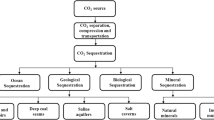
Abstract
The appeal of mineral carbonation (MC) as a process technology for scalable and long-term CO2 reduction, is that it is a solution that has the sequestration capacity to match the amount of CO2 emitted from energy generation and industrial activities [1,2,3]. Many inorganic materials such as minerals [4, 5], incineration ash [6, 7], concrete [8, 9] and industrial residues [10, 11] are potentially huge sinks for anthropogenic CO2 emissions. These materials are typically abundant sources of alkaline and alkaline-earth metal oxides, which can react naturally with CO2 to form inorganic carbonates and bicarbonates. In addition, their products are thermodynamically stable and relatively inert at ambient conditions. On paper, MC should be able to fully sequester all anthropogenic CO2 emissions, since the abundance of magnesium and calcium atoms on Earth far exceeds the total amount of carbon atoms [12, 13]. However, despite the apparently favorable pre-conditions, we still observe a net accumulation of CO2 in the atmosphere because the rates of reaction to form (bi)carbonates in nature are too slow compared to the current rate at which CO2 is being emitted [14, 15]. If left to their own devices, thousands of years are needed to achieve any substantial sequestration of CO2 [16]. This is clearly not rapid enough to solve the pressing problem of climate change that is already affecting us now. Therefore there is a need to employ mineral carbonation as an artificial method to accelerate the rates of CO2 sequestration. In this chapter, we will take a look into the chemistry and thermodynamics of mineral carbonation and discuss some of the main obstacles to large scale MC implementation. Additionally, we highlight the types of starting materials from which basic alkaline-earth metal oxides can be obtained and discuss how their abundance and properties affect MC performance. We will also give a short review of current research in the area to develop MC into viable and economic processes, with some focus on the main categories of process designs and their working principles. We will then look at MC from a techno-economic standpoint and assess the opportunities to integrate MC into the existing industrial and environmental landscape. Lastly, we conclude the chapter with a hypothetical scenario of MC deployment in Singapore, an economically developed but land-scarce country under threat by rising sea levels.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
4.1 Background and Key Concepts in Mineral Carbonation
In this section, we introduce and discuss several of the key concepts behind mineral carbonation. This is to provide the reader with sufficient background information to understand and appreciate the science and basic guiding principles of MC. Hopefully this will inspire the reader to utilize this knowledge to develop new ideas and exploit new opportunities that may emerge from it. Here, we will briefly explain the chemistry, reaction characteristics, thermodynamics, and material sources for mineral carbonation.
4.1.1 The Chemistry of Mineral Carbonation
There is often a common misconception that CO2 is an unreactive molecule that is difficult to functionalize and convert into other industrially relevant chemicals. This is demonstrably untrue, as CO2 is in fact capable of participating in a wide variety of reactions [17]. For example, we can easily observe its effects on the gradual chemical erosion of calcium and magnesium silicate materials at ambient conditions [18]. At its very core, mineral carbonation is a simple reaction between an acid (CO2) and a base (usually alkaline or alkaline-earth metal oxides/hydroxides):
This reaction occurs spontaneously in nature, and can be considered as a subset of natural weathering processes [18]. This phenomenon is responsible for the formation of limestone and Mg/Ca salinity in the environment. In a natural setting, rain and flowing water can dissolve CO2 and other acidic gases from the air. When CO2 is dissolved in water, it is hydrated and carbonic acid is formed:
When this acidic water comes into contact with minerals with high Mg or Ca content, the dissolved CO2 reacts with the alkaline-earth metals and forms soluble aqueous bicarbonate species. For example, when wollastonite (a calcium-rich silicate mineral) reacts with carbonic acid, aqueous calcium bicarbonate is formed:
These dissolved bicarbonates are then carried off downstream and are eventually re-precipitated as solid carbonates when the local conditions change (i.e. warmed, pH raised, CO2 partial pressure lowered, evaporated etc.):
The net result is that the original mineral is altered and magnesium or calcium carbonate sediments are formed elsewhere:
The dissolved aqueous bicarbonates may also be carried off by rivers into the ocean as well, where the calcium can be taken up by marine organisms to form shell structures or coral which eventually settle on the ocean floor or wash up on seashores as beach sand.
The chemistry and speciation of CO2 in aqueous solutions are ultimately governed by the pH, which in turn depends on many factors such as the temperature, total dissolved solids (TDS) content, and the partial pressure of CO2 in equilibrium with the solution etcetera [19]. As carbonic acid is a diprotic acid, it can be deprotonated once and twice into the bicarbonate and carbonate anions respectively. Also, since carbonic acid is a weak acid, the individual deprotonations occur gradually over a range of pH values (as opposed to a clear, sharp and complete deprotonation within a narrow pH range for strong acids). This leads to the observation that different inorganic carbon species can potentially coexist in the solution, especially at moderate pH values (between 4 and 12). The speciation can be visualized in a Bjerrum plot, showing the relative amount of each species at equilibrium within the solution at any given pH value [20]. As shown in Fig. 4.1, in a closed system, the bicarbonate anion is the predominant inorganic carbon species at neutral pH. Dissolved CO2 (simplified as carbonic acid here) makes up most of the remaining minor fraction, and carbonate anions are nearly non-existent.
Bjerrum plot showing the speciation of inorganic carbon species in aqueous solutions versus pH, within a closed system. Plot obtained and modified from GWCarb v1.0 Carbonate Speciation Tool [20]
At higher pH values, the second deprotonation occurs more extensively, and carbonate anions begin to predominate under these conditions. At lower pH values, the bicarbonate anion is re-protonated and forms carbonic acid, which has a strong tendency to revert to water and gaseous or dissolved CO2.
The pKa values for the two deprotonation reactions are:
As can be seen from the equations above, the distribution of carbon species in solution is highly dependent on the pH value. An increase in [H+] shifts the speciation towards more protonated species such as bicarbonates and carbonic acid. Conversely, increasing the pH encourages more deprotonated species to form (in other words increasing [HCO3−] or [CO32−]) in order to maintain the equilibrium within the system.
A fundamental understanding of the factors affecting this equilibrium and how they determine the behavior of inorganic carbon species in solution is important for the effective design of mineral carbonation processes. Firstly, it is known that the carbonate/bicarbonate system acts as a buffer to resist drastic changes in ocean chemistry [21]. The oceans are a huge carbon sink that absorbs significant quantities of CO2 emitted into the atmosphere every year [22]. The amount of CO2 absorbed into the oceans is estimated to be around 7.5 billion tonnes of CO2 annually. Yet despite this, the average oceanic pH has “merely” dropped 0.1 pH units from pre-industrial values thanks to the exceptional buffering capacity of the carbonate/bicarbonate system [23]. However, this apparently small change has already begun to affect the efficiency of shell and coral formation in many biological systems. It has also been predicted that the ocean pH will drop by another 0.3–0.4 units by the year 2100 under business-as-usual scenarios, with irreversible and devastating consequences on marine biota [24].
Secondly, many bicarbonate compounds are found only in aqueous solutions. Notable exceptions are sodium, potassium and ammonium bicarbonate, which can exist as solids under ambient conditions. Even so, these salts are notoriously hygroscopic and thermally unstable. For example, NH4HCO3 decomposes to release ammonia, water and CO2 when mildly heated, thus making it an unsuitable reservoir to reliably store captured CO2 [25]. Carbonates in general are often more stable than their bicarbonate counterparts, meaning that they are less inclined to re-release the CO2 after sequestration. Therefore it is desirable that the captured CO2 is sequestered as a carbonate species. However, in order to facilitate the rapid formation of carbonate products within an aqueous phase, a high concentration of carbonate anions must be present, implying the need to adjust the solution pH to high values to shift the speciation and increase its concentration.
Thirdly, since we know that many bicarbonate compounds are relatively temperature sensitive, it is possible to dissociate and precipitate them as carbonates from aqueous solutions simply by heating them [26, 27]. As the solution is heated, some bicarbonate anions dissociate to give water and CO2, which is less soluble at high temperatures and escapes the solution as a gas. As CO2 is removed from the aqueous system, the equilibrium is perturbed and this results in the formation of carbonates which are also usually much less soluble. Thus, as the carbonate product precipitates, it is removed from the aqueous system and a new equilibrium in the aqueous phase is re-established. This is in fact what leads to the formation of lime scale in kettles and boilers that come into contact with hard water.
The behaviors listed above are crucial to our understanding of the working principles of mineral carbonation, and they are the basis of the chemistry that supports nearly all reasoning that goes into the design, analysis and development of MC processes.
4.1.2 The Rates and Mechanisms of Mineral Carbonation
Although the conversion of CO2 into carbonates and bicarbonates occurs naturally in the environment, the rates of reaction are still too slow to match current rates of CO2 emissions from industrial activities. The imbalance in the rates of CO2 emission and sequestration leads to an accumulation of the greenhouse gas in the atmosphere, at an average rate of approximately +2 ppm per year in the 21st century. Thus, the acceleration of mineral carbonation reactions will enable CO2 to be removed from the atmosphere in a quicker manner, and hopefully tip the balance back to a more manageable equilibrium. As with any set of chemical reactions, the rates of mineral carbonation can be analyzed through the lens of conventional reaction engineering principles.
In nature, mineral carbonation is technically a three-phase reaction, which involves the dissolution of CO2 (a gas) and alkaline-earth metal cations from minerals (a solid) into water (a liquid) where they react to form the solid carbonate product [28, 29]. The rate limiting step is usually the CO2 dissolution step, since CO2 is only very slightly soluble in pure water (around 1.5 grams per liter at 1 bar CO2 pressure) [30]. The very low concentration of CO2 in the atmosphere (which currently stands at around 410 ppm or 0.041%) further retards the dissolution kinetics.
The dissolution of CO2 into a liquid phase also depends on several other factors such as pH and temperature. As with many other gases, higher temperatures decrease the solubility of CO2 in water, and vice versa. However, the effect of temperature on CO2 solubility under normal conditions is usually negligible, since the solubility value does not change much within the range of ambient temperatures commonly encountered in nature [31]. A higher pH in the aqueous solution helps to stabilize dissolved CO2 as (bi)carbonates, and prevents degassing from the liquid phase. In addition, higher pH values reduce the CO2 chemical potential at the liquid side of the gas-liquid interface, thus eliminating mass transfer resistances and speeds up the uptake of CO2 into solution [32,33,34,35].
On the other hand, the natural dissolution of the alkaline-earth cations from minerals is comparatively more favored due to the common presence of organic acids in groundwater, which promotes the dissolution reaction [36, 37]. The organic acids in groundwater originate from the metabolism and decomposition of terrestrial organisms, and are occasionally supplemented by inorganic species such as nitric and sulfuric acid from acid rain. However, as we will see later, as a result of the mineral structures and dissolution mechanisms, these rates gradually taper off and slow down significantly as time goes by.
A simplified reaction network chart showing the relationships and interactions between various carbon and alkaline-earth metal species is given in Fig. 4.2.
As mentioned previously, when CO2 gas comes into contact with liquid water, it can dissolve and speciate into various compounds in solution. These interactions can be roughly described as the CO2 dissolution (1a), CO2 hydration (1b) and sequential deprotonation (1c and 1d) reactions. All five species are often found to coexist, and their abundance and equilibrium positions are highly dependent on environmental conditions under which they are present.
On the other hand, the alkaline-earth metal oxides in silicate minerals have to be liberated from the mineral matrix for the carbonation reaction to occur. In nature, this dissolution is usually promoted by the presence of weak acids in the water in contact with the minerals. For example, the hydration of dissolved CO2 yields carbonic acid, which can react with and leach out calcium or magnesium cations from the minerals (2a). As shown in Fig. 4.2, this results in a dilute solution of metal bicarbonates. Under weakly acidic conditions, the leaching reactions are often quite selective and only the more alkaline components in the mineral are dissolved. Stronger acids can also be employed to accelerate and intensify the metal dissolution reactions (2b), though these come at a cost of loss in selectivity and more difficult reagent regeneration steps, as will be shown later.
The dissolution of alkaline-earth metal oxides from silicate minerals also suffers from a secondary problem, which is the gradual passivation of the mineral surface by unreactive silica residues left behind after acid leaching [38, 39]. Many studies have been done on the actual mechanisms of acid leaching of minerals, and the common consensus is that the dissolution reaction follows a shrinking core model (SCM). In the SCM, metal silicates react with acid to give the soluble metal salt and unreacted silica. The selective removal of the metal atoms from the mineral matrix results in the formation of vacancies in the material, effectively forming a layer of porous silica on the surface of the particle. At first, the layer is thin enough to allow acid species to rapidly penetrate and access the metal oxides situated deeper in the particle. However, as the reaction proceeds, the silica layer becomes increasingly thick, and the increased tortuosity and mass transfer resistance slows down the rate of acid diffusion into the particle. As a result, the dissolution rates drop off sharply and the reaction conversions plateau as a result. Figure 4.3 depicts the changes in spatial composition of the mineral particle according to SCM as the leaching reaction proceeds.
