Abstract
When we eat or drink, separate sensory systems carry taste, smell, irritation, and texture signals to the brain, where these signals are packaged into a composite flavor sensation. Each sensory system has specialized receptors that respond to a specific stimuli that can be chemical (taste, odor, irritant) or mechanical (texture) in nature. Variability in these sensory inputs can arise from genetics, environmental exposure, diseases, and aging. This variability influences the separate sensory inputs and composite flavor sensations with downstream implications for what we like and chose to eat, such as the quality of the eating experience, and our overall health. In some cases, sensory inputs can be altered or distorted (e.g., phantom sensations). Simple standardized measures are available for screening, such as in-depth assessment of separate sensory systems and integrated flavor sensations.
Access provided by Autonomous University of Puebla. Download reference work entry PDF
Similar content being viewed by others
Introduction
Every time we eat or drink, we experience the flavors evoked by foods and beverages. While our attention to food flavors varies with the eating context (e.g., savoring a gourmet dinner vs. eating a donut on the run), these percepts arise from the integration of multiple sensory inputs from anatomically and physiologically distinct systems. In turn, our affective and hedonic responses to these perceptual events can drive eating behavior (see Hayes chapter, Influence of Sensation and Liking on Eating and Drinking, in this handbook). This chapter provides a general overview on the chemical senses – smell, taste, and chemical touch (i.e., olfaction, gustation, and chemesthesis) – as well as oral somatosensation. While discussions of flavor are classically centered on the chemical senses, it is important to note that mouthfeel, texture, temperature, and astringency are also critical to the eating experience and are perceived via somatosensory inputs. Thus, some researchers include overall texture as part of flavor, while others may treat it operationally as a related but separate phenomenon (see Delwiche 2003). Also, it is important to keep in mind that even pure chemicals are not pure stimuli in terms of the sensations they give rise to, for example, concentrated table salt can elicit both salty taste and oral burn, while menthol can evoke cooling sensations and a minty odor.
This chapter is one of the three related chapters that explore connections between chemosensory biology, flavor perception, affective responses, food choices, and dietary behavior, including individual differences, and chemosensory dysfunction. Here, the biological foundations of chemosensation and flavor perception and assessment of their function are reviewed.
Mechanisms of Olfaction, Gustation, Chemesthesis, and Oral Somatosensation
We smell food odors through the nostril as we breathe or sniff (orthonasal olfaction) as well as through the mouth as we chew and manipulate substances in the mouth (retronasal olfaction) . With a functional sense of smell and some prior experience, one can detect subtle differences in wine varieties, recognize a specific off note in a dairy product, or identify the flavor of a gourmet jelly bean solely with the olfactory cues. This process starts when volatile chemicals reach olfactory sensory neurons located in the olfactory epithelium at the top of the nasal cavity. The olfactory sensory neurons have long hair-like extensions (i.e., cilia) to increase their surface area. Specialized receptors are expressed on these cilia. These specialized receptors are one type of G-protein-coupled receptors (GPCRs) that are a part of a larger superfamily of membrane proteins. In 2004, Linda Buck and Richard Axel received the Nobel Prize in Physiology for their work on the genetic basis of olfactory receptors and the pattern recognition system across cells (Buck and Axel 1991). In humans, there are ~400 different olfactory receptor (OR) genes (Malnic et al. 2004), and each of these is an uninterrupted region 1000 bases (1 kb) long. The number of OR genes in other species is even larger, as a substantial number of the potential OR genes in humans are nonfunctional pseudogenes, possibly due to the relaxation of selective pressure concurrent with the evolution of color vision. By some estimates, the OR gene family may comprise 3% of the entire human genome (Olender et al. 2008).
In humans, the OR genes have numerous common variants (polymorphisms) (Mainland et al. 2014), leading to a potentially unique olfactory repertoire for each individual (Behrens et al. 2018). In one study that examined 356 OR genes believed to be functional (selected from a total of 851 different locations in the human genome), an average of 273 were expressed in the olfactory epithelium, and of these, only 90 were found in all participants (Verbeurgt et al. 2014). Each olfactory sensory neuron is believed to express a single olfactory receptor (Buck 2005), and the olfactory sensory neurons that express a specific OR are spatially distributed across the olfactory epithelium. All the olfactory sensory neurons that express a specific OR then project via the olfactory nerve (cranial nerve 1) to a common glomerulus in the olfactory bulb. Thus, a single OR is finely tuned to a specific motif on a ligand, and the aggregate pattern of activation across the glomeruli encodes the sensation that gives rise to a specific percept.
The considerable number of receptors, coupled with this combinatorial code across the glomeruli, implies that humans can respond to an extremely diverse range of potential odorants. To be odor active, chemical stimuli must be of low molecular weight, volatile, and hydrophobic and be able to bind to GPCR ORs. Common flavors may be comprised of tens or even hundreds of different volatile compounds. For example, one recent estimate suggests that strawberry aroma/flavor contains 360 different volatile compounds (Yan et al. 2018). Historical attempts to systematically determine relationships between chemical structure and perception were further complicated by factors like chirality (i.e., molecular handedness). For example, two otherwise chemically identical stimuli (D- and L-isomers of carvone) have completely different percepts: the D-isomer smells like caraway, while the L-isomer smells like spearmint (Pickenhagan 1989).
Just as smell occurs when a volatile chemical (an odorant) reaches and activates a specialized receptor in the nose, the same general process occurs when a soluble chemical (a tastant) reaches an activates specialized receptors in the mouth. However, unlike smell, these receptors are found on specialized epithelia cells, not true neurons. These taste receptor cells (TRCs) are bundled in small grape-like clusters called taste buds that are found in papillae on the tongue or on other oral surfaces including the roof of the mouth and the throat. The taste buds contain 50–150 cells that form a discrete ovoid structure. These cells are divided into basal cells (from which new taste cells originate) and three types of elongated bipolar cells (dark, intermediate, and light), which have microvilli that extend through a taste pore into the oral environment. The microvilli contain the taste receptors. To reach the microvilli, tastants dissolve in saliva and a mucus layer for transport to taste receptors (individuals with diminished salivary production can show impaired taste perception). Taste receptors for sweet, bitter, and umami/savory stimuli occur via GPCRs, while sour and salty tastes occur via ion channels. In contrast to the GPCRs encoded by the OR genes, there far fewer taste receptor (TAS) genes: in humans, the TAS1 family has 3 members, while the TAS2 family has 25. The T1R proteins (receptors) encoded by the TAS1 genes form heterodimers to transduce sweet (T1R2/T1R3) and savory (T1R1/T1R3) stimuli. The T2R proteins encoded by the TAS2 genes provide the ability to detect a wide range of structurally diverse chemicals that humans describe as bitter (Behrens et al. 2018). Notably, the TAS2R receptor genes contain polymorphisms that alter receptor functioning and explain individual differences in bitter perception (e.g., Hayes et al. 2011). Genetic variation in bitter taste receptors translates into differences in ability to taste bitters in the diet, dietary behaviors toward food/beverages with these bitter compounds, and diet-related diseases associated with ingesting these foods/beverages (Tepper et al. 2014). Despite having a single heterodimeric protein, the sweet receptor appears to have multiple binding sites, which allows it to respond to diverse chemicals of varying size and shape (DuBois 2016; Reyes et al. 2019). The bitter taste receptors may also show similar complexity (Fierro et al. 2019). The ion channels responsible for sour and salty taste are less understood, although some candidates have been identified recently. For sourness, the OTOP1 proton-selective ion channel appears to be involved in sour taste transduction, at least in mice (Teng et al. 2019). Regarding saltiness, lower concentrations of salt are sensed in mice via amiloride-sensitive epithelial sodium channels (ENaCs) (Vandenbeuch et al. 2008); however, other amiloride-insensitive channels also play a role in salt perception (Roebber et al. 2019). Additional work is needed to determine if these mechanisms apply in humans.
Once a taste receptor is activated, the taste receptor cell needs to transmit this signal to a neuron to carry the signal to the central nervous system. The chorda tympani branch of cranial nerve VII (CN VII) innervates fungiform papilla on the tongue tip, while the glossopharyngeal nerve (cranial nerve IX; CN IX) innervates the circumvallate and foliate papillae on the posterior side of the tongue, respectively; the superior laryngeal branch of the vagus nerve (cranial nerve X; CN X) carries taste signals from the throat (Snyder and Bartoshuk 2016). Humans show large variation in both the number of fungiform papillae and the number of taste buds located within these papillae (Miller and Reedy 1990). These sources of variation may to relate to sensory abilities: some reports suggest greater numbers result in great taste intensity although other studies fail to find this effect. Notably, the peripheral taste system is highly redundant: unlike olfaction, damage to a single nerve does not result in total loss of function, emphasizing the evolutionary importance of taste function as a gatekeeper of ingestive behavior. All three of the cranial nerves mentioned above project to the nucleus of the solitary tract (NST). This region also receives information from the somatosensory and the olfactory systems. From the NST, signals travel to the ventrobasal thalamus and then to the taste cortex, orbitofrontal cortex, amygdala, and lateral hypothalamus.
