Abstract
Type I collagen is the predominant protein in the body and the extracellular matrix, where it gives rise to the vast diversity of tissue form and function. Within the extracellular matrix, this natural polymer exists as the fibrillar scaffolding that not only dictates tissue-specific structure and mechanical properties but also interacts with cells and other biomolecules to orchestrate complex processes associated with tissue development, homeostasis, and repair. For this reason, the hierarchical self-assembly of collagen molecules and their inherent biochemical and biophysical signaling capacity have been a long-standing subject of study across multiple disciplines, including structural biochemistry, biomechanics, biomaterials and tissue engineering, computational modeling, and medicine. This review works to capture some of the major discoveries and innovative technologies related to the supramolecular assembly of collagen in vivo and in vitro, with a focus on motivating their integration and application for advanced tissue fabrication and regenerative medicine therapies.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Tissue Engineering and Regenerative Medicine: The Goal and Challenge
The fields of tissue engineering and regenerative medicine, which operate at the interface of engineering and life sciences, have evolved over the last three decades with the goal of restoring damaged or dysfunctional tissues and organs through the development of biological substitutes and/or the promotion of tissue regeneration. Miniaturized in vitro human tissue systems are also highly sought after as an alternative to animals for cosmetic and chemical toxicity testing, high-throughput/high-content drug screening, and basic research. One foundational element of such efforts has been development of biomaterials that recreate the extracellular matrix (ECM) component of tissues. The ECM constitutes non-living material produced and secreted by cells within which they are distributed and organized. It represents a composite material, largely composed of an insoluble collagen-fibril scaffold surrounded by an interstitial fluid phase, giving tissues both poroelastic and viscoelastic properties [84]. More specifically, applied deformation to the composite will intrinsically lead to fluid flow that homogenizes scaffold pore pressure. At the same time, the composite will undergo viscoelastic deformation, exhibiting both viscous (liquid) and elastic (solid) characteristics [137]. ECM is found in all tissues and organs, providing not only the essential physical structure that organizes and supports cellular constituents but also crucial biochemical and biomechanical signaling required for tissue morphogenesis, homeostasis, and remodeling. In fact, a dynamic and reciprocal dialogue exists between cells and their surrounding ECM, such that multi-scale tissue architecture and function are integrated [216]. As such, the ability to recapitulate this natural scaffold and dynamic cell-ECM interactions has been a focused effort of tissue engineering and regenerative medicine even prior to the formal definition of these fields.
When tissue engineering emerged as a new field in the early 1990s, emphasis was placed on the use of synthetic polymers for development of porous scaffolds to mimic the structural features of ECM [115]. Synthetic materials received preference over natural polymers, such as collagen, largely owing to advantages associated with cost, batch-to-batch reproducibility, mechanical stability, as well as amenability to customization, processing, and scale-up manufacturing. Furthermore, at the time, medical devices containing candidate synthetic materials had already received FDA-approval, documenting their biocompatibility and paving the way for translation into the clinic. To date, extensive effort has been invested in the design and manufacturing of synthetic biomaterials that are biocompatible (non-toxic to cells) and possess the structural and mechanical properties of a target tissue. Another fundamental design criteria was that the biomaterial should be biodegradable, allowing host cells to progressively deposit site-appropriate replacement tissue over time [64, 107, 201]. However, in recent times, concerns have been raised regarding the immune-mediated foreign-body responses elicited by synthetic materials [46, 98] as well as their lack of biological signaling capacity [71, 88]. As a result, design criteria for next-generation biomaterials are changing, moving away from merely providing bulk structure and mechanical properties to strategies that guide biological processes underlying tissue regeneration [3, 71, 128].
Despite this initial focus on synthetics, others targeted the use of natural materials, including intact ECMs prepared from various tissues and their component molecules (e.g., collagen, fibrin, glycosaminoglycans). Here, the goal was to capitalize on the biological signaling capacity inherent to these molecules and their assemblies for purposes of inducing site-appropriate tissue regeneration. Interestingly, evaluation of the present-day tissue engineering and regenerative medicine market, shows that biologically-derived materials (e.g., decellularized tissues) and natural polymers, specifically type I collagen, account for the majority of translated technologies [8]. Within this context, this chapter focuses on type I collagen and its use for tissue engineering and regenerative medicine applications. We start by providing a historical overview of milestone discoveries related to collagen biochemistry and collagen-based biomaterials, highlighting their impact on research and medicine. The next section describes what is known regarding the biosynthesis and hierarchical self-assembly of type I collagen as it occurs within the body. This is followed by a brief description of collagen biomechanics and the more recent discovery of collagen’s participation in mechanobiology signaling, which collectively have contributed new and important design criteria for cell-instructive biomaterials. We then rigorously define and compare various collagen preparations, lending support to the notion that “all collagens or collagen-containing materials are not alike.” We then hone in on collagen advancements and applications that support next-generation, multi-scale design and custom fabrication of collagen scaffolds and tissues. Finally, we conclude with a look to the future, where this natural polymer interfaces with other tissue engineering and regeneration advancements, including stem cells (adult, induced pluripotent), computational modeling, and advanced manufacturing, to address today’s challenges and unmet clinical needs.
2 Collagen Biomaterials: The History
Scientific inquiry and applications of collagen as a tool and in medicine date back millennia. Figure 1 provides a timeline, outlining some of the major milestones in the development and application of collagen biomaterials. The word collagen is Greek, from the roots “κόλλα” (glue) and “γέν” (to make), so called because the first application of denatured collagen (gelatin) was as an adhesive for wood furnishings [63]. The first medical application of collagen as an implantable biomaterial was likely “catgut” suture, which was documented as early as 150 A.D. by Galen of Pergamon [41, 124]. Despite the moniker, these collagenous threads were typically formed from decellularized sheep intestine, not cats. Although catgut sutures were used for centuries, it wasn’t until the late 19th century that their production was perfected, with the development of chromic acid-based sterilization procedures by Lister and MacEwen [67, 121]. Catgut persisted into modern use, though largely supplanted by resorbable synthetic products due to their ease of manufacturing and sterilization. Despite the common usage of collagen over this early time period, it’s unique structure as a semiflexible, triple helical rod was not determined until the 1950s. Ramachandran and others used fiber diffraction analysis and model building, together with early amino acid composition and sequence data, to elucidate that the three component polypeptide chains, each in an extended left-handed polyproline II-helix conformation, were supercoiled in a right-handed manner about a common axis [166].
Timeline of key developments in the history of collagen biomaterials. a Assembly and reinforcement of glutaraldehyde-treated aortic valve xenograft onto supports (reprinted with permission from Zudhi et al. [226]). b Man-made bioprosthetic valves prepared from glutaraldehyde-treated bovine pericardial tissue (reprinted with permission from Society for Cardiothoracic Surgery in Great Britain and Ireland). c Freeze-dried collagen-glycosaminoglycan sponge. d Processing and sterilization of catgut sutures using the Kuhn procedure (reprinted with permission from Dietz et al. [56]). e Living-skin equivalent prepared from fibroblast-contracted collagen matrix (reprinted with permission from Bell et al. [15]). f Vascular graft fashioned from decellularized small intestine submucosa (reprinted with permission from Badylak et al. [10])
In the mid-1960s, another historical milestone was reached for collagen biomaterials—the use of biological tissue valves derived from porcine or bovine sources. The very first xenograft (porcine) aortic valve replacement in a human patient was performed in 1965 by Carpentier and his team [18]. It was later discovered that stent reinforcements and treatment of these valves with exogenous glutaraldehyde crosslinking reduced their antigenicity and degradation, dramatically improving clinical success rates [127, 226]. The first clinical use of an “engineered” or man-made heart valve followed in 1971, when Marian Ion Ionescu introduced the novel concept of constructing heart valves by attaching glutaraldehyde-treated bovine pericardium to a support frame [90]. This application of a replenishable collagen tissue source for valve design and manufacturing has contributed significantly to the evolution of the heart valve industry. Today, innovative, non-invasive trans-catheter approaches involving stented pericardial tissue are paving the way for expanded valve applications and patient populations, including children [187].
More widespread use of collagen for tissue-engineered medical products came with the isolation and decellularization of porcine small intestine submucosa (SIS), developed at Purdue University in the late 1980s [10]. Here, the design strategy was to remove all cellular components while maintaining the complex molecular composition, architecture, mechanical properties, and biological activity inherent to the naturally-occurring ECM. With a focus on inducing tissue regeneration, SIS became one of the first major tissue engineering industry success stories [122, 123], with Cook Biotech continuing to expand its portfolio of wound management and surgical reconstruction products based on this technology. Today, a number of decellularized tissue products populate the market, including those derived from multiple animal tissue sources (porcine and bovine small intestine, dermis, and urinary bladder) as well as human tissue sources (dermis and placenta). It is notable that AlloDerm, produced by LifeCell, was the first decellularized human dermal tissue on the market, receiving initial FDA approval in 1992 for treatment of burns [204].
As an alternative to these top-down approaches to tissue design, others have applied bottom-up strategies, focused on applications of purified collagen in both insoluble fibrillar and soluble, fibril-forming (self-assembling) formats. Improvements in biotechnology and development of scalable extraction procedures, such as those developed by Miller and Rhodes [134], facilitated large-scale production of high-purity collagens, paving the way for their use in tissue engineering and medicine. One of the first and most successful products created from insoluble fibrillar collagen was the “collagen-glycosaminoglycan membrane,” which was initially developed by Yannas and Burke for management of skin wounds [51, 219, 220]. These scaffolds were created by freeze drying a viscous slurry of purified bovine hide particulate and chondroitin 6-sulfate from shark cartilage followed by chemical crosslinking. Design criteria including pore size, mechanical properties, and degradation (resorption) rate were modulated, with the goal of retarding wound contraction while carefully controlling host cell infiltration and tissue deposition. This technology was acquired by Integra, which successfully entered the burn market with the first dermal regeneration template in 1995. Integra’s tissue-engineered products have become a significant commercial success with many applications, including burns, diabetic ulcers, and dental wounds. One might argue this is, in large part, owing to the design control afforded by their fabrication process.
Insoluble fibrillar collagen also served as the starting material for injectable soft tissue fillers products that reached popularity for cosmetic applications in the late twentieth century [188]. More specifically, Zyderm and its chemically crosslinked counterpart Zyplast consisted of insoluble bovine dermal collagen dispersed in phosphate-buffered saline, which contained lidocaine as a local anesthetic. Because these injectable collagens required multiple injections and chemical crosslinking to enhance their stability in vivo, they are no longer on the market and have been superseded by hyaluronic acid products [99]. Lyophilized collagen sponges, again which comprise insoluble fibrillar collagen, have also been used as drug or growth factor carriers. One particular example of a mainstay collagen-based drug delivery device is InFuse bone graft, which received approval in the early 2000s. This product involves the application of recombinant human bone morphogenetic protein-2 (rhBMP-2) to a lyophilized collagen sponge prior to implantation into bone defects [33, 65].
Some of the first descriptions of in vitro collagen self-assembly, also referred to as fibrillogenesis or polymerization, came in 1952 by Gross and Schmitt as well as Jackson and Fessler in 1955 [73, 92]. Collagen self-assembly refers to the spontaneous and precise multi-scale aggregation of collagen molecules to form longitudinal staggered arrays, giving rise to insoluble fibrous networks with a characteristic banding pattern. Additional details regarding this process as it occurs in vivo and in vitro can be found in Sects. 3 and 5, respectively.
Although some earlier studies identified the ability of cells to interface with collagen, it was Bell and co-workers, in 1979, who reported that human dermal fibroblasts encapsulated within a reconstituted collagen matrix reorganized the fibrous scaffold into a “dermal equivalent” following culture in vitro [13]. This landmark discovery, which came at the infancy of tissue engineering, eventually gave rise to Apligraf, the first “living” dermal-epidermal skin product [15]. Apligraf was produced by culturing human keratinocytes on the surface of the contracted collagen-fibroblast dermal layer. It received initial FDA approval in 1998 and remains on the market to date with indications for venous leg and diabetic foot ulcers that are not responding to conventional therapy.
Although self-assembling collagens have received considerable attention for development of 3D in vitro tissue systems, tissue-engineered constructs, and drug delivery vehicles, translation into medically useful products has been limited to date. There have been and continue to be numerous commercial products consisting of acid-soluble collagen in lyophilized or solution format for research or cell culture applications. These formulations represent single collagen molecules (monomeric collagen) extracted and purified from various tissue sources; however, little focus is given to self-assembly as a functional and standardizable collagen property [112]. As a result, significant product-to-product and lot-to-lot variation exists in the time required for collagen self-assembly (polymerization kinetics) as well as the physical properties (microstructure and mechanical properties) of self-assembled construct [5, 112]. Other persistent challenges of monomeric collagens include long polymerization times (>30 min), low mechanical integrity of formed constructs, and rapid degradation following culture in vitro and/or implantation in vivo [97].
Increased attention on self-assembling collagens came in the late 1990s and early 2000s, with the emergence of recombinant collagens, collagen mimetic peptides, and oligomeric collagen. Advancements in recombinant technology and peptide synthesis facilitated the pursuit of recombinant human collagen (rhCOL) and synthetic collagen-mimetic peptides (CMPs) as potential alternative collagen sources [4]. Today, rhCOL has been produced in plant, insect, yeast, and bacterial systems which co-express the necessary enzymes to create stable collagen triple helices; however, only a subset of these can self-assemble into fibrils [4, 177]. The first report of tissue-derived oligomeric collagen for tissue engineering applications came in 2010 [47, 112]. Unlike monomeric collagens, oligomers represented aggregates of collagen molecules (e.g., trimers) that retained their natural intermolecular crosslinks [12]. Published work shows that oligomers overcome many of the limitations of conventional monomeric formulations, with rapid polymerization, dramatically improved mechanical integrity, and resistance to proteolytic degradation both in vitro and in vivo (see Sect. 5 for specific details).
Collagen has a storied history as the preeminent biomaterial of the body and medicine. The current landscape has led to a variety of collagen formats and formulations, which are routinely categorized as crosslinked tissues, decellularized ECMs (dECMs), insoluble fibrillar collagens, and self-assembling collagens. There exists great promise and potential at the interface of self-assembling collagens, bioinspired multi-scale tissue design, and scalable manufacturing processes for advanced tissue design and fabrication. Additionally, unraveling the mechanisms by which this natural polymer guides fundamental cell behaviors through biochemical and biophysical signaling will continue to inspire approaches to promote tissue regeneration.
3 Hierarchical Design of Collagen In Vivo
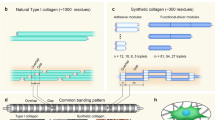
Understanding the unique hierarchical organization of type I collagen and its associated physical properties, interactions with other biomolecules, and metabolism (turnover) is fundamental to its use in the fabrication of next-generation biomaterials and tissue-engineered medical products. As the most prevalent protein, collagen is widely distributed throughout the body, where it is found in load-bearing tissues (e.g., skin, bone, tendon, cartilage, and blood vessels), organs (e.g., bladder, stomach, and intestine), and other connective tissues (e.g., pericardium, fat, and placenta). Collagen molecules are produced by cells and deposited within the extracellular space, where they self-assemble in a multi-scale fashion to give rise to the fibrillar scaffold of the tissue ECM. As shown in Fig. 2, this supramolecular assembly involves several aggregation steps: first from single polypeptide chains to a stable triple helical molecule, then to microfibrils, fibrils, and fibers, and finally to macro-scale tissues. Although its primary sequence is identical across tissues, post-translational modifications and formation of intermolecular crosslinks contribute to diversification of collagen building blocks, ECM collagen-fibril networks, and therefore tissue-specific form and function [61, 109].
