Abstract
Seafloor hydrothermal sulfides, which are expected to be a future resource for metals, could be a potential source for metal contamination in the seawater around mining sites. In this chapter, we illustrate the potential for metal leaching of both non-oxidized (non-exposed to atmosphere; before and during exploitation) and oxidized (exposed to atmosphere; after lifting and recovery) hydrothermal sulfides to seawater under different temperatures and redox conditions. One of the crucial findings was that metal dissolution behaviors differed significantly according to the specific areas and/or the initial oxidation states of the sulfide surfaces. Once the non-oxidized sulfide chips were ground to particulates and mixed with seawater, Zn and Pb were preferentially released even though these metals were included as minor components of the sulfides. For Zn, the dissolution rate increased under the oxic and higher temperature (20 °C) conditions when compared to the anoxic and lower temperature (5 °C) conditions, but the absolute rate was relatively moderate. These findings suggest that instantaneous metal release from sulfides into seawater will not occur before or during the crushing and lifting processes of seafloor mining. In contrast to the non-oxidized sulfides, the oxidized sulfides rapidly released large amounts of various metals and metalloids (e.g., Mn, Fe, Zn, Cu, As, Sb, and Pb) into seawater. The different metal dissolution behaviors between the non-oxidized and oxidized hydrothermal sulfides suggest the importance of the implementation of appropriate environmental measures to prevent leakage of the lifted sulfides to the marine surface.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Toxic metal release from hydrothermal sulfides is a potential problem that could be caused by seafloor metal-mining operations. Metal sulfides, which are present in abundance in submarine hydrothermal deposits, have become a major source of metal contamination via release of metal cations by oxidation of the sulfides (Tsang and Parry 2004; Cook et al. 2009; Hageman et al. 2015). These released metal cations are easily introduced into aquatic ecosystems, where they gradually accumulate in the bodies of living organisms (Eggleton and Thomas 2004; Simpson and Spadaro 2016).Thus, the anthropogenic releases of sulfide minerals and metal-contaminated seawater into marine environments by mining operations are likely to have negative effects on marine ecosystems.
Different metal dissolution potentials of sulfides are predicted in each mining process because the reaction rates can vary depending on oxidation states of the sulfide surface (non-oxidized or oxidized) and marine environmental conditions such as benthic and surface environments (Bilenker et al. 2016). Natural hydrothermal sulfides beneath the seafloor are often covered by insoluble oxides and/or sulfates and unconsolidated sediments known as gossan (Feely et al. 1987; Fallon et al. 2017). Under such states, the oxidative dissolution of hydrothermal sulfides is suppressed by the insoluble layer. However, metal release is likely to occur when sulfides are crushed and their fresh interfaces are exposed to seawater by seafloor mining operations (Fig. 1a). After the crushed sulfides are lifted with seawater from the seafloor to a mining support vessel (Fig. 1b), the sulfide surface will be gradually oxidized by atmospheric oxygen and rainwater during onboard storage (Fig. 1c). If the oxidized sulfides are discharged as tailings either operationally or accidentally from the vessel to the surface seawater, the fine particulates of hydrothermal sulfide in the surface plume are likely to release toxic metals (Fig. 1d). To enable adequate and realistic evaluation of the impact of such mining activities on marine environments, it is important to clarify the potential for metal leaching and dissolution processes of both non-oxidized and oxidized hydrothermal sulfides into seawater under different environmental conditions. In this chapter, we show the results of seawater leaching experiments using various hydrothermal sulfide samples and discuss the possibility of metal release in each mining process.
2 Metal Leaching Potential and Possible Mechanism of Dissolution from Non-oxidized Hydrothermal Sulfides into Seawater
Few studies have investigated dissolution of metals from single sulfide minerals (e.g., pyrite, sphalerite, and galena) into seawater, even though this topic has been thoroughly investigated in low ionic strength water within the context of terrestrial mining (e.g., Steger and Desjardins 1980; Goldhaber 1983; Michael et al. 1986). Bilenker et al. (2016) recently determined the oxidation rate of pyrrhotite and chalcopyrite in seawater under different PO2 and temperature conditions and concluded that the metal dissolution and acid generation caused by seafloor mining will be limited because the oxidation rates are significantly low. However, natural hydrothermal sulfides comprise mixtures of various sulfide minerals; therefore, the metal dissolution rate from these sulfides into seawater is likely to be significantly different from the single mineral oxidation rate (Tsang and Parry 2004). Accordingly, to enable realistic evaluation of the impact of seafloor mining operations, it is necessary to investigate the potential for metal leaching from hydrothermal sulfides into seawater rather than that from individual sulfide minerals.
