Abstract
The unique role of hyaluronan (HA) in mammal life can be easily understood considering that the first reaction in mammals involves this molecule. In fact, the spermatozoa, before fertilization, use a specific hyaluronidase to digest a large stratum of HA surrounding the oocyte in order to reach it. HA is an extracellular matrix polymer with extraordinary structure and functions: it is a simple, linear, and unbranched polymer chain without sulfate or phosphate groups. Nevertheless, it has a key role in several physiological and pathophysiological processes in mammals. HA is ubiquitous in mammalian tissues with several specific functions, such as influencing cell proliferation and migration, angiogenesis, and inflammation. Considering the simple structure of HA, to exert several important functions in tissues, this polymer can only be modified in its concentration and size. Hence, HA content in tissues is carefully controlled by different mechanisms, including covalent modification of the synthetic enzymes and epigenetic control of their gene expression. HA function is also critical in several diseases including cancer, diabetes, and chronic inflammation. Among these biological roles, the biophysical properties of HA allow its use as a hydrogel in regenerative medicine, cosmetics, and drug delivery. HA takes advantage from its capacity to form gels even at the concentration of 1% producing non-immunogenic scaffolds with very intriguing mechanical properties. The HA hydrogels are useful tools in regenerative medicine as biocompatible material for advanced therapeutic uses, also as drug delivery system.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
1 Introduction
1.1 Structure
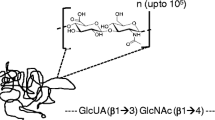
Hyaluronan (HA) is the simplest glycosaminoglycan (GAG) present in nature. Even though characterized by a simple linear chain, it has several important biomechanical properties. First it has terrific hydrophilic properties, in fact, 1 g of this polymer can interact with several liters of water, and this influences most of the biomechanical properties of the polymer (Laurent and Fraser 1992). HA is a polymer constituted by disaccharide units of d-glucuronic acid (GlcUA) linked to N-acetyl-d-glucosamine (GlcNAc) with a glucuronic beta 1–3 linkage between GlcUA and GlcNAc and a hexosaminidic bond beta 1–4 between GlcNAc and GlcUA (Fig. 4.1, panel A). The disaccharide unit can interact with 25 water molecules and is repeated till 25,000 times; hence, the HA chain reaches a molecular mass of millions of Dalton generating a linear polymer with a molecular mass ranging from 5 × 05 to 5 × 06 Da and more (Fig. 4.1, panel B) (Fraser et al. 1997; Viola et al. 2015b). The synthesis of HA is peculiar: it is the only GAG produced on the cell membrane and immediately extruded in the extracellular matrix (ECM) without any protein linkage. The other GAGs are produced inside the Golgi and secreted in the ECM or exposed on the cell membrane linked to the protein core constituting proteoglycans.
HA chemical structure. (a) The hyaluronan disaccharide is composed of d-glucuronic acid and d-N-acetylglucosamine linked together with β-1-3 and β-1-4 glycosidic bonds, respectively. This disaccharide can be repeated up to thousands of times to give high molecular mass HA. (b) Schematic representation of HA disaccharide by using the symbol nomenclature for graphical representations of glycans (Varki et al. 2015)
The enzymes involved in HA synthesis are located on the cellular membrane and are structurally organized to extrude the polymer outside of the cell in the ECM. These enzymes are called hyaluronan synthases (HASes) and in mammals are present as three different isoforms: HAS1, 2, and 3. The isoforms are coded on different chromosomes, and these genes could be a product of an ancestral gene duplication, which can explain the specific evolutionary path of this molecule (Spicer and McDonald 1998). Until now, scarce information is available on the structure of these enzymes, as they have never been crystallized. HA is widely distributed in mammals and in few bacteria and considering the other GAGs or structural-related carbohydrate polymers as chitin and cellulose, interestingly, appeared late in the evolution (Csoka and Stern 2013).
HASes use the UDP-sugar precursors (UDP-GlcUA and UDP-GlcNAc) to produce HA chain picking up these molecules from the cytoplasm. In these enzymes, the presence of a double catalytic domain to interact with the two different substrates generating the disaccharide units necessary to create the polymer is of remarkable interest. The kinetic of the HASes has been extensively studied, even though, without crystallography information, all kinetic explanations are still hypothetical (Weigel and DeAngelis 2007).
The presence of three different enzymes to produce HA raised the question if each specific enzyme shows unique kinetic properties, and it has been proposed that the different enzymes can produce polymers with different length and at different rates (Itano and Kimata 2002; Viola et al. 2015a, b; Vigetti et al. 2015). Beside the chain length, a large body of evidences supports the idea that the HASes also differ between each other in the regulation of their catalytic activity. Only HAS2 has several covalent regulations, as phosphorylation (Suzuki et al. 1995; Vigetti et al. 2011a), O-GlcNAcylation (Vigetti et al. 2012), and ubiquitination (Karousou et al. 2010). Indeed, in the case of HAS3, its activity is regulated by its sorting to the cell membrane by interaction with Rab10 (Deen et al. 2014).
To exert its biological properties, HA requires specific interactions with receptors which not only regulate the cell-ECM interactions but trigger intracellular signaling. Beside receptors, other proteins can interact with HA and regulate tridimensional ECM structure. In general, all proteins that interact with HA are defined “hyaloadherins” including not only receptors but also proteoglycans and other ECM molecules (Tammi et al. 2011). Proteoglycans as aggrecan, neurocan, brevican, and versican can be included among these ECM molecules interacting with HA-forming networks that act as an architectural scaffold (Laurent and Fraser 1992). The biological functions of HA in tissues are due to its interactions with these “hyaloadherins.” Such proteins are receptors (i.e., CD44, RHAMM, LYVE-1 , Layilin, Stabilin 1, and HARE) and, in fact, can trigger specific intracellular signaling (for a review on this issue, see (Day and Prestwich 2002)) or receptors that mediate endocytosis and degradation of HA. Proteins can interact with HA by using the LINK module or Bx7B motif (Day and Prestwich 2002; Baggenstoss et al. 2017). Interestingly, TLR2/4 was recently described as an HA-binding receptor, even though it does contain neither the LINK module nor the BX7B motif. TLR2/4 is necessary to trigger the response after LMW-HA stimulation; however this process is still controversial (Cyphert et al. 2015). Although the physical direct interaction between TLR and HA is still not experimentally demonstrated, it is reasonable that the polyanionic nature of HA mimics the canonical ligands of TLR2/4 as lipopolysaccharides. HA and PG LiNk protein family (HAPLN1–4) has been described to interact with HA and PG stabilizing such multicomponent complexes (Day and Prestwich 2002).
The most common HA receptor is CD44, a proteoglycan widely distributed in different cells and particularly concentrated on the membranes of inflammatory and cancer cells (Toole 2004). Another important HA receptor related to cell motility is RHAMM. RHAMM is an acronym for receptor for hyaluronan-mediated motility; the receptor is also known as CD168. RHAMM has been found in several cell types, including cancer and endothelial cells (Hardwick et al. 1992; Savani et al. 2001). Interaction between HA and RHAMM triggers a signaling pathway not completely described which includes ras oncogene activity (Hall and Turley 1995; Hofmann et al. 1998; Tolg et al. 2006). It is also known that RHAMM and CD44 share ERK1/2 phosphorylation cascade activation (Tolg et al. 2006).
Even though HA is typically present in mammalian ECM, it is also described in few bacteria, which probably imported the genes from mammals during evolution. It is noteworthy that HA-producing bacteria use three genes, which encode UDP-glucose pyrophosphorylase (hasC), UDP-glucose dehydrogenase (hasB), and HA synthase (hasA), and these genes are arranged in an operon (Weigel and DeAngelis 2007; Weigel et al. 2013).
Interestingly, microorganisms such as Streptococcus uberis, Streptococcus equisimilis, Streptococcus pyogenes, and Pasteurella multocida (pathogens for humans and other vertebrates) evolved with the capacity to produce a HA capsule which acts as a shield-protecting bacteria from the immune system (Lee and Spicer 2000). Bacterial and vertebrate HA present the same structure with the same biomechanical properties and absence of immunogenic activity, which represents a major advantage in medical applications.
In the last decade, HA started to be considered more than a passive molecule, characterized by remarkable mechanical properties, including space filler and molecular sieving. Due to its biological properties, this GAG emerged among the most biologically active relevant molecules in the body. HA plays a key role in several physiological processes including development (Vigetti et al. 2006), wound healing (Motolese et al. 2013), cell migration (Vigetti et al. 2009b), and proliferation (Vigetti et al. 2011b). It is also involved in several pathologies, such as cancer (Toole 2004), vascular diseases (Merrilees et al. 2011; Bollyky et al. 2012; Vigetti et al. 2014d), and diabetes (Bollyky et al. 2012).
1.2 Discovery of Hyaluronan
The first description of HA was done by Carl Meyer (Meyer and Palmer 1934), who purified the polymer from corpus vitreous of the eye. In the next years, HA was found in other tissues, and today it is known to be present in almost all mammalian tissues. The first described property for HA is its dramatic capacity to interact with water, and, because of that, most of the initial hypotheses for HA role in tissues were related to this hydrophilic characteristic. Hence, the main function attributed to HA is ECM space filler and organizer. HA presence in synovial fluid suggested its role as a lubricant in the joints. This observation is based on the first clinical application of HA in visco-supplementation in joint disease. Until now, this therapy represents one of the most common HA applications in humans. The polymer rheology is strongly dependent on its size and concentration, and pharmaceutical companies are induced to produce specific HA solutions with defined rheology for specific applications. The use of bioreactors with genetically modified bacteria for the production of highly pure HA opened the field to HA-focused biotechnology. This technology dramatically changed the field and is now considered obsolete for the presence of tissue contaminants.
The human body contains more than 15 g HA, mainly located in the dermis. HA turnover is fast; about 30% of the molecule is replaced daily (Stern 2004). The human body finely regulates HA production, and the polymer is mainly removed through the lymphatic system to the liver. Polymer size is critical for HA biological function. In healthy tissues, a typical HA size is, approximately, 1 × 103 kDa, and this is considered high molecular weight HA (HMWHA). The relationship between size and biological functions is confirmed by recent literature describing a very HMWHA in naked mole rat (Tian et al. 2013), an animal with an incredible longevity and cancer resistance.
HA fast degradation rate is due to the activity of a class of enzymes called hyaluronidases, hydrolases which degrade HA in small fragments, named oligosaccharides (Stern 2004; Stern et al. 2007). It was recently demonstrated that HA oligosaccharides exert important biological functions in tissues (Stern et al. 2006). HA fragments are usually internalized into cells and destroyed within lysosomes. When oligosaccharide concentration exceeds the cell capacity to properly remove these fragments from ECM, they accumulate and trigger important biological processes, inducing angiogenesis and inflammation and changing cell behavior (Bohaumilitzky et al. 2017). Besides hyaluronidase activity, there are other processes of HA depolymerization, including exposure to UV radiation and free radicals. Moreover, tissue HA content is critical, and its reduction, such as in aging, dramatically influences the viscoelastic properties of the tissues. Several years ago, it was described that HA decrease in synovial fluid represents joint disease marker (Dicker et al. 2014). Figure 4.2 summarizes HA metabolic roles.
Schematic representation of HA metabolism. HA is synthesized by HAS1, 2, or 3, located in the plasma membrane that uses cytosolic UDP-GlcUA and UDP-GlcNAc as precursors. HASes catalyze the formation of a high molecular weight HA (HMWHA) that can remain associated to the enzyme (contributing to form the pericellular coat) or can be released in to ECM. During inflammation HA can be covalently bond with the heavy chains (HC) of inter-alpha-inhibitor (I-alpha-I). TSG6 catalyzes the transfer reaction of HC to the hydroxyl group of C6 of GlcNAc. Modified HA can form aggregates that under microscopy look like cables that possess the ability to interact and recruit monocytes via CD44. HA can be fragmented both via enzymatic reactions (catalyzed by different HA-degrading enzymes) and via nonenzymatic reactions (i.e., UV and ROS). Low molecular weight HA can be further internalized in the cell and completely degraded in lysosomes throughout receptor CD44 and HARE. Alternatively, LMW-HA can also trigger inflammation via activation of TLRs and NF-kB
1.3 Biological Properties of Hyaluronan
HA presents a simple linear structure and exerts several functions by the sole modification of its size and concentration (Stern et al. 2006; Dicker et al. 2014). As previously described, high molecular weight and concentration can protect animals from aging and cancer development (Tian et al. 2013). Interestingly, HA fragments present biological relevance and often an opposite effect compared to the high molecular weight intact molecule (Erickson and Stern 2012).
