Abstract
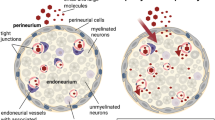
Peripheral nerves and nerve roots comprise of three structural compartments: the outer epineurium consisting of longitudinal arrays of collagen fibers responsible for structural integrity and the inner perineurium consisting of multiple concentric layers of specialized epithelioid myofibroblasts that surround the innermost endoneurium which consists of myelinated and unmyelinated axons embedded in a looser mesh of collagen fibers. Axons are responsible for signal transduction to and from the central nervous system required for normal physiological processes and are targeted by the immune system in autoimmune disorders. A highly regulated endoneurial microenvironment is required for normal axonal function. This is achieved by tight junction-forming endoneurial microvessels that control ion, solute, water, nutrient, macromolecule and leukocyte influx and efflux between the bloodstream and endoneurium, and the innermost layers of the perineurium that control interstitial fluid component flux between the epineurium and endoneurium. Endoneurial microvascular endothelium is considered the blood-nerve barrier (BNB) due to direct communication with circulating blood. The mammalian BNB is considered the second most restrictive vascular system after the blood-brain barrier (BBB). Guided by human in vitro studies using primary and immortalized endoneurial endothelial cells that form the BNB, in situ studies in normal and pathologic human peripheral nerves, and representative animal models of peripheral nerve autoimmune disorders, knowledge is emerging on human BNB molecular and functional characteristics, including its array of cytokines/cytokine receptors, selectins, and cellular adhesion and junctional complex molecules that may be employed during normal immune surveillance and altered in autoimmune diseases, providing potential targets of efficacious immunotherapy.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Anatomy of Human Peripheral Nerves
Human peripheral nerves serve to facilitate afferent and efferent communication between the central nervous system (brain and spinal cord) and the periphery (internal and external organs, such as the gastrointestinal tract and skin, respectively, secretory organs, and muscle) required for normal physiological processes needed to healthy bodily function. Human peripheral nerves comprise of three compartments: the outer epineurium which consists of longitudinal arrays of collagen fibers that are important for maintaining the structural integrity of the peripheral nerve, the inner perineurium which consists of concentric layers of specialized cells, and the innermost endoneurium which consists of a looser mesh of collagen fibers. A nerve fascicle consists of the endoneurium and its surrounding perineurium, initially described in 1876 (Fig. 1a) [1,2,3,4].
Digital light photomicrograph of a normal adult sural nerve (plastic embedded semi-thin axial section stained with Toluidine Blue and counterstained with basic fuchsin) showing the three compartments in peripheral nerves and endoneurial microvessels (EMV) that form the BNB (a) and an indirect fluorescent digital photomicrograph of a normal adult sural nerve (cryostat thick section stained with fluoresceinated Ulex europaeus agglutinin-1) showing epineurial macrovessels (solid arrow) and endoneurial microvessels (broken arrow) (b)
The epineurium consists of arteries, arterioles, venules, and veins that are considered collectively as epineurial macrovessels. The macrovessels are derived from and communicate with the extrinsic vascular supply to individual peripheral nerves known as the vasa nervorum. Lymphatic vessels are also present within the epineurium. The perineurium consists of specialized epithelioid myofibroblasts that form concentric layers, consisting of single cells, around the endoneurium (1–15 layers dependent on nerve diameter), forming fascicles, as well as smaller diameter macrovessels that communicate with the epineurium and endoneurium. The endoneurium consists of axons that are responsible for electrical impulse signal transduction to and from the central nervous system. These axons are myelinated or unmyelinated, are dependent on axonal size and function, and are aligned in the longitudinal axis of the peripheral nerve [1,2,3,4,5].
Schwann cells are the glial cells in peripheral nerves responsible for myelinating segments of large and small diameter axons needed to facilitate rapid salutatory action potential conduction, or surround bundles of small diameter unmyelinated axons (known as a Remak bundle), providing physiological support to these axons [6]. Motor neurons (axonal cell bodies) are located in the brain (for cranial nerves) and spinal cord (for somatic nerves), while sensory neurons are located in collections of cell bodies called ganglia (e.g., dorsal root ganglia for somatic nerves). The endoneurium also consists of capillary-like microvessels that lack smooth muscle walls (Fig. 1b), as well as rare resident leukocytes (macrophages and mast cells) and fibroblasts [1,2,3,4,5].
The sciatic nerve is the largest nerve in mammals, compromising of 50–80 fascicles in adult humans in the mid-thigh region (and as many as 140 fascicles in the gluteal region) [2, 7, 8] and 1–4 fascicles in adult mice and rats [9,10,11]. The commonly studied human sural nerve typically consists of 8–10 fascicles in adults [12]. It is important to recognize the rodent sciatic nerve consists of a thin epineurial layer with loose connective tissue in contrast with the more extensive and fibrous human epineurium. This significant structural difference between human and rodent peripheral nerves is important when extrapolating in vivo or in situ experimental observations made in rodents to human peripheral nerves, particularly with reference to nerve injury and local drug delivery (e.g., anesthetics and analgesics).
Identification and Definition of Blood-Nerve Barrier
The importance of maintaining a highly regulated ionic microenvironment to facilitate axonal impulse conduction in peripheral nerves is intuitive and led to the proposal of a blood-nerve barrier (BNB) akin to the blood-brain barrier (BBB). In vivo permeability studies performed in different animal species following intravenous Evans blue albumin and fluoresceinated albumin or dextran administration demonstrated restricted macromolecules within endoneurial microvessel lumens without extravasation into the endoneurium despite diffuse entry into the epineurium (which was in contrast with the diffuse lack of brain parenchymal entry), implying that restrictive interfaces exist in peripheral nerves and nerve roots [13,14,15,16,17].
Subsequent ultrastructural assessment of human peripheral nerves demonstrated that the impermeable endoneurial microvessels consist of endothelial cells that form tight intercellular junctions and share their basement membrane with surrounding pericytes, lack fenestrations, and possess very few 50–100 nm pinocytic vesicles. This was in contrast with permeable epineurial macrovessels that contain a layer of endothelial cells that possess fenestrations and are surrounded by a smooth muscle wall. Furthermore, the innermost concentric perineurial cell layers (i.e., closest to the endoneurium) are connect by intercellular tight junctions, lack fenestrations, and possess pinocytic vesicles (with higher density in the outermost layers). Thus, the internal microenvironment of the endoneurium is deemed to be regulated by tight junction-forming endoneurial endothelial cells and the cell layers of the innermost perineurium [2, 3, 5].
Endoneurial endothelial cells are in direct contact with circulating blood, including hematogenous leukocytes, while perineurial cells are in contact with interstitial fluid from the epineurium and endoneurium. As a consequence, endoneurial endothelial cells form the BNB, while perineurial cells form critical interfaces between the endoneurial and epineurial interstitial fluid compartments which are also important for maintaining peripheral nerve homeostasis. Since cross-talk between the systemic immune system and peripheral nerves largely depends on hematogenously derived circulating leukocytes, it is important to understand the structural, molecular, and functional characteristics of the human BNB in health in order to elucidate biologically relevant alterations that may occur in disease states such as peripheral nerve autoimmune disorders.
