Abstract
The 2013 outbreak of Ebola virus disease (EVD) in West Africa constituted a major humanitarian crisis. The outbreak numbered over 28,500 cases, more than 10 times the number cumulatively registered from all previous EVD outbreaks combined, with at least 11,000 deaths, and resulted in billions of dollars of lost economic growth to an already impoverished region. The unprecedented scale of West Africa 2013 took the world by surprise and laid bare deficiencies in our response capacity to complex humanitarian disasters of highly infectious and lethal pathogens. However, the magnitude of West Africa 2013 also provided a unique opportunity and obligation to better understand not only the biology and epidemiology of EVD, but also the many scientific, economic, social, political, ethical, and logistical challenges in confronting emerging infectious diseases in the modern era.
This article is dedicated to the many health workers who sacrificed their time, energy and, all too often their lives, to combat the Ebola virus disease outbreak in West Africa (Bausch et al. 2014).
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
1 First Case and Early Spread
In early December 2013, a 2-year-old boy in the village of Méliandou in the remote, largely deforested, and resource-poor Prefecture of Guéckédou, Republic of Guinea, fell sick with fever, vomiting, and black stool, dying a few days later (WHO 2014a; Baize et al. 2014; Mari Saez et al. 2014) (Figs. 1 and 2). The boy was reported to have previously played in a hollow tree housing a colony of the insectivorous bat Mops condylurus, a possible Ebola virus (EBOV) reservoir (Mari Saez et al. 2014). Over the following weeks and months, multiple waves of transmission and disease occurred in family members, healthcare workers (HCWs) who cared for them, and persons with contact with corpses during funeral rituals. On March 21, 2014, Guinean health authorities notified the World Health Organization (WHO) of a “rapidly evolving outbreak.” On March 23, EBOV infection was confirmed on patient samples sent to Biosafety Level 4 laboratories in Lyon, France, and Hamburg, Germany, and an outbreak of Ebola virus disease (EVD) was declared. Unbeknownst to all at the time, at least 49 cases with multiple but often poorly defined chains of transmission had already occurred in Guinea and the virus had already slipped across the border, smoldering quietly at first, into neighboring Liberia and Sierra Leone (WHO 2014b, 2015c).

Adapted from WHO Ebola Response Roadmap Situation Reports with publically available data. World Health Organization
Epidemiologic curve of the West Africa 2013 Ebola virus disease (EVD) outbreak. The dashed-vertical lines indicate key events during the outbreak: a First suspected case in Méliandou, Guinea, b Laboratory confirmation of EVD and disease reported by Guinean Health Authorities, c WHO declares Public Health Emergency of International Concern, d U.S. President Obama announces major initiative to help control EVD in Liberia; Creation of the United Nations Mission for Ebola Emergency, e Publication of preliminary results from first EVD Phase III vaccine efficacy study (rVSIV-EBOV), f Publication of preliminary results of first EVD Phase III therapeutic efficacy trial (convalescent plasma).

Reprinted with permission from Bausch and Rojek (2016)
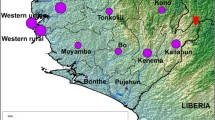
Map of West Africa showing the epicenter of the 2013–2016 outbreak of Ebola virus disease (red) and imported cases (orange and arrows). The village of Meliandou, Guinea, where the first case is thought to have occurred, is indicated by a blue star. The total number of cases seen in each country is shown in parentheses.
2 Virus Introduction
Five members of the Filoviridae family are known to cause disease in humans: EBOV (formerly designated Zaire Ebola virus), Bundibugyo, Sudan, and Taï Forest viruses of the genus Ebola virus and Marburg and Ravn viruses of the genus Marburg virus, with characteristic geographic distributions (Table 1 and Fig. 3). Prior to 2013, only a single case of EVD had been reported in West Africa, due to Taï Forest virus (Formenty et al. 1999a, b). When EVD appeared in neighboring Guinea in 2013, most experts predicted that Taï Forest virus would again be the culprit, and thus were surprised when the causative virus turned out to be a new variant of EBOV, subsequently named Makona after the Makona River in Guinea, close to the border with Liberia and Sierra Leone (Baize et al. 2014). How EBOV, which had never been noted outside of the Congo Basin of Equatorial Africa, found its way to West Africa remains unknown (Bausch and Schwarz 2014). Migration and transmission from infected bats, putative EBOV reservoirs, is considered the most likely modality (Mari Saez et al. 2014; Bausch and Schwarz 2014). Comparative phylogeographic analysis suggests that fruit bats of three species are theoretically capable of dispersing EBOV directly from the Congo Basin to Guinea: African straw-colored fruit bats (Eidolon helvum), hammer-headed fruit bats (Hypsignathus monstrosus), and Egyptian fruit bats (Rousettus aegyptiacus), although definitive evidence of EBOV infection in any type of bat anywhere has yet to be found (Hassanin et al. 2016).
Epidemiological and phylogenetic analyses during and after the West Africa EVD outbreak are consistent with all cases stemming from a single introduction of the Makona variant in Guinea in 2013 (Kuhn et al. 2014). This finding is consistent with most other EVD outbreaks, which generally result from a single introduction from the wild followed by amplification exclusively through human-to-human transmission. Molecular clock dating analyses suggest that the Makona variant diverged from other EBOVs only about a decade ago (Holmes et al. 2016). Also due to EBOV, sequence analysis makes clear that the much smaller outbreak of EVD that occurred in the Democratic of the Republic of the Congo in 2014 was a separate event unrelated to the outbreak in West Africa.
3 Underlying Geopolitical, Social, and Cultural Factors
There has been much speculation and extensive discussion in the literature regarding the causes for the unprecedented size of West Africa 2013 (Moon et al. 2015; Bausch and Rojek 2016). The outbreak’s roots are undoubtedly multifactorial, entailing a complex web of interrelated social, cultural, ecologic, and economic determinants viewed in the context of the overall geopolitical history of the region (Table 2). Many of these factors and challenges have been encountered in previous EVD outbreaks, but certainly not on the scale and with the intensity noted in West Africa.
