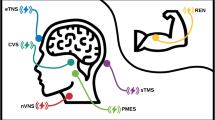
Abstract
Neuromodulation constitutes an entirely new discipline within headache therapy and its development was motivated largely by the unmet needs of chronic headache patients. The early, invasive strategies were primarily reserved for difficult-to-treat patients with a high headache burden. Newer, minimally- and non-invasive approaches have partly unmasked the complexity of defining common indications for all forms of neurostimulation. As the number of options available to clinicians expands, the field is becoming increasingly fragmented, partly a consequence of the lack of guidelines for the design of trials of neuromodulation. Such guidelines exist for drug trials in migraine, cluster headache (CH) and tension-type headache [1–4]. Although there are similarities between conducting trials in drugs and neuromodulation, major differences are obvious. No clear consensus has been agreed on how clinical trials in neuromodulation should be conducted, and common reporting standards are lacking. There is considerable heterogeneity in the methodology applied in published trials which represents a significant obstacle in the attempt to compare outcomes [5].
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Refractory headache
- Occipital nerve stimulation
- Deep brain stimulation
- Sphenopalatine ganglion stimulation
- Sham device
1 Introduction
Neuromodulation constitutes an entirely new discipline within headache therapy and its development was motivated largely by the unmet needs of chronic headache patients. The early, invasive strategies were primarily reserved for difficult-to-treat patients with a high headache burden. Newer, minimally- and non-invasive approaches have partly unmasked the complexity of defining common indications for all forms of neurostimulation. As the number of options available to clinicians expands, the field is becoming increasingly fragmented, partly a consequence of the lack of guidelines for the design of trials of neuromodulation. Such guidelines exist for drug trials in migraine, cluster headache (CH) and tension-type headache [1,2,3,4]. Although there are similarities between conducting trials in drugs and neuromodulation, major differences are obvious. No clear consensus has been agreed on how clinical trials in neuromodulation should be conducted, and common reporting standards are lacking. There is considerable heterogeneity in the methodology applied in published trials which represents a significant obstacle in the attempt to compare outcomes [5].
Considerable attention has been diverted to the philosophical paradox of pain—how an organic process can be subject to such strong subjective influences [6]. Keeping this in mind, headache can be very challenging to investigate. Firstly, the pain can fluctuate significantly in the course of minutes or hours, making defining the exact beginning of the attack difficult. Further, there is significant between- and within-subject variation. Whether invasive or non-invasive, all neuromodulation approaches have presented unique difficulties when tested clinically and in many cases, despite rigorous pre-trial efforts, in-the-field testing has uncovered issues which could not have been anticipated. This chapter will focus on methodological difficulties in developing studies to investigate neuromodulation therapies in the two disorders best investigated in headache, migraine and CH. Three areas will be covered: (1) defining the sample, (2) designing the trial and (3) interpreting results.
2 Defining the Sample
Some efforts have been made to define the subgroup of headache patients who may benefit from invasive neuromodulation approaches. Expert opinion consensus has been published by the American and European headache societies. The term intractable headache has now been replaced by “refractory” headache, highlighting the difficulty to treat these patients with pharmacological approaches only [7,8,9]. The immediate implications that the term refractory headaches may have in clinical practice is to consider neuromodulation treatments as soon as their condition becomes refractory. However, apart from clinical trial designs, referrals to neuromodulation centres are normally triggered once patients have failed a multitude of oral and/or injectable treatments [10, 11]. Although not guided by research data, the absence of many neuromodulation centres, the high costs of the devices and scepticism about the available efficacy evidence lead clinicians to delay consideration of these therapies.
2.1 Diagnosis
For all patients enrolled in neuromodulation trials, the diagnosis of a primary headache disorder should be made by a headache neurologist according to established criteria [12]. A full history should be taken and a complete physical and neurological examination as well as neuroimaging performed [13]. In most neuromodulation trials, it is required that patients have a stable headache diagnosis, including sub-classification (chronic/episodic) for at least 2 years. This aspect is crucial since it has been demonstrated that migraine can fluctuate between the chronic and episodic pattern without being modulated by medications [14]. The presence of medication overuse headache (MOH) should be ruled out as MOH, especially in migraine patients, has been historically considered a negative predictor. Clinical trials assessing novel headache therapies normally exclude patients with a primary headache and MOH [15]. This has also been the case for ONS trials with the exception of the Precision Implantable Stimulator for Migraine (PRISM) study where MOH was not an exclusion criterion; hence, patients with CM with and without MOH were implanted [16, 17]. Although the study did not show overall statistically significant superiority of ONS compared to sham, when the group without MOH was analysed separately, the efficacy outcome of ONS was statistically superior to sham. This may suggest, similarly to many oral preventive treatments, that MOH is a negative predictor of response to invasive neurostimulation therapies. However, in a more recent small, open-label feasibility study, high-frequency cervical spinal cord stimulation (HF-10 SCS) was tested in extremely challenging-to-treat CM and MOH patients. At 6-month follow-up, an average reduction of seven headache days/month was reported. In responders, there was an average reduction of 12.9 headache days/month. Furthermore, 50% of patients experienced 30% reduction in headache days and almost 60% of patients’ migraine became episodic. The study showed that a great proportion of patients managed to reduce the intake of abortive treatments (predominantly triptans) [18].