Multi-scale synthesis and assembly of collagen as occurs in vivo. (A) Collagen genes are transcribed from DNA into RNA. (B) Translation of component polypeptide alpha chains by ribosomes and translocation into the rough endoplasmic reticulum. (C) Hydroxylation of alpha chains by lysyl hydroxylases. (D) Folding of trimeric procollagen molecule. (E) Transfer of procollagen to Golgi for additional post-translational modification and packaging for exocytosis. (F) Enzymatic cleavage of propeptide ends yielding tropocollagen molecules. (G) Crosslinking of tropocollagen molecules by lysyl oxidases to form oligomers. (H) Self-assembly of collagen molecules into D-banded fibrils. (I) Fibrils merge to form fibers and networks, giving rise to complex tissue architecture
3.1 Biosynthesis of Collagen
The biosynthesis and folding of collagen as it occurs within the cell has been the topic of extensive study since the 1950s. It represents a highly complex process involving various post-translation events, including hydroxylation, glycosylation, trimerization, and crosslinking, so only the fundamentals are covered here. For more comprehensive coverage, the reader is referred to recent reviews [36, 100, 183]. Type I collagen is a trimeric protein composed of two α1 and one α2 polypeptide chains. Each of these chains contains the hallmark Gly-X-Y repeat, where X and Y can be any amino acid but are usually proline and hydroxyproline, respectively. This repeating sequence results in the formation of left-handed helices by component polypeptide chains, the interaction of which results in an overall right-handed triple helical structure. The full-length, processed tropocollagen molecule, which represents the fundamental building block of tissues, is approximately 300 nm in length and about 1.5 nm in diameter. Mutations in any of the component α chains, particularly ones that cause problems with folding and crosslinking, have significant consequences on tissue architecture and function, such as the heritable disease osteogenesis imperfecta (OI), a potentially lethal brittle bone disease [141].
As outlined in Fig. 2, synthesis begins with transcription and translation of individual soluble protocollagen chains. Within the endoplasmic reticulum, protocollagen α chains are strategically hydroxylated on proline and lysine residues by specific hydroxylase enzymes. These hydroxylation reactions are important not only for protein folding, but also for downstream intra- and inter-molecular crosslinking. Processed polypeptides then fold and assemble into the procollagen molecule, which contains a central triple-helical region, flanked by non-helical telopeptide and propeptide domains on each end. Terminal propeptides, most notably the one found at the carboxy terminus, and the Gly-X-Y repeats, are critical to proper protein folding [36, 118]. This folding and trimerization process is further assisted by molecular chaperones and enzymes [144, 212]. Additional post-translational processing of procollagen molecules includes the addition of carbohydrate moieties prior to translocation to the Golgi apparatus, where modification of N-linked oligosaccharides is known to occur.
Secretion of procollagen from cells is similar to that of other extracellular proteins, where molecules passing through the Golgi are packaged into secretory vesicles prior to moving to the cell surface for release by exocytosis. After secretion, amino- and carboxy-terminal propeptides are cleaved by multiple C– and N– terminal proteinases. This conversion is critical for proper self-assembly of fibrils, since tropocollagen has a drastically decreased critical aggregation concentration [36]. In fact, defects in N-terminal proteinase ADAMTS2 (a disintegrin and a metalloproteinase with thrombospondin repeats 2) have been shown to lead to the dermatosparaxis variant of Ehlers-Danlos syndrome, which is characterized by fragile, hyperextensible soft tissues [141].
3.2 In Vivo Collagen Self-assembly and Crosslinking
In contrast to the intracellular biosynthetic pathways described above, the precise mechanisms underlying collagen fibril assembly and tissue-specific organization are less well defined. Various models have been proposed to describe the progressive assembly of micro-fibrils, fibrils, fibers, and fiber bundles; however, significant mechanistic gaps that lack corroborating experimental evidence remain. There is, however, strong support suggesting that molecular aggregation begins within secretory vesicles, with the rest of the assembly process occurring exterior to the cell [36]. An important element of collagen assembly and stabilization is the formation of crosslinks, catalyzed by members of the lysyl oxidase (LOX) family. It is here where divergent theories exist, with lysyl oxidase often portrayed as a “welding” mechanism for already assembled collagen fibers. However, the isolation and properties of soluble collagen oligomers, representing stable crosslinked collagen molecules (e.g., trimers), together with what appears as a strategic tissue-specific distribution of crosslink chemistries (Fig. 3) challenges this notion [61]. Furthermore, it has been documented experimentally, that LOX is unable to penetrate the fibril surface, despite the presence of crosslinking throughout the fibril [48]. Based upon these findings and our experience with collagen oligomers, we are a proponent of the theory where collagen assembles as prefibrillar aggregates of staggered monomers, with LOX binding and catalyzing the formation of oligomers [48]. In turn, these early oligomer precursors serve as nucleation sites and direct the progressive molecular packing and assembly that ultimately gives rise to tissue-specific ECMs.
Structures of mature, trivalent collagen intermolecular crosslinks and their associated tissue-specific distribution (based on Eyre and Wu [61])
Naturally-occurring intra- and inter-molecular collagen crosslinks, which impart mechanical strength to collagen assemblies, have been extensively studied since the 1960s. These bonds form not only between collagen molecules of the same type in homopolymeric fibrils but also between different types of collagen molecules that give rise to heteropolymeric structures [61]. The significant contribution of different crosslinks chemistries in tissue-specific structure and function can be gleaned by analyzing their distribution (Fig. 3), where crosslink number and type appear to be associated with mechanical loading and collagen turnover [60, 61]. Furthermore, these crosslink chemistries, like the primary sequence of collagen, are well conserved across species. Finally, evidence that crosslink content is a critical determinant of collagen fibril ultrastructure, ECM microstructure, and tissue mechanical properties is derived from numerous hereditable diseases as well as in vitro and in vivo studies where specific crosslinking enzymes (e.g., lysyl oxidase or lysyl hydroxylase) are selectively inhibited or genetically knocked out [80, 125, 156]. Our own in vitro work with purified soluble oligomers shows the profound effect of these crosslinked collagen building blocks on the supramolecular assembly, including assembly kinetics, fibril-fibril associations, scaffold mechanical properties and persistence (resistance to proteolytic degradation), and modulation of fundamental cellular processes, such as vessel morphogenesis and tumor cell invasion.
The bulk of research defining the basic pathways of collagen crosslinking was performed over 3 decades ago, with the identification of new crosslink chemistries and their implications continuing today. For detailed reviews, see [7, 61, 174]. In brief, major collagen crosslinks are derived from the oxidative deamination of ε-amino groups of specific lysine and hydroxylysines by LOX within non-triple helical telopeptides regions of the molecules. In turn, the resulting aldehydes react with lysine or hydroxylysine residues within the central triple-helical region of adjacent molecules to form intermediate divalent crosslinks of the aldol, hydroxyaldol, or ketoimmine varieties. Upon maturation, these divalent crosslinks convert into more stable trivalent crosslinks such as the histidine derivative histidinyl-hydroxylysino-norleucine (HHL) which is prominent in skin and hydroxylysyl pyrrole which is prevalent in bone.
3.3 Supramolecular Collagen Assemblies
The supramolecular assembly of collagen is not random but ordered, and much of the process is inherent to the post-translationally modified molecule itself. While residual propeptides have an inhibitory effect on fibril formation, telopeptides are required for proper molecular registry and alignment [184]. The generally accepted Petruska model of collagen fibril structure is a repeated lattice, where collagen molecules are present in a head-to-tail quarter staggered array generating a characteristic banding pattern with 67 nm D-spacing (Fig. 2). While this general value of D-spacing is most commonly found in the literature, there is ample evidence suggesting that a distribution of values occurs throughout tissues, and may vary with age as observed with estrogen depletion in osteoporosis [62, 205]. Additionally, atomic force microscopy, x-ray diffraction, and crystallography studies have elucidated more complex 3D structures within fibrils, including polar ends, tilted or twisted molecules, and crystalline and disordered regions [87, 102, 152]. Oligomers may serve to nucleate formation of branch sites or connections between fibrils during self-assembly, providing an additional mechanism of stabilization [209]. In turn, these fibrils and their networks merge as well as entangle with each other to form larger composite structures such as fibers, bundles or fibrils, and lamellae. Tissues contain an array of higher-order collagen network structures that might be recreated in tissue engineering to give rise to improved functional outcomes. For example, skin is well-known for its anisotropic basket weave structure that contributes to its multiaxial tensile strength [29, 116, 117]. Tendons are composed of criss-crossing fibers densely bundled in parallel, making them ideally suited for their load-bearing function [16]. Other unique structures include the orthogonal lattice of the cornea [133] and the parallel lamellae in osteons of bone [59].
The high conservation of collagen primary sequence and crosslink chemistries across species illustrate their importance as determinants of tissue form and function [61]. Collagen molecules also contain many critical functional domains (motifs) that allow adhesion of cells, binding to other ECM molecules and growth factors, and control of proteolytic degradation. In fact, one fundamental reason why synthetic polymers have failed to displace collagen as a leading tissue engineering material is because of the immense biological activity held in its multifunctional domains. A comprehensive summary and diagram of these various domains has been provided by Sweeney and co-workers [195]. Reciprocal binding interactions between collagen, growth factors, heparin, fibronectin, and other matrix components lends further stability, fluid retention, and biological signaling capacity to the ECM [113]. Collagen is recognized by several cell surface receptors including integrins, discoidin domain receptor (DDR) receptor tyrosine kinases, glycoprotein VI for platelet adhesion, and the immunomodulatory leukocyte-associated immunoglobulin-like receptor 1 (LAIR1) [119]. Of these, integrins are exquisitely mechanosensitive and a prime target for tissue engineering and regeneration design.
4 Biomechanics and Mechanobiology of Collagen
Energy storage, transmission, and dissipation are some of the key mechanical functions provided by ECMs, contributing to bulk tissue mechanical properties as well as guiding cellular behavior through mechanochemical transduction. The hierarchical structure of collagen lends itself to both experimental and computational approaches for deciphering structure-function relationships at the various size scales as well as determining how forces are transmitted between the matrix and resident cells.
4.1 Scaffold and Tissue Biomechanics
To date, measurements of mechanical properties have been made on single molecules, individual collagen fibrils, collagen fibers, as well as native and engineered collagen tissues, with atomic force microscopy (AFM) serving as an important tool at the smaller size scales [23, 173, 208]. From these efforts, the elastic modulus, which provides a measure of rigidity or stiffness, and the fracture strength for a single tropocollagen molecule, has been estimated at 6–7 and 11 GPa, respectively, supporting its role as a “rigid rod” [31]. As we move up size scales, the mechanical properties of fibrils, fibers, and tissues are somewhat less and largely a function of their nano- and micro-structural organization.
The diversity of tissue mechanical properties is a manifestation and optimization of collagen structure at its various size scales. In general, collagenous tissues exhibit a non-linear stress-strain behavior with characteristic strain-stiffening, where the network becomes more rigid with increased deformation [136]. The small strain region, also known as the toe region, corresponds to removal of crimp, both at the molecular and fibrillar levels. The following phase of mechanical testing is a linear region, where the stiffness of collagen fibrils increases considerably with extension. This region has been associated with stretching of collagen triple helices or of the crosslinks between helices, implying a side-by-side gliding of neighboring molecules. Finally, at failure, a disruption of component fibrils occurs. It is well established that initial loading curves for collagenous tissues are different from subsequent loadings, therefore conventionally tissues are “preconditioned” via application of several loading and unloading cycles prior to measurement of mechanical properties. Preconditioning assists in reducing the contributions weak bonds/entanglements and the subsequent reorientation of component fibrils [83, 194]. The stress-strain response is also sensitive to strain rate, a characteristic of viscoelastic materials. Other behaviors exhibited by tissues and other viscoelastic materials include hysteresis—time-based dependence of a material’s output on its history, stress-relaxation—decrease in stress in response to a persistent strain (deformation), and creep—tendency to deform in response to a persistent stress [138, 145, 146].
Experimental studies on intact tissues and engineered collagen-fibril constructs as well as computational simulations indicate that key determinants of tissue viscoelastic and poroelastic properties include intrinsic stiffness of the constituent fibrils, interfiber connectivity (branching, bundling), fibril/fiber dimensions (length and diameter), and interactions between insoluble collagen fibrils, other ECM components, and the surrounding interstitial fluid. For example, when fibrils are aligned in parallel to the applied force, constructs fail at lower strain but higher stress values than those with more random fibril organizations. With aligned fibrils, low levels of deformation are required for their recruitment and reorganization in the axis of extension, where they are able to bear load. By contrast, with randomly organized fibrils, higher deformation levels are required for fibril reorganization and not all fibrils are positioned to bear load due to bending or buckling. In addition, while fibril diameter and length certainly contribute to bulk mechanical properties, fibril connectivity is likely the most important determinant, with native and engineered tissues with increased fibril connectivity (branching) and stronger fibril-fibril associations (bundling) able to store increased elastic energy. Supporting this notion we find Young’s modulus values for tendon, where fibrils and fibers are parallel aligned are 43–1600 MPa, while reported values are 21–39 MPa for dermis with its basket weave construction and 0.6–3.5 MPa for artery and vein with their layered laminae [131]. The high tear-resistance of skin also has been attributed to unique features of collagen networks, namely fibril straightening and reorientation, elastic stretching and interfibrillar sliding, which redistribute stresses and do not allow tear propagation [218]. While these molecular level events associated with preyield deformation of tissues are fairly well established, those that occur from the yield point to tissue failure (post yield) are less well defined. A number of studies on the overloading of tendon have documented fibril dissociation into their fine subfibrillar components [108, 200], while others report events associated with molecular unfolding [202, 203].
4.2 Mechanobiology and Functional Tissue Engineering
Since the early days of tissue engineering, significant focus has been placed creating constructs that matched the physical characteristics of natural tissues, such as geometry and structure, or the mechanical measures, such as Young’s modulus (stiffness) or failure strength. However, with the advent of mechanobiology, it is now recognized that cells can sense and respond to mechanical cues at the molecular and micro-scale levels, just as easily as they do chemical ones. Now, tissue design has shifted from simply mimicking the physical properties (e.g., architecture, mechanical properties) of tissue to focusing on creating biomaterials that provide the correct mechanochemical signals to direct cell phenotype and function as well as tissue morphogenesis [76]. This viewpoint was formalized as “functional tissue engineering” in 2000 by a United States National Committee on Biomechanics subcommittee. Their main goal was to increase awareness of the importance of engineered tissue biomechanics by identifying criteria for mechanical requirements and encouraging tissue engineers to incorporate biomechanics into their design process [34]. This encourages a more multi-scale design approach to tissue engineering and regeneration strategies, which is more focused on guiding the cell response, including therapeutic cell populations within the construct as well as host cells. This perspective is further bolstered by advancements in the stem cell area, where plentiful numbers of multi-potential cell populations can be harvested directly from tissues (e.g., fat, bone marrow, blood) or developed from induced pluripotent stem cells, which are created by reprogramming skin or blood cells into an embryonic-like pluripotent state.