4 A Failed Response
In the absence of effective therapeutics and vaccines (a work in progress—see below), control of EVD outbreaks is almost completely based on the classic control measures of thorough surveillance for case identification, isolation, and care in the setting of sound infection prevention and control (IPC) practices; contact tracing; and safe burials, all enhanced by effective social mobilization and public education campaigns, and coordinated through a partnership between the national government, WHO, and other international stakeholders. The international community and the governments they supported were accustomed to success in this approach, generally ending outbreaks after a few hundred cases within a few months, and indeed certainly anticipated the same result in West Africa (Table 3). However, a tragically different scenario played out.
Due to a preexisting health project, the nongovernmental organization Médecins Sans Frontières (MSF) was present at the epicenter of the EVD outbreak in Guinea even before its onset. On March 31, seven days after the EVD outbreak was declared, MSF warned of an EVD outbreak unprecedented in magnitude and distribution. Although many international organizations, including WHO, the International Federation of Red Cross and Red Crescent Societies, the U.S. Centers for Disease Control and Prevention, the European Union, and UNICEF, quickly joined MSF and national partners in the three West African countries to mount outbreak response operations, this early effort would prove to be too slow, piecemeal, and disorganized to prevent what would ultimately become a major humanitarian crisis. The shortcomings in the international response have been extensively discussed in the literature, especially with regard to WHO who, although engaged in outbreak control activities since the beginning, did not formally declare the outbreak to be a Public Health Emergency of International Concern, as outlined under International Health Regulations, until August 8, 2014 (Bausch and Rojek 2016) (Fig. 1).
The dramatic rise in cases in West Africa, projections of millions of cases if an aggressive response was not mounted (Meltzer et al. 2014), and increasing numbers of imported cases into surrounding countries in West Africa, the United States, and Europe, finally stirred the international community to more concerted action. Responses generally aligned with historical connections between the United States and European countries and their colonial-era African counterparts. In September 2014, United States President Obama committed to the construction of seventeen 100-bed Ebola Treatment Units (ETUs) in Liberia, deployment of up to 3000 medical military and support personnel, and support to train 500 HCWs a week. The United Kingdom and France soon followed with commitments to combat EVD in their ex-colonies of Sierra Leone and Guinea, respectively.
Ultimately, a vast array of at least 100 government and nongovernmental organizations, including over 5000 military personnel, contributed to the outbreak response, establishing over 70 ETUs, 800 community care centers, a vast network of over 50 laboratories (generally providing reliable diagnostic results within 24 h after receipt of a specimen), and an extensive surveillance and contact tracing operation across the three implicated countries. However, the response remained agonizingly slow, hampered by the logistical challenges of operationalizing work in the poorest countries in the world with fledging governments and poor infrastructure. The dispersal of cases across three West Africa countries, and in both remote rural and densely populated urban areas, ultimately presented too many “battle fronts,” outstripping both local and international capacities. Beds for patients with EVD (Table 4), HCWs to care for them, and field workers to undertake surveillance and contact tracing were woefully insufficient. Consequently, highly infectious patients remained untreated in the community and patients who were admitted to the drastically understaffed ETUs could expect little more than palliative care. Even after laboratories began being rapidly established, the steep increase in the number of samples exceeded local diagnostic capacities in many areas until well into the outbreak. With cases of EVD in HCWs mounting, some ETUs opted to enhance safety by proscribing close contact with patients, including the very controversial measure of not placing IVs for fluid repletion. This move, while perhaps unavoidable, likely further undermined the local population’s already shaky faith in the response operation.
Coordination of the outbreak response was a major challenge, with each organization largely acting independently or in bilateral concert with the government. In August 2014, the United Nations appointed a Special Envoy on Ebola, followed by the creation in September 2014 of a coordination body, the United Nations Mission for Ebola Emergency, headquartered in Ghana (Fig. 1). Opinions vary on the efficacy of these measures. Without doubt, the enormous scale and complexity of the outbreak, and the sheer number of organizations involved (far more than had ever been involved in an EVD outbreak before and at times compounded by historical frictions between them) made coordination a substantial challenge.
Although HCW infections have occurred in virtually every EVD outbreak to date (Table 1), prior to West Africa 2013, they were relatively uncommon once international support and resources arrived to assist with establishing ETUs and appropriate IPC measures. In contrast, one of the tragic consequences of the poor baseline infrastructure and failed response West Africa 2013 was EBOV infection in over 900 HCWs during the outbreak, including two cases contracted in the United States and one in Spain, with over 500 deaths (WHO 2015a). In the vast majority of HCW infections, no clear exposure risk, such as a needle stick or blood splash to mucous membranes, could be identified. The high number of HCW infections engendered speculation that EBOV Makona is more transmissible than other variants, although no supporting data for this theory are available.
Although IPC entails many diverse measures, many of which were inadequate during the outbreak, most of the focus has been on the issue of personal protective equipment (PPE). In addition to shortages of PPE, especially early in the outbreak, the situation was complicated by the diversity of PPE types advocated by different groups, causing significant confusion with training and safe use (Bausch and Rojek 2016; WHO 2008; Franklin 2016) (Fig. 4). Although PPE guidelines were eventually produced, which for the first time included technical specifications for PPE, the lack of evidence precluded a consensus on efficacy (Hersi et al. 2015). The procedure for doffing contaminated PPE, often considered a confusing and a vulnerable point for infection, is logically a focus of attention but, again, no data are available. Furthermore, it is not always clear the HCW infection resulted from exposure in the ETU. Local HCWs are members of the same communities where EBOV may be circulating during an outbreak, and thus may share many of the same risks. There are also many anecdotal reports of HCWs seeing patients in their homes, where the uses of full PPE and other IPC measures are unlikely to be adequate (Faye et al. 2015; Brainard et al. 2016). More in-depth investigations are needed, and indeed are ongoing, to reveal vulnerable points for HCW infection in the care of EVD, and to develop evidence-based uniform PPE standards to protect them.