Dissolved alkaline-earth metal cations can react directly with carbonate anions in solution to precipitate solid carbonates (3). The precipitation reaction is usually near instantaneous, and yields can be close to 100% under the right reaction conditions. Any soluble counter-ions that are present in the solution (Na+, K+, NH4+, Cl−, SO42−, NO3− etc.) during the reaction will combine to give a salt product, which is discarded or regenerated into the respective acids and bases for further reuse. This technique for mineral carbonation, first through a dissolution step followed by a precipitation step in separate reactors (2b + 3), is traditionally termed as an indirect method [40]. The acids used in indirect carbonation processes are usually strong mineral acids, and the metal salt product is typically separated from the silica residue (and occasionally purified) prior to carbonation in a second reactor [41,42,43,44].
In contrast, direct carbonation methods supply pressurized CO2 into a single reactor containing the minerals, where the dissolution/precipitation reactions take place simultaneously [45, 46]. Here, a high CO2 pressure forces an increase in the absolute amount of bicarbonate and carbonate species, which can react to form aqueous metal bicarbonate salts (4a), which in turn forms solid carbonates when the conditions are changed, for example when the system is heated or the pH is raised. Raising the pH disproportionates the bicarbonate compounds into two carbonate molecules (4b), while heating degasses the bicarbonate solution and shifts the equilibrium towards more carbonate formation (4c). In effect, both changes in the pH and temperature drive the precipitation reaction by removing one mole equivalent of carbonate anions from the system.
Based on these observations, it should be apparent by now that the conditions for dissolution and precipitation in mineral carbonation are clearly contradictory. The various chemical species that react in mineral carbonation processes are involved in an intricate interplay that necessarily leads to sub-optimal performances, especially if the process is conducted in a single steady-state reactor.
4.1.3 The Thermodynamics of Mineral Carbonation
The sign (positive or negative) of the change in Gibbs free energy (ΔGrxn) is an indicator of the spontaneity of a reaction. A negative ΔGrxn value points to a spontaneous reaction, and vice versa. ΔGrxn is a function of the change in enthalpy (ΔHrxn) of a reaction, and also of the temperature (T) and change in entropy (ΔS) of the system:
Based on this equation, we can infer that reactions with certain characteristics lend themselves more easily to spontaneous occurrence. Reactions that are highly exothermic (negative ΔHrxn) tend to occur naturally, as are those that result in increased disorder in the system (positive ΔS, usually by increasing the number of chemical species or molecules) [47]. By applying our understanding of this basic interpretation of the second law of thermodynamics, we can make a few observations with regards to mineral carbonation. Firstly, since the chemical weathering of magnesium and calcium silicate minerals to form carbonates is known to occur spontaneously in nature, it logically follows that the ΔGrxn of the overall reactions is negative and therefore the products are less energetic than their starting materials. In other words the carbonates are obviously less energetic and more stable than CO2 and the starting mineral silicates [48].
Secondly, since the carbonation reaction is one that produces solid products from (partly) gaseous reactants, this suggests that the net entropy (disordering) of the reaction system is one that is decreased at the end. In other words, the ΔS value of carbonation reactions is almost always negative. Bearing in mind that the pre-requisite of a spontaneous reaction is that ΔGrxn is negative, it thus follows that carbonation reactions should have a highly negative value for ΔHrxn to compensate for the always positive (−TΔS) term [49]. Put differently, for ΔGrxn to be negative the following needs to be true when ΔS values are negative:
It is thus logical to infer that carbonation reactions should give out a relatively large amount of heat, based on what has been mentioned above. This is in fact what is observed in accelerated carbonation experiments [50]. On the other hand, it is also useful to consider the thermodynamic aspects of mineral carbonation from perspective of the carbon atom, i.e. what actually happens to it as it is converted from a component in an organic molecule to CO2, and finally to a component within a carbonate solid. An analysis of this energy level pathway will quickly illustrate one of the key strengths of mineral carbonation compared to other CO2 utilization technologies.
CO2 is formed when materials containing carbon are oxidized. The most prominent examples of these oxidation reactions include fuel combustion and the in vivo metabolism of glucose to produce energy. For instance, when an energetic molecule like methane is burned, the oxidation state of the carbon atom is increased from 4− to 4 + and a huge amount of heat energy is released:
This change in enthalpy is the dominant driving force that pushes the reaction forward, since the ΔS value of this reaction is miniscule (on the order of a few Joules per Kelvin, as the net number of molecules does not increase, nor is any phase change apparent). Also, since methane can be oxidized into CO2 through numerous combinations of reactions, it is often neither practical nor necessary to calculate and tabulate the thermodynamic components for all the possible combinations. What really matters in our analysis is how the “embodied” Gibbs free energy of formation in the carbon-containing species changes, and by how much. This concept can be illustrated by mapping the Gibbs free energy of formation of various carbon-based molecules, in relation to CO2. We can use the following equation to calculate the relative Gibbs free energy of formation of the various carbon-based compounds:
In the equation, ΔGr is the relative Gibbs free energy of formation of the product molecule, ΔGm is the Gibbs free energy of formation of one mole of the product molecule, Nm is the number of carbon atoms in the product molecule, and \(\Delta G_{{{\text{CO}}_{2} }}\) is the Gibbs free energy of formation of one mole of CO2. Implicit in the equation is the assumption that all the carbon atoms in the product molecule are obtained from the reformation of CO2. This is expressed in our calculations through the ΔGm/Nm term in the equation (i.e. one mole of CO2 can be converted into one mole of calcium carbonate, or half a mole of ethylene, or a quarter of a mole of butanol, and so forth). While this is clearly not realistic, this simplification is helpful to illustrate the relation between CO2 and its potential reformed products. A positive ΔGr indicates that the formation of that particular product molecule requires a net energy input, and vice versa.
As can be seen in Fig. 4.4, all of the organic molecules are situated above CO2 in the figure, meaning that if these molecules were to be synthesized from CO2, a net amount of energy input is always required, no matter how efficient the process is. In other words, the reformation of CO2 into organic compounds is always thermodynamically unfavorable. For example, once we account for the heats of formation for water and the effects of temperature, we find the difference in Gibbs free energy between methane and CO2 to be around 330 kJ/mol at STP. This value is the relative Gibbs free energy of formation between these two chemical species, and can be considered as the absolute minimum amount of energy input required to convert one mole of CO2 into methane.
On the other hand, the conversion of CO2 into the carbonate anion has a negative ΔGr, meaning that the conversion actually releases energy into the system. This value decreases further when it is stabilized by a counter-cation such as Mg, Ca or Na. As mentioned earlier, this large and negative ΔGr value entails the release of significant amounts of heat. If properly and effectively harnessed, this energy can supplement part of the overall energy consumption in mineral carbonation processes [51].
Obviously, a thermodynamic analysis of mineral carbonation processes should not be limited to the carbon and alkaline-earth metal species only. The utilization of additives and manipulation of reaction conditions necessarily implies an energy input, either in the form of chemical potentials (regeneration of acid and base catalysts), thermal energy (reactor heating, mineral thermal activation etc.) or physical work (agitation, grinding, CO2 compression etc.). Proper analysis and accounting of these embodied energies is crucial to ensure that any proposed mineral carbonation process is truly effective at sequestering more CO2 than it emits.
4.1.4 Raw Materials for Mineral Carbonation
As implied by the name, the most widely available raw materials for mineral carbonation are alkaline-earth silicate minerals that are highly abundant in the earth’s crust. The preferred silicate raw materials are those termed as ultramafic minerals, whose name refers to their high magnesium and ferric content (hence the term mafic) [52]. As a very general rule of thumb, ultramafic minerals typically contain around 40% MgO, 40% SiO2, 10% Fe2O3 and 10% other metal oxides in dry weight, though obviously this is subject to major fluctuations depending on the quality, locality and age of the mineral deposits. The amount of these minerals far exceed the total quantity of combustible carbon on the planet, meaning that theoretically it is possible to store all of the anthropogenic CO2 into these minerals even if all of the fossil fuels were burned for industrial uses or energy production [53]. These minerals are also rather widespread, with known deposits being found in easily accessible locations that are near to urban centers and major point sources of CO2 emissions (Fig. 4.5).
World map with red dots showing regions where ultramafic minerals are known to be commonly found. The amount of accessible ultramafic minerals is estimated at approximately 90 trillion tonnes, enough to store around 22 trillion tonnes of CO2. For comparison, the total amount of potential anthropogenic CO2 from proven reserves is calculated to be around 10 trillion tonnes [53]
In addition to the chemical composition of the minerals, their physical structure and crystalline habits also play determinative roles in their reactivity and suitability for mineral carbonation reactions. Olivine (Mg2SiO4) and serpentine (Mg3Si2O5(OH)4) are two groups of ultramafic minerals that are commonly studied to explore their potential as sources of magnesium for mineral carbonation. Olivine is an orthosilicate mineral; its structure contains individual isolated tetrahedral [SiO4]4− units that are coupled to interstitial cations (Mg2+, Fe2+, Ni2+ etc.). On the other hand, serpentine is a phyllosilicate mineral, and has parallel and interconnected sheets of silica that contain metal cations in between the layers (Fig. 4.6).
Examples of crystal structures of orthosilicate minerals (left) and phyllosilicate minerals (right). Orthosilicates have individual silica tetrahedrals coupled with interstitial divalent cations, while phyllosilicates have lamellar (sheeted layers) networks of interconnected silica, with the metal cations sitting in the centre
Olivine and serpentine are closely related, in the sense (loosely speaking) that one is the parent of the other. Olivine is an igneous rock, meaning that it originates from tectonic activity in the earth’s mantle or crust. It is one of the most common minerals on the planet, with most of it being found in sub-surface deposits that have little contact with the atmosphere. When olivine is exposed to the elements, the mineral quickly weathers to serpentine where it changes its structure and composition, and also undergoes hydration [54]. As such, serpentine is termed as a metamorphic rock, where it is changed from a previous different mineral to its current form.
Serpentine has three notable and distinct morphologies; these are antigorite, lizardite and chrysotile [51, 55, 56]. Antigorite and lizardite differ only slightly in terms of their silicate arrangements in the crystal habit, and their bulk structures remain fairly similar to each other. Both are green-grey minerals with a scaly texture, and have a Moh’s hardness index of around 2–4. Chrysotile on the other hand, is notably different from the other two, being a soft fibrous mineral that can exist in long white strands. In fact, chrysotile is better known as asbestos, a cheap and highly effective thermal insulator that was widely used prior to its identification as a carcinogenic material. Some reports have suggested that chrysotile is much more reactive compared to its other two counterparts [57, 58].
The metamorphosis of olivine into serpentine also incorporates water and hydroxyl groups into the altered crystal structure. These hydrates and hydroxyl groups in serpentine can be removed via heating, which increases the porosity of the material [59, 60]. The act of dehydration also often destroys the crystallinity of the mineral in the process and makes it more reactive. As a result, the utilization of serpentine in modern mineral carbonation processes usually starts with a thermal activation step, where the mineral is heated to around 600 to 700 °C for the dehydration reaction to occur. The dehydration step must be done in a controlled manner, as excessive temperatures or long heating times may result in the recrystallization of the mineral into forsterite, which is a member of the olivine family. Forsterite is much less reactive than activated serpentine, hence it is important to optimize the thermal activation step to maximize the reactivity of the minerals [61, 62].
In addition to natural minerals, many other artificial sources of alkalinity can also be used for mineral carbonation. Notable examples of these include red mud (the waste product from bauxite processing), steel slag, waste concrete, incineration bottom ash and many others [6, 46, 63,64,65,66,67,68,69,70,71,72].
In the Bayer process, sodium hydroxide is used to dissolve aluminum from bauxite, the primary ore from which it is obtained. The resulting slurry is filtered to obtain a sodium aluminate solution and the unreacted residue. The sodium aluminate is sent downstream to be processed into aluminum metal while the residue is discarded and accumulates in disposal ponds. This residue, known as red mud, is highly alkaline in nature as it still contains an excess of unrecovered sodium hydroxide in the filter cake. The red coloration arises from the presence of iron hydroxides in the residue. More than 150 million tonnes of red mud is generated every year as a byproduct of aluminum production. The CO2 uptake capacity of red mud, depending on its quality, is reported to be in the range of 0.03–0.08 t CO2/t red mud. This translates to around 4–12 million tonnes of CO2 sequestered, in addition to the benefits of treatment and neutralization of the toxic material. Although relatively small, this amount is still considerable and should not be overlooked [73].
Slag from iron and steel production is an even larger source of industrial waste materials for mineral carbonation. During the smelting of iron ore, lime is added to the melt to remove impurities in the mixture. The added lime reacts with the silica and other contaminants to form a solid calcium silicate phase, which floats on top of the molten iron in the furnace and is skimmed off. This slag is alkaline in nature and can be used as a source of calcium to bind with captured CO2. There are also other utilization routes for the material, especially in construction applications. With an average CaO content of around 45–50 wt%, iron and steel slags are excellent sinks for CO2 emissions. Annual production of steel and iron slag amounts to around 470–610 million tonnes, which corresponds to a CO2 sequestration capacity of approximately 143–186 million tonnes/a if fully utilized [74].