Lastly, patients with chronic migraine or CH may report a constant background headache which constitutes a special challenge with regard to the trial. If this is considered secondary to the primary disorder, it should resolve if effective neuromodulation is achieved; however, this may not always be the case. Furthermore, treatment of one headache disorder may lead to unmasking phenomena. Prior to enrolment, it may also be prudent to rule out other causes of secondary headache such as sleep disorders.
2.2 Severity
Several factors contribute to the combined headache burden: severity, frequency and duration of the attacks along with the ability of the patient to cope with the attacks. Although frequency and duration of headache episodes are somehow easy to measure with the aid of headache charts, the severity of pain is a multifaceted and entirely subjective characteristic, which encompasses emotional and cultural variables. This can make evaluation of headache severity quite complex in clinical trials. It is important, therefore, not to use headache severity as a primary outcome measure in headache neuromodulation clinical trials. In an RCT testing ONS in CM, the primary outcome of the study was comparing the proportion of responders, who were defined as patients with Visual Analogue Scale (VAS) scale reduction from baseline of ≥50%, in the active and sham groups at 12-week follow-up. The study showed no significant difference in the percentage of responders in the Active compared with the Sham group (95% lower confidence bound (LCB) of −0.06; p = 0.55). However, there was a significant superiority of active compared to sham device when the reduction in headache days was considered (Active Group = 6.1, baseline = 22.4; Control Group = 3.0, baseline = 20.1; p = 0.008) [19]. This suggests that change in severity in headache neuromodulation trials should be kept as a secondary end point at least until better tools to measure pain intensity become available.
2.3 Comorbidities
Headache patients have high rates of co-occurring disorders and diseases. These comorbidities could broadly be classified in different classes, which include: respiratory (i.e. asthma), cardiovascular (i.e. hypertension), psychiatric (i.e. anxiety, depression), pain (chronic pain conditions) and sleep (i.e. insomnia and restless legs syndrome). It has been recently demonstrated that migraine patients with several comorbidities are more likely to display a chronic pattern, allodynia, MOH and severe migraine disability [20]. It is therefore plausible that patients from this group may be more difficult to treat, hence be classified as refractory. Although this group of patients may be the one that needs non-pharmacological treatments most, in view of the migraine-related level of disability, most of the clinical trials’ exclusion criteria recommend avoiding implanting patients with unstable or severe psychiatric conditions and to avoid (as discussed above) patients with MOH [11, 19]. Given the high prevalence of psychiatric disorders of axis I and II in patients with refractory primary headaches, it is recommended by international guidelines that potential candidates for neurostimulation undergo a specialist psychiatric/psychological assessment [21]. When applied to trials, the psychological screening can help the clinician screen out those patients considered non-suitable for psychiatric reasons [18]. It is also known that psychiatric disorders such as severe anxiety and/or depression is a negative predictor of response to ONS [22], suggesting the pivotal importance of assessing potential candidate for invasive neurostimulation in an expert multidisciplinary environment before enrolling them into clinical trials [23]. Cardiovascular comorbidities can have a detrimental effect on the adverse events rate in neuromodulation clinical trials by leading to high infection rate and wound-healing issues. The same can be said for obesity and smoking habit [24].
Another challenging comorbidity in primary headache disorders include the presence of additional chronic pain conditions such as fibromyalgia, chronic spinal pain and/or complex regional pain syndrome (CRPS). Migraine patients with comorbid fibromyalgia report more depressive symptoms, higher headache intensity and are more likely to have severe headache-related disability as compared to controls without fibromyalgia [25]. It has also been noted in one of the authors’ clinical experience that devices implanted for headache relief in this group of patients may unmask the comorbid underlying pain condition ultimately resulting in the same levels of disability. The other way around may also be possible, where patients implanted with SCS for conditions like failed back surgery syndrome (FBSS) or CRPS, report a de novo onset of a headache disorder, which is often chronic and difficult to treat. The complex but fascinating relationship between primary headaches and other comorbidities clearly highlights the importance of specialized multidisciplinary centres where these patients should be assessed if considered refractory to treatments. Additional care is envisaged when recruiting these patients for neuromodulation trials.