The presence of HA oligos in specific conditions gained great interest, producing an increasing body of literature that describes HA fragments as ligands for toll-like receptors (TLR), triggering specific inflammatory pathways (Jiang et al. 2005, 2011; Iijima et al. 2011). In an opposite way, HMWHA shows anti-angiogenic, immune-suppressive, and anti-inflammatory activities, inducing tissue-reparative processes, as described in wound healing (Jiang et al. 2011; Motolese et al. 2013). HA oligosaccharides below 200 kDa show the capacity to induce inflammatory processes, as well as, angiogenesis through the interactions with specific receptors (Day and Prestwich 2002; Iijima et al. 2011). Considering the latter properties, HA oligosaccharides can be considered part of the alarmin protein family (Gariboldi et al. 2008).
The biological activities of both HMWHA and oligosaccharides are mediated by cell-surface receptors (Sherman et al. 1994). HA functions depend on its structural properties. Hydrophilic properties mediate water content control in all tissues. One of the most important HA biological functions is to maintain a well-hydrated ECM and a perfect environment for cell migration and proliferation (Vigetti et al. 2006). The presence of a well-hydrated and soft ECM is critical for embryo development; in fact, oocytes’ microenvironment is mainly constituted of HA (Fenderson et al. 1993). The unique viscoelastic properties of HA have critical roles in the biomechanical functions of various tissues, from corpus vitreous to derma (Fraser et al. 1997). It is of remarkable importance to consider that HA presents a gel form when its concentration is above 1%, a concentration often reached in mammalian tissues. The space between epidermal keratinocytes, the synovial fluid, and the Wharton jelly from umbilical cords are examples of gel-like structures formed by high HA concentration, in which this polymer plays the role of space filler and shock-absorbing macromolecule (Raio et al. 2005; Viola et al. 2015b).
HMWHA and HA oligosaccharides’ biological activities are mediated by their specific receptors. CD44, the most abundant HA receptor, triggers ERK 1/2 activation (Itano 2008; Jeyapalan et al. 2011). CD44/HA interactions have been studied in inflammatory cells, where HA is able to interact with inflammatory cell receptors (CD44) to block cells in the inflamed area (Jiang et al. 2005, 2007, 2011). The signaling cascade triggered by CD44/HA includes PI3K/PDK1/Akt activation and Ras phosphorylation, which involves RAF1, MEK, and, eventually, Erk1/2 (Vigetti et al. 2008b). CD44/HA complexes recruit other cytoplasmic proteins, such as ezrin, merlin, and erbB1/2 (Toole 2004).
HA binding to CD44 is critical for cell adhesiveness, triggering the phosphorylation of CD44 cytoplasmic domain, which is also required for cell migration and infiltration (Jiang et al. 2007). CD44 signaling also participates in wound healing, influencing fibroblast migration into the wounded area from perilesional stroma (Clark et al. 2004). It is evident that CD44 does not directly regulate cell migration; however, its activation by HMWHA promotes the wound healing process. Moreover, the directionality of cell migration is strongly dependent on CD44 expression and on HA gradient in the ECM environment (Acharya et al. 2008).
RHAMM has been described in cancer cells and in endothelial cells (Hardwick et al. 1992; Savani et al. 2001). Several studies report that RHAMM plays a critical role in cell migration and inflammation and in tissue healing (Tolg et al. 2003; Zaman et al. 2005).
Stabilin 2, also known as HARE, an acronym for “hyaluronan receptor for endocytosis,” was initially described in endothelial cells in lymph nodes, the spleen, and the liver (Zhou et al. 2000; Nonaka et al. 2007). More recently, HARE has been described in the eye, brain, kidney, and heart. HARE also interacts with other GAGs, such as chondroitin sulfates (A, B, C, D, and E) (Harris et al. 2007). HARE is mainly implicated in HA endocytosis and localizes close to clathrin clusters in the cell membrane. Finally, HARE in endocytosis is well reported; however, its involvement in lysosomal pathways is still not clear, and information on its activity at molecular level is scarce (Zhou et al. 2002, 2003).
Lymphatic vessel endothelial hyaluronan receptor 1 (LYVE 1 ) is a HA receptor specifically expressed on lymphatic endothelial cells, also found in lymph nodes and sinusoidal endothelial cells in the liver (Prevo et al. 2001; Mouta Carreira et al. 2001; Wróbel et al. 2005). Due to this peculiar cell distribution, LYVE 1 is generally considered a molecular marker for lymphatic endothelial cells (Akishima et al. 2004). LYVE 1 interacts with HA, and the resulting complex receptor/HA can be internalized and digested in lysosomes (Johnson et al. 2007). LYVE 1 main function is HA absorption and transfer from tissues to the lymph acting as a regulator of tissue hydration. LYVE 1 abrogation from lymphatics generated animals with normal physiology, indicating that other still unknown receptors may rescue LYVE 1 activity in mammals (Gale et al. 2007).
A large body of evidence supports the role of toll-like receptors (TLRs) in HA signaling. TLRs belong to the innate immunity system and are expressed on the plasma membrane of all mammalian cells (Aderem and Ulevitch 2000; Takeda et al. 2003). Humans produce ten TLRs, and those involved in HA signaling are TLR2 and 4 (Jiang et al. 2011), TLR2 being able to interact with mycobacteria and Gram-positive bacteria, whereas TLR4 recognizes lipopolysaccharide (LPS). In macrophages, HA triggers, via TLR2 or TLR4, a signaling cascade that promotes expression of chemokine genes, and this process is strongly dependent on MyD88 (Jiang et al. 2011). The signaling triggered by TLR/HA strongly depends on HA size. TLR clustering on the cell membrane is promoted by interaction with HMWHA, and this complex induces cell survival. In an opposite fashion, HA oligosaccharides induce inflammatory stress and cell death (Jiang et al. 2011). It is important to note that the direct interaction between HA and TLR is not completely resolved and further experiments must be performed. HA oligosaccharides also induce dendritic cell maturation and TNF-alpha synthesis through the phosphorylation of MAPK and NF-kB nuclear translocation. HA oligosaccharides are also involved in transplant rejection demonstrating their involvement in alloimmunity (Tesar et al. 2006). The synthesis of IL-8 and MMP2 is also stimulated by HA/TLR4 interaction (Voelcker et al. 2008). Moreover, during osteoclast differentiation, HA/TLR4 interaction blocks the signaling triggered by macrophage colony-stimulating factor (M-CSF) (Chang et al. 2007). The role of HA oligosaccharides in the gut has been recently described as inducers of beta-defensin-2 synthesis in intestinal epithelium (Hill et al. 2012). HA oligosaccharide role in defensin synthesis has remarkable importance in tissue metabolism and wound healing. In fact, defensins are a family of small proteins with antibiotic properties and capacity to stimulate tissue regeneration (Gariboldi et al. 2008; Hill et al. 2012).
In tissues, HA degradation rate regulates HA concentration. Mammals present six hyaluronidases (Hyal-1, -2, -3, -4, P1, and PH20) which catalyze the hydrolysis of linkage bonds beta1-4 between hexosamine and glucuronic acid residues. The hyaluronidases (Hyals) are classified as endo-beta-N-acetylglucosaminidases according to their hydrolytic mechanisms (Csoka et al. 2001; Stern et al. 2007). All six Hyals in human are β, 1–4 endogalactosaminidases. The genes HYAL1, HYAL2, and HYAL3 code for the enzymes Hyal-1, Hyal-2 , and Hyal-3 (Csoka et al. 2001; Soltés et al. 2006). These genes are tightly clustered on chromosome 3p21.3. Hyal-1 and -2 are the major hyaluronidases found in tissues. Hyal-1 is found in lysosomes, whereas Hyal-2 is a GPI-anchored protein with extracellular activity (Csoka et al. 2001). Hyal-2 produces HA fragments with a size of about 20 kDa. Hyal-1 appears to be a lysosomal protein which cleaves HA into small disaccharides. The role and activities of Hyal-3 are still elusive, and few information is available on this enzyme, such as its report from KO mice-based experiments (Dumaresq-Doiron et al. 2012).
Other three genes HYAL4, HYAL1, and SPAM1 (sperm adhesion molecule1) are clustered on chromosome 7q31.3. Hyal-4 is a pseudogene transcribed, but not translated, in the human, and PH-20 is the enzyme that digests oocyte-surrounding HA, facilitating ovum fertilization (Cherr et al. 2001). The Hyals (1–4) can work in acidic environment (about pH 3 and 4), whereas PH-20 and other hyaluronidases from insects and snakes’ venoms are active at neutral pH (Girish and Kemparaju 2007). Hyal 1 , common in mammalian tissues, is active intracellularly. Finally, Hyal-1 deficiency leads to a genetic disease called mucopolysaccharidosis type IX (Martin et al. 2008).
Hyaluronidase 2 (Hyal 2) is a GPI-anchored receptor operating in acidic microenvironment (Bourguignon et al. 2004). This enzyme degrades HMWHA into low molecular weight HA (LMW-HA) (about 20 kDa) (Stern 2004) which is internalized and further digested to smaller HA oligosaccharides (oligo-HA) by Hyal 1 .
Interestingly, bacteria present several hyaluronidases which act as lyases (Csoka et al. 2001; Csoka and Stern 2013). In mammals, Hyal 1 and 2 activities are synergic; in fact, Hyal 2 degrades HA to fragments of 20 kDa, and, then, Hyal 1 degrades these fragments into smaller fragments of about 800 Da. HA polymer degradation could also be due to the action of free radicals that break HA polymer in fragments of unspecific size (Soltés et al. 2006). HA minimal size able to trigger cell response has been extensively addressed. It has been reported that 4-6 disaccharide units (4-6mers) oligomers are responsible for NF-kB signaling and metalloprotease synthesis (Voelcker et al. 2008); oligomers ranging from 4 to 16 disaccharides are able to activate dendritic cells via TLR receptors (Taylor et al. 2004; Takahashi et al. 2005).
Recently, a new hyaluronidase has been described: TMEM2, which is a transmembrane protein with strong hyaluronidase activity (Yamamoto et al. 2017; Yamaguchi et al. 2018). TMEM2 specifically degrades HMWHA into ∼5-kDa fragments. Another hyaluronidase called CEMIP/KIAA1199 (also known as HYBID) has been recently identified with HA-degrading activity (Nagaoka et al. 2015; Yoshida et al. 2018). Interestingly, this enzyme has a key role in cancer development, in skin biology, and in cell senescence (Zhang et al. 2014b; Yoshida et al. 2018).
2 The Magic Glue: Hyaluronan and Its Oligosaccharide Chemical Structure
HA is a GAG polymer constituted by disaccharide units of d-glucuronic acid (GlcUA) linked to N-acetyl-d-glucosamine (GlcNAc) with a glucuronic beta 1–3 linkage between GlcUA and GlcNAc and a hexosaminidic beta bond 1-4 between GlcNAc and GlcUA. These units are repeated, approximately, 25,000 times to a molecular mass of millions of Daltons ranging from 5 × 105 to 5 × 106 Da and more (Fraser et al. 1997; Viola et al. 2015b). The biotechnological properties of HA are unique and dependent on the structure of the polymer. HA structure is based on the presence of beta linkages which allows HA bulky groups (the hydroxyls, the carboxylate moiety, and the anomeric carbon on the adjacent sugar) to be in sterically favorable equatorial positions, favoring chemical modification. All axial positions, less favorable for chemical derivatization, are occupied by simple hydrogen atoms (Tamer 2013). When the polymer is in aqueous solution, a network of hydrogen bonds is established and maintains HA stiffness. The axial small hydrogens form a relatively hydrophobic environment, whereas the equatorial groups are more hydrophilic and interact with solvent, creating a twisting ribbon structure. In solution, HA forms an extended random coil structure, which can interact with other chains. Even at very low concentrations, soluble HA chains can entangle in each other. This phenomenon starts at 1 mg/mL and is a spontaneous process (Morris et al. 1980). HA helical chain in solution can bind 1000 times its weight in water (Cowman and Matsuoka 2005). At 1% HA forms a hydrogel with soft properties which allows handling using syringe with needles; it can be defined as a “quasi-plastic material” (Narins et al. 2003). Moreover, HA chains in solution can form double helices after and during entanglement (Scott et al. 1991). These physical aspects indicate HA hydrogel a perfect product with lubricant properties that can be used for replacing synovial fluid or in surgery to prevent postsurgical adhesion formation after abdominal surgery procedures.