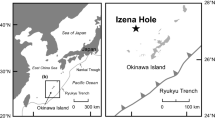
We recently reported the metal dissolution rate from non-oxidized (i.e., non-exposed to atmosphere) hydrothermal sulfides into seawater (Fuchida et al. 2018). In that study, a metal dissolution experiment was conducted onboard using sulfide samples collected by seafloor drilling from an active hydrothermal mound at the Izena Hole, middle Okinawa Trough by D/V Chikyu. The sulfide samples were powdered under inert gas to avoid oxidation by atmospheric air and then immediately subjected to experiments under different temperature (5 °C and 20 °C) and redox (oxic and anoxic) conditions. For that study, oxic and anoxic conditions were considered high (air-saturated, initial at room temperature: 5–6 mg/L) and low (degassed and nitrogen-purged, initial at room temperature: 1 mg/L) dissolved oxygen concentrations, respectively. Metal concentrations in the reacted solution were quantified by inductively coupled plasma-mass spectrometry (ICP-MS). The details of experimental methods are described in Fuchida et al. (2018).
Chemical compositions and mineral assemblages of the four sulfide samples (CKL-1, CKL-2, CKL-3, and CKL-4) used for the experiments are shown in Table 1. These sulfide samples contained various metals (i.e., Mn, Fe, Cu, Zn, Cd, and Pb), whereas Zn and Pb were preferentially released from the sulfide samples into seawater under all experimental conditions. Large monotonic increases in Zn concentrations were observed for two samples (CKL-3 and CKL-4), and their dissolution rates per unit surface area (mol m−2 s−1) were clearly higher under the oxic and 20 °C condition than the anoxic and 5 °C condition (Fig. 2). The final concentrations of Zn in seawater differed according to the sulfide sample; however, they did not correlate with those in the sulfide samples under all experimental conditions (see Fig. 6 in Fuchida et al. 2018). This lack of correlation indicates that simple oxidation reaction of sphalerite (i.e., ZnS + 2O2→ Zn2+ + SO42−) made a minor contribution to Zn dissolution from hydrothermal sulfides into seawater.
Time-course changes in concentrations of dissolved Zn for (a) CKL-1, (b) CKL-2, (c) CKL-3, and (d) CKL-4 under different redox and temperature conditions (Modified after Fuchida et al. 2018)
Several studies have reported that galvanic effects generated between different sulfide minerals can greatly accelerate their oxidative dissolution (Kwong et al. 2003; Tsang and Parry 2004; Fallon et al. 2017). Sulfide minerals generally have semiconducting properties, and direct contact between sulfide minerals with different resting potentials produces galvanic effects (Cruz et al. 2005; Liu et al. 2008; Fallon et al. 2017). The minerals with the highest and lowest rest potentials act as a cathode and anode, respectively. Cathodic minerals can be galvanically protected, whereas anodic minerals are easily dissolved through electronic interactions (Chopard et al. 2017). The resting potentials for some sulfide minerals measured in 1.0 M H2SO4 solution at room temperature are listed in Table 2 (Kwong et al. 2003 and references therein). Sphalerite and galena have relatively low resting potentials when compared to disulfide minerals (i.e., pyrite and chalcopyrite) and can be anodically dissolved when they come into contact with anodic disulfide minerals.
Scanning electron microscopy (SEM ) backscattered images of the particulate fragments of hydrothermal sulfides before the experimentation showed the presence of different mineral couplings (i.e., adjacent placement of iron disulfide and other sulfide minerals) in the high metal release samples (i.e., CKL-3 and CKL-4). In contrast, such couplings were less abundant or coated with silicates in other samples with less Zn and Pb releases (i.e., CKL-1 and CKL-2, see Fig. 5 in Fuchida et al. 2018). These microscopic observations implied that the galvanic couplings of sphalerite and galena with iron disulfides greatly affected the Zn and Pb dissolution from the hydrothermal sulfides into seawater.
