Abstract
Limited reserves of fossil fuel resources and negative environmental impacts increased energy demands toward renewable energy technologies. Bioenergy is one of the solutions, and biogas production from wastes and residues by anaerobic digestion (AD) is a promising technology. Municipal solid wastes, sludge from wastewater treatment plants, agricultural plant wastes, forestry residues and manure are the widely used sources in AD for biogas production. Aquatic plants can be evaluated as a renewable energy source. If waste and residues of these plants are not utilized in beneficial use, greenhouse gases (GHG) will be emitted through landfilling or direct combustion. Wastes should be converted to biogas with a high yield to decrease the quantity of wastes and biogas with a high-energy content. Substrate to inoculum ratio, temperature regime, C/N ratio, pH, volatile fatty acid and ammonia content are important process parameters for AD. Modified Gompertz, Cone and first-order equations are widely used model equations for kinetic parameters that are used in kinetic models (Monod, modified Andrew, Ratkowsky) for identification of optimum substrate concentration and temperature for each specific feed. This chapter evaluates effective process parameters on AD of aquatic plants for biogas production and application of kinetic analysis for assignment of optimum conditions.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
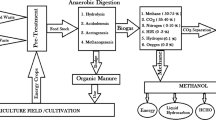
Increasing energy demands, decreasing resources of fossil fuels and concern about environmental protection are the main reasons to use renewable and environmentally benign energy sources [1]. Biomass is one of the renewable sources having advantages of wide availability and great energy potential. While the energy potential of biomass in 2016 was about 50 EJ that was 14% of the world’s final energy use, its realistic potential was estimated as 150 EJ by 2035 [2]. Most of the biomass potential was originated from agricultural residues and wastes, energy crops, forestry products and residues. Comparing with these agricultural and terrestrial plants, aquatic plants are accepted as prominent renewable energy resource since they are harvested with high yields and significant contributors to future biomass potential [3, 4]. Water lettuce, water hyacinth and salvinia as aquatic plants are very aggressive invader plants that are used in phytoremediation but they can form a layer over the rivers, lakes or ponds and threaten the irrigation, navigation systems and aquatic life. To overcome these negative impacts of aquatic plants, herbicides are used to suppress their vegetation or they are piled and then burned. These applications are not environmentally friendly preferences. Instead, aquatic plants can be evaluated in energy production such as biogas production by AD. Produced biogas can be supplied to a variety of uses including electricity, heat and power generation. AD also provides waste minimization and remaining solid residue after AD can be used as biofertilizer.
Hydrolysis, acidogenesis, acetogenesis and methanogenesis are the main reaction steps in AD. Simple sugars, sucrose, glucose and fructose, are formed by hydrolysis of carbohydrates. Proteins and lipids decay to amino acids and long-chain fatty acids, respectively. Fermentation reactions of acidogenic bacteria convert simple sugars, amino acids and long-chain fatty acids to intermediate compounds (VFAs) such as acetic acid, propionic acid, butyric acid and valeric acid. Intermediate compounds are metabolized into acetic acid, carbon dioxide and hydrogen by acetogenic bacteria. Two varieties of methanogens are active in the final step which is methanogenesis. One of the groups directs formation of methane by reduction of CO2 using H2 as the electron donor, and other group cleaves acetic acid into CH4 and CO2 [5].
Composition of the substrate is important for AD as every fraction is not decomposed easily. Lignocellulosic substrate is mainly consisting of cellulose, hemicellulose, lignin, extractives and inorganics. Main fractions of holocellulose (cellulose and hemicellulose) are easily decomposed by microorganisms during AD. But lignin forms a rigid structure inside holocellulose, and it retards decomposition of substrate [6]. High holocellulose content and low lignin content of aquatic plants are advantages for biogas production [7].
Biogas production from aquatic plants such as water lettuce, water hyacinth, cabomba and salvinia was investigated by using AD [3, 8,9,10,11,12,13]. The pilot-scale digestions resulted in biogas yield (approximately 50% of methane content) as 267 L biogas kg−1 VS and 221 L biogas kg−1 VS for water hyacinth and cabomba, respectively [8]. Biogas potential of water hyacinth was changing between 200 and 300 L biogas kg−1 VS with almost 70% of methane content [9]. Vaidyanathan et al. [10] obtained a higher yield of biogas with water hyacinth as 671 L biogas kg−1 VS with 64% of methane content. AD of water lettuce was performed in laboratory-scale digesters with digested cattle manure as inoculum and gas yields were found in the range of 533–707 L kg−1 VS with the average methane content of 58–68% at temperatures of 29.5–37.5 °C after thirteen days [11]. Ratios of carbon to nitrogen (C/N) and carbon to phosphorus (C/P), content of hemicellulose, pH and buffering capacity of substrate [12], digestion temperature, concentration of substrate (that is total solid (TS) or volatile solid (VS) content) and inoculum type are the effective parameters for biogas production [14]. Serez [15] investigated AD of water hyacinth (Fig. 1) with waste sludge at laboratory and pilot scales (Fig. 2) by changing substrate concentration and digestion temperature. Elsewhere, a similar study with only lab-scale batch digester was performed by using water lettuce as substrate [7].