HA hydrogel formation represents the most important property, allowing this molecule to be used as an innovative biocompatible product in several applications (Burdick and Prestwich 2011). HA solution viscosity increases with concentration, and its elasticity increases with polymer size. The relationship between elasticity and viscosity is two critical parameters used in commercial hyaluronan gel preparation (Edsman et al. 2012). The organ surface takes advantage from the rheological properties of HA, as in cartilage and in muscle bundles acting as osmotic buffer regulating the water content in the tissues (Simkovic 2013). From these viscoelastic properties arise the biomechanical characteristics for hydrogel medical applications (Jha et al. 2009; Bonafè et al. 2014). HA gels are useful tools for scaffold preparations in regenerative medicine. They are biodegradable, biocompatible, and bioresorbable. HA scaffolds induce cell differentiation and growth (Itano 2008) and improve wound healing without nonspecific absorption of proteins enhancing tissue growth and repair (Seyfried et al. 2005a, b; Damodarasamy et al. 2014). Preparation of highly purified HA is based on the applications of modern biotechnology procedures using bioreactors with genetically modified bacteria. The availability of large amounts of highly pure HA polymer introduces the possibility to treat arthritic joints, restoring lubrication and replacing the rheological properties of synovial fluid. HA chains can be chemically modified using adipic hydrazide, tyramide, benzyl ester, glycidyl methacrylate, thiopropionyl hydrazide, or bromoacetate, either at carboxylic acid of glucuronic acid or at the C-6 hydroxyl group of the N-acetylglucosamine (Darr and Calabro 2009). HA hydrogels can be used as drug delivery systems, as proposed in ophthalmology and otolaryngology (Lim et al. 2002; Välimäki 2015). HA hydrogels’ main function is to facilitate in situ drug release by HA degradation (Yang et al. 2012). In other in vivo models, the reabsorption of HA matrix is the mechanism used to produce new tissue in situ, stimulating cells with growth factors, such as BMP2 in cartilage (Jha et al. 2009).
3 Hyaluronan Biosynthesis Control
HA has a peculiar distribution in the human body with a rapid turnover. HA content is strictly regulated by cells in a finely tuned balance between synthetic and catabolic activities. Part of the polymer could also be removed from tissues by lymphatic system, which transport HA to liver for final degradation. Most oligosaccharides produced by hyaluronidase digestion are removed by cells through receptor-mediated internalization.
3.1 Formation of UDP-GlcNAc and UDP-Glucuronic Acid
The regulation of the HA synthesis includes mechanisms still poorly understood. One of them is substrate availability. It was demonstrated that substrate availability (UDP-sugars) influences HA synthesis. Altering UDP-sugars’ availability in cytoplasm influenced HA production as well as expression of HAS2 and 3 (Vigetti et al. 2003b, 2006).
UDP-sugar precursors are molecules with a high-energy cost, competing with glycolysis and other catabolic pathways for their synthesis (see Fig. 4.3, panel A). Hence, GAG synthesis is possible in tissues with a good oxygen supply, as oxygen allows the two oxidative reactions necessary for UDP-GlcUA synthesis (Fig. 4.3, panel B). The synthesis of UDP-GlcUA is a critical step for all GAGs, except keratan sulfate (KS), which does not contain uronic acid. In fact, KS is usually common in tissues with poor oxygen supply or even without vascularization. The synthesis of UDP-GlcUA requires the action of the UDP-glucose dehydrogenase (UGDH), which produces UDP-GlcUA from precursor UDP-Glc (Fig. 4.3, panel B). This reaction is possible in the presence of NAD, which is transformed in NADH during the double oxidation of the C-6 of UDP-Glc. This uncommon reaction has a remarkable role in terms of cell energy balance. From this point of view, the costs of UDP-GlcUA synthesis are completely balanced by the re-oxidation in mitochondria of the two NADH molecules produced by the UDP-GlcUA synthesis. HA synthesis reaction stoichiometry indicates that the disaccharide units contain one molecule of GlcUA and one GlcNAc with a ratio of 1:1. The five ATP obtained by the re-oxidation of two NADH molecules in mitochondria balance the energy cost of HA-unsulfated backbone synthesis.
(a) Schematic representation of the metabolism of dietary monosaccharides (in red) in the formation of activated sugar nucleotides used in biosynthesis of various glycans. UDP-sugars used for GAG and PG biosynthesis are shown in green. All reactions take place in the cytosol except for reaction boxed in blue. In this scheme almost all the enzymes have been omitted except for the critical enzymes involved in UDP-GlcUA synthesis (UGPP and UGDH), UDP-GlcNAc synthesis through the hexosamine biosynthetic pathway (GFAT), and UDP-GalNAc (epimerase) synthesis. These latter UDP-sugars will be further chemically modified in HS/heparin, CS, and KS biosynthesis. (b) Conversion of UDP-glucose to UDP-glucuronic acid by UGDH. In the scheme is highlighted the involvement of 2 NAD in the oxidation of the C6 of glucose with the generation of 2 NADH. The regeneration of NAD via the mitochondrial metabolism is critical to supply energy. The big red arrows highlight the double oxidation on C6
The modulation of UDP-GlcUA availability by overexpressing or silencing UGDH in cytoplasm can dramatically influence HA production, as well as expression of HAS2 and 3 (Vigetti and Passi 2014; Vigetti et al. 2003b, 2006, 2014b, c).
4-methylumbelliferone confirmed this aspect as it binds UDP-GlcUA, reducing the availability of this precursor (Viola et al. 2008; Vigetti et al. 2009b; Piccioni et al. 2012). Another way to regulate HA synthesis involves the activity of adenosine monophosphate-activated protein kinase (AMPK), the cell’s energy level sensor. It was demonstrated that the activation of AMPK can phosphorylate HAS2 threonine 110 residue, blocking enzyme activity (Vigetti et al. 2011a). It is remarkable that this kind of regulation is specific only for HAS2 and does not influence the other two enzymes. Moreover, this regulation only affects HA production, leaving other GAGs’ synthesis unaltered. This observation correlates a cell’s energy level with its specific capacity to produce HA.
3.2 Regulation of the UDP-Sugar Precursors Concentration in Cytoplasm
As discussed previously the first HA synthesis regulation level is UDP-sugar availability in cytoplasm. The substrate for glycosyltransferases used to synthesize all the glycans (i.e., GAGs, as well as, glycoproteins and glycolipids) is nucleotide-activated sugars. UDP is the most common nucleotide used to activate sugars in animal cells, as it is found linked to glucose (Glc), galactose (Gal), N-acetylated glucosamine (GlcNAc ), N-acetylated galactosamine (GalNAc), glucuronic acid (GlcA), and xylose (Xyl). Only two additional nucleotides are used in animals, GDP that is linked to mannose (Man) and fucose (Fuc) and CMP that is linked to sialic acid (Sia) (Fig. 4.3, panel A) (Yarema and Bertozzi 2001). It is to be noted that all the substrates for glycoconjugate reactions are sugar nucleotides generated mainly in the cytosol, with the exception for the synthesis of UDP-Xyl and CMP-Sia that take place in the Golgi and in the nucleus, respectively (Fig. 4.3, panel A). Further, these sugar-nucleotide precursors are transported in the ER/Golgi by specialized transporters (Gerardy-Schahn et al. 2001; Hadley et al. 2014). This point is crucial, as it allows the creation of two precursor pools: one in the cytosol and the other inside the ER/Golgi. Although the real concentration of the sugar nucleotides in these two pools is not easily measurable, it is reasonable to suppose that ER/Golgi precursors would be in higher concentration than in the cytosol, as transporters have low Km values, ensuring an efficient supply of such precursors in the ER/Golgi lumen. On the other hand, the cytosolic pool could be directly affected by nutrients, as clearly described for the concentration of UDP-GlcNAc (Marshall et al. 2004).
4-methylumbelliferone, a drug that binds the UDP-GlcUA reducing the availability of this precursor, is another way to confirm this metabolic aspect (Vigetti et al. 2009a; Piccioni et al. 2012). The activation of AMPK by AICAR induces the phosphorylation of a threonine (Thr 110) in HAS2, blocking enzyme activity (Vigetti et al. 2011a). AMPK activity acts only on HAS2 and not on HAS1 and 3, confirming the specific regulation of HASes. Moreover, AMPK activity plays a role only for HA synthesis, and not for the synthesis of other GAGs, indicating that HA is the only GAG affected by energy modulation mechanisms, correlating cell energy level with HA synthesis.
The concentration of the second UDP-sugar precursor of HA synthesis (UDP-GlcNAc) has an important role in this context; however, it participates in a different mechanism. UDP-GlcNAc influences HA synthesis not only as precursor but also because HAS2 itself is a target of O-GlcNAcylation, a protein covalent modification described by Torres and Hart in 1984 (Torres and Hart 1984). The cytosolic levels of UDP-GlcNAc are finely regulated by the hexosamine pathway. When UDP-GlcNAc is elevated in the cytoplasm, it induces the activity of the enzyme O-GlcNAc transferase (OGT), which catalyzes the β-O-linkage of one residue of N-acetylglucosamine (GlcNAc) to serine 221 of HAS2 (Vigetti et al. 2012). As previously discussed for the AMPK regulation, the O-GlcNAcylation is a covalent regulation specific for HAS2 and does not affect other HASes and the synthesis of the other GAGs. O-GlcNAcylation is a protein covalent modification widely described in several physiological and pathological conditions including cancer and chronic diseases (Dias and Hart 2007; Hart et al. 2007). It is interesting to note that protein O-GlcNAcylation is related to general metabolic conditions, and UDP-GlcNAc is defined as a “general sensor” of energy level in mammalian cells (Lewis and Hanover 2014). In the case of HAS2, the mechanism used by cells to regulate HA synthesis through O-GlcNAcylation affects enzyme stability on the membrane. HAS2 is usually active on the cell membrane for 17 min, whereas after O-GlcNAcylation, the enzyme can remain active on the cell membrane for more than 5 h, increasing HA content in the ECM (Vigetti et al. 2012). The regulation sites of the enzyme are reported in Fig. 4.4.
Schematic representation of human HAS2. HAS2 protein, which belongs to Class I HASes, is schematized in the plasma membrane and the eight transmembrane helices represented as cylinders. The large intracellular loop between transmembrane helices two and four contains the catalytic site. Moreover, the three critical residues modified by phosphorylation, O-GlcNAcylation, and ubiquitination that modulate protein activity, stability, and dimerization are shown in different colors
3.3 Hyaluronan Synthases
HASes are classified in two classes. Class I HASes are multiple transmembrane proteins that are present in bacteria and animals. Typically, bacterial enzymes contain six membrane-associated helices, whereas animals’ HASes have eight transmembrane domains (Fig. 4.4). Pasteurella multocida expresses a peculiar HAS that belongs to Class II family; it has only one transmembrane domain at the C-terminal of the protein and has a completely different mechanism of catalysis (for a review see (Weigel and DeAngelis 2007)).
The literature suggests that several growth factors and cytokines regulate the expression of the HASes (Jacobson et al. 2000; Pienimaki et al. 2001; Karvinen et al. 2003; Pasonen-Seppänen et al. 2003) by signaling pathways triggered by specific receptors (Wang et al. 2005).
3.4 Epigenetic Regulation of Hyaluronan Synthase
Besides direct covalent modification of enzymes, HAS2 gene transcription epigenetic control has been described involving a long noncoding RNA. It was recently described a HAS2 antisense (HAS2-A1) which can increase HAS2 transcription acting as a gene activator in cis (Vigetti et al. 2014a, d). The presence of antisense transcript is common in different cell models. This epigenetic regulation was described in an in vivo approach based on murine and human tissue specimen (Vigetti et al. 2014c). Noteworthy, HAS2-A1 activity involves NF-kB signaling cascade through P65. The interaction of this nucleoprotein with antisense promoter may explain the correlation between inflammatory signals and HAS2 expression.
