Abstract
Sub-Saharan Africa is prone to outbreaks of emerging and re-emerging infectious diseases, especially those transmitted between vertebrate animals and humans. Some of these diseases include: Ebola, Rift Valley Fever, and plague. In recent years, a number of sub-Saharan African countries have experienced outbreaks of either Ebola or Lassa fever, which tend to spread across national borders. Yet, the reaction of governments in many of the affected African countries has often been characterized by an inability to promptly, effectively, and efficiently address these outbreaks. In this chapter we discuss the role of African governments in the fight against emerging and re-emerging infectious diseases.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
1 Historical Perspectives
One of the earliest records of disease outbreak in Africa dates back to the period 165–180 AD, when an unknown disease with symptoms similar to smallpox occurred in northern Africa (UMMC 1999). This was followed by plague disease which ravaged human populations from 746 to 747 AD. According to Alchon (2003), the plague is caused by a bacterium, Yersinia pestis. Humans can get the plague following a bite by a rodent flea that is a carrier of the bacterium. Alternatively they could be infected by handling an animal that is infected with it. Plague continued to ravage human populations up to the early nineteenth century, when the discovery and use of modern antibiotics brought the disease under control. However, human plague infections still occur significantly in parts of Africa and Asia (CDC 2017). The end of the nineteenth century and the beginning of the twentieth century saw the appearance of the influenza disease. Particularly severe were the global influenza pandemics of 1889–1890 and 1918–1920, during which one million and 75 million people, respectively, died. (Great Britain Local Government Board 1893; Patterson and Pyle 1991). Less severe influenza pandemics occurred between 1957 and 1958 (Asian flu, with two million deaths), and between 1957 and 1958 (Hong Kong flu, with 1million deaths) (Paul 2008).
1.1 Cholera
Cholera that has been responsible for significant high morbidity and mortality throughout human history (Hays 2005). Millions of people died during the first cholera pandemic from 1816 to 1826, and seventh cholera pandemics which occurred in 1975. Cholera remains a disease of public health concern especially in Africa and Asia, with an on-going massive cholera epidemic in Yemen since 2016 (WHO 2017a).
1.2 Yellow Fever
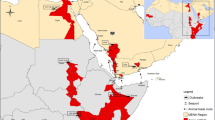
Yellow Fever (YF) is a mosquito-borne virus disease, with an incubation period of 3–7 days, and an initial malaria-like acute onset. The disease runs a mild to fatal course, with either remission in 4–5 days or, death within 7–10 days (WHO 2016a). The severe disease is characterised by hepato-renal and neurotropic manifestations with or without haemorrhage. Mortality can be as high as 20–50% in hepato-renal disease. It is believed that YF originated in Africa since 3000 BC (CDC 2017). Through the slave trade (1526–1687), the disease was introduced into the Americas (Haddow 2012). Maya manuscripts dating back to 1648 contain the description of a disease with symptoms similar to yellow fever. Severe YF epidemics were reported to have occurred in two waves in the Americas. The first wave, from 1668 to 1699, swept through New York, Boston and Charleston. The second wave which ran from 1793 to 1878 occurred in Philadelphia, Savannah, New Orleans, Norfolk, Texas. Louisiana and Mississippi. The disease was introduced to Europe between 1700 and 1730; first in Spain and subsequently in the French and English sea ports. The first documented report of YF in Africa was in 1768 in St. Louis, Senegal. Outbreaks of the disease were subsequently reported across West Africa from Fernando Po to the Gambia between 1842 and 1878. In 1930, two YF vaccines were discovered and used in mass vaccination exercises between 1934 and 1953 to control outbreaks In West Africa (Tomori 2004; WHO 2016a). It was not until 1948 that the World Health Organization (WHO) commenced the official reporting of YF outbreaks (Fig. 11.1a, Tomori 2015b). However following attainment of independence by many of the West African countries, mass YF campaigns were stopped and the disease re-emerged with reports of major outbreaks between 1960 and 1990 in West Africa and as far east as Ethiopia (Vainio and Cutts 1998; Garske et al. 2014). Further spread of the disease was reported with outbreaks in Central and East Africa between 1990 and 2003 (Fig. 11.1b; Tomori 2015b). Following the introduction by the WHO, of an YF control strategy in West Africa, YF outbreaks escalated in Central and East Africa with reduced incidence in West Africa (WHO 2010). The YF control strategy used a three prong approach – a preventive mass campaign, introduction of YF vaccine into the routine immunization programme and an improved surveillance system for rapid YF outbreak detection. Consequently, the period 2008–2014, coincided with a definite shift of YF outbreaks to Central and East Africa. In 2016, a severe urban YF outbreak which began in Luanda, Angola, later spread to the Democratic Republic of Congo, with infection and self-limiting exportation to China, Kenya and Mauritania (WHO 2017b).
(a) Seventy years of reporting yellow fever outbreaks to the World Health Organization. (b) Shift of yellow fever Oubreaks from west to east and central Africa. (Tomori 2015b)
1.3 Lassa Fever
Lassa fever is endemic in many countries in West Africa including: The Republic Benin, Ghana, Guinea, Liberia, Mali, Sierra Leone, and Nigeria (CDC 2015). Lassa fever is an acute viral haemorrhagic illness and the causative agent, the Lassa virus, is transmitted to humans through contact with food or other household items that have been contaminated with the urine or faeces of a rodent that is infected with the virus. There is also the possibility Person-to-person and laboratory transmission especially in hospitals that do not have adequate infection prevention and control measures The incubation period of the disease ranges from 7 to 21 day. Case-fatality rate for Lassa fever is 1% (Richmond and Baglole 2003), whilst observed case-fatality rate among patients hospitalized with severe Lassa fever is approximately 15%.