Examples of various types of personal protective equipment used during the care of patients with Ebola virus disease during the 2013–2016 outbreak in West Africa. The equipment shown are for demonstration only, and should not be construed as implying as advocating or confirming efficacy for any specific equipment.
5 Virus Evolution During the Outbreak
Whole genome sequencing was performed on hundreds of samples from West Africa 2013, a far greater number than had been sequenced from all previous EVD outbreaks combined (Gire et al. 2014; Park et al. 2015; Matranga et al. 2014). There are conflicting reports and considerable controversy over whether EBOV Makona evolved genetically more rapidly during West Africa 2013 relative to the causative viruses of other EVD outbreaks (Holmes et al. 2016) and whether virus adaptation to humans occurred (Li et al. 2016; Urbanowicz et al. 2016). What is clear, however, is that the prolonged course of the outbreak provided sufficient time for the emergence of at least three distinct viral lineages (Gire et al. 2014; Urbanowicz et al. 2016; Carroll et al. 2015; Quick et al. 2016; Simon-Loriere et al. 2015). Most of these major lineages circulated locally, with only sporadic cross-border transmission.
The highest level of EBOV genetic amino acid diversity generated during West Africa 2013 occurred in the EBOV glycoprotein (GP) (Holmes et al. 2016; Ning et al. 2017). Because of its key role in virus–host interactions, with the potential for altered interaction with the EBOV host receptor Niemann-Pick C1, GP sequence variation is of particular interest. In the laboratory, minor changes in the GP have been shown to impact viral entry into cells from different mammalian species (Ning et al. 2017). Pseudo-typed virion particles incorporating synthetically generated amino acid substitutions observed during the outbreak more efficiently entered human cells, with possible implications for viral fitness, host specificity, and transmissibility (Urbanowicz et al. 2016). Early in the West Africa outbreak, a variant in lineage SL2 emerged with sequence changes in the GP receptor-binding site (Holmes et al. 2016).
Despite evidence for a degree of EBOV evolution during West Africa 2013, no clear phenotypic significance (i.e., changes in transmissibility, virulence, antigenicity, or influence on the efficacy of diagnostic assays, vaccines, or therapeutics) has been noted between the lineages of EBOV Makona or between Makona and other variants of EBOV; the range of reported case fatality rates in West Africa (31–76%), calculated basic reproduction number R 0 (1.5–2.5), and duration of virus shedding are comparable to those noted in previous outbreaks, with no evidence for heritable changes during the course of the outbreak (Holmes et al. 2016; Bausch and Rojek 2016). An EBOV vaccine developed against the 1995 Kikwit variant of EBOV was 100% protective against the Makona variant in both animal models (Marzi et al. 2015a) and a Phase III trial in humans (Henao-Restrepo et al. 2016), suggesting that the genetic differences between the two virus variants did not result in significant differences in immunogenicity. Nor have laboratory studies shown EBOV Makona to be appreciably different from other variants of EBOV with regard to virulence in nonhuman primates (Marzi et al. 2015b), entry into cells (Dunham et al. 2015; Hofmann-Winkler et al. 2015), or detection via polymerase chain reaction assays (Sozhamannan et al. 2015). One exception, however, is the results from experiments in humanized laboratory mice, in which a longer mean-time-to-death was noted with EBOV Makona compared to the 1976 Yambuku variant (Bird et al. 2016), leading to speculation that the seemingly high attack rates in West Africa 2013 could be the result of prolonged virus shedding and thus opportunity for transmission. Despite high-profile speculation to the contrary, there is no evidence or reason to believe that EBOV Makona or any other EBOV variant or even any other Ebola virus has or will evolve naturally to be capable of aerosol or airborne transmission (Osterholm et al. 2015).
6 A Heavy Toll Before Final Outbreak Control
The EVD outbreak in West Africa would ultimately last 3 years and officially result in over 28,616 cases and 11,310 deaths (numbers widely considered underestimates), eclipsing by far and by every measure all previous EVD outbreaks combined (Table 1). In addition to the toll in terms of cases counted and lives lost, the outbreak resulted in billions of dollars in lost economic growth in West Africa (Bank TW 2016), upward of 3500 orphaned children (UNICEF 2016), delayed or impaired child development since school was canceled for a year, widespread job loss resulting in economic and food insecurity, and deep but less easily measurable mental health and sociocultural impacts. Furthermore, as the region’s resources were funneled to EVD, an estimated 10,000+ excess deaths occurred due to untreated malaria, HIV/AIDS, and tuberculosis (Parpia et al. 2016). Reductions in vaccination coverage and a rise in teenage pregnancy were also noted (Elston et al. 2015). In addition to the cases in Africa, there were 27 cases, 5 of which were fatal, imported, or medically evacuated to the United States and Europe (Fig. 2) (System Ebola Epidemiology Team IM 2014; WHO 2015b)—a pittance compared to the massive humanitarian disaster in West Africa, but a situation that nevertheless fomented considerable panic and expenditure of resources in those industrialized areas of the world.
The success of EVD outbreak control measures is highly dependent on community engagement to arrive at a common understanding of the nature of the disease threat and cooperation with the plan for control. A combination of historical distrust of authority and slow and poor messaging conspired to impede such engagement, understanding, and cooperation in many communities during West Africa 2013, providing a major impediment. Whether the major factors in ultimate control of the outbreak were the classic response measures implemented by national and international public health agencies or a more grassroots autonomous behavioral adaptation of the indigenous population is a matter of debate, and certainly varies by country and community. In some cases, additional measures were implemented, such as quarantine of affected villages and exit and entrance screening for fever and other clinical manifestations of EVD of travelers between affected regions and in airports, often controversially and with uncertain effect. One novel control method that may have had a significant impact on transmission in the later stages of the outbreak was vaccination (see below and chap. 9 by Higgs).
