Abstract
The autologous blood injection intracerebral hemorrhage (ICH) model has been used successfully in rats due to the fact that they are large enough to accommodate all of the procedures and protocols and that measurement of pathophysiological parameters in the rat brain can be extrapolated to humans. In this chapter, we present a step-by-step procedure to model intracerebral hemorrhage by injecting autologous whole blood. This model can be readily modified to investigate the effects of infusing different compounds other than autologous whole blood.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Model Selection
Blood injection is a suitable model for studying intracerebral hemorrhage. Autologous blood obtained from the femoral artery is infused into the right caudate nucleus to simulate hemorrhage (Fig. 1). Advantages of this model include:
-
1.
In vivo observation of the pathological changes of intracerebral hemorrhage and the results of therapeutic manipulations;
-
2.
A relative homogeneity within strains owing to inbreeding;
-
3.
A small brain size well suited to immunohistochemical and biochemical studies;
-
4.
The ability to study a variety of physiologic parameters that are altered by the hematoma, including intracranial pressure, cerebral blood flow, cerebral perfusion pressure, and neurological function;
-
5.
Ease of reproducibility and rarity of complications;
-
6.
The ability to measure blood-brain permeability, brain edema, and brain atrophy and extrapolate those measurements to the human situation;
-
7.
Modification of the procedure to test the neurotoxic and neuroprotective effects of a virtually unlimited number of infused compounds.
Materials
Rats weighing 250–300 g are used in this model of intracerebral hemorrhage. The materials recommended for the surgical procedure include:
-
1.
Kopf stereotactic frame
-
2.
Infusion pump (Harvard)
-
3.
Dremel drill with 1 mm drill bit
-
4.
Moria spring scissors with very sharp points
-
5.
Scalpel handle with scalpel blades
-
6.
Fine iris scissors
-
7.
Curved Mayo scissors
-
8.
Dumont forceps
-
9.
Dumont angled forceps
-
10.
Adson forceps with teeth
-
11.
Heiss retractor
-
12.
Hemostats
-
13.
Needle holder
-
14.
Cotton tipped applicators
-
15.
1 mL syringes
-
16.
Polyethylene tubing (PE-50)
-
17.
23-gauge and 26-gauge needles
-
18.
5-0 black braided silk (Deknatel)
-
19.
3-0 Dermalon suture
Procedure
-
1.
Inject sodium pentobarbital (50 mg/kg, i.p.) to anesthetize the rat.
-
2.
Apply ophthalmic ointment to both eyes.
-
3.
Use a heating pad with feedback regulator to maintain body temperature at 37 °C.
-
4.
Use a hair clipper to remove hair from the scalp and right groin regions and apply Betadine to the exposed skin.
-
5.
Make a 2 cm incision in the groin at the region of the right femoral artery.
-
6.
Use the curved Mayo scissors to cut away fat to expose the artery, being careful to avoid cutting any major blood vessels.
-
7.
Expose the femoral artery and vein using cotton tipped applicators and fine iris scissors to cut away fascia.
-
8.
Separate 1.5 cm of the femoral artery from the femoral vein using angled forceps and a cotton tipped applicator, being careful not to tear the artery.
-
9.
Ligate the distal end of the artery using 5-0 black braided silk and clip one end of the silk with a hemostat and place to the side to help expose the artery.
-
10.
Place a thread of silk under the proximal end of the artery and clip both ends with a hemostat to temporarily occlude blood flow.
-
11.
Using spring scissors, prick the artery to create an opening for the catheter without severing the blood vessel.
-
12.
Make sure the PE-50 catheter is trimmed to a point and attached to a 23-gauge needle and 1 mL syringe filled with saline.
-
13.
Insert the tubing into the opening with the pointed side down using two pairs of forceps to hold the tubing and the artery in place.
-
14.
Use a loop of silk to cinch the tubing in place.
-
15.
Inject a small amount of saline to ensure that blood is being pumped into the tubing.
-
16.
Exchange the saline-filled syringe for an empty syringe and release the tension on the proximal occluding thread to fill the syringe with 0.2 mL of blood.
-
17.
Using another syringe that has been filled with heparin and then emptied completely, withdraw a 0.2 mL blood sample to analyze glucose, hematocrit and blood gases.
-
18.
After sampling the blood and measuring blood pressure if appropriate, put the tension back on the proximal end of the artery, then gently slide the PE-50 out enough so that the loop originally cinching the tubing to the artery slides off, then tighten it to ligate the artery.
-
19.
Remove the silk on the proximal end and cut all the loose ends of silk, then suture the skin using two to three 3-0 Dermalon sutures.
-
20.
Place the rat into the Kopf stereotactic frame and make a 2 cm sagittal incision over the midline of the scalp.
-
21.
Using cotton tipped applicators, clear away the fascia to expose the bregma, then position the Heiss retractor under the skin to keep the area exposed.
-
22.
Measure 4.0 mm laterally to the right and 0.1 mm anterior to the bregma and mark the area for the cranial burr hole, making sure to stay posterior to the coronal suture.
-
23.
Holding the Dremel drill with 1 mm drill bit vertically over the mark, slowly drill through the cranium, stopping when the pressure is no longer felt in order avoid tearing the dura.
-
24.
