Abstract
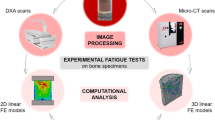
In recent years, imaging methods have started to be used for scientific purposes as well as diagnostic purposes as technology develops. Possibilities that have revolutionary quality have been presented for scientific researches within the features that are operating principle of micro-computed tomography (micro-CT), image quality, and three-dimensional reconstruction. In bone researches, samples in certain size are transferred to the computer environment without degenerating structural integrity and being exposed to chemical processing and microarchitecture which can be examined in every axis. Many bone analysis can be performed with conventional histomorphological methods by using micro-CT. Stretching tests can be applied to the samples such as analysis of advanced finite element by using micro-CT images.
Many parameters such as pathological tissues formed during healing, following either normal bone tissue or notably trabecular thickness (Tb.Th), trabecular number (Tb.N), trabecular separation (Tb.Sp), bone volume (BV), total tissue volume (TV), trabecular bone ratio (BV/TV), structural model index that shows numeric characteristics of trabecular as 3D (SMI), trabecular bone connections, number of trabecular nodes in each tissue volume (N.Nd/TV), and bone mineral density, belong to callus tissue which can be counted fast and in a secure manner.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
6.1 Bone Tissue
The human body is composed of 74 bones forming the axial skeleton, 26 of which are located in the spine, 22 of which are in the head skeleton, 25 of which are in chest wall which form the ribs and sternum, and 1 of which is the hyoid bone. Sixty-four pieces of upper extremity bones and 62 pieces of lower extremity bones which consist of 126 pieces of bones compose the appendicular skeleton. When six pieces of ear bones (ossicula auditoria) are added to these, a total of 206 bones form the human skeleton. The bones, which are the most rigid structure of the body after the teeth, weight approximately 15% of an adult human body, and the total weight of it is around approximately 5–6 kg [1].
Bone tissue is made of two main substances: organic substances (30–40%) which give flexibility to the structure of it and inorganic salts (60–70%) which give rigidity to the structure. The inorganic portion of the bone forms calcium phosphate (CaPO4) (85%), calcium carbonate (CaCO3) (10%), magnesium phosphate (MgPO4) (1.5%), calcium fluoride (CaF4), calcium chloride (CaCl), and alkali salts. Ninety-nine percent of body calcium is found in the bones, and this acts as a reservoir for metabolic calcium metabolism, whereas the phosphate content in bone structure is under the control of hormones and cytokines [1, 2].
Osteoblasts develop from mesoderm-derived stem cells and are found in bone marrow and some other connective tissues. Osteoblasts are responsible for the synthesis and mineralization of bone matrix; which then differentiate into osteocytes. The osteocytes are the essential structures that form the bone tissue, and they are dispersed within the bone matrix and are connected to each other by extensions to form a meshwork appearance. A bone volume of 1 mm3 contains approximately 700–900 osteocytes which are the most important cells that provide bone viability. The death of these cells triger the bone resorption as osteoclastic activity. Osteoclasts are responsible for the bone destruction during growth and formation [1, 2].
Bone tissue usually consists of bone substance (substantia ossea) and bone marrow (medulla ossea). The bone membrane (periosteum), which provides the feeding and repair of the bone, surrounds all the faces of the bones except for the parts of the bones participating in articular surfaces. Arteries that supply the bone (nutrient arteries) enter the bone from the periosteum, and then the periosteal arteries supply the cortical bone by entering the bone from many points. For this reason, if the periosteum is damaged the bone loses its supply and ability to repair. The ligaments, the tendons, and the muscles that connect bones to each other attached to the bones via the periosteum. Another task of periosteum is to enable the transverse growth of the bone [2].
The inner side of the bones are covered with a membrane called endosteum. The nutrient artery, which generally in the middle of the shaft enters from different points of each bone, and passes through the cortical bone obliquely that supplies the trabecular portion and bone marrow. Metaphyseal and epiphyseal arteries supply the ends of each bone. The veins accompany to the arteries, but the veins are numerous than the arteries within the red bone marrow. In periosteum, there are many receptors that are responsible for nerve impulse which receive sense from periosteum following the vessels. Periosteum consists of two layers; an outer fibrous layer (stratum fibrosum) and inner osteogenic layer (stratum osteogenicum). The fibrous layer of the periosteum continues with the fibrous layer of the articular capsule at the articular surfaces. The osteogenic layer of the periosteum is rather rich than vessels and enables the transverse growth of the bone. The periosteum, as it contains too many receptors, is very sensitive to trauma [3].