7 Sequelae, Virus Persistence, and Recrudescence
One of the unforeseen issues in West Africa 2013 was the high frequency of sequelae, virus persistence, and, more rarely, recrudescence in EVD survivors. Although a host of both short- and long-term post-EVD sequelae has been noted dating back to the first recognized outbreak in Zaire (current Democratic Republic of the Congo) in 1976, little attention was typically afforded to survivors, in part due to the limited infrastructure for study in the outbreak areas (Vetter et al. 2016b). However, early anecdotal observations and subsequent more formal study on the estimated over 15,000 EVD survivors from West Africa 2013 reveal a wide range of medical and psychosocial challenges, including persistent arthralgia, ocular complications (including potentially sight-threatening uveitis that may result in early cataract formation) abdominal pain, extreme fatigue, and anorexia, sleep and memory disturbances, anxiety disorders, depression, post-traumatic stress disorder, and survivors’ guilt in not only survivors, but also other family and community members (Vetter et al. 2016b). The challenges facing survivors created an urgent moral imperative to provide clinical care, only partially met by national programs with international support, including clinical care guidelines for EVD survivors developed by (WHO 2016a).
The underlying pathogenesis of EVD sequelae is not well understood, but anecdotal observations increasingly suggest that at least some relate to persistent virus in selected immunologically protected tissue compartments and fluids, including the testes/semen, chambers of the eye, cerebrospinal fluid (CSF), and the fetus, placenta, and amniotic sac/fluid of women infected during pregnancy (Vetter et al. 2016a, b). EBOV RNA has been found by RT-PCR in a host of body fluids for weeks or even months after resolution of acute disease and clearance of virus from the blood (Fig. 5), although the significance of these findings is often unknown since, in most cases, infectious virus could not be isolated by cell culture after a few weeks after disease onset.

Updated and reprinted with permission from Vetter et al. (2016b)
Virus persistence after the day of disease onset in various body compartments in survivors of Ebola virus disease as detected by reverse-transcription polymerase chain reaction (RT-PCR, green) and cell culture (blue). Red bars represent the day of the first negative RT-PCR detection in the patient’s blood, when available.

Republic of the Congo. Reprinted with permission from Bausch and Rojek (2016)
Sizes and population densities of Guinea, Liberia, and Sierra Leone combined compared with the Democratic Republic of the Congo. To illustrate the difference in size, the three West African countries are shown superimposed on the Democratic.
Virus persistence in the semen is of most concern since it has occasionally resulted in sexual transmission, sometimes initiating small case clusters in the wake of the acute outbreak (Eggo et al. 2015; Christie et al. 2015; Mate et al. 2015). Albeit in low copy numbers and in a small minority of EVD survivors, EBOV RNA has been detected in the semen up to a year or more and infectious virus by cell culture up to 82 days after acute disease (Deen et al. 2015). A few cases of recrudescence associated with prolonged virus persistence have been noted, including uveitis with EBOV isolated from the aqueous humor of the eye at 14 weeks after disease onset (Varkey et al. 2015) and severe meningitis with seizures with isolation of virus from the CSF 9 months after resolution of acute disease (Jacobs et al. 2016). Anecdotal reports exist of recrudescent disease and viremia in West Africa, in some cases thought to be related to underlying HIV-1 infection, although this association remains to be validated (Howlett et al. 2016). A few cases have been noted in which women infected with EBOV during pregnancy, possibly with no or atypically mild disease, have recovered and remained pregnant, only to spontaneously abort a macerated and nonviable fetus in subsequent weeks or months (Bower et al. 2015; Caluwaerts et al. 2015). EBOV RNA was found in the products of conception, although cell culture results confirming the presence of infectious virus were generally not reported (Bower et al. 2015; Caluwaerts et al. 2015; Baggi et al. 2014; Oduyebo et al. 2015). With the exception of sexual transmission, no cases of secondary transmission resulting from EVD survivors have been suspected. Nevertheless, the possibility of virus persistence and renewed transmission from EVD survivors illustrate the need for continued non-stigmatizing but heighted surveillance even after the immediate threat of EVD from more common modes of transmission has been extinguished.
8 Research During the Outbreak
As the gravity of the situation in West Africa rose, the global community felt increasingly compelled to consider use of various experimental therapeutics and vaccines. Albeit unwelcome, the magnitude of the outbreak provided an important opportunity and obligation for prospective clinical research that had never before been possible. In August 2014, WHO convened a meeting in Geneva, Switzerland, of the diverse stakeholders, including representatives from the ministries of health, pharmaceutical companies, drug regulatory agencies, nongovernmental organizations providing clinical care, and experts in virology and medical ethics. WHO then quickly created a Scientific and Technical Advisory Committee for Ebola Experimental Interventions to guide the process, which required consideration not only of the evidence for safety and efficacy, but also the anticipated feasibility and utility of conducting clinical trials in the setting of limited production capacities or intermittent drug availability. While various therapeutic trials were undertaken (Table 5), the many complex scientific, logistical, and sociocultural challenges could not be met quickly enough to take full advantage of the large case numbers potentially affording statistical power. By the time most therapeutic trials were implemented, case counts in West Africa had fallen to a level insufficient to meet clinical endpoints. There was also an opportunity missed to enroll more patients in clinical trials in resource-rich settings.