The different solubility of each metallic compound in seawater also influences the metal composition of the leachates. The solubility of ferric ion is estimated to be only 0.2–0.3 nM in seawater. This is because ferrous ions (Fe2+) released from sulfide minerals are instantaneously oxidized to ferric ions (Fe3+) and precipitated as FeO(OH) in seawater (Liu and Millero 1999). The finding that the Fe concentration was less than the detection limit in our leachates was likely because of the low solubility of FeO(OH). Lead also has low solubility in seawater (3 μM) and can be removed by formation of PbSO4 and PbCO3 (Angel et al. 2016). In our experiment, the Pb concentrations in the seawater reacted with CKL-2 and CKL-4 increased initially but then greatly decreased from 1 to 10 h (Fuchida et al. 2018). This decrease can be explained by the formation of insoluble precipitates. Conversely, the Zn concentration is predicted to be high, even in seawater, because most of the secondary Zn minerals are highly soluble (Kwong et al. 2003). Thus, the removal of Fe and Pb by precipitate formation (i.e., secondary mineral formation) could play a large role in the preferential increase of Zn in seawater after the reaction with hydrothermal sulfides.
The metal concentrations in seawater were higher under the oxic and 20 °C conditions than the anoxic and 5 °C conditions in our experiment. This was likely because both the galvanic dissolution of sulfide minerals was promoted and the metal solubility increased at higher temperature and PO2 condition. Based on these results, metal dissolution from hydrothermal sulfides can be greatly accelerated when the minerals are transported to areas with warm oxic conditions, such as surface environments, although little metal dissolution from crushed sulfides is expected to occur under cold anoxic conditions, such as deep seafloor environments.
3 Evaluation of Leachable Metals and Metalloids from Hydrothermal Sulfides After Exposed to Atmosphere into Seawater
Metal leaching potential from “oxidized” hydrothermal sulfides into seawater has been reported in several studies, even though our study is the only one known to have investigated metal release from non-oxidized hydrothermal sulfides to date (Fuchida et al. 2018). The “oxidized” was taken to indicate that the samples were exposed to air and/or oxic water for a long time; the original assemblage of constituent minerals could be partly changed to be secondary/altered phase. The results of a leaching experiment using the oxidized sulfide samples showed different metal release patterns from those of non-oxidized sulfide samples and indicated that various metals and metalloids can be rapidly released in large quantities from oxidized sulfide samples into seawater. Here, we show the results of leaching experiments conducted using various oxidized sulfides and the characteristics of metal compositions in the leachates.
Two studies (i.e., Simpson et al. 2007; Parry 2008) have investigated the metal leaching potentials of seafloor hydrothermal sulfides collected from active/inactive chimney structures in the East Manus Basin hydrothermal field (Papua New Guinea) as part of the Solwara 1 project. In these studies, blocks (large chips of 25 mm diameter) of sulfide samples containing large amounts of metals (Mn, Fe, Cu, Zn, and Pb) and As (Table 3a) were reacted with natural seawater at a liquid-to-solid ratio of 10:1 for a maximum of 24 h. Representative results of the leaching experiments are shown in Table 3b. Large amounts of Zn and Mn followed by Cu, Pb, and As were released from those oxidized samples; however, the metal and metalloid compositions in the leachates were significantly different from those in the sulfide samples.
Our recent study reported the metal leachability of four different hydrothermal sulfides collected from the Okinawa Trough hydrothermal fields (Japan) (sample no. 1–4 in Table 4) (Fuchida et al. 2017). We used fine particulate matters (<1/16 mm) of those samples, which would contribute to plume formation, although previous studies used large block sample chips (<25 mm). Approximately 3 g powdered sample was stirred into 30 mL artificial seawater (Daigo’s SP, Nihon Pharmaceutical Co. Ltd., Tokyo, Japan; pH = 8.2; dissolved oxygen = 5.7 mg/L) in an acid-cleaned polypropylene centrifuge tube (50 mL) for a liquid-to-solid ratio of 20:1, and then the tube was reciprocally shaken at 200 rpm per min at 25 °C for 5 min, 6 h, or 18 h. After shaking, the solid phase was separated by centrifugation and collected by filtration through a polyvinylidene difluoride membrane filter (0.45 μm). The leachate was preserved in a polypropylene tube with HNO3. Metals and metalloids present at detectable levels in the leachates (i.e., Mn, Fe, Cu, Zn, As, Cd, Sb, and Pb) were selected after screening by ICP-atomic emission spectroscopy and quantified by ICP-MS (modified after Fuchida et al. 2017).