Characteristics of the Human BNB in Health
Basic knowledge of the structural, molecular, and functional characteristics of the human BNB in health and disease is emerging, guided by data from the human BBB and studies performed on peripheral nerve biopsies in situ and primary and immortalized human endoneurial endothelial cells in vitro; however, our knowledge is far from complete. Structurally, human endoneurial endothelial cells that form the BNB possess electron-dense intercellular tight junctions in situ and in vitro (Fig. 2) [3, 7]. In vitro, these tight junctions consist of occludin, members of the claudin family such as claudin-5, as well as cytoplasmic adaptors such as members of the zonula occludens (ZO) family, e.g., ZO-1 and ZO-2 (also known as tight junction proteins 1 and 2, respectively), based on immunocytochemistry of confluent cultures [7, 18,19,20], while claudin-5 and ZO-1 had been previously demonstrated in situ [21,22,23]. Data has emerged over the past 15 years on the importance of the intercellular junctional complex, consisting of tight, adherens, and gap junctions and their associated adaptor proteins and interacting cytoskeletal components in normal specialized endothelial and epithelial cell function [22, 24,25,26,27,28,29].
Digital electron ultramicrographs from an adult sural nerve (a) and cultured semipermeable transwell inserts (b) showing human endoneurial endothelial cells with electron-dense intercellular tight junctions (black arrows). A red blood cell (RBC) is present in the lumen of the endoneurial microvessel
Recent work elucidating the normal adult human BNB transcriptome based on conserved transcripts expressed by early- and late-passage primary human endoneurial endothelial cells and laser-capture microdissected endoneurial microvessels from four histologically normal adult sural nerve biopsies demonstrated expression of 133 intercellular junctional complex molecules (22 tight junction or junction-associated, 45 adherens junction or junction-associated, and 52 cell junction-associated or adaptor molecules), with in situ protein expression of α1 catenin, cadherin-5, cadherin-6, claudin-4, claudin-5, crumbs cell polarity complex component lin-7 homolog A, gap junction protein A1, multiple PDZ domain crumbs cell polarity complex component, protocadherin-1, vezatin, ZO-1, and zyxin demonstrated on endoneurial microvessels by indirect fluorescent immunohistochemistry [22]. This complexity may exist to provide significant molecular redundancy needed to maintain a structurally normal BNB due to its essential homeostatic role in normal peripheral nerve function.
Restrictive intercellular tight junction formation is a critical observation that differentiates endoneurial microvascular endothelial cells from epineurial macrovascular endothelial cells in human peripheral nerves. Endoneurial endothelial cells express receptors for specific mitogens such as glial-derived neurotrophic factor (GDNF, GFRα1), vascular endothelial growth factor (VEGF, VEGFR2), basic fibroblast growth factor (bFGF, FGFR1), transforming growth factor-β (TGF-β, TGFRI/II), and glucocorticoids (GR) [18, 19, 30,31,32], implying that autocrine or paracrine mitogen secretion by endothelial cells, Schwann cells, pericytes, mast cells, or endoneurial fibroblasts could regulate BNB composition and function in health. Schwann cells, the glial cells of the peripheral nervous system present in the endoneurium, have been shown to secrete GDNF in vitro and in vivo [33, 34], and GDNF has been demonstrated to influence restrictive human BNB characteristics in vitro at low nanomolar concentrations in a dose-dependent manner via RET-tyrosine kinase-mitogen-activated protein kinase (MAPK) signaling and enhance murine BNB restrictive characteristics in vivo following non-transecting nerve injury using a tamoxifen-inducible conditional knockout model [30, 35]. This suggests that GDNF is an essential paracrine regulator of BNB formation that may also have an important role during BNB formation during development and maintenance in health, with some redundancy demonstrated in vitro by other less efficacious mitogens, such as basic fibroblast growth factor.
In addition to the junctional complex, specialized influx and efflux transporters that regulate ionic, water, molecular, nutrient, drug, and xenobiotic entry into or removal from the peripheral nerve endoneurium exist at the human BNB, controlling the endoneurial microenvironment. In vitro, these include alkaline phosphatase, glucose transporter-1 (also known as SLC2A1), monocarboxylate transporter-1 (also known as SLC16A1), creatine transporter (also known as SLC6A8), large amino acid transporter-1 (also known as SLC7A5), γ-glutamyl transpeptidase, and p-glycoprotein (also known as ABCB1) expressed by primary and immortalized human endoneurial endothelial cells (messenger RNA or protein) [7, 32], with glucose transporter-1 previously demonstrated on human endoneurial microvessels in situ [36].
The human BNB transcriptome demonstrated 509 transporter transcripts, including 196 members of the solute carrier transport family, 76 cation channel, 33 members of the ATP-binding cassette family, 14 zinc transporter, 13 anion channel, 4 solute carrier organic transporter, and 3 aquaporin molecules. ABCA8, ABCB1, AQP1, SLC1A1, SLC2A1, SLC3A2, SLC5A6, SLC16A1, and SLC19A2 were demonstrated on BNB-forming endoneurial endothelial cells in normal human sural nerve biopsies by indirect immunohistochemistry in situ [22]. The extensive repertoire of transcripts that comprise the healthy human BNB cellular components (i.e., cell junction, cell part, extracellular matrix, extracellular region, macromolecular complex, membrane, organelle, and synapse) and their protein classes has been recently published, recognizing that not all transcripts undergo translation to functional protein. Although there are major similarities, structural differences and molecular heterogeneity in the composition of the BNB probably exist between different species [5, 37], limiting the degree of extrapolation feasible between data derived from animal models in vitro and in vivo and the human BNB. Figure 3 depicts a schematic figure summarizing essential structural and molecular components of the human BNB.
Human BNB Physiology
The human BNB, similar to other specialized tight junction-forming microvascular systems such as the BBB, blood-retina barrier, and blood-testis barrier, is expected to possess high transendothelial electrical resistance (TEER), low permeability to solutes and macromolecules, and low transendothelial water flux (hydraulic conductivity). In support of this, comparative animal studies have determined that the BNB is the second most restrictive microvascular tissue barrier in mammals, after the BBB. Unlike the human BBB, supported by the glia limitans (which consists of astrocyte and microglial foot processes), there is no physical support of the BNB by Schwann cells. It has not been conclusively established whether endoneurial microvascular pericytes (that lack intercellular junctions and share a basement membrane with endoneurial endothelial cells) provide trophic support to the human BNB.
The human BNB TEER in vivo is unknown; however, it is expected to be >1500 Ω.cm2, based on BBB data [38,39,40,41]. Similarly, its permeability coefficients and hydraulic conductivity in vivo are also unknown, although some work has been published in other mammalian and nonmammalian species evaluating solute permeability and interstitial fluid flux in peripheral nerves following intravenous electrolyte and tracer injections, followed by timed nerve procurement [17, 42,43,44]. Human BNB TEER has been measured to be as high as ~180 Ω.cm2 in confluent cultures by a voltohmmeter applying a direct current across transwell inserts and as high as ~900 Ω when recorded in specialized culture wells with gold electrodes using a fixed alternating current at 4000 Hz via electrical cell impedance sensing [7, 20, 32, 35].