Between 1969, when the disease was first discovered in Nigeria, and 2000, Lassa fever disease in Nigeria was limited to a few states in northern Nigeria occurring usually during the dry season. However, from 2001, the disease was reported in 10–21 of the 36 states in Nigeria and occurring throughout the year. For example, in 2016, there were over 1000 cases and 120 deaths reported from 28 states. In 2017 up to week 46, 19 States have reported 985 suspected cases and 126 deaths (NCDC 2017a), whereas in 1989, there were cases and deaths in 2 states, 29 cases and 12 deaths associated with nosocomial infections in a hospital Fisher-Hoch et al. (1995).
1.4 Ebola Virus Disease (EVD)
Ebola virus disease (EVD), previously known as Ebola haemorrhagic fever, is a severe, often fatal illness in humans (CDC 2017b). The average case fatality rate for EVD is around 50% with case fatality rates often varying from 25% to 90% (WHO 2018). The Ebola virus is transmitted to humans via wild animals and spreads in the human population through human-to-human transmission. Early supportive care with rehydration and treatment of symptoms has the potential to improve survival. Till date, there is no licensed proven treatment for EVD but there exist a range of blood, immunological and drug therapies that are currently are under development. The first EVD outbreaks occurred in 1976 in remote villages in the Democratic Republic of Congo and Sudan and 602 cases and 431 deaths were recorded (Table 11.1) (CDC 2017c). Between 1976 and 2012, there were 22 EVD outbreaks in Africa, with 17,851 cases and 1159 deaths reported in 22 countries (DR Congo (5), Uganda (5) Congo (4), Gabon (4), Sudan (2), RSA (1) and CIV (1). The most severe and devastating epidemic of EVD occurred in West Africa (from 2014 to 2015. A total of 28,639 cases and 11,322 deaths were recorded in the 6 affected countries (CDC 2017c) – Guinea (3811 cases and 2543 deaths), Sierra Leone (14,214 cases and 3956 deaths), Liberia (10,675 cases and 4809 deaths), Nigeria (20 cases and 8 deaths), Mali (8 cases and 6 deaths) and Senegal (1 case and 0 death). Seven additional cases with one death, were exported to the USA (4 cases and 1 death) and one case each with no death in Spain, UK and Italy. An EVD outbreak in 2014, independent of the West African outbreaks, occurred in the DRC with 66 cases and 49 deaths. Eight cases and 4 deaths were recorded in the eighth EVD outbreak in DRC which occurred between May and July 2017 (CDC 2017c). The ninth EVD outbreak in the Democratic Republic of the Congo, started in April 2018 in the Bikoro Health Zone. Since the onset (on 4 April 2018), of the recent outbreak, 50 confirmed or probable EVD cases and 25 deaths (giving a case fatality rate 50.0%) have been reported. (WHO 2018).
Effective control of an EVD outbreak would require a holistic approach involving but not limited to interventions such as: case management; infection prevention and control practices; surveillance and contact tracing; a good laboratory service; safe burial practices; and social mobilisation. Also, history shows that the role of community engagement in the management of EVD outbreaks cannot be overemphasised. These measures, however, often come up against countervailing socio-cultural beliefs, rituals and practices, such as those connected with death, burial, afterlife and welfare of the community (WHO 2018).
A recent WHO-led clinical trial aimed at testing a potential vaccine against EVD saw the participation reported that the candidate vaccine was highly protective against the Ebola virus (WHO 2017c). This vaccine is yet to receive regulatory approval. However, there is a commitment to conduct emergency ring vaccinations in the unfortunate event of an EVD outbreak in selected African countries including: the Democratic Republic of Congo; Sierra Leone; Liberia; Gabon; Guinea Bissau; the Republic of Guinea; Ivory Coast; Mali; Niger; Nigeria; Senegal; South Sudan; and Uganda. These emergency ring vaccinations will be carried out under the umbrella of the Expanded Access/Compassionate use. A ring EVD vaccination exercise was launched in affected Health Zones of the 2018 EVD outbreak in the DRC (WHO 2018). The exercise targeted health professionals, persons who have been in contact with confirmed EVD cases, as well as contacts of these contacts (Ibid).
1.5 Cerebrospinal Meningitis
Cerebrospinal meningitis is a bacterial disease caused by several microorganisms; among which is, Neisseria Meningitides (Nm), the most common cause of bacterial meningitis. The disease is highly contagious and often occurs as seasonal epidemics with the greatest burden being in in the meningitis belt of sub-Saharan Africa (WHO 2017d). In the meningitis belt, the highest morbidity due to cerebral meningitis is often recorded during the dry season, as the climatic and living conditions (e.g. crowding) as well as population movements tend to favour increased disease transmission. Between 1995 and 2004, outbreaks in the meningitis belt (Table 11.2) have resulted in close to 700,000 cases and 60,000 deaths (WHO 2005, 2014, 2015, 2016b, 2017d; Horn 1951; Mohammed et al. 2000; NCDC 2013, 2014, 2015, 2016a, b, 2017b). The largest recorded outbreak, in 1996, caused 250,000 cases and almost 25,000 deaths, with Nigeria alone reporting 109, 500 cases and 11,717 deaths (Mohammed et al. 2000). With the introduction, in 2010, of the Men-A conjugate vaccine (MenAfrivac) for mass preventive immunization campaigns in the meningitis belt, the occurrence of the disease has declined significantly (SAGE 2014). However, the risk of other types of Nm persists, with a recent outbreak in Nigeria, recording 14,005 cases and 1114 deaths (NCDC 2017b).