While there is disappointment that therapeutic trials during West Africa 2013 did not produce definitive evidence of an efficacious drug for EVD, the experience cannot be considered completely futile. Many difficult but valuable lessons were learned regarding the challenges of inconsistent reproducibility of in vitro experiments, poorly predictive animal models, and the operational demands of conducting trials overseas in an ETU during an outbreak without any preexisting research infrastructure. Rigorous debate continues regarding the scientific and ethical merit of the various clinical trial designs used in this outbreak. Nevertheless, numerous drug candidates progressed through Phase I, II, and III clinical trials at an unprecedented pace and the recognition that some agents are ineffective, along with promising interim results for a few, provide a starting point for prioritization in future outbreaks. However, much work remains to be done to capitalize on the lessons learned from West Africa 2013 and make the accelerated pace of therapeutic trials during outbreaks the norm, including prioritizing drug candidates, working out trial designs, prepositioning protocols and ethics committee reviews, and setting logistical frameworks for rapid operationalization.
As with therapeutics, the urgency of West Africa 2013 thrust vaccines for EVD from a conventional protracted research and development timeline into high gear. After rapid Phase I and II clinical trials were undertaken at various sites in the United States, Europe, and Africa (outside the EVD epidemic zone), a large Phase III trial of an experimental vaccine composed of a recombinant vesicular stomatitis Indiana virus expressing the EBOV GP was implemented in Guinea with a ring vaccination approach. The trial showed 100% vaccine efficacy (Henao-Restrepo et al. 2016) and was employed in the later stages of the outbreak to help stem spread from reintroduced virus from sexual transmission. Adverse effects were frequent but mostly minor, although vaccine-induced arthritis, dermatitis, and vasculitis were reported (Henao-Restrepo et al. 2016; Huttner et al. 2015).
In addition to the aforementioned prospective clinical investigations on therapeutics and vaccines, a vast amount of information was generated from informal observations and empiric experience with the large number of cases. This included noting relatively rare clinical presentations—at times challenging accepted case definitions—sequelae, and modes of transmission (Bausch and Rojek 2016). In particular, the spectrum of disease and transmission modes in pregnant women and their offspring brought many new and often unforeseen challenges (Akerlund et al. 2015). The 20 medically evacuated cases to the United States and Europe were generally cared for in advanced medical settings that allowed for more detailed clinical observation and laboratory analysis of both acute disease and sequelae than was typically possible in West Africa (Uyeki et al. 2016).
Although, as discussed above, clinical trials generally evolved too slowly to provide firm conclusions on efficacy, it is interesting to note that the CFR in the 27 cases who received care in the United States and Europe was only 18.5% (Uyeki et al. 2016), compared to 31–76% reported from West Africa 2013, depending upon the specific ETU and time during the outbreak. It is unknown whether this discrepancy in outcome relates to use of experimental therapies (of which 85% of patients in high-resource setting received one or more), better fluid and electrolyte monitoring and organ support (including mechanical ventilation and renal replacement therapy), genetic predisposition, and/or diminished comorbidities relative to the West African population. Observational studies on sequelae and persistence in EVD survivors, including the Partnership for Research on EBOV in Liberia (PREVAIL) III, a large multiyear controlled cohort study of EVD being undertaken in Liberia, promise to eventually yield a wealth of information (NIH 2015).
Significant progress was made on development and validation of new laboratory diagnostic platforms for nucleic acid detection of EBOV as well as progress on rapid tests (Dhillon et al. 2015). West Africa 2013 also set a new benchmark in providing real-time large-scale molecular epidemiologic data to guide response efforts during an outbreak (Holmes et al. 2016; Matranga et al. 2014). Such high-resolution genetic analysis is generally available only retrospectively. However, despite often difficult conditions, toward the end of the West Africa outbreak, novel field-applicable genome sequencing platforms were developed and deployed that were capable of generating results in less than 24 h (Holmes et al. 2016; Quick et al. 2016). These genomic data may allow accurate ongoing estimates of various important outbreak parameters, including of reproduction numbers R 0 and R 1, to determine the impact of specific interventions such as border closures and quarantine; elucidation of transmission chains and virus provenance when classically collected field epidemiologic data are unclear, including identification of super-spreaders and cases where sexual or other transmission from survivors is suspected; identification of signatures of host adaptation; identification and monitoring of diagnostic targets; and characterization of responses and resistance to treatments and vaccines (Matranga et al. 2014; Quick et al. 2016; Mate et al. 2015; Whitmer et al. 2016; Lau et al. 2017; Keita et al. 2016; Diallo et al. 2016).
9 Conclusions and Future Challenges
The unprecedented scale of West Africa 2013 took the world by surprise and sadly added another tragic event to a region already struggling to escape decades of poverty and war. The outbreak also shook the international response community, laying bare deficiencies in our response capacity to complex humanitarian crises involving highly infectious and lethal pathogens. Although much remains to be learned, West Africa 2013 also represents a tragic but nevertheless watershed moment in our understanding not only of the biology and epidemiology of EVD, but equally important, the many economic, social, political, ethical, and logistical challenges in confronting emerging diseases in the modern era. As the global population surges and becomes more interconnected, the risk of such outbreaks is destined to increase. In the absence of redoubled efforts to build capacity for surveillance and response, outbreaks such as West Africa 2013 threaten to become the “new norm.” One need not look much further for the proof than to the Zika virus disease outbreak that swept through the Caribbean and Latin America starting in 2015.
West Africa 2013 has challenged the world to respond better. The pressure is on to capitalize on the lessons learned during the outbreak both from failures and the glimpses of innovation and research progress to create a new norm of comprehensive surveillance and organized response. In response to numerous internal as well as external evaluations (Moon et al. 2015), WHO has created a new Health Emergencies Programme designed to streamline response operations to such crises under one clear line of authority (WHO 2016b). However, the funding to fully implement the new program is still in question. Furthermore, reform and improved performance must extend far beyond only WHO. Many national governments and independent stakeholders have also created rapid response teams. Whether this revamped structure and national capacity will result in a more effective response to the next outbreak remains to be seen. Coordination of the many different partners and programs during the next outbreak will undoubtedly remain one of the key challenges.
