Abstract
We present the first character-based phylogenetic hypothesis for cunaxid genera and subfamilies based on 47 morphological characters scored from adult females. The phylogeny suggests that two of the subfamilies are not monophyletic, and although bootstrap support was not high enough to confidently redefine the subfamilies, it presents a testable hypothesis that will hopefully spur phylogenetic investigations of Cunaxidae using molecular techniques.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Cunaxidae (Acariformes: Trombidiformes: Prostigmata: Eupodina: Bdelloidea) (Fig. 4.1a–d) are cosmopolitan predatory mites that are commonly encountered in terrestrial habitats, including forest leaf litter and soil, tree holes, grasslands, agriculture fields, and anthropogenically disturbed areas such as house dust and stored food products (Skvarla et al. 2014). They are generalist predators that employ active and ambush hunting techniques to feed on a variety of active and sessile prey, including, Psocoptera, phytophagous mites, nematodes, scale insects, and arthropod eggs (Ewing and Webster 1912; Walter and Kaplan 1991; Smiley 1992; Walter and Proctor 1999; Castro and Moraes 2010). Cunaxids fail to survive when offered only plant material, and although one report of honeydew feeding exists, it is unknown if they can survive solely on honeydew (Zaher et al. 1975; Walter and Proctor 1999; Skvarla et al. 2014). Smiley (1992) and Skvarla et al. (2014) reviewed the biology, biogeography, morphology, and taxonomy and systematics of the family.
Cunaxidae and Bdellidae. a Cunaxoidinae. b Pulaeus. c Armascirus. d Rubroscirus. e, f Bdellidae. (a) © Scott Justis, (b–d) originally published in Skvarla et al. (2014), (e) © Graham Montgomery, (f) © Alice Abela. All photos used with permission
Cunaxidae is a relatively small family consisting of approximately 436 species, which are organized in 27 genera and 6 subfamilies (Table 1). Most authors consider Cunaxidae and Bdellidae (Fig. 4.1e, f) to be sister taxa that comprise Bdelloidea based on a number of derived characters, including the presence of trichobothria on leg segments, a solenidia present on the palp tibiotarsi, and unique leg segmentation consisting of a divided femur and fused tibiotarsus (Smiley 1992; Skvarla et al. 2014), although this classification is not universally accepted (Lindquist, pers. comm.). Bdelloidea are sometimes considered to be sister to the aquatic and mostly marine Halacaroidea, and are generally placed in the cohort Eupodina within the suborder Prostigmata (e.g., Norton et al. 1993; Lindquist 1996).
Cunaxid subfamilies have traditionally been proposed with little or no cladistic backing, which creates two major problems. First, basal taxa may be grouped together because they have fewer derived characters or share pleisomorphic characters lost in derived taxa. Second, highly derived taxa that stem from within established groupings may be classified separate from those groups, leading to paraphyly of the larger group. Both of these situations lead to classifications that do not reflect evolutionary history and are thus misleading.
Testing the validity of cunaxid subfamilies is compounded by the fact that no rigorous phylogenetic hypotheses of higher classifications (i.e., Prostigmata, Eupodina, Bdelloidea) exist, which makes outgroup selection difficult. For example, Norton et al. (1993) and Lindquist (1996) provided cladograms of Prostigmata, but the relationships were based on unpublished analyses and data (Proctor 1998). Numerous studies (morphological: O’Connor 1984; molecular: Dabert et al. 2010; Pepato et al. 2010; Pepato and Klimov 2015; Dabert et al. 2016; Xue et al. 2017) have recovered a monophyletic Prostigmata, but none have focused on the group and suffer from limited taxon sampling; additionally, recent molecular studies have recovered various phylogenetic hypotheses, some of which do not support recognized or hypothesized groups or relationships, including a monophyletic Euopdina (Dabert et al. 2010; Pepato and Klimov 2015; Dabert et al. 2016; Xue et al 2017), a sister-group relationship between Halacaroidea and Bdelloidea (Pepato and Klimov 2015; Dabert et al. 2016), or monophyletic Bdelloidea (Pepato and Klimov 2015).