1.6 Other Disease Outbreaks
Apart from the above listed diseases, Africa is home to other outbreaks of diseases, such as cholera, plague, Dengue haemorrhagic fever and Typhoid. The WHO African Region (AFRO) publishes a weekly bulletin on disease outbreaks and other emergencies in African countries. The edition of the bulletin for week 50 (10–15 December 2017) reports 2 new events (Fig. 11.2), 47 ongoing events, 41 disease outbreaks and 8 humanitarian crises (WHO-AFRO 2017a, b). On the cholera outbreaks in Zambia and Tanzania, the WHO AFRO weekly bulletin reports that“…the incidence of cholera in Zambia is rapidly increasing, particularly in Lusaka District where transmission is intense. In Tanzania, the cholera outbreak is spreading to new areas, including Dar es Salam and Ruvuma Region. Cholera case fatality rates have remained very high in both countries, as well as in the Democratic Republic of the Congo, ranging from 3–5% (exceeding the 1% mark set by WHO)” (ibid). The bulletin declares that national authorities are failing in their duties and recommends that “the ongoing cholera outbreaks in Zambia and Tanzania require specific attention from the national authorities and partners” (ibid). Meanwhile, response efforts to the cholera outbreak in the Democratic Republic of the Congo need to be scaled up and maintained. On the continuing deterioration of the “humanitarian crisis in the Democratic Republic of the Congo, the bulletin joins UNICEF in warning that “more than 400 000 severely malnourished children could die within months unless emergency interventions are undertaken” (ibid).
Outbreak and emergency situation in Africa. Left- Week 21 (20–26 May 2017) and Right -Week 50 (10–15 December 2017). (WHO-AFRO 2017a)
In Nigeria, the WHO is monitoring outbreaks of Lassa Fever, Yellow Fever, Cholera, Monkey pox, as part of the humanitarian crises caused by the “protracted conflict which has resulted in widespread population displacement, restricted access to basic social services, including healthcare and protection needs, and a deepening humanitarian crisis” (WHO-AFRO 2017a, b). Furthermore, in 2017, UNICEF estimates that about 400,000 children in north-east Nigeria are at risk of severe acute malnutrition and without treatment, it is estimated that one in five of those children are likely to die (Vittozzi 2017). The UNICEF report concluded that the malnutrition situation in northeast Nigeria remains critical. The number of cases of children suffering from Severe Acute Malnutrition is extremely high, with the crisis in Borno state most acute. In 2016, working with partners, UNICEF treated 160,000 children suffering from Severe Acute Malnutrition in Borno, Yobe and Adamawa states. Although UNICEF has made significant progress in reaching children and their families with healthcare, treatment for malnutrition, safe water, sanitation and hygiene services, education and child protection, a persistent lack of funding continues to hamper the response effort (Vittozzi 2017).
The state of affairs presented in the WHO AFRO bulletin for week 50 of 2017 is not an isolated event. It is a description of the day to day, common and regular endemic state of disease outbreaks and humanitarian disasters occurring all over Africa. How did Africa get to this miserable, deplorable, poor and wretched state of health? How come there is this pervading powerlessness and inability to control even the commonest diseases? It is an indictment of national authorities in Africa, who in perpetual fits of misplaced priorities, divert national resources to executing projects which fail to provide those basic essentials and facilities needed to provide better health and better protection for their citizens from the scourge of preventable and controllable disease outbreaks. Year in, year out, African countries lurch and stumble in dazed stupor as Ebola decimates sections of our populations, leaving survivors to the pain, agony and eventual annihilation by any of cholera, Lassa fever, malaria and numerous other diseases that have become contributors to the underdevelopment of African states. We must appreciate that the long history of poor governance in many independent Africa countries is the root cause of the deplorable state of humanitarian disasters arising from disease outbreaks.
2 Has Africa Always Been Unable to Take Care of Her Health and Incapable of Mounting effective Response for Combating Disease Outbreaks?
If Africa was ever able, it must have been only during the period when many African countries were under colonial subjugation/rule. For close to 50 years or more, many African countries have ostensibly operated as independent nations, free from colonial rule. Yet with every disease outbreak, African countries surrender their independence to donors and partners, as they seek assistance and aid to control even common disease outbreaks. Since 1976, when EVD was first discovered in Africa, and before the West African outbreak, there have been 24 other outbreaks, with a total of 2900 cases and 1583 deaths (CDC 2017a). Despite the number of EVD outbreaks in Africa, the West African outbreak, in terms of morbidity and mortality, was ten times worse that all of the EVD outbreaks reported in Africa since 1976. When the West African EVD humanitarian disaster was declared over by the WHO, there were 28,712 cases and 11,372 deaths (CDC 2017c). Several reasons were given for Africa’s inability to learn from the lessons of the past and her inability to prevent repeats of histories of devastating disease outbreaks. According to Tomori (2015a, b), some of the reasons include:
-
Unreliable disease surveillance system in most countries resulting in failure of early detection of outbreaks;
-
Official national and community denial of the occurrence of most epidemics;
-
Fragile and weak heath systems in most African countries leading to limited capacity to manage and contain epidemics;
-
Cultural practices that favour the spread of the disease;
-
Easy and uncontrolled mass movement of people between rural areas and urban centres, and across national and international borders;
-
An unashamed dependency on foreign assistance.