Waste concrete is generated when buildings are demolished, and a large portion of the waste is recycled as aggregates in new construction projects. However, there is still a fraction of waste concrete that is disposed of every year, and these can be utilized as carbon sinks for CO2 as well. Concrete contains significant amounts of calcium, and is capable of capturing CO2 on its own as it settles and hardens. The natural carbonation of concrete occurs on the surface, which gradually retards the penetration of CO2 into the deeper layers and maintains a highly basic environment in the interior. The alkaline pH in the interior sections of concrete helps to prevent corrosion of the steel reinforcing bars in the structure, ensuring that the construction remains structurally sound for a long time. When buildings are demolished, the concrete is broken down into smaller pieces, exposing the interior parts to air and binding CO2 to the calcium content in the concrete waste [75].
Incineration bottom ash (IBA) is also a potential source of alkalinity for the sequestration of CO2 through carbonation. Combustion and gasification of the organic content in municipal solid waste (MSW) leave behind a solid ash residue. This ash consists of non-volatile materials such as ceramics, glass, metals and metal oxides. A rudimentary magnetic separation step is usually employed to remove most of the ferrous metals in the ash for recycling. The remains typically contain around 10–25% of alkaline-earth oxide content, though this is highly variable and will change depending on fluctuations in municipal waste compositions. In order to meet environmental standards, the flue gas from incineration is usually scrubbed with lime to remove NOx and SOx prior to release into the atmosphere. The lime is usually added in excess, to prevent the pollutants from escaping into the environment. The spent lime is not recycled, and is typically mixed with the incineration bottom ash. This further increases the calcium content in the ash residue, and may be made available for the capture and sequestration of CO2. The calcium oxide content in the mixed ash can be as high as 50% by weight, though a sizeable portion of this may already be bound as nitrates, carbonates and sulfates. Joseph et al. estimates that globally, a total of around 1.3 billion tonnes of MSW is generated every year. Of this, 10% (130 million tonnes) are incinerated and the rest are landfilled. The incinerated MSW is reduced in mass and volume, and generally the ash residue is around 10% of the original weight of the disposed waste (equating to around 13 million tonnes of IBA). This also means that IBA in effect acts as a renewable source of alkalinity for CO2 capture and sequestration via carbonation. A very rough calculation would suggest that IBA from MSW incineration would then have the potential to absorb around 8–10 million tonnes of CO2 per year at current rates of MSW disposal and incineration. The carbonation of IBA would also stabilize the material and inhibit the leaching of heavy metals from the ash to a certain degree, thus reducing the environmental impacts of its disposal [68, 76,77,78].
A comparison of the indicative compositions of the abovementioned materials is given in Table 4.1.
4.2 Overview of Basic Process Designs for Mineral Carbonation
In the second part of the chapter, we will discuss the practical application of the theoretical understanding and concepts as described in Part One. Based on these ideas, many chemical processes have been developed to exploit and accelerate the reactions, in order to enable mineral carbonation to become a feasible pathway for large scale CO2 capture and sequestration. This section will act both as a review of recent developments in mineral carbonation process designs, and also as a study in how the previously introduced concepts are exploited and applied in practical examples for mineral carbonation.
Traditionally speaking, there are two schools of mineral carbonation technologies that were developed in parallel; these are the direct (or sometimes called “pressure”) carbonation and indirect (occasionally described as “chemically enhanced”) carbonation routes [40, 79]. These process designs are not strictly incompatible or inherently better than the other, and sometimes a process can even contain a little bit of both routes such that they may defy categorization into the conventional ways that we usually classify mineral carbonation processes.
4.2.1 Pressure Carbonation
Pressure carbonation, as the name implies, involves mineral carbonation reactions that are conducted under pressure. In one of the earliest iterations of pressure carbonation processes, pure CO2 and steam were supplied at high pressure (115–150 bars) into a heated autoclave to react with activated serpentine (i.e. milled and heat-treated). This process was developed by NETL in the mid-2000s [45, 80,81,82]. The reaction was designed as a one-pot process, where the CO2 and activated serpentine were converted to silica and magnesium carbonate:
Other types of minerals such as olivine and wollastonite were also used as well, and the products were obtained as a mixture. The reaction conversions were in the range of 36–92%, depending on the type of raw materials used. The authors reported that lizardite was the least reactive with conversions around 40%. Olivine, wollastonite and antigorite were much more effective for mineral carbonation, attaining conversions in excess of 80%. In addition to CO2 and the minerals, 1 mol/L NaCl and 0.64 mol/L NaHCO3 were also added to the reaction system promote the mineral carbonation reactions. The authors also concluded that the best reaction conditions for various mineral types were as follows: olivine (185 °C, 150 bar, with additives); wollastonite (100 °C, 40 bar without additives); and for activated serpentine (155 °C, 115 bar, with additives). A simplified flow diagram for this process is given in Fig. 4.7.
In the NETL process, high pressures and temperatures were necessary to push CO2 into the supercritical phase to facilitate mass transfer between the reactants. The high pressure also shifts the CO2 speciation equilibrium in the system, by forcing more CO2 to convert into carbonic acid and thus lowering its pH (think of it as a supercharged club soda). This acidified environment, combined with the high temperatures, helped to leach out the alkaline contents in the mineral and made them available for reaction. The shifted equilibrium also resulted in more carbonate and bicarbonate anions being present in the system, which rapidly combines with the leached alkaline-earth cations to form solid carbonates. The mixture exits the reactor after 1 h of reaction time to be filtered. The water and unreacted CO2 are recycled and recompressed, and the carbonate and silica product mixture is separated.
We now know that in pressure carbonation, there are two distinct reactions that occur that ultimately result in the precipitation of inorganic carbonates from the reaction of CO2 with minerals. These two reactions are contradictory, and their optimum reaction conditions are opposites of each other [83]. As described earlier in the first part of the chapter, the first reaction is a dissolution reaction, where an alkaline-earth metal oxide is extracted (preferably) into an aqueous phase. Acidic conditions and lower temperatures (dissolution is typically an exothermic reaction) are favorable for this. However, for practical purposes, the dissolution reaction is often conducted under moderate heating, since the gains in reaction rates far outweigh the benefits from the small driving force due to heat removal. Once dissolved, the alkaline-earth metals can be precipitated from solution as well. Precipitation is favored under high pH and temperature conditions, where carbonate anions predominate and easily react to form insoluble solids.
With a better understanding of the basic mechanisms of mineral carbonation, it is tempting to draw a conclusion that the one-pot reactor method for direct carbonation is inefficient. The conditions applied in the process are not conducive for either reaction, and both compete against each other. Thus, a more rational design is needed for a direct mineral carbonation process. From this analysis, researchers at Royal Dutch Shell came up with an improved and more refined version of the process developed by NETL. In their version of the direct carbonation process, the fundamental reaction mechanisms remained more or less the same: pressurized CO2 is supplied into a heated reactor to extract magnesium from activated serpentine. The extracted magnesium is then precipitated to give magnesium carbonates, and the fluid phase is recycled [84,85,86].
However, the main difference was that the individual reactions were conducted in separate reactors, and this allowed them to isolate the reactants from each other and optimize the conditions in order to promote the desired reactions in each step. By compartmentalizing the reactions, the reactions could be conducted under less harsh conditions. In their design, 5 bars of CO2 pressure was sufficient to allow reasonably high degrees of Mg extraction as bicarbonate species (compared to operating conditions of >100 bars for the NETL process) in the first step. In the second step, near-total recoveries of magnesium (as hydrated carbonates) were achievable at sub-boiling temperatures up to 90 °C. One version of their process also had the dissolution reaction occur in two stages (two separate reactors) to maximize the conversion of Mg into the solution [86]. The entire process was designed as a counter-current setup, where activated serpentine minerals enter from one end and is gradually depleted in magnesium content, while flue gas enters from the other end and is enriched with CO2 and then consumed before it exits the system. The counter-current design in the process ensures that the driving force in each reactor is maximized to enhance reaction efficiencies. A simplified diagram of their refined process is given in Fig. 4.8.
Diagram of refined direct carbonation process as proposed by researchers from Shell: note the overall counter-current design embedded in the system to maximise the driving forces (chemical potential gradients) between reactions. The liquid phase is recycled instead of being discarded. R1: Dissolution reactor; R2: optional dissolution enhancement reactor; R3: precipitation reactor
As can be seen, the refined process by Shell is perhaps more elegant, and clearly has an advantage in terms of operating conditions.
4.2.2 Chemically-Assisted Carbonation
Chemically-assisted carbonation processes are another sub-category of mineral carbonation technologies. The basic sequence of dissolution-precipitation still applies in chemically-assisted carbonation, although there are some notable differences between the two process categories in several aspects. Firstly, the reactions are accelerated primarily via the addition of chemical energy instead of mechanical or thermal energies. Secondly, chemically-assisted processes typically proceed at much lower temperatures and pressures (mild heating and ambient pressure). Thirdly, raw minerals can be used directly without the need for intensive comminution and heat treatment. Fourthly, a regeneration step is usually needed to convert the spent salt by-products into reactants again for recycling back into the process [87,88,89,90].
A common feature of chemically-assisted carbonation processes are the use of highly energetic chemical species such as strong acids and bases [67, 91]. These are added to the system to react with CO2 or the alkaline-earth containing species to form a reactive intermediate. Many chemically-assisted carbonation processes follow a general template which involves dissolution steps to bring the (bi)carbonate and alkaline-earth species into the same aqueous system followed by rapid precipitation of the solid carbonate product. For example, a common way to facilitate the dissolution reaction is to use aqueous HCl to dissolve Mg from serpentine into the aqueous phase:
The reaction is technically a neutralization reaction, where HCl is consumed and converted into a dissolved chloride salt (MgCl2). Also, since silica is practically inert against acid attacks, it remains as a solid residue in the mixture. A filtration step usually follows, where the liquid and solid phases are separated. On the other hand, CO2 can be contacted with an alkali such as sodium hydroxide to form carbonate or bicarbonate species:
This reaction is clearly also a neutralization reaction, where the overall effect is to form a salt (Na2CO3) from stronger acid/base parents. Following these steps, the aqueous salt products are mixed and the precipitation reaction takes place almost immediately:
In the case shown here, sodium chloride is obtained as a spent by-product, which obviously may not be reused directly in the process. The spent by-product has to be regenerated in an additional step, where the salt is converted into its acid and base parents. The regeneration step is often the most energy intensive step in the entire chemically-assisted carbonation process. As such, recent trends have tended towards the use of reagents that can be thermally regenerated at lower temperatures. On the other hand, electrochemical regeneration methods are uncommon, but not unheard of [92,93,94]. A systematic analysis of process thermodynamics can help chemists and engineers to determine the most suitable combination of materials and reactants for a proper design of chemically-assisted carbonation processes.
Since there are near-infinite combinations of acids and bases that can be used to accelerate the dissolution reactions, there is a plethora of various chemically-assisted carbonation processes that have been reported in the literature [19, 40, 79, 95, 96]. The wide array of reagent choices and combinations available for a chemist to use in a process can seem bewildering at first, and it is impossible to properly study each and every possible combination. Fortunately, there are some general rules of thumb and process design heuristics for this:
-
Begin with the identification of a salt compound that is easily dissociated or hydrolyzed at relatively low temperatures, and then determine if the acid and base parents are sufficiently reactive and soluble in an aqueous phase.
-
The intermediate salt compounds (i.e. MgCl2, Na2CO3 etc.) should be highly soluble to minimize the amount of water needed in the system.
-
Heating usually helps in the alkaline-earth dissolution steps, but is not mandatory. On the other hand, ambient or low temperatures generally facilitate the CO2 absorption step.
-
Inorganic acids such as HCl, H2SO4 and HNO3 are commonly used to dissolve alkaline-earth metal oxides from minerals. The reason for this is that their conjugate bases are often more resistant to deterioration during the thermal regeneration of their spent salts compared with organic acids.
-
Monoprotic bases are commonly used to absorb CO2, since their (bi)carbonate salts are usually highly soluble.
-
Impurities in the feed materials will have significant effects on the quality of carbonate products, and have to be accounted for when estimating the mass and energy balance for the process.
-
Multiple separation/purification steps are often included in the process design for economic reasons. These steps allow the recovery of high purity products that can be sold to recover the (usually) higher costs of chemically-assisted carbonation processes.
As mentioned earlier, the bulk of chemically-assisted carbonation processes described in literature follow a similar acid leaching/CO2 absorption/carbonate precipitation sequence. The large number of interesting processes based on this template are too numerous to list and describe in detail. In lieu of that, we would like to bring the reader’s attention to some of the more recent and unique processes that deviate from this basic structure. These will serve as examples to highlight and explain how the rules of thumb come into play in chemically-assisted carbonation processes, and also how others have sought to bypass the major limitations of the standard processes through innovative process design.
Researchers in Abo Akademi, Finland have developed a process (the “AA route”) utilizing ammonium sulfate as a leaching reagent for mineral carbonation [43, 97,98,99]. Ammonium sulfate is easily dissociable at moderately low temperatures. When mixed and heated with milled serpentine, the sulfate salt melts and decomposes to form ammonium bisulfate and gaseous ammonia:
The molten and acidic bisulfate salt reacts with the serpentine mineral to form magnesium sulfate and release more ammonia gas. The resultant mixture comprising MgSO4, SiO2, unreacted bisulfates and impurities is then washed with water. The wash water forms a sulfate salt solution which is filtered off, and the prior degassed ammonia is recombined with the solution to precipitate magnesium hydroxides:
As a result, ammonium sulfate is regenerated as an aqueous solution, and separated from magnesium hydroxide for reuse. The regenerated solution is evaporated to recover solid (NH4)2SO4, and magnesium hydroxide is used as the sorbent for CO2 capture and carbonation. The carbonation reaction in this case is conducted at high temperature and pressures to increase the reaction rates. A simplified illustration of the AA route is given in Fig. 4.9.
