Abstract
Abundantly available chitin/chitosan and their derivatives are full of useful bioactivities. They have numerous applications in industries like food, wastewater treatment, pharmaceuticals, agriculture, cosmetics etc. However, their insolubility in water plays spoilsport in way of their use as cost-effective biomolecules for various sectors. Breakage of chitosan to smaller oligosaccharides solves this problem to larger extent preferably using highly specific enzymes. It is well known that that bioactivities of oligosaccharides improve upon hydrolysis to lower molecular weight chitosan i.e. chitooligosaccharides. Availability and production of anti-oxidant chitooligosaccharides by non-chemical approach is desirable for consumer satisfaction. Bioprocessing of chitin/chitosan generated from marine waste to be used as bioactive chitooligosaccharides, can reduce both environmental and human health hazards to a great extent.
Here we review (1) biocatalytic approaches for chitooligosaccharides production, (2) bioprocess strategies for large scale production, (3) functionalization and (4) anti-oxidant activity of chitooligosaccharides. Specific and non-specific biocatalysts are used for chitooligomer preparation either by hydrolysis and transglycosylation approaches. Cellulase enzymes have been found to be most frequently used non-specific enzymes for chitosan hydrolysis but microbial chitosanases show excellent performance for chitooligosaccharides production both in terms of yield and specificity. Transglycosylation also have been found to be promising for chitooligosaccharides production especially at small scale. Combination reactors have been found to be most suitable for upscaling of chitooligomer production. Immobilized packed column with ultrafiltration membrane reactors are used for simultaneous hydrolysis and separation of chitooligomers. Chemically synthesized derivatives of chitooligomers have been reported in many studies by introducing carboxyl, quaternized amino, amino ethyl, sulfate, gallyl and many more groups. Amino ethyl, Gallyl, sulphated, phenolic acid conjugated and carboxylated derivatized chitooligomers have shown anti-oxidant activity. Anti-oxidant activity of chitooligomers and relation with their structure and polymerisation has been well established. Chitooligomers longer than trimer show good activity while best activity has been reported in degree of polymerisation from 10 to 12. Acetylation of chitooligomers leads to improvement in anti-oxidant activity than their deacetylated version.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Anti-oxidant
- Oligosaccharides
- Chito-oligosaccharides
- Chitosanase
- Chitin
- Chitosan
- Radical-scavenging
- DPPH
- Bioactivity
3.1 Introduction
Chitin is the second most abundant renewable biopolymer after cellulose in nature which could be used as starting material for various industries (Kaur and Dhillon 2015). Chitin and chitosan have been converted to oligosaccharides because lower solubility of chitosan in water possess difficulty in their application for various purposes and oligosaccharides are shown to have better bioactivity than their chitosan/chitin polymers. Chitosans with degree of polymerization less than 20 and an average molecular weight less than 3.9 KDa are called chitosan oligosaccharides (Mourya et al. 2011). Chitooligosaccharides can be homochitooligomers or heterochitooligomers. Homochitooligomers contain either only glucosamine or D-glucosamine units, whereas heterochitooligomers contain both types of monomers and can be a mixture of various oligomers having various degree and position of polymerisation/acetylation. Hetero-chitooligosaccharides with degree of polymerisation less than 10 are soluble in water but for chitooligosaccharides having degree of polymerisation more than 10, solubility depends on pH of the solution and degree of acetylation. Pharmaceutical companies, food industries and researchers preferably uses chitooligosaccharide in hetero form (Il’ina and Varlamov 2015). Chitooligosaccharides have same or variation in degree of acetylation and the sequence of acetylated/deacetylated residues, however these can be considered as homologs (Kim 2011).
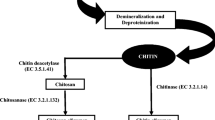
Chitin/chitosan conversion to their oligosaccharides can be achieved physically, chemically or bio-catalytically (Aam et al. 2010). Ultrasonic and gamma irradiation have been used for physical depolymerisation. Fully deacetylated chitosan was depolymerised with ultrasonic irradiation to produce chitosan oligos with degree of polymerisation between 2 and 11 and maximum concentration of trimers (Popa-Nita et al. 2009). Gamma irradiation has been used for reducing the viscosity of chitosan in acetic acid solution and producing dimers, trimers and tetramers of chitin/chitosan polymer (Choi et al. 2002). Chitosan hydrolysis using various acids like hydrochloric acid, electrolyte acid, nitrous acid, phosphoric acid, fluoric acid etc. and hydrogen peroxide or persulfate using oxidative/reductive methods have also been demonstrated (Lodhi et al. 2014). Milder acids like lactic acid, trichloroacetic acid, formic acid, acetic acid etc. has also been studied for their degradative action on chitosan (Ando and Kataoka 1980; Yamaguchi et al. 1982; Il’ina and Varlamov 2004). However, chemical reactions are difficult to control leading to synthesis of spurious or secondary products which hinder the downstream processing and therefore are not eco-friendly. Enzymatic processes are considered to be most feasible and attractive for chitooligosaccharide preparation either by hydrolysis of chitin/chitosan and related substrates or by synthesis of larger oligomers by transglycosylation methods. Biocatalytic methods reduce the use of toxic chemicals, are easier to control and do not generate any harmful waste as in case of chemical methods. Various specific and non-specific biocatalysts used for chitooligosaccharide production and their reported anti-oxidant activity has been covered in this chapter (Fig. 3.1).