The third chemosensory system that contributes to flavor perception is chemesthesis (i.e., chemical touch). Sometimes referred to as oral irritation or the trigeminal sense, the term chemesthesis was coined in 1990 to describe the panoply of sensations that arise from chemical stimuli that activate the somatosensory system (Green 2016). Chemesthesis includes thermal sensations like the burn from capsaicin in chilies or the cooling from menthol but also mechanical sensations like the buzzing from Sichuan buttons (hao jiao). For the mouth and nose, these signals are largely carried by the trigeminal nerve (cranial nerve V; CN V) but also the glossopharyngeal nerve (cranial nerve IX; CN IX). In the nasal cavity, trigeminal receptors occur throughout, with the highest density toward the posterior nasal region (Poletti et al. 2019). The practical distinction between true smell and nasal irritation is blurred somewhat as many putative odorants also stimulate trigeminal receptors (Filiou et al. 2015). Still, the careful distinction between chemesthesis and oral somatosensation depends not on the anatomy, as the same nerves are generally involved, but rather on the nature of the stimulus (i.e., chemical vs. physical/mechanical). Thus, activation of the polymodal TRPV1 (transient receptor potential vanilloid 1) receptor on trigeminal neurons gives rise to hot painful burning sensations, regardless of whether the stimulus is the capsaicin in a habanero-laced salsa or a scalding hot cup of tea. Indeed, one study suggests a 4.9 uM capsaicin solution generates the same burning sensations as 52C water (Kapaun and Dando 2017). Likewise, the buzzing sensation from Sichuan buttons matches a vibrational frequency of 50 hz (Hagura et al. 2013).
Oral touch also plays a key role in the perception of foods and drinks, as somatosensory mechanoreceptors mediate sensations like grittiness (i.e., particle size), mouthfeel, and creaminess. Fat moving across the tongue during eating or licking stimulates trigeminal nerve fibers within fungiform papillae to provide sensations that are perceived as creamy or oily (Prutkin et al. 2000). Tactile acuity on the tongue is at least as good, if not better, than on the fingertip (Miles et al. 2018), and this leads to the ability to detect exceedingly small differences in particle sizes within foods, on the order of a few microns (Breen et al. 2019). The structures in the oral cavity that detect mechanosensation are similar to those found in hairless skin; like the fingers, the oral cavity contains Merkel’s disk receptors (edge and point detection) and Ruffini endings (stretch), as well as Meissner corpuscles (pressure and flutter). However, it is not clear if the mouth contains Pacinian corpuscles (pressure and vibration).
Astringency has classically been considered a chemically initiated tactile event (Breslin et al. 1993) – that is, it is assumed drying and roughing sensations are purely mechanical phenomena caused by increased friction following delubrication. Saliva contains multiple proteins that lubricate the oral cavity, and when acids or polyphenols react with and precipitate these proteins, lubrication is lost, and the mouth feels dry and rough (see Bajec and Pickering 2008). Indeed, those who are less able to replenish their salivary proteins report more astringency from tannic acid (Fleming et al. 2016). However, recent data also suggest some astringent stimuli may also activate specifically tuned chemoreceptors (Schobel et al. 2014), which would explain how high-fat foods like chocolate can still trigger dry and rough sensations, despite lubricity from the fat (Fleming et al. 2016). If confirmed, these findings would suggest astringency may be, at least in part, chemesthetic in nature, and not merely a simple mechanical consequence of delubrication.
Integration of Olfaction, Gustation, Chemesthesis, and Oral Somatosensation
Separate anatomical systems carry smell, taste, chemesthesis, and oral somatosensation from the periphery to the brain for packing or sensory integration with visual sensory input into a composite flavor sensation in the insular cortex (Gogolla 2017) and the orbitofrontal cortex (Rolls 2015). Eating an ice cream cone exemplifies the integration of physiologically distinct peripheral messages (Green 1984). When licking a scoop of coffee Oreo ice cream, sugar binds to the TAS1R2/R3 dimer and depolarizes the cell. The chorda tympani nerve (CN VII) carries the sweet signal centrally, while the trigeminal nerve carries information on coolness, creaminess, and texture of the ice cream and added cookies. As the ice cream is moved back through the mouth, more taste receptors are stimulated with recruitment of branches of cranial nerves IX and X, and mechanical action releases and pumps volatile odorants to the olfactory epithelium, where they bind to olfactory receptors, activating a specific pattern of glomeruli that we perceive as coffee and Oreo percepts from our prior learned experience. The visual, taste, olfactory, and touch signals are carried to the brain on separate pathways that are integrated by the orbitofrontal cortex into a composite flavor message and, with the amygdala and in the anterior cingulate cortex, to produce a hedonic and reward signal, and then to areas in the cortex for decisions about eating the ice cream (I will have more) and hypothalamus for satiety and fullness signals (Rolls 2015).
Sensory integration occurs when we perceive foods and beverages that are complex mixtures of stimuli for taste, smell, and touch receptors, and this process is mostly seamless and unitary: when I sip my Coca-Cola, I think “Ah, a Coke” without specifically noting the sweetness of the sugars, the bite of the phosphoric acid, the tingle of the carbon dioxide, or the individual contributions of the vanilla, cinnamon, nutmeg, and citrus aromas. Perceptual interactions occur frequently, with different combinations of stimuli showing enhancement or suppression. For combinations of prototypical taste stimuli, mixture suppression in the norm (e.g., Keast and Breslin 2003) although super-additivity (synergy) is seen for some combinations of sweeteners (Reyes et al. 2019) or savory (umami) (Zhang et al. 2008) stimuli. For cross-modal interactions, enhancement is commonly observed, as taste and retronasal olfactory input work synergistically to enhance overall perceived intensity from the oral cavity. Enhanced taste intensity (typically sweetness) in conjunction with a congruent food odor has been repeatedly observed (Duffy et al. 2016). For example, Bartoshuk and colleagues have aimed to breed better fruits and vegetables by enhancing the sweet volatiles to enhance the overall sweetness without adding sugar or debittering to remove important nutrients (Bartoshuk and Klee 2013). A similar phenomenon also is seen when adding vanilla to milk (Wang et al. 2019) or adding sweet-smelling volatiles to highly phenolic juice from the aronia berry (Duffy et al. 2016). However, enhancing sweetness via cross modal interactions may not be sufficient to increase liking, if other attributes like astringency are not reduced (e.g., Duffy et al. 2016). Increasing viscosity typically decreases taste intensity, and this appears a perceptual, not physiochemical effect, as the volatile concentration does not change with alteration in viscosity (Cook et al. 2003; Hollowood et al. 2002). In summary, given all the complex interactions that can occur, attention must be paid to the overall percept of a food product to assure it is acceptable.
There are clinical and experimental examples of altered oral sensations that occur with changes to sensory information received from the taste-related cranial nerves. One sudden source of taste loss occurs with severing of the chorda tympani nerve (cranial nerve VII) during middle ear surgery, such as to remove an acoustic neuroma (Kveton and Bartoshuk 1994). Early clinical reports showed this damage could influence more than just taste perception. In 1965, Bull (1965) observed two individuals with chorda tympani damage from middle ear surgery (out of three) who complained of alterations in true taste, oral somatosensation, and retronasal olfaction, including inability to differentiate between coffee and tea, but also that foods, such as bread and chocolate, were “doughy” and “greasy,” respectively.
The interactions between multiple sensory systems in the mouth can be shown experimentally with injection of a small amount of anesthesia into the middle ear to temporarily remove taste inputs that occur via the chorda tympani nerve (CN VII). This anesthesia does not numb the mouth overall but only serves to remove taste input from the anterior tongue. Anesthesia to CN VII on one side abolishes taste on that side simultaneously increasing the intensity of taste on the other side from a separate cranial nerve (cranial nerve IX) (Lehman et al. 1995; Yanagisawa et al. 1997). This phenomenon is thought to indicate release of inhibition. CN VII normally dampens down input from CN IX, but when input from CN VII is lost, this inhibition disappears, resulting in greater intensity from regions of the mouth innervated by CN IX and phantom oral sensations. Sensory interactions between taste and flavor is seen after modifications of taste with certain plant extracts (i.e., Gymnema sylvestre (“gymnema”) or Synsetalum dulcificum (“miracle fruit”)) (Hudson et al. 2018).
The chemosensory system shows plasticity or the ability to generate new cells, maturation, and programmed death. Neuroplasticity in the olfactory system, or the ability to make new functional neurons (neurogenesis), can occur throughout life (Brann and Firestein 2014) and is key to maintaining olfactory function throughout life. Extrinsic (e.g., exposure to microorganisms) and intrinsic (e.g., growth factors, sex hormones) factors can stimulate neurogenesis in the olfactory system. Olfactory sensory neurons that express olfactory receptors can regenerate across the lifespan, in contrast to sensory systems like vision and audition, which can generally only form new synapses during critical developmental windows (Coppola and White 2019). The ability of olfactory sensory neurons to regenerate these connections has fueled the concept of olfactory training to stimulate plasticity in response to damage as a means to recover the sense of smell (Hummel et al. 2018).
For taste, early experiences with diet may influence peripheral and central development. Children born of mothers who experienced dehydration during pregnancy due to excessive vomiting (hyperemesis gravidarum) report greater preference for salt during infancy (Crystal and Bernstein 1998) that can persist into adulthood (Leshem 2009). Sex hormones also appear to influence taste function. Taste function varies across the menstrual cycle (Prutkin et al. 2000), rising to a peak at the first trimester of pregnancy to the lowest point by the third trimester (Duffy et al. 1998), and then declines across menopause. Hormones and neuropeptides (e.g., insulin, ghrelin, and cholecystokinin) that regulate metabolism also influence taste perception and have implication for food preferences, diet behaviors, and risk of chronic diseases (Loper et al. 2015).