Solute permeability to sodium fluorescein (molecular mass 376 Da) and 70 KDa fluoresceinated dextran (dextran-70-FITC) across primary and immortalized human endoneurial endothelial cells is typically <5% of input at 15 minutes using static transwell systems in vitro, with higher values (~3–15-fold) seen with sodium fluorescein when directly compared to dextran-70-FITC using the same batch of endothelial cells in concurrent experiments [7, 20, 32]. Human BNB transendothelial water flux under the influence of hydrostatic pressure, otherwise known as hydraulic conductivity, has been measured in vitro (~2.0 × 10−7 cm/s/cm H2O) using a customized transwell diffusion chamber-bubble track system [45]. Consistent with prior observations, the human BNB was the second most restrictive human or mammalian microvascular endothelial cell type after the BBB in terms of water flux [17, 43,44,45].
Hematogenous leukocyte trafficking across microvascular endothelium in vivo (based on intravital microscopy) or in vitro under flow is a sequential coordinated process that involves leukocyte attraction from circulating blood to the endothelial cell luminal surface (mediated by specific chemokines bound to glycosaminoglycans on the endothelium and chemokine receptors expressed by leukocytes), rolling (mediated by selectins expressed on the endothelium and their glycoproteins or carbohydrate moiety counterligands expressed on leukocytes), leukocyte arrest and haptotaxis on the endothelial cell surface (mediated by chemokines and chemokine receptors), integrin activation and firm adhesion (via leukocyte integrin binding to endothelial cell adhesion molecules) that induces a conformation change in leukocyte shape from round to flat with formation of pseudopodia, and leukocyte transmigration via the paracellular (i.e., through intercellular junctions) or transcellular (i.e., through endothelial cells) routes followed by basement membrane disruption at the abluminal surface (via secretion of specific matrix metalloproteases) required for complete passage into tissues [46,47,48,49,50,51,52]. There is in vitro data using a flow-dependent leukocyte-BNB trafficking model providing evidence that this sequential process (also known as the multistep paradigm of leukocyte trafficking) occurs in peripheral nerves [53,54,55].
The presence of rare endoneurial macrophages , mast cells, and T lymphocytes in normal human peripheral nerve endoneurium implies some physiological cross talk between the systemic immune compartment and peripheral nerves at the BNB. The human BNB transcriptome supports the expression of human leukocyte antigen (or major histocompatibility complex) class I and II molecules in normal healthy endoneurial microvessels in situ [22], suggesting that the human BNB may directly participate in innate and adaptive immune responses in peripheral nerves (Tables 1 and 2). Furthermore, specific chemokine transcripts were also expressed by the normal healthy adult BNB based on this transcriptome. These include CCL2, CCL14, CCL28, CXCL3, CXCL12, CXCL16, and CX3CL1 [22].
These chemokines could facilitate the interaction of hematogenous monocytes (CCL2, CCL14, CX3CL1), T lymphocytes (CCL2, CX3CL1), natural killer T cells (CXCL16), and neutrophils (CXCL3) with endoneurial microvascular cells during normal immunosurveillance or part of an early immune response to injury, while CXCL12 and CCL28 may be important in endothelial cell migration and vascular repair. A more complex array of chemokines including CXCL9, CXCL10, and CXCL11 that facilitate CXCR3+ CD4+ T-helper 1 lymphocyte migration were expressed by the basal human BNB in vitro [22, 55], implying some degree of endothelial cell activation in vitro or dysregulated chemokine expression in situ.
Endoneurial microvascular endothelial cells also express selectins (e.g., P-selectin, E-selectin) and cell adhesion molecules (e.g., intercellular adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1), fibronectin Type III connecting segment) under basal conditions that were upregulated or underwent alternative splicing following stimulus with physiological concentrations of pro-inflammatory cytokines tissue necrosis factor-α (TNF-α) and interferon-γ (IFN-γ) in vitro (Fig. 4) [55]. The constitutive expression of these cell adhesion molecules known to facilitate leukocyte adhesion and transmigration supports the notion the endoneurial microvessels participate in cross talk between subsets of circulating leukocytes that are components of systemic immune compartment and peripheral nerves.
Composite indirect fluorescent digital photomicrographs showing cellular adhesion molecule expression by confluent primary human endoneurial endothelial cells under basal and physiological cytokine-activated states in vitro (B indicates expression under basal culture conditions; CA indicates expression following cytokine activation with 10 U/mL TNF-α and 20 U/mL IFN-γ for 24 hours) A, C, E, G and I indicate cellular adhesion molecule expression under basal cultures conditions, while B, D, F, H and J indicate upregulated expression following physiological cytokine stimulus in vitro
Structural and Functional Changes at the BNB Associated with Autoimmune Disorders
Increased permeability of or leukocyte trafficking at the human BNB, commonly cited as “BNB breakdown,” has been pathologically associated with peripheral nerve autoimmune disorders, with a paper reporting downregulation of BNB tight junction protein claudin-5 and translocation of ZO-1 by immunohistochemistry in sural nerve biopsies of patients with chronic inflammatory demyelinating polyradiculoneuropathy (CIDP), without change in occludin expression [21]. It is important to recognize that claudin-5 was also expressed on epineurial macrovessels that do not form the restrictive tight junctions [21], as well as immature endoneurial microvessels during development [23], calling to question its role in mediating restrictive junction barrier function in human peripheral nerves. Importantly, this commonly held viewpoint implies that the human BNB is relatively passive during autoimmune disorders affecting peripheral nerves.
Recent data demonstrating the complexity of the restrictive junction components and possible redundancy of tight junction-forming molecules involved in the human BNB [22] suggest that downregulation of a single tight junction-forming molecule or reduction in TEER or increase in solute permeability demonstrated in vitro following administration of sera from GBS or CIDP patients [56,57,58] may be an insufficient structural or functional change at the human BNB in vivo during autoimmune disorders. In support of this, physiological cytokine stimulus of confluent primary human endoneurial endothelial cells grown on transwell inserts with TNF-α and IFN-γ over a 100-fold range did not alter TEER in vitro [55]. Ultrastructural examination of endoneurial microvessels within the inflammatory milieu from patients with GBS and CIDP demonstrates intact electron-dense intercellular tight junctions, with similar electron-dense contacts between infiltrating leukocytes and endothelial cells (Fig. 5) [59, 60]. These observations should provide the impetus for further studies to better understand biologically relevant structural and functional alterations at the human BNB during peripheral nerve autoimmune disorders relative to healthy nerves.
Composite digital electron ultramicrographs demonstrating intact electron-dense intercellular tight junctions (solid black arrows) between endoneurial endothelial cells within the inflammatory milieu in a GBS (a) and CIDP (b) patient sural nerve biopsy, with electron-dense intercellular contacts observed between infiltrating leukocytes and endothelial cells (c, white arrows) and endoneurial microvessel basement membrane duplication (d, black asterisk)
Endoneurial microvessel basement membrane thickening/duplication (Fig. 5d) has been described in association with CIDP and peripheral nerve vasculitis (which typically affects epineurial arteries or arterioles and rarely involves endoneurial capillary-like vessels with resultant endoneurial ischemia) [60,61,62]. The functional implications of the basement membrane alterations are undetermined; however, this may reflect an adaptive or maladaptive response to chronic and persistent endothelial cell/pericyte pro-inflammatory cytokine exposure or hypoxia/ischemia as a compensatory or reactive means of maintaining BNB functional integrity.