2.4 Medications
The cut-off number or preventive treatments that need to be failed before considering a patient suitable for neuromodulation vary between guidelines [21, 26, 27]. The average range is between two and three failed preventive classes before considering a patient as refractory and this cut-off has been used in the three RCT using ONS in CM [16, 17, 19]. However, in clinical practice, by the time they may be considered for invasive neurostimulation, patients normally have failed to respond or tolerate many more than three preventive treatments. Furthermore, since Onabotulinum toxin A (Botox) has been recognized as the standard of care in CM, neuromodulation candidates must be Onabotulinum toxin A non-responders. This level of refractoriness is reflected in more recent open label ONS and SCS studies in migraine. In a study by Miller et al. the mean number of preventive treatments prior to ONS in a CM cohort was 9.36 (±2.61). In a recent open-label feasibility study using cervical SCS for refractory CM, the mean number of treatments failed at baseline was 11.7 (±3.2) [11, 22]. With the advent of anti-CGRP monoclonal antibodies (mab), it is likely that the type of treatments to be failed before considering patients refractory and suggesting neuromodulation will include three to four oral preventive treatments, Botox, and anti-CGRP mab.
The role of concomitant oral preventive treatments intake in potential candidates for invasive neurostimulation is also a matter of debate. It is likely that by the time patients with refractory headaches reach a neurostimulation clinical trial, they will have failed most of the medications with some evidence, so many will likely be off preventive drugs. However, if on partly effective medications, it is normal practice to keep medication stable for a period, typically 1 month, before implantation and during a possible experimental period. However, there are caveats to this approach, as it assumes an additive effect of neurostimulation on top of pre-existing pharmaceutical treatment. Such an additive effect remains completely speculative and unproven. Further, the metabolism of drugs does not occur at a constant rate; thus, it may be likely that while the administered dose is kept stable, the circulating dose may in fact be changing through the trial which complicates the interpretation of results. Contrary to pharmaceutical trials, it is unlikely that neuromodulation may interact directly with the metabolism of drugs. It may, however, lower the therapeutic threshold, rendering previously ineffective treatments effective which should be captured in secondary outcomes.
3 Designing a Neuromodulation Trial in Primary Headaches
Designing neuromodulation trials in headache patients carries several challenges. Once the diagnosis of a chronic stable headache disorder is made, patient selection is critical. The role of MOH has already been discussed. Additionally, the level of refractoriness needed to be reached before suggesting surgery, guided by the number of treatments failed and the subjective level of disability, is still a matter of debate. It is unclear whether to explore the full effect of neurostimulation devices, it is better to include patients who just met the suggested cut-off of failed medications (two to three drugs/classes of drugs) or whether the therapies should be limited for those very refractory patients who had a much higher number of treatments. Other important factors to take into account when planning such trials include the need of a stimulation trial (if possible), which is standard practice in chronic pain neuromodulation trials. In headache studies, a stimulation trial has been employed by some and not by others, without discernible differences [11, 18, 19, 22]. Another challenge includes the difficulty in creating a reliable sham, especially for tonic, low-frequency paraesthesia-producing devices. Different efficacy outcomes and length of follow-up for the primary endpoint are also all critical factors to properly address.
3.1 Outcome Measures
Direct effects of headache therapies can be divided into acute and preventive effects. This also applies to neuromodulation. The mainstay of evaluating the effect of a treatment on attacks is the prospectively recorded headache diary. The challenge with this solution is adherence. In response, some trials have employed alternative strategies such as retrospective recall over varying intervals. The problem with this method is recall bias. A more flexible solution in trials assessing acute efficacy may be incorporation of an electronic diary programmed into the neurostimulation device itself [28]. Using such an approach, greater detail can be captured during specified stages of the trial and fewer during other stages, ensuring continued adherence.
IHS guidance for trials in migraine suggests the use of migraine attacks and migraine days as primary efficacy outcomes [29]. More recently, a revision of the guidelines has been published. The suggested primary efficacy endpoint in CM clinical trials were change in migraine days, change in moderate-to-severe headache days, or responder rate. The two non-selected end points should then be considered secondary endpoints [30]. The primary outcomes selected in the three ONS trials conducted in CM were: change in migraine days (although MOH was allowed), responder rate, and VAS score reduction. The outcomes of these trials was less dramatic than wished. However, if MOH was excluded and the primary outcome was change in headache or migraine days, then all the three trials would have been superior to sham and perhaps the development and use of ONS in migraine would have moved forward [16, 17, 19].