2.1 Capacity Building for National Ownership
Africa has experienced no less than 24 episodes of EVD outbreaks, since the first outbreak was reported in 1976 (CDC 2017b). One would imagine that by now, African countries would have learned the lessons and develop their capacity and competence to deal with EVD or other disease outbreaks. However, and unsurprisingly, Africa has, for the past 41 years relied heavily on international aid and assistance to solve a majority of its health crisis (Tomori 2015a, b). Unfortunately, these long years of reliance on international aid to support the continent’s response to disease outbreaks has not yielded the desired result on building human and infrastructural capacity in Africa to effectively respond to disease outbreaks. This is aptly illustrated in a diary entry (published) of a WHO expert who has been at the forefront of several outbreaks of EVD and other viral haemorrhagic fever in Africa. He writes “We received the first emails on the Guinea event on Friday 14 March. During the weekend, I was travelling in the DRC for a training course on how to take blood samples in Ebola. It was a 3-day training course”(Formenty 2015). It is almost heart-breaking to read that in 2014, African health care professionals need to travel out of their countries to receive training on ‘how to take blood samples in Ebola’. Although the DRC often provides technical assistance to other African countries during outbreaks of EVD Muyembe-Tamfum et al. (2012), by and large, Africa has relied on foreign assistance and aid to solve her disease outbreak problems, and failed to create an enabling and a conducive local environment for her human resource capacity to function effectively (Tomori 2015a, b).
Arguably, foreign aid, by its nature and character, be it for economic purposes or health research, always tend to benefit the donor country more than the recipient country. A Director of a UK funded research centre in Africa, wrote this piece in an annual report of the center: “Perhaps the most important achievement of the Unit during the past five years has been the training of a group of dedicated scientists and clinicians who have gone on to establish their own highly successful groups elsewhere in the tropics and in the UK and who continue to maintain the high reputation of the UK in tropical medicine” (Greenwood 1995). Unfortunately, this is not an isolated case in Africa. A cursory review of the activities of externally funded health research centres in Africa reveals that researchers of the donor countries tend to benefit more from research collaborations than their African counterparts (Adubifa 2004). Although the dynamics may be slowly changing, in many of the international conferences that I have attended and for which issues concerning diseases of African origin, and diseases endemic in Africa are being discussed, most, if not all, of the participating researchers and presenters are non-Africans. Equally, outbreak of emerging and re-emerging infectious diseases have become opportunities for researchers in high income and research intense countries to fine-tune their skills thereby enabling them to be at the forefront of solving Africa’s health problems, and by extension, doing for Africa what African researchers should be doing for Africa (Tomori 2015a, b). When African researchers are engaged in these collaboration, they are assigned the role sample collectors and eventually end up as impotent contributors to the control of disease outbreaks in their own countries and globally (Tomori 2015a, b).
For Africa to be prepared to manage and contain future outbreaks of EVD, the current heavy reliance and nature of external aid and assistance for health research collaboration must change (ibid). Africa must reorganize her priorities, and invest in health research and research capacity strengthening. Also, African governments must create an environment that allows its researchers to function with some degree of relevance and independence. On the other hand, high income countries should consider relinquishing their dominance and control of global disease surveillance and establish research collaborations that are based on mutual appreciation and respect for all partners (Tomori 2014). If African countries and high income countries where to do the suggested, the chances of Africa adequately responding to outbreaks will be maximised (Tomori 2015a, b).
3 African Governments and People’s Health
While governments and scientific communities in the high income countries often devote considerable resources to addressing emerging infectious diseases, the reverse is the case in the resource-constrained countries of Africa, despite the fact that the region is especially prone to outbreaks of the diseases. In 2001, African Heads of State signed the Abuja Declaration and where member states pledged to increase their health budgets to at least 15% of the their annual national budget OAU (2001). Ten years after the Abuja Declaration, the African Union reported that only six AU member states had met set benchmark (Olajide 2010). Following an assessment by the WHO, 15 years after the Commitment was made, it was concluded that “Most African governments have increased the proportion of total public expenditure allocated to health in the early 2000s. The average level of per capita public spending on health rose from about US$70 in the early 2000s to more than US$160 in 2014. Domestic resources for health in Africa accounted for about 76 [per cent] in 2014, and external aid has increased from 13 [per cent] to 24 [per cent] of total health expenditure over the same period” (WHO 2016c). However, the report also estimated that of every US$100 of State fund, only US$16 is allocated to health, of which less than US$4 goes to the right health services. It also observed that funding of the health sector is inconsistent and unpredictable (ibid).