The biggest benefit of the AA route is that it allows the amount of water used in the process to be properly controlled and minimized to avoid excessive energy use. The steps requiring the highest temperatures (dissolution, gas-solid carbonation) are substantially free of water, which helps to minimize energy use associated with vaporization.
The regeneration step can also be improved by preventing the formation a salt product that has high thermodynamic stability such as NaCl, or has acid/base parents that are hard to separate (NH4Cl → NH3 and HCl, for example). Researchers at the Institute of Chemical and Engineering Sciences (ICES), Singapore have developed a chloride-based process based on this concept [89]. The process is depicted in Fig. 4.10.
In the ICES process, serpentine mineral is first reacted with aqueous HCl to dissolve magnesium out from the material. The chloride solution is then purified by removing residual silica and any co-extracted metals from the aqueous phase. After that, the resultant magnesium chloride solution is concentrated and pyrolyzed to obtain MgO, and HCl gas is regenerated as well. Heat can be recovered from the steam generated in the pyrolysis step, and the condensed water is used to absorb HCl to regenerate an acid solution for recycling back into the process. MgO from the pyrolysis step is brought forward for reaction in a carbonate or bicarbonate solution, which regenerates a base (typically Na+- or NH4+-based) for CO2 capture from flue gas.
In this process, regeneration step is done “halfway” into the process, before the two intermediates interact to form the carbonate product and stable salt by-product. This effectively isolates the acid and base components from each other, and avoids complicated regeneration and separation regimes usually associated with the lysis of highly stable chemical compounds. An illustration of the concept in thermodynamic terms is given in Fig. 4.11.
Energy changes from the perspective of the chloride species in the chemically-assisted mineral carbonation system. Blue arrows depict: a Formation of MgCl2 from HCl in the dissolution step; and b conversion of MgCl2 into NaCl in the carbonation step. Regeneration of the hydrochloric acid from MgCl2 is energetically less demanding than from NaCl
The processes mentioned above adhere to and cleverly exploit many of the rules of thumb mentioned earlier in this section, yet do not resemble any of the “standard” chemically-assisted process templates. This goes to show that designing a process for mineral carbonation is an art as much as it is science.
4.2.3 Other Carbonation Processes
Many other less conventional mineral carbonation processes have been reported. These do not conform strictly to the categories as described previously, yet they do adhere to the general principles and mechanisms of mineral carbonation reactions.
The concept of bio-catalyzed mineral carbonation has been studied by several research groups. These processes revolve around the use of an enzyme called carbonic anhydrase to increase the concentrations of carbonate anions in aqueous systems, in order to facilitate the actual carbonation reaction [100,101,102]. Carbonic anhydrase is an enzyme that is commonly found in many organisms, and its primary function is to quickly convert dissolved carbonic acid or bicarbonate species into hydrogen cations and carbonate anions:
As mentioned previously in the first section in the chapter, the rates of the actual carbonation reaction are dependent on the concentrations of the carbonate anions and alkaline-earth cations in the system. This reaction is often limited by the rate of CO2 dissolution, hydration and deprotonation in the aqueous phase, thus the use of carbonic anhydrase can help to overcome one of the biggest rate-limiting obstacles to mineral carbonation. A simple and effective way to exploit the benefits of this biomimetic carbonation mechanism is to introduce carbonic anhydrase into hard water, which already contains relatively high levels of magnesium or calcium cations in solution. The added enzyme will quickly convert the bicarbonate counter-ions into carbonate anions, which then interact and bind with the dissolved alkaline-earth metals and precipitate from solution. Power et al. have reported an increase in carbonation rates of up to 240% using carbonic anhydrase, and this could be improved even more by increasing the supply of CO2 into the system [103].
However, since enzymes are proteins, they have a very narrow range of ideal operating conditions and are also very sensitive to changes in local conditions. Fortunately, the optimum operating conditions for enzyme usage are often under ambient conditions, and generally do not require additional energy or material inputs to the system. On the other hand, there are also several major drawbacks of using a biomimetic reaction system for mineral carbonation. Enzymes are susceptible to significant changes in pH and temperature, and will undergo irreversible denaturation and permanently lose their function once this occurs. Secondly, an enzyme is a catalyst, which does not alter the equilibrium of the system. This means that the reactor must be optimized in such a way that the systemic equilibrium is favored towards product formation, and not allowing the products to revert into the reactants. This usually means a continuous removal of the solid carbonate products, or a constant supply of excess reactants into the reactor to ensure that the equilibrium is pushed towards the right. Other major drawbacks of using enzymes are that they are costly to replace, and not really volume efficient compared with other mineral carbonation processes. Therefore, while enzyme mediated mineral carbonation is an attractive prospect from an energy use standpoint, it remains to be seen if the process can be implemented in a practical and economic manner.
Apart from bio-catalysis, the hydration and conversion of CO2 into reactive aqueous species can also be accomplished using metal-based catalysts. Nickel and zinc complexes have been reported to be reasonably efficient for CO2 hydration reactions, though some have questioned their effectiveness in real life situations [104,105,106]. They can also be applied for the reverse reaction, where dissolved bicarbonates are decomposed (ultimately) into gaseous CO2 to precipitate solid carbonates from solution (see Fig. 2).
Another interesting way to conduct mineral carbonation is to couple it with other existing reaction systems, either as a complement or to improve the main process. One notable possibility is the integration and utilization of mineral carbonation for sorption-enhanced water-gas shift reactions [107]. The water-gas shift reaction involves the conversion of carbon monoxide and steam into carbon dioxide and hydrogen gas.
While the water-gas shift reaction can achieve reasonably high conversions under specific conditions, it is still an equilibrium-controlled reaction where a high concentration of products can retard the forward reaction. By continuously removing the CO2 byproduct from the product stream, two benefits are realized simultaneously: Firstly, the equilibrium in the system is shifted and higher conversions are achievable. Secondly, the hydrogen gas is purified as well, from a maximum of 50 v/v% in a non-sorption enhanced system, to more than 90 v/v% with proper removal of CO2. Depending on the reaction conditions, the removal of CO2 is typically effected through the use of in situ chemisorbents such as calcium oxide or magnesium oxide [108].
4.3 Techno-economic Aspects of Mineral Carbonation
In this section of the chapter, we will look at the general technical and economic aspects of mineral carbonation and see how these can influence the practicality and implementation of MC technologies on an industrial scale. More specifically, there are three major techno-economic aspects that are of interest to quickly evaluate and screen for viable process alternatives for mineral carbonation. These are the CO2 penalty, capital costs (CapEx) and operating costs (OpEx). These helpful indicators allow researchers to conduct a reality check on their ideas, and rapidly pinpoint major issues with their proposed processes. In addition, this section will also briefly discuss how mineral carbonation can be integrated with material upgrading processes that can generate multiple revenue streams to offset the costs associated with the sequestration process itself.
4.3.1 The CO2 Penalty
As with all other green technologies, a mineral carbonation process is clearly bound by some material and energy use limits to ensure that there is a net sequestration of CO2 when the process is implemented. In other words, an MC process must be designed in such a way that it does not emit more greenhouse gases than it sequesters throughout its life cycle. However, it is inevitable that a certain amount of energy has to be supplied to enable the reactions to take place. These energies can take the form of electrical, mechanical or thermal energies [109].
The use of fossil fuel-derived energy to drive these reactions will result in CO2 emissions that will offset the gains in an MC process. These emissions reduce the efficiency of mineral carbonation, via the imposition of a CO2 penalty on the process. One simple way of defining the CO2 penalty is to state it as the amount of CO2 emitted for every unit of CO2 sequestered.
The units can be in terms of mass per mass (i.e. t/t) CO2, though it can also be defined as a percentage value for a more straightforward indication. In this form, the equation can be used as a gauge of the efficiency of an MC process, but its usefulness is rather limited. The equation can be expanded to take into account the amount of energy use in a mineral carbonation process, and also the type of fuel the energy is derived from.
In this form, the amount of CO2 emitted from the process is expressed as the total sum of the products of the energy use (EU) in each process step, their respective emission factors (EF) and the efficiency (Eff) of that particular unit operation involved. The units of energy use are typically in joules (or its multiples, i.e. kJ, MJ, GJ etc.) or watt-hours (also its multiples, i.e. kWh, MWh etc.). It is often more convenient to express thermal energy use in units of joules, and electrical or mechanical energy use in watt-hours, since these are the units that are most commonly encountered when discussing the respective energy outputs. An emission factor is a measure of the amount of CO2 emitted when a unit of energy is produced and consumed [110]. As such, the units associated with an emission factor are in the form of mass of CO2 per energy unit (i.e. t CO2 per GJ, kg CO2/kWh etc.). Multiplication of the amount of energy use with an emission factor would thus give the amount of CO2 emitted from a particular process step, and their sum is then compared against the amount of CO2 sequestered as a result of implementing the MC process.
This ratio, the CO2 penalty, is therefore an indication of the efficiency of an MC process. Logically, any combination of process steps that result in 100% or more will mean that the process will have no net sequestration of CO2, and in fact will be better off without its implementation. It thus follows that this number should be as low as possible. With the near-infinite combinations of process steps and reaction conditions that can be implemented in an MC process, it is up to the researcher to determine the energy use in their own process designs. However, there are some approximate rules of thumb that are readily available for reference, and these usually relate to process steps that are common to nearly all mineral carbonation processes. A table listing the approximate energy consumption for these steps is shown below (Table 4.2).
These numbers serve as a rough guideline to quickly estimate the energy use in each process step in mineral carbonation. They are not meant to be precise, as slight changes in the system variables will yield a different value for each item listed above. For example, CO2 entering a compression train at a relatively high pressure (let’s say 50 bars) and compressed to supercritical state will obviously consume less energy than when it’s coming in at 1 bar, and depending on the impurities present, it may also vary by an order of magnitude or more. Another example is that similar classes of minerals from different sources (i.e. serpentine from China vs from Finland) will have slight differences in grindability and thermal dehydration properties, and also mean that they have grinding and activation energies that are particular to that sampling of materials. If a more thorough and precise study of the energy use of a mineral carbonation process is needed, researchers are advised to conduct experiments to measure the relevant mass and energy balances. In addition, some values such as the energy requirements for CO2 compression and salt solution heating can also be referenced from published standards or data curves available in the literature.
On the other hand, the emission factors for energy use are much more straightforward and easier to estimate. If renewable energy is used to drive any of the process steps, then the related emission factors can be safely taken to be zero [110]. However, if fossil fuels are used to supply energy into the process, then some care must be taken to calculate their impact on the CO2 penalty of the process. In general, energy is supplied as heat or mechanical energy, which is in turn derived from electricity generated from power plants. As a general rule, the use of electricity in a mineral carbonation process should be kept to a minimum for a couple of reasons [19]. Firstly, by diverting its electricity output into MC processes, the power plant has to generate more electricity to compensate for the reduced amount that can be sold to household or other industrial consumers. This lowers the profitability of the plant and indirectly leads to increased CO2 output at the source [113]. Secondly, fossil fuel-derived electrical energy has much higher emission factors compared with thermal energy use. The most efficient commercially available turbines level out at around 50–60% efficiency, meaning that every joule of energy supplied as heat from fossil fuel combustion yields at most about half a joule of electrical energy and the rest is lost as waste heat. Additionally, in most countries, electricity is generated and supplied through a grid that links various power plants. These plants have different designs, efficiencies and may even run on different fuel types. As such, the emission factor for electricity use in a particular country is highly dependent on its fuel mix, and the value is usually available from environmental reports prepared by non-governmental sources.
In addition to the type of energy used, the kind of fossil fuel used to generate that energy also plays a major role in determining the value of the emission factors. Common fuels include natural gas, oil, coal, municipal solid waste and biomass, and each has a characteristic and representative emission factor related to the fuel type. Natural gas and biomass have the lowest emission factors; crude oil is somewhere in the middle, and coal and municipal solid waste have the highest CO2 emissions per unit energy supplied. Approximate or indicative values for the emission factors of various fuel types can be found in Table 4.3.
For illustration purposes, let us apply the concepts that we have just discussed to evaluate a process for obtaining activated serpentine for use in mineral carbonation. This typically involves grinding the mineral to 100 microns and heat activation at 700 °C. For convenience we assume that all the energy requirements are supplied entirely by coal. It is also assumed that four tonnes of minerals thusly prepared are needed to sequester one ton of CO2. Finally, we ignore all efficiency factors in the unit operations and assume that these are 100% efficient. The CO2 penalty of this process is therefore:
Based on these estimates, we find that a coal-powered process for mineral grinding and heat activation will incur a CO2 penalty of around 24%. Further downstream unit operations to actually react CO2 with the activated minerals will also add to the CO2 penalty. It can also be seen that the use of cleaner sources of energy will greatly help with lowering the CO2 penalty, making the mineral carbonation process much more efficient and capable of sequestering larger amounts of CO2.