When approaching tissue fabrication, whether in the body or man-made, it is important to understand how hierarchical collagen construction contributes to not only tissue-level mechanical properties but also transmission of loads across size scales to cells and vice versa. Biophysical cues such as those originating from the ECM microstructure and mechanical properties are now recognized as major signaling sources, regulating growth and differentiation of cells [126]. It’s important to note that this transmission of biophysical signals is a two-way street, evoked by the contractile machinery of resident cells or by loads applied externally. This exchange of biophysical information is further facilitated by the physical connectivity between cells and collagen fibrils, which in large part is mediated through specific cell surface receptor proteins known as integrins. It was Donald Ingber that first depicted the dynamic force balance that exists between cells and their ECM using the popular tensegrity model, where cytoskeleton and ECM form a single, tensionally integrated structural system [89]. It is at this interface where specific design criteria and constraints for advanced tissue fabrication continue to emerge. While certainly a difficult task, sophisticated methods designed to probe biophysical and biomolecular responses of living cells within tissues continue to assist in elucidation of the mechanochemical signaling that occurs from tissue level through the ECM to the cell nucleus.
5 Collagens as a Natural Polymer for Custom Tissue Fabrication
Because type I collagen is one of most commonly used biomaterials in both research and clinical settings, there exists a wide variety of formulations, as alluded to in Sects. 1 and 2. Most collagen-based products used clinically represent processed intact tissues (e.g., dECMs) or insoluble fibrillar collagenin various formats (e.g., sponge, particulate), with only a few products prepared from self-assembling collagens. This section focuses on advancements related to self-assembling collagen formulations and their potential for multi-scale tissue design. We begin with molecular and micro-level design control, identifying how specific collagen building blocks, assembly conditions, and exogenous crosslinking affect the microstructure of engineered biomaterials and tissues. This is followed by a description of higher-level fabrication and manufacturing techniques for controlling macro-scale properties, including 3D geometry and physical properties (e.g., mechanical strength and stiffness). Special emphasis is placed on the cellular response, whether in vitro or in vivo, documenting its dependence upon multiple size scale features, extending from molecular to macroscopic.
5.1 Micro-scale Design Control
The first fundamental level of design control for collagen materials resides at the molecular level. Molecular level features largely determine the achievable range of chemical, biological, and physical attributes of resulting scaffolds and tissue constructs; however, user control at this level is often overlooked.
5.1.1 Molecular Building Blocks
The molecular make-up, structure, and self-assembly capacity of various collagen building blocks are summarized in Table 1, where extraction, processing and reconstitution techniques are known to be a source of variation. Insoluble fibrillar collagen, which is the starting material for many freeze-dried collagen and collagen-glycosaminoglycan sponge products, represents a particulate of undissociated collagen fibers isolated and purified from comminuted tissues. While this form of collagen does not self-assemble or offer molecular and fibril-microstructure control, it does aggregate to form a viscous gel or slurry when swollen in acid or hydrated in phosphate buffered saline, which has proven useful for various medical applications. As documented by Yannas and Burke and others, insoluble fibrillar collagen supports cell adhesion and offers design control of larger scale material features such as particulate content, porosity, and resorption rate [51, 219, 220].
Unlike fibrillar collagen, other collagen building blocks do have the capacity to self-assemble or form fibrils in vitro, providing control over molecular and fibril microstructure features. The ability of relatively pure collagen molecules to spontaneously form fibrils when brought to physiologic conditions (pH and ionic strength) and warmed was first reported by Gross in the 1950s and has since been the subject of extensive research [73, 74]. Collagen is routinely extracted and purified from various tissue sources (rat tail tendon or calf skin) using either dilute acid or enzymatic digestion (pepsin), yielding a solution composed predominantly of single molecules (monomers) [5]. Historically, crosslinked oligomers and insoluble molecular aggregates that accompanied monomers were viewed as undesirable by-products, especially for studies focused on collagen molecule structure and fibril assembly [143]. In fact, enzymatic digestion, secondary purification strategies, or young or lathrytic animals were routinely used to minimize or eliminate these components [39, 132, 134, 142]. Acetic acid extraction followed by salt precipitation is one of the most common approaches used to generate telocollagen, which represents full length tropocollagen molecules with telopeptide regions intact [25]. The addition of pepsin to the extraction mixture increases yield but causes cleavage of telopeptide regions, giving rise to atelocollagen [179]. More recently, a sodium citrate extraction process was applied to porcine dermis, generating a high fraction of soluble oligomeric collagen for biomaterials development [12, 112]. Oligomers represent aggregates of individual collagen molecules (e.g., trimers) that retain their natural intermolecular crosslinks.
Monomeric collagens, specifically telocollagen and atelocollagen, continue to be the most commonly used self-assembling collagens because of their relatively facile extraction and commercial availability. However, the shortcomings of these preparations are well established and commonly cited by users, including lot-to-lot variability in purity and self-assembly capacity, long polymerization times (often >30 min), lack of user control, low mechanical strength, and poor stability in vitro and in vivo [1, 5, 112]. When comparing telocollagen and atelocollagen, it has been shown that telopeptide preservation is important for the thermal stability of the collagen triple helix and the organized arrangement of collagen molecules into fibrils [82, 183]. The loss of the telopeptide regions in atelocollagen significantly hinders and slows assembly kinetics, resulting in less organized fibrils that vary in size and lack natural D-banding pattern [21, 79, 179]. This difference in molecular chemistry and fibril microstructure also affects matrix physical properties and proteolytic resistance, with atelocollagen generating weaker (i.e., Young’s modulus and yield strength) constructs that are more prone to rapid dissolution and proteolytic degradation [81, 112].
Oligomers, a more recently-discovered collagen building block, appear to play a critical role in collagen self-assembly, both in vitro and in vivo. Over the past decade, oligomer preparations have proven to be quite robust and reproducible, exhibiting rapid polymerization (<1 min at 37 °C) and generating distinct fibril microstructures compared to telocollagen and atelocollagen formulations [12, 112, 190]. Since oligomers retain intermolecular crosslinks, they exhibit a higher average molecular weight compared to monomers and a distinct protein and peptide banding pattern [12, 112]. In addition, the presence of crosslinked oligomers induces fibrillar as well as suprafibrillar assembly, resulting in networks with high fibril-fibril connectivity and branching. These higher-order assembly properties support formation of collagen scaffolds that not only retain their shape but exhibit a much broader range of physical properties and slow turnover (Fig. 4) [21, 112, 190]. In particular, collagen oligomer scaffolds demonstrate significantly increased shear, tensile, and compressive moduli compared to their monomeric counterparts (Fig. 4c–e). Since these parameters increase linearly or quadratically with oligomer concentration, differences become even greater at high concentration. The improved stability and mechanical integrity exhibited by oligomer effectively eliminates the need for exogenous crosslinking, which is routinely applied to constructs produced from monomeric collagens [77, 136].
Self-assembly of collagen and comparison of different collagen formulations. a Hierarchical, multi-scale assembly of type I collagen as occurs in vivo and in vitro with polymerizable monomer (atelocollagen and telocollagen) and oligomer formulations (reprinted with permission from Blum et al. [21]). b Representative images of oligomer, atelocollagen, and telocollagen constructs before (3.5 mg/mL) and after (24.5 mg/mL) confined compression (86% strain or 7×), demonstrating differences in shape retention and mechanical properties. Scale bars = 2 mm (reprinted with permission from Blum et al. [21]). c–e Comparison of mechanical properties, including (c) shear storage modulus (G’), unconfined compressive modulus (EC), and tensile modulus (ET), for oligomer (PSC) and commercial atelocollagen (PureCol) and telocollagen (Sigma, BD-RTC) formulations (reprinted with permission from Kreger et al. [112])
Other approaches for generating purified collagen molecule preparations, especially human, include recombinant technology or peptide synthesis. Production of collagen molecules and peptides via these techniques supports design control at the molecular level (Fig. 5a), which is especially useful for elucidating relationships between specific molecular motifs/domains and functional properties [26]. To date, researchers have successfully genetically modified mammalian, bacterial, and plant systems to produce recombinant human procollagen, from which self-assembling collagen formulations can be derived [96, 155, 176, 197]. One of the challenges associated with recombinant collagen production has been the ability to introduce and co-express various genes involved in collagen post-translational modifications, including prolyl-4-hydroxylase and lysyl hydroxylase, which are necessary for triple-helix stabilization [217]. To date, a number of groups have overcome this obstacle, successfully generating stable procollagen triple helices [111, 165, 167, 170, 189] Since procollagen molecules are unable to undergo self-assembly due to the presence of propeptide ends, endopeptidase treatment (e.g., pepsin, ficin) is routinely applied to yield fibril-forming recombinant human atelocollagens [11, 26]. At present, atelocollagen produced recombinantly yields thinner fibrils with less mechanical integrity than their tissue-derived counterparts (Fig. 5b) [211]. Researchers focused on recombinant collagen development for biomedical applications continue to work to scale their processes to support more cost-effective, large-scale production [26, 167].
Examples of the utility of recombinant collagens. a Schematic representation of recombinant bacterial collagen construct, showing examples of possible sequence manipulations (reprinted with permission from Brodsky and Ramshaw [26]). b Scanning electron microscopy of fibrils formed from purified recombinant human atelocollagen produced in tobacco plants. Scale bar = 5μm (reprinted with permission from Stein et al. [189])
Collagen mimetic peptides (CMPs) produced using synthetic chemistry methods are another means of achieving molecular-level design control [191]. Relatively short sequences, roughly 30 amino acids in length, are synthesized with the goal of forming homo- or hetero-trimeric collagen helices, which in turn self-assemble into fibrils. The majority of sequences consist of amino acid triplet repeats found within the helical region of collagen, capitalizing on electrostatic forces to drive molecular assembly. While a number of groups have created CMPs that generate fibrils, creating peptides that mimic the various levels of collagen supramolecular assembly, including staggered alignment, has been a challenge [111]. In 2009, Chaikof, Conticello and co-workers reported a CMP that, in part, formed fibrils with a regular D-spacing pattern; however, the periodicity was about 18 nm rather than the characteristic 67 nm observed in native collagen fibrils (Fig. 6a) [170]. Building off this work, O’Leary and colleagues prepared a new CMP, where arginine residues were replaced with lysine and glutamate residues were replaced with aspartate, to give the sequence (Pro-Lys-Gly)4(Pro-Hyp-Gly)4(Asp-Hyp-Gly)4 [154]. These CMPs showed improved fibril- and hydrogel-forming characteristics, giving rise to shape-retaining gels as shown in Fig. 6b. Finally, although functional domains, such as integrin binding sequences (e.g. GFOGER), can be engineered into CMPs, size constraints inherent to peptide synthesis (about 60 amino acids or less) preclude the inclusion of all functional collagen domains, thereby limiting overall biosignaling capacity [70].
Notable examples of self-assembling CMPs. a Schematic of CMP and associated Coulombic forces between cationic and anionic blocks that yield self-assembled fibrils. Transmission electron microscopy image of CMP fibril shows D-periodicity with D = 17.9 nm. Natural type I collagen has D ≈ 67 nm (reprinted with permission from Shoulders and Raines [183]). b Chemical structure of the common amino-acid triplets used to generate CMPs. Photo and scanning electron microscopy image show shape-retaining fibrillar gel (1%) formed following self-assembly of CMPs consisting of (Pro-Lys-Gly)4(Pro-Hyp-Gly)4(Asp-Hyp-Gly)4 (reprinted with permission from O’Leary et al. [154])
5.1.2 Polymerization Conditions
In addition to the various collagen building blocks described above, there are a wide variety of external means by which collagen self-assembly can be modulated to create hydrogels, matrices, and scaffolds with distinct structural and physical properties. This section summarizes various conditions, such as concentration, pH, temperature, and ionic strength, that have been used to modulate collagen assembly kinetics and outcomes. These conditions can be carefully controlled to modulate fibril density, fiber length, fibril diameter, fibril-fibril associations (e.g., branching), and pore size, all of which, in turn, determine functional physical properties, including strength, stiffness, fluid and mass transport, and proteolytic degradation. When cells are encapsulated in these self-assembled collagen matrices, they quickly adhere to the collagen fibrils, sensing and responding to differences in microstructure mechanical properties [72, 160, 171]. Through this mechanotransduction signaling, fundamental cell behavior is modulated, including cell-induced matrix contraction and remodeling, morphogenesis, proliferation, migration, and differentiation. Additionally, these microstructure features dictate how external mechanical loads are transmitted from the construct or macro-level to resident cells [14].
A landmark study by Wood and Keech in 1960 showed that increasing collagen concentration or temperature, and decreasing pH and ionic strength, accelerated the formation of individual collagen fibrils [213]. Additionally, they reported that higher temperatures, increased pH, and lower ionic strengths yielded thinner fibrils; however, no correlation was made between structural and mechanical properties of resulting fibrils was provided [213]. The effects of temperature, pH, and ionic strength on fibril assembly of telocollagen have been confirmed by many, and studies have expanded to include more detailed mechanical characterization [2, 6, 168, 175, 178]. In addition to the effects on self-assembly kinetics, increasing the temperature or pH of the reaction results in decreased pore size and fibril diameter, which have been shown to increase compressive, tensile, and shear storage moduli [2, 6, 168, 175]. The effect of ionic strength on matrix mechanics seems to be dependent on pH and temperature, thus making distinctive trends difficult to decipher [2].
Collagen concentration is another primary means by which many researchers vary matrix mechanics, since increasing collagen concentration leads to increased fibril density which increases matrix stiffness (compressive, tensile and shear) [112, 160, 175]. In attempts to independently control collagen fibril density and matrix stiffness, many have created composite systems, sometimes termed interpenetrating networks, composed of mixtures such as gelatin and collagen [17], alginate and collagen [50], polyethylene glycol and collagen [120]. Whittington and co-workers identified another approach for independently controlling fibril density and matrix stiffness which did not rely on non-collagenous agents. Here, the total content and ratio of type I collagen oligomers to monomers were used to independently vary fibril density and the extent of fibril-fibril branching, both of which are known determinants of in vivo ECM stiffness [209, 210].
Another way in which researchers have attempted to gain design control of collagen self-assembly is motivated by the fact that collagen fibrillogenesis and assembly in vivo is guided by other collagenous and non-collagenous proteins and proteoglycans of the ECM. For example, fibronectin and collagen assembly in vivo are known to be reciprocally dependent such that interruption of one decreases the other [101]. However, early experimental evidence from Brokaw and co-workers suggested that in vitro, the addition of fibronectin only affected collagen self-assembly kinetics, with no changes in the resulting microstructure [27]. On the other hand, it has also been shown that co-polymerization of fibronectin with collagen increases the tensile strength of formed matrices, supporting the notion that fibronectin affects collagen fibril organization and microstructure [68]. Type V collagen also affects in vivo collagen assembly, where it is thought to serve as a nucleation site. Loss-of-function mutations are embryonic lethal, characterized by lack of collagen fibril formation in the mesenchyme [101]. While type I collagen can self-assemble in vitro without type V collagen, Birk et al. showed that the presence of collagen V during in vitro self-assembly yielded heterotypic fibrils with decreased diameter and altered D-periodicity [19]. More recently, Piechocka et al. demonstrated that these relatively minor changes in microstructure caused drastic decreases in shear storage modulus [158]. These authors propose that this discrepancy between in vitro results and in vivo mechanisms may be due to the fact that the type V collagen used in vitro is pepsin treated and lacks the N-propeptide region which is present during in vivo ECM assembly. One final example demonstrating how other ECM components guides collagen assembly and mechanics involves glycosaminoglycans and proteoglycans, which consist of glycosaminoglycans attached to a core protein. Interestingly, polymerization of monomeric collagen in the presence of the glycosaminoglycan dermatan sulfate resulted in increased lateral fibril aggregation and decreased tensile properties compared to control matrices. On the other hand, co-polymerization of collagen and the dermatan sulfate containing proteoglycan decorin yielded highly interconnected, long, thin fibrils with increased tensile strength [66, 159, 169]. Collectively, these studies highlight the impact of other ECM components on the hierarchical organization of collagen. Discrepancies between in vivo and in vitro results, as well as between studies reveal the sensitive nature of these reactions and their dependence of specific molecular features and reaction conditions. Continued elucidation of mechanisms underlying supramolecular collagen assembly both in vivo and in vitro will continue to inspire tissue engineering and regeneration design strategies.