Many African governments still ascribe their lack of adequate funding for public health activates to the rampant poverty in their countries. But a look at the recent operations of many African countries will suggest that the real culprits are corruption and prolonged misplaced priorities. The AIDS and Rights Alliance for Southern Africa, a regional network of non-governmental organizations, runs a campaign that draws attention to the spending choices of African governments (AIDS and Rights Alliance for Southern Africa 2014). The alliance reports that some African governments, would rather devote exorbitant sums of national spending to frivolous expenditures rather than allocate it to health, education, and other basic services. Some examples include $500,000 expenditure on a luxury car for the king of Swaziland; A 48 million dollar spending for a private presidential jet for Uganda; $27 million dollars for a bronze statue in Senegal (Walker 2010); and the proposed new city gate to Abuja, which is estimated would cost the government of Nigeria, the huge sum of 395 million dollars (Agbo 2013). None of these nations has met its 2001 commitment in the Abuja declaration.
In most African countries, implementation of disease surveillance systems for the early detection of emerging pathogens remains defective (Tomori 2014). A recent assessment of disease surveillance and response in Kaduna State, Nigeria, showed that approximately 38% of the state’s health facilities in Kaduna had no standard case definition for priority diseases, 71% lacked access to a computer and printer, and 81% did not analyse the data collected in their health facilities (Abubakar et al. 2013). Poor surveillance and data management can increase the time between the beginning of an outbreak and the first reported case, thereby limiting response. African governments have the capacity to provide such basic services, yet corruption and misplaced priorities makes it almost impossible for them to provide such services. For example, the African Union estimates that corruption costs African economies about $150 billion each year. Undoubtedly, a minute fraction of that amount could provide many African countries with efficient disease surveillance systems (Tomori 2014). African countries will have to wake up from their slumber and dependency.
4 Way Forward
A short while before and immediately after the WHO declared that the EVD outbreak in West Africa had been contained, many agencies, governments, and institutions all from outside Africa had already put together resources to assess lessons learnt from the EVD outbreaks and to map out strategies in the unfortunate event of another epidemics (Bell et al. 2016). However, the reports from Africa where mainly related to misuse of EVD funds (Paye-Layleh 2015; Sahid 2015). This was at a time when international agencies and donor governments were being blamed for reacting too slowly and not providing enough donations to combat the EVD disaster (Nierle and Jochum 2014; Grépin 2015). The questions that come to mind are: Is Africa prepared for the next EVD epidemic? What lessons have African countries learnt from the 2014 to 2015 EVD outbreak? Will Africa still be helpless and totally dependent on international agencies for assistance to control the future epidemic, which will surely come? Will scientists and governments from high income countries continue to control the processes of global disease surveillance system without meaningfully engaging African countries? Of course, the answers of a right thinking pessimist would be “no” to the first two questions and “yes” to the last two. For the contrary to occur, Africa must accept that her self-imposed poverty status is not due to a lack of resources, but rather to their misuse, and that it can be reversed (Tomori 2015a, b). This requires purposeful leadership and rightly guided followership. African leaders must regard health as a human right and the citizens must demand this right from their governments. Without good governance, there will never be a purposeful and citizen oriented leadership. With poor governance, funds allocated for health, education and other development activities are likely to be wasted, yielding poor outcomes. In addition, aid funds are more likely to be sucked into the vortex of corruption along with local and national resources (Paye-Layleh 2015; Sahid JS 2015). Civil society, and other stakeholders, must be frontline advocates for the establishment and institutionalization of good governance. During the first annual African governance forum, Kofi Anan, former UN Secretary General, said: ‘there is no single issue of greater importance to the economic and political future of Africa than good governance, and it must command the full and lasting attention of Africans” (ECA 1997). The comment was made 20 years ago, and it is even more relevant today for the development and health of the people of Africa.
4.1 Purposeful Leadership
African Governments must provide a conducive and enabling environment with adequate resources for African researchers working together under the One Health concept to focus on local processes of pathogen emergence. Pathogens emerge under widely varying environmental, demographic, and socioeconomic circumstances (Tomori 2014). A pathogen’s ability to emerge or re-emerge depends on several factors including but not limited to genetic changes or adaptation in the pathogen; environmental conditions; land use patterns;, international trade patterns, the neglect of public health services, and bioterrorism (Daszak et al. 2012). These factors and conditions interact differently in different parts of the world and therefore, the first step toward preventing and controlling outbreaks of emerging and re-emerging diseases will be to gain a thorough understanding of local processes of pathogen emergence. Once such an understanding is gained then governments, institutions, and researchers and healthcare professionals in Africa would have to commit themselves to clearly defined and proactive roles in the fight against emerging and re-emerging infectious diseases.
4.2 African Health Researchers
The African researcher or scientist would have to evaluate the needs of the countries where they operate and direct their research activities towards issues that are relevant to their and can positively impact on the daily life of populations in their countries of operation (Tomori 2015a, b). African researchers must take on the extra roles of guide, teacher, mentor, and beacon to their society. Therein lies the true relevance of the African researcher. International aid will not build the capacity for such desired relevance; it would have to be funded and groomed with home-based resources and through governments with the right priorities (ibid).
4.3 National Governments
It is also essential for all African countries to take “ownership” of their national disease surveillance systems as well as prevention and control of epidemics. This will allow for effective country-specific response measures (Tomori 2014). This will entail making a genuine political commitment providing adequate resources both financial and human. Taking a proactive role in combating disease will also require that African governments: implement appropriate emergency response plans; coordinate collaborative interactions between human and veterinary health surveillance systems; build and sustain the capacity of local health personnel to respond to disease outbreaks; ensure private sector involvement and establish a multidisciplinary approach to disease control (Tomori 2014).