Biocatalytic production of chitooligosaccharides and their anti-oxidant activity. Chitin from waste from seafood industry is the source of chitosan which is depolymerized (enzymatic or chemical) to produce chitooligosaccharides with a range of molecular weight, degree of polymerization and deacetylation. These chitooligosaccharides have been found to exert antioxidant activity in vitro, in vivo and in plants
3.2 Biocatalysts of Chitosan Oligosaccharides
3.2.1 Specific Biocatalysts
Chitosan enjoys broad substrate specificity as it is susceptible to number of carbohydrases, proteases and chitinases reported from fungi and bacteria (Kim and Rajapakse 2005; Sinha et al. 2016a). Microbial chitosanases from fungi and bacteria have shown excellent performances in hydrolysing chitosan of various degree of deacetylation and these enzymes have also been reported in virus, animals and plants (Hamed et al. 2016). However, chitosanases reported from microbial sources are very few and are expensive to be used at industrial scale due to high cost of extraction, concentration and purification. There are specific and non-specific enzymes which affect the types and structure of glycosidic bonds in chitosan hydrolysis. Random distribution of four types of glycosidic bonds in the structure of chitosan determines their hydrolysis by enzymes. These could be between two deacetylated units (D-D), one acetylated and one deacetylated (A-D), one deacetylated and one acetylated (D-A) and two acetylated units (A-A) and their hydrolysis depends on presence of reducing/non-reducing ends and degree of deacetylation. Lysozyme from egg white has been found to be specific towards two acetylated (A-A) units and Bacillus chitosanase has been found to be specific towards glycosidic bonds two deacetylated units (D-D) (Vårum et al. 1996). Chitinases act on partially deacetylated chitosan by detecting the presence of N-acetyl glucosamine (GlcNAc) moiety in the sequence of chitosan (Aiba 1994). Chitosan and other non-specific enzymatic hydrolysis with respect to microbial source of enzyme and product obtained, have been summarised in Table 3.1.
3.2.2 Non-Specific Enzymatic Hydrolysis
As specific enzymes are not available in bulk for commercial preparation and are not cost effective hence non-specific biocatalytic preparations of chitooligomers are being explored for this purpose. Lipases, cellulases (Wu and Tsai 2004), papain, lysozyme, hemicellulases, protease, pectinases, pepsin, pronase, chitinases and many other non-specific enzymes have been reported for hydrolysis of chitosan (Abdel-Aziz et al. 2014). Cellulase is the frequently used non-specific enzyme being reported for chitosan hydrolysis and this is explained by similarity between structure of chitin, chitosan and cellulose due to presence of β, 1-4 glycosidic bond between glucose subunits. The presence of acetamide group in chitin and amino group in chitosan at C-2 hydroxyl position is found to have no role in enzyme substrate reaction. Wide variety of cellulases, especially bifunctional chitosanase-cellulase from various microbial sources have been reportedly used for production of chitooligomers from chitosan (Xia et al. 2008). All these enzymes belong to glycosyl hydrolase family mainly GH-5, GH-7 and GH-8 and few have been found to be superior to even chitosanases enzymes for chitosan hydrolysis.
3.2.3 Transglycosylation Activity for Synthesis of Chitooligosaccharides
Apart from hydrolysis of chitosan, chitooligomers are also synthesised using transglycosylation activity of biocatalysts. Chemical and enzymatic methods of chitooligosaccharides synthesis have been proposed and has been reviewed extensively (Yang and Biao 2014; Li et al. 2016). Large number of steps related to protection and deprotection make chemical glycosylation methods cumbersome as steps increases with the size of oligosaccharides, so synthesis of trimer and larger oligosaccharides are not considered feasible. However, biocatalytic methods allow regio-selectivity, milder reaction and hassle-free ways (no protection and deprotection step) of glycosidic bond formation. Formation of new glycosidic bonds between donor and acceptor saccharides can also be established by few glycosyl hydrolases apart from their usual activity of glycosidic bond hydrolysis (Li et al. 2016). Active-site architecture of transglycolytic enzymes in their full efficiency hinders correct positioning of water molecule and promotes/favours binding of incoming carbohydrate molecule through strong interaction of aglycon subsites. Released chitooligosaccharides is transferred to suitable acceptor to form a new glycosidic bond to synthesize chitooligosaccharide. Stereo- and size specific preparation of higher chitooligosaccharide have been achieved using this activity of chitosanase/chitinolytic enzymes and other glycosidases. An exo-chitosanase enzyme having transglycosylation activity was isolated from Aspergillus fumigatus IIT-004 which was immobilized on nanofibers and employed for chitosan hydrolysis. However, synthesis of chitodimer was also achieved using this enzyme when reaction conditions were changed (Sinha et al. 2016b). Purified chitinase from Trichoderma reesei KDR-11 has been shown to convert dimer and hexamer of N-acetyl glucosamine (GlcNAc)2 (55.7%) and (GlcNAc)6 (39.6%) from tetramer of N-acetyl glucosamine (GlcNAc)4 (100%) using a transglycosylation reaction (Usui et al. 1990). Lysozyme from hen egg-white lysozyme has been used for synthesis of chitooligosaccharide (4-12) polymerisation (Akiyama et al. 1995).
3.2.4 Bioprocess Strategies for Chitooligosaccharides Production/Synthesis
Upscaling of COS production has been tried mainly in three types of bioreactor settings like batch, column and ultrafiltration reactor (Vidanarachchi et al. 2010). Batch reactor is most common where enzyme (chitosanase from Bacillus pumilus BN-262) is mixed with substrate (1% chitosan) and glycosidic bonds are allowed to break under optimized pH, temperature and time. Nonetheless, it has certain drawbacks like lower yield, lack of continuous production and higher cost due to lack of enzyme reusability. Packed immobilized enzyme in a column reactor through which substrate is passed continuously, has been suggested for continuous production but its use has been limited by poor affinity of immobilized enzyme towards substrates. Enzyme immobilization has been tested with various carriers for column packing but chitosanase bonded on chitin has shown better activity than other matrices (Kim and Rajapakse 2005). Ultrafiltration membrane with cut off of 3 KDa has been used for production of relatively higher oligomers of chitosan (trimer to hexamer) (Jeon and Kim 2000). Eleven batches of hydrolysis could be achieved with same amount of enzyme used in batch condition, thus at relatively lower cost, chitooligosaccharide with higher degree of polymerisation (chitotrimer to chitohexamer) could be achieved. In continuous reactor, a dual reactor system was proposed where ultrafiltration membrane was attached to chitin packed column with immobilized chitosanase enzyme system (Jeon and Kim 2000). Chitosanase enzyme was physically adsorbed on the chitin but showed less affinity and lower reaction rate towards substrates than free enzyme. Optimized permeation rate of 4 ml/min was determined where the 80% of product contained larger chitooligosaccharides (trimer to hexamer). Membrane of 10 KDa was used in ultrafiltration membrane reactor for selective fractionation of chitooligosaccharide which resulted from hydrolysis of partially hydrolysed chitosan and this was controlled by changing flow rate. Monomer production from chitooligosaccharide was stopped by controlling the chitosan hydrolysis which in turn halted product inhibition. In this study, membrane fouling in ultrafiltration membrane was removed by partially hydrolysing chitosan before applying to the reactors. Chitooligosaccharides preparation by continuous hydrolysis of chitosan in ultrafiltration membrane reactor along with immobilized column reactor utilising Bacillus chitosanase has also been reported from a different study where the product was used for radical scavenging activity studies (Park et al. 2003).