In addition to testing the current subfamilial classification scheme, well-supported phylogenetic hypotheses allow stories to be told about evolutionary trends and character evolution. For example, Smiley (1992) considered Parabonzia the most basal cunaxid genus as they share a number of characteristics with Bdellidae, including 6–9 pairs of setae on the subcapitulum and “a five segmented palpus which resembles the palpi of the Bdellidae”. He also considered cunaxids with 3–segmented palps the most derived, having “body sizes [that] are smaller and…adaptations to exploit different habitats and smaller prey.” However, without a well-supported phylogenetic hypothesis, these opinions cannot be corroborated.
4.1 Materials and Methods
This manuscript is modified from Chapter VI of Skvarla’s (2011) unpublished MS thesis. Terminology has been updated and follows Skvarla et al. (2014).
4.1.1 Cladistic Analysis
The analysis included every genus of Cunaxidae (27 genera) and Bdellidae (13 genera) as ingroup taxa. Anystidae, Eupodidae, Tydeidae, and Labidostommatidae were chosen as outgroup taxa as they represent many of the larger groups (e.g., Anystina, Eupodina) and morphological diversity within Prostigmata. Halacarioidea, which has been suggested as the sister group of Bdelloidea, was excluded from the analysis as their many adaptations to an aquatic lifestyle make determining homologies difficult or impossible. 47 characters were scored from adult females; males and non-adult stages were excluded as they are unknown from most species of Cunaxidae.
Characters states were obtained from literature and, when possible, confirmed with specimens. Characters present in at least two taxa, and thus potentially synapomorphic, were included. Characters were equally weighted and unordered. Characters were coded as polymorphic when more than one character state existed within a single taxa. In some instances, an apparently polymorphic character (e.g., tarsal lobes absent; present and small; present and large) was divided into two characters (character 1: tarsal lobes absent or present; character 2: if present, tarsal lobes small or large) as an underlying relationship appeared to exist between the characters.
Character states that could not be determined were coded with a question mark. The uncertainty of a character was due to the complete absence of the character in the taxa in question (such as the number of setae on the palp femurogenu when the femora and genua of a particular taxa are not fused) or to uncertainty as to the homology of the character across taxa. A question mark was also scored when the state of a character could not be determined from the literature.
A heuristic search for 1000 most parsimonious cladograms was carried out using Mesquite 2.74 (Maddison and Maddison 2010). Trees were rearranged by subtree pruning and regrafting. A strict consensus tree was calculated using the 1000 most parsimonious cladograms. Bootstap values, consistency index (C.I.) and retention index (R.I.) were calculated using WinClada 1.00.08 (Nixon 2002).
4.1.2 Characters and Character States
Gnathasoma
-
I.
Shape of gnathasoma: normal (0); elongated (1).
-
II.
Setae hg1: not geniculate (0); geniculate (1).
-
III.
Number of subcapitular setae: 2 (0); 4 (1); 5 or more (2).
-
IV.
Pedipalps extend beyond distal end of subcapitulum by at least the last two segments: no (0); yes (1).
-
V.
Pedipalp ends in a claw: no (0); yes (1).
-
VI.
Pedipalp fixed digit: absent (0); present (1).
-
VII.
Pedipalp femora divided: no (0); yes (1).
-
VIII.
Pedipalp femora and genua fused: no (0); yes (1).
-
IX.
Pedipalp tibiae and tarsi fused: no (0); yes (1).
-
X.
Femoral apophysis: absent (0); present (1).
-
XI.
Apophysis between genua and tibiae: absent (0); present (1).
-
XII.
Number of setae on femurogenua: 5 (5); 6 (6).
-
XIII.
Number of setae on basifemora: 1 (0); more than 1 (1).
-
XIV.
Shape of basifemoral seta if only 1 seta present: simple (0); spine-like (1).
-
XV.
Shape of telofemoral seta: simple (0); spine-like (1); multi-branched (2).
-
XVI.