4.4 Global Health Collaborations
Making the world safer from emerging and re-emerging infectious diseases requires global collaboration (Tomori 2014). It will entail a global collaborative efforts to develop and implement policies, for pathogen control and spread. It will require the sharing of real-time surveillance information that could support the detection of zoonotic diseases in animal populations before they appear in human populations. Also the contributions of Science-based nongovernmental organizations should be harnessed. This is because some of these organisations have a wide geographic reach and could help provide comprehensive surveillance and response capabilities (Tomori 2014). The fight against emerging and re-emerging disease is a complex and difficult one and would require genuine commitment from the different global health actors.
5 Conclusion
African countries individually and collectively through the AU, the WHO African Region, and other international and regional groups, have by resolutions and agreements, focused actions and adopted measures for the promotion and protection of the health of Africans. The enthusiasm of African leaders (including Ministers of Health) for adopting resolutions and making declarations on good governance was far less than for health and health matters. Over the past 10 years, African leaders have, on the average, adopted four resolutions/declarations per year on health and less than one on good governance (African Union 2017; WHO Afro 2017b). The surfeit of health resolutions, demonstrating more political “whiff” than will and commitment has led to Africa’s health indices remaining much below accepted and acceptable standards. The clear lesson is that the health of African citizens will continue to suffer in the absence of good governance.
On a final positive note, two survivors of the EVD outbreak in Sierra Leone Ebola have sued the Sierra Leone government over mismanaged funds (Reuters 2017). This is a step in the right direction and a demonstration of positive use of citizen power. Our governments must be held accountable for their negligence and failure especially on issues adversely affecting the health of the people. On reflection, it appears that some of our leaders, through poor governance, may have produced more severe and longer lasting devastation than have been caused by past disease epidemics in Africa.
References
Abubakar, A. A., Sambo, M. N., Idris, S. H., Sabitu, K., & Nguku, P. (2013). Assessment of integrated disease surveillance and response strategy implementation in selected Local Government Areas of Kaduna state. Annals of Nigerian Medicine, 7(1), 14–19.
Adubifa, A. O. (2004). An assessment of science and technology capacity building in sub-Saharan Africa (Special Paper Series 19). Nairobi: African Technology Policy Studies Network (ATPS).
African Union. (2017). OAU/AU treaties, conventions, protocols & charters. https://au.int/en/treaties. Accessed 19 Sept 2017.
Agbo, C. (2013). Nigeria: New Abuja city gate to gulp N64 billion. http://allafrica.com/stories/201310150454.html. Accessed 21 Dec 2017.
AIDS and Rights Alliance for Southern Africa. (2014). The right to health. Available at: www.arasa.info/index.php/info/fact-sheets. Accessed 26 Jan 2019.
Alchon, S. A. (2003). A pest in the land: New world epidemics in a global perspective (p. 21). University of New Mexico Press. ISBN:0-8263-2871-7.
Bell, B. P., Damon, I. K., Jernigan, D. B., Kenyon, T. A., Nichol, S. T., O’Connor, J. P., & Tapperoet, J. W. (2016). Overview, control strategies, and lessons learned in the CDC response to the 2014–2016 Ebola epidemic. MMWR Supplement, 65(3), 4–11.
CDC. (2015). Lassa fever. https://www.cdc.gov/vhf/lassa. Accessed 19 Dec 2017.
CDC. (2017). Plague. https://www.cdc.gov/plague/. Accessed 19 Dec 2017.
CDC. (2017a). Yellow fever timeline of activities. https://www.cdc.gov/travel-training/local/HistoryEpidemiologyandVaccination/HistoryTimelineTranscript.pdf. Accessed 21 Dec 2017.
CDC. (2017b). Ebola virus disease. https://www.cdc.gov/vhf/ebola. Accessed 21 Dec 2017.
CDC. (2017c). Outbreaks chronology- Ebola virus disease. https://www.cdc.gov/vhf/ebola/outbreaks/history/chronology.html#modalIdString_outbreaks. Accessed 21 Dec 2017.
Daszak, P., Zambrana-Torrelio, C., Bogich, T. L., Fernandez, M., Epstein, J. H., Murray, K. A., & Hamilton, H. (2012). Interdisciplinary approaches to understanding disease emergence: The past, present, and future drivers of Nipah virus emergence. PNAS, 110(Suppl 1), 3681–3688.
Economic Commission for Africa. (1997). ECA First African Governance Forum. http://www.africa.upenn.edu/Urgent_Action/apic_72097.html. Accessed 19 Sept 2017.
Fisher-Hoch, S. P., Tomori, O., Nasidi, A., Perez-Oronoz, G. I., Fakile, Y., Hutwagner, L., & McCormick, J. B. (1995). Review of cases of nosocomial Lassa fever in Nigeria: The high price of poor medical practice. British Medical Journal, 311(7009), 857–859.
Formenty, P. (2015). Ebola diaries: First signals–March 2014. http://www.who.int/features/2015/ebola-diaries-formenty/en/. Accessed 19 Dec 2017.