A chitosanolytic α-amylase enzyme from Bacillus amylolyquefaciens was covalently immobilized on glyoxal agarose beads and was assessed in batch and fixed bed reactors for continuous production of chitooligosaccharide with activity recovery of 25% (Moriano et al. 2016). Here, improved thermostability of the immobilized enzyme and conversion yield of 73% was obtained. Also, chitotriose and chitobiose were found to be the major products and conversion yield dropped by an increase in the dilution rate. In yet another study, polyacrylonitrile nanofibrous membrane (PANNFM) based chitosanase enzyme from Aspergillus sp. was used for selective fractionation of chitodimer to hexamer by varying the reaction temperature (Sinha et al. 2012b).
3.2.5 Production of Functionalized Chitooligosaccharides
Not only chitooligosaccharide but their derivatives have also shown antioxidant activity in different biological systems (Table 3.2). Various functional groups like hydroxyl and amino groups in the chitosan backbone have been added by enzymatic approaches to improve its application in various fields as it leads to changes in physicochemical properties. Amide coupling reaction has been used for conjugates preparation like phenolic and gallic acid conjugates (Liaqat and Rengin 2018). Phenolic acid compounds have tendency to donate H atom which enhanced the potential of conjugated anti-oxidant compounds, similarly, gallic acid conjugated chitooligosaccharides have also shown anti-oxidant capacity (Vo et al. 2017) Also, quaternization, alkyltion, thiolation, hydroxyalkylation, carboxyalkylation are some of the methods by which chitooligosaccharides can be modified (Mourya and Inamdar 2009). In a study on functionalized chitooligosaccharide, it was observed that chitooligosaccharides have better anti-oxidant activity than their O- and N-carboxymethyl substituted counterparts, while reducing power was greatest in O-carboxymethyl substituted chitooligosaccharide. In case of quaternized carboxymethyl chitooligosaccharide (QCMCOS), anti-oxidant activity was directly related to degree of substitution. Substitution with quaternary ammonium and carboxymethyl group also enhanced their thermal stability and degree of crystallinity (Li et al. 2012).
3.3 Anti-Oxidant Activity
Antioxidant molecules play an important part in neutralization of oxidative stress in living systems since they bind free radicals. Free radicals, also known as Reactive Oxygen Species or ROS, are chemical species with an unpaired electron in its outer orbital and therefore they are highly reactive e.g. hydroxyl free radical (OH°). ROS harms the cell membrane by lipid peroxidation and impairs cellular machinery by DNA and protein oxidation. Free radicals are produced under normal as well as pathological conditions and levels of free radicals are balanced by the endogenous antioxidants produced by the living systems during normal physiological state (Valko et al. 2007). Imbalance between their level leads to damage to the biological components which is one of the underlying cause of diseases and disorders such as cancer, arteriosclerosis, stroke, heart attack, Alzheimer’s, ageing etc. (Pham-Huy et al. 2008).
Initiation of free radical injury can be caused by ionising radiations, inflammatory conditions, excess metal ions in the body and drugs/chemicals such as acetaminophen, carbon-tetrachloride. Generally, these are overcome by body’s defence mechanism which comprises of antioxidants (vitamins A, C, E, glutathione), enzymes (superoxide dismutase; catalase; glutathione peroxidase (GSH-PX) and metal carrier proteins (transferrin, ceruloplasmin) (Yu 1994). However, it has been postulated that external supply of antioxidants to the body will help in relieving it from diseases and disorders caused by oxidative stress (Sindhi et al. 2013). Thus, there is ongoing research towards discovery and synthesis of both artificial and natural antioxidant molecules which may assist patients in conquering their symptoms. Chitosan and chito-oligosaccharides have been extensively researched as natural antioxidants which are not only inexpensive but also biodegradable. The various antioxidant capacity assay along with their principle and end-product determination have been summarised in Table 3.3.
3.3.1 Anti-Oxidant Activity of Chitooligosaccharides
In view of growing interest in identification of natural anti-oxidants, anti-oxidant activity of chitooligosaccharides has been explored in various biological systems (Table 3.4). Chitooligosaccharide and their conjugates possess greater anti-oxidant activity as compared to chitosan. Although, molecular mechanism of antioxidant activity of chitooligosaccharides is unclear but it is suggested that amino group in chitooligosaccharides react with unstable free radicals in order to make them stable, resulting in its anti-oxidant activity. Radical scavenging activity or anti-oxidant activity of chitooligosaccharide has been correlated with molecular weight, degree of polymerisation, degree/fraction of deacetylation and N-acetylation and chitosan substrate source (Anraku et al. 2018). In a systematic study on seven chitosan samples with molecular weight in the range of 2 to 300 KDa it was concluded that anti-oxidant properties of chitosan and chitooligosaccharide were inversely proportional to their molecular weight (Laokuldilok et al. 2017; Chang et al. 2018). Chitooligosaccharides with active hydroxyl and amino groups helps to scavenge free radicals and low molecular weight chitooligosaccharide with lower degree of polymerisation have more such groups available for reaction. Chitobiose and chitotriose were found to possess better anti-oxidant and reducing activity than chitooligosaccharides with higher degree of polymerisation (Chen et al. 2003). Chitooligosaccahrides with degree of polymerisation (10-12) show best anti-oxidant activity while trimers and higher degree of polymerisation shows good anti-oxidant activity (Li et al. 2012). Partially acetylated version of chitotrimer was compared with deacetylated version and it was seen that acetylated chitosan has greater anti-oxidant activity proving that degree of acetylation has a role in anti-oxidant activity (Liaqat and Rengin 2018).
