Abstract
More than 500,000 spinal operations are performed annually in the USA. Unanticipated coagulopathy during spine surgery is uncommon; however, substantial blood loss remains a feared complication of increasingly complex and longer-duration procedures. Significant intraoperative coagulopathy, defined as recurrent microvascular bleeding despite local hemostatic measures or decreased clot formation of blood pooled within the surgical field, has been reported in up to 16% of patients undergoing major spinal surgery. This bleeding can result in serious consequences including early termination of the procedure, postoperative hematoma formation, and increased in-hospital mortality.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Overview
More than 500,000 spinal operations are performed annually in the USA. Unanticipated coagulopathy during spine surgery is uncommon; however, substantial blood loss remains a feared complication of increasingly complex and longer-duration procedures. Significant intraoperative coagulopathy, defined as recurrent microvascular bleeding despite local hemostatic measures or decreased clot formation of blood pooled within the surgical field, has been reported in up to 16% of patients undergoing major spinal surgery. This bleeding can result in serious consequences including early termination of the procedure, postoperative hematoma formation, and increased in-hospital mortality. Therefore, it is imperative to identify risk factors for coagulopathy during preoperative assessment and take appropriate preventative action (Table 39.1).
While congenital bleeding disorders cannot be overlooked, those with severe manifestations are likely to be detected prior to adulthood. As the vast majority of patients will have undergone routine preoperative evaluation of PT, aPTT, and platelet count, coagulopathy during spinal surgery is generally due to some preexisting platelet dysfunction (i.e., known or unidentified use of platelet inhibitors) or an acquired problem with coagulation during the surgery such as dilutional coagulopathy. Other comorbid conditions with systemic impacts on coagulation may include hepatic or renal failure, malignancy, and collagen vascular disorders. In addition, there is evidence to suggest that certain spinal conditions, such as idiopathic scoliosis, may be associated with a degree of intrinsic platelet dysfunction.
An acquired problem with coagulation during surgery, such as a dilutional coagulopathy , can contribute to significant bleeding. Blood loss in large instrumented spine cases or resections of vascular spinal metastases can approach a patient’s estimated blood volume. This loss is typically replaced with a combination of crystalloid, intraoperative autotransfused blood, and/or allogenic blood products. Continuing crystalloid administration without appropriate replacement of clotting factors and/or platelets can lead to severe coagulopathy over the course of long procedures. Coagulation factors typically remain functional down to concentrations approximately one-third of normal. However, this threshold is reached upon replacement of an entire blood volume. Of note, though coagulopathy may occur secondary to dilution of either coagulation factors or platelets, there is evidence suggesting that coagulation factor dilution and disturbed fibrin polymerization are of greater concern than thrombocytopenia.
It is extremely important to prevent hypothermia, especially in the setting of a concurrent acidosis, as both factors are strongly associated with coagulopathy. Other severe but uncommon causes of coagulopathy include allergic and immunologic reactions (e.g., transfusion, drug reaction) or disseminated intravascular coagulation (DIC) . DIC is of particular concern in patients with multiple traumatic injuries, especially severe head injury, and in patients with widespread metastases. During spine surgery, exposed bone may act as a source of tissue plasminogen activator and urokinase which may lead to activation of the fibrinolytic system and subsequent DIC.
Possible platelet dysfunction as a cause of coagulopathy is often difficult to assess preoperatively and is even more difficult to assess intraoperatively. Platelet aggregation studies and bleeding times have variable predictive value preoperatively and are not useful in the intraoperative setting. Assessment of surgical bleeding by measuring the viscoelastic properties of whole blood by techniques such as thromboelastography (TEG) has not been well described other than in cardiac and liver transplant surgery, though they have potential for application in spine surgery. This technique measures the entire clotting process from fibrin formation to fibrinolysis. Because whole blood is used, the plasmatic coagulation system interacts with platelets and red cells, providing useful information on platelet function at the patient’s temperature. However, there is a difference between in vitro and in vivo coagulation as viscoelastic coagulation tests measure coagulation under static conditions (no flow) in a cuvette (not an endothelialized blood vessel).