Lastly, let us remember that, while important, science and technological advancement alone will never be sufficient; poverty and lack of the fundamental human right to health consistently underlie outbreaks of emerging pathogens (Nations 1948). EVD is but the proverbial “canary in the coal mine,” indicative of the world’s most vulnerable populations. We must advocate for and work toward restitution of the right to health in low- and middle-income countries (LMICs). This will entail much more than simply building a laboratory or conducting a research project. Local educational institutions must be strengthened and career opportunities created to stop the “brain drain” of HCWs to high-income countries and produce future “home grown” leaders in the health sciences. Novel and technology-appropriate approaches to local problems must be sought, as well as the funding mechanisms that enable their execution. Furthermore, after the major struggle to implement the quantity of medical care necessary in West Africa 2013, the outbreak rightly brought up the issue of quality of care. Implicit in this is a just rejection of a perhaps long-held but implicit acceptance of disparate qualities of care between patients in LMICs and resource-rich countries, an archaic notion whose time must now be passed. Regardless of country of origin or personal wealth, patients should have the right to HCWs with the right training for their condition and who implement evidence-based standards of care. Of course, this gap between rich and poor cannot be closed overnight. There is much work to be done with regard to both scientific research to generate the best evidence and advocacy and organization to ensure thorough and equitable implementation. Responsibility falls also on LMICs to create strong and transparent governmental and public health administrative frameworks capable of capitalizing on international collaboration and support. Long after West Africa 2013 is over, these will be our true measures of success.
References
Akerlund E, Prescott J, Tampellini L (2015) Shedding of Ebola virus in an asymptomatic pregnant woman. N Engl J Med 372(25):2467–2469
Baggi FM, Taybi A, Kurth A, Van Herp M, Di Caro A, Wolfel R et al (2014) Management of pregnant women infected with Ebola virus in a treatment centre in Guinea, June 2014. Euro Surveill: Bull Eur Sur Les Mal Transm = European Commun Dis Bull 19(49)
Baize S, Pannetier D, Oestereich L, Rieger T, Koivogui L, Magassouba N et al (2014) Emergence of Zaire Ebola virus disease in Guinea. N Engl J Med 371(15):1418–1425
Bank TW (2016) The world bank fact sheet. The world bank
Bausch DG, Rojek A (2016) West Africa 2013: re-examining Ebola. Microbiol Spectr 4(3)
Bausch DG, Schwarz L (2014) Outbreak of Ebola virus disease in Guinea: where ecology meets economy. PLoS Negl Trop Dis 8(7):e3056
Bausch DG, Bangura J, Garry RF, Goba A, Grant DS, Jacquerioz FA et al (2014) A tribute to Sheik Humarr Khan and all the healthcare workers in West Africa who have sacrificed in the fight against Ebola virus disease: Mae we hush. Antiviral Res 111C:33–35
Bird BH, Spengler JR, Chakrabarti AK, Khristova ML, Sealy TK, Coleman-McCray JD et al (2016) Humanized mouse model of Ebola virus disease mimics the immune responses in human disease. J Infect Dis 213(5):703–711
Bower H, Grass JE, Veltus E, Brault A, Campbell S, Basile AJ et al (2015) Delivery of an Ebola virus-positive stillborn infant in a rural community health center, Sierra Leone, January 2015. Am J Trop Med Hyg
Brainard J, Pond K, Hooper L, Edmunds K, Hunter P (2016) Presence and persistence of Ebola or Marburg virus in patients and survivors: a rapid systematic review. PLoS Negl Trop Dis 10(2):e0004475
Caluwaerts S, Fautsch T, Lagrou D, Moreau M, Modet Camara A, Gunther S et al (2015) Dilemmas in managing pregnant women with Ebola: 2 case reports. Clin Infect Dis: Off publ Infect Dis Soc Am
Carroll MW, Matthews DA, Hiscox JA, Elmore MJ, Pollakis G, Rambaut A et al (2015) Temporal and spatial analysis of the 2014–2015 Ebola virus outbreak in West Africa. Nature 524(7563):97–101
Christie A, Davies-Wayne GJ, Cordier-Lasalle T, Blackley DJ, Laney AS, Williams DE et al (2015) Possible sexual transmission of Ebola virus—Liberia, 2015. MMWR Morb Mortal Wkly Rep 64(17):479–481
Deen GF, Knust B, Broutet N, Sesay FR, Formenty P, Ross C et al (2015) Ebola RNA persistence in semen of Ebola virus disease survivors—preliminary report. N Engl J Med
Dhillon RS, Srikrishna D, Garry RF, Chowell G (2015) Ebola control: rapid diagnostic testing. Lancet Infect Dis 15(2):147–148
Diallo B, Sissoko D, Loman NJ, Bah HA, Bah H, Worrell MC et al (2016) Resurgence of Ebola virus disease in Guinea linked to a survivor with virus persistence in seminal fluid for more than 500 days. Clin Infect Dis: Off Publ Infect Dis Soc Am 63(10):1353–1356
Dunham EC, Banadyga L, Groseth A, Chiramel AI, Best SM, Ebihara H et al (2015) Assessing the contribution of interferon antagonism to the virulence of West African Ebola viruses. Nat Commun 6:8000
Eggo RM, Watson CH, Camacho A, Kucharski AJ, Funk S, Edmunds WJ (2015) Duration of Ebola virus RNA persistence in semen of survivors: population-level estimates and projections. Euro surveillance: Bull Eur Sur Les Mal Transm = Eur Commun Dis Bull 20(48)