Disorders of Smell, Taste, and Somatosensation
Chemosensory disorders impair quality of life and can make it difficult to maintain a healthy diet and overall health. The reverse is also true, as many chemosensory disorders can be prevented through healthy behaviors and improved overall health. Indeed, population-based studies indicate that physical activity is associated with lower risk of olfactory dysfunction (Hoffman et al. 2016; Schubert et al. 2013), and a healthy diet consistent with public guidance such as the US Dietary Guidelines (https://health.gov/dietaryguidelines/2015/) prevents chronic diseases and obesity that are associated with chemosensory disorders. Further, smoking (Duffy et al. 2019) and excessive alcohol intake (Hoffman et al. 2016) each associate with olfactory impairment. Healthy behaviors including physician-recommended vaccinations, maintaining oral health, and healthy living environments can prevent viral infections and exposures that are associated with chemosensory dysfunction.
Clinically, disorders of smell are far more common than taste or oral somatosensory disorders. Notably, patient complaints of “taste loss” are almost always olfactory in nature, as the colloquial usage “taste” (i.e., flavor) differs from its use as narrow technical jargon. As noted previously, most individuals do not separate and distinguish true taste from smell, chemesthesis, or oral somatosensation when a food or beverage is consumed. Because of this understandable semantic confusion, patients often complain of “taste loss” when the fundamental cause is a disruption of olfaction, as this dysfunction is most apparent during eating. Olfaction is more vulnerable than taste to loss with aging because of its anatomical structure and because aging associates with changes in peripheral and central components. Olfactory information is carried only by a single nerve (cranial nerve I), while taste is transmitted by multiple branches of three separate nerves (cranial nerves VII, IX, X). Olfactory sensory neurons pass through fine holes in the cribriform plate at the top of the nasal cavity. These neurons are directly exposed to environmental insults such as toxins and infectious agents, and their connections to glomeruli in the olfactory bulb at the base of the brain may even be severed with head trauma. These axons may fail to regenerate, causing loss of smell, or worse yet, regrow incorrectly, connecting to the wrong glomerulus, resulting in stimuli taking on the wrong olfactory quality (i.e., a parosmia).
Infection or trauma can cause generalized anosmia (i.e., inability to smell). In population-based studies, the prevalence of measured olfactory dysfunction ranges from as low as 3.8% (Schubert et al. 2012, 2015) in the Beaver Dam Offspring Study to 12.4% in the NHANES (Hoffman et al. 2016) and up to 19.1% in the Skövde study (Bramerson et al. 2004). Increased rates of olfactory dysfunction are seen in older adults (Hoffman et al. 2016; Murphy et al. 2002; Pinto et al. 2015), males (Boesveldt et al. 2011; Doty et al. 2011; Menon et al. 2013; Pinto et al. 2015; Roberts et al. 2016; Schubert et al. 2013), certain ethnic/racial minorities (Hoffman et al. 2016; Pinto et al. 2015), and those with lower income/educational attainment (Hoffman et al. 2016; Schubert et al. 2012). However, it should also be noted that many individuals retain a good sense of smell well into their seventh or eighth decade; rather, the prevalence of olfactory dysfunction (i.e., the number of people in the population with some sort of dysfunction) increases with aging, due to increased opportunity for trauma or damage from infection. That is, increasing prevalence with aging should not be interpreted as an overall gradual decline within an individual with aging.
Total ageusia (“taste blindness”) is rarely seen. Data from the University of Pennsylvania Smell and Taste Center serve as compelling evidence in support of this statement. In over 1,000 individuals presenting to this Center with “taste loss,” less than 1% had measurable taste impairment, while 32% had severe olfactory dysfunction (Deems et al. 1991; Pribitkin et al. 2003). The inability to distinguish sour from salty and bitter is more common (Cruickshanks et al. 2009; Welge-Lussen et al. 2011), although this may be a semantic labeling issue rather than a biological deficit (McAuliffe and Meiselman 1974). Because common conditions can influence both smell and taste function (e.g., mucus quality, viral infection, head trauma, cognitive function), individuals can suffer from simultaneous smell, taste, and oral sensory disorders (Walliczek-Dworschak et al. 2017).
Clinicians must use measures of self-report with functional testing to understand and be able to treat the disorder.
Smell
Individuals suffer from diminished (hyposmia) or absent ability to perceive and identify a few (specific anosmia) or all tested (general anosmia) odorants (Murphy et al. 2003). Specific anosmias are largely due to genetic variation across individuals. For example, 6% of adults are estimated to have a specific anosmia to the musky compounds galoxide and androstenone (Amoore 1977; Bremner et al. 2003), which is explained by polymorphisms in the OR7D4 gene (Keller et al. 2007). These genetic variations are widespread and involve multiple olfactory receptor genes (Trimmer et al. 2019). Altered olfactory perception or dysosmia also exists. Dysosmia can be the distortion of odor quality (parosmia, e.g., smelling burnt paper instead of baby powder) or a phantom olfactory sensation with no apparent olfactory stimulus (i.e., olfactory hallucinations, termed phantosmia) (Murphy et al. 2003). Based on nationally representative data (NHANES 2011–2014), the prevalence of phantosmia was estimated at 6.5% of the population (Bainbridge et al. 2018).
As noted above, odorants reach receptors on olfactory sensory neurons via two routes: orthonasally (via the nostrils) and retronasally (from the mouth via the nasopharynx). Either route can be disrupted, causing olfactory dysfunction. Prior to placing food in the mouth, we may perceive food odors through passive breathing (i.e., orthonasally) and sniffing, which can increase perceived intensity. Foods and beverages that are cold or contain odorants trapped in the food matrix may provide little olfactory stimulation until they are warmed and/or released in the mouth. Notably, odorants delivered retronasally are perceived (localized) as occurring in the mouth, rather than the nose. This perceptual localization is attributed to concurrent touch and taste sensations taste (Snyder and Bartoshuk 2016). Patients report retronasal olfactory impairment with loss of taste from the anterior tongue that is medically (Bull 1965; MacCarthy-Leventhal 1959) or experimentally (Fast et al. 2000) induced. Conversely, individuals who have heightened taste response report greater retronasal olfactory intensity from model foods and beverages (Pickering et al. 2006), as the taste cortex is needed to integrate retronasal smell into a flavor percept (Blankenship et al. 2019). Taste and touch sensations may help to maintain the ability to perceive food flavor (and thus quality of life) even if the sense of smell is impaired (Oleszkiewicz et al. 2019).
The olfactory epithelium has olfactory sensory neurons, supporting cells, and basal cells. The supporting cells secrete mucus to protect against foreign agents, and basal cells serve to generate new olfactory receptor cells. Odorants must dissolve in mucus for them to interact with the receptors expressed by olfactory sensory neurons; this mucus also contains odorant-binding proteins that carry and concentrate hydrophobic odors, as well as xenobiotic-metabolizing enzymes that transform odorants (Heydel et al. 2013). There is genetic variation in odor-binding proteins that is associated with variability in olfactory ability, differentiating normal ability from hyposmia in healthy participants (Sollai et al. 2019). The metabolizing enzymes also may clear odors from olfactory receptors to increase olfactory acuity (Heydel et al. 2013). The size of the olfactory epithelial area varies. Individuals with congenital anosmia (i.e., born without a sense of smell) have reduced or absent olfactory epithelium (Moran et al. 1992). The nasal microbiome may also be key to developing a normal olfactory epithelium. Variability in this microbiome is associated with differences in olfactory ability in otherwise healthy individuals (Koskinen et al. 2018).
Taste
Individuals can also show diminished (hypogeusia) or absent (ageusia) taste perception. As noted above, total ageusia is very rare. The ability to taste with whole-mouth stimulation is maintained despite regional loss of input from damage to individual nerves that innervate different regions of the oral cavity (i.e., taste has redundant wiring). Individuals can suffer from taste altered perception, which is termed dysgeusia. Chronic dysgeusia (a persistent taste sensation) can result from a true stimulus in the mouth (e.g., the taste of an oral infection) or when stimuli reach taste receptors from the blood stream (e.g., persistent bitterness from some medications). Dysgeusia can also describe phantom sensations generated by spontaneous neuronal activity in the absence of a stimulus (analogous to phantom limb sensations) (Snyder and Bartoshuk 2016). Such sensations are often described as being metallic. A human metallic taste receptor has not yet been identified, but other data suggest metallic sensations in the mouth may be oral (taste or chemesthetic) in nature and not merely due to retronasal olfaction (Lawless et al. 2004) .
Oral and Nasal Somatosensation
Oral somatosensation also can be altered. Individuals experience diminished touch sensations (numbness) and response to chemical irritants (desensitization). Individuals also perceive pain or hypersensitivity to stimuli, such as chronic smokers reporting intense sensations from concentrated salt (Duffy et al. 2019) as well as and oral pain syndromes (e.g., burning mouth syndrome (Imamura et al. 2019)). Interestingly, there is a rare syndrome called empty nose syndrome where patients perceive obstruction in their nasal passages, yet upon examination, the passages are clear. Trigeminal impairments could be part of the cause of this syndrome (Gill et al. 2019).