Even if several aspects of HASes activity are still elusive, it is evident that the complexity of activity regulation depends on the general metabolic conditions of the cells. HA synthesis regulation is carried out by direct and different covalent modifications at protein level, as well as by epigenetic modifications at gene expression level (Vigetti et al. 2014d).
4 Biological Role of Hyaluronan in Mammalian Tissues
4.1 Hyaluronan in Musculoskeletal Tissue
One of the first HA medical applications is in intra-articular injections to attenuate damage to cartilage in inflamed joints (Plaas et al. 2011). The complete mechanism of action is not fully understood, probably due to the combination of lubricant and anti-inflammatory properties of HMWHA (Jiang et al. 2011). HA could stimulate chondrocyte metabolism, restoring cartilage ECM to physiological deposition; nevertheless, this hypothesis should be confirmed by more robust experimental and clinical data. Alternative approaches foresee to use of stem cells with the aim to regenerate damaged tissues. Interestingly many scaffolds used to maintain stem cells in the affected area and to increase stem cell viability are HA hydrogels that probably trigger stem cells specific signals, favoring survival and proliferation. A similar approach is used for skin regeneration with interesting results (Burdick and Prestwich 2011).
4.2 Hyaluronan in the Skin
The skin contains the largest amount of HA in human body, particularly concentrated in the dermis. A dramatic reduction of HA is common in aging. HA decrease implies a diminished skin thickness and hydration (Meyer and Stern 1994; Tigges et al. 2014). Interestingly, the naked mole rat, the only rodent with the exceptional life span of about 30 years, produces an extremely large HA of over 20 million Dalton within the skin that protects this animal from cancers (Tian et al. 2013). Moreover, these animals are active and fertile for a very long time. On the other hand, HA in the skin is not always protective; in fact Chinese Shar-Pei dogs’ characteristic of wrinkled skin derives from mutations in HAS2 promoter that greatly increase HAS2 expression. These animals suffer from periodic fever syndrome (Olsson et al. 2011).
In epidermis, HA has a critical role in keratinocyte differentiation and is also involved in the scarless wound healing process, stimulating keratinocyte migration (Aya and Stern 2014). It is of remarkable importance to note that oligosaccharides have a dramatic effect on skin beta-defensin-2 secretion both in mice and in human (Gariboldi et al. 2008).
4.3 Hyaluronan in Cardiovascular Tissue
HA is critical in several pathophysiological conditions of the cardiovascular system. Normally, HA is present mainly in artery adventitia; nevertheless, in early stages of atherosclerosis, HA accumulates in the media layer, favoring migration and proliferation of smooth muscle cells and contributing to vessel thickening (Viola et al. 2015a). HA can, therefore, participate in immune cell recruitment to the vessel wall, favoring inflammation. HA is the major component of the endothelial glycocalyx, and when its content is reduced, immune cell adhesion increases and favors shear stress (Nagy et al. 2010; Fischer 2018). During acute inflammation, as well as angioplasty or, in animal models, after wire or balloon injuries, HA is involved in neointima ECM formation, and the inhibition of HA synthesis dramatically reduces neointima formation, as demonstrated in conditional HAS2 KO mice (Kashima et al. 2013).
4.4 Hyaluronan in Neural Tissue
The central nervous system has a HA-rich ECM that contributes to brain hydration in physiological conditions (Perkins et al. 2017). HA is present in the perineuronal net where it, in collaboration with other sulfated GAGs, such as chondroitin 4-sulfate, regulates neuron excitability via binding with divalent cations (Vigetti et al. 2008a). Recent findings showed that lack of HA in the brain causes reduced extracellular space volume, increasing the concentration of neurotransmitters and affecting normal synapsis function, as described in a mouse model of epilepsy (Arranz et al. 2014).
4.5 HA in Respiratory System
HA present in the lungs contributes to the mechanical and viscoelastic properties of the tissues influencing stiffness and elasticity. In asthmatic patients or after cigarette smoking, HA fragmentation is evident and can be involved in chronic inflammation via the TLR2/4 and NF-kB pathway and macrophage recruitment that eventually contribute to fibrosis (Lauer et al. 2015; Tighe and Garantziotis 2018). HA receptor RHAMM (but not CD44) has been described to have a pivotal role in hyperoxia-mediated neonatal lung injury, disrupting alveolar development (Liao et al. 2015).
4.6 Hyaluronan in Development
During development, gut rotation is a critical event. ECM and HA play a pivotal role in controlling such gut movements that take place in fetal development (Kurpios et al. 2008). In adult, patients with chronic intestinal inflammation (i.e., Crohn’s disease and ulcerative colitis) accumulate HA in colon nonvascular space dramatically increasing the recruitment of immune cells, contributing to the infiltration of leukocytes (Rieder et al. 2011; de la Motte 2011). In a rat model of inflammatory bowel disease, HA is altered not only in the mucosa but also in neurons of the enteric nervous system, where it could regulate physiological functions, such as cell motility (Filpa et al. 2017). HA is also involved in liver fibrosis representing a key molecule for therapy in pathophysiology (Neuman et al. 2016). HA has a critical role in development. Silencing of the main enzyme HAS2 in mouse leads to severe cardiac malformation that causes embryo death (Camenisch et al. 2000). The conditional abrogation of Has2 in different tissues also revealed its importance in skeletal growth, patterning, chondrocyte maturation, and joint formation in the developing limb (Matsumoto et al. 2009). HAS1 and HAS3 knockout mice do not show defects and have no effect on development. Recent data on HAS1 and HAS3 KO mice are investigated to detect specific HAS isoform functions, as reported, for instance, in the skin (Wang et al. 2014) or in hematopoiesis (Goncharova et al. 2012). In other model organisms, as the amphibian Xenopus laevis, lack of HAS3 impairs gastrulation (Vigetti et al. 2003a). In recent years, zebrafish has been used as a convenient model to study development and human pathologies, and HA has been described as a critical molecule for organogenesis and, interestingly, necessary for tail regeneration (Bakkers et al. 2004; Chanmee et al. 2015, 2016); Ouyang et al. 2017).
5 Hyaluronan in Pathology
5.1 Hyaluronan in Tumors
Aggressive malignancies present an increased HA content surrounding cells and inside them as well (Tammi et al. 2008; Chanmee et al. 2016). Upregulation of HASes and Hyals has been described in several malignancies, as well as an increased activity of HA receptors (Udabage et al. 2005). In many animal models, the inhibition of HA synthesis via 4-MU reduced tumor growth and metastasis, suggesting that HA metabolism could represent a good target for new therapeutic strategies (Nagy et al. 2015; Edelman et al. 2017).
Many tumors present an increased HA UDP-sugar precursor concentration, which is in accordance with an augmented HA synthesis (Oikari et al. 2018). Moreover, UDP-GlcUA is used in the liver to detoxify chemotherapy drugs, such as epirubicin, and, for this reason, an increased HA synthesis reducing UDP-GlcUA availability could favor drug resistance.
5.2 New Insights into Hyaluronan Role in Human Pathology
The use of hyaluronidases in therapy is a recent evidence that shows interesting application in the treatment of solid tumors where hyaluronidase treatment improves chemotherapy bioactivity (Infante et al. 2018). Hyaluronidase role in human pathology and in cancer is still not clear, and several controversial data are reported in literature. However, these contradictory data indicate that Hyal 1 and Hyal 2 might promote or suppress tumor development, suggesting that Hyals activity might be part of a finely tuned system which includes HA synthesis and degradation (McAtee et al. 2014).
It was previously discussed that HA is normally present in healthy tissue in high molecular weight. However, in some specific pathological conditions, such as inflammation or reactive oxygen species (ROS) production (Jiang et al. 2005, 2011), the amount of HA fragments with different low molecular weights increase, and these fragments show diverse biological activities (Stern et al. 2006).
ROS degrade HA chains generating biologically active fragments. Usually, ROS are present in injured tissues, in inflamed areas and in tumor microenvironment. They may provide a mechanism for generating HA fragments in vivo and may further exaggerate the inflammatory state.
The accumulation of the HA fragments should be carefully avoided, as they could trigger an inflammatory response. This is the reason why the removal of digested fragments from the ECM through internalization after their interactions with receptors, such as CD44, is a critical step for inflammation development. In fact, HA fragments can be recognized by specific receptors that could influence cell behavior according to their size (Iijima et al. 2011). From this point of view, HA oligosaccharides could be classified as matrikines (Maquart et al. 1999) as they have all characteristics of ECM active fragments. Oligosaccharides produced by the catabolic activity of hyaluronidase, if not rapidly internalized by the cells, can diffuse through tissues and bind to HA receptors on adjacent cells, acting as intracellular signals such as NF-kB and Erk.
6 Hyaluronan as Drug Delivery System and in Cosmetics
6.1 Hyaluronan as Drug Delivery System
HA is involved in several therapies in regenerative medicine and in cosmetics. In wound healing or in surgery as anti-adherence agent (Tsai et al. 2005), HA shows an effective activity due to its capacity to improve cell growth and angiogenesis.
HA hydrogel can be also used as a drug delivery system (Hsu et al. 2018; Jiang et al. 2018). The polymer chain chemistry can be easily modified with adipic hydrazide, tyramide, benzyl ester, glycidyl methacrylate, thiopropionyl hydrazide, or bromoacetate, either at carboxylic acid of glucuronic acid or at the C-6 hydroxyl group of the N-acetylglucosamine (Darr and Calabro 2009).
HA has attracted significant interest in development of drug delivery systems because of its intrinsic physicochemical and biological properties. As previously described, HA water solubility, viscoelasticity, non-immunogenicity, biocompatibility, and biodegradability are key aspects for consideration when applying this polymer as a vehicle for drug delivery (Saravanakumar et al. 2014). HA interacts with specific receptors (e.g., CD44) on disease-related cells, such as cancer cells and activated macrophages, followed by receptor-mediated endocytosis. With these unique features, HA has been extensively used for development of the targetable carriers to deliver therapeutic and imaging agents (Huang and Huang 2018). Many reports present an extensive use of HA in the controlled release and targeted drug delivery systems. However, most studies are performed only in vitro, and in vivo data are scarce (Huang and Huang 2018).
As previously discussed, HA presents hydroxyl, carboxyl, and N-acetyl groups suitable for chemical modification. Therefore, HA and its derivatives act as drug carriers; contribute to drug concentration, sustained release, and transdermal absorption; and improve drug targeting. The chemical coupling of cytotoxic drug to HA improves drug pharmacokinetic profile, prolongs drug distribution, and reduces time for drug effect (Zhang et al. 2014a). Basically, HA allows low plasma drug concentration increasing drug concentration in affected tissue (Ossipov 2010; Ossipov et al. 2010). HA and its derivatives have been widely used in various drug delivery systems, such as nanoparticle drug delivery system, gel drug delivery system, cationic polymer gene carrier system, nano-emulsion delivery system, polyelectrolyte microcapsule drug delivery system, microsphere drug delivery system, and film delivery system, and these various techniques enhanced flexibility (Saravanakumar et al. 2014). HA hydrogels are methods of gene delivery and have been widely used, especially in tissue engineering as HA forms a system that controls gene delivery for tissue regeneration.
One of the first drug delivery systems based on HA was the creation of an amphipathic vector with polyethyleneimine (PEI). The hyaluronic acid-PEI (HAP) for gene delivery by periodic acid oxidation is one of the first systems developed in this context (Yao et al. 2010). This vector protects DNA from nuclease degradation, isolates DNA from the complex, and is less toxic. The high transfection rate of HAP in cancerous HepG2 cells promoted more effective cell drug uptake. Another molecule coupled with HA was spermine, developed to improve transfection efficiency of encapsulated DNA. Synthetic hyaluronic acid-polylysine (PLL) conjugate is recognized by HARE receptor in the sinusoidal epithelium of liver cells (Asayama et al. 1998; Rosso et al. 2013). This complex was designed to induce the ε-amino group of HA-terminal PLL to form a comb-type copolymer by reducing the amino group. This copolymer has been used to form a complex with DNA after intravenous injection in the animal model. This system showed that the copolymer was mainly concentrated in the sinusoidal cells of the liver affecting gene expression. The first HA gene delivery application was HA-adipic acid dihydrazide (ADH) hydrogels used to protect DNA from enzyme degradation and for sustained release of DNA (Shoham et al. 2013). This injectable HA/ADH hydrogel worked as a vessel for protecting preadipocytes during and at short term after delivery to native tissues. This system was used in research toward regenerative medicine in tissue reconstructions. Therefore, HA can act as a non-viral vector for gene drugs and could be targeted to tumor cells through CD44 receptor-mediated endocytosis working as antitumor delivering gene drug (Huang and Huang 2018).