Elston JW, Moosa AJ, Moses F, Walker G, Dotta N, Waldman RJ et al (2015) Impact of the Ebola outbreak on health systems and population health in Sierra Leone. J Public Health
Faye O, Boelle PY, Heleze E, Faye O, Loucoubar C, Magassouba N et al (2015) Chains of transmission and control of Ebola virus disease in Conakry, Guinea, in 2014: an observational study. Lancet Infect Dis 15(3):320–326
Formenty P, Hatz C, Le Guenno B, Stoll A, Rogenmoser P, Widmer A (1999a) Human infection due to Ebola virus, subtype Cote d’Ivoire: clinical and biologic presentation. J Infect Dis 179(Suppl 1):S48–S53
Formenty P, Boesch C, Wyers M, Steiner C, Donati F, Dind F et al (1999b) Ebola virus outbreak among wild chimpanzees living in a rain forest of Cote d’Ivoire. J Infect Dis 179(Suppl 1):S120–S126
Franklin SM (2016) A comparison of personal protective standards: caring for patients with Ebola virus. Clin Nurse Spec 30(2):E1–E8
Gire SK, Goba A, Andersen KG, Sealfon RS, Park DJ, Kanneh L et al (2014) Genomic surveillance elucidates Ebola virus origin and transmission during the 2014 outbreak. Science (New York, NY) 345(6202):1369–1372
Hassanin A, Nesi N, Marin J, Kadjo B, Pourrut X, Leroy E et al (2016) Comparative phylogeography of African fruit bats (Chiroptera, Pteropodidae) provide new insights into the outbreak of Ebola virus disease in West Africa, 2014–2016. CR Biol 339(11–12):517–528
Hayden FG, Friede M, Bausch DG (2017) Experimental therapies for Ebola virus disease: what have we learned? J Infect Dis
Henao-Restrepo AM, Camacho A, Longini IM, Watson CH, Edmunds WJ, Egger M et al (2016) Efficacy and effectiveness of an rVSV-vectored vaccine in preventing Ebola virus disease: final results from the Guinea ring vaccination, open-label, cluster-randomised trial (Ebola Ca Suffit!). Lancet
Hersi M, Stevens A, Quach P, Hamel C, Thavorn K, Garritty C et al (2015) Effectiveness of personal protective equipment for healthcare workers caring for patients with filovirus disease: a rapid review. PLoS ONE 10(10):e0140290
Hofmann-Winkler H, Gnirss K, Wrensch F, Pohlmann S (2015) Comparative analysis of host cell entry of Ebola virus from Sierra Leone, 2014, and Zaire, 1976. J Infect Dis 212(Suppl 2):S172–S180
Holmes EC, Dudas G, Rambaut A, Andersen KG (2016) The evolution of Ebola virus: insights from the 2013–2016 epidemic. Nature 538(7624):193–200
Howlett P, Brown C, Helderman T, Brooks T, Lisk D, Deen G et al (2016) Ebola virus disease complicated by late-onset encephalitis and polyarthritis Sierra Leone. Emerg Infect Dis 22(1):150–152
Huttner A, Dayer JA, Yerly S, Combescure C, Auderset F, Desmeules J et al (2015) The effect of dose on the safety and immunogenicity of the VSV Ebola candidate vaccine: a randomised double-blind, placebo-controlled phase 1/2 trial. Lancet Infect Dis 15(10):1156–1166
Jacobs M, Rodger A, Bell DJ, Bhagani S, Cropley I, Filipe A et al (2016) Late Ebola virus relapse causing meningoencephalitis: a case report. Lancet
Keita M, Duraffour S, Loman NJ, Rambaut A, Diallo B, Magassouba N et al (2016) Unusual Ebola virus chain of transmission, Conakry, Guinea, 2014–2015. Emerg Infect Dis 22(12):2149–2152
Kuhn JH, Andersen KG, Baize S, Bao Y, Bavari S, Berthet N et al (2014) Nomenclature- and database-compatible names for the two Ebola virus variants that emerged in Guinea and the Democratic Republic of the Congo in 2014. Viruses 6(11):4760–4799
Lau MS, Dalziel BD, Funk S, McClelland A, Tiffany A, Riley S et al (2017) Spatial and temporal dynamics of superspreading events in the 2014–2015 West Africa Ebola epidemic. Proc Natl Acad Sci USA 114(9):2337–2342
Li X, Zai J, Liu H, Feng Y, Li F, Wei J et al (2016) The 2014 Ebola virus outbreak in West Africa highlights no evidence of rapid evolution or adaptation to humans. Sci Rep 6:35822
Mari Saez A, Weiss S, Nowak K, Lapeyre V, Zimmermann F, Dux A et al (2014) Investigating the zoonotic origin of the West African Ebola epidemic. EMBO Mol Med 7(1):17–23
Marzi A, Robertson SJ, Haddock E, Feldmann F, Hanley PW, Scott DP et al (2015a) EBOLA VACCINE. VSV-EBOV rapidly protects macaques against infection with the 2014/15 Ebola virus outbreak strain. Science (New York, NY) 349(6249):739–742
Marzi A, Feldmann F, Hanley PW, Scott DP, Gunther S, Feldmann H (2015b) Delayed disease progression in cynomolgus macaques infected with Ebola virus makona strain. Emerg Infect Dis 21(10):1777–1783
Mate SE, Kugelman JR, Nyenswah TG, Ladner JT, Wiley MR, Cordier-Lassalle T et al (2015) Molecular evidence of sexual transmission of Ebola virus. N Engl J Med
Matranga CB, Andersen KG, Winnicki S, Busby M, Gladden AD, Tewhey R et al (2014) Enhanced methods for unbiased deep sequencing of Lassa and Ebola RNA viruses from clinical and biological samples. Genome Biol 15(11):519
Meltzer MI, Atkins CY, Santibanez S, Knust B, Petersen BW, Ervin ED et al (2014) Estimating the future number of cases in the Ebola epidemic–Liberia and Sierra Leone, 2014–2015. MMWR Suppl 63(3):1–14
Moon S, Sridhar D, Pate MA, Jha AK, Clinton C, Delaunay S et al (2015) Will Ebola change the game? Ten essential reforms before the next pandemic. The report of the Harvard-LSHTM Independent Panel on the Global Response to Ebola. Lancet 386(10009):2204–2221