Chitooligosaccharides of five types were prepared using reactor where membrane of cut off 10, 5, 3 and 1 KDa was used for fractionation of various molecular weight chitooligosaccharide. Size of chitooligosaccharides varied according to pore size of membrane used. DPPH (1,1-diphenyl-2-picrylhydrazyl) radical scavenging ability was found to be present in all types of chitooligosaccharide and mechanism involved pairing of odd electrons of the DPPH (1,1-diphenyl-2-picrylhydrazyl) radicals. They also showed radical scavenging activities for all the free radicals but showed most effectiveness on DMPO (5,5-dimethyl-1-pyrroline N-oxide)-OH scavenging. Among all the fractions, those containing mol wt. 3–10 KDa showed highest radical scavenging activity. In another study, radical scavenging ability of chitosan of various molecular weight (30, 90 and 120 KDa) was compared with that of an established synthetic anti-oxidant butylated hydroxytoluene (BHT) and an equivalent efficiency of 85% was obtained. Here also the lowest molecular weight chitosan showed highest activity (Hamed et al. 2016). Radical scavenging ability of chitooligosaccharides has also been studied in in vivo condition in a mouse model on high fat diet. Chitooligosaccharide showed potent anti-oxidant activity by protecting mice from oxidative stress (Qu and Han 2016) by reducing level of certain enzymes (Glutathione peroxidase, superoxide dismutase, catalase) in liver, serum and stomach which increases considerably in case of stress due to high fat diet. Gallic-acid conjugated chitooligosaccharides were been found to exert anti-inflammatory and anti-cancer effect on human lung epithelial cells (A549). It also depicted DPPH (1,1-diphenyl-2-picrylhydrazyl) radical scavenging and H2O2 induced DNA damage protection (Vo et al. 2017). Effects of chitooligosaccharide supplementation on performance, blood characteristics, relative organ weight, and meat quality in broiler chickens was analysed by Zhou et al. (2009). Butylated hydroxytoluene (BHT), butylated hydroxyanisole (BHA) and tertiary butyl hydroquinone (TBHQ) are some of the reported synthetic anti-oxidants which are used in food industry to prevent oxidation and reduce rancidity and loss of flavours. However, because of health hazards related with these compounds natural and safe compounds are preferable and chitooligosaccharides has been found to be a suitable alternative of these compounds (Kim and Thomas 2006). Supplementation of chitooligosaccharide was reviewed on processing and storage quality of foods of animal and aquatic origin such as milk, meat, fish, eggs, sea foods, etc. (Singh 2016). Therefore, there is an increasing interest in antioxidants, particularly in those intended to arrest the presumed harmful effects of free radicals in the living systems, as well as the deterioration of fats and other constituents of foodstuffs (Rao et al. 2006).
3.3.2 Anti-Oxidant Activity of Chitooligosaccharides in Plants
Due to their strong antioxidant activity, chitooligosaccharide has been studied in plants for their agricultural application. Chitooligosaccharides at 1–10 mg/L with acetylation degree of 65% and molecular weight of 5–10 KDa significantly activated OPD (o-phenylenediamine) oxidation by wheat seedlings (Khairullin et al. 2001). Cabrera et al. (2006) reported effect of the degree of polymerization, degree of acetylation and concentration of chitooligosaccharide on defence activation in Arabidopsis thaliana suspension-cultured cells. Similarly chitooligosaccharides were able to induce nitric oxide (NO) generation followed by up-regulation in the activities of defence-related enzymes through an oligochitosan induced Ser/Thr protein kinase (OIPK)-dependent or independent pathway (Zhang et al. 2011). Plant mineral nutrient dynamics was studied in hydroponically grown plants by Chatelain et al. (2014) and their use in phytoremediation and biofortification programs is promoted (Vasconcelos 2012). Application of chitooligosaccharide as a commercial preservative to improve the longevity of cut roses has also been studied (Jing and Li 2015). This was due to the decreased superoxide anion, hydrogen peroxide and malondialdehyde levels in the cut roses which protected them from withering.
3.4 Conclusion
In spite of making a lot of progress in the research area of “COS as an anti-oxidant” very few industries have used them in the area of food, pharmaceuticals and cosmetics. Their appearance as promising anti-oxidant biomolecules are being hindered by their non-availability on larger scale as obtaining chitooligosaccharide in highly purified form in bulk is still considered a difficult task. Downstream processing of enzymatically produced chitooligosaccharides is one of the key areas which is attracting the attention of researchers for getting purified fraction of chitooligosaccharide in terms of defined degree of polymerization and exact known sequence of monomer (both acetylated and deacetylated). More studies are required to establish relation between the anti-oxidant activity and degree of deacetylation/polymerisation and sequence in order to predict the connection between biological activity and their structure. Studies on in vitro and in vivo activity of chitooligosaccharides after characterisation are required for their establishment as an anti-oxidant molecule. However, most of these studies have been done on chitooligosaccharide mixtures which contained chitooligosaccharides molecule with various degree of polymerisation. Establishment of anti-oxidant activity due to any particular type of chitooligosaccharide was found to be difficult and needs to be explored further. Efficient biocatalytic production of chitooligosaccharides with good yield and cost effectiveness are desirable. Downstream processing studies leading to separation and purification of chitooligosaccharide with single degree of polymerization along with their in vitro and in vivo effect is another area where more investigation is needed. Apart from antioxidant activity, chitooligosaccharides has been implicated in medicinal uses due to their antimicrobial effect (Park et al. 2004), neuroprotective effect (Huang et al. 2015) and anti-tumour effect (El-Sayed et al. 2017). Recently nanoparticles of chitooligosaccharides and their conjugates have shown a promise as a drug delivery vehicle (Lu et al. 2015; Xu et al. 2016) and more research is needed for evaluating the anti-oxidant potential of nanoparticulate forms of chitooligosaccharide.