Cheliceral fixed digit: absent (0); present (1).
-
XVII.
Number of cheliceral seta(e): 0 (0); 1(1), more than 1 (2).
-
XVIII.
Number of adoral setae: 0 (0); 1 (1); 2 (2).
Dorsal idiosoma
-
XIX.
Eyes: absent (0); present (1).
-
XX.
Naso: absent (0); present (1).
-
XXI.
Number of dorsal trichobothria. 0 (0); 1 (1); 2(2).
-
XXII.
Hysterosomal median plate: absent (0); present (1).
-
XXIII.
Hysterosomal median plate fused to protersomal shield if median plate present: no (0); yes (1).
-
XXIV.
Idiosomal plates and shields patterned with reticulations: no (0); yes (1).
-
XXV.
Cupule ia: absent (0); present (1).
-
XXVI.
Cupule im: absent (0); present (1).
-
XXVII.
Cupule ip: absent (0); present (1).
-
XXVIII.
Setae f2: absent (0); present (1).
Ventral idiosoma
-
XXIX.
Coxae I-II fused into sternal shield: no (0); yes (1).
-
XXX.
Number of setae on coxae I: 3 or fewer (0); more than 3 (1).
-
XXXI.
Number of setae on coxae II: 3 or fewer (0); more than 3 (1).
-
XXXII.
Number of setae on coxae III: 3 or fewer (0); more than 3 (1).
-
XXXIII.
Number of setae on coxae IVI: 3 or fewer (0); more than 3 (1).
-
XXXIV.
Internal genital setae: absent (0); present (1).
-
XXXV.
Cupule ih: absent (0); present (1).
-
XXXVI.
Number of setae on genital plates: 4 (0); more than 4 (1).
Legs
-
XXXVII.
Tibiae I trichoborhtium: absent (0); present (1).
-
XXXVIII.
Tibiae II trichoborhtium: absent (0); present (1).
-
XXXIX.
Tarsus III trichoborhtium: absent (0); present (1).
-
XL.
Tibiae IV trichoborhtium: absent (0); present (1).
-
XLI.
Tarsus IV trichoborhtium: absent (0); present (1).
-
XLII.
Tasri constricted distally, forming lobes: no (0); yes (1).
-
XLIII.
Tarsal lobes, if present: small (0); large (1).
-
XLIV.
Leg tibiae divided into basi- and telofemora: no (0); yes (1).
-
XLV.
Shape of empodium: pad-like (0); 4-rayed (1).
-
XLVI.
Ambulacral claw sculpturing: smooth (0); rippled (1).
-
XLVII.
Number of setae complementing anal plates: 1 (1); 2 (2); more than 2 (3).
The coded matrix run in this analysis is presented in Fig. 4.2.
4.2 Results and Discussion
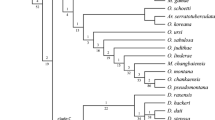
The heuristic search resulted in 1000 cladograms with a length of 131 (CI = 52; RI = 86). The strict consensus of these cladograms is given in Fig. 4.3. Bootstrap values >50 are presented over each branch.
4.2.1 Monophyly of Bdelloidea and Cunaxidae
The strict consensus cladogram suggests that Bdelloidea is a monophyletic lingeage and that Cunaxidae and Bdellidae are sister clades, rather than a single clade in which one family grades into the other. However, Halacaroidea was excluded from the analysis and the number of outgroups was extremely limited. Inclusion of additional Prostigmata outgroups or Halacaroidea within a molecular phylogeny may change one or both of these conclusions.
4.2.2 Validity of Subfamilies
Parabonzia was recovered as the most basal cunaxid genus and sister to the rest of the family. This is not surprising as Parabonzia shares many characteristics with Bdellidae (e.g., non-raptorial pedipalps). The grouping of Parabonzia with Bonzia in Bonziinae was not recovered. This subfamily is identified primarily by the presence of a multi-branced seta on the palp telofemora, which suggests that the multi-branched seta is plesiomorphic or evolved independently.