The chemical compositions and mineral assemblages of the sulfide samples are shown in Table 4. As the results of reaction, significant amounts of metals and metalloids were released into the seawater (Fig. 3); the metals and metalloid concentrations in the leachates increased significantly within 5 min of shaking and showed little change between 5 min and 6 or 18 h of shaking. A similar rapid initial release of metals from oxidized sulfide samples within 5 min was also reported by Parry (2008) (Table 3b). The chemical compositions of the leachates differed depending on the mineral assemblages and chemical compositions of the sulfide samples. Specifically, Fe−Zn−Pb-rich, Ba-rich, and Fe-rich mineral samples released abundant amounts of Zn + Pb, As + Sb, and Zn + Cu into seawater, respectively. There were no correlations between the chemical compositions of leachates and those of sulfide samples, similar to the results reported by Simpson et al. (2007) and Parry (2008).
Metal and metalloid concentrations in both leachate (n = 3± SD) and sulfide samples (n = 2) (Fuchida et al. 2017)
For further discussion of leachable metal and metalloid compositions in different hydrothermal sulfides, we conducted leaching experiments using ten sulfide (chimney) samples with the same method of our previous study (liquid-to-solid ratio of 20:1 and reacted for 6 h) (Fuchida et al. 2017). These mineral samples were collected from different hydrothermal sites of the Okinawa Trough (sample nos. 5–10 and 12–14) and Izu-Ogasawara Arc (sample no. 11). The chemical compositions of leachates and sulfide samples and mineral assemblages are summarized in Table 4, and the metals and metalloids are indicated in four colors (red, blue, yellow, and white) in Fig. 4 based on their concentrations in both the leachate and sulfide samples.
Among all released metals and metalloids, Zn was the most easily leached from oxidized hydrothermal sulfides, regardless of chemical compositions and mineral assemblages of sulfide samples (represented by red in Fig. 4). These results agree with those of our (Fuchida et al. 2017) and other previous studies (Simpson et al. 2007; Parry 2008). Several samples released high amounts of Mn and Cd, despite their being present in low levels in the sulfide samples (represented by yellow in Fig. 4). For example, Mn and Cd could be co-released with Zn into seawater because they are generally contained in Zn-bearing sulfide minerals (i.e., sphalerite) as impurities (Cook et al. 2009). Moreover, Pb was released in high levels into seawater when high amounts of Pb-bearing minerals such as galena and anglesite were present in the sulfide samples, e.g., Fe–Zn–Pb-rich and Zn–Pb-rich samples. For several samples from which high levels of Zn and Pb were released, the pH of their leachates decreased to four to six after the reaction (Table 4). Zn- and Pb-sulfide minerals are known to be acid soluble (Heidel et al. 2013), indicating that dissolution of these sulfide minerals would be promoted as pH decreases (i.e., acid generation).
Arsenic was released from Ba-rich samples (Ba-rich and Zn–Ba-rich) because Ba-rich sample is often associated with As-bearing sulfide minerals such as realgar (As4S4) and orpiment (As2S3). Antimony was also released with As from Ba-rich samples, whereas Zn–Ba-rich samples did not release Sb.
In contrast to Zn and Pb, Fe and Cu were released at lower levels, even though they were present in high abundance in the sulfide samples (represented by blue in Fig. 4). Fe and Cu in the solid samples were mainly present as disulfide minerals, i.e., pyrite and chalcopyrite, based on XRD analysis. In addition, Fe was often contained in the sphalerite as an impurity, and as a result it can be released with Zn and other impurities (i.e., Mn and Cd) into seawater. However, Fe was absent in most of the leachates, while Zn, Mn, and Cd were present, indicating that Fe can be rapidly removed from seawater by the formation of insoluble oxyhydroxides.