Assessing Chemosensory Disorders
There are a variety of questions and psychophysical tools available to assess chemosensory complaints, as well as screen for and fully measure taste and olfactory functioning. If a chemosensory dysfunction is suspected, additional physical examinations, including otolaryngologic, neurological, and dental evaluations, can assess probable causes of the disorder.
Determining if the Complaint Is Sensory or Non-sensory in Origin
Because of the tight integration of multiple sensory systems in flavor perception as well as the role of prior experience in our affective responses, individuals may complain about the “taste” of foods for a number of reasons unrelated to chemosensory dysfunction. Loss of pleasure from eating and loss of appetite can occur separately from altered sensory signals from foods and beverages. Simple questions can help to distinguish dysfunction that is sensory in nature from other non-sensory problems.
Self-Report of Chemosensory Disorders
Table 1 lists interview questions for assessment of chemosensory disturbances. Self-reported health status can provide important insight to understand how an individual evaluates and acts on symptoms as well as provides a historical complement to a single measure of function. Some clinicians favor quantitative assessment over self-report; however, asking about perceived changes provides additional information about how individuals attend to their health and health-related behaviors. Self-report also is the only way to collect information on phantom chemosensations or altered function such as dysgeusia, phantosmia, and parosmia. Among those seeking treatment for chemosensory disorders, greater nutritional risk (e.g., weight changes) is seen in those who reported the disorder changed their interest in eating or felt that eating exacerbated the disorder (Mattes et al. 1990).
Self-rating of sensory function requires an individual to evaluate their own sense of well-being, rate a perceptual experience, and, in relation to aging, compare current function to that of an earlier age. Young and older subjects are equally unable to assess their olfactory abilities correctly, but each age group makes a different type of error in self-assessment: young patients underestimate their abilities, while older patients seem unaware of the deficit (White and Kurtz 2003). Discordance between self-reported and measured olfactory function may stem from the fact that olfactory testing is rarely part of routine health assessments. Individuals may notice a sudden loss (as with an insult such as head trauma) or a problem temporarily related to the sense of smell more easily than a gradual loss of function. Still, with dramatic changes in function, an individual may notice the change even if it occurs gradually. Finally, some people may neither assign value to their sense of smell nor exhibit overall health-seeking tendencies. Therefore, they may just assume that their sense of smell is adequate without really giving it much thought.
Standardized Survey Questions with US Nationally Representative Data for Comparison
The National Health and Nutrition Examination Survey (NHANES) included a chemosensory component for the first time in its 2011–2014 waves. NHANES is a continual, cross-sectional evaluation of the nutrition and health of the USA in a nationally representative sample (Centers for Disease Control and Prevention 2013); the protocols and data are available for review and analysis. The NHANES chemosensory component had a home interview with a Chemosensory Questionnaire (CSQ) that included items regarding self-reported olfactory, flavor and taste ability, problems such as phantom smells and dysgeusia as well as symptoms, changes notice with aging, medical treatments, and presence of related risk factors for chemosensory dysfunction that is available online (CDC 2013). These questions were content-validated by experts in chemosensation and tested to ensure consistency in participant understanding, processing, and interpretation (Hoffman et al. 2016). Combining items into an alteration score can increase the ability to detect normal function and dysfunction (i.e., specificity and sensitivity, respectively) (Hoffman et al. 2016; Rawal et al. 2014), although many people do not notice milder dysfunction (e.g., hyposmia). For example, only about 1/3 of smokers self-reported olfactory alteration despite having evidence of hyposmia upon examination (Duffy et al. 2019). Poorer sensitivity is expected of conditions, such as olfactory dysfunction, which are rarely measured (Oksanen et al. 2010).
Assessing the Complaint of Dysgeusia
Individuals who experience dysgeusia find it highly disturbing, and it can be difficult to diagnosis correctly. Unfortunately, much of the medical literature confuses taste and flavor, particularly in the cancer literature (Boltong et al. 2011), without accurately reporting dysgeusia. In practice, clinicians can use a combination of questions and testing methods to diagnose the origins of dysgeusia, as described elsewhere (Bartoshuk et al. 2005). First, determine if the patient has a persistent taste (salty, sweet, sour, bitter, etc.). Does gently swishing the mouth with water and expectorating or eating diminish the dysgeusia? If so, the source may be a stimulus in the mouth (e.g., infection, medication). Alternatively, a physician or dentist can apply a topical anesthesia to test the effect on the dysgeusia. If a topical anesthetic abolishes the persistent taste, then it may be due to a stimulus in the mouth. Addressing the source of the taste or changing the medication may alleviate the dysgeusia. If the sensation is not changed by rinsing or with a topical anesthetic, it may be a centrally mediated phantom sensation. There is evidence that dysgeusias and phantom oral pains may respond to stimulating taste, with a mild irritant, lozenge, or eating (Bartoshuk et al. 2005) or pharmacologically with low doses of clonazepam (Heckmann et al. 2012). The rationale for this treatment is that the oral pain is centrally mediated and possibly controlled by the trigeminal nucleus of the medulla. Taste appears to inhibit oral pain through gamma-aminobutyric acid (GABA), an inhibitory neurotransmitter. Clonazepam, a GABA receptor agonist, intensifies the inhibition normally provided by GABA and thus can suppress the oral pain and taste phantoms. An individual who has intensification of a dysgeusia from a topical anesthetic should have further medical and dental evaluation.
Rapid Assessment of Screening for Taste and Olfactory Function
The sense of taste is the easiest to test with stimuli readily available food stimuli (table sugar, salt, white vinegar for sour, and instant coffee crystals or Angostura Bitters for bitter). Please see the NHANES chemosensory protocol manual for a standardized script (Centers for Disease Control and Prevention 2013). Patients who report a taste intensity below “weak” probably have taste damage.
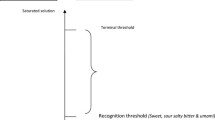
Smell should be screened via both orthonasal and retronasal routes (i.e., via the nostrils and via the mouth, respectively). The simplest assessment is to use an identification task and add on an intensity measure as well. One odor identification test that uses common stimuli you can obtain at a typical grocery store is the Connecticut Chemosensory Clinical Research Center Test (Cain et al. 1988). This low-cost screening test uses baby powder, chocolate, cinnamon, coffee, mothballs, peanut butter, and Ivory® soap, as well as Vicks VapoSteam®, as a trigeminal probe. The stimuli should be visually concealed from participants: putting the stimulus in a jar and covering with cotton gauze or cotton rounds or cotton balls work well. The stimuli should be refreshed weekly. Unseal the jar right before testing and hold under the participant’s nose. Using a word bank, have the participant identify the odors; the word bank should include the 8 stimuli and 8 other distractor items, for a total of 16 choices (Cain et al. 1988). Because odor identification tasks measure both sensory function and cognitive ability (Lehrner et al. 1999), provide correct feedback, and present misidentified items a second time to minimize cognitive effects. If a participant misses four out of the seven non-trigeminal odors (even after giving correct feedback and presenting missed items a second time), they have probable hyposmia and should be referred for more in-depth evaluation by an otolaryngologist. If the stimuli for the olfactory testing are kept fresh, an intensity task can be added to the identification task with very minimal additional time. After the participant smells the odor, first ask an intensity judgment on the gLMS (Fig. 1), and then ask for the odor identification. Again, after excluding the trigeminal control (Vicks VapoSteam), participants who report more than half of the odor stimuli as less than moderate in intensity likely have depressed olfactory function. Commercially produced screening kits are also available, as a scratch-and-sniff (Brief Smell Identification Test) or capped markers (Sniffin’ Sticks). The scratch-and-sniff version also comes in 2, 4-odor packets (Pocket Smell Test), and results can be compared with US nationally representative NHANES data (Centers for Disease Control and Prevention 2013).
The general labeled magnitude scale (Bartoshuk et al. 2004) for testing intensities
To test retronasal olfaction, orally sampled jelly beans work well, and gourmet jelly beans work especially well, as their flavors can be highly distinctive. To distinguish taste from retronasal olfaction, have the participant pinch their nostrils, and then have them put the jelly bean into the mouth and chew fully. Then, have the participant unplug the nose. Plugging the nose allows only the taste and chemesthetic input (for jelly beans, this will be primarily sweetness and sourness unless cinnamon- or chili-flavored jelly beans are used). Opening the nostrils at the end of chewing should cause a rapid retronasal transport of odorants to the olfactory epithelium. Typically, this causes an “aha” moment. If the participant does not notice a stark difference between the plugged and unplugged nose, this suggests they have impaired retronasal olfaction. If more precision is required, have the participant judge the intensity of the sweetness of the jelly bean with the nose plugged on the gLMS (Fig. 1) and then the jelly bean sweetness and flavor with the nose unplugged. A rating below “moderate” that does not increase in perceived sweetness would suggest depressed retronasal olfaction. For screening, we have used chocolate, coffee, and cherry jelly bean flavors, as well as Tabasco®-flavored jelly beans, as a trigeminal probe (Hubert et al. 2019). Jelly beans also can be used in an identification task. Select the jelly bean flavors that might be most familiar to your participants, and set up a word bank that includes the correct labels and an equal number of distractors. Be sure to give feedback and retest missed items to maximize olfactory effects and minimize cognitive effects. Misidentification of more than half of the jelly beans probably would constitute olfactory impairment.