BNB Endothelial-Leukocyte Interactions in Immune-Mediated Neuropathies
While it is unresolved whether systemic immune system activation (e.g., by infections, minor surgery or trauma) with primary attack of peripheral nerves and nerve roots (through the process of “molecular mimicry”) [63, 64] or endogenous activation of the innate immune system in peripheral nerves (e.g., by viruses) [65] with secondary selective adaptive immune system activation in genetically susceptible individuals is responsible for tissue-specific autoimmunity, or whether suspected circulating polyclonal anti-myelin protein, anti-axonal nodal protein, and anti-ganglioside or anti-glycolipid autoantibodies can cross the human BNB in vivo, a pathologic hallmark of autoimmune neuropathies is the infiltration of subpopulations of hematogenous leukocytes in peripheral nerves and nerve roots, commonly demonstrated in situ on patient nerve biopsies [61].
In GBS and CIDP, leukocyte infiltration is associated with demyelination, axonal degeneration, or both. In peripheral nerve vasculitis, leukocyte infiltration is associated with vascular wall infiltration, transmural vasonecrosis, and endoneurial ischemia. In HIV-associated distal sensory polyneuropathy (DSP), although not considered an autoimmune neuropathy, clusters of leukocytes are also seen within the endoneurium, associated with axonal loss. Since endoneurial microvessels that form the BNB provide the main route of entry for hematogenous leukocytes from circulation into the endoneurium, leukocyte-endothelial cell interactions are important in the pathogenesis of peripheral nerve autoimmune disorders. In support of this, hematogenous leukocytes interacting with the endoneurial microvessels that form the BNB have been observed in untreated patients with GBS, CIDP, and HIVDSP in situ (Fig. 6).
Composite digital indirect fluorescent photomicrographs showing interaction between hematogenous leukocytes and endoneurial microvessels in GBS (a; CD11b+), CIDP (b; CD49d+), and HIV-DSP (c; CD68+ CCR5+) patient sural nerve biopsies (yellow cells shown with white arrows). S100β+ myelinating Schwann cells associated with axons (green) are also depicted in a. The outline of an endoneurial microvessel in longitudinal section is shown with the white lines in c
Using a flow-dependent leukocyte-BNB trafficking model in vitro, untreated GBS, CIDP, and HIV-DSP patient peripheral blood mononuclear leukocytes (PBMLs) firmly adhere to the surface of confluent primary endoneurial endothelial cells and undergo paracellular transmigration at higher rates that normal healthy donor PBMLs in vitro [53, 55], supporting the notion that leukocyte trafficking at the BNB is pathogenically relevant to autoimmune peripheral neuropathies and potentially HIV-DSP.
Subpopulation Leukocyte Infiltration in Immune-Mediated Neuropathies
The major challenges in definitively ascertaining the phenotypic characteristics of infiltrating leukocytes in autoimmune neuropathies include disease heterogeneity, the scarcity of pathologic patient biopsies for large-scale comparative analyses, the frequent analysis of sural nerves that may be partially involved in the disease process but practically safer to biopsy in patients rather than clinically and electrophysiologically affected motor nerves, the paucity or multifocal nature of inflammatory infiltrates reducing the likelihood of detecting pathogenic leukocytes in small specimens, and the selection and ascertainment biases intrinsic to immunohistochemistry studies.
The expression of HLA class II molecules, interleukin 1-beta (IL-1β), IFN-γ, TNF-α, CCL2, CXCL10, and ICAM-1 on endoneurial endothelial cells has been described in peripheral nerve biopsies of GBS patients. Similarly, HLA-DR, interleukin-2 (IL-2), IFN-γ, TNF-α, CXCL10, and ICAM-1 have also been expressed at the human BNB in situ in CIDP patient nerve biopsies at higher levels compared to control nerves, supporting the notion that local activation of the adaptive immune response at the BNB may be pathogenically significant in GBS and CIDP [66,67,68,69,70,71,72,73,74,75,76]. In a single study, chemokine receptors CCR1 and CCR5 were demonstrated on endoneurial macrophages with CCR2, CCR4, and CXCR3 expressed on infiltrating T lymphocytes in GBS and CIDP patient sural nerve biopsies [76]. Another study demonstrated increased numbers of CCR2+ mononuclear cells in GBS patient nerve biopsies [69].
Guided by in vitro observations implying a role for leukocyte integrin CD11b (also known as αM-integrin or Mac-1)-ICAM-1 interactions in mediating pathogenic leukocyte trafficking at the human BNB under hydrodynamic forces mimicking in vivo capillary flow rates [55], expression of clusters of infiltrated CD11b+ leukocytes interacting with endoneurial endothelial cells that accumulate within untreated GBS patient sural nerve biopsy endoneurium has been shown (Fig. 7) [59]. Similarly, CD49d+ (also known as α4-integrin or very late antigen-4) mononuclear leukocytes in CIDP patient sural nerve biopsy endoneurium [53] and CCR5+ and CD11d+ (also known as αD-integrin) mononuclear leukocytes in untreated HIV-DSP patient sural nerve biopsies have been demonstrated (Fig. 6), consistent with a prior report indicating a predominance of CCR5-dependent and macrophage tropic HIV-1 virus based on sequence analysis and evaluation of infectious recombinant viruses containing peripheral nerve-derived C2V3 sequences in autopsied sural and peroneal nerves in decedent HIV+ individuals [77].
Composite digital indirect fluorescent photomicrographs depicting subpopulations of hematogenous leukocytes that have infiltrated into sural nerve endoneurium in untreated GBS (a–d)-, CIDP (e, f)-, and HIV-DSP (g–j)-affected patients, the sciatic nerves of representative murine GBS (k, l) and CIDP (m, n) animal models, and the effect of targeted molecular inhibition in the mouse models. Clusters of infiltrated monocytes/macrophages (a), T lymphocytes (b), B lymphocytes (c), and CD11b+ leukocytes in a region of demyelination (d; green depicts S100β+ myelinating Schwann cells associated with axons) are shown in GBS patients, and clusters of infiltrated monocytes/macrophages (e) and CD49d+ leukocytes (f) are shown in CIDP patients, with CCR5+ monocytes/macrophages (g), CD4+ T lymphocytes (h), CD8+ T lymphocytes (i), and CD11d+ leukocytes (j) shown in HIV-DSP patients. The sciatic nerve of an untreated severe EAN-affected mouse shows intense endoneurial infiltrates of CD11b+ leukocytes (k) with a significant reduction in infiltrates seen in another mouse treated with a function-neutralizing rat anti-mouse CD11b monoclonal antibody (l). The sciatic nerve of an untreated SAPP-affected mouse shows intense CD45+ leukocyte infiltrates (m) that are significantly reduced in another mouse treated with a fibronectin-connecting segment 1 peptide (n) early in the disease course. Examples of infiltrated leukocytes are depicted with either black or white arrows in the photomicrographs
Peripheral nerve vasculitis is typically associated with leukocyte infiltration of epineurial macrovascular endothelium walls, rather than direct involvement with endoneurial microvessels that form the BNB. However, strong expression of HLA class I and class II molecules on affected vascular endothelial cells has been described, typically associated with prominent CD4+ and fewer CD8+ T lymphocytes and CD68+ macrophages. CD22+ B lymphocytes and CD16+ natural killer cells are less commonly observed in vasculitic neuropathy than T lymphocytes and macrophages. T lymphocyte infiltrates in vasculitic neuropathy are heterogeneous based on T-cell receptor Vβ utilization, similar to descriptions in CIDP, supporting the polyclonal nature of these conditions [74, 75, 78,79,80,81].