Abbreviations
- ABTS:
-
2,2′-azino-bis-3-ethylbenzthiazoline-6-sulfonic acid
- BHA:
-
butylated hydroxyanisole
- BHT:
-
butylated hydroxytoluene
- CAT:
-
chloramphenicol acetyltransferase
- COS:
-
chitooligosaccharides
- DCFH-DA:
-
dichloro-dihydro-fluorescein-diacetate
- DD:
-
degree of deacetylation
- DMPO:
-
5,5-dimethyl-1-pyrroline N-oxide
- DNA:
-
deoxyribonucleic acid
- DNA:
-
deoxyribonucleic acid
- DPPH:
-
2,2-Diphenyl-1-picrylhydrazyl
- ESR:
-
electron spin resonance
- FRAP:
-
ferric reducing power
- GSH-PX:
-
glutathione peroxidase
- KDa:
-
kilo Dalton
- PANNFM:
-
polyacrylonitrile nanofibrous membrane
- QCMCOS:
-
quaternised carboxymethyl chitooligosaccharide
- ROS:
-
reactive oxygen species
- SOD:
-
superoxide dismutase
- TBARS:
-
thiobarbituric acid reactive substances
- TBHQ:
-
tertiary butyl hydroquinone
References
Aam BB, Heggset EB, Norberg AL, Sørlie M, Vårum KM, Eijsink VGH (2010) Production of chitooligosaccharides and their potential applications in medicine. Mar Drugs 8:1482–1517. https://doi.org/10.3390/md8051482
Abdel-Aziz SM, Kahil T, Keera AA (2014) Kinetic behavior of free and in situ immobilized chitosanases produced by the fungus Mucor rouxii. World Appl Sci J 30:1–09. https://doi.org/10.5829/idosi.wasj.2014.30.01.13980
Aiba S (1994) Preparation of N-acetylchitooligosaccharides by hydrolysis of chitosan with chitinase followed by N-acetylation. Carbohydr Res 265:323–328. https://doi.org/10.1016/0008-6215(94)00243-6
Akiyama K, Kawazu K, Kobayashi A (1995) A novel method for chemo-enzymatic synthesis of elicitor-active chitosan oligomers and partially N-deacetylated chitin oligomers using N-acylated chitotrioses as substrates in a lysozyme-catalyzed transglycosylation reaction system. Carbohydr Res 279:151–160. https://doi.org/10.1016/0008-6215(95)00288-X
Ando T, Kataoka S (1980) Acylations of chitin with acid anhydrides in trichloroacetic acid systems. Kobunshi Ronbunshu 37:1–7. https://doi.org/10.1295/koron.37.1
Anraku M, Gebicki JM, Iohara D, Hisao T, Kaneto U, Toru M, Hirayamaa F, Otagiri M (2018) Antioxidant activities of chitosans and its derivatives in in vitro and in vivo studies. Carbohydr Polym 199:141–149. https://doi.org/10.1016/j.carbpol.2018.07.016
Cabrera JC, Messiaen J, Cambier P, Van Cutsem P (2006) Size, acetylation and concentration of chitooligosaccharide elicitors determine the switch from defence involving PAL activation to cell death and water peroxide production in Arabidopsis cell suspensions. Physiol Plant 127:44–56. https://doi.org/10.1111/j.1399-3054.2006.00677.x
Chang SH, Wu CH, Tsai GJ (2018) Effects of chitosan molecular weight on its antioxidant and antimutagenic properties. Carbohydr Polym 181:1026–1032. https://doi.org/10.1016/J.CARBPOL.2017.11.047
Chatelain PG, Pintado ME, Vasconcelos MW (eds) (2014) Evaluation of chitooligosaccharide application on mineral accumulation and plant growth in Phaseolus vulgaris. Plant Sci 215:134–140. https://doi.org/10.1016/j.plantsci.2013.11.009
Chen AS, Taguchi T, Sakai K, Kikuchi K, Wang MW, Miwa I (2003) Antioxidant activities of chitobiose and chitotriose. Biol Pharm Bull 26:1326–1330. https://doi.org/10.1248/bpb.26.1326
Cho SY, Lee JH, Song MJ, Park PJ, Shin ES, Sohn JH (2010) Effects of chitooligosaccharide lactate salt on sleep deprivation-induced fatigue in mice. Biol Pharm Bull 33:1128–1132. https://doi.org/10.1248/bpb.33.1128
Choi WS, Ahn KJ, Lee DW, Byun MW, Park HJ (2002) Preparation of chitosan oligomers by irradiation. Polym Degrad Stab 78:533–538. https://doi.org/10.1248/bpb.33.1128
de Assis CF, Araújo NK, Pagnoncelli MGB, da Silva Pedrini MR, de Macedo GR, dos Santos ES (2010) Chitooligosaccharides enzymatic production by Metarhizium anisopliae. Bioprocess Biosyst Eng 33:893–899. https://doi.org/10.1007/s00449-010-0412-z
de Assis CF, Costa LS, Melo-Silveira RF, Oliveira RM, Pagnoncelli MGB, Rocha HAO, Macedo GR, Santos ES (2012) Chitooligosaccharides antagonize the cytotoxic effect of glucosamine. World J Microbiol Biotechnol 28:1097–1105. https://doi.org/10.1007/s11274-011-0910-4
Dou J, Tan C, Du Y, Bai X, Wang K, Ma X (2007) Effects of chitooligosaccharides on rabbit neutrophils in vitro. Carbohydr Polym 69:209–213. https://doi.org/10.1016/j.carbpol.2006.09.029
El-Sayed ST, Omar NI, El Sayed ESM, Shousha WG (2017) Evaluation antioxidant and cytotoxic activities of novel chitooligosaccharides prepared from chitosan via enzymatic hydrolysis and ultrafiltration. J Appl Pharm Sci 7:50–55. https://doi.org/10.7324/JAPS.2017.71107
Eom TK, Senevirathne M, Kim SK (2012) Synthesis of phenolic acid conjugated chitooligosaccharides and evaluation of their antioxidant activity. Environ Toxicol Pharmacol 34:519–527. https://doi.org/10.1016/j.etap.2012.05.004
Feng T, Du Y, Li J, Wei Y, Yao P (2007) Antioxidant activity of half N-acetylated water-soluble chitosan in vitro. Eur Food Res Technol 225:133–138. https://doi.org/10.1007/s00217-006-0391-0
Fernandes JC, Eaton P, Nascimento H, Gião MS, Ramos ÓS, Belo L (2010) Antioxidant activity of chitooligosaccharides upon two biological systems: erythrocytes and bacteriophages. Carbohydr Polym 79:1101–1106. https://doi.org/10.1016/j.carbpol.2009.10.050