Nations U (ed) (1948) The universal declaration of human rights. UN Gen Assem, New York, United Nations
NIH (2015) Study of Ebola survivors opens in Liberia [cited 2016 April 21]. http://www.nih.gov/news-events/news-releases/study-ebola-survivors-opens-liberia
Ning YJ, Deng F, Hu Z, Wang H (2017) The roles of Ebolavirus glycoproteins in viral pathogenesis. Virol Sin. 32(1):3–15
Oduyebo T, Pineda D, Lamin M, Leung A, Corbett C, Jamieson DJ (2015) A pregnant patient with Ebola virus disease. Obstet Gynecol
Osterholm MT, Moore KA, Kelley NS, Brosseau LM, Wong G, Murphy FA et al (2015) Transmission of Ebola viruses: what we know and what we do not know. mBio 6(2):e00137
Park DJ, Dudas G, Wohl S, Goba A, Whitmer SL, Andersen KG et al (2015) Ebola virus epidemiology, transmission, and evolution during seven months in Sierra Leone. Cell 161(7):1516–1526
Parpia AS, Ndeffo-Mbah ML, Wenzel NS, Galvani AP (2016) Effects of response to 2014–2015 Ebola outbreak on deaths from Malaria, HIV/AIDS, and tuberculosis, West Africa. Emerg Infect Dis 22(3):433–441
Quick J, Loman NJ, Duraffour S, Simpson JT, Severi E, Cowley L et al (2016) Real-time, portable genome sequencing for Ebola surveillance. Nature 530(7589):228–232
Simon-Loriere E, Faye O, Faye O, Koivogui L, Magassouba N, Keita S et al (2015) Distinct lineages of Ebola virus in Guinea during the 2014 West African epidemic. Nature 524(7563):102–104
Sozhamannan S, Holland MY, Hall AT, Negron DA, Ivancich M, Koehler JW et al (2015) Evaluation of signature erosion in Ebola virus due to genomic drift and its impact on the performance of diagnostic assays. Viruses 7(6):3130–3154
System Ebola Epidemiology Team IM (2014) Update: Ebola virus disease epidemic—west Africa, November 2014. MMWR Morbidity and mortality weekly report 63(46):1064–1066
UNICEF (2016) Children hardest hit New York, USA [cited 2017 April 23]. https://www.unicef.org/emergencies/ebola/75941_76202.html
Urbanowicz RA, McClure CP, Sakuntabhai A, Sall AA, Kobinger G, Muller MA, et al (2016) Human adaptation of Ebola virus during the West African outbreak. Cell 167(4):1079–1087 e5
Uyeki TM, Mehta AK, Davey RT Jr, Liddell AM, Wolf T, Vetter P et al (2016) Clinical management of Ebola virus disease in the United States and Europe. N Engl J Med 374(7):636–646
Varkey JB, Shantha JG, Crozier I, Kraft CS, Lyon GM, Mehta AK et al (2015) Persistence of Ebola virus in ocular fluid during convalescence. N Engl J Med
Vetter P, Fischer WA, 2nd, Schibler M, Jacobs M, Bausch DG, Kaiser L (2016a) Ebola virus shedding and transmission: review of current evidence. J Infect Dis
Vetter P, Kaiser L, Schibler M, Ciglenecki I, Bausch DG (2016b) Sequelae of Ebola virus disease: the emergency within the emergency. Lancet Infect Dis
Whitmer SL, Albarino C, Shepard SS, Dudas G, Sheth M, Brown SC et al (2016) Preliminary evaluation of the effect of investigational Ebola virus disease treatments on viral genome sequences. J Infect Dis 214(suppl 3):S333–S341
WHO (2008) Interim infection control recommendations for care of patients with suspected or confirmed Filovirus (Ebola, Marburg) Haemorrhagic Fever
WHO (2014a) Ground zero in Guinea: the Ebola outbreak smoulders—undetected—for more than 3 months. http://www.who.int/csr/disease/ebola/ebola-6-months/guinea/en/
WHO (2014b) Ebola virus disease in Guinea. http://www.afro.who.int/en/clusters-a-programmes/dpc/epidemic-a-pandemic-alert-and-response/outbreak-news/4063-ebola-hemorrhagic-fever-in-guinea.html
WHO (2015a) Ebola Situation Report—4 November 2015. http://apps.who.int/ebola/current-situation/ebola-situation-report-4-November-2015
WHO (2015b) Ebola situation report—2 December 2015. http://apps.who.int/ebola/current-situation/ebola-situation-report-2-December-2015
WHO (2015c) Ebola virus disease (EVD) in West Africa: an extraordinary epidemic. Releve epidemiologique hebdomadaire/section d’hygiene du secretariat de la societe des nations = weekly epidemiological record/ health section of the secretariat of the league of nations. 90(10):89–96
WHO (2016a) Clinical care for survivors of Ebola virus disease. Interim guidance Geneva, Switzerland: WHO [cited 2016 January 24, 2016]. http://apps.who.int/csr/resources/publications/ebola/guidance-survivors/en/index.html
WHO (2016b) WHO health emergencies program geneva, Switzerland: WHO [cited 2017 April 23]. http://www.who.int/about/who_reform/emergency-capacities/emergency-programme/en/
Acknowledgements
The author thanks Ian Crozier and Jens Kuhn for critical review of the manuscript and Lara Schwarz for administrative assistance.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Bausch, D.G. (2017). West Africa 2013 Ebola: From Virus Outbreak to Humanitarian Crisis. In: Mühlberger, E., Hensley, L., Towner, J. (eds) Marburg- and Ebolaviruses. Current Topics in Microbiology and Immunology, vol 411. Springer, Cham. https://doi.org/10.1007/82_2017_69
Download citation
DOI: https://doi.org/10.1007/82_2017_69
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-68946-3
Online ISBN: 978-3-319-68948-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)