Expression of CD58 (also known as lymphocyte function-associated antigen-3; a cell adhesion molecule typically expressed on antigen-presenting cells such as macrophages and binds to CD2 on T lymphocytes) and CD86 (a protein expressed on antigen-presenting cells that provides costimulatory signals necessary for T-cell activation and survival) on affected vascular endothelial cells have also been described, with the former also expressed by Schwann cells [75]. Variable focal expression of hypoxia-inducing factors (HIFs), HIF-1α, HIF-1β, and HIF-2α, as well as VEGF, VEGFR, and erythropoietin receptor was seen on endoneurial microvessels in a small percentage of nerve biopsies from patients with vasculitic neuropathy at higher rates than control sural nerve biopsies [82, 83].
Recent work elucidating the normal adult BNB transcriptome provides molecular targets putatively involved in cross-talk between the innate (Table 1) and adaptive (Table 2) immune responses in peripheral nerves. Validation of these proposed molecules and their associated signaling networks, as well as future single cell transcriptomics and proteomics studies, could provide avenues to more comprehensively elucidate molecular changes at the human BNB in situ and characterize the different infiltrated leukocyte subpopulations associated with specific peripheral nerve autoimmune disorders required to better understand the pathogenesis of these conditions and also understand how HIV-infected leukocytes could gain access into peripheral nerves. The ultimate goal is to devise targeted efficacious molecular therapies for autoimmune neuropathies and prevent the development of consequential chronic neuropathic pain.
Animal Models and Targeted Inhibition of Pathogenic Leukocyte Trafficking
Despite the limitations of autoimmune neuropathy animal models and species differences in BNB function and the inflammatory cascade [84, 85], experimental observations made in representative animal models guided by data derived from human in situ leukocyte-BNB interactions in autoimmune neuropathies could provide further insights into the pathogenesis of these disorders and the adaptive or pathological changes that occur at the BNB during autoimmunity. Animal models could also aid dissect the mechanisms by which the systemic immune system engages with peripheral nerves and nerve roots during normal physiologic states and the earliest signaling pathways associated with tissue-specific autoimmune disorders.
Experimental autoimmune neuritis (EAN, an established model of GBS) in the Lewis rat implicated important roles of CD11a (also known as αL-integrin or lymphocyte function-associated antigen-1) in disease induction [86] and CCL3 and partially CCL2 in pathogenic leukocyte trafficking [87]. Pharmacologic blockade and germline gene knockout of CCR2 (expressed by monocytes/macrophages and a subset of T lymphocytes which most commonly binds to CCL2) ameliorated disease in a severe murine EAN model associated with markedly attenuated leukocyte trafficking into the sciatic nerves [9], while germline CCR5 knockout did not modulate disease in a less severe murine EAN model associated with compensatory increase in sciatic nerve CCL4 and CXCL10 expression [88]. Integrin blockade with a depleting function-neutralizing rat anti-mouse CD11b monoclonal antibody administered after clinically discernible disease onset was efficacious in the severe murine EAN model (Fig. 7) [59], providing further insight into the molecular determinants of pathogenic leukocyte trafficking in acute autoimmune neuropathies in vivo.
Chronic relapsing EAN animal models have been employed to understand CIDP pathogenesis; however, these models are generally limited by variable disease onset and severity. A severe murine chronic demyelinating neuritis model has been established in the autoimmune disease-susceptible CD86 (also known as B7-2)-deficient non-obese diabetic mouse strain, known as spontaneous autoimmune peripheral polyneuropathy (SAPP) that recapitulates features of severe CIDP [89, 90]. In this model associated with a cell and humoral autoimmune response to myelin protein zero [91], peptide blockade of fibronectin connecting segment 1 (which serves as an endothelial counterligand for CD49d or α4-integrin) ameliorated disease to a similar magnitude as functional neutralizing rat anti-mouse monoclonal CD49d and VCAM-1 antibodies, associated with reduced leukocyte infiltration into the sciatic nerves (Fig. 7) [53], providing further insight into the molecular determinants of pathogenic leukocyte trafficking in chronic autoimmune neuropathies in vivo.
Future Directions
The human BNB, formed by endoneurial microvascular endothelial cells, is a critical interface hypothetically essential to the cross-talk between components of the systemic immune system and peripheral nerves and nerve roots in health during normal immune surveillance and in disease states that manifest as autoimmune neuropathies. The molecular determinants and signaling pathways responsible for hematogenous leukocyte interaction with and trafficking across the human BNB in health and disease are incompletely understood, with advances being made using a near-physiological flow-dependent leukocyte-endothelial cell trafficking model and animal models of peripheral nerve autoimmune disorders, critically supported by observational in situ data obtained from human peripheral nerve biopsies. Applying bioinformatics analyses to transcriptomic and proteomic data derived from normal and pathologic peripheral nerves at the batch or single cell level to establish biologically relevant networks/signaling pathways could accelerate our knowledge of the essential structural and functional characteristics of the human BNB in health, alterations, or adaptations in autoimmune disorders and aid discover molecular targets for disease-specific therapeutic modulation in this group of disorders that takes into account the unique biology of the BNB and the peripheral nervous system.
Abbreviations
- BBB:
-
Blood-brain barrier
- BNB:
-
Blood-nerve barrier
- CIDP:
-
Chronic inflammatory demyelinating polyradiculoneuropathy
- DSP:
-
Distal sensory polyneuropathy
- EAN:
-
Experimental autoimmune neuritis
- FITC:
-
Fluorescein isothiocyanate
- GBS:
-
Guillain-Barré syndrome
- GDNF:
-
Glial-derived neurotrophic factor
- HIFs:
-
Hypoxia-inducing factors
- HIV:
-
Human immunodeficiency virus
- HLA:
-
Human leukocyte antigen
- ICAM-1:
-
Intercellular adhesion molecule-1
- IFN-γ:
-
Interferon-γ
- IL-1β:
-
Interleukin-1β
- IL-2:
-
Interleukin-2
- MAPK:
-
Mitogen-activated protein kinase
- RET:
-
“rearranged upon transformation”
- RNA:
-
Ribonucleic acid
- SAPP:
-
Spontaneous autoimmune peripheral polyneuropathy
- TEER:
-
Transendothelial electrical resistance
- TGF-β:
-
Transforming growth factor-β
- VCAM-1:
-
Vascular cell adhesion molecule-1
- VEGF:
-
Vascular endothelial cell growth factor
- ZO:
-
Zonula occludens
References
Olsson Y. Microenvironment of the peripheral nervous system under normal and pathological conditions. Crit Rev Neurobiol. 1990;5:265–311.