6.2 Types of Bone Tissue
There are two types of bone tissue according to their mechanical and biological characteristics: primary (immature) coarse fibrous bone and secondary (mature) lamellar bone [4]. Immature bone creates an embryological skeleton, and it continuously replaces with mature bone during the development. During the repair process of fractures, the immature bone forms the premature fracture repair tissue similar procedure how happens during the development process. The impaction and resorption process in the immature bone are faster, fibrils in the matrix that is collagen-structured exhibit an irregular sequence, and mineralization of the matrix forms an irregular shape when it is compared to the mature bone. The formation of the immature bone is being irregular that allows fracture callus to be distinguished from the mature bone tissue by creating an irregular image. The immature bone is softer and more easily deformed than the mature bone due to the irregularity and weakness of the sequence in the collagen fibers, depending on the high water content and irregular mineralization [4]. There are two types of tissue in the mature bone. A tight (cortical, compact) bone on the outer surface, and cancellous (trabecular, spongy) bone part is located on the inner surface. Compact bone forms the outer layer that provides a connection with the Havers channels and Volkmann channels that contain vascular structures that extend inwardly. The blood in the Havers channels supplies the osteocyte cells through numerous small canals. The osteon structure of the long bones is aligned in a manner coherent with the long axis of the bone. The structure that tightly connects to the periosteum is the Sharpey’s fibers. The cancellous bone in the inner layer is similar to the structurally tight bone and has a trabecular structure. Matured osteon units are only present in thickened trabeculae. The facing inward layer of the trabecules is covered with resting osteoblast cells [3, 5].
The term bone quality is frequently used in the literature to describe the structural properties of the bone. Bone quality is often associated with the trabecular bone structure [6]. Trabecular bone is the primary anatomic and functional structure of the cancellous bone. Bone quality is particularly important for implants placed on the bone. Besides, the amount of the cortical bone affects the primary stabilization of the implant; the role of trabecular bone in implant success is very important because the vast majority of an implant placed in the bone remains inside of the cancellous bone and is in direct contact with the implant. Bone contact and osseointegration can be measured by histomorphometric analysis. The histomorphometric analysis is a long-lasting and expensive method that can damage the analyzed sample and not allow to use the same sample for another evaluation. Due to these disadvantages, three-dimensional microtomography techniques have been put into use as nondestructive, rapid, and reliable methods with the aim of analyzing the microarchitecture of cortical and trabecular bones [6,7,8,9].
6.3 Ossification (Osteogenesis)
Osteogenesis may occur in two different ways.
The first one is; undifferentiated mesenchymal cells which merge in the form of layers and turn into osteoblasts with “intramembranous ossification” that develops by mineralizing and forming organic matrix. There is no cartilage model in this type of ossification. Flat bone formation, healing fractures with stable distraction methods, and long bone healing in children are examples of this type of ossification. The first point where the ossification begins in the mesenchymal tissue concentration is called the primary ossification center.
The second type of ossification is; “endochondral ossification.” Long and short bones are ossified through this way. In this type of ossification, initially small bone models of hyaline cartilage is being form during the development of bone. The cartilage model is replaced by bone tissue over time with endochondral ossification. Unstable fractures heal in this way.