Hamed I, Özogul F, Regenstein JM (2016) Review: regenstein industrial applications of crustacean by-products (chitin, chitosan, and chitooligosaccharides). Trends Food Sci Technol 48:40–50. https://doi.org/10.1016/j.tifs.2015.11.007
Huang R, Rajapakse N, Kim SK (2006) Structural factors affecting radical scavenging activity of chitooligosaccharides (COS) and its derivatives. Carbohydr Polym 63:122–129. https://doi.org/10.1016/j.carbpol.2005.08.022
Huang HC, Hong L, Chang P, Zhang J, Lu SY, Zheng BW, Jiang ZF (2015) Chitooligosaccharides attenuate Cu2+-induced cellular oxidative damage and cell apoptosis involving Nrf2 activation. Neurotox Res 27:411–420. https://doi.org/10.1007/s12640-014-9512-x
Il’ina AV, Varlamov VP (2004) Hydrolysis of chitosan in lactic acid. Appl Biochem Microbiol 40:300–303. https://doi.org/10.1023/B:ABIM.0000025956.98250.30
Il’ina AV, Varlamov VP (2015) In vitro antitumor activity of heterochitooligosaccharides (a review). Prikl Biokhim Mikrobiol 51:5–14. https://doi.org/10.7868/S0555109915010067
Je JY, Park PJ, Kim SK (2004) Free radical scavenging properties of hetero chitooligosaccharides using an ESR spectroscopy. Food Chem Toxicol 42:381–387. https://doi.org/10.1016/j.fct.2003.10.001
Jeon YJ, Kim SK (2000) Continuous production of chitooligosaccharides using a dual reactor system. Process Biochem 35:623–632. https://doi.org/10.1016/S0032-9592(99)00118-1
Jing H, Li H (2015) Chitooligosaccharide prolongs vase life of cut roses by decreasing reactive oxygen species. Kor J Hort Sci Technol 33:383–389. https://doi.org/10.7235/hort.2015.14188
Karadeniz F, Artan M, Kong CS, Kim SK (2010) Chitooligosaccharides protect pancreatic β-cells from hydrogen peroxide-induced deterioration. Carbohydr Polym 82:143–147. https://doi.org/10.1016/j.carbpol.2010.04.046
Kaur S, Dhillon GS (2015) Review: recent trends in biological extraction of chitin from marine shell wastes. Crit Rev Biotechnol:44–61. https://doi.org/10.3109/07388551.2013.798256
Khairullin RM, Yarullina LG, Troshina NB, Akhmetova IE (2001) Chitooligosaccharide-induced activation of o-phenylenediamine oxidation by wheat seedlings in the presence of oxalic acid. Biochemist 66:286–289. https://doi.org/10.1023/A:1010247712723
Kim S K (ed) (2011) Chitin, chitosan, oligosaccharides and their derivatives: biological activities and applications. CRC Press, Boca Raton, 225p. https://doi.org/10.1201/EBK1439816035
Kim SK, Rajapakse N (2005) Review: enzymatic production and biological activities of chitosan oligosaccharides (COS). Carbohydr Polym 62:357–368. https://doi.org/10.1016/j.foodchem.2006.01.038
Kim KW, Thomas RL (2006) Antioxidative activity of chitosans with varying molecular weights. Food Chem 101:308–313. https://doi.org/10.1016/j.foodchem.2006.01.038
Koryagin AS, Erofeeva EA, Yakimovich NO, Aleksandrova EA, Smirnova LA, Mal’kov AV (2006) Analysis of antioxidant properties of chitosan and its oligomers. Bull Exp Biol Med 142:461–463. https://doi.org/10.1007/s10517-006-0392-9
Laokuldilok T, Potivas T, Kanha N, Surawang S, Seesuriyachan P, Wangtueai S (2017) Physicochemical, antioxidant, and antimicrobial properties of chitooligosaccharides produced using three different enzyme treatment. Food Biosci 18:28–33. https://doi.org/10.1016/j.fbio.2017.03.004
Li X, Liu BO, Wang X, Han Y, Su H, Zeng X (2012) Synthesis, characterization and antioxidant activity of quaternized carboxymethyl chitosan oligosaccharides. J Macromol Sci A 49:861–868. https://doi.org/10.1080/10601325.2012.714679
Li K, Xing R, Liu S, Li P (2016) Advances in preparation, analysis and biological activities of single chitooligosaccharides. Carbohydr Polym 139:178–190. https://doi.org/10.1016/j.carbpol.2015.12.016
Liaqat F, Rengin E (2018) Review: chitooligosaccharides and their biological activities. Carbohydr Polym 184:243–259. https://doi.org/10.1016/j.carbpol.2017.12.067
Liu HT, Li WM, Xu G, Li XY, Bai XF, Wei P (2009) Chitosan oligosaccharides attenuate hydrogen peroxide-induced stress injury in human umbilical vein endothelial cells. Pharmacol Res 59:167–175. https://doi.org/10.1016/j.phrs.2008.12.001
Lodhi G, Yon SK, Hwang JW, Kim SK, Jeon YJ, Je JY (2014) Chitooligosaccharide and its derivatives: preparation and biological applications. Biomed Res Int:1–13. https://doi.org/10.1155/2014/654913
Lu X, Guo H, Zhang Y (2012) Protective effects of sulfated chitooligosaccharides against hydrogen peroxide-induced damage in MIN6 cells. Int J Biol Macromol 50:50–58. https://doi.org/10.1016/j.ijbiomac.2011.09.020
Lu C, Park MK, Lu C, Lee YH, Chai KY (2015) A mussel-inspired chitooligosaccharide based multidentate ligand for highly stabilized nanoparticles. J Mater Chem B 3:3730–3737. https://doi.org/10.1039/c5tb00114e
Luo Z, Dong X, Ke Q, Duan Q, Shen L (2014) Chitooligosaccharides inhibit ethanol-induced oxidative stress via activation of Nrf2 and reduction of MAPK phosphorylation. Oncol Rep 32:2215–2222. https://doi.org/10.3892/or.2014.3463