Secondary outcomes normally include the use of rescue medication, improvements in the use of preventive medications and headache-related disability and quality-of-life scales such as the HIT-6 or SF-36 questionnaires [31, 32]. In lieu of standardized reporting measures, the availability of these validated tools has allowed some comparison across different study populations and neuromodulation modalities. It should also be noted that these may not be validated for specific headache syndromes. Moving forward, patient-reported outcome measures may see increased use [33].
3.2 Establishing a Control
Randomized controlled trials for neuromodulation in headache are scarce and this is not without reason. Firstly, neuromodulation has been mostly reserved for refractory patients, which constitute a minority of chronic headache patients. Further, the challenges associated with sham surgery, the difficulty in blinding and small patient populations have proven difficult obstacles to overcome. This may be changing, especially with the introduction of less invasive forms of neuromodulation.
Control conditions can include a crossover between active stimulation and sham/placebo stimulation; comparison to standard of care; sub-perception or sub-effective stimulation; randomly inserted treatment or sham/placebo for acute attacks. Careful consideration of the duration of the different phases of the study is necessary. The only double-blinded RCT testing hypothalamic DBS in CH randomized patients to either sham or active stimulation for 1 month only and found no difference between the two groups [34]. However, in the open-label phase of this study, and in the >60 patients who have been published, the majority of patients obtained at least a 50% reduction in attack frequency. It is obvious that the crossover period was simply too short to capture the slower onset of effective preventive therapy. The way to deal with this is obviously to increase the duration of the experimental period. One argument against crossover trials is the need for washout periods. At the time of writing, there are no clear indications of how long such periods should be for neurostimulation. Certainly, for the preventive effect, it seems that the washout should be longer than the one employed in the only RCT in CH [35], and it may very well be that in migraine it should be longer than in CH.
3.3 Placebo and Blinding
The placebo effect in headache can be quite strong and there are examples of RCTs with placebo being more effective than the active treatment [36]. SPGS, ONS, nVNS and supraorbital nerve stimulation can all be felt, while DBS cannot. Whether investigating a possible acute effect or a preventive one, a major concern when stimulation elicits a sensory experience is that patients will be able to discern different stimulation settings—full stimulation, some form of sham stimulation or no stimulation at all. It has also been suggested that sub-threshold stimulation can produce pain relief [37]. So far, the control used in headache has involved sham stimulation (changed frequency, changed amplitude), some form transcutaneous stimulation and sub-perception stimulation (amplitude is reduced to below sensory levels). Often, patients can detect even minute changes in these parameters. In some non-headache applications, this issue has been dealt with by application of an anaesthetic agent. While such an approach could be used in the case of non-invasive transcutaneous approaches such as nVNS, where a gel is applied, it is difficult to imagine how this would be feasible in other cases. It is also unknown how such application of an anaesthetic could affect the effect of the neurostimulation. The ongoing ICON trial, investigating ONS in CH, employs 100% amplitude in the active group compared to 30% amplitude in the control group [38]. It will be very interesting to see if this design maintains effective blinding.
A further challenge is to prevent exchange of individual experiences of the sensation of the neurostimulation between patients. This could give rise to specific expectations of what (effective) stimulation should feel like. Thus, in the age of social media, being treatment-naïve may not be sufficient, as patients may have read or heard about the experiences of other patients in the trial. Therefore, on inclusion, patients should be encouraged not to share their experiences with potential or other enrolled candidates in the trial, while it is ongoing. This issue may be mitigated somewhat in a multicentre set-up. In a setting where stimulation can be felt, random assignment to sham or treatment with partial crossover from the beginning may be preferable, as patients remain naïve to the sensation of full therapy while in the placebo group. Sham implants have not been used in the headache field; the ethical implications are substantial and if control conditions can be obtained using some form of altered stimulation parameters it may be unnecessary.
The nature of headache and the inherent subjective component of perceived pain results in a placebo response in the range of 20–40% [39]. In such a setting, rigorous blinding becomes imperative, as open label and single-blinded trials otherwise complicate interpretation of results. In any case, whether the stimulation can be felt or not, the optimal procedure in the titration period remains repeated presentation of varying stimulation parameters by a neutral technician, not the patient or evaluator. This may aid in minimizing the cues or suggestions pertaining to the programming parameters. At the end of the trial, blinded investigators can be asked regarding their opinion concerning treatments groups (active vs. placebo). These data, together with treatment response, may provide information about how successful blinding was.