HA was also tested for siRNA delivery. HA cross-linking could be formed by disulfide bonds, which can be degraded by glutathione in the cytoplasm (Lee et al. 2007). Moreover, to confirm the mechanism of internalization, the efficiency of cell absorption and gene silencing was much higher in the CD44-overexpressed cell lines compared to cell lines with lower CD44 expression (Lee et al. 2007; Jang et al. 2014). The HAP conjugate was also developed to deliver siRNA through LYVE-1-mediated targeting cells (Jang et al. 2014).
A recent achievement is the production of complex polyethylene glycol (PEG) and HA, which resulted in a very useful tool for its pharmacokinetic properties. In fact, in physiological conditions, PEGylated HA nanoparticles (HA-NPs) formed self-assembled nanoparticles (217–269 nm in diameter) with negatively charged surfaces (Choi et al. 2011). The nanoparticles uptake is mediated by CD44, resulting in a high tumor targetability of PEGylated HA-NPs. These data were obtained in vitro and in vivo modes, supported by intravital tumor imaging. In these experiments, rapid extravasation into the tumor tissue was observed, indicating that PEGylated HA-NPs can be useful as a means for cancer therapy and diagnosis (Choi et al. 2011). PEGylation presents advantages in terms of biodistribution but may present some problems associated with long-term therapies. HA emerged as a good vehicle for drug delivery; in fact, each individual HA chain conjugates with several different peptides, making it possible for polypeptide drugs to exert multiple effects (Jiang et al. 2012). The coupling procedures for HA are very mild and use the carboxylic group as acceptor. This strongly supports the use of HA as drug delivery carrier. To prolong release time of protein drugs, HA hydrogels have been extensively studied producing hybrid hydrogel system showing both simple drug loading and controlled release with no denaturation of the protein drugs (Hirakura et al. 2010). One example of HA use in protein release by a cross-linked HA hydrogel was the product generated to release erythropoietin (EPO) (Motokawa et al. 2006). HA-drug conjugates are widely tested as they can improve drug solubility and change drug distribution and its half-life in vivo. These events can increase drug concentration in tumor tissue by enhancing the osmotic retention effect augmenting the efficacy of therapy (Fan et al. 2015; Dosio et al. 2016). The antitumor complex HA-paclitaxel was developed to improve the antitumor effect of this compound, and in vivo and in vitro tests proved the concept identifying CD44 as a receptor for this complex. HA-paclitaxel can effectively inhibit tumor growth in human cancer xenografts via HA-mediated mechanism (Galer et al. 2011). HA was also tested with liposomes (El Kechai et al. 2015), HA-modified polylactic acid-glycolic acid copolymer nanoparticles (HCDs) were prepared to increase drug uptake in breast cancer cells (Park et al. 2011). From these examples, HA has several advantages in cancer therapy and in drug release in general; these aspects are related to its good biocompatibility, easily chemically modifiable groups, and targeting of tumor cells via CD44. Nevertheless, some problems are still present in HA application in therapy, for instance, the number of molecules linked to the chain has to be carefully controlled as an excess of linking material can modify HA solubility and interaction with HA receptors. Moreover, as HA receptor is largely present in the liver, in intravenous treatment a great part of the complex can be blocked in this organ. These aspects limit widespread use of HA as a drug delivery system and require an improved tumor active targeting to ameliorate efficacy. The preliminary data from clinical trials indicate that industrialization and wide clinical application of HA as drug delivery system are still to be properly developed.
6.2 Hyaluronan in Cosmetics
In cosmetics HA polymer is commonly present in the products commercially available, and this is due to HA activity as a moisturizing agent. It has been demonstrated that elastic properties of the skin have been improved by the regular application of HA on skin even though the biological effects on keratinocytes are not completely understood at molecular level (Gariboldi et al. 2008). Nevertheless, even robust scientific data are still necessary to completely understand HA role in topic cutaneous application, several sunscreen products containing HA showed important anti-free radicals’ action with a protective activity against ultraviolet irradiation (Manuskiatti and Maibach 1996). In plastic surgery HA gels with entanglements or chemical linkages between chains are widely used as filler to treat facial lines and wrinkles (Maytin 2016; Fallacara et al. 2017). The success of this application is due to the greater tolerability of HA filler compared to collagen products (Duranti et al. 1998; Narins et al. 2003; Pao and Mancini 2014). Even the use of HA as filler is largely diffused in aesthetic medicine, it must be considered that HA is not only a filler (Maytin 2016). The filler technology addressed mainly the problem related to the HA integrity and the viscoelastic properties of the hydrogels used. In the market there are several formulations of HA filler that have chemical bonds between chains (cross-linkers) or natural entanglements between HA chains. HA concentration, the number of HA bonds, and polymer size influence hydrogel viscoelastic properties and gave the producers the possibility to develop dozens of different fillers. Duration, filler effect, syringe application, and skin rejuvenation are all aspects that characterize different filler products. HA filler advantages are its easy modulation considering the skin area, the effect target, and the possibility to be rapidly digested by hyaluronidases if the product generates adverse reactions (inflammation, granuloma).
Eventually, HA is also present in nutraceutical market, as beverages, food, and confectioneries which have been approved as health food material worldwide. Nevertheless, its use is still unclear as these macromolecules are completely digested in the gut.
7 Concluding Remarks
HA represents a key molecule in the ECM from the beginning of embryo development to advanced age. The aging of mammals is characterized by a marked reduction of HA content in tissues. In other acute and chronic pathologies, HA role emerged as a critical aspect in disease outcome. Randomized controlled trials have successfully proved the remarkable HA properties. Trials currently in progress approach various therapeutic strategies, including healing burns, surgery, and chronic wound healing (Voigt and Driver 2012). In diabetes, in chronic inflammatory diseases and in pulmonary and kidney fibrosis, HA represents a potential therapeutic target (Rieder et al. 2011; Wang et al. 2011; Petrey and de la Motte 2014; Hascall et al. 2014). The HA interactome and viscoelastic properties indicate that HA will be more than an ECM molecule. HA synergistic activity with bioactive molecules and drugs is close to be ready for clinical applications, even if few technical questions remain to be addressed. From this point of view, the HA hydrogels will be a common tool in engineered tissues and in regenerative medicine.
References
Acharya PS, Majumdar S, Jacob M, Hayden J, Mrass P, Weninger W et al (2008) Fibroblast migration is mediated by CD44-dependent TGF beta activation. J Cell Sci 121(Pt 9):1393–1402
Aderem A, Ulevitch RJ (2000) Toll-like receptors in the induction of the innate immune response. Nature 406(6797):782–787
Akishima Y, Ito K, Zhang L, Ishikawa Y, Orikasa H, Kiguchi H et al (2004) Immunohistochemical detection of human small lymphatic vessels under normal and pathological conditions using the LYVE-1 antibody. Virchows Arch 444:153–157
Arranz AM, Perkins KL, Irie F, Lewis DP, Hrabe J, Xiao F et al (2014) Hyaluronan deficiency due to Has3 knock-out causes altered neuronal activity and seizures via reduction in brain extracellular space. J Neurosci 34:6164–6176
Asayama S, Nogawa M, Takei Y, Akaike T, Maruyama A (1998) Synthesis of novel polyampholyte comb-type copolymers consisting of a poly(L-lysine) backbone and hyaluronic acid side chains for a DNA carrier. Bioconjug Chem 9:476–481
Aya KL, Stern R (2014) Hyaluronan in wound healing: rediscovering a major player. Wound Repair Regen 22:579–593
Baggenstoss BA, Harris EN, Washburn JL, Medina AP, Nguyen L, Weigel PH (2017) Hyaluronan synthase control of synthesis rate and hyaluronan product size are independent functions differentially affected by mutations in a conserved tandem B-X7-B motif. Glycobiology 27:154–164
Bakkers J, Kramer C, Pothof J, Quaedvlieg NE, Spaink HP, Hammerschmidt M (2004) Has2 is required upstream of Rac1 to govern dorsal migration of lateral cells during zebrafish gastrulation. Development 131:525–537
Bohaumilitzky L, Huber AK, Stork EM, Wengert S, Woelfl F, Boehm H (2017) A trickster in disguise: hyaluronan’s ambivalent roles in the matrix. Front Oncol 7:242. https://doi.org/10.3389/fonc.2017.00242
Bollyky PL, Bogdani M, Bollyky JB, Hull RL, Wight TN (2012) The role of hyaluronan and the extracellular matrix in islet inflammation and immune regulation. Curr Diab Rep 12:471–480
Bonafè F, Govoni M, Giordano E, Caldarera CM, Guarnieri C, Muscari C (2014) Hyaluronan and cardiac regeneration. J Biomed Sci 21:100. https://doi.org/10.1186/s12929-014-0100-4
Bourguignon LY, Singleton PA, Diedrich F (2004) Hyaluronan-CD44 interaction with Rac1-dependent protein kinase N-gamma promotes phospholipase Cgamma1 activation, Ca(2+) signaling, and cortactin-cytoskeleton function leading to keratinocyte adhesion and differentiation. J Biol Chem 279:29654–29669
Burdick JA, Prestwich GD (2011) Hyaluronic acid hydrogels for biomedical applications. Adv Mater 23:H41–H56
Camenisch TD, Spicer AP, Brehm-Gibson T, Biesterfeldt J, Augustine ML, Calabro A et al (2000) Disruption of hyaluronan synthase-2 abrogates normal cardiac morphogenesis and hyaluronan-mediated transformation of epithelium to mesenchyme. J Clin Invest 106:349–360
Chang EJ, Kim HJ, Ha J, Ryu J, Park KH, Kim UH et al (2007) Hyaluronan inhibits osteoclast differentiation via Toll-like receptor 4. J Cell Sci 120(Pt 1):166–176
Chanmee T, Ontong P, Kimata K, Itano N (2015) Key roles of hyaluronan and its CD44 receptor in the stemness and survival of cancer stem cells. Front Oncol 5:180. https://doi.org/10.3389/fonc.2015.00180
Chanmee T, Ontong P, Itano N (2016) Hyaluronan: a modulator of the tumor microenvironment. Cancer Lett 375:20–30
Cherr GN, Yudin AI, Overstreet JW (2001) The dual functions of GPI-anchored PH-20: hyaluronidase and intracellular signaling. Matrix Biol 20:515–525
