Abstract
Tanning industry is receiving a lot of attention due to its pollution levels and discharge legislations toward the environment. Tanerry wastewater treatment by using electrochemical oxidation with SnO2/Ti and PbO2/Ti anodes. The tannery wastewater was pretreated using biological method with aerotank process. SnO2/Ti and PbO2/Ti anodes effectively treated COD and total nitrogen over 85% after 90 min of electrolysis. The treatment efficiency in tannery wastewater of SnO2/Ti anode is greater than PbO2/Ti anode.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
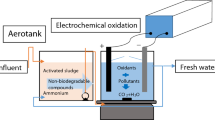
Tanning is an industry that pollutes the environment, including solids, liquids, and gaseous media. Unwanted organic substances such as hair, fat, and meat in the original materials (fresh skin, salted skin) are removed with the excess chemicals with inorganic and organic waste to the wastewater (Dinh et al. 2013). The decomposition of organic matter in the original material creates a foul smell characteristic in the production area and the surrounding area. Evaporation and boiler emissions also contribute negatively to the state of the environment. The pollutants in tannery wastewater require a combination of treatments such as mechanical, physical, chemical like fenton, and biological methods. However, due to economic and technical constraints, the majority of pollutants after treatment at the factories are still high, the basic pollution parameters such as BOD5, COD, NH3, and color do not still not meet the effluent discharge standards. Recently, the electrochemical oxidation has been extensively studied for the application of many types of wastewater treatment. Electrochemicals open up new avenues for the treatment of harmful organic pollutants, which can oxidize toxic organic pollutants with phenolic microorganisms into organic substances that can be completely oxidized to CO2 and water. This technology consists of the direct and/or indirect oxidation of organic matter contained in wastewater in a suitable anode or in a solution of an electrochemical device. However, in the process of electrolysis, the side reactions that make up oxygen can occur and reduce the oxidation efficiency. The electrochemical treatment of industrial wastewater is increasingly in concern because it has its own advantages such as simple equipment, high capacity for medium and small scale, low initial investment, electrical speed control and easy automation, requires very little or no chemicals in the process, environmental friendly, and high selectivity.
2 Materials and Methods
All the experiments were performed at room temperature and analysis followed the Standard Method for the examination of Water and Wastewater (USA). At regular time intervals, wastewater samples were withdrawn and collected in beaker for further analysis. pH, color, and conductivity were measured by using Metrohm 900 multimeter, Switzerland. Chemical oxygen demand (COD) was measured by using Lovibond RD125 Thermoreactor Closed Reflux Titrimetric Method, England. Total Nitrogen (TN) was measured by using TOC Shimadzu 00936, Japan. Total Chromium in the wastewater was analyzed by using an atomic absorption spectrometer (Analytic Jena Contraa 300, Germany). Total \({{\text{NO}}_{3}}^{ - }\), \({{\text{NH}}_{4}}^{ + }\), and Cl− were measured by using ion chromatography (Metrohm IC 883, Switzerland).
3 Results and Discussion
3.1 Effect of pH
The result of treatment of pollutants in tanning effluents with SnO2/Ti and PbO2/Ti anodes corresponding to difference pH is presented in Fig. 1. As shown in Fig. 2a, the color treatment efficiency of the SnO2/Ti electrode was quite high, up to 60.7% at pH 8, equivalent to a drop of color to 186 Pt–Co. Increasing or decreasing pH of the wastewater compared to pH 8 resulted in a reduction of treatment efficiency. The color treatment efficiency in the base medium is higher than the acidic environment. The COD removal efficiency of SnO2/Ti anode when changing the pH of the wastewater gave similar results to the color treatment, which is due to the colorant compounds in the wastewater treatment is mainly the organic residue remaining after biological pretreatment (e.g., tannic acid). When chemically oxidized organic compound into easily digestible secondary products, like either CO2 or H2O, is followed by a reduction in COD and color. The COD and TN treatment efficiencies when using SnO2/Ti electrode gave the best at pH 8 with 51.8 and 55.2%, equivalent to 149 and 77.4 mg/L, respectively. Similar to the color processing efficiency, total COD and nitrogen removal efficiency were lowest at pH 3 with 37.7 and 24.6%. The pH values significantly influence on the effluent treatment efficiency of the SnO2/Ti electrode. The color, COD, and TN removal efficiencies are best in neutral medium (pH 7), which results in higher processing efficiency in the acid or base mediums. This result agreed with the study of Xiuping Zhu et al. (2009) reported the N–NH3 treatment by changing pH that neutral N–NH3 was more effective than in acidic environments. This is explained by the fact that nitrogen in tannery wastewater after biological pretreatment mainly exists in the N–NH3 form and is readily decomposed in the base medium according to the following reaction:
As another aspect showed in Fig. 2b, the color treatment efficiency of the PbO2/Ti electrode was highest at pH 7 with 56.3%, corresponding a reduction to 196 Pt–Co. Effectively, color treatment in acidic environments showed better performance than in the base environment. The lowest color treatment efficiency was achieved at pH 11 when the treatment efficiency was only 31.6%, equivalent to the color reduction to 299 Pt–Co. Similarly with the color efficiency, COD and TN efficiencies also were best achieved at pH 7 with a COD removal efficiency 47.1%, equivalent to a reduction to 165 mg/L, the TN removal efficiency was 52.81% equivalent to the reduction to 78.2 mg/L. COD removal efficiency was low at pH 3 and pH 11 with 31.6 and 31.8%, respectively. Meanwhile, the TN treatment efficiency was lowest at pH 3, reaching only 35.67%. After treatment, the pH of the wastewater was reduced.