Chuang et al. [13] experimented anaerobic digestion of water hyacinth using pig manure as inoculum to produce hydrogen and methane. They selected substrate concentration and incubation temperature as 10–80 g L−1 and 25–65 °C, respectively. Optimum substrate concentration and temperature for maximum yields of methane and hydrogen were searched by application of kinetic analysis. Biogas yield increased by increasing substrate concentration but up to 60 g L−1. Excessive organic feed (80 g L−1) showed adverse effect on AD. Optimum temperatures for maximum hydrogen and methane productions were 47.8 and 62.5 °C, respectively. Optimum conditions depend on the types of substrate and inoculum in addition to reactor type used in AD.
Kinetic analysis is used for modeling and scaling up the reactor employed, identifying optimum conditions that should be defined for each specific feed. Before application of kinetic analysis, kinetic parameters should be assigned by model equations such as modified Gompertz, Cone and first-order kinetics [16, 17]. Experimental and predicted values should be compared in terms of correlation coefficient and fitting errors. Kinetic parameters of model equation giving the best fit are used further in kinetic models. The influence of substrate concentration was included in Monod and modified Andrew models while the impact of temperature was investigated in Ratkowsky model [7, 13].
2 Effect of Process Parameters on AD
2.1 Substrate to Inoculum Ratio
Concentration of substrate and inoculum in AD must be in an ideal balance to avoid excessive loading of organic material and to stabilize bacterial activity. Low concentration of substrate causes low methane production, while high concentration results in total inhibition or long lag-phase time for acclimation [18]. The substrate to inoculum ratio is important for methanogenesis, and optimum value is determined by achieving the highest methane production. In the literature, the highest amount of methane production was reached at a substrate to inoculum ratio of 0.5 based on VS (volatile solid) for the fresh human fecal and the digested sewage sludge as inoculum [19]. The use of higher inoculum concentration generated higher methane production rate requiring lower adaptation time in AD of swine wastewater with sewage sludge as inoculum (substrate to inoculum ratio of 1:1 based on VS) [14]. Ratio of substrate to inoculum can be expressed as total solid (TS) or volatile solid (VS).
Serez [15] reported AD of water hyacinth by changing substrate concentration at constant waste sludge concentration and at digestion temperature and then by changing digestion temperature at constant substrate and waste sludge concentrations (Table 1).
In that study, lab-scale batch studies were performed, and variation of cumulative biogas production obtained at different water hyacinth concentrations was shown in Fig. 3a. According to the report, the highest biogas yield was found as 66.1 mL g−1 VS at 50 g TS L−1 of substrate concentration. Low value of biogas yield is due to the low concentration of inoculum used. Madenoğlu et al. [7] performed further studies with water lettuce and using higher inoculum concentration. They used waste sludge as inoculum and mixed with substrate (water lettuce) with different ratios based on TS concentration. Change in cumulative biogas production (mL g−1 VS) with time at varying substrate (30, 40 and 50 g TS L−1) and waste sludge concentrations (3.4 and 6.8 g TS L−1) was investigated at a constant digestion temperature of 35 °C (Fig. 4a, b). Whereas there was a slight change by increasing substrate concentration from 30 to 50 g TS L−1, a remarkable increase was obtained by increasing waste sludge concentration from 3.4 to 6.8 g TS L−1. Biogas production was increased twofold (from 168.8 to 321 mL g−1 VS) by increasing waste sludge concentration (from 3.4 to 6.8 g TS L−1) at a constant substrate concentration (30 g TS L−1). In addition, methane content was almost same as 72.5% which corresponds 122.4 mL CH4 g−1 VS and 232.7 mL CH4 g−1 VS. The reason of sharp increase in biogas yield with a high sludge concentration was due to the fact that an increase in microorganism quantity resulted in better biodegradability.

Adapted from Serez [15]
Variation of cumulative biogas production with a water hyacinth concentration (at waste sludge concentration of 3.4 g TS L−1 and 35 °C) and b digestion temperature (at water hyacinth concentration of 50 g L−1 and waste sludge concentration of 3.4 g TS L−1).

Adapted from Güngören Madenoğlu et al. [7], with permission from John Wiley and Sons
Variation of cumulative biogas production with a water lettuce concentration (at waste sludge concentration of 3.4 g TS L−1 and 35 °C), b water lettuce concentration (at waste sludge concentration of 6.8 g TS L−1 and 35 °C) and c digestion temperature (at water lettuce concentration of 50 g L−1 and waste sludge concentration of 6.8 g TS L−1).