Choi KY, Min KH, Yoon HY, Kim K, Park JH, Kwon IC et al (2011) PEGylation of hyaluronic acid nanoparticles improves tumor targetability in vivo. Biomaterials 32:1880–1889
Clark RA, Lin F, Greiling D, An J, Couchman JR (2004) Fibroblast invasive migration into fibronectin/fibrin gels requires a previously uncharacterized dermatan sulfate-CD44 proteoglycan. J Invest Dermatol 122:266–277
Cowman MK, Matsuoka S (2005) Experimental approaches to hyaluronan structure. Carbohydr Res 340:791–809
Csoka AB, Stern R (2013) Hypotheses on the evolution of hyaluronan: a highly ironic acid. Glycobiology 23:398–411
Csoka AB, Frost GI, Stern R (2001) The six hyaluronidase-like genes in the human and mouse genomes. Matrix Biol 20:499–508
Cyphert JM, Trempus CS, Garantziotis S (2015) Size matters: molecular weight specificity of hyaluronan effects in cell biology. Int J Cell Biol 2015:1–8
Damodarasamy M, Johnson RS, Bentov I, MacCoss MJ, Vernon RB, Reed MJ (2014) Hyaluronan enhances wound repair and increases collagen III in aged dermal wounds. Wound Repair Regen 22:521–526
Darr A, Calabro A (2009) Synthesis and characterization of tyramine-based hyaluronan hydrogels. J Mater Sci Mater Med 20:33–44
Day AJ, Prestwich GD (2002) Hyaluronan-binding proteins: tying up the giant. J Biol Chem 277:4585–4588
de la Motte CA (2011) Hyaluronan in intestinal homeostasis and inflammation: implications for fibrosis. Am J Physiol Gastrointest Liver Physiol 301:G945–G949
Deen AJ, Rilla K, Oikari S, Kärnä R, Bart G, Häyrinen J et al (2014) Rab10-mediated endocytosis of the hyaluronan synthase HAS3 regulates hyaluronan synthesis and cell adhesion to collagen. J Biol Chem 289:8375–8389
Dias WB, Hart GW (2007) O-GlcNAc modification in diabetes and Alzheimer’s disease. Mol BioSyst 3:766–772
Dicker KT, Gurski LA, Pradhan-Bhatt S, Witt RL, Farach-Carson MC, Jia X (2014) Hyaluronan: a simple polysaccharide with diverse biological functions. Acta Biomater 10:1558–1570
Dosio F, Arpicco S, Stella B, Fattal E (2016) Hyaluronic acid for anticancer drug and nucleic acid delivery. Adv Drug Deliv Rev 97:204–236
Dumaresq-Doiron K, Edjekouane L, Orimoto AM, Yoffou PH, Gushulak L, Triggs-Raine B et al (2012) Hyal-1 but not Hyal-3 deficiency has an impact on ovarian folliculogenesis and female fertility by altering the follistatin/activin/Smad3 pathway and the apoptotic process. J Cell Physiol 227:1911–1922
Duranti F, Salti G, Bovani B, Calandra M, Rosati ML (1998) Injectable hyaluronic acid gel for soft tissue augmentation. A clinical and histological study. Dermatol Surg 24:1317–1325
Edelman R, Assaraf YG, Levitzky I, Shahar T, Livney YD (2017) Hyaluronic acid-serum albumin conjugate-based nanoparticles for targeted cancer therapy. Oncotarget 8:24337–24353
Edsman K, Nord LI, Ohrlund A, Lärkner H, Kenne AH (2012) Gel properties of hyaluronic acid dermal fillers. Dermatol Surg 38:1170–1179
El Kechai N, Bochot A, Huang N, Nguyen Y, Ferrary E, Agnely F (2015) Effect of liposomes on rheological and syringeability properties of hyaluronic acid hydrogels intended for local injection of drugs. Int J Pharm 487:187–196
Erickson M, Stern R (2012) Chain gangs: new aspects of hyaluronan metabolism. Biochem Res Int 2012:1–9
Fallacara A, Manfredini S, Durini E, Vertuani S (2017) Hyaluronic acid fillers in soft tissue regeneration. Facial Plast Surg 33:87–96
Fan X, Zhao X, Qu X, Fang J (2015) pH sensitive polymeric complex of cisplatin with hyaluronic acid exhibits tumor-targeted delivery and improved in vivo antitumor effect. Int J Pharm 496:644–653
Fenderson BA, Stamenkovic I, Aruffo A (1993) Localization of hyaluronan in mouse embryos during implantation, gastrulation and organogenesis. Differentiation 54:85–98
Filpa V, Bistoletti M, Caon I, Moro E, Grimaldi A, Moretto P et al (2017) Changes in hyaluronan deposition in the rat myenteric plexus after experimentally-induced colitis. Sci Rep 7. https://doi.org/10.1038/s41598-017-18020-7
Fischer JW (2018) Role of hyaluronan in atherosclerosis: current knowledge and open questions. Matrix Biol. https://doi.org/10.1016/j.matbio.2018.03.003
Fraser JR, Laurent TC, Laurent UB (1997) Hyaluronan: its nature, distribution, functions and turnover. J Intern Med 242:27–33
Gale NW, Prevo R, Espinosa J, Ferguson DJ, Dominguez MG, Yancopoulos GD et al (2007) Normal lymphatic development and function in mice deficient for the lymphatic hyaluronan receptor LYVE-1. Mol Cell Biol 27:595–604
Galer CE, Sano D, Ghosh SC, Hah JH, Auzenne E, Hamir AN et al (2011) Hyaluronic acid-paclitaxel conjugate inhibits growth of human squamous cell carcinomas of the head and neck via a hyaluronic acid-mediated mechanism. Oral Oncol 47:1039–1047
Gariboldi S, Palazzo M, Zanobbio L, Selleri S, Sommariva M, Sfondrini L et al (2008) Low molecular weight hyaluronic acid increases the self-defense of skin epithelium by induction of beta-defensin 2 via TLR2 and TLR4. J Immunol 181:2103–2110
Gerardy-Schahn R, Oelmann S, Bakker H (2001) Nucleotide sugar transporters: biological and functional aspects. Biochimie 83:775–782
Girish KS, Kemparaju K (2007) The magic glue hyaluronan and its eraser hyaluronidase: a biological overview. Life Sci 80:1921–1943
Goncharova V, Serobyan N, Iizuka S, Schraufstatter I, de Ridder A, Povaliy T et al (2012) Hyaluronan expressed by the hematopoietic microenvironment is required for bone marrow hematopoiesis. J Biol Chem 287:25419–25433
Hadley B, Maggioni A, Ashikov A, Day CJ, Haselhorst T, Tiralongo J (2014) Structure and function of nucleotide sugar transporters: current progress. Comput Struct Biotechnol J 10:23–32
Hall CL, Turley EA (1995) Hyaluronan: RHAMM mediated cell locomotion and signaling in tumorigenesis. J Neuro-Oncol 26:221–229
Hardwick C, Hoare K, Owens R, Hohn HP, Hook M, Moore D et al (1992) Molecular cloning of a novel hyaluronan receptor that mediates tumor cell motility. J Cell Biol 117:1343–1350
Harris EN, Kyosseva SV, Weigel JA, Weigel PH (2007) Expression, processing, and glycosaminoglycan binding activity of the recombinant human 315-kDa hyaluronic acid receptor for endocytosis (HARE). J Biol Chem 282:2785–2797
Hart GW, Housley MP, Slawson C (2007) Cycling of O-linked beta-N-acetylglucosamine on nucleocytoplasmic proteins. Nature 446:1017–1022
Hascall VC, Wang A, Tammi M, Oikari S, Tammi R, Passi A et al (2014) The dynamic metabolism of hyaluronan regulates the cytosolic concentration of UDP-GlcNAc. Matrix Biol 35:14–17
Hill DR, Kessler SP, Rho HK, Cowman MK, de la Motte CA (2012) Specific-sized hyaluronan fragments promote expression of human β-defensin 2 in intestinal epithelium. J Biol Chem 287:30610–30624
Hirakura T, Yasugi K, Nemoto T, Sato M, Shimoboji T, Aso Y et al (2010) Hybrid hyaluronan hydrogel encapsulating nanogel as a protein nanocarrier: new system for sustained delivery of protein with a chaperone-like function. J Control Release 142:483–489
Hofmann M, Assmann V, Fieber C, Sleeman JP, Moll J, Ponta H et al (1998) Problems with RHAMM: a new link between surface adhesion and oncogenesis? Cell 95:592–593
Hsu MF, Tyan YS, Chien YC, Lee MW (2018) Hyaluronic acid-based nano-sized drug carrier-containing Gellan gum microspheres as potential multifunctional embolic agent. Sci Rep 8(1):731. https://doi.org/10.1038/s41598-018-19191-7
Huang G, Huang H (2018) Application of hyaluronic acid as carriers in drug delivery. Drug Deliv 25:766–772
Iijima J, Konno K, Itano N (2011) Inflammatory alterations of the extracellular matrix in the tumor microenvironment. Cancers (Basel) 3:3189–3205
Infante JR, Korn RL, Rosen LS, LoRusso P, Dychter SS, Zhu J et al (2018) Phase 1 trials of PEGylated recombinant human hyaluronidase PH20 in patients with advanced solid tumours. Br J Cancer 118(2):e3. https://doi.org/10.1038/bjc.2017.438
Itano N (2008) Simple primary structure, complex turnover regulation and multiple roles of hyaluronan. J Biochem 144:131–137
Itano N, Kimata K (2002) Mammalian hyaluronan synthases. IUBMB Life 54:195–199
Jacobson A, Brinck J, Briskin MJ, Spicer AP, Heldin P (2000) Expression of human hyaluronan synthases in response to external stimuli. Biochem J 348(Pt 1):29–35
Jang YL, Ku SH, Jin S, Park JH, Kim WJ, Kwon IC et al (2014) Hyaluronic acid-siRNA conjugate/reducible polyethylenimine complexes for targeted siRNA delivery. J Nanosci Nanotechnol 14:7388–7394
Jeyapalan Z, Deng Z, Shatseva T, Fang L, He C, Yang BB (2011) Expression of CD44 3′-untranslated region regulates endogenous microRNA functions in tumorigenesis and angiogenesis. Nucleic Acids Res 39:3026–3041
Jha AK, Hule RA, Jiao T, Teller SS, Clifton RJ, Duncan RL et al (2009) Structural analysis and mechanical characterization of hyaluronic acid-based doubly cross-linked networks. Macromolecules 42:537–546
Jiang D, Liang J, Fan J, Yu S, Chen S, Luo Y et al (2005) Regulation of lung injury and repair by Toll-like receptors and hyaluronan. Nat Med 11:1173–1179
Jiang D, Liang J, Noble PW (2007) Hyaluronan in tissue injury and repair. Annu Rev Cell Dev Biol 23:435–461
Jiang D, Liang J, Noble PW (2011) Hyaluronan as an immune regulator in human diseases. Physiol Rev 91:221–264
Jiang T, Zhang Z, Zhang Y, Lv H, Zhou J, Li C et al (2012) Dual-functional liposomes based on pH-responsive cell-penetrating peptide and hyaluronic acid for tumor-targeted anticancer drug delivery. Biomaterials 33:9246–9258
Jiang Z, Dong X, Yan X, Liu Y, Zhang L, Sun Y (2018) Nanogels of dual inhibitor-modified hyaluronic acid function as a potent inhibitor of amyloid β-protein aggregation and cytotoxicity. Sci Rep 8(1):3505. https://doi.org/10.1038/s41598-018-21933-6
Johnson LA, Prevo R, Clasper S, Jackson DG (2007) Inflammation-induced uptake and degradation of the lymphatic endothelial hyaluronan receptor LYVE-1. J Biol Chem 282:33671–33680
Karousou E, Kamiryo M, Skandalis SS, Ruusala A, Asteriou T, Passi A et al (2010) The activity of hyaluronan synthase 2 is regulated by dimerization and ubiquitination. J Biol Chem 285:23647–23654
Karvinen S, Pasonen-Seppänen S, Hyttinen JM, Pienimäki JP, Törrönen K, Jokela TA et al (2003) Keratinocyte growth factor stimulates migration and hyaluronan synthesis in the epidermis by activation of keratinocyte hyaluronan synthases 2 and 3. J Biol Chem 278:49495–49504
Kashima Y, Takahashi M, Shiba Y, Itano N, Izawa A, Koyama J et al (2013) Crucial role of hyaluronan in neointimal formation after vascular injury. PLoS One 8(3):e58760. https://doi.org/10.1371/journal.pone.0058760
Kurpios NA, Ibañes M, Davis NM, Lui W, Katz T, Martin JF et al (2008) The direction of gut looping is established by changes in the extracellular matrix and in cell:cell adhesion. Proc Natl Acad Sci USA 105:8499–8506
Lauer ME, Dweik RA, Garantziotis S, Aronica MA (2015) The rise and fall of hyaluronan in respiratory diseases. Int J Cell Biol 2015:1–15