Mendis E, Kim MM, Rajapakse N, Kim SK (2007) An in vitro cellular analysis of the radical scavenging efficacy of chitooligosaccharides. Life Sci 80:2118–2127. https://doi.org/10.1016/j.lfs.2007.03.016
Montilla A, Ruiz-Matute AI, Corzo N, Giacomini C, Irazoqui G (2013) Enzymatic generation of chitooligosaccharides from chitosan using soluble and immobilized glycosyltransferase (Branchzyme). J Agric Food Chem 60:10360–10367. https://doi.org/10.1021/jf403321r
Mourya VK, Inamdar NN (2009) Trimethyl chitosan and its applications in drug delivery. J Mater Sci Mater Med 20:1057–1079. https://doi.org/10.1007/s10856-008-3659-z
Mourya VK, Inamdar NN, Choudhari YM (2011) Chitooligosaccharides: synthesis, characterization and applications. Polym Sci Ser A 53:583–612. https://doi.org/10.1134/S0965545X11070066
Ngo DN, Lee SH, Kim MM, Kim SK (2009) Production of chitin oligosaccharides with different molecular weights and their antioxidant effect in RAW 264.7 cells. J Funct Foods 1:60–198. https://doi.org/10.1016/j.jff.2009.01.008
Ngo DH, Qian ZJ, Ngo DN, Vo TS, Wijesekara I, Kim SK (2011) Gallyl chitooligosaccharides inhibit intracellular free radical-mediated oxidation. Food Chem 128:974–981. https://doi.org/10.1016/j.foodchem.2011.03.128
Ngo D, Ngo D, Vo T, Ryu B, Van TQ, Kim S (2012) Protective effects of aminoethyl-chitooligosaccharides against oxidative stress and inflammation in murine microglial BV-2 cells. Carbohydr Polym 88:743–747. https://doi.org/10.1016/j.carbpol.2012.01.037
Nidheesh T, Pal GK, Suresh PV (2015) Chitooligomers preparation by chitosanase produced under solid state fermentation using shrimp by-products as substrate. Carbohydr Polym 121:1–9. https://doi.org/10.1016/j.carbpol.2014.12.017
Oh SH, Ryu BM, Ngo DH, Kim WS, Kim DG, Kim SK (2017) 4-hydroxybenzaldehyde-chitooligomers suppresses H2O2-induced oxidative damage in microglia BV-2 cells. Carbohydr Res 440–441:32–37. https://doi.org/10.1016/j.carres.2017.01.007
Park PJ, Je JY, Kim SK (2003) Free radical scavenging activity of chitooligosaccharides by electron spin resonance spectrometry. J Agric Food Chem 51:4624–4627. https://doi.org/10.1021/jf034039
Park PJ, Lee HK, Kim SK (2004) Preparation of hetero-chitooligosaccharides and their antimicrobial activity on Vibrio parahaemolyticus. J Microbiol Biotechnol 14:41–47. Retrieved from: http://www.jmb.or.kr/journal/main.html?mod=vol&tops=&year=2004
Pham-Huy LA, He H, Pham-Huy C (2008) Free radicals, antioxidants in disease and health. Int J Biomed Sci 4:89–96. https://doi.org/10.1073/pnas.0804252105
Popa-Nita S, Lucas JM, Ladavière C, David L, Domard A (2009) Mechanisms involved during the ultrasonically induced depolymerization of chitosan: characterization and control. Biomacromolecules 10:1203–1211. https://doi.org/10.1021/bm8014472
Qu D, Han J (2016) Investigation of the antioxidant activity of chitooligosaccharides on mice with high-fat diet. Rev Bras Zootec 45:661–666. https://doi.org/10.1590/S1806-92902016001100004
Rao MS, Chander R, Sharma A (2006) Radiation processed chitosan a potent antioxidant. Food Technol 27:188–194. 10.1.1.563.3945. http://barc.gov.in/publications/nl/2006/200610-24.pdf
Salgaonkar N, Prakash D, Nawani NN, Kapadnis BP (2015) Comparative studies on ability of N-acetylated chitooligosaccharides to scavenge reactive oxygen species and protect DNA from oxidative damage. Indian J Biotechnol 14:186–192. http://nopr.niscair.res.in/handle/123456789/31805
Santos-Moriano P, Woodley JM, Plou FJ (2016) Continuous production of chitooligosaccharides by an immobilized enzyme in a dual-reactor system. J Mol Catal B Enzym 133:211–217. https://doi.org/10.1016/j.molcatb.2016.09.001
Sindhi V, Gupta V, Sharma K, Bhatnagar S, Kumari R, Dhaka N (2013) Review: potential applications of antioxidants. J Pharm Res 7:828–835. https://doi.org/10.1016/j.jopr.2013.10.001
Singh P (2016) Review: effect of chitosans and chitooligosaccharides on the processing and storage quality of foods of animal and aquatic origin. Nutr Food Sci 46:51–81. https://doi.org/10.1108/NFS-08-2015-0092
Sinha S, Tripathi P, Chand S (2012a) A new bifunctional chitosanase enzyme from Streptomyces sp. and its application in production of antioxidant chitooligosaccharides. Appl Biochem Biotechnol 167:1029–1039. https://doi.org/10.1007/s12010-012-9546-6
Sinha S, Dhakate SR, Kumar P, Mathur RB, Tripathi P, Chand S (2012b) Electrospun polyacrylonitrile nanofibrous membranes for chitosanase immobilization and its application in selective production of chitooligosaccharides. Bioresour Technol 115:152–157. https://doi.org/10.1016/j.biortech.2011.11.101
Sinha S, Chand S, Tripathi P (2014) Microbial degradation of chitin waste for production of chitosanase and food related bioactive compounds. Appl Biochem Microbiol 50(2):125–133. https://doi.org/10.1134/S0003683814020173
Sinha S, Chand S, Tripathi P (2016a) Enzymatic production of glucosamine and chitooligosaccharides using newly isolated exo-β-d-glucosaminidase having transglycosylation activity. 3 Biotech 6(1):1–9. https://doi.org/10.1007/s13205-015-0330-5