A certain percentage of coagulopathy cannot be anticipated or avoided. Significant intraoperative bleeding mandates rapid assessment and treatment. This requires close communication between the anesthesia and surgery teams, including the decision to delay or abort further surgery for resuscitation as necessary.
Prevention
A complete review and reassessment, including obtaining a past medical history, current and recent medication use, family history, and surgical and anesthesia history, is essential to avoid intraoperative bleeding difficulties. Excessive bruising and/or unusual bleeding with attempts at intravenous or arterial access may suggest the possibility of clotting difficulties. Baseline studies typically include coagulation labs (PT/INR, aPTT), hemoglobin (Hb) levels, and platelet count. Bleeding times and other studies of platelet aggregation are of questionable utility in the preoperative setting. There must also be direct discussion between the surgical and anesthetic team prior to beginning complex cases, as the consent or booking description may not completely convey the complexity, length, or potential for blood loss. This discussion will help determine the need for large-bore vascular access, arterial line monitoring, blood products, and/or cell saver. Consideration should also be given to staging procedures, especially if combined anterior and posterior approaches are planned.
Intraoperative fluid replacement strategies are highly controversial. Restrictive, “goal-directed” fluid administration has been increasingly advocated as a means to prevent dilutional coagulopathy, as opposed to a solely formula-based approach. Suggested parameters include a rate of 4 mL/kg/h, with additional goal-directed boluses of 250 mL (up to a total of 1500 mL) given for periods of hypotension and tachycardia and for urine output dropping below 0.5 mL/kg/h for 2 or more hours. Vasopressors and/or furosemide may be used in patients not responding to these boluses. There is controversy regarding the value of administering fluids as colloid or crystalloid. Concerns exist over an increased risk of coagulopathy with the use of hetastarch in normal saline. Regardless of the fluid replacement strategy employed, the use of warming devices and the exclusive infusion of warmed fluids are recommended to maintain normothermia in long cases.
Further measures can be taken preoperatively and intraoperatively to help minimize the risk of significant blood loss. When positioning a patient prone, it is important to avoid abdominal compression that may cause engorgement of epidural veins, potentially exacerbating blood loss and contributing to a coagulopathy. It is advisable to have patients with vascular spinal metastases such as renal cell carcinoma undergo preoperative embolization to potentially reduce intraoperative blood loss. In these patients it is our practice to place the pedicle screws and contour the rods prior to proceeding with tumor resection. This allows for provisional stabilization of the spine in the event that massive bleeding necessitates premature termination of the case. With large incisions it is also important to use packing to control local bleeding in portions of the incision not immediately being addressed to avoid continuous blood loss. The use of vasopressor agents during surgery for the purpose of maintaining cord perfusion in cases of spinal cord compression, for example, may mask reduced intravascular volume. Indirect measures of adequate perfusion such as urine output and possibly lactate levels should be assessed. Regular reassessment of hemoglobin levels is advised, as a loss of RBC mass may have adverse effects on coagulation due to altered blood rheology. Periodic monitoring of electrolytes is also warranted, as coagulopathy can occur in the setting of electrolyte derangements. Of these, hypocalcemia is of particular concern given the complex interactions between ionized calcium and the negatively charged vitamin K-dependent clotting factors. Hypocalcemia may also be worsened by volume replacement with colloid solutions or infusion of citrated blood products.