Measuring Taste and Olfactory Functioning
Thresholds provide a measure of sensitivity but critically may or may not reflect the ability to perceive stimuli at concentrations relevant to eating, at least for taste (Pangborn and Pecore 1982; Webb et al. 2015). The food and beverage world is one of suprathreshold sensation, rarely tapping sensations close to threshold (except perhaps when chemosensation is used to detect faint off flavors or spoilage). Conversely, suprathreshold tasks measure perception of ecologically relevant stimuli and include perceived intensity measures and identification tasks. For olfaction, threshold versus suprathreshold tests may help in the diagnosis of disorders that impair peripheral olfactory function (e.g., chronic rhinosinusitis) versus those that impair central odor processing and memory (e.g., Alzheimer’s disease) (Wu et al. 2018). Discrimination testing also may help track improvement in olfactory function related to peripheral olfactory disease (Wu et al. 2018). This is a nonverbal test where the participant smells a reference odor and then needs to match that reference odor out of four additional odor probes.
Measures of perceived intensity are preferred for the assessment of taste and can be useful in the assessment of olfaction if the testing stimuli are assured for quality and freshness. Identification tasks are not useful for the sense of taste as there is only four or five qualities and there is common confusion between sour and bitter (McAuliffe and Meiselman 1974). The perceived intensity method must (1) allow subjects to express the full range of their sensations (reducing ceiling effects); (2) avoid the use of a standard to assign a particular value or the limits of the scale; (3) use a scale that allows for ratio comparison of relative intensities within individuals; and (4) provide a valid way to compare perceived intensity across subjects (Bartoshuk et al. 2006; Hayes et al. 2013). The gLMS measure described above with standardized instructions and practice with non-taste or smell stimuli meets these four criteria. For identification tasks, the method must attempt to separate sensory influences from cognitive influences. Chemosensory tasks that utilize foods and beverages may have the most application in the exploration of the relationship between chemosensation and nutrition.
Comprehensive olfactory testing generally requires both threshold and identification tasks, including some measure of retronasal ability. Commercial odor identification tests are available, including The University of Pennsylvania Smell Identification Test (UPSIT) (Doty et al. 1984) and Sniffin’ Sticks (Rumeau et al. 2016). Olfactometers also are available – these devices standardize the air flow, concentration, timing of when the odor is delivered and for how long, and when it is shut off. Research grade olfactometers have a high level of precision but are quite expensive; less expensive olfactometers that provide a reasonable level of stimulus control are also commercially available. Inclusion of an intensity judgment task extends the utility of the odor identification tests described above. The Sniffin’ Sticks also comes with threshold modules of either n-butanol, which has a trigeminal component, or 2-phenylethanol, which is a floral odor and has no trigeminal component. The UPSIT is a commercially available test of olfactory functioning (www.sensonics.com). This test includes 40 “scratch-and-sniff” odors and a multiple-choice format; a brief 12-item test is also available. Normative data for these tests determine the level of olfactory functioning (i.e., anosmia, hyposmia) according to age range and sex. These tests are also available for different cultural groups. Individuals with evidence of olfactory dysfunction should be evaluated by an otolaryngologist based on medical history, the examination with nasal endoscopy, full olfactory testing, and imaging (CT scan, MRI, and EEG) (Boesveldt et al. 2017).
Taste tests are designed to assess dysfunction but also can test the wide range of normal ability, including those who experience “pastel” oral sensations (also referred to as nontasters) versus those who experience “neon” oral sensation (also referred to as supertasters). Thresholds for a particular compound may be useful to test a mechanism of taste receptor binding or in sensory analysis to assess for off-tastes. Taste thresholds are difficult to measure, requiring precise control of the stimulus and the non-stimulus for non-taste attributes (e.g., temperature), and are slow and labor-intensive. Perceived intensity measures, particularly that probe the cranial nerves that innervated taste, offer a useful measure of taste function as initially described by Bartoshuk et al. (1994) for full testing of cranial nerves VII, IX, and X and updated with improved intensity scaling in the National Institutes of Health Toolbox (Coldwell et al. 2013) and procedurally in the NHANES protocol. In the full spatial taste testing protocol, the researcher draws cotton swabs soaked in taste solutions (sweet (1 M sucrose), salty (1 M sodium chloride), sour (32 mM citric acid), and bitter (1 mM quinine hydrochloride)) unilaterally across different areas of taste nerve innervation before a measure of intensity with whole-mouth stimulation (Sipiora et al. 2000). The NHANES protocol is a briefer measure of taste function: it only includes the salt and bitter probes with bilateral stimulation of the tongue tip and the whole mouth. Participants report the intensity of the taste on the gLMS and the quality. Adding in a water probe may be able to provide additional information on dysgeusia if the participant reports a taste from the water applied to areas of taste nerve innervation. A brief test of the ability to taste the bitterness or propylthiouracil (PROP) can be added to the spatial taste test as a screening for the most common genetic variation in taste (Hubert et al. 2019) that has been associated with diet and health outcomes (Tepper et al. 2017). For further discussion of different functional measures of taste, see (Webb et al. 2015).
Biological Measures of Smell, Taste, and Somatosensation
These measures rely less on psychophysical testing and instead on the electrophysical measures of brain potentials, magnetic responses, and changes in blood flow in response to chemical stimuli. Olfactory event-related potentials (OERP) involve providing an odorant and asking the participant to respond to the stimulus while minimizing other visual, somatosensory, or auditory sensory inputs. The OERP provides information on the length of time it takes to show significant changes in neural electrical response to the odor (i.e., latency) and the magnitude of neural response (i.e., amplitude). However, interpreting the OERP is challenged by differentiating signals from noise via complex data analyses. Comparison of individuals with normal smell versus anosmia in an automated OERP correctly identified 75% of anosmics (Guducu et al. 2019), suggesting additional refinement would be required before clinical use is indicated.
Electrogustometry is frequently used in research and practice, but it only measures taste thresholds, which, as noted above, have limited utility in relation to eating behavior. The method usually involves localized testing by applying a mild electrical current to the surface of the tongue. However, the stimulation also captures multiple qualities of taste as well as irritation. Thus, electrogustometry may be somewhat misnamed in that it may not detect taste damage but can determine the integrity of cranial nerve VII (Snyder and Bartoshuk 2016). Electrophysical recording from the tongue also has been reported for assessing the ability to taste the bitter stimulus propylthiouracil (Sollai et al. 2017). This method involves placing silver electrodes on the dorsal and ventral surface of the tongue and analyzing bioelectrical potential variations for peripheral taste responses. Assessing fungiform papillae density on the tongue tip or taste buds within these papillae is difficult and requires magnification and a contrast agent. Even with an operating microscope, 10× magnification, blue food coloring, and the ability to video record the images for in-depth reviewing, it is challenging to identify fungiform papilla from filiform papilla (non-taste) because of their varying shapes and sizes (Miller and Reedy 1990). Assessing taste bud number requires 40× magnification of a few papillae and looking for shadows indicating taste pores on slow-motion replay. Confocal laser scanning microscopy is a newer technique that can examine singular papilla or area on the tongue over time with more accuracy (Saito et al. 2017).
Conclusion
Taste, smell, and oral somatosensation contribute to food enjoyment and nutritional health by receiving sensory input from foods and beverages. A significant amount of research has advanced our knowledge of the basic mechanisms of chemosensory perception from stimulus receptor binding, transduction, nerve transmission, and central nervous packaging into an integrated perceptual experience. Directed questioning, psychophysical testing, and biological assessment identify normal variation in chemosensory function and alterations associated with genetic differences, development, and exposures as well as diseases across the lifespan. Most common is alteration of olfactory perception and changes to olfactory component of food flavor. Poorly oral health may further increase the risk of olfactory dysfunction by reducing retronasal processing of olfactory food flavor. Loss of true taste as perceived with the whole mouth is rare because of the tremendous redundancy in cranial nerves that carry taste sensations from the periphery to the brain. However, recent evidence shows that localized loss of taste from individual areas innervated by cranial nerves can alter oral sensations from foods and beverages and change food preferences and patterns of food intake. Localized losses of taste, if extreme enough, can result in dysgeusia and oral pain syndromes that impair the quality of life and the ability to obtain oral nourishment. Individuals who complain of olfactory loss and oral sensory disturbances or complain that eating is not enjoyable deserve a thorough assessment. Chemosensory disorders can improve if the underlying cause is treatable, such as through the modification of medications or alleviation of the underlying condition (see Chap. 61, “Causes of Smell, Taste, and Oral Somatosensory Disorders Affecting Eating and Drinking” by Duffy in this handbook). Cross-disciplinary opportunities exist across the food, nutrition, and health spheres to personalize eating recommendations matched with chemosensory variation to support healthy eating and the quality of the eating experiences.
References
Amoore, J. (1977). Specific anosmia and the concept of primary odors. Chemical Senses and Flavor, 2, 267–281.
Bainbridge, K. E., Byrd-Clark, D., & Leopold, D. (2018). Factors associated with phantom odor perception among US adults: Findings from the National Health and Nutrition Examination Survey. JAMA Otolaryngology. Head & Neck Surgery, 144(9), 807–814.