Sinha S, Chand S, Tripathi P (2016b) Recent progress in chitosanase production of monomer-free chitooligosaccharides: bioprocess strategies and future applications. Appl Biochem Biotechnol 180:883–899. https://doi.org/10.1007/s12010-016-2140-6
Sun T, Yun Z, Jing X, Xuhong Y (2011) Antioxidant activity of N-acyl chitosan oligosaccharide with same substituting degree. Bioorg Med Chem Lett 21:798–800. https://doi.org/10.1016/j.bmcl.2010.11.097
Usui T, Hidenori M, Kiyoshi I (1990) Enzymic synthesis of useful Chito-oligosaccharides utilizing transglycosylation by chitinolytic enzymes in a buffer containing ammonium sulfate. Carbohydr Res 203:65–77. https://doi.org/10.1016/0008-6215(90)80046-6
Valko M, Leibfritz D, Moncol J, Cronin MTD, Mazur M, Telser J (2007) Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol 39:44–84. https://doi.org/10.1016/j.biocel.2006.07.001
Vårum KM, Holme HK, Izume M, Stokke BT, Smidsrød O (1996) Determination of enzymatic hydrolysis specificity of partially N-acetylated chitosans. Biochim Biophys Acta, Gen Subj 1291(1):5–15. https://doi.org/10.1016/0304-4165(96)00038-4
Vasconcelos MW (2012) Chitosan and chitooligosaccharide utilization in phytoremediation and biofortification programs: current knowledge and future perspectives. Front Plant Sci 5:1–4. https://doi.org/10.3389/fpls.2014.00616
Vidanarachchi J, Kurukulasuriya M, Kim SK (2010) Chitin, chitosan, and their oligosaccharides in food industry. In: Chitin, chitosan, oligosaccharides and their derivatives. CRC Press, Boca Raton, pp 543–560. https://doi.org/10.1201/EBK1439816035-c38
Vo TS, Ngo DH, Bach LG, Ngo DN, Kim SK (2017) The free radical scavenging and anti inflammatory activities of gallate-chitooligosaccharides in human lung epithelial A549 cells. Process Biochem 54:188–194. https://doi.org/10.1016/j.procbio.2017.01.001
Wu GJ, Tsai GJ (2004) Cellulase degradation of shrimp chitosan for the preparation of a water-soluble hydrolysate with immunoactivity. Fish Sci 70:1113–1120. https://doi.org/10.1111/j.1444-2906.2004.00912.x
Xia W, Liu P, Liu J (2008) Advance in chitosan hydrolysis by non-specific cellulases. Bioresour Technol:6751–6762. https://doi.org/10.1016/j.biortech.2008.01.011
Xie C, Xin W, Cimin L, Qinhua W, Zhiyong F, Li S (2016) Chitosan oligosaccharide affects antioxidant defense capacity and placental amino acids transport of sows. BMC Vet Res 12(1):1–8. https://doi.org/10.1186/s12917-016-0872-8
Xu Y, Wang L, Li YK, Wang CQ (2016) Reduction and pH dual-responsive nanoparticles based chitooligosaccharide-based graft copolymer for doxorubicin delivery. Colloids Surf A Physicochem Eng Asp 497:8–15. https://doi.org/10.1016/j.colsurfa.2016.01.049
Yamaguchi R, Arai Y, Itoh T (1982) A microfibril formation from depolymerized chitosan by n-acetylation. Agric Biol Chem 46:2379–2381. https://doi.org/10.1080/00021369.1982.10865442
Yang Y, Biao Y (2014) Recent advances in the synthesis of chitooligosaccharides and congeners. Tetrahedron 70:1023–1046. https://doi.org/10.1016/j.tet.2013.11.064
Yang Y, Shu R, Shao J, Xu G, Gu X (2006) Radical scavenging activity of chitooligosaccharide with different molecular weights. Eur Food Res Technol 222:36–40. https://doi.org/10.1007/s00217-005-0028-8
Yu BP (ed) (1994) Cellular defenses against damage from reactive oxygen species. Physiol Rev 74(1):139–162. https://doi.org/10.1152/physrev.1994.74.1.139
Yuan W (2009) Antioxidant activity of chito-oligosaccharides on pancreatic islet cells in streptozotocin-induced diabetes in rats. World J Gastroenterol 15(11):1339. https://doi.org/10.3748/wjg.15.1339
Zhang H, Zhao X, Yang J, Yin H, Wang W, Lu H, Du Y (2011) Nitric oxide production and its functional link with OIPK in tobacco defense response elicited by chitooligosaccharide. Plant Cell Rep 30:1153–1162. https://doi.org/10.1007/s00299-011-1024-z
Zhang Y, Zhou X, Lusha J, Du X, Sang Q, Chen F (2017) Enzymatic single-step preparation and antioxidant activity of hetero-chitooligosaccharides using non-pretreated housefly larvae powder. Carbohydr Polym 172:113–119. https://doi.org/10.1016/j.carbpol.2017.05.037
Zhou TX, Chen YJ, Yoo JS, Huang Y, Lee JH, Jang HD (2009) Effects of chitooligosaccharide supplementation on performance, blood characteristics, relative organ weight, and meat quality in broiler chickens. Poult Sci 88:593–600. https://doi.org/10.3382/ps.2008-00285
Acknowledgement
Corresponding author acknowledges Department of Science & Technology, Government of India for financial support vide reference no (SR/WOS-A/LS-1004/2015) and (SR/WOS-A/LS-129/2009) under Women Scientist Scheme.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Jaiswal, S., Tripathi, P., Sinha, S. (2019). Biocatalytic Production of Hetero-Chitosan Oligosaccharides as Anti-oxidants. In: Crini, G., Lichtfouse, E. (eds) Sustainable Agriculture Reviews 35. Sustainable Agriculture Reviews, vol 35. Springer, Cham. https://doi.org/10.1007/978-3-030-16538-3_3
Download citation
DOI: https://doi.org/10.1007/978-3-030-16538-3_3
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-16537-6
Online ISBN: 978-3-030-16538-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)