Garske, T., Van Kerkhove, M. D., Yactayo, S., Ronveaux, O., Lewis, R. F., Staples, J. E., Perea, W., Ferguson, N. M., & Committee, Y. F. E. (2014). Yellow Fever in Africa: Estimating the Burden of Disease and Impact of Mass Vaccination from Outbreak and Serological Data. PLoS Medicine, 11(5), e1001638.
Great Britain Local Government Board. (1893). Further report and papers on epidemic influenza, 1889–92: With an introduction by the medical officer of the Local Government Board. H. M Stationery Office.
Greenwood, B. M. (1995). From the Director: Gambia Medical Research Council Unit; Annual Report for 1995.
Grépin, K. A. (2015). International donations to the Ebola virus outbreak: Too little, too late? BMJ, 350, h376.
Haddow, A. J. (2012). The Natural History of Yellow Fever in Africa. Proceedings of the Royal Society of Edinburgh Section B: Biology, 70(03), 191–227.
Hays J. N. (2005). Epidemics and pandemics: Their impacts on human history (pp. 513). Santa Barbara: ABC-CLIO.
Horn, D. W. (1951). The epidemic of cerebrospinal fever in the northern provinces of Nigeria, 1949–1950. Journal Royal Sanitary Institute, 71(5), 573–589.
Mohammed, I., Nasidi, A., Alkali, A. S., Garbati, M. A., Ajayi-Obe, E. K., Audu, K. A., Abdulmumini, U., & Suleiman Abdullahi, S. (2000). A severe epidemic of meningococcal meningitis in Nigeria May 2000. Transactions of the Royal Society of Tropical Medicine and Hygiene, 94(3), 265–270.
Muyembe-Tamfum, J. J., Mulangu, S., Masumu, J., Kayembe, J. M., Kemp, A., & Paweska, J. T. (2012). Ebola virus outbreaks in Africa: Past and present. The Onderstepoort Journal of Veterinary Research, 79, 6–13.
Nierle, T., & Jochum, B. (2014). Ebola: The failures of the international outbreak response. http://www.msf.org/article/ebola-failures-internationaloutbreak-response. Accessed 21 Dec 2017.
Nigeria Centre for Disease Control. (2013). Federal Ministry of Health–Weekly Epidemiological Report: 2:52.
Nigeria Centre for Disease Control. (2014). Federal Ministry of Health–Weekly Epidemiological Report: 4 (1).
Nigeria Centre for Disease Control. (2015). Federal Ministry of Health – Weekly Epidemiological Report. 4: 52.
Nigeria Centre for Disease Control. (2016a) Federal Ministry of Health – Weekly Epidemiological Report: 6:5.
Nigeria Centre for Disease Control. (2016b). Federal Ministry of Health Weekly Epidemiology Report: 6:49.
Nigeria Centre for Disease Control. (2017a). Federal Ministry of Health Lassa fever outbreak weekly Situation Report No. 46–December 1, 2017.
Nigeria Centre for Disease Control. (2017b). Federal Ministry of Health – Nigeria Meningitis Outbreak, situation as of week ending 28 April 2017.
OAU. (2001). Abuja declaration on HIV/AIDS, tuberculosis and other related infectious diseases-OAU/SPS/ABUJA/3. http://www.un.org/ga/aids/pdf/abuja_declaration.pdf. Accessed 19 Dec 2017.
Olajide, A. (2010). Ten year review: Abuja Declaration on health financing in Africa. http://www.who.int/pmnch/media/membernews/2011/20110329_abujadeclaration.pdf. Accessed 18 Dec2017.
Patterson, K. D., & Pyle, G. F. (1991). The geography and mortality of the 1918 influenza pandemic. Bulletin of the History of Medicine, 65(1), 4–21.
Paul W. E. (2008). Fundamental immunology (pp. 1603). Philadelphia: Lippincott Williams & Wilkins.
Paye-Layleh, J. (2015). Liberia audit report questions $673,000 in Ebola spending. http://www.nytimes.com/aponline/2015/04/15/world/africa/ap-af-ebola-westafrica.html?_r=0. Accessed 21 Dec 2017.
Reuters. (2017). Ebola victims sue Sierra Leone government over mismanaged funds. https://www.reuters.com/article/us-health-ebola-leone/ebola-victims-sue-sierra-leone-government-over-mismanaged-funds-idUSKBN1E92NE. Accessed 21 Dec 2017.
Richmond, J. K., & Baglole, D. J. (2003). Lassa fever: Epidemiology, clinical features and social consequences. The BMJ, 327(7426), 1271–1275.
SAGE. (2014). Roll out of the meningococcal A conjugate vaccine through mass vaccination campaigns in countries of the African meningitis belt current status, vaccine coverage, epidemiological and economic impact. http://www.who.int/immunization/sage/meetings/2014/october/2.DJINGAREY_Session6_SAGE_Oct2014_FINAL_21Oct2014.pdf. Accessed 12 Dec 2017.
Sahid, J. S. (2015). Sierra Leone’s missing Ebola millions. http://www.irinnews.org/analysis/2015/03/30. Accessed 20 Dec 2017.
Tomori, O. (2004). Yellow fever: The recurring plague. Critical Reviews in Clinical Laboratory Sciences, 41(4), 391–427.
Tomori, O. (2014). Winning the battle against emerging pathogens. A Nigerian Response. Bulletin of the Atomic Scientists, 70, 14–17.