5.1.3 Exogenous Crosslinking
Mechanical integrity, metabolic turnover, and degradation resistance are properties afforded to in vivo collagen assemblies, in part, by the formation of natural intra- and inter-molecular crosslinks as described in Sect. 3.2. These natural crosslinks are controlled via post-translational modifications and enzymatic reactions that occur within and outside the cell, respectively, making them difficult to recreate in vitro [61]. The application of oligomeric collagen allows tissue-engineered constructs to capture some of the performance characteristics imparted by natural intermolecular crosslinks. However, for materials produced from insoluble fibrillar collagen or self-assembling monomeric collagens, the development and application of exogenous physical and chemical crosslinking is commonplace to improve mechanical properties and proteolytic resistance [78].
Glutaraldehyde is one of the most commonly employed chemical crosslinking agents [37]. It is well established that glutaraldehyde enhances collagenous material stiffness, strength, and resistance to proteolytic degradation through formation of intramolecular and intermolecular crosslinks by non-specifically reacting with lysine and hydroxylysine residues on collagen [52]. Despites its widespread use, glutaraldehyde is far from ideal as its crosslinks are transient and release of glutaraldehyde monomers over time is cytotoxic [78, 207]. Additionally, calcification of glutaraldehyde crosslinked tissues upon implantation remains a challenge [180, 181].
Dehydrothermal treatment (DHT) and ultraviolet (UV) radiation have been examined as alternatives to glutaraldehyde since the 1980s [135, 207]. DHT and UV crosslinking methods are thought to be advantageous because they do not introduce any exogenous toxic chemicals; however, these treatments can induce partial denaturation or fragmentation of collagen [78]. Carbodiimide treatment is another technique used to form amide-type bonds within collagen. Here, the only by-product is urea, which can be washed away after crosslinking [78]. The combination of 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide hydrochloride with N-hydroxysuccinimide (EDC/NHS) is the most commonly used strategy and has been applied both during and after self-assembly of monomeric collagen to enhance scaffold strength [224]. Interestingly, when EDC crosslinking was applied to scaffolds created from oligomeric collagen, it did not enhance the mechanical properties thus suggesting that the presence of the natural intermolecular crosslink outweighs the effect of these unnatural chemistries [150]. It is important to note that owing to their non-specificity and cytotoxicity, the majority of exogenous crosslinking strategies are incompatible with self-assembled collagen constructs formed in the presence of cells.
Enzymatically crosslinking collagen with LOX and transglutaminase or generating advanced glycation end-products (AGE) with sugars such as ribose are crosslinking strategies that are reported to be more compatible with cells. However, these methods only modestly improve mechanical strength and are reported to be cost-prohibitive for large/clinical scale applications [78, 192]. Despite being non-cytotoxic in the short term, non-enzymatic glycation, as occurs during ageing and pathological processes such as diabetes, has been linked to reactive oxygen species production and cellular inflammation via the receptor for advanced glycation end products (RAGE) pathways, suggesting this method of crosslinking may be suboptimal for many engineered products intended to for permanent tissue replacements [186]. Finally, genipin, a plant-derived chemical used in traditional Chinese medicine is another collagen crosslinker that has been shown to be cell-compatible at low concentrations [192, 193]. However, genipin crosslinking turns crosslinked collagenous materials blue and upon in vivo implantation induces inflammation and an associated foreign body response, although the extent is reduced compared to glutaraldehyde [40, 55].
Collectively, molecular and microscale features, including molecular composition, endogenous or exogenous crosslinking, and fibril ultrastructure and architecture, are important considerations when designing next-generation tissue engineering and regenerative medicine strategies. This is especially true when working to promote a regenerative phenotype since cells naturally interface with collagen at these levels and can readily detect and respond to changes at these size scales.
5.2 Meso- and Macro-scale Design Control
The ECM component of tissues has a complex construction with spatial gradients, anisotropies, and higher-order structures. By contrast, the majority of constructs formed by encapsulation of cells within self-assembling collagens in vitro represent isotropic random fibril networks, and are often limited in concentration or fibril density due to the solubility and phase behavior of collagen. For accurate recreation of tissues, the density and spatial organization of the collagen-fibril ECM is an important design consideration. Historically, these meso-scale features have been difficult to control, making the engineering of functional tissue replacements challenging. Recent years have seen the rise of process engineering and manufacturing techniques to address these challenges.
5.2.1 Compression
Initial efforts to convert polymerized collagen-fibril matrices into constructs with tissue-like histology and consistency relied on the remodeling properties of cells to densify or compact surrounding collagen fibrils. More specifically, collagen-fibril matrices seeded with fibroblasts and cultured up to 2 weeks yielded contracted or condensed dermal-like tissue equivalents [15]. Seeding keratinocytes on the surface of these dermal equivalents resulted in the formation of a multilayered epidermis, yielding a tissue-engineered living skin, which ultimately was produced by Organogenesis and gained FDA approval in 1998 for management of diabetic ulcers and hard-to-heal venous ulcers. Persistent drawbacks to this product include its costly manufacturing process, limited shelf-life (5–10 days) and the slight risk of disease transmission, all of which are due to the requirement of allogeneic cells to contract and further mature the ECM and finished product [182].
In 2005, Brown described a process designed to “engineer tissue-like constructs without cell participation.” This “cell-independent” approach involved polymerization of monomeric collagen in the presence or absence of cells followed by plastic compression (PC) in an unconfined format and/or capillary fluid flow into absorbent layers to reduce the interstitial fluid content [30]. Here, low-loads (50–60 g or 1.1 kPa) are applied to the top surface of a collagen matrix to achieve significant fluid reduction (approximately 85–99.8% compressive strain) through a supporting nylon mesh (Fig. 7a). The resulting densified collagen sheets, which measure 20–200 μm in thickness are still fragile, requiring spiraling and multiple compressions to facilitate handling and further mechanical testing. Tensile strength and modulus values of 0.6 ± 0.11 MPa and 1.5 ± 0.36 MPa, respectively, have been supported with 85% viability of encapsulated cells [20]. Additional compression of spiraled constructs improves mechanical integrity but reduces cellular viability [20, 42]. This technology contributed to development of the RAFT 3D Cell Culture System by TAP Biosystems (now part of Sartorius Stedim Biotech Group), which applies their patented absorber technology to monomeric rat tail collagen to create densified tissue constructs for research applications.
Densification of collagen-fibril constructs through plastic deformation achieved with unconfined compression and absorption. a Plastic compression is achieved by applying known weights to low-density collagen-fibril matrices to achieve fluid reduction through a supporting nylon mesh into an absorbent layer (based on Brown et al. [30]). b Dermo-epidermal skin substitute produced by densification of monomeric type I collagen in the presence of human dermal fibroblasts. Seven days following culture the collagen-fibroblast construct was seeded with human keratinocytes (reprinted with permission from Braziulis et al. [24])
An adaptation of this PC technology was reported by Reichmann’s group for generation of an autologous tissue-engineered skin. This work involved custom-fabrication of a large (7 × 7 cm) compression chamber, fashioned to support weights on top and absorbent filter paper on the bottom [24]. This device was used to cast square polymerized collagen matrices containing human dermal fibroblasts, which in turn were compressed to 0.5–0.6 mm thickness and then transferred to culture dishes. Following 7 days of culture and maturation in vitro, a high density of keratinocytes was applied and cultured for an additional 7 days. To date, analyses of histological outcomes as well as gene expression of relevant dermal and epidermal markers have been conducted [24, 151]; however, mechanical properties testing has yet to be reported. This tissue-engineered autologous dermo-epidermal skin graft, referred to as denovoSkin (Fig. 7b), has obtained orphan drug designation as a treatment for burns by Swissmedic, European Medicine Agency, and the FDA. Reports indicate that this product can be safely and conveniently handled by surgeons, and matures into high quality skin in animal models as well as recently performed clinical studies [151].
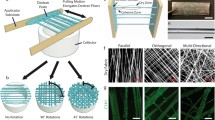
Expanded efforts on this front, include work by Voytik-Harbin and collaborators where scalable plastic compression processes have been applied to type I oligomeric collagen, providing increased versatility in product design and geometry as well as predictive meso-scale control [21, 149]. As mentioned previously, type I oligomeric collagen exhibits not only fibrillar but also suprafibrillar assembly, yielding highly interconnected collagen-fibril scaffolds with substantially improved proteolytic resistance and mechanical integrity compared to standard monomeric collagens. In this work, plastic compression was applied in a confined, rather than an unconfined, format to increase fibril density via controlled fluid removal (Fig. 8a). Interestingly, this approach was not applicable to monomeric matrices due to the inability of the resultant fibril microstructure to sustain or support associated compressive and fluid shear forces [21]. This fabrication process provided control of the final solid fibril content (fibril density) of the compressed construct through modulation of starting volume and concentration of the oligomer solution together with the applied compressive strain [149]. Additionally, strain rate was used to control steepness of fibril density gradient, and placement of porous polyethylene foam and associated porous-solid boundary conditions defined high-order spatial fibril organization (e.g., alignment). Finite element analysis confirmed this process to be dependent upon the fluid flow induced during compression, with steepness of gradient formation dependent on strain rate [149]. These early findings support the notion that controlled, plastic compression together with computational models can be used for predictive design and scalable manufacture of a diverse array of precision-tuned tissue constructs. To date, this fabrication method has been applied for the development of cartilage constructs for laryngeal reconstruction [28, 225], articular cartilage constructs with continuous fibril density gradients that recapitulate the different histological zones in native cartilage (Fig. 8c) [149], acellular and cellular dermal replacements (Fig. 8b) [149], as well as an in vitro model of cardiac fibrosis [215].
Controlled confined compression for fabrication of acellular and cellular constructs with and without continuous structural gradients a Schematic depicting controlled confined compression process for densification of collagen-fibril constructs. A low-density collagen-fibril matrix is formed in a mold and then compressed at a controlled strain rate to achieve a specified strain. Fluid flow is directed through the porous boundary (adapted from Blum et al. [21]). b Densified sheet formed via controlled confined compression of type I oligomeric collagen. Scale bar = 2 mm (reprinted with permission by Blum et al. [21]). c Gradient densification of collagen-fibril matrices as achieved via controlled confined compression. Type I oligomer matrices were compressed with a porous platen, directing fluid flow through an upper porous boundary condition. Confocal reflection microscopy revealed a gradient in fibril density, with a high density of fibrils aligned parallel to the construct surface near the top progressing to a low-density region of randomly organized fibrils near the bottom. Scale bar = 100 μm (reprinted with permission from Novak et al. [149]). Encapsulated cells responded to their local microenvironment as a result of densification, as detected 1 week with confocal microscopy (green = F-actin; blue = nucleus). Cells in the high-density region developed a spindle shape and were oriented parallel to the fibrils, while cells in the low-density regions displayed a more rounded morphology. Scale bar = 10μm. Such gradients in collagen microstructure and cell morphology/phenotype are reminiscent of the gradient layers found in articular cartilage
5.2.2 Electrospinning
Electrospinning is a fiber-forming process that applies a large electric field between a polymer solution reservoir and a collection plate to form polymer fibers with nanometer-scale diameters. More specifically, when a sufficiently high voltage is applied to a liquid polymer droplet, the body of liquid becomes charged, and electrostatic repulsion counteracts the surface tension and the droplet is stretched. At a critical point a stream of liquid erupts from the surface forming a fiber. This fiber elongates and thins, and the solvent evaporates, as it moves towards the grounded collector where it is deposited. Published work on the electrospinning of collagen dates back nearly two decades [85, 86, 130]. In this case, materials are designed to mimic the geometry (e.g., diameter) of collagen fibrils or fibers found in vivo within the extracellular matrix. Since that time a large number of design variables including solvents, molecular make-up of collagen, collagen concentration and viscosity, applied electric field, flow rates, collection distance, and collection strategies (plates, rotating mandrels) have been explored [35, 130]. At present, this technique has been used to generate collagen-based scaffolds of varying geometries (tubes, mats) and architectures (randomly oriented, aligned, high porosity, low porosity) for various tissue applications including bone [163], nerve [164], blood vessel [22, 196], and skin [162, 172]. For more details, the readers are encouraged to see DeFrates et al. [54]. A major limitation associated with present-day electrospinning is its requirement for volatile solvents (e.g., fluoroalcohols), which denature the native structure of collagen yielding gelatin. Furthermore, resulting materials lack collagen fiber ultrastructure (axial periodicity and D-banding) and therefore display altered biological and physical properties compared to native collagen assemblies. To address these issues, electrospinning of collagen is routinely performed in the presence of other synthetic [polycaprolactone, poly(lactic-co-glycolic acid)] or natural (elastin) polymers or in conjunction with physical or chemical crosslinking (e.g., N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride and NHS, and glutaraldehyde) to improve mechanical integrity.
To overcome the persistent challenges associated with the electrospinning of collagen, alternative manufacturing processes are continued to be developed for creation of collagen fibers. For example, Polk and co-workers [161] described volatilization of collagen using a high-speed compressed air jet such as that produced by a common airbrush. This process which they termed pneumatospinning was used to form non-woven meshes of randomly organized and aligned fibrils, approximately 200 nm in diameter. Interestingly, pneumatospun and electrospun fibers formed from acetic acid showed similarity in size, strength, and cytocompatibility. However, like electrospun fibers, pneumatospun fibers were not stable in aqueous media in absence of chemical crosslinking.
5.2.3 3D Bioprinting
In the early 1990s, 3D printing emerged as an additive manufacturing technique for production of 3D objects based on computer-assisted design [49]. With advantages of mass production and fine tuning of spatial-dimensional properties, this process has been adapted for purposes of developing functional tissues and organ constructs. Such constructs are being fashioned for use as in vitro model systems for basic research or drug screening [110, 147], delivery of pharmaceutical agents (genes, drugs) or cells [93, 185], and tissue-engineered medical products for tissue replacement or reconstruction [153]. Bioprinting involves sequential layer-by-layer deposition of biomaterials in the presence and absence of specific cell populations in predetermined spatial-dimensional patterns with millimeter or nanometer scale resolution. In this way, porosity, permeability and mechanical properties, and cell-cell and cell-ECM associations within the construct may be controlled. Of the various 3D printing technologies, direct ink writing and inkjet printing have received the most widespread use for bioprinting applications. For direct ink writing, high viscosity hydrogels in the presence or absence of cells are extruded to obtain 3D structures with or without a carrier. By contrast, inkjet bioprinting applies low viscosity solutions or suspensions as droplets.
A critical component of bioprinting are the “bioinks”, which typically are polymeric materials that are used to deposit cells and/or serve as the extracellular scaffold. Ideally, bioink materials need to exhibit (i) good printability, (ii) biocompatibility for maintaining cell viability without eliciting immune reactions, (iii) cell-friendly curability, (iv) mechanical stability with shape retainability, (v) predictive biodegradability including mechanism (hydrolysis or proteolytic degradation) and kinetics, and (vi) predictable material-cell interface with ability to promote fundamental cellular behaviors (adhesion and remodeling, migration, proliferation, differentiation) and processes (morphogenesis) [95]. While bioink materials used to date satisfy a subset of these design requirements, bioink development and characterization remains a high-priority activity, together with optimization of the bioink-bioprinter interface [139].