The results of our experiments and previous studies revealed that oxidized hydrothermal sulfides have high metal leaching potentials and that the compositions of leachable metals and metalloids differ significantly from those of solid samples. The chemical compositions and mineral assemblages of hydrothermal sulfides are highly dependent on the geological settings and fluid chemistry (e.g., Von Damm 1995); thus, the chemical compositions of leachates are expected to differ among different types of the hydrothermal sulfide samples. However, our results using 14 sulfide samples indicate that Zn, Pb and As are generally relatively more leachable than Fe and Cu, even if they are present at high levels in the sulfide samples. Therefore, this information would be useful for estimation of reference values for the maximum amounts of leachable metals and metalloids that might be leached from hydrothermal sulfides.
4 Metal Release from Hydrothermal Sulfides During Mining Operations
Our and other leaching experimental results demonstrated that metal dissolution from both non-oxidized and oxidized hydrothermal sulfides into seawater can occur. One of the crucial findings of these studies is that the metal dissolution behavior in seawater differed significantly during the initial oxidation of sulfide surfaces (i.e., oxidized or non-oxidized). For example, metal dissolution from non-oxidized samples was relatively gradual (Fig. 2), while that from oxidized samples was rapid, occurring within a few minutes (Fig. 3). Furthermore, the main components of metals released from non-oxidized samples were only Zn and Pb, whereas the oxidized samples released various metals and metalloids. Physicochemical parameters of seawater such as redox conditions and temperature also control the metal dissolution rate; thus, different metal contents of sulfide-contact seawater are predicted in between the surface and seafloor environment and between mining processes.
Based on the leaching experimental results of non-oxidized sulfide, severe metal releases from the minerals would not likely occur near the seafloor due to its low temperature, at least in the short term, even if a benthic plume with a large quantity of fine sulfide particulates was formed by a seafloor mining operation (Fig. 1a). Once the crushed sulfides are lifted to the vessel through the riser pipe, it is predicted that the metal release would be accelerated because of changes in the environmental condition to oxic and higher temperature (Fig. 1b). However, the reaction rate would be rather slow relative to that of fully oxidized sulfides. In cases in which sulfides lifting from the seafloor to the vessel occur within several tens of minutes, the metal concentration in the seawater with the crushed sulfides would not increase greatly.
However, once the sulfides are exposed to air and rainwater after lifting, their surface will be gradually oxidized and transformed into more soluble compounds. If these well-oxidized sulfides are discharged and/or accidentally spilled from the vessel into the oxic and warm surface environment, a rapid dissolution reaction of the minerals would occur. Moreover, if the discharged minerals were composed of very fine particulates for lifting up from seafloor, their suspension and metal dissolution reaction would persist in the plume for a long time.
When the collected hydrothermal sulfides are carried to shore by shuttle barge and temporarily stored on port for processing (Collins et al. 2013), the exposure of the sulfide to air-oxygen is continued for a long period of time. If the well-oxidized sulfides are accidentally discharged to soil and groundwater on port and other places, the metal contamination will extend to shore and land areas. Therefore, it should pay particular attention to the treatment of the oxidized sulfides during the mining activity.
5 Summary
We described the results of leaching experiments using both non-oxidized and oxidized hydrothermal sulfides. Metal dissolution behaviors differed depending on initial oxidation states of the sulfide surface, mineral configurations (galvanic couplings), specific surface areas, and other physical parameters (e.g., temperature and redox potential), indicating that metal leachability and their major released elements will differ depending on marine environmental conditions, which can vary drastically according to the sequential progression of the mining process. The properties of metal dissolution from hydrothermal sulfides into seawater are summarized as follows:
-
1.
Zn and Pb were the main components in the seawater that reacted with non-oxidized sulfides that were underwater, whereas large amounts of various metals and metalloids were released from oxidized sulfides exposed to atmosphere.
-
2.
Metals dissolution from non-oxidized sulfides was greatly accelerated at high temperature and oxic conditions close to the surface relative to the low temperature and anoxic conditions close to the seafloor.
-
3.
The dissolution of metals from non-oxidized sulfides was gradual, while that from oxidized sulfides was rapid, occurring within several minutes.
-
4.
The presence of iron disulfide minerals (e.g., pyrite and marcasite) would induce galvanic metal dissolution from hydrothermal sulfides into seawater.