Bajec, M. R., & Pickering, G. J. (2008). Astringency: Mechanisms and perception. Critical Reviews in Food Science and Nutrition, 48(9), 858–875.
Bartoshuk, L. M., & Klee, H. J. (2013). Better fruits and vegetables through sensory analysis. Current Biology, 23(9), R374–R378.
Bartoshuk, L. M., Kveton, J. F., Yanagisawa, K., & Catalanotto, F. A. (1994). Taste loss and taste phantoms: A role of inhibition in taste. In K. Kurihara, N. Suzuki, & H. Ogawa (Eds.), Olfaction and taste XI (pp. 557–560). New York: Springer.
Bartoshuk, L. M., Duffy, V. B., Green, B. G., Hoffman, H. J., Ko, C. W., Lucchina, L. A., … Weiffenbach, J. M. (2004). Valid across-group comparisons with labeled scales: The gLMS versus magnitude matching. Physiology & Behavior, 82(1), 109–114.
Bartoshuk, L. M., Snyder, D. J., Grushka, M., Berger, A. M., Duffy, V. B., & Kveton, J. F. (2005). Taste damage: Previously unsuspected consequences. Chemical Senses, 30(Suppl 1), i218–i219.
Bartoshuk, L. M., Duffy, V. B., Hayes, J. E., Moskowitz, H. R., & Snyder, D. J. (2006). Psychophysics of sweet and fat perception in obesity: Problems, solutions and new perspectives. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 361(1471), 1137–1148.
Behrens, M., Briand, L., de March, C. A., Matsunami, H., Yamashita, A., Meyerhof, W., & Weyand, S. (2018). Structure-function relationships of olfactory and taste receptors. Chemical Senses, 43(2), 81–87.
Blankenship, M. L., Grigorova, M., Katz, D. B., & Maier, J. X. (2019). Retronasal odor perception requires taste cortex, but orthonasal does not. Current Biology, 29(1), 62–69. e63.
Boesveldt, S., Lindau, S. T., McClintock, M. K., Hummel, T., & Lundstrom, J. N. (2011). Gustatory and olfactory dysfunction in older adults: A national probability study. Rhinology, 49(3), 324–330.
Boesveldt, S., Postma, E. M., Boak, D., Welge-Luessen, A., Schopf, V., Mainland, J. D., … Duffy, V. B. (2017). Anosmia – A clinical review. Chemical Senses, 42, 513.
Boltong, A., Keast, R. S., & Aranda, S. K. (2011). A matter of taste: Making the distinction between taste and flavor is essential for improving management of dysgeusia. Support Care Cancer, 19(4), 441–442.
Bramerson, A., Johansson, L., Ek, L., Nordin, S., & Bende, M. (2004). Prevalence of olfactory dysfunction: The Skövde population-based study. Laryngoscope, 114(4), 733–737.
Brann, J. H., & Firestein, S. J. (2014). A lifetime of neurogenesis in the olfactory system. Frontiers in Neuroscience, 8, 182.
Breen, S. P., Etter, N. M., Ziegler, G. R., & Hayes, J. E. (2019). Oral somatosensatory acuity is related to particle size perception in chocolate. Scientific Reports, 9(1), 7437. https://doi.org/10.1038/s41598-019-43944-7.
Bremner, E. A., Mainland, J. D., Khan, R. M., & Sobel, N. (2003). The prevalence of androstenone anosmia. Chemical Senses, 28(5), 423–432.
Breslin, P. A. S., Gilmore, M. M., Beauchamp, G. K., & Green, B. G. (1993). Psychophysical evidence that oral astringency is a tactile sensation. Chemical Senses, 18(4), 405–417.
Buck, L. B. (2005). Unraveling the sense of smell (Nobel lecture). Angewandte Chemie (International Ed. in English), 44(38), 6128–6140.
Buck, L., & Axel, R. (1991). A novel multigene family may encode odorant receptors: A molecular basis for odor recognition. Cell, 65(1), 175–187.
Bull, T. R. (1965). Taste and the chorda tympani. The Journal of Laryngology and Otology, 79, 479–493.
Cain, W. S., Gent, J. F., Goodspeed, R. B., & Leonard, G. (1988). Evaluation of olfactory dysfunction in the Connecticut Chemosensory Clinical Research Center. Laryngoscope, 98, 83-88.
CDC. (2013). National Health and Nutrition Examination Survey (NHANES). 2011–2012 Data documentation, codebook, and frequencies: Taste and smell disorders [Internet]. Centers for Disease Control and Prevention (CDC), National Center for Health Statistics (NCHS). [September 2013]. Available from: http://www.cdc.gov/nchs/nhanes/nhanes2011-2012/CSQ_G.htm
Centers for Disease Control and Prevention. (2013). National Health and Nutrition Examination Survey (NHANES). Retrieved from https://www.cdc.gov/nchs/data/nhanes/nhanes_13_14/Taste_Smell.pdf
Coldwell, S. E., Mennella, J. A., Duffy, V. B., Pelchat, M. L., Griffith, J. W., Smutzer, G., Cowart, B. J., Breslin, P. A. S., Bartoshuk, L. M., Hastings, L., Beauchamp, G. K., O’Mahony, M. A., Victorson, D., & Hoffman, H. J. (2013). NIH Toolbox for Assessment of Neurological and Behavioral Function: Assessments for Gustation. Neurology, 12;80(11 Suppl 3), S20–4.
Cook, D. J., Linforth, R. S., & Taylor, A. J. (2003). Effects of hydrocolloid thickeners on the perception of savory flavors. Journal of Agricultural and Food Chemistry, 51(10), 3067–3072.
Coppola, D. M., & White, L. E. (2019). Forever young: Neoteny, neurogenesis and a critique of critical periods in olfaction. Journal of Bioenergetics and Biomembranes, 51(1), 53–63.
Cruickshanks, K. J., Schubert, C. R., Snyder, D. J., Bartoshuk, L. M., Huang, G. H., Klein, B. E., … Moy, G. S. (2009). Measuring taste impairment in epidemiologic studies: The beaver dam offspring study. Annals of the New York Academy of Sciences, 1170, 543–552.
Crystal, S. R., & Bernstein, I. L. (1998). Infant salt preference and mother’s morning sickness. Appetite, 30(3), 297–307.
Deems, D., Doty, R., Settle, R., Moore-Gillon, V., Shaman, P., Mester, A., … Snow, J. J. (1991). Smell and taste disorders: An analysis of 750 from the University of Pennsylvania Smell and taste Center. Arch Otolaryng Head Neck Surg, 117(5), 519–528.
Delwiche, J. F. (2003). Attributes believed to impact flavor: An opinion survey. Journal of Sensory Studies, 18(4), 347–352.
Doty, R., Shaman, P., & Dann, M. (1984). Development of the University of Pennsylvania smell identification test: A standardized microencapsulated test of olfactory function. Physiology & Behavior, 34, 489–502.
Doty, R. L., Petersen, I., Mensah, N., & Christensen, K. (2011). Genetic and environmental influences on odor identification ability in the very old. Psychology and Aging, 26(4), 864–871. https://doi.org/10.1037/a0023263.
DuBois, G. E. (2016). Molecular mechanism of sweetness sensation. Physiology & Behavior, 164(Pt B), 453–463.
Duffy, V. B., Bartoshuk, L. M., Striegel-Moore, R., & Rodin, J. (1998). Taste changes across pregnancy. Annals of the New York Academy of Sciences, 855, 805–809.
Duffy, V. B., Rawal, S., Park, J., Brand, M. H., Sharafi, M., & Bolling, B. W. (2016). Characterizing and improving the sensory and hedonic responses to polyphenol-rich aronia berry juice. Appetite, 107, 116–125. https://doi.org/10.1016/j.appet.2016.07.026.
Duffy, V. B., Glennon, S. G., Larsen, B. A., Rawal, S., Oncken, C., & Litt, M. D. (2019). Heightened olfactory dysfunction and oral irritation among chronic smokers and heightened propylthiouracil (PROP) bitterness among menthol smokers. Physiology & Behavior, 15(201), 111–122.
Fast, K., Bartoshuk, L., Kveton, J., & Duffy, V. (2000). Unilateral anesthesia of the chorda tympani nerve suggests taste may localize retronasal olfaction. Chemical Senses, 25, 614–615 (abstract).
Fierro, F., Giorgetti, A., Carloni, P., Meyerhof, W., & Alfonso-Prieto, M. (2019). Dual binding mode of “bitter sugars” to their human bitter taste receptor target. Scientific Reports, 9(1), 8437.
Filiou, R. P., Lepore, F., Bryant, B., Lundstrom, J. N., & Frasnelli, J. (2015). Perception of trigeminal mixtures. Chemical Senses, 40(1), 61–69.
Fleming, E. E., Ziegler, G. R., & Hayes, J. E. (2016). Salivary protein levels as a predictor of perceived astringency in model systems and solid foods. Physiology & Behavior, 163, 56–63.
Gill, A. S., Said, M., Tollefson, T. T., & Steele, T. O. (2019). Update on empty nose syndrome: Disease mechanisms, diagnostic tools, and treatment strategies. Current Opinion in Otolaryngology & Head and Neck Surgery, 27(4), 237–242.