3.2 Effect of Current Density
Figure 2 shows the experimental result of tannery wastewater treatment using electrochemical oxidation at SnO2/Ti and PbO2/Ti anodes with different current densities. The current densities significantly affect tannery wastewater after biological pretreatment with SnO2/Ti anode. Increasing the current density increases the treatment efficiency of pollutants in wastewater. As shown in Fig. 2a with SnO2/Ti anode, the color treatment efficiency rapidly increased as the current density increased from 16.7 to 100 mA/cm2 then decreased at 133.3 mA/cm2. In particular, at current density 16.7 mA/cm2, the treatment efficiency was 41.6%, equivalent to the reduction of 279 Pt–Co. When increasing the current density to 33.3, 66.6, and 100 mA/cm2, the color treatment efficiency achieved 59, 82.4, and 87%, respectively. At the current density 133.3 mA/cm2, the color treatment efficiency reduced to 78.7%, equivalent to 102 Pt–Co. The trend of decreasing COD and total Nitrogen is quite similar to color, however at a smaller scale. This indicates the stable and comprehensive treatment of SnO2/Ti anode after biological pretreatment of tannery wastewater.
4 Conclusion
In this study, SnO2/Ti and PbO2/Ti anodes can thoroughly treat the pollutants in the tannery wastewater after activated sludge pretreatment. SnO2/Ti and PbO2/Ti anodes effectively treated COD and total nitrogen to up to 85% after 90 min of electrolysis at a current density of 66.7 mA/cm2. From the above results, it was found that the pH of the wastewater directly affected the efficiency of the treatment. SnO2/Ti electrode gave higher efficiency in wastewater with neutral pH to base, while PbO2/Ti electrode offered higher processing efficiency in acidic to neutral wastewater. The current density and the rate of agitation affected the treatment efficiency of the two electrodes, while the duration of the treatment is inversely proportional to the depletion of pollutants in the effluent. SnO2/Ti anode has higher treatment efficiency, lower energy consumption, and lower cost compared to PbO2/Ti anode in tannery wastewater treatment; however, its stability is not quite good. The combination of biological pretreatment and electrochemical oxidation can treat the tannery wastewater to achieve the Vietnamese discharge standard for effluents.
References
Benhadji, A., Ahmed, M. T., & Maachi, R. (2011). Electrocoagulation and effect of cathode materials on the removal of pollutants from tannery wastewater of Rouïba. Desalination, 277, 128–134.
Deghles, A., & Kurt, U. (2016). Treatment of raw tannery wastewater by electrocoagulation technique: Optimization of effective parameters using Taguchi method. Desalination and Water Treatment, 57, 14798–14809.
Dinh, H. T. (2013). Light manufacturing in Viet Nam (pp. 1–89). The World Bank.
Lofrano, G., Meriç, S., Zengin, G., & Orhon, D. (2013). Chemical and biological treatment technologies for leather tannery chemicals and wastewaters: A review. Science of the Total Environment, 461, 265–281.
Zhu, X., Ni, J., & Lai, P. (2009). Advanced treatment of biologically pretreated coking wastewater by electrochemical oxidation using boron-doped diamond electrodes. Water Research, 43, 4347–4355.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this paper
Cite this paper
Le Luu, T., Tien, T.T., Duong, N.B., Phuong, N.T.T. (2020). Tannery Wastewater Treatment After Biological Pretreatment by Using Electrochemical Oxidation. In: Naddeo, V., Balakrishnan, M., Choo, KH. (eds) Frontiers in Water-Energy-Nexus—Nature-Based Solutions, Advanced Technologies and Best Practices for Environmental Sustainability. Advances in Science, Technology & Innovation. Springer, Cham. https://doi.org/10.1007/978-3-030-13068-8_39
Download citation
DOI: https://doi.org/10.1007/978-3-030-13068-8_39
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-13067-1
Online ISBN: 978-3-030-13068-8
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)