To date a number of synthetic, nature-derived, and natural biomaterials have been used for a myriad of bioprinting activities and have been the subject of recent comprehensive reviews [53, 95, 139]. Here, we focus on the application of various collagen-based formulations, especially those that exhibit self-assembly. As stated previously, the use of collagen is advantageous because of its inherent biocompatibility and biosignaling capacity. However, a persistent limitation with conventional monomeric collagens has been their poor mechanical properties and long polymerization times, contributing to poor shape retaining properties and printing resolution. To circumvent these problems, collagen and its denatured counterpart gelatin have been modified by introducing new functional groups or used in conjunction with other biomaterials.
Gelatin methacryloyl (GelMA) represents one of the most popular bioinks, offering fast polymerization, good biocompatibility as well as tunable mechanical properties (for recent reviews see [223]). GelMA is a chemically-modified version of gelatin that exhibits photopolymerization (gelation) upon exposure to light irradiation in the presence of photoinitiators. Gelatin is distinct from native collagen in that it represents a mixture of collagen peptides and single-stranded polypeptide chains produced by collagen hydrolysis and denaturation. Although gelatin retains arginine-glycine-aspartic acid (RGD) sequences that promote cell attachment as well as target sequences for matrix metalloproteases, it does not maintain collagen’s native triple helical structure and therefore inherent fibril-forming capacity. Introduction of methacryloyl groups confers to gelatin the capacity to be photocrosslinked with the assistance of photoinitiators and exposure to light. Many physical parameters of GelMA hydrogels, such as mechanical properties, pore sizes, degradation rates, and swell ratio can be readily tailored by changing the degree of methacryloyl substitution, GelMA prepolymer concentration, initiator concentration, and light exposure time [43, 148].
More recently, tissue- and organ-derived dECMs that retain collagen’s fibril-forming capacity have been gaining increased use as bioinks for 3D bioprinting applications [106]. While traditionally intact dECMs derived from various allogeneic and xenogeneic tissue sources have been used clinically (surgical mesh, wound management), recent studies have focused on adapting these materials for tissue-specific 3D bioprinting applications [94, 157]. Creation of dECM bioinks involves application of various decellularization methods to remove cells from tissues and organs. The resulting dECMs are then exposed to acid-treatment in the presence or absence of pepsin, yielding a complex mixture of self-assembling collagen as well as other ECM components (glycosaminoglycans, proteoglycans, growth factors, fibronectin). It is noteworthy that that dECM bioink composition can vary widely since it depends largely on the decellularization and solubilization protocols employed. Furthermore, removal of cells and their associated components is essential so to avoid elicitation of immune-mediated responses when used in vivo [9]. At present, most dECM bioinks form soft hydrogels, therefore the use of exogenous crosslinking in commonplace. To date dECM bioinks have been derived from various tissues and organs including heart, liver, fat, cartilage, skeletal muscle, skin, and vascular tissue. For a more comprehensive review of dECM bioink use in 3D bioprinting see [45].
5.2.4 Extrusion, Electrochemical Processes, and Magnetic Fields
One of the first applications of flow to induce preferential alignment of collagen fibrils was provided by Elsdale and Bard in 1972 [58]. This method, referred to as the “draining method”, involved pipetting polymerizable collagen into a dish and placing the dish at an incline to achieve gravity-induced flow and aligned bundles of fibrillar collagen. These findings have been extended to more scalable, industrial processes such as extrusion. Extrusion is formally defined as the act or process of shaping a material by forcing it through a die. In the late 1980s and early 1990s, Kato and co-workers described a scalable process for collagen fiber production that involved extrusion of acid-swollen dispersions of insoluble fibrillar collagen through polyethylene tubing into a phosphate-based buffer reservoir to induce gelation (Fig. 9a) [103, 105]. The resultant fibers were then transferred to isopropyl alcohol followed by air drying under tension. Chemical and physical crosslinking resulted in fibers with ultimate tensile strength values that were comparable to those for rat tail tendon fibers (24–66 MPa). When fashioned and implanted as tendon and ligaments, implants showed inflammatory reactions, degradation profiles, and neotissue formation that varied with type of crosslinking [57, 69, 104, 206]. More recently, a similar approach was applied to soluble telocollagen and atelocollagen formulations, yielding “strings” of flow-aligned collagen fibrils (Fig. 9b) [91, 114]. Although this process could be applied to yield a wide variety of geometries and patterns, including sheets, meshes, and tubes, functional mechanical properties for tissue engineering applications have yet to be achieved.
Extrusion processes for production of collagen threads and aligned collagen. a Collagen fiber formation from acidic dispersions of insoluble fibrillar collagen (based on Kato et al. [103]). Collagen dispersions are extruded into a sodium phosphate based fiber formation buffer. Resulting threads are sequentially dehydrated in isopropyl alcohol, washed in water, and air dried prior to spooling. b Wet spinning of collagen fibers (adapted from Caves et al. [38]). An acidic solution of collagen monomers is aggregated into a gel-like fiber by mixing with a buffered PEG solution. The extruded fiber is dehydrated in ethanol prior to collection on a spool. Resulting threads are exposed to phosphate buffer to induce formation of D-banded collagen fibrils, rinsed, and then air dried prior to spooling. c In-flow collagen fibril formation and alignment (based on Brookes et al. [28]). Neutral solutions of oligomer collagen in the presence or absence of cells are extruded through a die, resulting in alignment of self-assembled collagen fibrils and resident cells. d Electrochemical aggregation and alignment of collagen. Soluble collagen molecules are placed within an electrochemical cell consisting of two parallel electrode wires. Isoelectric focusing occurs with application of DC voltage resulting in molecular accumulation into compacted threads. Formation of D-banded collagen fibrils occurs when resulting collagen thread is placed in phosphate buffered saline. e Magnetic alignment of collagen as occurs when neutralized collagen solutions are placed within high strength magnetic fields. Mechanical torque on molecules results in alignment orthogonal to the applied field
Taking advantage of the rapid polymerization and improved mechanical integrity of type I oligomeric collagen, Brookes and co-workers described methods of extruding self-assembling oligomer solutions in the presence of muscle progenitor cells for creating engineered skeletal muscle for laryngeal reconstruction (Fig. 9c). This process yielded mechanically stable constructs with aligned cells surrounded by highly aligned collagen fibrils [28]. Resident muscle progenitor cells readily fused, forming multi-nucleated myotubes upon culture in vitro. When used for laryngeal muscle reconstruction in a rat hemi-laryngectomy model, these tissue-engineered muscle constructs integrated with the surrounding host tissue in absence of a significant inflammatory response. Furthermore, functional muscle regeneration and maturation occurred over a 3 month period marked by progressive increases in striations, innervation, and functional motor unit activity [28]. Other recently reported methods for achieving aligned cellularized collagen constructs include the multi-step process referred to as gel aspiration-ejection [129]. Here, isotropic, densified collagen constructs are aspirated into a syringe and then ejected through capillary tubes, 0.3–0.9 mm in diameter. Initial in vitro studies showed that constructs formed by this process containing mesenchymal stem cells showed accelerated osteoblast and neuronal differentiation when cultured in the appropriate differentiation medium formulations.
Methods other than extrusion have been used to create anisotropic collagen constructs. Specifically, collagen monomer solutions have been exposed to electrochemical processes, where isoelectric focusing is used to drive aggregation of collagen molecules (Fig. 9d). While this process does not produce the staggered arrangement of molecules observed in native collagen fibrils, D-spacing can be achieved with exposure to phosphate buffered saline [44]. Follow-up processing of these electrochemically aligned collagen threads by sequential treatment with genipin crosslinking, peracetic acid/ethanol exposure, and heparinization in EDC/NHS yields heparinized sutures that can be used for growth factors such as platelet derived growth factor [221, 222]. On the other hand, application of large magnetic fields to polymerizable collagens, which was first described in the 1980s [140, 199], orients collagen molecules and associated fibril-forming counterparts perpendicular to the applied field (Fig. 9e). This outcome is largely attributed to diamagnetism of the peptide bond [214]. Since that time, magnetic fields have been applied to generate anisotropic constructs for mechanistic studies of cell contact guidance [75] as well as generating tissue-engineered constructs cartilage [150], cornea [32, 198], and peripheral nerve replacement [32, 198] and regeneration. Notable findings from this work, was that orthogonal patterns of collagen fibrils, similar to those found in native cornea stroma, could be generated by polymerization-rotation-polymerization of sequential layers of collagen in the presence of a magnetic field [198]. Resident cells, whether grown in culture or infiltrating from surrounding tissue following implantation, align by contact guidance along the long axis of the fibrils. Interestingly, magnetically aligned constructs produced with atelocollagen and telocollagen showed improved handling and mechanical properties upon exogenous crosslinking [44]. However, magnetically aligned constructs produced with oligomeric collagen showed no significant change in mechanical integrity upon chemical crosslinking [150]. This observation was attributed to the fact that compared to monomers, oligomer produces more mechanically stable fibril microstructures with increased connectivity between fibrils (interfibril branching).
6 Conclusion and Future Directions
To date, tissue engineering and regenerative medicine approaches involving collagen-based scaffolds, cells and combinations thereof have led to a number of new, FDA-approved therapies. However, many would say that the field, in general, has still not lived up to promises and enthusiasm generated early on. The ability to replace or regenerate damaged or diseased tissues and organs remains one of the great challenges and unmet needs facing medicine and society. Continued translation and commercialization of next generation therapies must forge new pathways that interface biomolecules and cells, scalable manufacturing processes, and regulatory policy. Careful consideration of the scientific, regulatory, and business hurdles is paramount in streamlining translation and maximizing clinical impact. Integration of computational modeling for predictable, customizable design will facilitate precision medicine, applications, which works to account for the inevitable variability in health status and intrinsic healing/remodeling potential between patients. Translating these biomedical advances to medical successes will help fulfill the long-standing promise of tissue engineering and regenerative medicine to patients, clinicians, investors, and society.
References
Abraham, L.C., Zuena, E., Perez-Ramirez, B., Kaplan, D.L.: Guide to collagen characterization for biomaterial studies. J. Biomed. Mater. Res. B Appl. Biomater. 87(1), 264–285 (2008)
Achilli, M., Mantovani, D.: Tailoring mechanical properties of collagen-based scaffolds for vascular tissue engineering: the effects of pH, temperature and ionic strength on gelation. Polymers 2(4), 664–680 (2010)
Aguado, B.A., Grim, J.C., Rosales, A.M., Watson-Capps, J.J., Anseth, K.S.: Engineering precision biomaterials for personalized medicine. Sci. Transl. Med. 10(424), eaam8645 (2018)
An, B., Kaplan, D.L., Brodsky, B.: Engineered recombinant bacterial collagen as an alternative collagen-based biomaterial for tissue engineering. Front. Chem. 2, 40 (2014)
Antoine, E.E., Vlachos, P.P., Rylander, M.N.: Review of collagen I hydrogels for bioengineered tissue microenvironments: characterization of mechanics, structure, and transport. Tissue Eng. Part B Rev. 20(6), 683–696 (2014)
Antoine, E.E., Vlachos, P.P., Rylander, M.N.: Tunable collagen I hydrogels for engineered physiological tissue micro-environments. PLoS ONE 10(3), e0122500 (2015)
Avery, N.C., Bailey, A.J.: Restraining cross-links responsible for the mechanical properties of collagen fibers: natural and artificial. In: Fratzl, P. (ed.) Collagen Structure and Mechanics, pp. 81–110. Springer, New York (2008)