6.4 Healing of Bone Tissue
The deterioration of the anatomical integrity of the bone as a result of mechanical forces coming from outside or inside of the body is called a fracture. The most remarkable feature of the fracture healing is that the repair tissue formed during the healing is in good quality bone tissue without developing the scar tissue when it is compared with the healing of the other tissues. Therefore, when defining the process of fracture healing, it can be called regeneration instead of restoration or repair [10]. The fracture developed in the bone leads to inflammation, repair, and remodeling process. Immediately after the development of the fracture, the inflammation process begins and the repair starts subsequently. Damaged cells and bone matrix in the process of repair will be recovered after that remodeling phase that is more long-lasting in comparison with the other two processes begins. The cells grow rapidly and the matrix synthesis constitutes the repair process in the area where the fracture fragments meet [11]. Inflammatory cytokines such as lymphocytes and macrophages are immigrating to the fracture site. The cytokines secreted by the inflammatory cells provide the formation of new vessels [11]. As inflammation decreases, necrotic dead tissues are removed, and fibroblasts appear in the region and begin to produce callus that is a new bone matrix and fracture union tissue. The fracture is a hematoma that initiates the progression of all these stages, allows the release of the cytokines, and emerges by the development of fracture, and there are scientific studies showing that the healing process has been disrupted by the loss of fracture’s hematoma [12, 13]. There are studies about the mineral density that indicated the low density in an extremity where fracture develops even after a well fracture union [14, 15]. The fracture healing is divided into two types in terms of the method; direct fracture healing and indirect fracture healing. Direct fracture healing is a type of fracture healing that is anatomically a reduced healing in the event that the tight detection is applied. Indirect fracture healing is a type that is seen more common than direct fracture healing. It is seen after the fractures that aren’t or can’t be reduced anatomically without being performed tightly [16]. The stages of fracture healing are histomorphologically divided into sections as inflammation, soft callus, hard callus, and remodelation. These four phases of fracture healing are intertwined; however, it should be stated that this staging is based on clinical and microscopic observations of fractures with soft tissue cover and with relatively low-energy mechanisms [10].
6.5 Use of Micro-CT in Bone Researches
Imaging methods have been used for scientific purposes as well as for diagnostic purposes as technology evolves, in recent years. The voxel range of micro-CT is almost one million times smaller than normal tomography. It obtains the 3D image of the object with detectors that have a high-resolution. In addition, micro-CT allows the visualization of the three-dimensional structures of anatomical structures and the necessary measurements can be made without using the histological sample preparation steps. The integrity of the samples is not impaired and is not subjected to any chemical effect on the specimen (Fig. 6.1).
Several parameters such as bone trabecular thickness (Tb.Th), trabecular number (Tb.N), trabecular separation (Tb.Sp), bone volume (BV), total tissue volume (TV), trabecular bone ratio (BV/TV), structural model index that shows numeric characteristics of trabecular as three-dimensional (SMI), trabecular bone connections, number of trabecular nodes in each tissue volume (N.Nd/TV), and bone density that can be determined depending on hydroxyapatite amount can be calculated quickly and reliably by high-resolution 3D micro-CT.
In the literature, the use of micro-CT has become widespread in the studies that are done in order to evaluate implants and assessment of implant-bone circumference. Many researchers have used three-dimensional microtomography in order to assess implant and the bone surrounding the implant (union between the implant surface and bone osseointegration) and obtain approximately the same results. Although some analyses can be made regarding the implant and surrounding bone by 3D micro-computed tomographies, these analyses can also be affected negatively by metal artifacts and, miscalculation of bone density that is caused by titanium implant absorbing more X-rays than the bone and scatters it to the surrounding tissue [17,18,19,20]. Moreover, it is also possible to evaluate the stress of the implant on the trabecular and cortical bone by performing finite element analysis with digital data obtained by micro-CTs [20].
Mulder et al. used the micro-CT method in order to examine the structure of pig condyle, the mineralization, and the trabecular bone development. According to their study, the remodeling on the anterior and posterior aspect of the mandible condyle was different from each other and that the remodeling of the posterior aspect of the condyle was larger. The study showed that trabecular bone volume and thickness is more in the body of the mandible and the quantity of mineralization in the anterior and posterior side of the condyle is the same, but more mineralization in the body of the mandible [19].
Tissue engineering is a multidisciplinary approach that forms a lost tissue or organ again which aims them to function. The use of micro-computed tomography in tissue engineering has become popular in recent years in order to investigate the materials forming the tissue skeleton. Micro-CT is mainly widely used in examining the material structure that constitutes the skeleton of the tissue and the amount of bone growth in materials such as polymeric and calcium phosphate in evaluation as in vitro [21]. Three-dimensional data presents more effective information compared to the two-dimensional inspection. Micro-computed tomography allows getting information about the material of lost tissue skeleton as well as investigation of the acquired tissue [22].
