gradient in polyacrylamide hydrogels by a simple diffusion-controlled hydrolysis of amide groups. Materials 3(4). https://doi.org/10.3390/ma3042393
Yamauchi K, Goda T, Takeuchi N, Einaga H, Tanabe T (2004) Preparation of collagen/calcium phosphate multilayer sheet using enzymatic mineralization. Biomaterials 25(24):5481–5489. https://doi.org/10.1016/j.biomaterials.2003.12.057
Acknowledgements
We would like to thank and acknowledge the financial support from Indo-French Centre for the Promotion of Advanced Research, IFCPAR/CEFIPRA (Project No. 5608-1).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this paper
Cite this paper
Radhakrishnan, S., Nagarajan, S., Bechelany, M., Kalkura, S.N. (2020). Collagen Based Biomaterials for Tissue Engineering Applications: A Review. In: Frank-Kamenetskaya, O., Vlasov, D., Panova, E., Lessovaia, S. (eds) Processes and Phenomena on the Boundary Between Biogenic and Abiogenic Nature. Lecture Notes in Earth System Sciences. Springer, Cham. https://doi.org/10.1007/978-3-030-21614-6_1
Download citation
DOI: https://doi.org/10.1007/978-3-030-21614-6_1
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-21613-9
Online ISBN: 978-3-030-21614-6
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)