Emerging data using different electrical stimulation waves have obtained promising albeit preliminary results in primary headache, predominantly migraine. High-frequency (10 kHz), paraesthesia-free, high cervical SCS is emerging as a safe and potentially effective treatment in refractory CM with and without MOH [11, 18]. The absence of paraesthesia constitutes an appealing factor that could overcome the limitation of tonic, low-frequency stimulation in clinical trials and allows the design of reliable sham devices, which will be able to maintain correct blinding.
3.4 Programming, Unknown Stimulation Parameters, and Accommodation
As with all novel therapies, first-in-human studies of neurostimulation present unique challenges as optimal stimulation parameters initially are largely unknown. These are specific for each mode of neurostimulation and the effect of changing pulse width, frequency, and amplitude is not directly comparable. In cases such as ONS and SPGS, transcutaneous stimulation prior to implant can shed some light on effective parameters [40]. The titration or optimization period should be shorter than the experimental period and the investigator and patient should be unaware of the stimulation settings. Again, with these considerations in mind, the optimal setting in the titration period includes a blinded implanter, a blinded evaluator and finally a blinded or neutral programmer. There is some variation in how much time is allowed for healing post-surgery and when to attempt first programming. The rationale for allowing time for the surgical field to heal is that oedema and inflammation may create suboptimal settings for neuromodulation and that as swelling subsides, the effective electrical field may change, requiring reprogramming. Failed, suboptimal initial attempts at programming may also compromise the patients’ faith in the treatment.
4 Interpreting Results
Neurostimulation is especially prone to positive bias, as expectations are particularly increased to a novel, high-tech treatment [41]. Challenges regarding response evaluation in these trials in headache are similar to those described for pharmaceutical trials [1, 2]. For expected preventive effects, the primary outcome can be headache days with moderate–severe intensity, number of headache days, or number of attack episodes. Clear definitions for these exist, but careful planning is necessary to avoid missing a signal. For acute effects pain freedom at various intervals has been used. One problem with regard to this approach is rebound headache which is known to be an issue in CH [42]. Another end point which may be worth considering is that of phenotype conversion. This may be secondary, however, as the delineation between episodic and chronic to an extent remains nosological without an obvious pathophysiological correlate. Should this be included, it is important to decide whether the definition should include patients who are attack-free but receiving preventive treatment, including prophylactic neurostimulation [43].
Typically, a 50% improvement defines a responder in controlled trials of drugs in migraine [1]. However, use of a 30% response may be justified in chronic, medically refractory patients, especially if this response is sustained over a considerable period of time [2, 44]. A unique challenge arises if there is an unanticipated mixed preventive/acute response. This was seen in the CH-1 pathway trial of SPGS in CH [28] where the preventive effect was unanticipated at the time of writing the protocol; hence, a post-hoc analysis was performed to capture both the acute and preventive signals. This method of capturing a combined acute and preventive response was also used in the subsequent open-label trial.
An issue to be especially aware of when investigating treatments which, due to a variety of reasons, are reserved for patients with a high headache burden is that these patients may be referred to tertiary centres and consequently included in these trials during exacerbations [45, 46]. In these patients, there may be an element of regression towards the mean during follow-up periods since the natural history of headache includes periods of worsening. As time passes, frequency and intensity may decrease which should not be interpreted as treatment response. As discussed above, the solution to this problem is longer baseline periods—with appropriate ethical considerations. In most trials, the status of chronicity and refractoriness must have been present for at least 2 years which seems prudent. Further, if the preventive response is genuinely due to the neurostimulation, frequency should increase if stimulation is seized.
Another clinical factor which has not been a problem for pharmaceutical trials is the issue of side-shift. Unilaterality is reported to be around 60% in migraine patients with around 40% being alternating unilateral [47]. In CH, spontaneous side shift has been reported to be around 15% [48]. In one cohort of chronic CH patients receiving ONS, infrequent contralateral attacks were reported in 5/14 patients [49]. For interventions targeting particular anatomical structures, permanent side-shift is quite important but sporadic contralateral attacks less so. Retrospective collection of data in this regard may be particularly prone to bias, as patients may be less likely to remembering the odd contralateral attack, or indeed whether their last cluster occurred on the opposite side. It has been suggested that a side-shift of attacks may actually indicate effective, unilateral, targeted treatment [50], but it is not likely that stimulation on one side would affect contralateral attacks. Thus, laterality of attacks must strictly be established prior to implant. A clear distinction should be made between patients who have previously experienced contralateral attacks and patients who, after lateralized stimulation, experience contralateral attacks or manifest side shift.