Comparing the results of water hyacinth and water lettuce at the same operating conditions, both aquatic plants gave the highest biogas yield at a substrate concentration of 50 g L−1, but biogas yield was found to be higher when water lettuce was used as substrate (Figs. 3a and 4a).
2.2 Temperature Regime
Temperature is one of the most important parameters affecting not only bacterial activity but also biodegradation rate and methane yield. AD can be performed at four different temperature regimes that are psychrophilic (15–25 °C), mesophilic (20–40 °C), thermophilic (50–65 °C) and hyperthermophilic (65–75 °C). Thermophilic conditions are more advantageous as reaction rates are higher at a high digestion temperature yielding higher productivity, but biogas yield can be lower because of the tendency of acidification. High-energy input, negative impacts for environmental changes and lower stability, lower methanogenesis are the other disadvantages of thermophilic conditions. Even though mesophilic conditions show better process stability, low biodegradation and low methane content occur in this regime [20]. In psychrophilic regime, organic materials can be digested at ambient temperature. While energy requirement is lower, biodegradation, methane production and stability can be also lower compared to mesophilic condition because of the negative effect of temperature fluctuation in the environment. Wei et al. [21] emphasized that biogas production at thermophilic condition (55 °C) was more than double at psychrophilic (15 °C) condition. In addition, the passive disintegration of solid under thermophilic condition was easier than at psychrophilic condition [22]. In the hyperthermophilic regime, persistent biomaterials, proteins and lipids can be treated, but methane production can stop because of proliferation of acidogenic communities [12]. Lee et al. [23] co-digested waste activated sludge with kitchen garbage at two-phased hyperthermophilic conditions. High-performance treatment was achieved by acidogenesis at 70 °C and by methanogenesis at 55 °C. High protein solubilization of sludge was confirmed by the presence of specific bacteria (Coprothermobacter sp.) at 70 °C. Wang et al. [24] described a relation between temperature and C/N ratio. The increase of C/N ratios reduced the ammonia formation, but maximum methane production potential was achieved with C/N ratios of 25:1 and 30:1 at 35 and 55 °C, respectively. Effect of ammonia inhibition can be reduced by increasing the C/N ratio of feed when temperature increased.
Effect of digestion temperature was investigated at constant water hyacinth and waste sludge concentrations as summarized in Table 1 [15]. Change in cumulative biogas production with time at varying digestion temperatures (35, 45, and 55 °C) was shown in Fig. 3b for water hyacinth concentration of 50 g TS L−1 and waste sludge concentration of 3.4 g TS L−1. The highest biogas yield as 144.2 mL g−1 VS was obtained at 55 °C. Even though methane content reached up to 70% at 55 °C, biogas yield at this temperature was not high enough. Therefore, further studies were carried out with higher inoculum concentration and temperature.
Madenoglu et al. [7] studied on the effect of mesophilic and thermophilic temperatures on biogas yield of water lettuce. Effect of digestion temperatures (35, 45, 55 and 65 °C) on cumulative biogas production (mL g−1 VS) was investigated at constant substrate and waste sludge concentrations of 50 g TS L−1 and 6.8 g TS L−1, respectively (Fig. 4c). The maximum biogas yield reached was 289 mL g−1 VS at 35 °C while the minimum yield at 65 °C as 162 mL g−1 VS in which methane content was only 50.4%. Bacterial activity was almost diminished at 65 °C. Although the methane content was satisfactory as 79% at 45 and 55 °C, maximum biogas yields obtained were not as high as at 35 °C.
AD of water hyacinth with waste sludge was investigated using a pilot-scale batch digester [25]. Effect of digestion temperature (35, 45, and 65 °C) was searched at a water hyacinth concentration of 20 g TS L−1 and using a waste sludge concentration of 1.7 g TS L−1. Similar to lab-scale studies, the highest biogas and methane yields were obtained at 35 °C as 176.9 and 108.8 mL g−1 VS (61.5% of methane), respectively (Figs. 5 and 6). Yields of pilot-scale studies [25] with water hyacinth were found to be lower compared with the lab-scale studies [15] since both concentrations of the substrate and inoculum were lower in pilot-scale studies.
2.3 C/N Ratio
The C/N ratio defines the performance of digestion process as anaerobic bacteria need nutrients to build its cell structure and to growth. High value of C/N ratio shows a low protein solubilization rate and low total ammonium nitrogen (TAN). Insufficient nitrogen to build cell of bacteria leads to failure in microbial activity and lower biogas yield. Substantially, low ratio of C/N in substrate increases the ammonia inhibition effect that is toxic for methanogens and carbon source cannot be evaluated and digested effectively. Ammonia inhibition can be controlled by adjustment of C/N ratio. The optimum C/N ratio for AD was recommended between 20 and 35, and the ratio of 25 was the most commonly used value [26,27,28].