Dharan et al. [23] compared the effect of porous titanium granules on sinus augmentation by using different graft materials and using micro-computed tomography. For this, they made comparisons by using the parameters such as bone volume (BV), total tissue volume (TV), trabecular bone ratio (BV/TV), trabecular number (Tb.N), trabecular separation (Tb.Sp), trabecular thickness (Tb.Th), structural model index that shows numeric characteristics of trabecular as 3-dimension (SMI), trabecular bone connections, the number of trabecular nodes (N.Nd/TV), and bone density in each tissue volume. They emphasized that porous titanium granules provide space for the new bone formation and this may be effective in long-term success [23].
6.6 Use of Micro-CT in the Research of Bone Healings
Bone samples can be scanned in micro-CT with dry or distilled water in the tube. Dry scanning is a classic and fast method for micro-CT, and it is widely used for samples that are not affected by heat. The sample can be fixed to the center of the stage with a radiolucent substance, following the scan operation is performed. The wet screening is recommended if samples are wanted not to be affected by heat or if the samples are to be subjected to histopathological analysis after the micro-CT procedure. Additionally, wet scanning absolutely must be made in a tube in order to be able to make an analysis of the bone mineral density (g/mm3).
Generally, aluminum or copper filters are used in order to review the bone samples and to minimize radiologic artifacts during scanning. The samples are being scanned at 360° rotation, and high-resolution scans 33 μm or the smaller cross section are recommended for resulting the analysis correctly. The resulting images automatically are converted to 8-bit gray axial images by the software. Bone components are determined on axial images, and upper and lower limits are determined for analysis. ROIs (regions of interest) are selected by determining bone regions in axial images that lie within these limits. The white and black pixel ranges in the ROI are determined by setting histogram as semiautomatic, and global thresholding process is performed between these reference ranges for the automatic analyses in the next stage. If it needs to be expressed more clearly, the gray image of the ROIs in the respective range is pixelated as black and white dots. Total tissue volume, total bone volume, bone surface area, and the percentage rate of bone tissue further can be measured, and analysis of trabeculae structure mentioned earlier can be performed (Fig. 6.2). Please see chapter 5.
A robust old and mature bone can easily be distinguished from the healing tissue in the newly formed cartilage or immature callus during the global thresholding procedure. Similarly, the outer cortical bone can be differentiated easily trabeculae of bone located inside by selecting the correct field during the process of ROI. All these bone tissues can be analyzed together or separately (Fig. 6.3).
Several studies were made accrodinf to fracture calculus. Shefelbine evaluated fractures by using micro-computed tomography and images with the finite element analysis method. In this study, A torsional fracture in the mouse femurs was made and, following scans were performed from fracture calluses to determine three-dimensional geometry and material properties for finite element models in the third and fourth weeks following recovery. The area, stiffness, and mineral density of these calluses were calculated [24].
Bosemark also examined the anabolic use with autograft treatment in bone fractures using micro-CT. He performed the same type of autograft implementation by creating the same type of fracture indifferent mice groups using an anabolic substance or physiological salt water [25].
Reynolds et al. also compared the treatments with autograft and allograft in a similar study on mice using micro-CT. They made a volumetric analysis of the callus tissue and estimated biomechanical predictions [26].
Ezirganli et al. investigated the different types of bone graft effect of the healing on New Zealand rabbits. They analyzed the amount of new ossification with the micro-CT method and supported their studies with histomorphological tests. They declared that there were no statistical differences between the two methods during their studies [27].
Morgan et al. suggested that quantitative data from the newly formed callus to be obtained using micro-CT are more reliable than other methods. They followed different experimental conditions that changed postfracture recovery with micro-CT imaging and torsion test. In their research, total tissue volume (TV), bone volume (BV), trabecular bone ratio (BV/TV), bone mineral content (BMC), tissue mineral density (TMD), standard deviation (σTMD), active mineral density polar moment of inertia (Jeff), torsional strength, and torsional rigidity were calculated [28].
Yang et al. analyzed the callus tissue characteristics by micro-CT and Fourier transform by infrared imaging spectroscopy (FT-IRIS) in their research. Tissue mineral density (TMD) and bone volume fraction (BVF) analysis were performed in their research and stated both techniques are comparable [29].