Reina MA, Lopez A, Villanueva MC, de Andres JA, Leon GI. [Morphology of peripheral nerves, their sheaths, and their vascularization]. Rev Esp Anestesiol Reanim. 2000;47:464–475.
Reina MA, Lopez A, Villanueva MC, De Andres JA, Maches F. [The blood-nerve barrier in peripheral nerves]. Rev Esp Anestesiol Reanim. 2003;50:80–86.
Mizisin AP, Weerasuriya A. Homeostatic regulation of the endoneurial microenvironment during development, aging and in response to trauma, disease and toxic insult. Acta Neuropathol. 2011;121:291–312.
Bell MA, Weddell AG. A descriptive study of the blood vessels of the sciatic nerve in the rat, man and other mammals. Brain J Neurol. 1984;107(Pt 3):871–98.
Monk KR, Feltri ML, Taveggia C. New insights on Schwann cell development. Glia. 2015;63:1376–93.
Yosef N, Xia RH, Ubogu EE. Development and characterization of a novel human in vitro blood-nerve barrier model using primary endoneurial endothelial cells. J Neuropathol Exp Neurol. 2010;69:82–97.
Sladjana UZ, Ivan JD, Bratislav SD. Microanatomical structure of the human sciatic nerve. Surg Radiol Anat. 2008;30:619–26.
Yuan F, Yosef N, Lakshmana Reddy C, Huang A, Chiang SC, Tithi HR, Ubogu EE. CCR2 gene deletion and pharmacologic blockade ameliorate a severe murine experimental autoimmune neuritis model of Guillain-Barre syndrome. PLoS One. 2014;9:e90463.
Tanaka K, Webster HD. Myelinated fiber regeneration after crush injury is retarded in sciatic nerves of aging mice. J Comp Neurol. 1991;308:180–7.
Christensen MB, Tresco PA. Differences exist in the left and right sciatic nerves of naive rats and cats. Anat Rec (Hoboken). 2015;298:1492–501.
Ochoa J, Mair WG. The normal sural nerve in man. I. Ultrastructure and numbers of fibres and cells. Acta Neuropathol. 1969;13:197–216.
Olsson Y. Studies on vascular permeability in peripheral nerves. I. Distribution of circulating fluorescent serum albumin in normal, crushed and sectioned rat sciatic nerve. Acta Neuropathol. 1966;7:1–15.
Olsson Y. Topographical differences in the vascular permeability of the peripheral nervous system. Acta Neuropathol. 1968;10:26–33.
Olsson Y. Studies on vascular permeability in peripheral nerves. IV. Distribution of intravenously injected protein tracers in the peripheral nervous system of various species. Acta Neuropathol. 1971;17:114–26.
Hultstrom D, Malmgren L, Gilstring D, Olsson Y. FITC-Dextrans as tracers for macromolecular movements in the nervous system. A freeze-drying method for dextrans of various molecular sizes injected into normal animals. Acta Neuropathol. 1983;59:53–62.
Poduslo JF, Curran GL, Berg CT. Macromolecular permeability across the blood-nerve and blood-brain barriers. Proc Natl Acad Sci U S A. 1994;91:5705–9.
Shimizu F, Sano Y, Abe MA, Maeda T, Ohtsuki S, Terasaki T, Kanda T. Peripheral nerve pericytes modify the blood-nerve barrier function and tight junctional molecules through the secretion of various soluble factors. J Cell Physiol. 2011;226:255–66.
Shimizu F, Sano Y, Saito K, Abe MA, Maeda T, Haruki H, Kanda T. Pericyte-derived glial cell line-derived neurotrophic factor increase the expression of claudin-5 in the blood-brain barrier and the blood-nerve barrier. Neurochem Res. 2012;37:401–9.
Ubogu EE. The molecular and biophysical characterization of the human blood-nerve barrier: current concepts. J Vasc Res. 2013;50:289–303.
Kanda T, Numata Y, Mizusawa H. Chronic inflammatory demyelinating polyneuropathy: decreased claudin-5 and relocated ZO-1. J Neurol Neurosurg Psychiatry. 2004;75:765–9.
Palladino SP, Helton ES, Jain P, Dong C, Crowley MR, Crossman DK, Ubogu EE. The human blood-nerve barrier transcriptome. Sci Rep. 2017;7:17477.
Pummi KP, Heape AM, Grenman RA, Peltonen JT, Peltonen SA. Tight junction proteins ZO-1, occludin, and claudins in developing and adult human perineurium. J Histochem Cytochem. 2004;52:1037–46.
Muller WA. Leukocyte-endothelial-cell interactions in leukocyte transmigration and the inflammatory response. Trends Immunol. 2003;24:327–34.
Hartsock A, Nelson WJ. Adherens and tight junctions: structure, function and connections to the actin cytoskeleton. Biochim Biophys Acta. 2008;1778:660–9.
Dejana E, Orsenigo F, Molendini C, Baluk P, McDonald DM. Organization and signaling of endothelial cell-to-cell junctions in various regions of the blood and lymphatic vascular trees. Cell Tissue Res. 2009;335:17–25.
Cichon C, Sabharwal H, Ruter C, Schmidt MA. MicroRNAs regulate tight junction proteins and modulate epithelial/endothelial barrier functions. Tissue Barriers. 2014;2:e944446.
Stamatovic SM, Johnson AM, Keep RF, Andjelkovic AV. Junctional proteins of the blood-brain barrier: new insights into function and dysfunction. Tissue Barriers. 2016;4:e1154641.
Sluysmans S, Vasileva E, Spadaro D, Shah J, Rouaud F, Citi S. The role of apical cell-cell junctions and associated cytoskeleton in mechanotransduction. Biol Cell. 2017;109:139–61.
Yosef N, Ubogu EE. GDNF restores human blood-nerve barrier function via RET tyrosine kinase-mediated cytoskeletal reorganization. Microvasc Res. 2012;83:298–310.
Reddy CL, Yosef N, Ubogu EE. VEGF-A165 potently induces human blood-nerve barrier endothelial cell proliferation, angiogenesis, and wound healing in vitro. Cell Mol Neurobiol. 2013;33:789–801.
Yosef N, Ubogu EE. An immortalized human blood-nerve barrier endothelial cell line for in vitro permeability studies. Cell Mol Neurobiol. 2013;33:175–86.
Trupp M, Ryden M, Jornvall H, Funakoshi H, Timmusk T, Arenas E, Ibanez CF. Peripheral expression and biological activities of GDNF, a new neurotrophic factor for avian and mammalian peripheral neurons. J Cell Biol. 1995;130:137–48.
Naveilhan P, ElShamy WM, Ernfors P. Differential regulation of mRNAs for GDNF and its receptors Ret and GDNFR alpha after sciatic nerve lesion in the mouse. Eur J Neurosci. 1997;9:1450–60.
Dong C, Ubogu EE. GDNF enhances human blood-nerve barrier function in vitro via MAPK signaling pathways. Tissue Barriers 2018;6(4):1–22.
Muona P, Jaakkola S, Salonen V, Peltonen J. Expression of glucose transporter 1 in adult and developing human peripheral nerve. Diabetologia. 1993;36:133–40.