4.3.2 The Economics of Mineral Carbonation
CO2 emissions are a negative externality of industrial activity; this means that their economic impacts are often not properly accounted for in conventional economic analyses [115]. In addition, since carbon capture and storage (CCS) technologies generally do not produce tangible products that can be sold to generate revenue, the financial motivations for capturing and sequestering CO2 are often weak and not influenced by market forces [113]. As such, without laws or taxation schemes that put a price on carbon emissions, it is difficult to determine what an appropriate or acceptable target cost is for CO2 sequestration.
Therefore the economic justifications for implementing CCS technologies are usually context-dependent, and are highly reliant on local government policies for carbon emissions pricing [116, 117]. The development and implementation of CCS technologies will be hampered by negligible carbon taxes, since this may mean that it is cheaper to simply pay the tax than to install and run expensive processes to prevent CO2 emissions. The reverse is also true; a high carbon tax incentivizes industry players to seek out cheaper alternatives to paying a hefty tax on excessive CO2 emissions. It is thus worth bearing in mind that any CCS process must have a CO2 sequestration cost that is less than the local carbon tax/price. Even so, we will see that this is not strictly true in some very special cases. Certain processes that capture and utilize CO2, such as enhanced oil recovery and CO2-to-chemicals will generate a saleable product and can bring economic benefits [118,119,120,121]. As a result, the drive to develop, adopt and implement them is of greater interest for the private sector. Similar to these, mineral carbonation processes can also be relatively independent from government policy on carbon pricing, and we will discuss this later in the chapter.
Since the targeted readers of this chapter are researchers, it is likely that any ideas the audience has in mind are in the relatively early stages of process development. At this stage, it is impossible to obtain precise or reliable costing data, seeing as the information available on hand is likely to be scant anyway. However, this does not mean that the proposed MC process cannot be evaluated properly. To this end, we will discuss some shortcut techniques to quickly estimate the capital expenditure (CapEx) and operating expenditure (OpEx) of MC processes in order to reach a ballpark figure. These techniques can also be generalized and applied to other carbon capture and storage/utilization processes as well.
The unit operations typically encountered in mineral carbonation processes are usually quite traditional; these often include milling, calcination, filtration, gas compression, conventional reactors etc. As such, the optimization, sizing and costing of MC processes are usually quite straightforward. Sizing equations and costing curves that correlate size and equipment costs are widely available, and these are easily found in textbooks or the internet. This aspect will not be discussed in-depth here.
While it is not that difficult to calculate the CapEx of a mineral carbonation process for a fixed scale, repeated calculations for scenarios with different plant capacities can be quickly become tedious. Fortunately, there are shortcut methods to extrapolate the CapEx from one set of data easily, using a lumped parameter approach. One particular method of note is called the six-tenths rule [122,123,124]. The six-tenths rule relates the sizes and costs of two plants at different scales through the following equation:
In the equation, S1 and S2 are the capacities of two similarly configured chemical plants. One of these is the capacity for which a chemical plant has been adequately designed and costed for (S1), while the other is the desired scale for which an estimated cost is to be obtained (S2). On the other side of the equation, C1 and C2 are the costs of the respective plants with the specified capacities. Since S1 is the evaluated and well characterized scenario, C1 is the known variable in the equation. As such, C2 can be obtained through a fairly straightforward calculation. However, there are several things that should be noted while using this equation:
-
The two plants being compared should differ only in scale. Their general designs should not vary too much; otherwise the results are very likely to be invalid.
-
This equation is applicable to within reasonable capacity limits only; it is usually not used to estimate the costs of two plants that are more than two orders of magnitude apart in size.
-
The equation can be used to size and cost individual pieces of equipment, though it is more useful to apply it to entire plants to minimize systematic errors in the results.
-
The value of the exponential factor can be variable. The reason a value of 0.6 is used is that it is a good approximation of the relationship between surface areas to volumes of equipment. When used to size individual pieces of equipment, the exponential can be anything from 0.2 to 0.8; recommended values for these can be found elsewhere [125].
-
There are limits to the maximum sizes of individual unit operations. The process scales cannot be magnified indefinitely, and once the maximum size of a unit operation is reached, an additional unit has to be added in parallel in order to enable further scaling. This clearly will change the process configuration and have a non-negligible impact on the results.
The six-tenths rule can be used to quickly conduct sensitivity analyses on how the CapEx will affect the economics of mineral carbonation, and also to determine the minimum scale of a viable MC process. Researchers familiar with this equation will also note that economies of scale are a major consideration for the implementation of MC technologies, since CapEx increases at a slower rate than the plant scale. This means that the relative contribution of CapEx to the costs of MC is much lower at larger scales, and therefore the unit costs of sequestered CO2 are also reduced.
On the other hand, the operating expenses (OpEx) for a mineral carbonation process can be calculated in a manner similar to the CO2 penalty as described earlier. The OpEx can be calculated by summing up the amounts of energy and materials used, multiplied by their unit costs:
Since the amounts of energy or materials consumed are usually quite static relative to the unit amounts of CO2 processed, OpEx tends to be independent of process scale and can be quite significant compare to CapEx. This is especially true for bulk chemical processing or at larger scales [126]. Furthermore, the unit costs of energy and materials for a MC process are obviously dependent on local conditions, and these will have to be determined via market research.
By applying these techniques, we can thus estimate the approximate unit costs of mineral carbonation. A very rough way to put all these together to estimate the costs of processing one unit of CO2 can be expressed as follows:
The units for each term in the equation above should be noted to be as follows: CapEx, $; Plant Capacity, tonnes/year; Operating Labor, $/year; OpEx, $/tonne CO2 sequestered. The coefficient 1.1 linked to the CapEx term takes into account maintenance, utilities, and insurance etc., while the coefficient 1.7 attached to the operating labor term considers admin, tech support and others. A plant life of 20 years is assumed here, which is similar to or typical for many large scale petrochemical plants. We emphasize again that these numbers are a very approximate guide and may vary depending on local situations.
A cursory analysis of the equation can provide a few insights into the costs of mineral carbonation. Firstly, MC processes can clearly benefit from economies of scale, since we have seen previously from the six-tenths rule that CapEx does not increase linearly with process scale. This is not surprising, as the principle holds true for most bulk chemical manufacturing processes. Secondly, operating labor costs tend to contribute to only a small fraction of the unit costs in modern bulk chemical plants. This is especially true as automation reduces the need for manual labor in many aspects of chemical manufacturing. Under these circumstances, the contribution of operating labor to the unit costs typically does not exceed 5–10% of the total costs. Thirdly, OpEx is usually the main contributor to the costs of bulk chemical manufacturing, since these are usually pegged to each unit of CO2 processed and less related to gains in efficiency through scaling. Therefore, we can conclude that MC process development should focus more on reducing operating costs more than anything else, since these costs are easy targets to quickly achieve major improvements.
4.3.3 Integrated Mineral Carbonation Processes
Even the most well-designed mineral carbonation process is ultimately still dependent on an external and often non-technical factor such as a carbon price. This puts the development of MC processes (and most green technologies in general) at the mercy of policymakers and funding agencies under pressure to deliver tangible outcomes from public spending. Furthermore, in the absence of a profit motive, there is a practically zero chance of attracting the attention of private sector entities and get them to take up the process for commercialization.
With this obstacle in mind, some startup companies have recently begun a trend that seeks to integrate mineral carbonation processes with a mineral upgrading side operation to upgrade residual wastes or carbonation by-products such as silica and transition metal oxides into value-added chemicals for sale [127,128,129,130]. The sale of these products can then finance the larger and more costly MC process, and may even turn a profit to attract investments into the technology. Generally speaking, the side processes to upgrade mineral carbonation residues tend to be rather energy consuming and emit non-negligible amounts of CO2 on their own. However, the coupled bulk carbonation process can help to offset the CO2 emissions from the upgrading process, and result in a net negative amount of emissions. With a proper techno-economic analysis to determine the appropriate scales for both processes, it may be possible to develop a symbiotic integrated process that brings both environmental and financial benefits to society. An illustration of the concept of an integrated mineral carbonation process is given in Fig. 4.12.
The relationship between the bulk carbonation and material upgrading processes has to take into account a few considerations. Firstly, the source of materials subject to upgrading has to be defined clearly. These materials can be the by-products of the bulk carbonation process, or a small quantity of raw or processed minerals diverted from the input at the start. Examples of such materials include magnetic fractions, Mg- or Ca-depleted residue after carbonation, or even crushed minerals straight from the mine.
Secondly, the nature of the material to be upgraded will play a large determining role in the design of the upgrading process, and whether it can generate sufficient revenue to turn a profit for the overall integrated process. Non-homogeneous and complex materials such as natural minerals or incineration ash will usually require complicated designs for the upgrading process, but they are also rich and diverse sources of highly valuable metals such as nickel and cobalt [131,132,133]. Simpler materials such as concrete waste or red mud have fewer components and thus require fewer separation steps, but they often lack the flexibility to adapt to changes in market demands (especially when the primary product sees a huge drop in prices). Generally speaking, a modular process where certain steps can be turned on or off in response to market trends would be most suitable for this task.
Thirdly, the techno-economic aspects of both segments of the integrated process must be congruent and balanced. This would mean that the bulk carbonation process must be sufficiently large in order to be able to sequester enough CO2 to offset the emissions from the upgrading process, yet not so massive that the costs are exorbitant and are difficult to be recovered. Similarly, the upgrading process must be relatively small (or as energy efficient as possible), yet able to generate enough revenue to bring economic benefits to the overall process.
The ubiquitous presence of some oxide species (silica, alumina and ferric oxide) in these materials means that they are common to many material upgrading processes. Depending on the quality and grades, refined versions of these products can be sold for relatively high prices. These are briefly discussed below.
4.3.3.1 Precipitated Silica
Precipitated silica is used in a variety of applications; among the largest ones is its use as a specialty additive into rubber products. Precipitated silica with different characteristics can be added during the manufacturing process to impart various desirable properties to the rubber; these include improvements in mechanical strength, heat resistance, friction control and visual appearance. The market demand for precipitated silica is estimated to be around 1.5–2 million tonnes a year. Although relatively small compared to the global need for CO2 reductions, this is nevertheless a large enough market to accommodate a portion of the value-added products from an integrated process (remember that the material upgrading segment in an integrated process is intended to be a small part in the overall scheme). Depending on the market and location, the price of precipitated silica usually falls in the range of $500–$1000 per ton [134, 135].
4.3.3.2 Pigment Grade Ferric Oxide
Metal oxides can also be recovered from the by-products of the bulk carbonation process. These include the oxides or salts of many transition metals, which are useful as catalysts, pigments and raw materials for metal and alloy production. Iron oxides are likely the most abundant and common species found in the residues of mineral carbonation. Low grade ferric oxide intended for iron or steel production will not command a high price, since there is a lot of competition from mined iron ores. However, pigment grade ferric oxide can be sold for $1300–$1500 per ton, although its market demand is much lower compared to the raw material intended for steel refining. The annual demand for pigment grade Fe2O3 is estimated to be in excess of 2 million tonnes [136].
4.3.3.3 Transition Metal Oxides and Alumina
Other transition metals (and their oxides or salts) that can be refined through a material upgrading process include nickel, chromium, manganese, cobalt, and copper. The relative abundance of these transition metals obviously varies according to the source material, but they are usually commonly found in slags, incineration ash and natural minerals. Needless to say, these are much more valuable products that have much higher selling prices compared to ferric oxide pigments or precipitated silica.
The bulk of alumina produced every year (>90%) goes into the production of aluminum metal; apart from that there are not many applications that involve its direct use in manufacturing processes. As with iron oxide, alumina obtained from mineral carbonation residues may face stiff competition from commercial Bayer process operations and thus may not be cost competitive in the traditional sense. However, there may be opportunities for process innovations that allow for the co-recovery of alumina with embedded or impregnated metal oxides. These composite materials may find use as catalysts in petrochemical plants, and is also possibly easier to produce compared to traditional catalyst syntheses [137].
4.3.3.4 Magnesium and Calcium Carbonates
Depending on the process choice and designs, highly pure magnesium or calcium carbonates can also be obtained from a bulk carbonation process. The sales of these carbonates can help to recoup the costs of mineral carbonation; however it must be noted that the amounts of pure carbonates produced as a result of mineral carbonation will greatly exceed their demand and therefore only a small part of these can be sold. These carbonates have a wide variety of industrial applications, though some of them (for example their use in soil pH adjustments or as refractory materials) will ultimately result in the release of the bound CO2 back into the atmosphere.
The differences between magnesium and calcium carbonates are generally minimal, especially when it comes to their use as fillers or additives in various manufactured goods. Despite this, these carbonates are not strictly interchangeable but dependent on the particular formulation to achieve the desired properties in the final product. As additives, both are used as whiteners in paper manufacturing, as well as fire-retardant fillers in plastics and rubbers. Food grade carbonates can be used in personal care products and cosmetics, as antacids, as well as excipients for pharmaceuticals. Obviously this would be much more challenging to produce (due to likely heavy metal contamination from minerals) to meet the stringent safety requirements in these applications.
However, it should be noted that unlike calcium carbonates, magnesium carbonates can come in a variety of hydrated species such as hydromagnesite (4MgCO3·Mg(OH)2·4H2O), nesquehonite (MgCO3·3H2O), artinite (MgCO3·Mg(OH)2·3H2O) and many other forms. This should be factored into the techno-economic analyses for MC processes, since the mass balances and economic factors can be rather different when the carbonate product is not exactly not MgCO3.