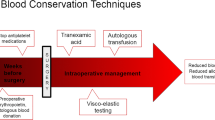
The preoperative and/or intraoperative administration of antifibrinolytic agents, such as aprotinin and lysine analogs tranexamic acid (TXA) and epsilon aminocaproic acid (EACA), has become increasingly widespread in elective spinal surgery. There is a growing body of evidence to support their routine use in a variety of orthopedic and neurosurgical procedures. These agents have been most extensively studied in the cardiovascular surgical literature, wherein all three have been found to significantly reduce intraoperative blood loss and postoperative transfusion rates. However, despite slightly superior efficacy, aprotinin has been associated with a higher risk of cardiovascular complications and death when compared to the lysine analogs, accounting for the increasingly widespread use of the latter. Dosing regimens and medical contraindications for antifibrinolytic agents remain variable and institution-dependent. While these agents show considerable promise and are the subject of further study, it must be noted that their use in spinal procedures remains off-label at this time.
Crisis Management
Once an intraoperative bleeding issue is identified, it is imperative that there is rapid assessment and treatment (Table 39.2). This requires close communication between the anesthesia and surgical teams.
Pathophysiology and Clinical Presentation
Rapid blood loss can be both the cause and result of coagulopathy. Intraoperative emergencies such as laceration of a large artery or vein can be life threatening and require immediate packing and vascular repair. Rapid blood loss can lead to hypotension and possibly DIC. Blood loss with vascular spinal lesions such as renal cell metastasis can be substantial despite preoperative embolization. Nevertheless, aggressive fluid resuscitation can cause a dilutional coagulopathy. Strong consideration should be given to aborting the originally intended procedure even if the injury is rapidly repaired and the patient is physiologically stable.
The development of excessive bleeding in complex cases can also be a gradual process with the development of recurrent microvascular bleeding. Sometimes, this is a subjective response by the surgical teams that comes with experience as “things just seem oozy.” Well-established communication between anesthesia and surgery teams will allow early identification and intervention in such circumstances. New-onset bleeding in previously hemostatic areas is most frequently dilutional in nature. Paradoxically, this is may be more likely to occur in cases where large-volume resuscitation has been required following rapid bleeding from other etiologies, such as a vascular injury. In these situations it is often advisable to stop and reassess before continuing.
General anesthesia masks symptoms of end-organ dysfunction, and many signs (hypotension, tachycardia, oliguria/hemoglobinuria) may be wrongly explained by other causes. DIC should be suspected at the first potential sign of end-organ dysfunction, as clinically apparent bleeding is a late manifestation that occurs only after consumption of coagulation factors. While exceedingly rare, consideration must also be given to the possibility of a transfusion reaction in all patients receiving blood products.
Patient Assessment
Any response to an intraoperative bleeding crisis must begin with an airway, breathing, and circulation assessment. Sites of intravenous or arterial access should be examined for evidence of new-onset bleeding and extremities examined for possible signs of ischemia or thrombosis. There should also be a simultaneous assessment of patient’s intake and output balance, especially with regard to estimated blood loss and volume replacement. Initial laboratory evaluation should include Hb level, platelet count, and coagulation labs (PT/INR, aPTT). While PT and aPTT may often be mildly abnormal, values in excess of 1.5 times controls are most sensitive for clinically evident coagulopathy.
Periods of hypoperfusion or hypotension (i.e., patient requiring pressor agents to maintain blood pressure) should prompt an immediate blood gas to check for acidosis. Lactate levels may be useful in the assessment of systemic hypoperfusion, as lactic acidosis can preclude the restoration of normal coagulation. Additional laboratory evaluation may require levels of lactate dehydrogenase (LDH), fibrinogen, and fibrin split products (D-dimer) to evaluate for hemolysis or DIC. While elevations are nonspecific, a D-dimer within normal limits along with a normal platelet count renders DIC highly unlikely. An adequate concentration of fibrinogen is critical for clot formation. Concern for a transfusion reaction should prompt retesting and cross-matching of blood samples for major incompatibility.
Intervention/Treatment
Treatment decisions must be based on the extent and etiology of coagulopathy. Temporary suspension of surgical activity is advised in order to achieve adequate hemostasis and resuscitation before proceeding with the operation.