Latker CH, Shinowara NL, Miller JC, Rapoport SI. Differential localization of alkaline phosphatase in barrier tissues of the frog and rat nervous systems: a cytochemical and biochemical study. J Comp Neurol. 1987;264:291–302.
Cohen-Kashi Malina K, Cooper I, Teichberg VI. Closing the gap between the in-vivo and in-vitro blood-brain barrier tightness. Brain Res. 2009;1284:12–21.
Lippmann ES, Azarin SM, Kay JE, Nessler RA, Wilson HK, Al-Ahmad A, Palecek SP, Shusta EV. Derivation of blood-brain barrier endothelial cells from human pluripotent stem cells. Nat Biotechnol. 2012;30:783–91.
Lippmann ES, Al-Ahmad A, Azarin SM, Palecek SP, Shusta EV. A retinoic acid-enhanced, multicellular human blood-brain barrier model derived from stem cell sources. Sci Rep. 2014;4:4160.
Wang YI, Abaci HE, Shuler ML. Microfluidic blood-brain barrier model provides in vivo-like barrier properties for drug permeability screening. Biotechnol Bioeng. 2017;114:184–94.
Poduslo JF, Curran GL, Dyck PJ. Increase in albumin, IgG, and IgM blood-nerve barrier indices in human diabetic neuropathy. Proc Natl Acad Sci U S A. 1988;85:4879–83.
Rechthand E, Rapoport SI. Regulation of the microenvironment of peripheral nerve: role of the blood-nerve barrier. Prog Neurobiol. 1987;28:303–43.
Rechthand E, Smith QR, Rapoport SI. Transfer of nonelectrolytes from blood into peripheral nerve endoneurium. Am J Physiol. 1987;252:H1175–82.
Helton ES, Palladino S, Ubogu EE. A novel method for measuring hydraulic conductivity at the human blood-nerve barrier in vitro. Microvasc Res. 2017;109:1–6.
Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7:678–89.
Man S, Ubogu EE, Ransohoff RM. Inflammatory cell migration into the central nervous system: a few new twists on an old tale. Brain Pathol. 2007;17:243–50.
Muller WA. How endothelial cells regulate transmigration of leukocytes in the inflammatory response. Am J Pathol. 2014;184:886–96.
Mempel TR, Scimone ML, Mora JR, von Andrian UH. In vivo imaging of leukocyte trafficking in blood vessels and tissues. Curr Opin Immunol. 2004;16:406–17.
Pai S, Danne KJ, Qin J, Cavanagh LL, Smith A, Hickey MJ, Weninger W. Visualizing leukocyte trafficking in the living brain with 2-photon intravital microscopy. Front Cell Neurosci. 2012;6:67.
Teixeira MM, Vilela MC, Soriani FM, Rodrigues DH, Teixeira AL. Using intravital microscopy to study the role of chemokines during infection and inflammation in the central nervous system. J Neuroimmunol. 2010;224:62–5.
Zenaro E, Rossi B, Angiari S, Constantin G. Use of imaging to study leukocyte trafficking in the central nervous system. Immunol Cell Biol. 2013;91:271–80.
Dong C, Greathouse KM, Beacham RL, Palladino SP, Helton ES, Ubogu EE. Fibronectin connecting segment-1 peptide inhibits pathogenic leukocyte trafficking and inflammatory demyelination in experimental models of chronic inflammatory demyelinating polyradiculoneuropathy. Exp Neurol. 2017;292:35–45.
Greathouse KM, Palladino SP, Dong C, Helton ES, Ubogu EE. Modeling leukocyte trafficking at the human blood-nerve barrier in vitro and in vivo geared towards targeted molecular therapies for peripheral neuroinflammation. J Neuroinflammation. 2016;13:3.
Yosef N, Ubogu EE. alpha(M)beta(2)-integrin-intercellular adhesion molecule-1 interactions drive the flow-dependent trafficking of Guillain-Barre syndrome patient derived mononuclear leukocytes at the blood-nerve barrier in vitro. J Cell Physiol. 2012;227:3857–75.
Kanda T. Biology of the blood-nerve barrier and its alteration in immune mediated neuropathies. J Neurol Neurosurg Psychiatry. 2013;84:208–12.
Kanda T, Yamawaki M, Mizusawa H. Sera from Guillain-Barre patients enhance leakage in blood-nerve barrier model. Neurology. 2003;60:301–6.
Shimizu F, Sawai S, Sano Y, Beppu M, Misawa S, Nishihara H, Koga M, Kuwabara S, Kanda T. Severity and patterns of blood-nerve barrier breakdown in patients with chronic inflammatory demyelinating polyradiculoneuropathy: correlations with clinical subtypes. PLoS One. 2014;9:e104205.
Dong C, Palladino SP, Helton ES, Ubogu EE. The pathogenic relevance of alphaM-integrin in Guillain-Barre syndrome. Acta Neuropathol. 2016;132:739–52.
Bosetti F, Galis ZS, Bynoe MS, Charette M, Cipolla MJ, Del Zoppo GJ, Gould D, Hatsukami TS, Jones TL, Koenig JI, Lutty GA, Maric-Bilkan C, Stevens T, Tolunay HE, Koroshetz W. Small Blood Vessels: Big Health Problems Workshop P: “Small Blood Vessels: Big Health Problems?”: Scientific Recommendations of the National Institutes of Health Workshop. J Am Heart Assoc. 2016:5.
Ubogu EE. Inflammatory neuropathies: pathology, molecular markers and targets for specific therapeutic intervention. Acta Neuropathol. 2015;130:445–68.
Eames RA, Lange LS. Clinical and pathological study of ischaemic neuropathy. J Neurol Neurosurg Psychiatry. 1967;30:215–26.
Dalakas MC. Pathogenesis of immune-mediated neuropathies. Biochim Biophys Acta. 2015;1852:658–66.
Mathey EK, Park SB, Hughes RA, Pollard JD, Armati PJ, Barnett MH, Taylor BV, Dyck PJ, Kiernan MC, Lin CS. Chronic inflammatory demyelinating polyradiculoneuropathy: from pathology to phenotype. J Neurol Neurosurg Psychiatry. 2015;
Ziganshin RH, Ivanova OM, Lomakin YA, Belogurov AA Jr, Kovalchuk SI, Azarkin IV, Arapidi GP, Anikanov NA, Shender VO, Piradov MA, Suponeva NA, Vorobyeva AA, Gabibov AG, Ivanov VT, Govorun VM. The pathogenesis of the demyelinating form of Guillain-Barre Syndrome (GBS): proteo-peptidomic and immunological profiling of physiological fluids. Mol Cell Proteomics. 2016;15:2366–78.
Matsumuro K, Izumo S, Umehara F, Osame M. Chronic inflammatory demyelinating polyneuropathy: histological and immunopathological studies on biopsied sural nerves. J Neurol Sci. 1994;127:170–8.
Mitchell GW, Williams GS, Bosch EP, Hart MN. Class II antigen expression in peripheral neuropathies. J Neurol Sci. 1991;102:170–6.
Steck AJ, Kinter J, Renaud S. Differential gene expression in nerve biopsies of inflammatory neuropathies. J Peripher Nerv Syst. 2011;16(Suppl 1):30–3.