Again, depending on the quality and location, the prices of these carbonates can vary significantly. Food or pharma grade magnesium carbonates can be expected to sell at $2000–$2500 per tonne, while lower grades can fetch prices of around $500–$1000 per tonne [138]. Calcium carbonate is unfortunately much cheaper (due to competition from abundant and easily-mined resources) and is typically sold at less than $300 per tonne [139].
4.4 Synergy Between Mitigation and Adaptation Actions Against Climate Change, via Mineral Carbonation
In this penultimate section, we will discuss some of the big-picture aspects of mineral carbonation. By envisioning a scenario where the technology is implemented on a moderately large scale, we will see why mineral carbonation has the potential to play a massive role in the fight against climate change. To this end, we will study a couple of hypothetical case studies involving the implementation of mineral carbonation technologies in Singapore, an affluent but land scarce island nation in Southeast Asia.
4.4.1 A Brief Overview of Singapore’s Circumstances
Singapore is a prosperous and highly developed country situated at the southern tip of the Malay Peninsula. It is one of the smallest sovereign nations in the world, consisting of one main island and 62 other much smaller islets that fall under its jurisdiction. The country is densely populated, with (at the time of writing) around 5.6 million residents living on approximately 722 km2 of land. Most of the population lives on the main island, though there are small communities that still inhabit the outlying islands. As a result, the main island is highly urbanized and has one of the highest population densities (~7800 p/km2) in the world. Most of the country is less than 5 m above sea level, and a large proportion of the key infrastructure (i.e. the city core, financial district, airport and seaport) lies in areas that are very vulnerable to rising sea levels.
Being extremely land-scarce, the country has continuously sought to increase its habitable land area by reclaiming land from the sea (see Fig. 4.13 for a map of modern Singapore). Since its separation from Malaysia in 1965, the country has reclaimed more than 130 square kilometers of land, first by using its own soil (from excavated hills and tunnel construction projects), and subsequently through imported sand from neighboring countries. More recently, the government has unveiled plans to further expand the total land area to 766 km2 to accommodate a projected increase of an additional 1 million residents by 2030 [140,141,142].

Figure is adapted from Wang et al. [142]
Elevation map of modern Singapore (2012), with blue outlines marking the original coastline as mapped in 1924.
The importation of these materials have led to rising tensions, and as of 2018 export bans on these aggregates are in effect in several countries. However, discrepancies between reported sand imports and the amounts consumed locally are also rather significant, which suggests that sand smuggling is a serious issue. This has also occasionally led to criticism from environmental groups [143,144,145,146].
Being situated on one of the busiest shipping routes that connect China and Europe, Singapore has traditionally been a busy shipping hub as well. It is also one of the most affluent countries, with a GDP per capita of around $98,000. Almost all (95%) of the electricity in Singapore is generated from natural gas-fired power plants; as such the country’s CO2 emissions from this sector are relatively low [147]. Despite having no natural resources of its own, Singapore is nevertheless the world’s third largest crude oil refining center. Most of the refining capacity is located on Jurong Island and Bukom Island, where the country’s chemical manufacturing industries are based. The government has pushed hard for Singapore to become a smart and clean city, and has invested heavily in high tech manufacturing industries. As a result, apart from oil refining and some chemical industries, there are practically no other heavy polluting industries (other than a handful of steel recycling and waste incineration plants) in the country. All these factors mean that Singapore contributes less than 0.1% of the world’s total CO2 emissions. Even so, the country’s per capita CO2 footprint is rather significant (slightly more than 10 tonnes per person per year), which is typical of residents of highly developed and affluent countries [148].
As a land scarce country, Singapore also does not have the luxury of allocating large amounts of precious land area for landfilling purposes. As a result, municipal and industrial waste is extensively recycled here, with overall recycling rates in excess of 60%. The remaining unrecycled waste is sent to four waste-to-energy incineration plants in the country, where it is burnt to generate some electricity and physically converted to incineration bottom ash (IBA) in order to reduce the disposal volumes. Despite the high recycling rates, up to half a million tonnes of IBA is still generated annually from the incineration of the remainder unrecycled solid waste. This IBA is then disposed of in Semakau Island, an artificial offshore island designed for this specific purpose. Semakau Island was built in 1999, and is expected to last until 2035 where it will become fully utilized and a new site will have to be found to handle additional IBA in the future [149].
Singapore is also a signatory of the Paris Agreement, and has pledged to reduce their CO2 emissions intensity (amount of CO2 emitted per unit GDP) by 36% from 2005 levels by the year 2030. To this end, the government has introduced a carbon tax beginning at $5/t CO2, which takes effect in 2019. The tax is planned to increase to around $15 - $20/t CO2 in the near future. The government’s advisory panel on climate change (NCCS) has also proposed a multi-pronged approach that involves the use of renewables such as solar power and improvements in energy efficiency in the housing, commercial and industrial sectors [150,151,152].
4.4.2 A Pilot Scale Mineral Carbonation Plant in Singapore
In the case of refining, power and chemical plants in Singapore, there is likely little room for improvement to reduce their CO2 emissions any further. This is because these plants are relatively modern and already tap on some of the more efficient technologies available during their construction and operation. In addition, these are large scale facilities that are relatively inflexible in changing their configuration or adapting retrofits onto their systems [153].
Mineral carbonation can help to reduce their CO2 emissions by capturing and converting the greenhouse gas into solid inorganic carbonates. The quality and quantity of CO2 emissions are quite diverse across the industries, and the choice of mineral carbonation technology would have to be tailored to the particular scenario in question. However, for discussion purposes we assume for the time being that there is a clear and mature MC process technology that is available and applicable to these varying flue gas streams. With this in mind, we can begin to look at the implementation of MC in Singapore from a high level perspective. A back of the envelope calculation can be very helpful to demonstrate the utility of mineral carbonation under these circumstances.
Let’s suppose that a decision has been made to construct and operate a pilot scale mineral carbonation plant in Singapore that captures and converts one million tonnes of CO2 annually. This amount is equivalent to roughly 1.3% of the total CO2 emissions in Singapore. The logistics of the project would depend a lot on the actual design and efficiency of the MC process, but we can roughly estimate the amount of material flows for this project. As mentioned previously, a good estimation of the overall mass balance would be to assume that four tonnes of minerals are needed to sequester one tonne of CO2 in mineral carbonation. This produces five tonnes of material in total. This also means that four million tonnes of minerals have to be imported every year from overseas, which is a relatively small amount in mining terms (for comparison, the port of Newcastle in Australia alone handles approximately 160 million tonnes of coal every year).
This amount of mixed carbonate products (five million tonnes) has an equivalent volume of around 3 million cubic meters, assuming that their packed density is around 1.6 tonnes per m3. With an average depth of 30 m in the surrounding waters of Singapore, these materials would be sufficient to reclaim 10 hectares of land every year, which is equivalent to the area of 14 football (soccer) fields. Of course, larger scales of mineral carbonation will produce more materials for land reclamation purposes, and using these materials for land reclamation in Singapore would reduce the country’s reliance on contentious foreign imports of sand.
On the other hand, incineration bottom ash can serve as a partial substitute for minerals for MC in Singapore. Although smaller in capacity, incineration bottom ash nevertheless represents a constant and readily available source of alkalinity to sequester CO2 from industrial emitters in Singapore. Based on our previous estimates, we can expect that the 500 kilotonnes of IBA generated every year in Singapore to be sufficient to capture up to around 350 kt CO2 and produce 850 kt of treated solids every year. The toxic metal leaching characteristics of these solids would ideally be inhibited, and this would allow them to be used in various construction industries with minimal risk to the population. This latter criterion is especially crucial in a small island country like Singapore, since any contamination of the soil or water by heavy metals would seriously impact its inhabitants due to the high population density of the country.
In addition to reclaiming land, these solid materials can also be used to construct levees and seawalls, and to raise floodwater-threatened infrastructure to adapt against storm surges and rising sea levels. The implementation of MC in this manner would thus not only mitigate climate change by reducing CO2 emissions, but also adapt against it by using it to defend against its worst effects.
Let’s suppose again that 1% of the total mineral imports were diverted into a material upgrading process to generate some revenue for the overall integrated MC process. This is equivalent to a small plant that processes 40,000 tonnes of minerals every year, which is miniscule compared with typical mining and refining operations. Assuming that the mixed raw materials can be refined into their pure components, we can expect that the upgrading process would produce around 16,000 tonnes of precipitated silica (assuming a price of $1000 per tonne, therefore worth a total of $16 million), 4000 tonnes of pigment grade iron oxide ($1500 per tonne, worth $6 million), and another 16,000 tonnes of MgO ($500 per tonne, worth $8 million). The sales of these products can thus generate up to $30 million dollars in revenue; in other words this will help to reduce the net costs of CCU by $30/t CO2 sequestered in this case. Of course, if the net costs of the integrated process are less than $30/t CO2, then the entire operation will actually be earning a gross profit. An overview of a hypothetical pilot scale mineral carbonation operation and its potential benefits is shown in Fig. 4.14.
It is also possible to upgrade incineration bottom ash in a manner similar to the mineral fraction in an integrated mineral carbonation process. Since IBA is relatively richer in valuable transition metal oxides, the volumes that need to be processed to obtain equivalent amounts of value-added products are often much smaller. However, IBA is also much more complex compared with natural minerals, and it may not be practical to separate and recover each and every element in the ash (at least not without incurring excessive costs).
It is worth bearing in mind that this scenario ignores the higher value transition metal products such as nickel, chromium, cobalt etc. that can be refined from the minerals. Their recovery and sales can generate even more revenue for the overall operations (whether their quantities and recovery costs are worth the effort will remain a debate for another time). It is also worth noting that the amounts of value-added products generated in this scenario are far smaller than the world’s annual market demand for them, meaning that there is almost no risk of market flooding and price destabilization for these products.
It should also be noted that this scenario is not particular to or designed specifically for Singapore’s specific circumstances. Since a majority of the world’s cities are densely populated, energy intensive and situated along major rivers or coasts, they face many of the same problems that affect Singapore. Thus, they will also benefit from the implementation of MC technologies as outlined previously in this section. To take this one step further, collaborative partnerships between large emitters and vulnerable regions threatened by rising water levels can mutually benefit both parties from the dual action of mitigation and adaptation afforded by MC technologies. Of course, these partnerships are not limited to the national level but can also involve international cooperation treaties.
4.5 Conclusions and Outlook
Mineral carbonation technologies can play an important role in our collective actions against climate change. Comparatively speaking, MC has numerous unique strengths that are not often found in other carbon capture and utilization technologies. These include:
-
Scalability: Mineral carbonation can be scaled to match the amounts of CO2 emissions from all anthropogenic activities on the planet. There is, in theory, sufficient alkaline-earth species that can be made available to react with all the CO2 from fossil fuel combustion.
-
Permanence: The solid carbonates formed from mineral carbonation are highly stable and not susceptible to decomposition under ambient conditions. This ensures that the products are permanent sinks to sequester CO2 without fear of re-emission.
-
Favorable thermodynamics: This is related to the second point above. On paper, the carbonation reaction has favorable thermodynamics, as the products are lower in energy than the starting reactants. This raises the possibility of harnessing this energy difference to drive the reactions forward, though this is much more difficult in practice.
-
Economic potential: The raw materials used for mineral carbonation often contain many valuable elements, and they can be refined into saleable products to enhance the overall economics of MC.
-
Synergetic with adaptation actions: The solid carbonate products can contribute to land reclamation and fortify against rising sea levels and storm surges. Proper utilization of these materials enables small island nations or city waterfronts to adapt against the worst effects of climate change.
However, there are still some obstacles that prevent MC from being ready for deployment on a wide scale. The natural reaction rates of mineral carbonation are very slow, and they have to be accelerated to ensure that the technologies are feasible and practical to suit the task of large scale CO2 removal and sequestration. To this end, an energy input is often needed, and these typically involve the use of chemicals, pressure or higher temperatures to enhance the reaction rates. This energy use results in a decrease in process efficiencies, and currently most MC processes still do not appear to meet the energy-use criteria needed to justify their widespread implementation.
While economies of scale can likely help to bring the costs of MC down, there are also financial-related obstacles to its large scale implementation. The recoverable amounts of value-added products from an upgrading process are limited by market forces, meaning that this aspect of the integrated process is not as scalable as bulk carbonation processes in general. Therefore, under these conditions, there are limited avenues for revenue generation from value-added byproducts to supplement the economics of MC. There will be a point where the value-added products will begin to flood the market and affect their selling prices, thus negating the entire point of generating revenue from these byproducts.
Since the idea of mineral carbonation was conceived more than twenty years ago, the field has gradually grown and broadened to include the use of other less conventional materials such as industrial waste or incineration ash as sources of alkalinity to sequester CO2. These materials represent the low hanging fruit from which alkaline calcium or magnesium species can be obtained easily, and thus possibly reduce the energy use associated with their extraction (compared to natural minerals). An added benefit is that carbonation can help to neutralize the effects of toxic heavy metal leaching from these materials as well. Although these schemes are much smaller in scope than conventional mineral carbonation processes (due to the limited amounts of waste available), their success in the short-to-medium term can help to pave the way towards adapting the learnt experience for large scale carbonation technologies using more abundant minerals.