BCCResearch: Tissue Engineering and Regeneration: Technologies and Global Markets (2016)
Badylak, S.F., Gilbert, T.W.: Immune response to biologic scaffold materials. Semin. Immunol. 20(2), 109–116 (2008)
Badylak, S.F., Lantz, G.C., Coffey, A., Geddes, L.A.: Small intestinal submucosa as a large diameter vascular graft in the dog. J. Surg. Res. 47(1), 74–80 (1989)
Baez, J., Olsen, D., Polarek, J.W.: Recombinant microbial systems for the production of human collagen and gelatin. Appl. Microbiol. Biotechnol. 69(3), 245–252 (2005)
Bailey, J.L., Critser, P.J., Whittington, C., Kuske, J.L., Yoder, M.C., Voytik-Harbin, S.L.: Collagen oligomers modulate physical and biological properties of three-dimensional self-assembled matrices. Biopolymers 95(2), 77–93 (2011)
Bell, E., Ivarsson, B., Merrill, C.: Production of a tissue-like structure by contraction of collagen lattices by human fibroblasts of different proliferative potential in vitro. Proc. Natl. Acad. Sci. 76(3), 1274–1278 (1979)
Bell, B.J., Nauman, E., Voytik-Harbin, S.L.: Multiscale strain analysis of tissue equivalents using a custom-designed biaxial testing device. Biophys. J. 102(6), 1303–1312 (2012)
Bell, E., Sher, S., Hull, B., Merrill, C., Rosen, S., Chamson, A., Asselineau, D., Dubertret, L., Coulomb, B., Lapiere, C., Nusgens, B., Neveux, Y.: The reconstitution of living skin. J. Invest. Dermatol. 81(1 Suppl), 2s–10s (1983)
Benjamin, M., Kaiser, E., Milz, S.: Structure-function relationships in tendons: a review. J. Anat. 212(3), 211–228 (2008)
Berger, A.J., Linsmeier, K.M., Kreeger, P.K., Masters, K.S.: Decoupling the effects of stiffness and fiber density on cellular behaviors via an interpenetrating network of gelatin-methacrylate and collagen. Biomaterials 141, 125–135 (2017)
Binet, J.P., Carpentier, A., Langlois, J., Duran, C., Colvez, P.: Implantation of heterogenic valves in the treatment of aortic cardiopathies. C. R. Acad. Sci. Hebd. Seances Acad. Sci. D 261(25), 5733–5734 (1965)
Birk, D.E., Fitch, J.M., Babiarz, J.P., Doane, K.J., Linsenmayer, T.F.: Collagen fibrillogenesis in vitro: interaction of types I and V collagen regulates fibril diameter. J. Cell Sci. 95(4), 649–657 (1990)
Bitar, M., Salih, V., Brown, R.A., Nazhat, S.N.: Effect of multiple unconfined compression on cellular dense collagen scaffolds for bone tissue engineering. J. Mater. Sci. Mater. Med. 18(2), 237–244 (2007)
Blum, K.M., Novak, T., Watkins, L., Neu, C.P., Wallace, J.M., Bart, Z.R., Voytik-Harbin, S.L.: Acellular and cellular high-density, collagen-fibril constructs with suprafibrillar organization. Biomater. Sci. 4(4), 711–723 (2016)
Boland, E.D., Matthews, J.A., Pawlowski, K.J., Simpson, D.G., Wnek, G.E., Bowlin, G.L.: Electrospinning collagen and elastin: preliminary vascular tissue engineering. Front. Biosci. 9, 1422–1432 (2004)
Bozec, L., Horton, M.: Topography and mechanical properties of single molecules of type I collagen using atomic force microscopy. Biophys. J. 88(6), 4223–4231 (2005)
Braziulis, E., Diezi, M., Biedermann, T., Pontiggia, L., Schmucki, M., Hartmann-Fritsch, F., Luginbuhl, J., Schiestl, C., Meuli, M., Reichmann, E.: Modified plastic compression of collagen hydrogels provides an ideal matrix for clinically applicable skin substitutes. Tissue Eng. Part C Methods 18(6), 464–474 (2012)
Brennan, M., Davison, P.F.: Role of aldehydes in collagen fibrillogenesis in vitro. Biopolymers 19(10), 1861–1873 (1980)
Brodsky, B., Ramshaw, J.A.: Bioengineered collagens. Fibrous Proteins: Structures and Mechanisms, pp. 601–629. Springer, Berlin (2017)
Brokaw, J.L., Doillon, C.J., Hahn, R.A., Birk, D.E., Berg, R.A., Silver, F.H.: Turbidimetric and morphological studies of type I collagen fibre self assembly in vitro and the influence of fibronectin. Int. J. Biol. Macromol. 7(3), 135–140 (1985)
Brookes, S., Voytik-Harbin, S., Zhang, H., Halum, S.: Three-dimensional tissue-engineered skeletal muscle for laryngeal reconstruction. Laryngoscope 128(3), 603–609 (2018)
Brown, I.A.: Scanning electron microscopy of human dermal fibrous tissue. J. Anat. 113(Pt 2), 159–168 (1972)
Brown, R.A., Wiseman, M., Chuo, C.B., Cheema, U., Nazhat, S.N.: Ultrarapid engineering of biomimetic materials and tissues: fabrication of nano- and microstructures by plastic compression. Adv. Funct. Mater. 15(11), 1762–1770 (2005)
Buehler, M.J.: Nature designs tough collagen: explaining the nanostructure of collagen fibrils. Proc. Natl. Acad. Sci. 103(33), 12285–12290 (2006)
Builles, N., Janin-Manificat, H., Malbouyres, M., Justin, V., Rovere, M.R., Pellegrini, G., Torbet, J., Hulmes, D.J., Burillon, C., Damour, O., Ruggiero, F.: Use of magnetically oriented orthogonal collagen scaffolds for hemi-corneal reconstruction and regeneration. Biomaterials 31(32), 8313–8322 (2010)
Burkus, J.K., Transfeldt, E.E., Kitchel, S.H., Watkins, R.G., Balderston, R.A.: Clinical and radiographic outcomes of anterior lumbar interbody fusion using recombinant human bone morphogenetic protein-2. Spine (Phila Pa 1976) 27(21), 2396–2408 (2002)
Butler, D.L., Goldstein, S.A., Guilak, F.: Functional tissue engineering: the role of biomechanics. J. Biomech. Eng. 122(6), 570–575 (2000)
Buttafoco, L., Kolkman, N.G., Engbers-Buijtenhuijs, P., Poot, A.A., Dijkstra, P.J., Vermes, I., Feijen, J.: Electrospinning of collagen and elastin for tissue engineering applications. Biomaterials 27(5), 724–734 (2006)
Canty, E.G., Kadler, K.E.: Procollagen trafficking, processing and fibrillogenesis. J. Cell Sci. 118(Pt 7), 1341–1353 (2005)
Carpentier, A., Lemaigre, G., Robert, L., Carpentier, S., Dubost, C.: Biological factors affecting long-term results of valvular heterografts. J. Thorac. Cardiovasc. Surg. 58(4), 467–483 (1969)
Caves, J.M., Kumar, V.A., Wen, J., Cui, W., Martinez, A., Apkarian, R., Coats, J.E., Berland, K., Chaikof, E.L.: Fibrillogenesis in continuously spun synthetic collagen fiber. J. Biomed. Mater. Res. B Appl. Biomater. 93(1), 7175–7182 (2009)
Chandrakasan, G., Torchia, D.A., Piez, K.A.: Preparation of intact monomeric collagen from rat tail tendon and skin and the structure of the nonhelical ends in solution. J. Biol. Chem. 251(19), 6062–6067 (1976)
Chang, Y., Tsai, C.C., Liang, H.C., Sung, H.W.: In vivo evaluation of cellular and acellular bovine pericardia fixed with a naturally occurring crosslinking agent (genipin). Biomaterials 23(12), 2447–2457 (2002)
Chattopadhyay, S., Raines, R.T.: Review collagen-based biomaterials for wound healing. Biopolymers 101(8), 821–833 (2014)
Cheema, U., Brown, R.A.: Rapid fabrication of living tissue models by collagen plastic compression: understanding three-dimensional cell matrix repair in vitro. Adv. Wound Care 2(4), 176–184 (2013)
Chen, Y.C., Lin, R.Z., Qi, H., Yang, Y., Bae, H., Melero-Martin, J.M., Khademhosseini, A.: Functional human vascular network generated in photocrosslinkable gelatin methacrylate hydrogels. Adv. Funct. Mater. 22(10), 2027–2039 (2012)
Cheng, X., Gurkan, U.A., Dehen, C.J., Tate, M.P., Hillhouse, H.W., Simpson, G.J., Akkus, O.: An electrochemical fabrication process for the assembly of anisotropically oriented collagen bundles. Biomaterials 29(22), 3278–3288 (2008)
Choudhury, D., Tun, H.W., Wang, T., Naing, M.W.: Organ-derived decellularized extracellular matrix: a game changer for bioink manufacturing? Trends Biotechnol. 36(8), 787–805 (2018)
Chung, L., Maestas Jr., D.R., Housseau, F., Elisseeff, J.H.: Key players in the immune response to biomaterial scaffolds for regenerative medicine. Adv. Drug Del. Rev. 114, 184–192 (2017)
Critser, P.J., Kreger, S.T., Voytik-Harbin, S.L., Yoder, M.C.: Collagen matrix physical properties modulate endothelial colony forming cell-derived vessels in vivo. Microvasc. Res. 80(1), 23–30 (2010)
Cronlund, A.L., Smith, B.D., Kagan, H.M.: Binding of lysyl oxidase to fibrils of type I collagen. Connect. Tissue Res. 14(2), 109–119 (1985)
Crump, S.S.: Apparatus and method for creating three-dimensional objects. 5,121,329, 9 June 1992
da Cunha, C.B., Klumpers, D.D., Li, W.A., Koshy, S.T., Weaver, J.C., Chaudhuri, O., Granja, P.L., Mooney, D.J.: Influence of the stiffness of three-dimensional alginate/collagen-I interpenetrating networks on fibroblast biology. Biomaterials 35(32), 8927–8936 (2014)
Dagalakis, N., Flink, J., Stasikelis, P., Burke, J.F., Yannas, I.V.: Design of an artificial skin. Part III. Control of pore structure. J. Biomed. Mater. Res. 14(4), 511–528 (1980)
Damink, L.O., Dijkstra, P.J., Van Luyn, M., Van Wachem, P., Nieuwenhuis, P., Feijen, J.: Glutaraldehyde as a crosslinking agent for collagen-based biomaterials. J. Mater. Sci. Mater. Med. 6(8), 460–472 (1995)
Datta, P., Barui, A., Wu, Y., Ozbolat, V., Moncal, K.K., Ozbolat, I.T.: Essential steps in bioprinting: from pre- to post-bioprinting. Biotechnol. Adv. 36(5), 1481–1504 (2018)
DeFrates, K.G., Moore, R., Borgesi, J., Lin, G., Mulderig, T., Beachley, V., Hu, X.: Protein-based fiber materials in medicine: a review. Nanomaterials (Basel) 8(7), 457 (2018)
Delgado, L.M., Bayon, Y., Pandit, A., Zeugolis, D.I.: To cross-link or not to cross-link? Cross-linking associated foreign body response of collagen-based devices. Tissue Eng. Part B Rev. 21(3), 298–313 (2015)
Dietz U.A., Kehl F., Hamelmann W., Weißer C.: On the 100th anniversary of sterile catgut Kuhn: franz Kuhn (1866–1929) and the epistemology of catgut sterilization. World J. Surg. 31(12), 2275–2283 (2007)
Dunn, M.G., Tria, A.J., Kato, Y.P., Bechler, J.R., Ochner, R.S., Zawadsky, J.P., Silver, F.H.: Anterior cruciate ligament reconstruction using a composite collagenous prosthesis. Am. J. Sports Med. 20(5), 507–515 (1992)
Elsdale, T., Bard, J.: Collagen substrata for studies on cell behavior. J. Cell Biol. 54(3), 626–637 (1972)
Evans, F.G., Vincentelli, R.: Relation of collagen fiber orientation to some mechanical properties of human cortical bone. J. Biomech. 2(1), 63–71 (1969)
Eyre, D.R., Paz, M.A., Gallop, P.M.: Cross-linking in collagen and elastin. Annu. Rev. Biochem. 53, 717–748 (1984)
Eyre, D.R., Wu, J.J.: Collagen cross-links. In: Collagen, vol. 247, pp. 207–229 (2005)
Fang, M., Goldstein, E.L., Turner, A.S., Les, C.M., Orr, B.G., Fisher, G.J., Welch, K.B., Rothman, E.D., Holl, M.M.B.: Type I collagen D-spacing in fibril bundles of dermis, tendon, and bone: bridging between nano- and micro-level tissue hierarchy. A.C.S. Nano 6(11), 9503–9514 (2012)
Fratzl, P.: Collagen: structure and mechanics, an introduction. Collagen, pp. 1–13. Springer, Berlin (2008)
Freed, L.E., Vunjak-Novakovic, G., Biron, R.J., Eagles, D.B., Lesnoy, D.C., Barlow, S.K., Langer, R.: Biodegradable polymer scaffolds for tissue engineering. Biotechnology (N. Y.) 12(7), 689–693 (1994)
Friedlaender, G.E., Perry, C.R., Cole, J.D., Cook, S.D., Cierny, G., Muschler, G.F., Zych, G.A., Calhoun, J.H., LaForte, A.J., Yin, S.: Osteogenic protein-1 (bone morphogenetic protein-7) in the treatment of tibial nonunions—A prospective, randomized clinical trial comparing rhOP-1 with fresh bone autograft. J. Bone Joint Surg. Am. 83a(Pt 2), S151–S158 (2001)
Garg, A.K., Berg, R.A., Silver, F.H., Garg, H.G.: Effect of proteoglycans on type I collagen fibre formation. Biomaterials 10(6), 413–419 (1989)
Gibson, T.: Evolution of catgut ligatures: the endeavours and success of Joseph Lister and William Macewen. Br. J. Surg. 77(7), 824–825 (1990)
Gildner, C.D., Lerner, A.L., Hocking, D.C.: Fibronectin matrix polymerization increases tensile strength of model tissue. Am. J. Physiol. Heart Circ. Physiol. 287(1), H46–H53 (2004)
Goldstein, J.D., Tria, A.J., Zawadsky, J.P., Kato, Y.P., Christiansen, D., Silver, F.H.: Development of a reconstituted collagen tendon prosthesis. A preliminary implantation study. J. Bone Joint Surg. Am. 71(8), 1183–1191 (1989)
Golser, A.V., Scheibel, T.: Routes towards novel collagen-like biomaterials. Fibers 6(2), 21 (2018)
Green, J.J., Elisseeff, J.H.: Mimicking biological functionality with polymers for biomedical applications. Nature 540(7633), 386–394 (2016)
Grinnell, F.: Fibroblast biology in three-dimensional collagen matrices. Trends Cell Biol. 13(5), 264–269 (2003)
Gross, J., Highberger, J.H., Schmitt, F.O.: Collagen structures considered as states of aggregation of a kinetic unit—the tropocollagen particle. Proc. Natl. Acad. Sci. 40(8), 679 (1954)
Gross, J., Kirk, D.: The heat precipitation of collagen from neutral salt solutions: some rate-regulating factors. J. Biol. Chem. 233(2), 355–360 (1958)
Guido, S., Tranquillo, R.T.: A methodology for the systematic and quantitative study of cell contact guidance in oriented collagen gels. Correlation of fibroblast orientation and gel birefringence. J. Cell Sci. 105(Pt 2), 317–331 (1993)
Guilak, F., Butler, D.L., Goldstein, S.A., Baaijens, F.P.: Biomechanics and mechanobiology in functional tissue engineering. J. Biomech. 47(9), 1933–1940 (2014)
Hall, M.S., Alisafaei, F., Ban, E., Feng, X., Hui, C.Y., Shenoy, V.B., Wu, M.: Fibrous nonlinear elasticity enables positive mechanical feedback between cells and ECMs. Proc. Natl. Acad. Sci. 113(49), 14043–14048 (2016)
Hapach, L.A., VanderBurgh, J.A., Miller, J.P., Reinhart-King, C.A.: Manipulation of in vitro collagen matrix architecture for scaffolds of improved physiological relevance. Phys. Biol. 12(6), 061002 (2015)
Helseth, D., Veis, A.: Collagen self-assembly in vitro. Differentiating specific telopeptide-dependent interactions using selective enzyme modification and the addition of free amino telopeptide. J. Biol. Chem. 256(14), 7118–7128 (1981)
Herchenhan, A., Uhlenbrock, F., Eliasson, P., Weis, M., Eyre, D., Kadler, K.E., Magnusson, S.P., Kjaer, M.: Lysyl oxidase activity is required for ordered collagen fibrillogenesis by tendon cells. J. Biol. Chem. 290(26), 16440–16450 (2015)