On the basis of these findings, it is estimated that little metal-rich seawater is produced when crushing and lifting hydrothermal sulfides during mining because the sulfides have not been oxidized and there is little opportunity or the minerals to be exposed to air-oxygen for a long period of time. However, once the minerals have been oxidized through contact between the lifted hydrothermal sulfides on the vessel and air, oxic seawater, and rainwater, various metals and metalloids can be released rapidly if the oxidized sulfides are spilled into the ocean. Thus, surface plumes of spilled material could cause more serious problems than benthic plumes produced by the crushing process of the seafloor mine. As reported in previous studies (Satoh et al. 2005; Caroppo et al. 2006; Fuchida et al. 2017), metals and metalloids released from hydrothermal sulfides could be toxic to marine organisms living in the surface environment, such as marine phytoplankton species, which are a significant primary producer in the ocean. Therefore, we suggest that disposal of tailings and mining wastes below the oxygen minimum zone may be better to minimize the impacts of metal leaching from hydrothermal sulfides on the marine surface environments. Also, adequate monitoring of water quality, drainage treatment, and impact assessment of accidental leakage of the tailings, mining wastes, and drainages to the surface environment are essential.
References
Angel, B. M., Apte, S., Batley, C. E., & Raven, M. D. (2016). Lead solubility in seawater: An experimental study. Environmental Chemistry, 13, 489–495.
Bilenker, L. D., Romano, G. Y., & McKibben, M. A. (2016). Kinetics of sulfide mineral oxidation in seawater: Implications for acid generation during in situ mining of seafloor hydrothermal vent deposits. Applied Geochemistry, 75, 20–31.
Caroppo, C., Stabili, L., Aresta, M., Corinaldesi, C., & Danovaro, S. (2006). Impact of heavy metals and PCBs on marine picoplankton. Environmental Toxicology, 21, 541–551.
Chopard, A., Plante, B., Benzaazoua, M., Bouzahzah, H., & Marion, P. (2017). Geochemical investigation of the galvanic effects during oxidation of pyrite and base-metals sulfides. Chemosphere, 166, 281–291.
Collins, P. C., Croot, P., Carlsson, J., Colaço, A., Grehan, A., Hyeong, K., Kennedy, R., Mohn, C., Smith, S., Yamamoto, H., & Rowden, A. (2013). A primer for the environmental impact assessment of mining at seafloor massive sulfide deposits. Marine Policy, 42, 198–209.
Cook, N. J., Ciobanu, C. L., Pring, A., Skinner, W., Shimizu, M., Danyushevsky, L., Saini-Eidukat, B., & Melcher, F. (2009). Trace and minor elements in sphalerite: A LA-ICPMS study. Geochimica et Cosmochimica Acta, 73, 4761–4791.
Cruz, R., Luna-Sánchez, R. M., Lapidus, G. T., González, I., & Monroy, M. (2005). An experimental strategy to determine galvanic interactions affecting the reactivity of sulfide mineral concentrates. Hydrometallurgy, 78, 198–208.
Eggleton, J., & Thomas, K. V. (2004). A review of factors affecting the release and bioavailability of contaminants during sediment disturbance events. Environment International, 30, 973–980.
Fallon, E. K., Petersen, S., Brooker, R. A., & Scott, T. B. (2017). Oxidative dissolution of hydrothermal mixed-sulphide ore: An assessment of current knowledge in relation to seafloor massive sulphide mining. Ore Geology Reviews, 86, 309–337.
Feely, R. A., Lewison, M., Massoth, G. J., Robertbaldo, G., Lavelle, J. W., Byrne, R. H., Vondamm, K. L., & Curl, H. C. (1987). Composition and dissolution of black smoker particulates from active vents on the Juan-De-Fuca Ridge. J Geophys Res-Solid, 92, 11347–11363.
Fuchida, S., Yokoyama, A., Fukuchi, R., Ishibashi, J.-i., Kawagucci, S., Kawachi, M., & Koshikawa, H. (2017). Leaching of metals and metalloids from hydrothermal ore particulates and their effects on marine phytoplankton. ACS Omega, 2, 3175–3182.