5 Conclusion
Neurostimulation offers some distinct advantages over pharmaceutical and injectable strategies. Completed trials and published data have revealed a number of caveats and investigators are taking these into consideration moving forward. It is likely that as further experience is gained with neurostimulation and it becomes available at an increasing number of centres, the indications for its use will expand. This use must of course be based on good clinical evidence and trials providing this evidence should take into consideration the unique benefits and challenges associated with working in the neurostimulation field.
References
Tfelt-Hansen P, Pascual J, Ramadan N, Dahlöf C, D’Amico D, Diener H-C, Hansen JM, Lanteri-Minet M, Loder E, McCrory D, Plancade S, Schwedt T, International Headache Society Clinical Trials Subcommittee. Guidelines for controlled trials of drugs in migraine: third edition. A guide for investigators. Cephalalgia. 2012;32(1):6–38.
Silberstein S, Tfelt-Hansen P, Dodick DW, Limmroth V, Lipton RB, Pascual J, Wang SJ, Task Force of the International Headache Society Clinical Trials Subcommittee. Guidelines for controlled trials of prophylactic treatment of chronic migraine in adults. Cephalalgia. 2008;28(5):484–95.
Bendtsen L, Bigal M, Cerbo R, Diener H, Holroyd K, Lampl C, Mitsikostas D, Steiner T, Tfelt-Hansen P, International Headache Society Clinical Trials Subcommittee. Guidelines for controlled trials of drugs in tension-type headache: second edition. Cephalalgia. 2010;30(1):1–16.
Lipton RB, Micieli G, Russell D, Solomon S, Tfelt-Hansen P, Waldenlind E. Guidelines for controlled trials of drugs in cluster headache. Cephalalgia. 1995;15(6):452–62.
Jürgens TP, Leone M. Pearls and pitfalls: neurostimulation in headache. Cephalalgia. 2013;33(8):512–25.
Coffey RJ, Lozano AM. Neurostimulation for chronic noncancer pain: an evaluation of the clinical evidence and recommendations for future trial designs. J Neurosurg. 2006;105(2):175–89.
Goadsby PJ, Schoenen J, Ferrari MD, Silberstein SD, Dodick D. Towards a definition of intractable headache for use in clinical practice and trials. Cephalalgia. 2006;26(9):1168–70.
Schulman EA, Peterlin BL, Lake AE, Lipton RB, Hanlon A, Siegel S, Levin M, Goadsby PJ, Markley HG. Defining refractory migraine: results of the RHSIS Survey of American Headache Society members. Headache. 2009;49(4):509–18.
Schulman EA, Lake AE, Goadsby PJ, Peterlin BL, Siegel SE, Markley HG, Lipton RB. Defining refractory migraine and refractory chronic migraine: proposed criteria from the refractory headache special interest section of the American Headache Society. Headache. 2008;48(6):778–82.
Miller S, Watkins L, Matharu M. Treatment of intractable chronic cluster headache by occipital nerve stimulation: a cohort of 51 patients. Eur J Neurol. 2017;24(2):381–90.
Giorgio L, Adnan AK, Stefano P, Roy C, Samuel W, David P. Safety and efficacy of cervical 10 kHz cervical spinal cord stimulation (SCS) for the management of refractory chronic migraine: a prospective, proof-of-concept open label study. J Headache Pain. 2018;19(Suppl 1):148.
Jürgens TP, Schoenen J, Rostgaard J, Hillerup S, Láinez MJ, Assaf AT, May A, Jensen RH. Stimulation of the sphenopalatine ganglion in intractable cluster headache: expert consensus on patient selection and standards of care. Cephalalgia. 2014;34(13):1100–10.
Leone M, May A, Franzini A, Broggi G, Dodick D, Rapoport A, Goadsby PJ, Schoenen J, Bonavita V, Bussone G. Deep brain stimulation for intractable chronic cluster headache: proposals for patient selection. Cephalalgia. 2004;24(11):934–7.
Serrano D, Lipton RB, Scher AI, Reed ML, Stewart WBF, Adams AM, Buse DC. Fluctuations in episodic and chronic migraine status over the course of 1 year: implications for diagnosis, treatment and clinical trial design. J Headache Pain. 2017;18(1):101.