Gogolla, N. (2017). The insular cortex. Current Biology, 27(12), R580–R586. https://doi.org/10.1016/j.cub.2017.05.010.
Green, B. (1984). Thermal perception on lingual and labial skin. Perception & Psychophysics, 36(3), 209–220.
Green, B. G. (2016). Introduction: what is chemesthesis? In D. Bolliet, S. McDonald, & J. E. Hayes (Eds.), Chemesthesis: Chemical touch in food and eating (pp. 1–7). Hoboken, New Jersey: John Wiley & Sons, Inc.
Guducu, C., Olcay, B. O., Schafer, L., Aziz, M., Schriever, V. A., Ozgoren, M., & Hummel, T. (2019). Separating normosmic and anosmic patients based on entropy evaluation of olfactory event-related potentials. Brain Research, 1708, 78–83.
Hagura, N., Barber, H., & Haggard, P. (2013). Food vibrations: Asian spice sets lips trembling. Proceedings of the Biological Sciences, 280(1770), 20131680.
Hayes, J. E., Wallace, M. R., Knopik, V. S., Herbstman, D. M., Bartoshuk, L. M., & Duffy, V. B. (2011). Allelic variation in TAS2R bitter receptor genes associates with variation in sensations from and ingestive behaviors toward common bitter beverages in adults. Chemical Senses, 36(3), 311–319.
Hayes, J. E., Allen, A. L., & Bennett, S. M. (2013). Direct comparison of the generalized Visual Analog Scale (gVAS) and general Labeled Magnitude Scale (gLMS). Food Quality and Preference, 28(1), 36–44.
Heckmann, S. M., Kirchner, E., Grushka, M., Wichmann, M. G., & Hummel, T. (2012). A double-blind study on clonazepam in patients with burning mouth syndrome. Laryngoscope, 122(4), 813–816.
Heydel, J. M., Coelho, A., Thiebaud, N., Legendre, A., Le Bon, A. M., Faure, P., … Briand, L. (2013). Odorant-binding proteins and xenobiotic metabolizing enzymes: Implications in olfactory perireceptor events. Anat Rec (Hoboken), 296(9), 1333–1345.
Hoffman, H. J., Rawal, S., Li, C. M., & Duffy, V. B. (2016). New chemosensory component to the U.S. National Health and Nutrition Examination Survey (NHANES), first-year results for measured olfactory dysfunction. Reviews in Endocrine & Metabolic Disorders, 17(2), 221–240.
Hollowood, T., Linforth, R., & Taylor, A. (2002). The effect of viscosity on the perception of flavour. Chemical Senses, 27, 583–591.
Hubert, P., Papasavas, P., Stone, A., Swede, H., Huedo-Medina, T., Tishler, D., & Duffy, V. (2019). Associations between weight loss, food likes, dietary behaviors and chemosensory function in bariatric surgery: A case-control analysis in women. Nutrients, 11(4), 804.
Hudson, S. D., Sims, C. A., Odabasi, A. Z., Colquhoun, T. A., Snyder, D. J., Stamps, J. J., … Bartoshuk, L. M. (2018). Flavor alterations associated with miracle fruit and Gymnema sylvestre. Chemical Senses, 43(7), 481–488.
Hummel, T., Stupka, G., Haehner, A., & Poletti, S. C. (2018). Olfactory training changes electrophysiological responses at the level of the olfactory epithelium. Rhinology, 56(4), 330–335.
Imamura, Y., Shinozaki, T., Okada-Ogawa, A., Noma, N., Shinoda, M., Iwata, K., … Svensson, P. (2019). An updated review on pathophysiology and management of burning mouth syndrome with endocrinological, psychological and neuropathic perspectives. Journal of Oral Rehabilitation, 46(6), 574–587.
Kapaun, C. L., & Dando, R. (2017). Deconvoluting physical and chemical heat: Temperature and spiciness influence flavor differently. Physiology & Behavior, 170, 54–61.
Keast, S. J. R., & Breslin, P. A. S. (2003). An overview of binary taste-taste interactions. Food Quality and Preference, 142(2), 111–124.
Keller, A., Zhuang, H., Chi, Q., Vosshall, L. B., & Matsunami, H. (2007). Genetic variation in a human odorant receptor alters odour perception. Nature, 449(7161), 468–472.
Koskinen, K., Reichert, J. L., Hoier, S., Schachenreiter, J., Duller, S., Moissl-Eichinger, C., & Schopf, V. (2018). The nasal microbiome mirrors and potentially shapes olfactory function. Scientific Reports, 8(1), 1296.
Kveton, J., & Bartoshuk, L. (1994). The effect of unilateral chorda tympani damage on taste. Laryngoscope, 104(1), 25-29.
Lawless, H., Schlake, S., Smythe, J., Lim, J., Yang, H., Chapman, K., & Bolton, B. (2004). Metallic taste and retronasal smell. Chemical Senses, 29, 25–33.
Lehman, C. D., Bartoshuk, L. M., Catalanotto, F. C., Kveton, J. F., & Lowlicht, R. A. (1995). Effect of anesthesia of the chorda tympani nerve of taste perception in humans. Physiology & Behavior, 57, 943–951.
Lehrner, J., Gluck, J., & Laska, M. (1999). Odor identification, consistency of label use, olfactory threshold and their relationship to odor memory over the human lifespan. Chemical Senses, 24, 337–346.
Leshem, M. (2009). The excess salt appetite of humans is not due to sodium loss in adulthood. Physiology & Behavior, 98(3), 331–337.
Loper, H. B., La Sala, M., Dotson, C., & Steinle, N. (2015). Taste perception, associated hormonal modulation, and nutrient intake. Nutrition Reviews, 73(2), 83–91.
MacCarthy-Leventhal, E. (1959). Post radiation mouth blindess. Lancet, 19, 1138–1139.
Mainland, J. D., Keller, A., Li, Y. R., Zhou, T., Trimmer, C., Snyder, L. L., … Matsunami, H. (2014). The missense of smell: Functional variability in the human odorant receptor repertoire. Nature Neuroscience, 17(1), 114–120.
Malnic, B., Godfrey, P. A., & Buck, L. B. (2004). The human olfactory receptor gene family. Proceedings of the National Academy of Sciences of the United States of America, 101(8), 2584–2589.
Mattes, R., Cowart, B., Schiavo, M., Arnold, C., Garrison, B., Kare, M., & Lowry, L. (1990). Dietary evaluation of patients with smell and/or taste disorders. The American Journal of Clinical Nutrition, 51, 233–240.
McAuliffe, W. K., & Meiselman, H. L. (1974). The roles of practice and correction in the categorization of sour and bitter taste qualities. Perception & Psychophysics, 16(2), 242–244.
Menon, C., Westervelt, H. J., Jahn, D. R., Dressel, J. A., & O’Bryant, S. E. (2013). Normative performance on the Brief Smell Identification Test (BSIT) in a multi-ethnic bilingual cohort: A project FRONTIER study. The Clinical Neuropsychologist, 27(6), 946–961.
Miles, B. L., Van Simaeys, K., Whitecotton, M., & Simons, C. T. (2018). Comparative tactile sensitivity of the fingertip and apical tongue using complex and pure tactile tasks. Physiology & Behavior, 194, 515–521.
Miller, I., & Reedy, F. (1990). Variation in human taste bud density and taste intensity perception. Physiology & Behavior, 47, 1213–1219.
Moran, D., Jafek, B., Eller, P., & Rowley, J. d. (1992). Ultrastructural histopathology of human olfactory dysfunction. Microscopy Research and Technique, 23(2), 103–110.
Murphy, C., Schubert, C., Cruickshanks, K., Klein, B., Klein, R., & Nondahl, D. (2002). Prevalence of olfactory impairment in older adults. JAMA, 288(18), 2307–2312.
Murphy, C., Doty, R., & Duncan, H. J. (2003). Clinical disorders of olfaction. In R. Doty (Ed.), Handbook of olfaction and gustation (2nd ed., pp. 461–478). New York: Marcel Dekker.
Oksanen, T., Kivimaki, M., Pentti, J., Virtanen, M., Klaukka, T., & Vahtera, J. (2010). Self-report as an indicator of incident disease. Annals of Epidemiology, 20(7), 547–554.
Olender, T., Lancet, D., & Nebert, D. W. (2008). Update on the olfactory receptor (OR) gene superfamily. Human Genomics, 3(1), 87–97.
Oleszkiewicz, A., Park, D., Resler, K., Draf, J., Schulze, A., Zang, Y., … Hummel, T. (2019). Quality of life in patients with olfactory loss is better predicted by flavor identification than by Orthonasal olfactory function. Chemical Senses, 44(6), 371–377.
Pangborn, R. M., & Pecore, S. D. (1982). Taste perception of sodium chloride in relation to dietary intake of salt. The American Journal of Clinical Nutrition, 35(3), 510–520.
Pickenhagan, W. (1989). Enantioselectivity in odor perception. In R. Teranishi, R. Buttery, & F. Shahidi (Eds.), Flavor chemistry: Trends and development (pp. 151–157). Washington, DC: American Chemical Society.
Pickering, G. J., Haverstock, G., & DiBattista, D. (2006). Evidence that sensitivity to 6-n-propylthiouracil (PROP) affects perception of retro-nasal aroma intensity. Journal of Food, Agriculture and Environment, 4(2), 15–22.