Fuchida, S., Ishibashi, J., Shimada, K., Nozaki, T., Kumagai, H., Kawachi, M., Matsushita, Y., & Koshikawa, H. (2018). Onboard experiment investigating metal leaching of fresh hydrothermal sulfide cores into seawater. Geochemical Transactions. https://doi.org/10.1186/s12932-018-0060-9., in press.
Goldhaber, M. B. (1983). Experimental study of metastable sulfur oxyanion formation during pyrite oxidation at pH 6-9 and 30 degrees C. American Journal of Science, 283, 193–217.
Hageman, P. L., Seal, R. R., Diehl, S. F., Piatak, N. M., & Lowers, H. A. (2015). Evaluation of selected static methods used to estimate element mobility, acid-generating and acid-neutralizing potentials associated with geologically diverse mining wastes. Applied Geochemistry, 57, 125–139.
Heidel, C., Tichomirowa, M., & Junghans, M. (2013). Oxygen and sulfur isotope investigations of the oxidation of sulfide mixtures containing pyrite, galena, and sphalerite. Chemical Geology, 342, 29–43.
Kwong, Y. T. J., Swerhone, G. W., & Lawrence, J. R. (2003). Galvanic sulphide oxidation as a metal-leaching mechanism and its environmental implications. Geochemistry: Exploration, Environment, Analysis, 3, 337–343.
Liu, X., & Millero, F. J. (1999). The solubility of iron in sodium chloride solutions. Geochimica et Cosmochimica Acta, 63, 3487–3497.
Liu, Q., Li, H., & Zhou, L. (2008). Galvanic interactions between metal sulfide minerals in a flowing system: Implications for mines environmental restoration. Applied Geochemistry, 23, 2316–2323.
Michael, A., McKibben, M. A., & Barnes, H. L. (1986). Oxidation of pyrite in low temperature acidic solutions: Rate laws and surface textures. Geochimica et Cosmochimica Acta, 50, 1509–1520.
Parry, D. L. (2008). Solwara 1 project elutriate report phase 1 and 2.
Satoh, A., Vudikaria, L. Q., Kurano, N., & Miyachi, S. (2005). Evaluation of the sensitivity of marine microalgal strains to the heavy metals, Cu, As, Sb, Pb and Cd. Environment International, 31, 713–722.
Simpson, S. L., & Spadaro, D. A. (2016). Bioavailability and chronic toxicity of metal sulfide minerals to benthic marine invertebrates: Implications for deep sea exploration, mining and tailings disposal. Environmental Science & Technology, 50, 4061–4070.
Simpson, S. L., Angel, B., Hamilton, I., Spadaro, D. A., & Binet, M. (2007). Water and sediment characterization and toxicity assessment for the Solwara 1 Project, CSIRO Land and Water Science Report. Coffey Natural Systems Pty Ltd.
Steger, H. F., & Desjardins, L. E. (1980). Oxidation of sulfide minerals. v. galena, sphalerite and chalcocite. Canadian Mineralogict, 18, 365–372.
Tsang, J. J., & Parry, D. L. (2004). Metal mobilization from complex sulfide ore concentrate: Effect of light and pH. Australian Journal of Chemistry, 57, 971–978.
Von Damm, K. L. (1995). Controls on the chemistry and temporal variability of seafloor hydrothermal fluids. Geophysical Monograph, 91, 222–247.
Acknowledgments
We are grateful to the crew members of the NT11-15, NT11-20, NT12-06, KY14-02, NT14-06, NT15-13, and KR15-16 cruises. This study was financially supported by the Cross-ministerial Strategic Innovation Promotion Program (SIP), Next-Generation Technology for Ocean Resource Exploration (funding agency: JAMSTEC). We thank Jeremy Kamen, MSc, from Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Fuchida, S., Ishibashi, Ji., Nozaki, T., Matsushita, Y., Kawachi, M., Koshikawa, H. (2019). Metal Mobility from Hydrothermal Sulfides into Seawater During Deep Seafloor Mining Operations. In: Sharma, R. (eds) Environmental Issues of Deep-Sea Mining. Springer, Cham. https://doi.org/10.1007/978-3-030-12696-4_8
Download citation
DOI: https://doi.org/10.1007/978-3-030-12696-4_8
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-12695-7
Online ISBN: 978-3-030-12696-4
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)