Lambru G, Andreou AP, Guglielmetti M, Martelletti P. Emerging drugs for migraine treatment: an update. Expert Opin Emerg Drugs. 2018. https://doi.org/10.1080/14728214.2018.1552939.
Dodick DW, Silberstein SD, Reed KL, et al. Safety and efficacy of peripheral nerve stimulation of the occipital nerves for the management of chronic migraine: long-term results from a randomized, multicenter, double-blinded, controlled study. Cephalalgia. 2015;35(4):344–58.
Lipton R, Goadsby PJ, Cady R, Aurora S, Grosberg B, et al. PRISM study: occipital nerve stimulation for treatment-refractory migraine. Cephalalgia. 2009;29(S1):30.
Arcioni R, Palmisani S, Mercieri M, et al. Cervical 10 kHz spinal cord stimulation in the management of chronic, medically refractory migraine: a prospective, open-label, exploratory study. Eur J Pain. 2016;20(1):70–8.
Silberstein SD, Dodick DW, Saper J, et al. Safety and efficacy of peripheral nerve stimulation of the occipital nerves for the management of chronic migraine: results from a randomized, multicenter, double-blinded, controlled study. Cephalalgia. 2012;32(16):1165–79.
Lipton RB, Fanning KM, Buse DC, et al. Identifying natural subgroups of migraine based on comorbidity and concomitant condition profiles: results of the Chronic Migraine Epidemiology and Outcomes (CaMEO) study. Headache. 2018;58(7):933–47.
Martelletti P, Katsarava Z, Lampl C, et al. Refractory chronic migraine: a consensus statement on clinical definition from the European Headache Federation. J Headache Pain. 2014;15(1):47.
Miller S, Watkins L, Matharu M. Long-term outcomes of occipital nerve stimulation for chronic migraine: a cohort of 53 patients. J Headache Pain. 2016;17(1):68.
Palmisani S, Al-Kaisy A, Arcioni R, Smith T, Negro A, Lambru G, Bandikatla V, Carson E, Martelletti P. A six year retrospective review of occipital nerve stimulation practice- controversies and challenges of an emerging technique for treating refractory headache syndromes. J Headache Pain. 2013;14:67.
Adelborg K, Szépligeti SK, Holland-Bill L, Ehrenstein V, Horváth-Puhó E, Henderson VW, Sørensen HT. Migraine and risk of cardiovascular diseases: Danish population based matched cohort study. BMJ. 2018;360:k96.
Whealy M, Nanda S, Vincent A, Mandrekar J, Cutrer FM. Fibromyalgia in migraine: a retrospective cohort study. J Headache Pain. 2018;19(1):61.
Mitsikostas DD, Edvinsson L, Jensen RH, Katsarava Z, Lampl C, Negro A, Osipova V, Paemeleire K, Siva A, Valade D, Martelletti P. Refractory chronic cluster headache: a consensus statement on clinical definition from the European Headache Federation. J Headache Pain. 2014;15:79.
Schulman EA, Lake AE 3rd, Goadsby PJ, et al. Defining migraine and refractory chronic migraine: proposed criteria from the Refractory Headache Specialist Interest Section of the American Headache Society. Headache. 2008;48(6):778–82.
Schoenen J, Jensen RH, Lantéri-Minet M, Láinez MJ, Gaul C, Goodman AM, Caparso A, May A. Stimulation of the sphenopalatine ganglion (SPG) for cluster headache treatment. Pathway CH-1: a randomized, sham-controlled study. Cephalalgia. 2013;33(10):816–30.
Tfelt-Hansen P, Block G, Dahlöf C, Diener HC, Ferrari MD, Goadsby PJ, Guidetti V, Jones B, Lipton RB, Massiou H, Meinert C, Sandrini G, Steiner T, Winter PB, International Headache Society Clinical Trials Subcommittee. Guidelines for controlled trials of drugs in migraine: second edition. Cephalalgia. 2000;20(9):765–86.
Tassorelli C, Diener HC, Dodick DW, Silberstein SD, Lipton RB, Ashina M, Becker WJ, Ferrari MD, Goadsby PJ, Pozo-Rosich P, Wang SJ, International Headache Society Clinical Trials Standing Committee. Guidelines of the International Headache Society for controlled trials of preventive treatment of chronic migraine in adults. Cephalalgia. 2018;38(5):815–32.
Kosinski M, Bayliss MS, Bjorner JB, Ware JE Jr, Garber WH, Batenhorst A, Cady R, Dahlöf CGH, Dowson A, Tepper S. A six-item short-form survey for measuring headache impact: the HIT-6™. Qual Life Res. 2003;12(8):963–74.