Tomori, O. (2015a). Will Africa’s future epidemic ride on forgotten lessons from the Ebola epidemic? BMC Medicine, 13, 116. https://doi.org/10.1186/s12916-015-0359-7.
Tomori, O. (2015b). Epidemiology and risk of yellow fever in current context. http://www.who.int/immunization/sage/meetings/2016/october/Session11-Part1-Epidemiology-and-risk-of-yellow-fever-in-current-context.pdf?ua=1. Accessed 25 Jan 2019.
University of Maryland Medical Center. (1999). Plague of Athens: Another medical mystery solved at University of Maryland. https://www.newswise.com//articles/plague-of-athens-medical-mystery-may-be-solved. Accessed 19 Dec 2017.
Vainio, J., & Cutts, F. (1998). Yellow fever. Geneva: World Health Organization (document WHO/EPI/GEN/98.11).
Vittozzi, K. (2017). 400,000 children in north-east Nigeria at risk of severe acute malnutrition. https://www.unicef.org/infobycountry/nigeria_94178.html. Accessed 21 Dec 2017.
Walker, P. (2010). Senegalese president unveils £17m African Renaissance statue. The Guardian. www.theguardian.com/world/2010/apr/04/senegal-african-renaissancestatue. Accessed 21 Dec 2017.
WHO. (2005). Enhanced surveillance of epidemic meningococcal meningitis in Africa: A three-year experience. http://www.who.int/wer/2005/wer8037.pdf. Accessed 21 Dec 2017.
WHO. (2010). Yellow fever initiative – providing an opportunity of a lifetime. Geneva: World Health Organization. http://www.who.int/csr/disease/yellowfev/YFIbrochure.pdf. Accessed 19 Dec 2017.
WHO. (2014). Meningococcal disease control in countries of the African meningitis belt. http://www.who.int/wer/2015/wer9013.pdf?ua=1. Accessed 21 Dec 2017.
WHO. (2015). Meningococcal disease control in countries of the African meningitis belt. http://www.who.int/wer/2016/wer9116.pdf?ua=1. Accessed 21 Dec 2017.
WHO. (2016a). Yellow fever. http://www.who.int/csr/disease/yellowfev/en/. Accessed 21 Dec 2017.
WHO. (2016b). Weekly epidemiological record, 31 March 2017. 92(13): 145–164. http://apps.who.int/iris/bitstream/10665/254901/1/WER9213.pdf?ua=1. Accessed 21 Dec 2017.
WHO. (2016c). Public financing for health in Africa: From Abuja to the SDGs. https://apps.who.int/iris/bitstream/handle/10665/249527/WHO-HIS-HGF-Tech.Report-16.2-eng.pdf?sequence=1. Accessed 26 Jan 2019.
WHO. (2017a). Cholera count reaches 500 000 in Yemen. http://www.who.int/mediacentre/news/releases/2017/cholera-yemen-mark/en/. Accessed 21 Dec 2017.
WHO. (2017b) The yellow fever outbreak in Angola and Democratic Republic of the Congo ends. http://www.afro.who.int/en/media-centre/pressreleases/item/9377-the-yellow-fever-outbreak-in-angolaand-democratic-republic-of-the-congo-ends.html. Accessed 21 Dec2017.
WHO. (2017c). Ebola ça suffit!- Phase III vaccine trial in Guinea. http://www.who.int/medicines/ebola-treatment/q-a_ebola-ca-suffit/en. Accessed 21 Dec 2017.
WHO. (2017d). Meningococcal meningitis. http://www.who.int/mediacentre/factsheets/fs141/en/. Accessed 21 Dec 2017.
WHO. (2018). Ebola virus disease: Democratic Republic of Congo external situation report 7. https://apps.who.int/iris/bitstream/handle/10665/272728/SITREP-EVD-DRC-20180601-eng.pdf?sequence=1&isAllowed=y. Accessed 01 June 2018.
WHO-AFRO. (2017a). Weekly bulletin on outbreaks and other emergencies week 50. http://apps.who.int/iris/bitstream/10665/259709/1/OEW50-1015122017.pdf. Accessed 21 Dec 2017.
WHO-AFRO. (2017b). Regional committee sessions. http://www.afro.who.int/about-us/governance. Accessed 19 Sept 2017.
WHO. (2019). Ebola situation reports: Democratic Republic of the Congo. https://apps.who.int/iris/bitstream/handle/10665/311972/SITREP_EVD_DRC-20190416-eng.pdf?utm_source=Newsweaver&utm_medium=email&utm_term=click+here+to+download+the+complete+situation+report&utm_content=Tag%3AAFRO%2FWHE%2FHIM+Outbreaks+Weekly&utm_campaign=WHO+AFRO+-+Situation+Report+-+Ebola+Virus+Disease+Outbreak+in+DRC+-+Sitrep+37+%282019%29
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Tomori, O. (2019). Disease Outbreaks in Africa and the Response of African Governments. In: Tangwa, G., Abayomi, A., Ujewe, S., Munung, N. (eds) Socio-cultural Dimensions of Emerging Infectious Diseases in Africa. Springer, Cham. https://doi.org/10.1007/978-3-030-17474-3_11
Download citation
DOI: https://doi.org/10.1007/978-3-030-17474-3_11
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-17473-6
Online ISBN: 978-3-030-17474-3
eBook Packages: MedicineMedicine (R0)