Wang et al. [29] investigated AD of multi-component substrates, using a mixture of dairy manure, chicken manure and wheat straw to obtain a high methane yield by adjusting C/N ratios. They concluded that C/N ratios of 25:1 and 30:1 had better digestion performances because of stable pH and low concentrations of TAN and free NH3. The optimum C/N ratio was 20 for co-digestion of algae with corn straw [30] while the optimum C/N ratio was 15.8 for co-digestion of food waste with cattle manure [31]. The optimum C/N ratio was 25 for the anaerobic co-digestion of rice straw and Hydrilla verticillata that is an aquatic weed [16]. It can be concluded that the optimum C/N ratio for AD depends on both substrate and the inoculum.
2.4 VFA, Ammonia and pH
Volatile fatty acids (VFAs) are formed as intermediates during AD of organics and mainly composed of acetic acid, propionic acid, butyric acid and valeric acid. Meanwhile, protein- or nitrogen-rich compounds are degraded to ammonia that are mainly in the forms of ammonium ion (NH4+) and free ammonia (NH3). The pH of the medium affects the progress of digestion and products. High organic loading causes accumulation of VFAs and results in a certain pH decrease and unsuccessful AD [32,33,34]. Presence of ammonia with high concentration results in higher pH. Buffering capacity of AD was improved by neutralization of VFAs with ammonia [35, 36]. The optimum pH range of AD process was recommended between 6.8 and 7.4 [37]. Anaerobic bacteria need different pH ranges for their growth. Optimum pH for acidogenesis bacteria is between 5.5 and 6.5 [38] while for methanogenesis bacteria between 6.5 and 8.2 [39]. As bacterial activity depends on pH values, two-stage AD was preferred for hydrolysis/acidification and acetogenesis/methanogenesis processes to increase the yield.
Madenoğlu et al. [7] investigated the effect of substrate (water lettuce) and inoculum (waste sludge) concentrations, and temperature on aqueous phase composition at the end of AD. Biogas formation was directly connected with degradation of compounds in the aqueous phase. Carbohydrate hydrolysis products were analyzed, and only glucose was identified among the products. Its concentration increased by increasing substrate concentration while low glucose concentration was handled by doubling waste sludge concentration from 3.4 to 6.8 g TS L−1. This situation was explained by a high-rate conversion of glucose to methane gas through VFAs with increased waste sludge concentration. Total VFAs concentration was defined as summation of acetic acid, propionic acid, butyric acid and iso-butyric acid. Continuous degradation of VFAs produced methane gas. Total VFAs increased by increasing substrate concentration. Parallel to biogas formation, total VFAs decreased by doubling waste sludge concentration from 3.4 to 6.8 g TS L−1. Total VFAs concentration decreased from 115 to 41 mg L−1 by doubling waste sludge concentration from 3.4 to 6.8 g TS L−1 at constant substrate concentration of 50 g TS L−1. Concentrations of VFAs were not high enough to change pH of the aqueous phase, and final pH was between 8.1 and 8.4 at a digestion temperature of 35 °C. Increase in final pH compared to initial pH of 7.0 was caused by the increase in total ammonia nitrogen (TAN) that was total of ammonium (NH4-N) and ammonia (NH3-N) content. At the end of AD, TAN, final NH4-N and NH3-N were found as 349–673 mg L−1, 329–635 and 19–65 mg L−1, respectively. Inhibitory effect of TAN concentration was in the range of 1.500 and 10.000 mg L−1, and toxicity level for bacteria was 30 g L−1 [40]. As their values were well below the limits, medium of bacteria was comfortable for their activities. Ammonia is prevailing at a pH greater than 9.25 while ammonium ion is at a pH less than 7.0 in the solution [41, 42]. The ratio of NH4+: NH3 was between 89.2:10.8 and 95.0:0.5 at 35 °C [7].
Separately, the effect of digestion temperature on aqueous solution of AD was also examined [7]. A high reaction rate expected at thermophilic conditions was not confirmed as biogas and methane yields decreased as a result of accelerated growth of acid-forming bacteria and suppressed activity of methanogenic bacteria. The concentrations of VFAs obtained, especially propionic acid, were 209 and 1856 mg L−1 at 55 and 65 °C, respectively. In the same temperature range, NH4+ concentrations ranged between 595 and 690 mg L−1. The pH of aqueous solution slightly increased from 7.0 to 7.7 during AD in thermophilic conditions due to the parallel increase of both VFAs and TAN. The ratio of NH4+: NH3 was between 90.7:9.3 and 97.7:2.3 at a temperature range of 35–65 °C. When ratio of NH4+: NH3 is high and pH of the medium exceeds 8, the performance of AD starts to decline that leads to a low gas formation [43, 44]. Additionally, high pH causes increase in concentrations of CO32− and S2− ions that give rise to elimination of trace metals which is necessary for bacterial activity [45]. Since bacterial activity is mostly inhibited at extremely thermophilic and hyperthermophilic conditions (>65 °C), methanogenic bacteria cannot convert VFAs into methane and excessive accumulation of VFAs cause a sharp drop in pH [13, 37].