Herrera-Perez, M., Voytik-Harbin, S.L., Rickus, J.L.: Extracellular matrix properties regulate the migratory response of glioblastoma stem cells in three-dimensional culture. Tissue Eng. Part A 21(19–20), 2572–2582 (2015)
Holmes, R., Kirk, S., Tronci, G., Yang, X., Wood, D.: Influence of telopeptides on the structural and physical properties of polymeric and monomeric acid-soluble type I collagen. Mater. Sci. Eng. C Mater. Biol. Appl. 77, 823–827 (2017)
Hosseini, S.M., Wilson, W., Ito, K., van Donkelaar, C.C.: How preconditioning affects the measurement of poro-viscoelastic mechanical properties in biological tissues. Biomech. Model. Mechanbiol. 13(3), 503–513 (2014)
Hu, Y.H., Suo, Z.G.: Viscoelasticity and poroelasticity in elastomeric gels. Acta Mech. Solida Sin. 25(5), 441–458 (2012)
Huang, L., Apkarian, R.P., Chaikof, E.L.: High-resolution analysis of engineered type I collagen nanofibers by electron microscopy. Scanning 23(6), 372–375 (2001)
Huang, L., Nagapudi, K., Apkarian, R.P., Chaikof, E.L.: Engineered collagen-PEO nanofibers and fabrics. J. Biomater. Sci. Polym. Ed. 12(9), 979–993 (2001)
Hulmes, D.J., Wess, T.J., Prockop, D.J., Fratzl, P.: Radial packing, order, and disorder in collagen fibrils. Biophys. J. 68(5), 1661–1670 (1995)
Ingber, D.E., Mow, V.C., Butler, D., Niklason, L., Huard, J., Mao, J., Yannas, I., Kaplan, D., Vunjak-Novakovic, G.: Tissue engineering and developmental biology: going biomimetic. Tissue Eng. 12(12), 3265–3283 (2006)
Ingber, D.E., Wang, N., Stamenovic, D.: Tensegrity, cellular biophysics, and the mechanics of living systems. Rep. Prog. Phys. 77(4), 046603 (2014)
Ionescu, M.I., Pakrashi, B.C., Holden, M.P., Mary, D.A., Wooler, G.H.: Results of aortic valve replacement with frame-supported fascia lata and pericardial grafts. J. Thorac. Cardiovasc. Surg. 64(3), 340–353 (1972)
Isobe, Y., Kosaka, T., Kuwahara, G., Mikami, H., Saku, T., Kodama, S.: Oriented collagen scaffolds for tissue engineering. Materials 5(3), 501–511 (2012)
Jackson, D.S., Fessler, J.H.: Isolation and properties of a collagen soluble in salt solution at neutral pH. Nature 176(4471), 69–70 (1955)
Jamroz, W., Szafraniec, J., Kurek, M., Jachowicz, R.: 3D printing in pharmaceutical and medical applications—Recent achievements and challenges. Pharm. Res. 35(9), 176 (2018)
Jang, J., Park, H.J., Kim, S.W., Kim, H., Park, J.Y., Na, S.J., Kim, H.J., Park, M.N., Choi, S.H., Park, S.H., Kim, S.W., Kwon, S.M., Kim, P.J., Cho, D.W.: 3D printed complex tissue construct using stem cell-laden decellularized extracellular matrix bioinks for cardiac repair. Biomaterials 112, 264–274 (2017)
Ji, S., Guvendiren, M.: Recent advances in bioink design for 3D bioprinting of tissues and organs. Front Bioeng. Biotechnol. 5, 23 (2017)
John, D.C., Watson, R., Kind, A.J., Scott, A.R., Kadler, K.E., Bulleid, N.J.: Expression of an engineered form of recombinant procollagen in mouse milk. Nat. Biotechnol. 17(4), 385–389 (1999)
Johnson, K.R., Leight, J.L., Weaver, V.M.: Demystifying the effects of a three-dimensional microenvironment in tissue morphogenesis. Methods Cell Biol. 83, 547–583 (2007)
Julier, Z., Park, A.J., Briquez, P.S., Martino, M.M.: Promoting tissue regeneration by modulating the immune system. Acta Biomater. 53, 13–28 (2017)
Kablik, J., Monheit, G.D., Yu, L., Chang, G., Gershkovich, J.: Comparative physical properties of hyaluronic acid dermal fillers. Dermatol. Surg. 35(Suppl 1), 302–312 (2009)
Kadler, K.E.: Fell muir lecture: collagen fibril formation in vitro and in vivo. Int. J. Exp. Pathol. 98(1), 4–16 (2017)
Kadler, K.E., Hill, A., Canty-Laird, E.G.: Collagen fibrillogenesis: fibronectin, integrins, and minor collagens as organizers and nucleators. Curr. Opin. Cell Biol. 20(5), 495–501 (2008)
Kadler, K.E., Holmes, D.F., Graham, H., Starborg, T.: Tip-mediated fusion involving unipolar collagen fibrils accounts for rapid fibril elongation, the occurrence of fibrillar branched networks in skin and the paucity of collagen fibril ends in vertebrates. Matrix Biol. 19(4), 359–365 (2000)
Kato, Y.P., Christiansen, D.L., Hahn, R.A., Shieh, S.J., Goldstein, J.D., Silver, F.H.: Mechanical properties of collagen fibres: a comparison of reconstituted and rat tail tendon fibres. Biomaterials 10(1), 38–42 (1989)
Kato, Y.P., Dunn, M.G., Zawadsky, J.P., Tria, A.J., Silver, F.H.: Regeneration of Achilles tendon with a collagen tendon prosthesis. Results of a one-year implantation study. J. Bone Joint Surg. Am. 73(4), 561–574 (1991)
Kato, Y.P., Silver, F.H.: Formation of continuous collagen fibres: evaluation of biocompatibility and mechanical properties. Biomaterials 11(3), 169–175 (1990)
Kim, B.S., Kim, H., Gao, G., Jang, J., Cho, D.W.: Decellularized extracellular matrix: a step towards the next generation source for bioink manufacturing. Biofabrication 9(3), 034104 (2017)
Kim, B.S., Mooney, D.J.: Development of biocompatible synthetic extracellular matrices for tissue engineering. Trends Biotechnol. 16(5), 224–230 (1998)
Knorzer, E., Folkhard, W., Geercken, W., Boschert, C., Koch, M.H.J., Hilbert, B., Krahl, H., Mosler, E., Nemetschekgansler, H., Nemetschek, T.: New aspects of the etiology of tendon-rupture—an analysis of time-resolved dynamic-mechanical measurements using synchrotron radiation. Arch. Orthop. Trauma Surg. 105(2), 113–120 (1986)
Knott, L., Bailey, A.J.: Collagen cross-links in mineralizing tissues: a review of their chemistry, function, and clinical relevance. Bone 22(3), 181–187 (1998)
Knowlton, S., Onal, S., Yu, C.H., Zhao, J.J., Tasoglu, S.: Bioprinting for cancer research. Trends Biotechnol. 33(9), 504–513 (2015)
Kotch, F.W., Raines, R.T.: Self-assembly of synthetic collagen triple helices. Proc. Natl. Acad. Sci. 103(9), 3028–3033 (2006)
Kreger, S.T., Bell, B.J., Bailey, J., Stites, E., Kuske, J., Waisner, B., Voytik-Harbin, S.L.: Polymerization and matrix physical properties as important design considerations for soluble collagen formulations. Biopolymers 93(8), 690–707 (2010)
Kubow, K.E., Vukmirovic, R., Zhe, L., Klotzsch, E., Smith, M.L., Gourdon, D., Luna, S., Vogel, V.: Mechanical forces regulate the interactions of fibronectin and collagen I in extracellular matrix. Nat. Commun. 6, 8026 (2015)
Lai, E.S., Anderson, C.M., Fuller, G.G.: Designing a tubular matrix of oriented collagen fibrils for tissue engineering. Acta Biomater. 7(6), 2448–2456 (2011)
Langer, R., Vacanti, J.P.: Tissue engineering. Science 260(5110), 920–926 (1993)
Lanir, Y., Fung, Y.C.: Two-dimensional mechanical properties of rabbit skin. I. Experimental system. J. Biomech. 7(1), 29–34 (1974)
Lanir, Y., Fung, Y.C.: Two-dimensional mechanical properties of rabbit skin. II. Experimental results. J. Biomech. 7(2), 171–182 (1974)
Lees, J.F., Tasab, M., Bulleid, N.J.: Identification of the molecular recognition sequence which determines the type-specific assembly of procollagen. EMBO J. 16(5), 908–916 (1997)
Leitinger, B., Hohenester, E.: Mammalian collagen receptors. Matrix Biol. 26(3), 146–155 (2007)
Liang, Y., Jeong, J., DeVolder, R.J., Cha, C., Wang, F., Tong, Y.W., Kong, H.: A cell-instructive hydrogel to regulate malignancy of 3D tumor spheroids with matrix rigidity. Biomaterials 32(35), 9308–9315 (2011)
Lister, J.: An address on the catgut ligature. Br. Med. J. 1(1050), 219–221 (1881)
Lysaght, M.J., Nguy, N.A., Sullivan, K.: An economic survey of the emerging tissue engineering industry. Tissue Eng. 4(3), 231–238 (1998)
Lysaght, M.J., Reyes, J.: The growth of tissue engineering. Tissue Eng. 7(5), 485–493 (2001)
Mackenzie, D.: The history of sutures. Med. Hist. 17(2), 158–168 (1973)
Maki, J.M., Sormunen, R., Lippo, S., Kaarteenaho-Wiik, R., Soininen, R., Myllyharju, J.: Lysyl oxidase is essential for normal development and function of the respiratory system and for the integrity of elastic and collagen fibers in various tissues. Am. J. Pathol. 167(4), 927–936 (2005)
Mammoto, T., Mammoto, A., Ingber, D.E.: Mechanobiology and developmental control. Annu. Rev. Cell Dev. Biol. 29, 27–61 (2013)
Manji, R.A., Lee, W., Cooper, D.K.C.: Xenograft bioprosthetic heart valves: past, present and future. Int. J. Surg. 23(Pt B), 280–284 (2015)
Mao, A.S., Mooney, D.J.: Regenerative medicine: current therapies and future directions. Proc. Natl. Acad. Sci. 112(47), 14452–14459 (2015)
Marelli, B., Ghezzi, C.E., James-Bhasin, M., Nazhat, S.N.: Fabrication of injectable, cellular, anisotropic collagen tissue equivalents with modular fibrillar densities. Biomaterials 37, 183–193 (2015)
Matthews, J.A., Wnek, G.E., Simpson, D.G., Bowlin, G.L.: Electrospinning of collagen nanofibers. Biomacromolecules 3(2), 232–238 (2002)
McKee, C.T., Last, J.A., Russell, P., Murphy, C.J.: Indentation versus tensile measurements of Young’s modulus for soft biological tissues. Tissue Eng. Part B Rev. 17(3), 155–164 (2011)
McPherson, J.M., Wallace, D.G., Sawamura, S.J., Conti, A., Condell, R.A., Wade, S., Piez, K.A.: Collagen fibrillogenesis in vitro: a characterization of fibril quality as a function of assembly conditions. Coll. Relat. Res. 5(2), 119–135 (1985)
Meek, K.M., Knupp, C.: Corneal structure and transparency. Prog. Retin. Eye Res. 49, 1–16 (2015)
Miller, E.J., Rhodes, R.K.: Preparation and characterization of the different types of collagen. Methods Enzymol. 82(Pt A), 33–64 (1982)
Ming-Che, W., Pins, G.D., Silver, F.H.: Collagen fibres with improved strength for the repair of soft tissue injuries. Biomaterials 15(7), 507–512 (1994)
Motte, S., Kaufman, L.J.: Strain stiffening in collagen I networks. Biopolymers 99(1), 35–46 (2013)
Muiznieks, L.D., Keeley, F.W.: Molecular assembly and mechanical properties of the extracellular matrix: a fibrous protein perspective. Biochim. Biophys. Acta 1832(7), 866–875 (2013)
Munster, S., Jawerth, L.M., Leslie, B.A., Weitz, J.I., Fabry, B., Weitz, D.A.: Strain history dependence of the nonlinear stress response of fibrin and collagen networks. Proc. Natl. Acad. Sci. 110(30), 12197–12202 (2013)
Murphy, S.V., Atala, A.: 3D bioprinting of tissues and organs. Nat. Biotechnol. 32(8), 773–785 (2014)
Murthy, N.S.: Liquid crystallinity in collagen solutions and magnetic orientation of collagen fibrils. Biopolymers 23(7), 1261–1267 (1984)
Myllyharju, J., Kivirikko, K.I.: Collagens and collagen-related diseases. Ann. Med. 33(1), 7–21 (2001)
Na, G.C., Butz, L.J., Bailey, D.G., Carroll, R.J.: In vitro collagen fibril assembly in glycerol solution: evidence for a helical cooperative mechanism involving microfibrils. Biochemistry 25(5), 958–966 (1986)
Na, G.C., Butz, L.J., Carroll, R.J.: Mechanism of in vitro collagen fibril assembly. Kinetic and morphological studies. J. Biol. Chem. 261(26), 12290–12299 (1986)
Nagata, K.: Hsp47: a collagen-specific molecular chaperone. Trends Biochem. Sci. 21(1), 22–26 (1996)
Nam, S., Hu, K.H., Butte, M.J., Chaudhuri, O.: Strain-enhanced stress relaxation impacts nonlinear elasticity in collagen gels. Proc. Natl. Acad. Sci. 113(20), 5492–5497 (2016)
Nam, S., Lee, J., Brownfield, D.G., Chaudhuri, O.: Viscoplasticity enables mechanical remodeling of matrix by cells. Biophys. J. 111(10), 2296–2308 (2016)
Nguyen, D.G., Pentoney Jr., S.L.: Bioprinted three dimensional human tissues for toxicology and disease modeling. Drug Discov. Today Technol. 23, 37–44 (2017)
Nichol, J.W., Koshy, S.T., Bae, H., Hwang, C.M., Yamanlar, S., Khademhosseini, A.: Cell-laden microengineered gelatin methacrylate hydrogels. Biomaterials 31(21), 5536–5544 (2010)
Novak, T., Seelbinder, B., Twitchell, C.M., van Donkelaar, C.C., Voytik-Harbin, S.L., Neu, C.P.: Mechanisms and microenvironment investigation of cellularized high density gradient collagen matrices via densification. Adv. Funct. Mater. 26(16), 2617–2628 (2016)
Novak, T., Voytik-Harbin, S.L., Neu, C.P.: Cell encapsulation in a magnetically aligned collagen-GAG copolymer microenvironment. Acta Biomater. 11, 274–282 (2015)
Oostendorp, C., Meyer, S., Sobrio, M., van Arendonk, J., Reichmann, E., Daamen, W.F., van Kuppevelt, T.H.: Evaluation of cultured human dermal- and dermo-epidermal substitutes focusing on extracellular matrix components: comparison of protein and RNA analysis. Burns 43(3), 520–530 (2017)
Orgel, J.P., Irving, T.C., Miller, A., Wess, T.J.: Microfibrillar structure of type I collagen in situ. Proc. Natl. Acad. Sci. 103(24), 9001–9005 (2006)
Ozbolat, I.T.: Bioprinting scale-up tissue and organ constructs for transplantation. Trends Biotechnol. 33(7), 395–400 (2015)
O’Leary, L.E., Fallas, J.A., Bakota, E.L., Kang, M.K., Hartgerink, J.D.: Multi-hierarchical self-assembly of a collagen mimetic peptide from triple helix to nanofibre and hydrogel. Nat. Chem. 3(10), 821–828 (2011)
Pakkanen, O., Hamalainen, E.R., Kivirikko, K.I., Myllyharju, J.: Assembly of stable human type I and III collagen molecules from hydroxylated recombinant chains in the yeast Pichia pastoris. J. Biol. Chem. 278(34), 32478–32483 (2003)
Pasquali-Ronchetti, I., Baccarani-Contri, M., Young, R.D., Vogel, A., Steinmann, B., Royce, P.M.: Ultrastructural analysis of skin and aorta from a patient with Menkes disease. Exp. Mol. Pathol. 61(1), 36–57 (1994)
Pati, F., Jang, J., Ha, D.H., Won Kim, S., Rhie, J.W., Shim, J.H., Kim, D.H., Cho, D.W.: Printing three-dimensional tissue analogues with decellularized extracellular matrix bioink. Nat. Commun. 5, 3935 (2014)
Piechocka, I.K., van Oosten, A.S., Breuls, R.G., Koenderink, G.H.: Rheology of heterotypic collagen networks. Biomacromolecules 12(7), 2797–2805 (2011)