Pinto, J. M., Wroblewski, K. E., Kern, D. W., Schumm, L. P., & McClintock, M. K. (2015). The rate of age-related olfactory decline among the general population of older U.S. adults. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 70(11), 1435–1441.
Poletti, S. C., Hausold, J., Herrmann, A., Witt, M., & Hummel, T. (2019). Topographical distribution of trigeminal receptor expression in the nasal cavity. Rhinology, 57(2), 147–152.
Pribitkin, E., Rosenthal, M. D., & Cowart, B. J. (2003). Prevalence and causes of severe taste loss in a chemosensory clinic population. The Annals of Otology, Rhinology, and Laryngology, 112(11), 971–978.
Prutkin, J. M., Duffy, V. B., Etter, L., Fast, K., Lucchina, L. A., Snyder, D. J., … Bartoshuk, L. M. (2000). Genetic variation and inferences about perceived taste intensity in mice and men. Physiology & Behavior, 61(1), 161–173.
Rawal, S., Hoffman, H. J., Chapo, A. K., & Duffy, V. B. (2014). Sensitivity and specificity of self-reported olfactory dysfunction in a home-based study of independent-living, healthy older women. Chemosensory Perception, 7(304), 108–116.
Reyes, M. M., Gravina, S. A., & Hayes, J. E. (2019). Evaluation of sweetener synergy in humans by isobole analyses. Chemical Senses, 44(8), 571–582.
Roberts, R. O., Christianson, T. J., Kremers, W. K., Mielke, M. M., Machulda, M. M., Vassilaki, M., … Petersen, R. C. (2016). Association between olfactory dysfunction and amnestic mild cognitive impairment and Alzheimer disease dementia. JAMA Neurology, 73(1), 93–101.
Roebber, J. K., Roper, S. D., & Chaudhari, N. (2019). The role of the anion in salt (NaCl) detection by mouse taste buds. The Journal of Neuroscience, 39(32), 6224–6232.
Rolls, E. T. (2015). Taste, olfactory, and food reward value processing in the brain. Progress in Neurobiology, 127-128, 64–90.
Rumeau, C., Nguyen, D. T., & Jankowski, R. (2016). How to assess olfactory performance with the Sniffin’ sticks test((R)). European Annals of Otorhinolaryngology, Head and Neck Diseases, 133(3), 203–206.
Saito, T., Ito, T., Ito, Y., Manabe, Y., & Sano, K. (2017). Relationship between gustatory function and average number of taste buds per fungiform papilla measured by confocal laser scanning microscopy in humans. European Journal of Oral Sciences, 125(1), 44–48.
Schobel, N., Radtke, D., Kyereme, J., Wollmann, N., Cichy, A., Obst, K., … Hatt, H. (2014). Astringency is a trigeminal sensation that involves the activation of G protein-coupled signaling by phenolic compounds. Chemical Senses, 39(6), 471–487.
Schubert, C. R., Cruickshanks, K. J., Fischer, M. E., Huang, G. H., Klein, B. E., Klein, R., … Nondahl, D. M. (2012). Olfactory impairment in an adult population: The beaver dam offspring study. Chemical Senses, 37(4), 325–334.
Schubert, C. R., Cruickshanks, K. J., Nondahl, D. M., Klein, B. E., Klein, R., & Fischer, M. E. (2013). Association of exercise with lower long-term risk of olfactory impairment in older adults. JAMA Otolaryngology. Head & Neck Surgery, 139(10), 1061–1066.
Schubert, C. R., Cruickshanks, K. J., Fischer, M. E., Huang, G. H., Klein, R., Tsai, M. Y., & Pinto, A. A. (2015). Carotid intima media thickness, atherosclerosis, and 5-year decline in odor identification: The beaver dam offspring study. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 70(7), 879–884.
Sipiora, M., Murtaugh, M., Gregoire, M., & Duffy, V. (2000). Bitter taste perception and severe vomiting during pregnancy. Physiology & Behavior, 69(3), 259–267.
Snyder, D. J., & Bartoshuk, L. M. (2016). Oral sensory nerve damage: Causes and consequences. Reviews in Endocrine & Metabolic Disorders, 17(2), 149–158.
Sollai, G., Melis, M., Pani, D., Cosseddu, P., Usai, I., Crnjar, R., … Tomassini Barbarossa, I. (2017). First objective evaluation of taste sensitivity to 6-n-propylthiouracil (PROP), a paradigm gustatory stimulus in humans. Scientific Reports, 7, 40353.
Sollai, G., Melis, M., Magri, S., Usai, P., Hummel, T., Tomassini Barbarossa, I., & Crnjar, R. (2019). Association between the rs2590498 polymorphism of Odorant Binding Protein (OBPIIa) gene and olfactory performance in healthy subjects. Behavioural Brain Research, 372, 112030.
Teng, B., Wilson, C. E., Tu, Y. H., Joshi, N. R., Kinnamon, S. C., & Liman, E. R. (2019). Cellular and neural responses to sour stimuli require the Proton Channel Otop1. Current Biology, 29, 3647. Available online, https://www.sciencedirect.com/science/article/pii/S0960982219311613.
Tepper, B. J., Banni, S., Melis, M., Crnjar, R., & Tomassini Barbarossa, I. (2014). Genetic sensitivity to the bitter taste of 6-n-propylthiouracil (PROP) and its association with physiological mechanisms controlling body mass index (BMI). Nutrients, 6(9), 3363–3381.
Tepper, B. J., Melis, M., Koelliker, Y., Gasparini, P., Ahijevych, K. L., & Tomassini Barbarossa, I. (2017). Factors influencing the phenotypic characterization of the oral marker, PROP. Nutrients, 9(12), E1275.
Trimmer, C., Keller, A., Murphy, N. R., Snyder, L. L., Willer, J. R., Nagai, M. H., … Mainland, J. D. (2019). Genetic variation across the human olfactory receptor repertoire alters odor perception. Proceedings of the National Academy of Sciences of the United States of America, 116(19), 9475–9480.
Vandenbeuch, A., Clapp, T. R., & Kinnamon, S. C. (2008). Amiloride-sensitive channels in type I fungiform taste cells in mouse. BMC Neuroscience, 9, 1.
Verbeurgt, C., Wilkin, F., Tarabichi, M., Gregoire, F., Dumont, J. E., & Chatelain, P. (2014). Profiling of olfactory receptor gene expression in whole human olfactory mucosa. PLoS One, 9(5), e96333.
Walliczek-Dworschak, U., Schops, F., Feron, G., Brignot, H., Hahner, A., & Hummel, T. (2017). Differences in the density of fungiform papillae and composition of saliva in patients with taste disorders compared to healthy controls. Chemical Senses, 42(8), 699–708.
Wang, G., Bakke, A. J., Hayes, J. E., & Hopfer, H. (2019). Demonstrating cross-modal enhancement in a real food with a modified ABX test. Food Quality and Preference, 77, 2006–2013.
Webb, J., Bolhuis, D. P., Cicerale, S., Hayes, J. E., & Keast, R. (2015). The relationships between common measurements of taste function. Chemosensory Perception, 8(1), 11–18.
Welge-Lussen, A., Dorig, P., Wolfensberger, M., Krone, F., & Hummel, T. (2011). A study about the frequency of taste disorders. Journal of Neurology, 258(3), 386–392. d.
White, T., & Kurtz, D. (2003). The relationship between metacognitive awareness of olfactory ability and age in people reporting chemosensory disturbances. The American Journal of Psychology, 116(1), 99–110.
Wu, D., Bleier, B. S., & Wei, Y. (2018). Temporary olfactory improvement in chronic rhinosinusitis with nasal polyps after treatment. European Archives of Oto-Rhino-Laryngology, 275(9), 2193–2202.
Yan, J. W., Ban, Z. J., Lu, H. Y., Li, D., Poverenov, E., Luo, Z. S., & Li, L. (2018). The aroma volatile repertoire in strawberry fruit: A review. Journal of the Science of Food and Agriculture, 98(12), 4395–4402.
Yanagisawa, K., Bartoshuk, L. M., Catalanotto, F. A., Karrer, T. A., & Kveton, J. F. (1997). Anesthesia of the chorda tympani nerve and taste phantoms. Physiology & Behavior, 63(3), 329–335.
Zhang, F., Klebansky, B., Fine, R. M., Xu, H., Pronin, A., Liu, H., … Li, X. (2008). Molecular mechanism for the umami taste synergism. Proceedings of the National Academy of Sciences of the United States of America, 105(52), 20930–20934.
Acknowledgments
This work was supported by the US Department of Food and Agriculture (USDA) Hatch Project Funds (#CONS00928 and Accession #1001056, and #PEN04708 and Accession #1019852).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this entry
Cite this entry
Duffy, V.B., Hayes, J.E. (2020). Biological Basis and Functional Assessment of Oral Sensation. In: Meiselman, H. (eds) Handbook of Eating and Drinking. Springer, Cham. https://doi.org/10.1007/978-3-030-14504-0_22
Download citation
DOI: https://doi.org/10.1007/978-3-030-14504-0_22
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-14503-3
Online ISBN: 978-3-030-14504-0
eBook Packages: Behavioral Science and PsychologyReference Module Humanities and Social SciencesReference Module Business, Economics and Social Sciences