Ware JE, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473–83.
García-Azorin D, Yamani N, Messina LM, Peeters I, Ferrili M, Ovchinnikov D, Speranza ML, Marini V, Negro A, Benemei S, Barloese M. A PRISMA-compliant systematic review of the endpoints employed to evaluate symptomatic treatments for primary headaches. J Headache Pain. 2018;19(1):90. https://doi.org/10.1186/s10194-018-0920-9.
Fontaine D, Lazorthes Y, Mertens P, Blond S, Géraud G, Fabre N, Navez M, Lucas C, Dubois F, Gonfrier S, Paquis P, Lantéri-Minet M, Geraud G, Fabre N, Navez M, Lucas C, Dubois F, Gonfrier S, Paquis P, Lanteri-Minet M. Safety and efficacy of deep brain stimulation in refractory cluster headache: a randomized placebo-controlled double-blind trial followed by a 1-year open extension. J Headache Pain. 2010;11(1):23–31.
Fontaine D, Christophe Sol J, Raoul S, Fabre N, Geraud G, Magne C, Sakarovitch C, Lanteri-Minet M. Treatment of refractory chronic cluster headache by chronic occipital nerve stimulation. Cephalalgia. 2011;31(10):1101–5.
Conforto AB, Amaro E, Gonçalves AL, Mercante JP, Guendler VZ, Ferreira JR, Kirschner CC, Peres MF. Randomized, proof-of-principle clinical trial of active transcranial magnetic stimulation in chronic migraine. Cephalalgia. 2014;34(6):464–72.
Wolter T, Kiemen A, Porzelius C, Kaube H. Effects of sub-perception threshold spinal cord stimulation in neuropathic pain: a randomized controlled double-blind crossover study. Eur J Pain. 2012;16(5):648–55.
Wilbrink LA, Teernstra OPM, Haan J, et al. Occipital nerve stimulation in medically intractable, chronic cluster headache. The ICON study: rationale and protocol of a randomised trial. Cephalalgia. 2013;33(15):1238–47.
Lewis JA. Migraine trials: crossover or parallel group? Neuroepidemiology. 1987;6(4):198–208.
Ansarinia M, Rezai A, Tepper SJ, Steiner CP, Stump J, Stanton-Hicks M, Machado A, Narouze S. Electrical stimulation of sphenopalatine ganglion for acute treatment of cluster headaches. Headache. 2010;50(7):1164–74.
Haines SJ, Haines SJ. Presidential address: ‘How do you know?,’. Clin Neurosurg. 1997;44:1–15.
Geerlings RP, Haane DY, Koehler PJ. Rebound following oxygen therapy in cluster headache. Cephalalgia. 2011;31(10):1145–9.
Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition. Cephalalgia. 2018;38(1):1–211.
Dworkin RH, Turk DC, McDermott MP, et al. Interpreting the clinical importance of group differences in chronic pain clinical trials: IMMPACT recommendations. Pain. 2009;146(3):238–44.
Barloese M, Lund N, Petersen A, Rasmussen M, Jennum P, Jensen R. Sleep and chronobiology in cluster headache. Cephalalgia. 2015;35(11):969–78.
Bekkelund SI, Müller KI, Wilhelmsen A, Alstadhaug KB. Photophobia and seasonal variation of migraine in a subarctic population. Headache. 2017;57(8):1206–16.
Kelman L. Migraine pain location: a tertiary care study of 1283 migraineurs. Headache. 2005;45(8):1038–47.
Meyer EL, Laurell K, Artto V, et al. Lateralization in cluster headache: a Nordic multicenter study. J Headache Pain. 2009;10(4):259–63.
Magis D, Gerardy P-Y, Remacle J-M, Schoenen J. Sustained effectiveness of occipital nerve stimulation in drug-resistant chronic cluster headache. Headache. 2011;51(8):1191–201.
Sjaastad O. Cluster headache syndrome. London: W.B. Saunders; 1992.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Barloese, M., Lambru, G. (2020). Methodological Difficulties in Clinical Trials Assessing Neuromodulation Devices in the Headache Field. In: Lambru, G., Lanteri-Minet, M. (eds) Neuromodulation in Headache and Facial Pain Management. Headache. Springer, Cham. https://doi.org/10.1007/978-3-030-14121-9_17
Download citation
DOI: https://doi.org/10.1007/978-3-030-14121-9_17
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-14120-2
Online ISBN: 978-3-030-14121-9
eBook Packages: MedicineMedicine (R0)