The subfamily Cunaxinae was recovered as a monophyletic lineage within a larger clade formed by the addition of three genera (Pseudobonzia, Neoscirula, and Neobonzia) currently classified within Coleoscirinae. This suggests that Cunaxinae is a valid subfamily, but should be redefined to accommodate the coleoscirine genera. The defining character of the larger clade is the absence of fusion between a hysterosomal plate (if it is present) with the protersomal plate and presence of 5-segmented palps (excluding Allocunaxa).
The second major clade recovered contains an unresolved basal polytomy formed by the remaining coleoscirine genera (Coleoscirus and Scutascirus), and a grade formed by the monobasic Orangescirulinae and Scirulinae into an unresolved Cunaxoidinae. This larger clade is defined by the expansion of the hysterosomal plate and fusion with the protersomal shield.
The lack of resolution and support in the phylogenetic hypothesis is problematic. There are trends that suggest the need for changes in classification, but resolution and support are too weak to confidently make those changes. For example, as previously mentioned, Bonzia is not recovered with Parabonzia, thus prompting the dissolution of Bonziinae. However, the placement of Bonzia within Cunaxidae is uncertain. With more data it may be recovered with Parabonzia as a monophyletic clade, as an independent lineage (as is suggested by this analysis), or within one of the two major clades.
The classification of the clade containing Cunaxoidinae depends on better resolution. If the basal polytomy is resolved and Coleoscirus and Scutascirus form a monophyletic lineage the clade could be broken into two subfamilies: Coleoscirus + Scutascirus and Orangescirulinae + Scirulinae + Cunaxoidinae. Alternately, it could be broken into four subfamilies, Coleoscirus + Scutascirus, Orangescirulinae, Scirulinae, Cunaxoidinae. If the basal polytomy is resolved and the coleoscirine genera are not monophyletic, that is form grade with Orangescirulinae and Scirulinae into Cunaxoidinae, Cunaxoidinae could redefined to include them, thus forming a single diverse subfamily, or they could be broken into five subfamilies (i.e., Coleoscirinae, Scutascirinae, Orangescirulinae, Scirulinae, and Cunaxoidinae), four of which would be monobasic.
4.2.3 Evolutionary Trends
While the strict consensus cladogram is not resolved enough to be a basis for classification changes, it does suggest directions in evolutionary trends. The most derived taxa in both larger clades (Allocunaxa and Cunaxoidinae) have palps in which the basifemora, telofemora, and genu have fused into a femurogenu, resulting in a 3-segmented pedipalp. This fusion in Cunaxinae may be more evolutionarily recent evolutionarily as species with fused pedipalpal segments still retains dark lines that indicate where the sutures are. Conversely, the palp segmentation of Cunaxoidinae can only be inferred through muscle attachment and setal placement as they lack sutures any external indication of the fused segments.
4.3 Conculsions
The morphological phylogenetic hypothesis presented here illustrates possible inconsistencies between current classification schemes and evolutionary history. However, a phylogenetic hypothesis with better support must be elucidated before such changes can be made with any kind of confidence. As the morphological characters presented in this study did not provide the needed resolution and support, molecular characters are the next logical step.
Change history
25 August 2019
In the original version of the book, the following belated corrections are to be incorporated.