Place the 0.2 mL sample of non-heparinized blood in the infusion pump with a 26-gauge needle with the beveled edge pointing away from the midline.
-
25.
Position the needle over the burr hole and insert the tip of the needle into the brain just until the beveled edge is no longer visible, then insert the needle 5.5 mm ventrally into the right caudate.
-
26.
Use the infusion pump to infuse 100 μL of autologous whole blood into the right basal ganglia at a rate of 10 μL/min, leaving the needle in place for 2 min once the infusion is complete.
-
27.
Retract the needle and fill the burr hole with bone wax.
-
28.
Suture the skin using two to three 3-0 Dermalon sutures.
Outcome Evaluations
-
1.
Magnetic resonance imaging: It is an intuitive way to evaluate brain injury by measuring brain swelling, T2 lesion and T2* lesion [1, 2].
-
2.
Brain water content: To examine brain edema formation, which is a major determinant of survival in patients with ICH. It is also a simple, reproducible, and inexpensive measure of brain injury [3,4,5].
-
3.
Blood-brain barrier (BBB) permeability: The commonly used assessors of BBB disruption are radioisotopes and Evans blue [6, 7]. Albumin leakage is used to evaluate BBB disruption as well.
-
4.
Brain genomics: To determine changes of gene expression [8].
-
5.
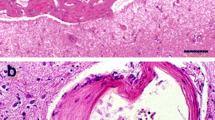
Histology: To evaluate the size, location, extent of the hemorrhage [9], neuronal death [10, 11], and brain atrophy [11, 12], coronal sections should be prepared (Figs. 2 and 3).
-
6.
Behavioral testing: Forelimb placing test, the forelimb use asymmetry test, and the corner turn test are sensitive measures of neurological deficits induced by intracerebral hemorrhage [9].
Limitations
-
1.
Lack of a ruptured blood vessel as the source of the ICH.
-
2.
Lacks the hematoma enlargement that occurs in ICH patients.
-
3.
Very low mortality rate.
References
Wu G, Xi G, Hua Y, Sagher O. T2* magnetic resonance imaging sequences reflect brain tissue iron deposition following intracerebral hemorrhage. Transl Stroke Res. 2010;1:31–4.
Dang G, Yang Y, Wu G, Hua Y, Keep RF, Xi G. Early erythrolysis in the hematoma after experimental intracerebral hemorrhage. Transl Stroke Res. 2017;8:174–82.
Yang GY, Betz AL, Chenevert TL, Brunberg JA, Hoff JT. Experimental intracerebral hemorrhage: relationship between brain edema, blood flow, and blood-brain barrier permeability in rats. J Neurosurg. 1994;81:93–102.
Xi G, Wanger KR, Hua Y, deCourten-Myers GM, Myers RE. Plasma but not packed red blood cells infused in pig cerebral white matter induces rapidly developing, marked edema. Soc Neurosci Abstr. 1996;22:1424.
Xi G, Keep RF, Hoff JT. Erythrocytes and delayed brain edema formation following intracerebral hemorrhage in rats. J Neurosurg. 1998;89:991–6.
Xi G, Hua Y, Bhasin RR, Ennis SR, Keep RF, Hoff JT. Mechanisms of edema formation after intracerebral hemorrhage: effects of extravasated red blood cells on blood flow and blood-brain barrier integrity. Stroke. 2001;32:2932–8.
Belayev L, Saul I, Busto R, Danielyan K, Vigdorchik A, Khoutorova L, et al. Albumin treatment reduces neurological deficit and protects blood-brain barrier integrity after acute intracortical hematoma in the rat. Stroke. 2005;36:326–31.
Lu A, Tang Y, Ran R, Ardizzone TD, Wagner KR, Sharp FR. Brain genomics of intracerebral hemorrhage. J Cereb Blood Flow Metab. 2006;26:230–52.
Hua Y, Schallert T, Keep RF, Wu J, Hoff JT, Xi G. Behavioral tests after intracerebral hemorrhage in the rat. Stroke. 2002;33:2478–84.
Wu J, Hua Y, Keep RF, Schallert T, Hoff JT, Xi G. Oxidative brain injury from extravasated erythrocytes after intracerebral hemorrhage. Brain Res. 2002;953:45–52.
Felberg RA, Grotta JC, Shirzadi AL, Strong R, Narayana P, Hill-Felberg SJ, et al. Cell death in experimental intracerebral hemorrhage: the “black hole” model of hemorrhagic damage. Ann Neurol. 2002;51:517–24.
Hua Y, Nakamura T, Keep RF, Wu J, Schallert T, Hoff JT, et al. Long-term effects of experimental intracerebral hemorrhage: the role of iron. J Neurosurg. 2006;104:305–12.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
O’Lynnger, T., Mao, S., Hua, Y., Xi, G. (2019). Blood Injection Intracerebral Hemorrhage Rat Model. In: Chen, J., Xu, Z., Xu, X., Zhang, J. (eds) Animal Models of Acute Neurological Injury. Springer Series in Translational Stroke Research. Springer, Cham. https://doi.org/10.1007/978-3-030-16082-1_18
Download citation
DOI: https://doi.org/10.1007/978-3-030-16082-1_18
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-16080-7
Online ISBN: 978-3-030-16082-1
eBook Packages: MedicineMedicine (R0)