In addition to that, by finding outlets for the utilization of the products from mineral carbonation, the prospects of implementing these technologies on a larger scale can be enhanced. One way to do this would be to engineer the properties of the carbonate and silica products to allow them to meet the requirements for direct utilization in construction applications, thus providing a minor (but nevertheless significant) economic incentive to help offset the costs. This approach enables mineral carbonation to support climate change adaptation efforts as well.
References
Lackner KS (2003) A guide to CO2 sequestration. Science 300(5626):1677–1678
Huijgen WJJ, Comans RNJ (2003) Carbon dioxide sequestration by mineral carbonation. Literature review. Energy research Centre of the Netherlands ECN2003
Zevenhoven R, Eloneva S, Teir S (2006) Chemical fixation of CO2 in carbonates: routes to valuable products and long-term storage. Catal Today 115(1–4):73–79
Geerlings H, Zevenhoven R (2013) CO2 mineralization—bridge between storage and utilization of CO2. Ann Rev Chem Biomol Eng 4(1):103–117
Sanna A, Maroto-Valer MM (2017) CO2 sequestration by ex-situ mineral carbonation
Rendek E, Ducom G, Germain P (2006) Carbon dioxide sequestration in municipal solid waste incinerator (MSWI) bottom ash. J Hazard Mater 128(1):73–79
Wee J-H (2013) A review on carbon dioxide capture and storage technology using coal fly ash. Appl Energy 106:143–151
Mo L, Panesar DK (2013) Accelerated carbonation—a potential approach to sequester CO2 in cement paste containing slag and reactive MgO. Cem Concr Compos 43:69–77
Xi F et al (2016) Substantial global carbon uptake by cement carbonation. Nat Geosci 9:880
Si C, Ma Y, Lin C (2013) Red mud as a carbon sink: variability, affecting factors and environmental significance. J Hazard Mater 244–245:54–59
Kirchofer A, Becker A, Brandt A, Wilcox J (2013) CO2 mitigation potential of mineral carbonation with industrial alkalinity sources in the United States. Environ Sci Technol 47(13):7548–7554
Mcdonough WF, Teisseyre ER, Majewski E (2000) Earthquake thermodynamics and phase transformations in the Earth’s interior
McDonough WF, Sun S-S (1995) The composition of the Earth. Chem Geol 120(3–4):223–253
Aresta M (2010) Carbon dioxide as chemical feedstock. Wiley
Berner RA (2003) The long-term carbon cycle, fossil fuels and atmospheric composition. Nature 426(6964):323
White AF (2003) 5.05—natural weathering rates of silicate minerals A2—Holland, Heinrich D. In: Turekian KK (ed) Treatise on geochemistry, Pergamon, Oxford, pp 133–168
Aresta M, Dibenedetto A, Angelini A (2013) The changing paradigm in CO2 utilization. J CO2 Utilization, 3:65–73
White AF, Brantley SL (2003) The effect of time on the weathering of silicate minerals: why do weathering rates differ in the laboratory and field? Chem Geol 202(3–4):479–506
Yuen YT, Sharratt PN, Jie B (2016) Carbon dioxide mineralization process design and evaluation: concepts, case studies, and considerations. Environ Sci Pollut Res 23(22):22309–22330
Taulis M (2012) GWCarb v1. 0: carbonate speciation tool
Stumm W, Morgan, JJ (2012) Aquatic chemistry: chemical equilibria and rates in natural waters. Wiley
Wanninkhof R et al (2013) Global ocean carbon uptake: magnitude, variability and trends. Biogeosciences 10(3):1983–2000
Zeebe RE, Zachos JC, Caldeira K, Tyrrell T (2008) Carbon emissions and acidification. Science 321(5885):51–52
Orr JC et al (2005) Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms. Nature 437:681
Wen N, Brooker MH (1995) Ammonium carbonate, ammonium bicarbonate, and ammonium carbamate equilibria: a Raman study. The J Phys Chem 99(1):359–368
Zhao Y, Zhu G (2007) Thermal decomposition kinetics and mechanism of magnesium bicarbonate aqueous solution. Hydrometallurgy 89(3–4):217–223
Keener TC, Frazier GC, Davis WT (1985) Thermal decomposition of sodium bicarbonate. Chem Eng Commun 33(1–4):93–105
Casey WH, Banfield JF, Westrich HR, McLaughlin L (1993) What do dissolution experiments tell us about natural weathering? Chem Geol 105(1–3):1–15
Oelkers EH, Gislason SR, Matter J (2008) Mineral carbonation of CO2. Elements 4(5):333–337
Dreybrodt W, Lauckner J, Zaihua L, Svensson U, Buhmann D (1996) The kinetics of the reaction CO2+ H2O→ H++ HCO3− as one of the rate limiting steps for the dissolution of calcite in the system H2O–CO2–CaCO3. Geochim Cosmochim Acta 60(18):3375–3381
Duan Z, Sun R (2003) An improved model calculating CO2 solubility in pure water and aqueous NaCl solutions from 273 to 533 K and from 0 to 2000 bar. Chem Geol 193(3–4):257–271
Cullinane JT, Rochelle GT (2004) Carbon dioxide absorption with aqueous potassium carbonate promoted by piperazine. Chem Eng Sci 59(17):3619–3630
Bishnoi S, Rochelle GT (2000) Absorption of carbon dioxide into aqueous piperazine: reaction kinetics, mass transfer and solubility. Chem Eng Sci 55(22):5531–5543
Ma’mun S, Svendsen HF, Hoff KA, Juliussen O (2007) Selection of new absorbents for carbon dioxide capture. Energy Convers Manag 48(1):251–258
Astarita G, Savage DW, Longo JM (1981) Promotion of CO2 mass transfer in carbonate solutions. Chem Eng Sci 36(3):581–588
Xie Z, Walther JV (1994) Dissolution stoichiometry and adsorption of alkali and alkaline earth elements to the acid-reacted wollastonite surface at 25 C. Geochim Cosmochim Acta 58(12):2587–2598
Brantley, SL (2008) Kinetics of mineral dissolution. Kinetics of water-rock interaction. Springer, pp 151–210
Teir S, Revitzer H, Eloneva S, Fogelholm C-J, Zevenhoven R (2007) Dissolution of natural serpentinite in mineral and organic acids. Int J Miner Process 83(1–2):36–46
Apostolidis C, Distin P (1978) The kinetics of the sulphuric acid leaching of nickel and magnesium from reduction roasted serpentine. Hydrometallurgy 3(2):181–196
Sanna A, Uibu M, Caramanna G, Kuusik R, Maroto-Valer M (2014) A review of mineral carbonation technologies to sequester CO2. Chem Soc Rev 43(23):8049–8080
Hemmati A, Shayegan J, Bu J, Yeo TY, Sharratt P (2014) Process optimization for mineral carbonation in aqueous phase. Int J Miner Process 130:20–27
Hemmati A, Shayegan J, Sharratt P, Yeo TY, Bu J (2014) Solid products characterization in a multi-step mineralization process. Chem Eng J 252:210–219
Zevenhoven R, Slotte M, Koivisto E, Erlund R (2017) Serpentinite carbonation process routes using ammonium sulfate and integration in industry. Energy Technol 5(6):945–954
Sanna A, Steel L, Maroto-Valer MM (2017) Carbon dioxide sequestration using NaHSO4 and NaOH: a dissolution and carbonation optimisation study. J Environ Manage 189:84–97
Gerdemann SJ, O’Connor WK, Dahlin DC, Penner LR, Rush H (2007) Ex situ aqueous mineral carbonation. Environ Sci Technol 41(7):2587–2593
Ghacham AB, Cecchi E, Pasquier L-C, Blais J-F, Mercier G (2015) CO2 sequestration using waste concrete and anorthosite tailings by direct mineral carbonation in gas–solid–liquid and gas–solid routes. J Environ Manage 163:70–77
Smith JM (1950) Introduction to chemical engineering thermodynamics. ACS Publications
Lackner KS, Wendt CH, Butt DP, Joyce EL Jr, Sharp DH (1995) Carbon dioxide disposal in carbonate minerals. Energy 20(11):1153–1170
Zevenhoven R, Kavaliauskaite I (2010) Mineral carbonation for long-term CO2 storage: an exergy analysis. Int J Thermodyn 7(1):23–31
Huijgen WJ, Ruijg GJ, Comans RN, Witkamp G-J (2006) Energy consumption and net CO2 sequestration of aqueous mineral carbonation. Ind Eng Chem Res 45(26):9184–9194
Goff F, Lackner K (1998) Carbon dioxide sequestering using ultramafic rocks. Environ Geosci 5(3):89–101
Alexander E, Wildman W, Lynn W (1985) Ultramafic (serpentinitic) mineralogy class 1. Mineral classification of soils, no. mineralclassifi, pp 135–146
Matter JM, Kelemen PB (2009) Permanent storage of carbon dioxide in geological reservoirs by mineral carbonation. Nat Geosci 2(12):837
Moody JB (1976) Serpentinization: a review. Lithos 9(2):125–138
Rinaudo C, Gastaldi D, Belluso E (2003) Characterization of chrysotile, antigorite and lizardite by FT-Raman spectroscopy. The Can Mineral 41(4):883–890
Groppo C, Rinaudo C, Cairo S, Gastaldi D, Compagnoni R (2006) Micro-Raman spectroscopy for a quick and reliable identification of serpentine minerals from ultramafics. Eur J Mineral 18(3):319–329
Evans BW (2004) The serpentinite multisystem revisited: chrysotile is metastable. Int Geol Rev 46(6):479–506
Lacinska AM et al (2016) Acid-dissolution of antigorite, chrysotile and lizardite for ex situ carbon capture and storage by mineralisation. Chem Geol 437:153–169
Farhang F, Rayson M, Brent G, Hodgins T, Stockenhuber M, Kennedy E (2017) Insights into the dissolution kinetics of thermally activated serpentine for CO2 sequestration. Chem Eng J 330:1174–1186
Benhelal E et al (2018) Study on mineral carbonation of heat activated lizardite at pilot and laboratory scale. J CO2 Utilization 26:230–238
Rashid MI et al (2018) ACEME: direct aqueous mineral carbonation of dunite rock. Environ Prog Sustain Energy
Dlugogorski BZ, Balucan RD (2014) Dehydroxylation of serpentine minerals: Implications for mineral carbonation. Renew Sustain Energy Rev 31:353–367
Yadav VS, Prasad M, Khan J, Amritphale S, Singh M, Raju C (2010) Sequestration of carbon dioxide (CO2) using red mud. J Hazard Mater 176(1–3):1044–1050
Bonenfant D et al (2008) CO2 sequestration by aqueous red mud carbonation at ambient pressure and temperature. Ind Eng Chem Res 47(20):7617–7622
Huijgen WJ, Witkamp G-J, Comans RN (2005) Mineral CO2 sequestration by steel slag carbonation. Environ Sci Technol 39(24):9676–9682
Santos RM, Van Bouwel J, Vandevelde E, Mertens G, Elsen J, Van Gerven T (2013) Accelerated mineral carbonation of stainless steel slags for CO2 storage and waste valorization: effect of process parameters on geochemical properties. Int J Greenhouse Gas Control 17:32–45
Dri M, Sanna A, Maroto-Valer MM (2013) Dissolution of steel slag and recycled concrete aggregate in ammonium bisulphate for CO2 mineral carbonation. Fuel Process Technol 113:114–122
Meima JA, van der Weijden RD, Eighmy TT, Comans RN (2002) Carbonation processes in municipal solid waste incinerator bottom ash and their effect on the leaching of copper and molybdenum. Appl Geochem 17(12):1503–1513
Lin WY, Heng KS, Sun X, Wang J-Y (2015) Accelerated carbonation of different size fractions of MSW IBA and the effect on leaching. Waste Manag 41:75–84
Lin WY, Heng KS, Sun X, Wang J-Y (2015) Influence of moisture content and temperature on degree of carbonation and the effect on Cu and Cr leaching from incineration bottom ash. Waste Manag 43:264–272
Pan S-Y, Chang E, Chiang P-C (2012) CO2 capture by accelerated carbonation of alkaline wastes: a review on its principles and applications. Aerosol Air Qual Res 12(5):770–791
Bobicki ER, Liu Q, Xu Z, Zeng H (2012) Carbon capture and storage using alkaline industrial wastes. Prog Energy Combust Sci 38(2):302–320
Paramguru R, Rath P, Misra V (2004) Trends in red mud utilization–a review. Mineral Process Extr Metall Rev 26(1):1–29
Shi C (2004) Steel slag—its production, processing, characteristics, and cementitious properties. J Mater Civ Eng 16(3):230–236
Papadakis VG, Vayenas CG, Fardis MN (1991) Fundamental modeling and experimental investigation of concrete carbonation. Mater J 88(4):363–373
Meima JA, Comans RN (1999) The leaching of trace elements from municipal solid waste incinerator bottom ash at different stages of weathering. Appl Geochem 14(2):159–171
Baciocchi R et al (2010) Accelerated carbonation of different size fractions of bottom ash from RDF incineration. Waste Manag 30(7):1310–1317
Li X, Bertos MF, Hills CD, Carey PJ, Simon S (2007) Accelerated carbonation of municipal solid waste incineration fly ashes. Waste Manag 27(9):1200–1206
Olajire AA (2013) A review of mineral carbonation technology in sequestration of CO2. J Petrol Sci Eng 109:364–392
O’Connor W, Dahlin D, Rush, G, Gerdemann, S, Penner, L, Nilsen D (2005) Aqueous mineral carbonation. Albany Research Center: Albany, OR