References
Castro TMMG, Moraes GJ (2010) Life cycle and behaviour of the predaceous mite Cunaxatricha tarsospinosa (Acari: Prostigmata: Cunaxidae). Exp App Acarology 50:133–139
Dabert M, Witalinski W, Kazmierski A, Olszanowski Z, Dabert J (2010) Molecular phylogeny of acariform mites (Acari, Arachnida): strong conflict between phylogenetic signal and long-branch attraction artifacts. Mol Phyl Evol 56(1):222–241
Dabert M, Proctor H, Dabert J (2016) Higher-level molecular phylogeny of the water mties (Acariformes: Prostigmata: Parasitengona: Hydrachnidiae). Mol Phyl Evol 101:75–90
Ewing HE, Webster RL (1912) Mites associated with oyster-shell scale (Lepidosaphes ulmi Linne). Psyche 19:121–134. https://doi.org/10.1155/1912/73282
Lindquist EE (1996) Phylogenetic relationships. In: Lindquist EE, Sabelis MW, Bruin J (eds) Eriophyoid mites—their biology, natural enemies and control. Elsevier Science, Amsterdam, pp 301–327
Maddison WP, Maddison DR (2010) Mesquite: a modular system for evolutionary analysis. Version 2.74. http://mesquiteproject.org
Nixon KC (2002) Winclada (BETA) ver. 1.00.08. Published by the author, Ithaca, NY
Norton RA, Kethley JB, Johnston DE, O’Connor BM (1993) Phylogenetic perspectives on genetic systems and reproductive modes of mites. In: Ebbert MA, Wrensch DL (eds) Evolution and diversity of sex ratio in insects and mites. Chapman & Hall, New York, pp 8–99
O’Connor, BM (1984) Phylogenetic relationships among higher taxa in the Acariformes, with particular reference to the Astigmata. In: Griffiths DA, Bowman CE (eds) Acarology VI, vol. 1. Ellis-Horwood Ltd., Chichester, pp 19–27
Pepato AR, Klimov PB (2015) Origin and higher-level diversification of acariform mites—evidence from nuclear ribosomal genes, extensive taxon sampling, and secondary structure alignment. BMC Evol Bio 15:178. https://doi.org/10.1186/s12862-015-0458-2
Pepato AR, Rocha CEF, Dunlop JA (2010) Phylogenetic position of the acariform mites: sensitivity to homology assessment under total evidence. BMC Evol Bio 10:235. https://doi.org/10.1186/1471-2148-10-235
Proctor H (1998) Trombidiformes. Trombidiform mites. Version 09 August 1998. http://tolweb.org/Trombidiformes/2568/1998.08.09. In: The tree of life web project. http://tolweb.org/
Smiley RL (1992) The predatory mite family Cunaxidae (Acari) of the world with a new classification. Indira Publishing House, West Bloomington, Michigan
Skvarla M (2011) Ozark Highland Cunaxidae (Acari: Prostigmata): descriptions and keys to genera found to occur in the region and a new phylogenetic hypothesis for the family. MS thesis, University of Arkansas, Fayetteville, Arkansas
Skvarla M, Fisher JR, Dowling APG (2014) A review of Cunaxidae (Acariformes, Trombidiformes): histories and diagnoses of subfamilies and genera, keys to world species, and some new locality records. ZooKeys 418:1–103
Walter DE, Kaplan DT (1991) Observations on Coleoscirus simplex (Acarina: Prostigmata), a predatory mite that colonizes greenhouse cultures of rootknot nematode (Meloidogyne spp.), and a review of feeding behavior in the Cunaxidae. Exp Appl Acarology 12:47–59
Walter DE, Proctor HC (1999) Mites: ecology, evolution, and behaviour. CABI Publishing, Wallingford, UK
Xue X-F, Dong Y, Deng W, Hong X-Y, Shao R (2017) The phylogenetic position of eriophyoid mites (superfamily Eriophyoidea) in Acariformes inferred from the sequences of mitochondrial genomes and nuclear small subunit (18S) rRNA gene. Mol Phyl Evol 109:271–282
Zaher MA, Soliman ZR, El-Bishlawy SM (1975) Feeding habits of the predaceous mite, Cunaxa capreolus (Acarina: Cunaxidae). Entomophaga 20(2):209–212
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Skvarla, M.J., Dowling, A.P.G. (2019). A Preliminary Phylogenetic Hypothesis for Cunaxidae (Acariformes: Trombidiformes: Prostigmata: Eupodina). In: Skvarla, M., Ochoa, R., Verle Rodrigues, J., Hutcheson, H. (eds) Contemporary Acarology . Springer, Cham. https://doi.org/10.1007/978-3-030-17265-7_4
Download citation
DOI: https://doi.org/10.1007/978-3-030-17265-7_4
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-17264-0
Online ISBN: 978-3-030-17265-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)