Laurent TC, Fraser JR (1992) Hyaluronan. FASEB J 6:2397–2404
Lee JY, Spicer AP (2000) Hyaluronan: a multifunctional, megaDalton, stealth molecule. Curr Opin Cell Biol 12:581–586
Lee H, Mok H, Lee S, Oh YK, Park TG (2007) Target-specific intracellular delivery of siRNA using degradable hyaluronic acid nanogels. J Control Release 119:245–252
Lewis BA, Hanover JA (2014) O-GlcNAc and the epigenetic regulation of gene expression. J Biol Chem 289:34440–34448
Liao J, Kapadia VS, Brown LS, Cheong N, Longoria C, Mija D et al (2015) The NLRP3 inflammasome is critically involved in the development of bronchopulmonary dysplasia. Nat Commun 6:8977. https://doi.org/10.1038/ncomms9977
Lim ST, Forbes B, Berry DJ, Martin GP, Brown MB (2002) In vivo evaluation of novel hyaluronan/chitosan microparticulate delivery systems for the nasal delivery of gentamicin in rabbits. Int J Pharm 231:73–82
Manuskiatti W, Maibach HI (1996) Hyaluronic acid and skin: wound healing and aging. Int J Dermatol 35:539–544
Maquart FX, Siméon A, Pasco S, Monboisse JC (1999) Regulation of cell activity by the extracellular matrix: the concept of matrikines. J Soc Biol 193:423–428
Marshall S, Nadeau O, Yamasaki K (2004) Dynamic actions of glucose and glucosamine on hexosamine biosynthesis in isolated adipocytes: differential effects on glucosamine 6-phosphate, UDP-N-acetylglucosamine, and ATP levels. J Biol Chem 279:35313–35319
Martin DC, Atmuri V, Hemming RJ, Farley J, Mort JS, Byers S et al (2008) A mouse model of human mucopolysaccharidosis IX exhibits osteoarthritis. Hum Mol Genet 17:1904–1915
Matsumoto K, Li Y, Jakuba C, Sugiyama Y, Sayo T, Okuno M et al (2009) Conditional inactivation of Has2 reveals a crucial role for hyaluronan in skeletal growth, patterning, chondrocyte maturation and joint formation in the developing limb. Development 136:2825–2835
Maytin EV (2016) Hyaluronan: more than just a wrinkle filler. Glycobiology 26:553–559
McAtee CO, Barycki JJ, Simpson MA (2014) Emerging roles for hyaluronidase in cancer metastasis and therapy. Adv Cancer Res 123:1–34
Merrilees MJ, Beaumont BW, Braun KR, Thomas AC, Kang I, Hinek A et al (2011) Neointima formed by arterial smooth muscle cells expressing versican variant V3 is resistant to lipid and macrophage accumulation. Arterioscler Thromb Vasc Biol 31:1309–1316
Meyer K, Palmer JW (1934) The polysaccharide of the vitreous humor. J Biol Chem 107:629–634
Meyer LJ, Stern R (1994) Age-dependent changes of hyaluronan in human skin. J Invest Dermatol 102:385–389
Morris ER, Rees DA, Welsh EJ (1980) Conformation and dynamic interactions in hyaluronate solutions. J Mol Biol 138:383–400
Motokawa K, Hahn SK, Nakamura T, Miyamoto H, Shimoboji T (2006) Selectively crosslinked hyaluronic acid hydrogels for sustained release formulation of erythropoietin. J Biomed Mater Res A 78:459–465
Motolese A, Vignati F, Brambilla R, Cerati M, Passi A (2013) Interaction between a regenerative matrix and wound bed in nonhealing ulcers: results with 16 cases. Biomed Res Int 2013:1–5
Mouta Carreira C, Nasser SM, di Tomaso E, Padera TP, Boucher Y, Tomarev SI et al (2001) LYVE-1 is not restricted to the lymph vessels: expression in normal liver blood sinusoids and down-regulation in human liver cancer and cirrhosis. Cancer Res 61:8079–8084
Nagaoka A, Yoshida H, Nakamura S, Morikawa T, Kawabata K, Kobayashi M et al (2015) Regulation of Hyaluronan (HA) metabolism mediated by HYBID (Hyaluronan-binding protein involved in HA depolymerization, KIAA1199) and HA synthases in growth factor-stimulated fibroblasts. J Biol Chem 290:30910–30923
Nagy N, Freudenberger T, Melchior-Becker A, Röck K, Ter Braak M, Jastrow H et al (2010) Inhibition of hyaluronan synthesis accelerates murine atherosclerosis: novel insights into the role of hyaluronan synthesis. Circulation 122:2313–2322
Nagy N, Kuipers HF, Frymoyer AR, Ishak HD, Bollyky JB, Wight TN et al (2015) 4-methylumbelliferone treatment and hyaluronan inhibition as a therapeutic strategy in inflammation, autoimmunity, and cancer. Front Immunol 6:123. https://doi.org/10.3389/fimmu.2015.00123
Narins RS, Brandt F, Leyden J, Lorenc ZP, Rubin M, Smith S (2003) A randomized, double-blind, multicenter comparison of the efficacy and tolerability of Restylane versus Zyplast for the correction of nasolabial folds. Dermatol Surg 29:588–595
Neuman MG, Cohen LB, Nanau RM (2016) Hyaluronic acid as a non-invasive biomarker of liver fibrosis. Clin Biochem 49:302–315
Nonaka H, Tanaka M, Suzuki K, Miyajima A (2007) Development of murine hepatic sinusoidal endothelial cells characterized by the expression of hyaluronan receptors. Dev Dyn 236:2258–2267
Oikari S, Kettunen T, Tiainen S, Häyrinen J, Masarwah A, Sudah M et al (2018) UDP-sugar accumulation drives hyaluronan synthesis in breast cancer. Matrix Biol 67:63–74
Olsson M, Meadows JR, Truvé K, Rosengren Pielberg G, Puppo F, Mauceli E et al (2011) A novel unstable duplication upstream of HAS2 predisposes to a breed-defining skin phenotype and a periodic fever syndrome in Chinese Shar-Pei dogs. PLoS Genet 7(3):e1001332. https://doi.org/10.1371/journal.pgen.1001332
Ossipov DA (2010) Nanostructured hyaluronic acid-based materials for active delivery to cancer. Expert Opin Drug Deliv 7:681–703
Ossipov DA, Piskounova S, Varghese OP, Hilborn J (2010) Functionalization of hyaluronic acid with chemoselective groups via a disulfide-based protection strategy for in situ formation of mechanically stable hydrogels. Biomacromolecules 11:2247–2254
Ouyang X, Panetta NJ, Talbott MD, Payumo AY, Halluin C, Longaker MT et al (2017) Hyaluronic acid synthesis is required for zebrafish tail fin regeneration. PLoS One 12(2):e0171898. https://doi.org/10.1371/journal.pone.0171898
Pao KY, Mancini R (2014) Nonsurgical periocular rejuvenation: advanced cosmetic uses of neuromodulators and fillers. Curr Opin Ophthalmol 25:461–469
Park JK, Shim JH, Kang KS, Yeom J, Jung HS, Kim JY et al (2011) Solid free-form fabrication of tissue-engineering scaffolds with a poly(lactic-co-glycolic acid) grafted hyaluronic acid conjugate encapsulating an intact bone morphogenetic protein-2/poly(ethylene glycol) complex. Adv Funct Mater 21:2906–2912
Pasonen-Seppänen S, Karvinen S, Törrönen K, Hyttinen JM, Jokela T, Lammi MJ et al (2003) EGF upregulates, whereas TGF-beta downregulates, the hyaluronan synthases Has2 and Has3 in organotypic keratinocyte cultures: correlations with epidermal proliferation and differentiation. J Invest Dermatol 120:1038–1044
Perkins KL, Arranz AM, Yamaguchi Y, Hrabetova S (2017) Brain extracellular space, hyaluronan, and the prevention of epileptic seizures. Rev Neurosci 28:869–892
Petrey AC, de la Motte CA (2014) Hyaluronan, a crucial regulator of inflammation. Front Immunol 5:101. https://doi.org/10.3389/fimmu.2014.00101
Piccioni F, Malvicini M, Garcia MG, Rodriguez A, Atorrasagasti C, Kippes N et al (2012) Antitumor effects of hyaluronic acid inhibitor 4-methylumbelliferone in an orthotopic hepatocellular carcinoma model in mice. Glycobiology 22:400–410
Pienimaki JP, Rilla K, Fulop C, Sironen RK, Karvinen S, Pasonen S et al (2001) Epidermal growth factor activates hyaluronan synthase 2 in epidermal keratinocytes and increases pericellular and intracellular hyaluronan. J Biol Chem 276:20428–20435
Plaas A, Li J, Riesco J, Das R, Sandy JD, Harrison A (2011) Intraarticular injection of hyaluronan prevents cartilage erosion, periarticular fibrosis and mechanical allodynia and normalizes stance time in murine knee osteoarthritis. Arthritis Res Ther 13:R46. https://doi.org/10.1186/ar3286
Prevo R, Banerji S, Ferguson DJ, Clasper S, Jackson DG (2001) Mouse LYVE-1 is an endocytic receptor for hyaluronan in lymphatic endothelium. J Biol Chem 276:19420–19430
Raio L, Cromi A, Ghezzi F, Passi A, Karousou E, Viola M et al (2005) Hyaluronan content of Wharton’s jelly in healthy and down syndrome fetuses. Matrix Biol 24:166–174
Rieder F, Kessler SP, West GA, Bhilocha S, de la Motte C, Sadler TM et al (2011) Inflammation-induced endothelial-to-mesenchymal transition: a novel mechanism of intestinal fibrosis. Am J Pathol 179:2660–2673
Rosso F, Quagliariello V, Tortora C, Di Lazzaro A, Barbarisi A, Iaffaioli RV (2013) Cross-linked hyaluronic acid sub-micron particles: in vitro and in vivo biodistribution study in cancer xenograft model. J Mater Sci Mater Med 24:1473–1481
Saravanakumar G, Deepagan VG, Jayakumar R, Park JH (2014) Hyaluronic acid-based conjugates for tumor-targeted drug delivery and imaging. J Biomed Nanotechnol 10:17–31
Savani RC, Cao G, Pooler PM, Zaman A, Zhou Z, DeLisser HM (2001) Differential involvement of the hyaluronan (HA) receptors CD44 and receptor for HA-mediated motility in endothelial cell function and angiogenesis. J Biol Chem 276:36770–36778
Scott JE, Cummings C, Brass A, Chen Y (1991) Secondary and tertiary structures of hyaluronan in aqueous solution, investigated by rotary shadowing-electron microscopy and computer simulation. Hyaluronan is a very efficient network-forming polymer. Biochem J 274(Pt 3):699–705
Seyfried NT, Blundell CD, Day AJ, Almond A (2005a) Preparation and application of biologically active fluorescent hyaluronan oligosaccharides. Glycobiology 15:303–312
Seyfried NT, McVey GF, Almond A, Mahoney DJ, Dudhia J, Day AJ (2005b) Expression and purification of functionally active hyaluronan-binding domains from human cartilage link protein, aggrecan and versican: formation of ternary complexes with defined hyaluronan oligosaccharides. J Biol Chem 280:5435–5448
Sherman L, Sleeman J, Herrlich P, Ponta H (1994) Hyaluronate receptors: key players in growth, differentiation, migration and tumor progression. Curr Opin Cell Biol 6:726–733
Shoham N, Sasson AL, Lin FH, Benayahu D, Haj-Ali R, Gefen A (2013) The mechanics of hyaluronic acid/adipic acid dihydrazide hydrogel: towards developing a vessel for delivery of preadipocytes to native tissues. J Mech Behav Biomed Mater 28:320–331
Simkovic I (2013) Unexplored possibilities of all-polysaccharide composites. Carbohydr Polym 95:697–715
Soltés L, Mendichi R, Kogan G, Schiller J, Stankovska M, Arnhold J (2006) Degradative action of reactive oxygen species on hyaluronan. Biomacromolecules 7:659–668
Spicer AP, McDonald JA (1998) Characterization and molecular evolution of a vertebrate hyaluronan synthase gene family. J Biol Chem 273:1923–1932
Stern R (2004) Hyaluronan catabolism: a new metabolic pathway. Eur J Cell Biol 83:317–325
Stern R, Asari AA, Sugahara KN (2006) Hyaluronan fragments: an information-rich system. Eur J Cell Biol 85:699–715
Stern R, Kogan G, Jedrzejas MJ, Soltés L (2007) The many ways to cleave hyaluronan. Biotechnol Adv 25:537–557
Suzuki M, Asplund T, Yamashita H, Heldin CH, Heldin P (1995) Stimulation of hyaluronan biosynthesis by platelet-derived growth factor-BB and transforming growth factor-beta 1 involves activation of protein kinase C. Biochem J 307(Pt 3):817–821