O’Connor WK, Dahlin DC, Rush G, Gerdemann SJ, Penner L (2004) Energy and economic considerations for ex-situ and aqueous mineral carbonation. Albany Research Center (ARC), Albany, OR
O’Connor WK, Dahlin DC, Rush G, Dahlin CL, Collins WK (2001) Carbon dioxide sequestration by direct mineral carbonation: process mineralogy of feed and products. Albany Research Center (ARC), Albany, OR
O’Connor WK, Dahlin DC, Nilsen DN, Walters RP, Turner PC (2000) Carbon dioxide sequestration by direct aqueous mineral carbonation. Albany Research Center (ARC), Albany, OR
Geerlings JJC, Wesker E (2010) Process for sequestration of carbon dioxide by mineral carbonation. Google Patents
Geerlings JJC, Van Mossel GAF, Veen BCMIT (2010) Process for sequestration of carbon dioxide. Google Patents
Werner M, Hariharan S, Mazzotti M (2014) Flue gas CO2 mineralization using thermally activated serpentine: from single-to double-step carbonation. Phys Chem Chemcal Phys 16(45):24978–24993
Sun Y, Yao M-S, Zhang J-P, Yang G (2011) Indirect CO2 mineral sequestration by steelmaking slag with NH4Cl as leaching solution. Chem Eng J 173(2):437–445
Bai P, Sharratt P, Yeo TY, Bu J (2011) Production of nanostructured magnesium carbonates from serpentine: implication for flame retardant application. J Nanoeng Nanomanuf 1(3):272–279
Bu J, Yeo TY, Sharratt P (2018) Method of producing metal carbonate from an ultramafic rock material. Google Patents
Alexander G, Maroto-Valer MM, Gafarova-Aksoy P (2007) Evaluation of reaction variables in the dissolution of serpentine for mineral carbonation. Fuel 86(1–2):273–281
Wang X, Maroto-Valer MM (2011) Dissolution of serpentine using recyclable ammonium salts for CO2 mineral carbonation. Fuel 90(3):1229–1237
Littau KA, Torres FE (2013) System and method for recovery of CO2 by aqueous carbonate flue gas capture and high efficiency bipolar membrane electrodialysis. Google Patents
Shuto D et al (2015) CO2 fixation process with waste cement powder via regeneration of alkali and acid by electrodialysis: effect of operation conditions. Ind Eng Chem Res 54(25):6569–6577
Van der Zee S, Zeman F (2016) Production of carbon negative precipitated calcium carbonate from waste concrete. The Can J Chem Eng 94(11):2153–2159
Naraharisetti PK, Yeo TY, Bu J (2019) New classification of CO2 mineralization processes and economic evaluation. Renew Sustain Energy Rev 99:220–233
Liu Q, Maroto-Valer MM, Sanna A (2017) Mineral carbonation technology overview. CO2 sequestration by ex-situ mineral carbonation: World Scientific, pp 1–15
Fagerlund J, Nduagu E, Romão I, Zevenhoven R (2012) CO2 fixation using magnesium silicate minerals part 1: process description and performance. Energy 41(1):184–191
Romão I, Nduagu E, Fagerlund J, Gando-Ferreira LM, Zevenhoven R (2012) CO2 fixation using magnesium silicate minerals. Part 2: energy efficiency and integration with iron-and steelmaking. Energy 41(1):203–211
Highfield J, Lim H, Fagerlund J, Zevenhoven R (2012) Activation of serpentine for CO2 mineralization by flux extraction of soluble magnesium salts using ammonium sulfate. RSC Adv 2(16):6535–6541
Mirjafari P, Asghari K, Mahinpey N (2007) Investigating the application of enzyme carbonic anhydrase for CO2 sequestration purposes. Ind Eng Chem Res 46(3):921–926
Power IM, Harrison AL, Dipple GM, Southam G (2013) Carbon sequestration via carbonic anhydrase facilitated magnesium carbonate precipitation. Int J Greenhouse Gas Control 16:145–155
Jo BH, Kim IG, Seo JH, Kang DG, Cha HJ (2013) Engineered Escherichia coli with periplasmic carbonic anhydrase as a biocatalyst for CO2 sequestration. Appl Environ Microbiol pp AEM. 02400-13
Power IM, Harrison AL, Dipple GM (2016) Accelerating mineral carbonation using carbonic anhydrase. Environ Sci Technol 50(5):2610–2618
Seo S, Perez GA, Tewari K, Comas X, Kim M (2018) Catalytic activity of nickel nanoparticles stabilized by adsorbing polymers for enhanced carbon sequestration. Sci Rep 8(1):11786
Ramsden JJ, Sokolov IJ, Malik DJ (2018) Questioning the catalytic effect of Ni nanoparticles on CO2 hydration and the very need of such catalysis for CO2 capture by mineralization from aqueous solution. Chem Eng Sci 175:162–167
Hu G, Xiao Z, Smith K, Kentish S, Stevens G, Connal LA (2018) A carbonic anhydrase inspired temperature responsive polymer based catalyst for accelerating carbon capture. Chem Eng J 332:556–562
Zevenhoven R, Virtanen M (2017) CO2 mineral sequestration integrated with water-gas shift reaction. Energy 141:2484–2489
Chein R-Y, Yu C-T (2017) Thermodynamic equilibrium analysis of water-gas shift reaction using syngases-effect of CO2 and H2S contents. Energy 141:1004–1018
Naraharisetti PK, Yeo TY, Bu J (2017) Factors influencing CO2 and energy penalties of CO2 mineralization processes. ChemPhysChem 18(22):3189–3202
UEPA (2014) Emission factors for greenhouse gas inventories. Stationary combustion emission factors. US Environmental Protection Agency
Balucan RD, Dlugogorski BZ, Kennedy EM, Belova IV, Murch GE (2013) Energy cost of heat activating serpentinites for CO2 storage by mineralisation. Int J Greenhouse Gas Control 17:225–239
Perry JH (1950) Chemical engineers’ handbook. ACS Publications
David J, Herzog H The cost of carbon capture. In: Fifth international conference on greenhouse gas control technologies, Cairns, Australia, 2000, pp 13–16
USEPA (1 November 2018) GHG emissions factors hub. Available: https://www.epa.gov/sites/production/files/2018-03/documents/emission-factors_mar_2018_0.pdf
Rezai A, Foley DK, Taylor L (2012) Global warming and economic externalities. Econ Theor 49(2):329–351
Wang Q, Chen X (2015) Energy policies for managing China’s carbon emission. Renew Sustain Energy Rev 50:470–479
Pezzey JC, Jotzo F, Quiggin J (2008) Fiddling while carbon burns: why climate policy needs pervasive emission pricing as well as technology promotion. Aust J Agric Resour Econ 52(1):97–110
Damen K, Faaij A, van Bergen F, Gale J, Lysen E (2005) Identification of early opportunities for CO2 sequestration—worldwide screening for CO2-EOR and CO2-ECBM projects. Energy 30(10):1931–1952
Mendelevitch R (2014) The role of CO2-EOR for the development of a CCTS infrastructure in the North Sea Region: a techno-economic model and applications. Int J Greenhouse Gas Control 20:132–159
Aresta M, Dibenedetto A, Angelini A (2013) Catalysis for the valorization of exhaust carbon: from CO2 to chemicals, materials, and fuels. Technological use of CO2. Chem Rev 114(3):1709–1742
Song C (2006) Global challenges and strategies for control, conversion and utilization of CO2 for sustainable development involving energy, catalysis, adsorption and chemical processing. Catal Today 115(1–4):2–32
Sinnott R (1999) Coulson & Richardson’s chemical enginering: volume 6/chemical engineering design. Elsevier Butterworth Heinemann
Lieberman MB (1984) The learning curve and pricing in the chemical processing industries. Rand J Econ 15(2):213–228
Tribe M, Alpine R (1986) Scale economies and the “0.6 rule”. Eng Costs Prod Econ 10(1):271–278
Whitesides RW (2005) Process equipment cost estimating by ratio and proportion. Course notes, PDH Course G, vol 127
Anderson J (2009) Determining manufacturing costs. CEP, pp 27–31
Liu W et al (2018) Optimising the recovery of high-value-added ammonium alum during mineral carbonation of blast furnace slag. J Alloys Compd
Pasquier L-C, Kemache N, Mocellin J, Blais J-F, Mercier G (2018) Waste concrete valorization; aggregates and mineral carbonation feedstock production. Geosciences 8(9):342
Chiang P-C, Pan S-Y (2017) Aggregates and high value products. Carbon dioxide mineralization and utilization. Springer, pp 327–334
Yeo TY, Bu J (2017) MC process scale and product applications. CO2 sequestration by ex-situ mineral carbonation: World Scientific, pp 133–165
Di Maria F, Micale C, Sordi A, Cirulli G, Marionni M (2013) Urban mining: quality and quantity of recyclable and recoverable material mechanically and physically extractable from residual waste. Waste Manag 33(12):2594–2599
Tunsu C, Petranikova M, Gergorić M, Ekberg C, Retegan T (2015) Reclaiming rare earth elements from end-of-life products: a review of the perspectives for urban mining using hydrometallurgical unit operations. Hydrometallurgy 156:239–258
Cossu R, Salieri V, Bisinella, V (2012) Urban mining: a global cycle approach to resource recovery from solid waste. CISA Publication
USGS (1 November 2018) 2015 Minerals Yearbook (Silica). Available: https://minerals.usgs.gov/minerals/pubs/commodity/silica/myb1-2015-silic.pdf
Bai P, Sharratt P, Yeo TY, Bu J (2014) A facile route to preparation of high purity nanoporous silica from acid-leached residue of serpentine. J Nanosci Nanotechnol 14(9):6915–6922
USGS 2015 Minerals Yearbook (Iron Oxide Pigments). Available: https://minerals.usgs.gov/minerals/pubs/commodity//iron_oxide/myb1-2015-feoxi.pdf
Ashok J, Das S, Yeo T, Dewangan N, Kawi S (2018) Incinerator bottom ash derived from municipal solid waste as a potential catalytic support for biomass tar reforming. Waste Manag 82:249–257
USGS (1 November 2018) 2015 Minerals yearbook (magnesium compounds). Available: https://minerals.usgs.gov/minerals/pubs/commodity/magnesium/myb1-2015-mgcom.pdf
USGS (1 November 2018) 2015 Minerals yearbook (lime). Available: https://minerals.usgs.gov/minerals/pubs/commodity/lime/myb1-2015-lime.pdf
Khoo HH et al (2011) Carbon capture and mineralization in Singapore: preliminary environmental impacts and costs via LCA. Ind Eng Chem Res 50(19):11350–11357
Lai S, Loke LH, Hilton MJ, Bouma TJ, Todd PA (2015) The effects of urbanisation on coastal habitats and the potential for ecological engineering: a Singapore case study. Ocean Coast Manag 103:78–85
Wang T, Belle I, Hassler U (2015) Modelling of Singapore’s topographic transformation based on DEMs. Geomorphology 231:367–375
Kog Y-C (2006) Environmental management and conflict in Southeast Asia–Land reclamation and its political impact
Franke M (2014) When one country’s land gain is another country’s land loss…: the social, ecological and economic dimensions of sand extraction in the context of world-systems analysis exemplified by Singapore’s sand imports. Working Paper, Institute for International Political Economy Berlin
Torres A, Brandt J, Lear K, Liu J (2017) A looming tragedy of the sand commons. Science 357(6355):970–971
Gavriletea M (2017) Environmental impacts of sand exploitation. Analysis of sand market. Sustainability 9(7):1118
Finenko A, Cheah L (2016) Temporal CO2 emissions associated with electricity generation: Case study of Singapore. Energy Policy 93:70–79
Lean HH, Smyth R (2010) CO2 emissions, electricity consumption and output in ASEAN. Appl Energy 87(6):1858–1864
Chan JKH (2016) The ethics of working with wicked urban waste problems: the case of Singapore’s Semakau Landfill. Lands Urban Plan 154:123–131
Deng Y, Li Z, Quigley JM (2012) Economic returns to energy-efficient investments in the housing market: evidence from Singapore. Reg Sci Urban Econ 42(3):506–515
Chung W, Hui Y, Lam YM (2006) Benchmarking the energy efficiency of commercial buildings. Appl Energy 83(1):1–14
Kannan R, Leong K, Osman R, Ho H (2007) Life cycle energy, emissions and cost inventory of power generation technologies in Singapore. Renew Sustain Energy Rev 11(4):702–715
Allcott H, Greenstone M (2012) Is there an energy efficiency gap? J Econ Perspect 26(1):3–28
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Yeo, T.Y., Bu, J. (2019). Mineral Carbonation for Carbon Capture and Utilization. In: Aresta, M., Karimi, I., Kawi, S. (eds) An Economy Based on Carbon Dioxide and Water. Springer, Cham. https://doi.org/10.1007/978-3-030-15868-2_4
Download citation
DOI: https://doi.org/10.1007/978-3-030-15868-2_4
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-15867-5
Online ISBN: 978-3-030-15868-2
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)