3 Model Equations for Kinetic Parameters
Modified Gompertz, Cone and first-order kinetic models [41, 46] can be used to simulate to estimate kinetic parameters for methane production. Modified Gompertz (Eq. 1), Cone (Eq. 2) and first-order (Eq. 3) model equations were fitted to cumulative methane production data and kinetic parameters (ym, U, λ, khyd, k and n) were calculated by the following equations:
The cumulative methane production is y(t) in mL/g VS, the maximum methane production potential is ym in mL/g VS, the maximum methane production rate is U in (mL/g VS)/h, e is 2.718, the lag-phase time is λ in h, the hydrolysis rate constant is khyd in 1/h, the shape factor is n, the rate constant is k in 1/h, and the incubation time is t in h. The volumetric overall methane production rate (Roverall, in mL CH4/L/h) can be calculated by (Eq. 4) as follows:
The total volume of the digestion mixture is V in mL. This equation includes process performance, rate and retardation in methane production.
All these kinetic models fitted to AD of water lettuce with waste sludge at different substrate concentrations and temperatures [7]. Cumulative methane productions well fitted only to modified Gompertz and Cone models since first-order kinetic models did not give a good fit. Correlation coefficient for both modified Gompertz and Cone models ranged between 0.94 and 0.99. In addition, the correlation coefficient is not satisfactory alone to decide on best fit of experimental and predicted values. Differences between these values were calculated for each condition, and it was emphasized that modified Gompertz model deviated from experimental values up to 32%. Cone model was found to be more flexible in order to fit experimental values as it contains a shape factor of “n”.
Kinetic models should be applied to find the best fit for AD of each substrate at different operating conditions as the composition of feed (substrate and inoculum) affects the best model selection. For instance, fish or meat wastes with high protein and fat contents do not give a good fit for the first-order kinetic model as lag-phase time is reasonably long. Kafle et al. [47] experienced that situation with fish wastes and decided that modified Gompertz model was the best model giving low fitting error. Similar results of Kafle et al. [47] were obtained by Zhan et al. [48] in AD of pig manure with dewatered sewage sludge as sludge can contain high nitrogen. Budiyono et al. [49] applied AD of carbohydrate-rich feed of vinasse, and they implied that the first-order kinetic model gave the best fit as the carbohydrates degraded quickly and biogas was produced in a short lag-phase time. Syaichurrozi [50] co-digested an aquatic plant (Salvinia) with rice straw to adjust C/N ratio in an optimum range and application of kinetic analysis which revealed that Cone model gave the lowest fitting error compared to modified Gompertz and first-order kinetic models.
4 Kinetic Models
Rate of substrate degradation and biogas (also methane) formation is interrelated with each other and directly affected by the substrate concentration. Kinetic models of Monod (or Michaelis–Menten) and modified Andrew highlight this relationship [51]. Limiting step was included in Monod model while inhibition effect of substrate concentration was only described by modified Andrew model. That is the reason why modified Andrew model is satisfactory at high concentrations of substrate. These models are not only used in methane production but also in hydrogen production by AD. Identification of digestion kinetics in AD is important for designing of digesters, understanding effect of process parameters and selecting optimum parameters for biogas production with a high yield [52].
4.1 Effect of Substrate Concentration
Monod (Eq. 5) and modified Andrew (Eq. 6) models were used to describe the effect of substrate concentration on methane production rate. Modified Gompertz equation (Eq. 1) was used to calculate methane production rate (R).
The methane production rate is R in mmol/L/d, the methane production rate constant is Rmax in mmol/L/d, the substrate concentration is S in g/L, the saturation constant is Ks in g/L, and the inhibition constant is Ki in g/L. The fitting parameters (Rmax, Ks and Ki) can be calculated by nonlinear regression method. In Monod model, Ks represents the affinity of the microorganisms to substrate.
Chen et al. [53] applied Monod model for AD of sucrose, food waste and non-fat dry milk (NFDM) with digested sludge and found high correlation coefficients as 0.858, 0.976 and 0.980, respectively. The values Ks of sucrose, food waste and NFDM were given as 1.4, 8.7 and 6.6 g COD L−1, respectively. Since the affinity of the microorganisms to substrate depends on carbohydrate content, the substrate with high carbohydrate content gave a lower Ks value. Madenoğlu et al. [7] fitted both Monod and modified Andrew models for AD of water lettuce with waste sludge and found good fit with high correlation coefficient for both models as 0.996. Chuang et al. [13] also applied these both models to AD of water hyacinth and obtained high correlation coefficient for both models as 0.998. High methane production rate constant (Rmax), saturation constant (Ks) and low inhibition constant (Ki) are favored for anaerobic digestion. Comparing these two studies, Madenoğlu et al. [7] reached higher Rmax (47.8 mmol/L/d > 37.3 mmol/L/d) and Ks (234.5 g/L > 24 g/L) but lower Ki (14,650 g/L < 973,087.5 g/L) values.