Pins, G.D., Christiansen, D.L., Patel, R., Silver, F.H.: Self-assembly of collagen fibers. Influence of fibrillar alignment and decorin on mechanical properties. Biophys. J. 73(4), 2164–2172 (1997)
Pizzo, A.M., Kokini, K., Vaughn, L.C., Waisner, B.Z., Voytik-Harbin, S.L.: Extracellular matrix (ECM) microstructural composition regulates local cell-ECM biomechanics and fundamental fibroblast behavior: a multidimensional perspective. J. Appl. Physiol. 98(5), 1909–1921 (2005)
Polk, S., Sori, N., Thayer, N., Kemper, N., Maghdouri-White, Y., Bulysheva, A.A., Francis, M.P.: Pneumatospinning of collagen microfibers from benign solvents. Biofabrication 10(4), 045004 (2018)
Powell, H.M., Boyce, S.T.: Engineered human skin fabricated using electrospun collagen-PCL blends: morphogenesis and mechanical properties. Tissue Eng. Part A 15(8), 2177–2187 (2009)
Prabhakaran, M.P., Venugopal, J., Ramakrishna, S.: Electrospun nanostructured scaffolds for bone tissue engineering. Acta Biomater. 5(8), 2884–2893 (2009)
Prabhakaran, M.P., Venugopal, J.R., Ramakrishna, S.: Mesenchymal stem cell differentiation to neuronal cells on electrospun nanofibrous substrates for nerve tissue engineering. Biomaterials 30(28), 4996–5003 (2009)
Przybyla, D.E., Chmielewski, J.: Metal-triggered radial self-assembly of collagen peptide fibers. J. Am. Chem. Soc. 130(38), 12610–12611 (2008)
Ramachandran, G.N., Kartha, G.: Structure of collagen. Nature 174(4423), 269–270 (1954)
Ramshaw, J.A., Werkmeister, J.A., Dumsday, G.J.: Bioengineered collagens: emerging directions for biomedical materials. Bioengineered 5(4), 227–233 (2014)
Raub, C.B., Suresh, V., Krasieva, T., Lyubovitsky, J., Mih, J.D., Putnam, A.J., Tromberg, B.J., George, S.C.: Noninvasive assessment of collagen gel microstructure and mechanics using multiphoton microscopy. Biophys. J. 92(6), 2212–2222 (2007)
Reese, S.P., Underwood, C.J., Weiss, J.A.: Effects of decorin proteoglycan on fibrillogenesis, ultrastructure, and mechanics of type I collagen gels. Matrix Biol. 32(7–8), 414–423 (2013)
Rele, S., Song, Y., Apkarian, R.P., Qu, Z., Conticello, V.P., Chaikof, E.L.: D-periodic collagen-mimetic microfibers. J. Am. Chem. Soc. 129(47), 14780–14787 (2007)
Rhee, S., Grinnell, F.: Fibroblast mechanics in 3D collagen matrices. Adv. Drug Del. Rev. 59(13), 1299–1305 (2007)
Rho, K.S., Jeong, L., Lee, G., Seo, B.M., Park, Y.J., Hong, S.D., Roh, S., Cho, J.J., Park, W.H., Min, B.M.: Electrospinning of collagen nanofibers: effects on the behavior of normal human keratinocytes and early-stage wound healing. Biomaterials 27(8), 1452–1461 (2006)
van der Rijt, J.A., van der Werf, K.O., Bennink, M.L., Dijkstra, P.J., Feijen, J.: Micromechanical testing of individual collagen fibrils. Macromol. Biosci. 6(9), 697–702 (2006)
Robins, S.P.: Biochemistry and functional significance of collagen cross-linking. Biochem. Soc. Trans. 35(Pt 5), 849–852 (2007)
Roeder, B.A., Kokini, K., Sturgis, J.E., Robinson, J.P., Voytik-Harbin, S.L.: Tensile mechanical properties of three-dimensional type I collagen extracellular matrices with varied microstructure. J. Biomech. Eng. 124(2), 214–222 (2002)
Ruggiero, F., Exposito, J.Y., Bournat, P., Gruber, V., Perret, S., Comte, J., Olagnier, B., Garrone, R., Theisen, M.: Triple helix assembly and processing of human collagen produced in transgenic tobacco plants. FEBS Lett. 469(1), 132–136 (2000)
Rutschmann, C., Baumann, S., Cabalzar, J., Luther, K.B., Hennet, T.: Recombinant expression of hydroxylated human collagen in Escherichia coli. Appl. Microbiol. Biotechnol. 98(10), 4445–4455 (2014)
Sapudom, J., Rubner, S., Martin, S., Kurth, T., Riedel, S., Mierke, C.T., Pompe, T.: The phenotype of cancer cell invasion controlled by fibril diameter and pore size of 3D collagen networks. Biomaterials 52, 367–375 (2015)
Sato, K., Ebihara, T., Adachi, E., Kawashima, S., Hattori, S., Irie, S.: Possible involvement of aminotelopeptide in self-assembly and thermal stability of collagen I as revealed by its removal with proteases. J. Biol. Chem. 275(33), 25870–25875 (2000)
Schmidt, C.E., Baier, J.M.: Acellular vascular tissues: natural biomaterials for tissue repair and tissue engineering. Biomaterials 21(22), 2215–2231 (2000)
Schoen, F.J., Levy, R.J.: Calcification of tissue heart valve substitutes: progress toward understanding and prevention. Ann. Thorac. Surg. 79(3), 1072–1080 (2005)
Shahrokhi, S., Arno, A., Jeschke, M.G.: The use of dermal substitutes in burn surgery: acute phase. Wound Repair Regen. 22(1), 14–22 (2014)
Shoulders, M.D., Raines, R.T.: Collagen structure and stability. Annu. Rev. Biochem. 78, 929–958 (2009)
Silver, F.H., Freeman, J.W., Seehra, G.P.: Collagen self-assembly and the development of tendon mechanical properties. J. Biomech. 36(10), 1529–1553 (2003)
Skeldon, G., Lucendo-Villarin, B., Shu, W.: Three-dimensional bioprinting of stem-cell derived tissues for human regenerative medicine. Philos. Trans. R. Soc. Lond. B Biol. Sci. 373(1750), 20170224 (2018)
Snedeker, J.G., Gautieri, A.: The role of collagen crosslinks in ageing and diabetes—the good, the bad, and the ugly. Muscles Ligaments Tendons J. 4(3), 303–308 (2014)
Soares, J.S., Feaver, K.R., Zhang, W., Kamensky, D., Aggarwal, A., Sacks, M.S.: Biomechanical behavior of bioprosthetic heart valve heterograft tissues: characterization, simulation, and performance. Cardiovasc. Eng. Technol. 7(4), 309–351 (2016)
Stegman, S.J., Tromovitch, T.A.: Implantation of collagen for depressed scars. J. Dermatol. Surg. Oncol. 6(6), 450–453 (1980)
Stein, H., Wilensky, M., Tsafrir, Y., Rosenthal, M., Amir, R., Avraham, T., Ofir, K., Dgany, O., Yayon, A., Shoseyov, O.: Production of bioactive, post-translationally modified, heterotrimeric, human recombinant type-I collagen in transgenic tobacco. Biomacromolecules 10(9), 2640–2645 (2009)
Stephens, C.H., Orr, K.S., Acton, A.J., Tersey, S.A., Mirmira, R.G., Considine, R.V., Voytik-Harbin, S.L.: In situ type I oligomeric collagen macroencapsulation promotes islet longevity and function in vitro and in vivo. Am. J. Physiol. Endocrinol. Metab. 315(4), E650–E661 (2018)
Strauss, K., Chmielewski, J.: Advances in the design and higher-order assembly of collagen mimetic peptides for regenerative medicine. Curr. Opin. Biotechnol. 46, 34–41 (2017)
Sundararaghavan, H.G., Monteiro, G.A., Lapin, N.A., Chabal, Y.J., Miksan, J.R., Shreiber, D.I.: Genipin-induced changes in collagen gels: correlation of mechanical properties to fluorescence. J. Biomed. Mater. Res. A 87(2), 308–320 (2008)
Sung, H.W., Chang, W.H., Ma, C.Y., Lee, M.H.: Crosslinking of biological tissues using genipin and/or carbodiimide. J. Biomed. Mater. Res., Part A 64(3), 427–438 (2003)
Susilo, M.E., Paten, J.A., Sander, E.A., Nguyen, T.D., Ruberti, J.W.: Collagen network strengthening following cyclic tensile loading. Interface Focus 6(1), 20150088 (2016)
Sweeney, S.M., Orgel, J.P., Fertala, A., McAuliffe, J.D., Turner, K.R., Di Lullo, G.A., Chen, S., Antipova, O., Perumal, S., Ala-Kokko, L., Forlino, A., Cabral, W.A., Barnes, A.M., Marini, J.C., San Antonio, J.D.: Candidate cell and matrix interaction domains on the collagen fibril, the predominant protein of vertebrates. J. Biol. Chem. 283(30), 21187–21197 (2008)
Tillman, B.W., Yazdani, S.K., Lee, S.J., Geary, R.L., Atala, A., Yoo, J.J.: The in vivo stability of electrospun polycaprolactone-collagen scaffolds in vascular reconstruction. Biomaterials 30(4), 583–588 (2009)
Tomita, M., Kitajima, T., Yoshizato, K.: Formation of recombinant human procollagen I heterotrimers in a baculovirus expression system. J. Biochem. 121(6), 1061–1069 (1997)
Torbet, J., Malbouyres, M., Builles, N., Justin, V., Roulet, M., Damour, O., Oldberg, A., Ruggiero, F., Hulmes, D.J.: Orthogonal scaffold of magnetically aligned collagen lamellae for corneal stroma reconstruction. Biomaterials 28(29), 4268–4276 (2007)
Torbet, J., Ronziere, M.C.: Magnetic alignment of collagen during self-assembly. Biochem. J. 219(3), 1057–1059 (1984)
Torp, S.: Effects of age and of mechanical deformation on the ultrastructure of tendon. In: Structure of Fibrous Biopolymers, pp. 223–250 (1975)
Vacanti, J.P., Langer, R.: Tissue engineering: the design and fabrication of living replacement devices for surgical reconstruction and transplantation. Lancet 354(Suppl 1), SI32–34 (1999)
Veres, S.P., Harrison, J.M., Lee, J.M.: Repeated subrupture overload causes progression of nanoscaled discrete plasticity damage in tendon collagen fibrils. J. Orthop. Res. 31(5), 731–737 (2013)
Veres, S.P., Lee, J.M.: Designed to fail: a novel mode of collagen fibril disruption and its relevance to tissue toughness. Biophys. J. 102(12), 2876–2884 (2012)
Wainwright, D.J.: Use of an acellular allograft dermal matrix (Alloderm) in the management of full-thickness burns. Burns 21(4), 243–248 (1995)
Wallace, J.M., Erickson, B., Les, C.M., Orr, B.G., Banaszak Holl, M.M.: Distribution of type I collagen morphologies in bone: relation to estrogen depletion. Bone 46(5), 1349–1354 (2010)
Wasserman, A.J., Kato, Y.P., Christiansen, D., Dunn, M.G., Silver, F.H.: Achilles tendon replacement by a collagen fiber prosthesis: morphological evaluation of neotendon formation. Scanning Microsc. 3(4), 1183–1197; discussion 1197–1200 (1989)
Weadock, K., Olson, R.M., Silver, F.H.: Evaluation of collagen crosslinking techniques. Biomater. Med. Devices Artif. Organs 11(4), 293–318 (1983)
Wenger, M.P., Bozec, L., Horton, M.A., Mesquida, P.: Mechanical properties of collagen fibrils. Biophys. J. 93(4), 1255–1263 (2007)
Whittington, C.F., Brandner, E., Teo, K.Y., Han, B., Nauman, E., Voytik-Harbin, S.L.: Oligomers modulate interfibril branching and mass transport properties of collagen matrices. Microsc. Microanal. 19(5), 1323–1333 (2013)
Whittington, C.F., Yoder, M.C., Voytik-Harbin, S.L.: Collagen-polymer guidance of vessel network formation and stabilization by endothelial colony forming cells in vitro. Macromol. Biosci. 13(9), 1135–1149 (2013)
Willard, J.J., Drexler, J.W., Das, A., Roy, S., Shilo, S., Shoseyov, O., Powell, H.M.: Plant-derived human collagen scaffolds for skin tissue engineering. Tissue Eng. Part A 19(13–14), 1507–1518 (2013)
Wilson, R., Lees, J.F., Bulleid, N.J.: Protein disulfide isomerase acts as a molecular chaperone during the assembly of procollagen. J. Biol. Chem. 273(16), 9637–9643 (1998)
Wood, G.C., Keech, M.K.: The formation of fibrils from collagen solutions. 1. The effect of experimental conditions: kinetic and electron-microscope studies. Biochem. J. 75(3), 588–598 (1960)
Worcester, D.L.: Structural origins of diamagnetic anisotropy in proteins. Proc. Natl. Acad. Sci. 75(11), 5475–5477 (1978)
Worke, L.J., Barthold, J.E., Seelbinder, B., Novak, T., Main, R.P., Harbin, S.L., Neu, C.P.: Densification of type I collagen matrices as a model for cardiac fibrosis. Adv. Healthc. Mater. 6(22), 1700114 (2017)
Xu, R., Boudreau, A., Bissell, M.J.: Tissue architecture and function: dynamic reciprocity via extra- and intra-cellular matrices. Cancer Metastasis Rev. 28(1–2), 167–176 (2009)
Yang, C., Hillas, P.J., Baez, J.A., Nokelainen, M., Balan, J., Tang, J., Spiro, R., Polarek, J.W.: The application of recombinant human collagen in tissue engineering. Biodrugs 18(2), 103–119 (2004)
Yang, W., Sherman, V.R., Gludovatz, B., Schaible, E., Stewart, P., Ritchie, R.O., Meyers, M.A.: On the tear resistance of skin. Nat. Commun. 6, 6649 (2015)
Yannas, I.V., Burke, J.F.: Design of an artificial skin. I. Basic design principles. J. Biomed. Mater. Res. 14(1), 65–81 (1980)
Yannas, I.V., Burke, J.F., Gordon, P.L., Huang, C., Rubenstein, R.H.: Design of an artificial skin. II. Control of chemical composition. J. Biomed. Mater. Res. 14(2), 107–132 (1980)
Younesi, M., Donmez, B.O., Islam, A., Akkus, O.: Heparinized collagen sutures for sustained delivery of PDGF-BB: delivery profile and effects on tendon-derived cells in-vitro. Acta Biomater. 41, 100–109 (2016)
Younesi, M., Knapik, D.M., Cumsky, J., Donmez, B.O., He, P., Islam, A., Learn, G., McClellan, P., Bohl, M., Gillespie, R.J., Akkus, O.: Effects of PDGF-BB delivery from heparinized collagen sutures on the healing of lacerated chicken flexor tendon in vivo. Acta Biomater. 63, 200–209 (2017)
Yue, K., Trujillo-de Santiago, G., Alvarez, M.M., Tamayol, A., Annabi, N., Khademhosseini, A.: Synthesis, properties, and biomedical applications of gelatin methacryloyl (GelMA) hydrogels. Biomaterials 73, 254–271 (2015)
Yunoki, S., Matsuda, T.: Simultaneous processing of fibril formation and cross-linking improves mechanical properties of collagen. Biomacromolecules 9(3), 879–885 (2008)
Zhang, H., Voytik-Harbin, S., Brookes, S., Zhang, L., Wallace, J., Parker, N., Halum, S.: Use of autologous adipose-derived mesenchymal stem cells for creation of laryngeal cartilage. Laryngoscope 128(4), E123–E129 (2018)
Zuhdi, N., Hawley, W., Voehl, V., Hancock, W., Carey, J., Greer, A.: Porcine aortic valves as replacements for human heart valves. Ann. Thorac. Surg. 17(5), 479–491 (1974)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Sohutskay, D.O., Puls, T.J., Voytik-Harbin, S.L. (2020). Collagen Self-assembly: Biophysics and Biosignaling for Advanced Tissue Generation. In: Zhang, Y. (eds) Multi-scale Extracellular Matrix Mechanics and Mechanobiology. Studies in Mechanobiology, Tissue Engineering and Biomaterials, vol 23. Springer, Cham. https://doi.org/10.1007/978-3-030-20182-1_7
Download citation
DOI: https://doi.org/10.1007/978-3-030-20182-1_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-20181-4
Online ISBN: 978-3-030-20182-1
eBook Packages: EngineeringEngineering (R0)