Orlikowski D, Chazaud B, Plonquet A, Poron F, Sharshar T, Maison P, Raphael JC, Gherardi RK, Creange A. Monocyte chemoattractant protein 1 and chemokine receptor CCR2 productions in Guillain-Barre syndrome and experimental autoimmune neuritis. J Neuroimmunol. 2003;134:118–27.
Mathey EK, Pollard JD, Armati PJ. TNF alpha, IFN gamma and IL-2 mRNA expression in CIDP sural nerve biopsies. J Neurol Sci. 1999;163:47–52.
Pollard JD, Baverstock J, McLeod JG. Class II antigen expression and inflammatory cells in the Guillain-Barre syndrome. Ann Neurol. 1987;21:337–41.
Pollard JD, McCombe PA, Baverstock J, Gatenby PA, McLeod JG. Class II antigen expression and T lymphocyte subsets in chronic inflammatory demyelinating polyneuropathy. J Neuroimmunol. 1986;13:123–34.
Putzu GA, Figarella-Branger D, Bouvier-Labit C, Liprandi A, Bianco N, Pellissier JF. Immunohistochemical localization of cytokines, C5b-9 and ICAM-1 in peripheral nerve of Guillain-Barre syndrome. J Neurol Sci. 2000;174:16–21.
Lindenlaub T, Sommer C. Cytokines in sural nerve biopsies from inflammatory and non-inflammatory neuropathies. Acta Neuropathol. 2003;105:593–602.
Van Rhijn I, Van den Berg LH, Bosboom WM, Otten HG, Logtenberg T. Expression of accessory molecules for T-cell activation in peripheral nerve of patients with CIDP and vasculitic neuropathy. Brain J Neurol. 2000;123(Pt 10):2020–9.
Kieseier BC, Tani M, Mahad D, Oka N, Ho T, Woodroofe N, Griffin JW, Toyka KV, Ransohoff RM, Hartung HP. Chemokines and chemokine receptors in inflammatory demyelinating neuropathies: a central role for IP-10. Brain J Neurol. 2002;125:823–34.
Jones G, Zhu Y, Silva C, Tsutsui S, Pardo CA, Keppler OT, McArthur JC, Power C. Peripheral nerve-derived HIV-1 is predominantly CCR5-dependent and causes neuronal degeneration and neuroinflammation. Virology. 2005;334:178–93.
Bosboom WM, Van den Berg LH, Mollee I, Sasker LD, Jansen J, Wokke JH, Logtenberg T. Sural nerve T-cell receptor Vbeta gene utilization in chronic inflammatory demyelinating polyneuropathy and vasculitic neuropathy. Neurology. 2001;56:74–81.
Collins MP, Arnold WD, Kissel JT. The neuropathies of vasculitis. Neurol Clin. 2013;31:557–95.
Engelhardt A, Lorler H, Neundorfer B. Immunohistochemical findings in vasculitic neuropathies. Acta Neurol Scand. 1993;87:318–21.
Leppert D, Hughes P, Huber S, Erne B, Grygar C, Said G, Miller KM, Steck AJ, Probst A, Fuhr P. Matrix metalloproteinase upregulation in chronic inflammatory demyelinating polyneuropathy and nonsystemic vasculitic neuropathy. Neurology. 1999;53:62–70.
Oka N, Kawasaki T, Mizutani K, Sugiyama H, Akiguchi I. Hypoxia-inducible factor 1alpha may be a marker for vasculitic neuropathy. Neuropathology. 2007;27:509–15.
Probst-Cousin S, Neundorfer B, Heuss D. Microvasculopathic neuromuscular diseases: lessons from hypoxia-inducible factors. Neuromuscul Disord. 2010;20:192–7.
Meyer zu Horste G, Hartung HP, Kieseier BC. From bench to bedside – experimental rationale for immune-specific therapies in the inflamed peripheral nerve. Nat Clin Pract Neurol. 2007;3:198–211.
Schafflick D, Kieseier BC, Wiendl H, Meyer Zu Horste G. Novel pathomechanisms in inflammatory neuropathies. J Neuroinflammation. 2017;14:232.
Archelos JJ, Maurer M, Jung S, Miyasaka M, Tamatani T, Toyka KV, Hartung HP. Inhibition of experimental autoimmune neuritis by an antibody to the lymphocyte function-associated antigen-1. Lab Invest. 1994;70:667–75.
Zou LP, Pelidou SH, Abbas N, Deretzi G, Mix E, Schaltzbeerg M, Winblad B, Zhu J. Dynamics of production of MIP-1alpha, MCP-1 and MIP-2 and potential role of neutralization of these chemokines in the regulation of immune responses during experimental autoimmune neuritis in Lewis rats. J Neuroimmunol. 1999;98:168–75.
Duan RS, Chen Z, Bao L, Quezada HC, Nennesmo I, Winblad B, Zhu J. CCR5 deficiency does not prevent P0 peptide 180-199 immunized mice from experimental autoimmune neuritis. Neurobiol Dis. 2004;16:630–7.
Salomon B, Rhee L, Bour-Jordan H, Hsin H, Montag A, Soliven B, Arcella J, Girvin AM, Padilla J, Miller SD, Bluestone JA. Development of spontaneous autoimmune peripheral polyneuropathy in B7-2-deficient NOD mice. J Exp Med. 2001;194:677–84.
Ubogu EE, Yosef N, Xia RH, Sheikh KA. Behavioral, electrophysiological, and histopathological characterization of a severe murine chronic demyelinating polyneuritis model. J Peripher Nerv Syst. 2012;17:53–61.
Louvet C, Kabre BG, Davini DW, Martinier N, Su MA, DeVoss JJ, Rosenthal WL, Anderson MS, Bour-Jordan H, Bluestone JA. A novel myelin P0-specific T cell receptor transgenic mouse develops a fulminant autoimmune peripheral neuropathy. J Exp Med. 2009;206:507–14.
Acknowledgments and Funding
Special thanks to past and current employees of the Shin J Oh Muscle and Nerve Histopathology Laboratory, the University of Alabama at Birmingham, for processing human tissue and generating histopathology slides from which digital photomicrographs are shown and current and past members and collaborators of the Neuromuscular Immunopathology Research Laboratory (NIRL) for digital photomicrographs and ultramicrographs of human cells and tissues and mouse tissues. Work described from the NIRL was supported by National Institutes of Health Grants R21 NS073702 (2011–2014), R21 NS078226 (2012–2015), R01 NS075212 (2012–2018), and a Creative and Novel Ideas in HIV Research Subaward P30 AI27767 (2012-2015), as well as institutional support from the Department of Neurology, the University of Alabama at Birmingham. The content is solely the responsibility of the author and does not necessarily represent the official views of the National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Ubogu, E.E. (2019). Structural and Functional Characteristics of the Human Blood-Nerve Barrier with Translational Implications to Peripheral Nerve Autoimmune Disorders. In: Mitoma, H., Manto, M. (eds) Neuroimmune Diseases. Contemporary Clinical Neuroscience. Springer, Cham. https://doi.org/10.1007/978-3-030-19515-1_8
Download citation
DOI: https://doi.org/10.1007/978-3-030-19515-1_8
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-19514-4
Online ISBN: 978-3-030-19515-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)