4.2 Effect of Temperature
The effect of digestion temperature on production of methane was defined by Ratkowsky equation [13]. The effect of temperature on methane production potential and rate was given by Eqs. 7 and 8, respectively. Modified Gompertz equation (Eq. 1) can be used to calculate methane production potential (P) and rate (R) as follows:
The methane production potential is P in mL, the methane production rate is R in mmol/L/d, A1 (mL0.5/°C), A2 (mL0.5/°C), B1 (mL/°Cd0.5) and B2 (mL/°Cd0.5) are all parameters in Ratkowsky model. The fitting parameters (A, B, Tmin and Tmax) can be calculated by nonlinear regression method. Minimum and maximum temperatures, Tmin (°C) and Tmax (°C), required for AD process can be determined from curve, and maximum point of the curve represents the optimum operating temperature, Topt (°C) (Fig. 7).
Optimum operating temperatures for AD of water lettuce with waste sludge was determined as almost 45 °C for P and R while minimum and maximum temperatures were 8.8 and 74.8 °C, respectively [7]. Fermentative hydrogen production from digested sludge was performed, and optimum temperatures were found by Ratkowsky model as 39.3 and 34.2 °C for P and R, respectively [54]. Optimum digestion temperature ranges of water hyacinth with pig manure were found as 47.8–57.5 °C and 50.0–62.5 °C for hydrogen and methane productions, respectively [13]. Selection of optimum temperature ranges was based on the values found in Eqs. 7 and 8.
5 Conclusions
Biogas production from agricultural wastes, residues and especially aquatic plants by AD provides a solution for waste minimization and sustainable alternative to fossil fuels. Effective parameters in AD process are substrate to inoculum ratio, temperature regime, C/N ratio, pH, VFAs and ammonia contents. Optimum C/N depends on digestion temperature. VFA and TAN contents affect pH of medium and activity of microorganisms. Biogas production with high yield and high methane content can be accomplished by selecting optimum conditions with application of kinetic analysis. Optimum parameters depend on each feed employed and should be specified by choosing suitable kinetic models. Effect of substrate concentration and temperature on kinetic models should be examined for identification of inhibition effect of high substrate concentration, minimum–maximum temperature range and optimum temperature for biogas production.
References
Liu X, Zhang S, Bae J (2017) The nexus of renewable energy-agriculture-environment in BRICS. Appl Energy 204:489–496
IRENA (2014) REmap 2030 global bioenergy supply and demand projections. Available via Google. https://www.worldenergy.org/wp-content/uploads/2016/10/World-Energy-Resources-Full-report-2016.10.03.pdf. Accessed 05 May 2019
Mishima D, Kuniki M, Sei K et al (2008) Ethanol production from candidate energy crops: Water hyacinth (Eichhornia crassipes) and water lettuce (Pistia stratiotes L.). Bioresour Technol 99:2495–2500
Wilkie A, Evans J (2010) Aquatic plants: an opportunity feedstock in the age of bioenergy. Biofuels 1:311–321
Meegoda JN, Li B, Patel K et al (2018) A review of the processes, parameters, and optimization of anaerobic digestion. Int J Environ Res Public Health 15:E2224
Wanwu LX, Khalid H, Zhu Z (2018) Methane production through anaerobic digestion: participation and digestion characteristics of cellulose, hemicellulose and lignin. Appl Energy 226:1219–1228
Güngören Madenoğlu T, Jalilnejad Falizi N, Kabay N et al (2019) Kinetic analysis of methane production from anaerobic digestion of water lettuce (Pistia stratiotes L.) with waste sludge. J Chem Technol Biotechnol 94:1893–1903
O’Sullivan C, Rounsefell B, Grinham A et al (2010) Anaerobic digestion of harvested aquatic weeds: water hyacinth (Eichhornia crassipes), cabomba (Cabomba Caroliniana) and salvinia (Salvinia molesta). Ecol Eng 36:1459–1468
Mallik MK, Singh UK, Ahmad N (1990) Batch digester studies on biogas production from Cannabis sativa, water hyacinth and crop wastes mixed with dung and poultry litter. Biol Waste 31:315–319
Vaidyanathan S, Kavadia KM, Shroff KC et al (1985) Biogas production in batch and semi-continuous digesters using water hyacinth. Biotechnol Bioeng 27:905–908
Nipaney PC, Panholzer MB (1987) Influence of temperature on biogas production from Pistia stratiotes. Biol Waste 19:267–274