Takahashi Y, Li L, Kamiryo M, Asteriou T, Moustakas A, Yamashita H et al (2005) Hyaluronan fragments induce endothelial cell differentiation in a CD44- and CXCL1/GRO1-dependent manner. J Biol Chem 280:24195–24204
Takeda K, Kaisho T, Akira S (2003) Toll-like receptors. Annu Rev Immunol 21:335–376
Tamer TM (2013) Hyaluronan and synovial joint: function, distribution and healing. Interdiscip Toxicol 6:111–125
Tammi RH, Kultti A, Kosma VM, Pirinen R, Auvinen P, Tammi MI (2008) Hyaluronan in human tumors: pathobiological and prognostic messages from cell-associated and stromal hyaluronan. Semin Cancer Biol 18:288–295
Tammi RH, Passi AG, Rilla K, Karousou E, Vigetti D, Makkonen K et al (2011) Transcriptional and post-translational regulation of hyaluronan synthesis. FEBS J 278:1419–1428
Taylor KR, Trowbridge JM, Rudisill JA, Termeer CC, Simon JC, Gallo RL (2004) Hyaluronan fragments stimulate endothelial recognition of injury through TLR4. J Biol Chem 279:17079–17084
Tesar BM, Jiang D, Liang J, Palmer SM, Noble PW, Goldstein DR (2006) The role of hyaluronan degradation products as innate alloimmune agonists. Am J Transplant 6:2622–2635
Tian X, Azpurua J, Hine C, Vaidya A, Myakishev-Rempel M, Ablaeva J et al (2013) High-molecular-mass hyaluronan mediates the cancer resistance of the naked mole rat. Nature 499:346–349
Tigges J, Krutmann J, Fritsche E, Haendeler J, Schaal H, Fischer JW et al (2014) The hallmarks of fibroblast ageing. Mech Ageing Dev 138:26–44
Tighe RM, Garantziotis S (2018) Hyaluronan interactions with innate immunity in lung biology. Matrix Biol. https://doi.org/10.1016/j.matbio.2018.01.027
Tolg C, Poon R, Fodde R, Turley EA, Alman BA (2003) Genetic deletion of receptor for hyaluronan-mediated motility (Rhamm) attenuates the formation of aggressive fibromatosis (desmoid tumor). Oncogene 22:6873–6882
Tolg C, Hamilton SR, Nakrieko KA, Kooshesh F, Walton P, McCarthy JB et al (2006) Rhamm-/- fibroblasts are defective in CD44-mediated ERK1,2 motogenic signaling, leading to defective skin wound repair. J Cell Biol 175:1017–1028
Toole BP (2004) Hyaluronan: from extracellular glue to pericellular cue. Nat Rev Cancer 4:528–539
Torres CR, Hart GW (1984) Topography and polypeptide distribution of terminal N-acetylglucosamine residues on the surfaces of intact lymphocytes. Evidence for O-linked GlcNAc. J Biol Chem 259:3308–3317
Tsai SW, Fang JF, Yang CL, Chen JH, Su LT, Jan SH (2005) Preparation and evaluation of a hyaluronate-collagen film for preventing post-surgical adhesion. J Int Med Res 33:68–76
Udabage L, Brownlee GR, Nilsson SK, Brown TJ (2005) The over-expression of HAS2, Hyal-2 and CD44 is implicated in the invasiveness of breast cancer. Exp Cell Res 310:205–217
Välimäki JO (2015) Pilot study of glaucoma drainage implant surgery supplemented with reticulated hyaluronic acid gel in severe glaucoma. Eur J Ophthalmol 25:140–144
Varki A, Cummings RD, Aebi M, Packer NH, Seeberger PH, Esko JD et al (2015) Symbol nomenclature for graphical representations of glycans. Glycobiology 25:1323–1324
Vigetti D, Passi A (2014) Hyaluronan synthases posttranslational regulation in cancer. In: Simpson MA, Heldin P (eds) Hyaluronan signaling and turnover, pp 95–119
Vigetti D, Viola M, Gornati R, Ori M, Nardi I, Passi A et al (2003a) Molecular cloning, genomic organization and developmental expression of the Xenopus laevis hyaluronan synthase 3. Matrix Biol 22:511–517
Vigetti D, Viola M, Gornati R, Ori M, Nardi I, Passi A et al (2003b) Molecular cloning, genomic organization and developmental expression of the Xenopus laevis hyaluronan synthase 3. Matrix Biol 22:511–517
Vigetti D, Ori M, Viola M, Genasetti A, Karousou E, Rizzi M et al (2006) Molecular cloning and characterization of UDP-glucose dehydrogenase from the amphibian Xenopus laevis and its involvement in hyaluronan synthesis. J Biol Chem 281:8254–8263
Vigetti D, Andrini O, Clerici M, Negrini D, Passi A, Moriondo A (2008a) Chondroitin sulfates act as extracellular gating modifiers on voltage-dependent ion channels. Cell Physiol Biochem 22:137–146
Vigetti D, Viola M, Karousou E, Rizzi M, Moretto P, Genasetti A et al (2008b) Hyaluronan-CD44-ERK1/2 regulate human aortic smooth muscle cell motility during aging. J Biol Chem 283:4448–4458
Vigetti D, Genasetti A, Karousou E, Viola M, Clerici M, Bartolini B et al (2009a) Modulation of hyaluronan synthase activity in cellular membrane fractions. J Biol Chem 284:30684–30694
Vigetti D, Rizzi M, Viola M, Karousou E, Genasetti A, Clerici M et al (2009b) The effects of 4-methylumbelliferone on hyaluronan synthesis, MMP2 activity, proliferation, and motility of human aortic smooth muscle cells. Glycobiology 19:537–546
Vigetti D, Clerici M, Deleonibus S, Karousou E, Viola M, Moretto P et al (2011a) Hyaluronan synthesis is inhibited by adenosine monophosphate-activated protein kinase through the regulation of HAS2 activity in human aortic smooth muscle cells. J Biol Chem 286:7917–7924
Vigetti D, Rizzi M, Moretto P, Deleonibus S, Dreyfuss JM, Karousou E et al (2011b) Glycosaminoglycans and glucose prevent apoptosis in 4-Methylumbelliferone-treated human aortic smooth muscle cells. J Biol Chem 286:34497–34503
Vigetti D, Deleonibus S, Moretto P, Karousou E, Viola M, Bartolini B et al (2012) Role of UDP-N-Acetylglucosamine (GlcNAc) and O-GlcNAcylation of hyaluronan synthase 2 in the control of chondroitin sulfate and hyaluronan synthesis. J Biol Chem 287:35544–35555
Vigetti D, Deleonibus S, Moretto P, Bowen T, Fischer JW, Grandoch M et al (2014a) Natural antisense transcript for hyaluronan synthase 2 (HAS2-AS1) induces transcription of HAS2 via protein O-GlcNAcylation. J Biol Chem 289:28816–28826
Vigetti D, Karousou E, Viola M, Deleonibus S, De Luca G, Passi A (2014b) Hyaluronan: biosynthesis and signaling. Biochim Biophys Acta, Gen Subj 1840:2452–2459
Vigetti D, Viola M, Karousou E, De Luca G, Passi A (2014c) Metabolic control of hyaluronan synthases. Matrix Biol 35:8–13
Vigetti D, Viola M, Karousou E, Deleonibus S, Karamanou K, De Luca G et al (2014d) Epigenetics in extracellular matrix remodeling and hyaluronan metabolism. FEBS J 281:4980–4992
Vigetti D, Karousou E, Viola M, Passi A (2015) Analysis of hyaluronan synthase activity. Methods Mol Biol 1229:201–208
Viola M, Vigetti D, Genasetti A, Rizzi M, Karousou E, Moretto P et al (2008) Molecular control of the hyaluronan biosynthesis. Connect Tissue Res 49:111–114
Viola M, Karousou E, D’Angelo ML, Caon I, De Luca G, Passi A et al (2015a) Regulated hyaluronan synthesis by vascular cells. Int J Cell Biol 2015:1–8
Viola M, Vigetti D, Karousou E, D’Angelo ML, Caon I, Moretto P et al (2015b) Biology and biotechnology of hyaluronan. Glycoconj J 32:93–103
Voelcker V, Gebhardt C, Averbeck M, Saalbach A, Wolf V, Weih F et al (2008) Hyaluronan fragments induce cytokine and metalloprotease upregulation in human melanoma cells in part by signalling via TLR4. Exp Dermatol 17:100–107
Voigt J, Driver VR (2012) Hyaluronic acid derivatives and their healing effect on burns, epithelial surgical wounds, and chronic wounds: a systematic review and meta-analysis of randomized controlled trials. Wound Repair Regen 20:317–331
Wang HS, Tung WH, Tang KT, Wong YK, Huang GJ, Wu JC et al (2005) TGF-beta induced hyaluronan synthesis in orbital fibroblasts involves protein kinase C betaII activation in vitro. J Cell Biochem 95:256–267
Wang KC, Yang YW, Liu B, Sanyal A, Corces-Zimmerman R, Chen Y et al (2011) A long noncoding RNA maintains active chromatin to coordinate homeotic gene expression. Nature 472:120–124
Wang Y, Lauer ME, Anand S, Mack JA, Maytin EV (2014) Hyaluronan synthase 2 protects skin fibroblasts against apoptosis induced by environmental stress. J Biol Chem 289:32253–32265
Weigel PH, DeAngelis PL (2007) Hyaluronan synthases: a decade-plus of novel glycosyltransferases. J Biol Chem 282:36777–36781
Weigel PH, Padgett-McCue AJ, Baggenstoss BA (2013) Methods for measuring Class I membrane-bound hyaluronan synthase activity. Methods Mol Biol 1022:229–247
Wróbel T, Dziegiel P, Mazur G, Zabel M, Kuliczkowski K, Szuba A (2005) LYVE-1 expression on high endothelial venules (HEVs) of lymph nodes. Lymphology 38:107–110
Yamaguchi Y, Yamamoto H, Tobisawa Y, Irie F (2018) TMEM2: a missing link in hyaluronan catabolism identified? Matrix Biol. https://doi.org/10.1016/j.matbio.2018.03.020
Yamamoto H, Tobisawa Y, Inubushi T, Irie F, Ohyama C, Yamaguchi Y (2017) A mammalian homolog of the zebrafish transmembrane protein 2 (TMEM2) is the long-sought-after cell-surface hyaluronidase. J Biol Chem 292:7304–7313
Yang JA, Kim ES, Kwon JH, Kim H, Shin JH, Yun SH et al (2012) Transdermal delivery of hyaluronic acid – human growth hormone conjugate. Biomaterials 33:5947–5954
Yao J, Fan Y, Du R, Zhou J, Lu Y, Wang W et al (2010) Amphoteric hyaluronic acid derivative for targeting gene delivery. Biomaterials 31:9357–9365
Yarema KJ, Bertozzi CR (2001) Characterizing glycosylation pathways. Genome Biol 2:REVIEWS0004
Yoshida H, Nagaoka A, Komiya A, Aoki M, Nakamura S, Morikawa T et al (2018) Reduction of hyaluronan and increased expression of HYBID (KIAA1199) correlate with clinical symptoms in photoaged skin. Br J Dermatol 179:136–144
Zaman A, Cui Z, Foley JP, Zhao H, Grimm PC, Delisser HM et al (2005) Expression and role of the hyaluronan receptor RHAMM in inflammation after bleomycin injury. Am J Respir Cell Mol Biol 33:447–454
Zhang X, Wang H, Ma Z, Wu B (2014a) Effects of pharmaceutical PEGylation on drug metabolism and its clinical concerns. Expert Opin Drug Metab Toxicol 10:1691–1702
Zhang Y, Jia S, Jiang WG (2014b) KIAA1199 and its biological role in human cancer and cancer cells (review). Oncol Rep 31:1503–1508
Zhou B, Weigel JA, Fauss L, Weigel PH (2000) Identification of the hyaluronan receptor for endocytosis (HARE). J Biol Chem 275:37733–37741
Zhou B, Weigel JA, Saxena A, Weigel PH (2002) Molecular cloning and functional expression of the rat 175-kDa hyaluronan receptor for endocytosis. Mol Biol Cell 13:2853–2868
Zhou B, McGary CT, Weigel JA, Saxena A, Weigel PH (2003) Purification and molecular identification of the human hyaluronan receptor for endocytosis. Glycobiology 13:339–349
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Passi, A., Vigetti, D. (2019). Hyaluronan: Structure, Metabolism, and Biological Properties. In: Cohen, E., Merzendorfer, H. (eds) Extracellular Sugar-Based Biopolymers Matrices. Biologically-Inspired Systems, vol 12. Springer, Cham. https://doi.org/10.1007/978-3-030-12919-4_4
Download citation
DOI: https://doi.org/10.1007/978-3-030-12919-4_4
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-12918-7
Online ISBN: 978-3-030-12919-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)