All homeostatic parameters affecting coagulation must be addressed. Buffering to physiologic pH is required at a pH less than 7.1 or a base deficit of 12.5. Optimal Hb for restoration of coagulation is higher than the one required for oxygen delivery, and transfusion to values of 10–11 g/dL may be necessary to support platelet function. Aggressive warming must also be pursued for core temperatures less than 34 °C.
Administration of fresh frozen plasma (FFP) is indicated for active bleeding following massive blood transfusion (more than one blood volume). Early implementation of a balanced transfusion protocol with both FFP and PRBC may limit the extent of transfusion-related coagulopathy. The extent of PT and/or aPTT elevations may be used to guide coagulation factor replacement, as they have been found to positively correlate with volumes of FFP required to maintain hemostasis. Cryoprecipitate or packed factor concentrates may be used, when available, if additional volume is undesirable or at fibrinogen concentrations less than 80–100 mg/dL. Platelets are often given prophylactically with normal counts even without documented coagulopathy on the presumption of altered platelet function; however, consideration must be given to the added expense, infectious risk, and risk of developing antibodies which could affect future transfusions.
Termination of the procedure to allow for optimal medical management of the coagulopathy is a complex decision that must be made in collaboration between the surgeon and the anesthesiologist. Factors to be considered include the patient’s overall medical condition, acuity of the condition for which the patient is undergoing surgery, anticipated length of remaining procedure, response to treatment thus far, and availability of critical care services in the immediate postoperative period. This decision will be highly individualized to the clinical scenario and requires communication between both teams to arrive at the best decision for the overall health and safety of the patient.
Key Points
-
Preoperative clinical and laboratory assessment, as well as close communication between the surgical and anesthetic teams, is essential to avoiding coagulation difficulties, as well as dealing with them effectively when they arise.
-
Early recognition of intraoperative bleeding difficulties should prompt a simultaneous and systematic evaluation and initiation of therapy and may require either temporarily or permanently stopping the surgical procedure to allow re-establishment of physiologic homeostasis.
Suggested Reading
Cheriyan T, Maier SP 2nd, Bianco K, Slobodyanyuk K, Rattenni RN, Lafage V, Schwab FJ, Lonner BS, Errico TJ. Efficacy of tranexamic acid on surgical bleeding in spine surgery: a meta-analysis. Spine J. 2015;15:752–61.
Drews RE. Critical issues in hematology: anemia, thrombocytopenia, coagulopathy, and blood product transfusions in critically ill patients. Clin Chest Med. 2003;24:607–22.
Lier H, Krep H, Schroeder S, et al. Preconditions of hemostasis in trauma: a review. The influence of acidosis, hypocalcemia, anemia, and hypothermia on functional hemostasis in trauma. J Trauma. 2008;65:951–60.
Ornstein E, Berko R. Anesthesia techniques in complex spine surgery. Neurosurg Clin N Am. 2006;17:191–203, v.
Practice guidelines for blood component therapy: a report by the American Society of Anesthesiologists Task Force on Blood Component Therapy. Anesthesiology. 1996;84:732–47.
Shen Y, Silverstein JC, Roth S. In-hospital complications and mortality after elective spinal fusion surgery in the United States: a study of the nationwide inpatient sample from 2001 to 2005. J Neurosurg Anesthesiol. 2009;21:21–30.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Milby, A.H., Halpern, C.H., Schuster, J.M. (2020). Coagulopathy in Spinal Surgery. In: Brambrink, A., Kirsch, J. (eds) Essentials of Neurosurgical Anesthesia & Critical Care. Springer, Cham. https://doi.org/10.1007/978-3-030-17410-1_39
Download citation
DOI: https://doi.org/10.1007/978-3-030-17410-1_39
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-17408-8
Online ISBN: 978-3-030-17410-1
eBook Packages: MedicineMedicine (R0)