Chunlan M, Yongzhong F, Xiaojiao W et al (2015) Review on research achievements of biogas from anaerobic digestion. Renew Sust Energ Rev 45:540–555
Chuang YS, Lay CH, Sen B et al (2011) Biohydrogen and biomethane from water hyacinth (Eichhornia crassipes) fermentation: effects of substrate concentration and incubation temperature. Int J Hydrogen Energ 36:14195–14203
Córdoba V, Fernández M, Santalla E (2018) The effect of substrate/inoculum ratio on the kinetics of methane production in swine wastewater anaerobic digestion. Environ Sci Pollut Res 25(22):21308–21317
Serez H (2016) Wastewater treatment using energy plants at ITOB industrial zone and biogas production by anaerobic fermentation of the energy plants. M.Sc. thesis, Ege University
Kainthola J, Kalamdhad AS, Goud VV (2019) Enhanced methane production from anaerobic co-digestion of rice straw and Hydrilla verticillata and its kinetic analysis. Biomass Bioenerg 125:8–16
Yang D, Deng LW, Zheng D et al (2015) Separation of swine wastewater into solid fraction, concentrated slurry and dilute liquid and its influence on biogas production. Fuel 144:237–243
González-Fernández C, García-Encina PA (2009) Impact of substrate to inoculum ratio in anaerobic digestion of swine slurry. Biomass Bioenerg 33:1065–1069
Fagbohungbe MO, Herbert BMJ, Li H et al (2015) The effect of substrate to inoculum ratios on the anaerobic digestion of human faecal material. Environ Technol Innov 3:121–129
Bowen EJ, Dolfing J, Davenport RJ et al (2014) Low-temperature limitation of bioreactor sludge in anaerobic treatment of domestic waste-water. Water Sci Technol 69:1004–1013
Wei RR, Cheng GW, Luo JJ et al (2010) Biogas and bio-energy production from anaerobic digestion of piggery manure at different temperatures. J Agric Mech Res 4:170–174
Kaparaju P, Angelidaki I (2008) Effect of temperature and active biogas process on passive separation of digested manure. Bioresour Technol 99:1345–1352
Lee M, Hidaka T, Tsuno H (2009) Two-phased hyperthermophilic anaerobic co-digestion of waste activated sludge with kitchen garbage. J Biosci Bioeng 108:408–413
Wang X, Lu X, Li F et al (2014) Effects of temperature and carbon-nitrogen (C/N) ratio on the performance of anaerobic co-digestion of dairy manure, chicken manure and rice straw: focusing on ammonia inhibition. PLoS ONE 9:e97265
Kabay N (2016) Optimization studies at MBR treatment system of ITOB Organized Industrial Zone and Biogas Production by Cultivating Energy Plants in Wastewater-SAN-TEZ Project Supported by Ministry of Science, Industry and Technology of Turkish Republic (Project No: 0330.STZ.2013-2)
Zhang T, Liu L, Song Z et al (2013) Biogas production by co-digestion of goat manure with three crop residues. PLoSOne 8:e66845
Punal A, Trevisan M, Rozzi A et al (2000) Influence of C:N ratio on the start-up of up-flow anaerobic filter reactors. Water Res 34:2614–2619
Yen HW, Brune DE (2007) Anaerobic co-digestion of algal sludge and wastepaper to produce methane. Bioresour Technol 98:130–134
Wang X, Yang G, Feng Y et al (2012) Optimizing feeding composition and carbon–nitrogen ratios for improved methane yield during anaerobic co-digestion of dairy, chicken manure and wheat straw. Bioresour Technol 120:78–83
Zhong W, Chi L, Luo Y et al (2013) Enhanced methane production from Taihu Lake blue algae by anaerobic co-digestion with corn straw in continuous feed digesters. Bioresour Technol 134:264–270
Zhang C, Xiao G, Peng L et al (2013) The anaerobic co-digestion of food waste and cattle manure. Bioresour Technol 129:170–176
Buyukkamaci N, Filibeli A (2004) Volatile fatty acid formation in an anaerobic hybrid reactor. Process Biochem 39:1491–1494
Cysneiros D, Banks CJ, Heaven S et al (2012) The effect of pH control and ‘hydraulic flush’ on hydrolysis and volatile fatty acids (VFA) production and profile in anaerobic leach bed reactors digesting a high solids content substrate. Bioresour Technol 123:263–271
Pham TN, Nam WJ, Jeon YJ et al (2012) Volatile fatty acids production from marine macroalgae by anaerobic fermentation. Bioresour Technol 124:500–503
Wang Q, Peng L, Su H (2013) The effect of a buffer function on the semi-continuous anaerobic digestion. Bioresour Technol 139:43–49
Banks CJ, Humphreys PN (1998) The anaerobic treatment of a lingo-cellulosic substrate offering little natural pH buffering capacity. Water Sci Technol 38:29–35