Nyman et al. evaluated the quantitative measurements of the rat femoral fracture repair on micro-CT images as well. They analyzed the callus formation at three separate points in the same long bones. They specified these three points separately by the ROI operation, and among them, they calculated the volume of total callus (TVcallus), the volume of mineralized callus (BVcallus), and the volume of total callus (BVcallus/TVcallus) in mineralized tissue fraction and callus (mBMDcallus) containing the mineralized tap volumetric BMD (g/cm3) [30].
Studies with human samples in the literature are also available. Thomsen et al. compared the stereological measurements of the trabecular bone structure with three-dimensional micro-CT images and two-dimensional histological sections in human proximal tibial bone biopsies. The study revealed high correlations between data that gained from conventional 2D cross section and 3D micro-CT and measurements of the bone structure. 3D micro-CT showed that bone structural evaluations can be used instead of conventional histological sections [31].
Figures 6.4 and 6.5 are related with callus formation in the healing process of long bone fractures and newly formed bone tissue with graft treatment (Figs. 6.4 and 6.5).
References
Moore KL, Dalley AF, Agur AM. Clinically oriented anatomy. Philadelphia, PA: Lippincott Williams & Wilkins; 2013.
Standring S. Gray’s anatomy E-book: the anatomical basis of clinical practice. Philadelphia, PA: Elsevier Health Sciences; 2015.
Buckwalter JA, Glimcher MJ, Cooper RR, Recker R. Bone biology. I: structure, blood supply, cells, matrix, and mineralization. Instr Course Lect. 1996;45:371–86.
Buckwalter JA, Einhorn TA, Marsh J. Bone and joint healing. Rockwood and Green’s fractures in adults. Philadelphia, PA: Lippincott, Williams, and Wilkins; 2001. p. 245–71.
Buckwalter JA, Glimcher MJ, Cooper RR, Recker R. Bone biology. II: formation, form, modeling, remodeling, and regulation of cell function. Instr Course Lect. 1996;45:387–99.
Fyhrie DP. Summary—measuring “bone quality”. J Musculoskelet Neuronal Interact. 2005;5(4):318–20.
Sakka S, Coulthard P. Bone quality: a reality for the process of osseointegration. Implant Dent. 2009;18(6):480–5. https://doi.org/10.1097/ID.0b013e3181bb840d.
Fanuscu MI, Chang TL. Three-dimensional morphometric analysis of human cadaver bone: microstructural data from maxilla and mandible. Clin Oral Implants Res. 2004;15(2):213–8.
Muller R, Van Campenhout H, Van Damme B, Van Der Perre G, Dequeker J, Hildebrand T, et al. Morphometric analysis of human bone biopsies: a quantitative structural comparison of histological sections and micro-computed tomography. Bone. 1998;23(1):59–66.
Marsh DR, Li G. The biology of fracture healing: optimising outcome. Br Med Bull. 1999;55(4):856–69.
Mark H, Penington A, Nannmark U, Morrison W, Messina A. Microvascular invasion during endochondral ossification in experimental fractures in rats. Bone. 2004;35(2):535–42.
Grundnes O, Reikeras O. The importance of the hematoma for fracture healing in rats. Acta Orthop Scand. 1993;64(3):340–2. https://doi.org/10.3109/17453679308993640.
Grundnes O, Reikeras O. The role of hematoma and periosteal sealing for fracture healing in rats. Acta Orthop Scand. 1993;64(1):47–9.
Van der Wiel HE, Lips P, Nauta J, Patka P, Haarman HJ, Teule GJ. Loss of bone in the proximal part of the femur following unstable fractures of the leg. J Bone Joint Surg Am. 1994;76(2):230–6.
Karlsson MK, Nilsson BE, Obrant KJ. Bone mineral loss after lower extremity trauma. 62 cases followed for 15-38 years. Acta Orthop Scand. 1993;64(3):362–4.
Mattox DE. Bone healing and grafting. Ear Nose Throat J. 1983;62(8):409–11.
Butz F, Ogawa T, Chang T-L, Nishimura I. Three-dimensional bone-implant integration profiling using micro-computed tomography. Int J Oral Maxillofac Implants. 2006;21(5):687–95.
Morinaga K, Kido H, Sato A, Watazu A, Matsuura M. Chronological changes in the ultrastructure of titanium-bone interfaces: analysis by light microscopy, transmission electron microscopy, and micro-computed tomography. Clin Implant Dent Relat Res. 2009;11(1):59–68.