Yokoyama H, Moriya N, Ohmori H et al (2007) Community analysis of hydrogen-producing extreme thermophilic anaerobic microflora enriched from cow manure with five substrates. Appl Microbiol Biotechnol 77:213–222
Kim J, Park C, Kim TH et al (2003) Effects of various pretreatments for enhanced anaerobic digestion with waste activated sludge. J Biosci Bioeng 95:271–275
Lee DH, Behera SK, Kim JW et al (2009) Methane production potential of leachate generated from Korean food waste recycling facilities: a lab-scale study. Waste Manage 29:876–882
Sung S, Liu T (2003) Ammonia inhibition on thermophilic anaerobic digestion. Chemosphere 53:43–52
Syaichurrozi I, Budiyono B, Sumardiono S et al (2013) Predicting kinetic model of biogas production and biodegradability organic materials: biogas production from vinasse at variation of COD/N ratio. Bioresour Technol 149:390–397
Markou G, Georgakakis D (2011) Cultivation of filamentous cyanobacteria (blue-green algae) in agro-industrial wastes and wastewaters: a review. Appl Energy 88:3389–3401
Hansen KH, Angelidaki I, Ahring BK (1998) Anaerobic digestion of swine manure: inhibition by ammonia. Water Res 32:5–12
Karlsson A, Ejlertsson J (2012) Addition of HCl as a means to improve biogas production from protein-rich food industry waste. Biochem Eng J 61:43–48
Callander IJ, Barford JP (1983) Precipitation, chelation, and the availability of metals as nutrients in anaerobic digestion. II. Applications. Biotechnol Bioeng 25:1959–1972
Zhen G, Lu X, Kobayashi T et al (2015) Mesophilic anaerobic co-digestion of waste activated sludge and Egeria densa: performance assessment and kinetic analysis. Appl Energy 148:78–86
Kafle GK, Kim SH, Sung KI (2012) Ensiling of fish industry waste for biogas production: a lab scale evaluation of biochemical methane potential (BMP) and kinetics. Bioresour Technol 127:326–336
Zhang W, Wei Q, Wu S et al (2014) Batch anaerobic co-digestion of pig manure with dewatered sewage sludge under mesophilic conditions. Appl Energy 128:175–183
Budiyono B, Syaichurrozi I, Sumardiono S et al (2014) Kinetic model of biogas yield production from vinasse at various initial pH: comparison between modified Gompertz model and first order kinetic model. Res J Appl Sci Eng Technol 7:2798–2805
Syaichurrozi I (2018) Biogas production from co-digestion Salvinia molesta and rice straw and kinetics. Renew Energ 115:76–86
Wang JL, Wan W (2009) Kinetic models for fermentative hydrogen production: a review. Int J Hydrogen Energy 34:3313–3323
Fernandez-Rodriguez MJ, Rincon B, Fermoso FG et al (2014) Assessment of two-phase olive mill solid waste and microalgae co-digestion to improve methane production and process kinetics. Bioresour Technol 157:263–269
Chen WH, Chen SY, Khanal SK (2006) Kinetic study of biological hydrogen production by anaerobic fermentation. Int J Hydrogen Energy 31(15):2170–2178
Wang JL, Wan W (2008) Effect of temperature on fermentative hydrogen production by mixed cultures. Int J Hydrogen Energy 33:5392–5397
Acknowledgements
The authors acknowledge the Scientific and Technological Research Council of Turkey (TÜBITAK, Project No: 114Y500) for the financial support. We also thank The Ministry of Science, Industry and Technology of Turkish Republic supporting our preliminary tests through the grant so-called SAN-TEZ (Project No: 0330.STZ.2013-2). We are grateful to IZSU Çiğli Advanced Biological Wastewater Treatment Plant, Izmir for giving us waste sludge for biogas production. We thank to Mr. G. Serin, M.Sc. students M. C. Akbas and B. Kaletas for assistance in laboratory studies. The authors acknowledge publisher “John Wiley and Sons” for permission of reuse of full article Güngören Madenoğlu et al. [7].
Author information
Authors and Affiliations
Corresponding authors
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this paper
Cite this paper
Güngören Madenoğlu, T. et al. (2020). Anaerobic Digestion of Aquatic Plants for Biogas Production. In: Dincer, I., Colpan, C., Ezan, M. (eds) Environmentally-Benign Energy Solutions. Green Energy and Technology. Springer, Cham. https://doi.org/10.1007/978-3-030-20637-6_12
Download citation
DOI: https://doi.org/10.1007/978-3-030-20637-6_12
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-20636-9
Online ISBN: 978-3-030-20637-6
eBook Packages: EnergyEnergy (R0)