Mulder L, Koolstra JH, de Jonge HW, van Eijden TM. Architecture and mineralization of developing cortical and trabecular bone of the mandible. Anat Embryol (Berl). 2006;211(1):71–8. https://doi.org/10.1007/s00429-005-0054-0.
Van Oosterwyck H, Duyck J, Sloten JV, Perre GV, Jansen J, Wevers M, et al. The use of microfocus computerized tomography as a new technique for characterizing bone tissue around oral implants. J Oral Implantol. 2000;26(1):5–12.
Cartmell S, Huynh K, Lin A, Nagaraja S, Guldberg R. Quantitative microcomputed tomography analysis of mineralization within three-dimensional scaffolds in vitro. J Biomed Mater Res A. 2004;69(1):97–104.
Hollister SJ, Lin C, Saito E, Lin C, Schek R, Taboas J, et al. Engineering craniofacial scaffolds. Orthod Craniofac Res. 2005;8(3):162–73.
Dursun E, Dursun CK, Eratalay K, Orhan K, Celik HH, Tözüm TF. Do porous titanium granule grafts affect bone microarchitecture at augmented maxillary sinus sites? A pilot split-mouth human study. Implant Dent. 2015;24(4):427–33.
Shefelbine SJ, Simon U, Claes L, Gold A, Gabet Y, Bab I, et al. Prediction of fracture callus mechanical properties using micro-CT images and voxel-based finite element analysis. Bone. 2005;36(3):480–8.
Bosemark P, Isaksson H, McDonald MM, Little DG, Tägil M. Augmentation of autologous bone graft by a combination of bone morphogenic protein and bisphosphonate increased both callus volume and strength. Acta Orthop. 2013;84(1):106–11.
Reynolds DG, Hock C, Shaikh S, Jacobson J, Zhang X, Rubery PT, et al. Micro-computed tomography prediction of biomechanical strength in murine structural bone grafts. J Biomech. 2007;40(14):3178–86.
Ezirganli S, Polat S, Baris E, Tatar I, Celik HH. Comparative investigation of the effects of different materials used with a titanium barrier on new bone formation. Clin Oral Implants Res. 2013;24(3):312–9. https://doi.org/10.1111/j.1600-0501.2011.02323.x.
Morgan EF, Mason ZD, Chien KB, Pfeiffer AJ, Barnes GL, Einhorn TA, et al. Micro-computed tomography assessment of fracture healing: relationships among callus structure, composition, and mechanical function. Bone. 2009;44(2):335–44.
Yang X, Ricciardi BF, Hernandez-Soria A, Shi Y, Camacho NP, Bostrom MP. Callus mineralization and maturation are delayed during fracture healing in interleukin-6 knockout mice. Bone. 2007;41(6):928–36.
Nyman JS, Munoz S, Jadhav S, Mansour A, Yoshii T, Mundy GR, et al. Quantitative measures of femoral fracture repair in rats derived by micro-computed tomography. J Biomech. 2009;42(7):891–7.
Thomsen JS, Laib A, Koller B, Prohaska S, Mosekilde L, Gowin W. Stereological measures of trabecular bone structure: comparison of 3D micro computed tomography with 2D histological sections in human proximal tibial bone biopsies. J Microsc. 2005;218(2):171–9.
Acknowledgment
All figures in this chapter were scanned and reconstructed with Skyscan 1275 (Skyscan, Kontich, Belgium) in Ankara University, Faculty of Dentistry, Micro-CT Laboratory which was founded by Ankara University Research Fund (Project No:17A0234001) and belongs to the courtesy of Orhan, K., Bilecenoglu B., and Ocak. M.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Bilecenoğlu, B., Ocak, M. (2020). Analysis of Fracture Callus Mechanical Properties Using Micro-CT. In: Orhan, K. (eds) Micro-computed Tomography (micro-CT) in Medicine and Engineering. Springer, Cham. https://doi.org/10.1007/978-3-030-16641-0_6
Download citation
DOI: https://doi.org/10.1007/978-3-030-16641-0_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-16640-3
Online ISBN: 978-3-030-16641-0
eBook Packages: MedicineMedicine (R0)