Abstract
Over the past decade, the rapidly expanding use of extracorporeal membrane oxygenation (ECMO) for the acute respiratory distress syndrome (ARDS) has outpaced the evidence. Before 2018, there had only been one large, randomized clinical trial involving relatively modern extracorporeal technology that evaluated the impact of ECMO on acute respiratory failure, predominately ARDS. Advances in ECMO technology, coupled with improvements in the management of ARDS, made it apparent that in order to clarify the role of ECMO for patients with severe forms of ARDS, further high-quality evidence would be needed [1]. The ECMO to Rescue Lung Injury in Severe ARDS (EOLIA) trial compared the impact of early venovenous ECMO (VV-ECMO) in patients with severe forms of ARDS against optimal conventional standard-of-care management [2]. Despite failing to meet the primary outcome of improved survival with ECMO at 60 days, the results of EOLIA are more nuanced than the trial conclusion might suggest. A comprehensive analysis of EOLIA provides valuable insights into the evolving role of ECMO and its future use in ARDS. This chapter will summarize the rationale for the trial, provide an in-depth interpretation of the results, and explore their implications on the role of ECMO in the management of ARDS.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
1 Introduction
Over the past decade, the rapidly expanding use of extracorporeal membrane oxygenation (ECMO) for the acute respiratory distress syndrome (ARDS) has outpaced the evidence. Before 2018, there had only been one large, randomized clinical trial involving relatively modern extracorporeal technology that evaluated the impact of ECMO on acute respiratory failure, predominately ARDS. Advances in ECMO technology, coupled with improvements in the management of ARDS, made it apparent that in order to clarify the role of ECMO for patients with severe forms of ARDS, further high-quality evidence would be needed [1]. The ECMO to rescue Lung Injury in severe ARDS (EOLIA) trial compared the impact of early venovenous ECMO (VV-ECMO) in patients with severe forms of ARDS against optimal conventional standard-of-care management [2]. Despite failing to meet the primary outcome of improved survival with ECMO at 60 days, the results of EOLIA are more nuanced than the trial conclusion might suggest. A comprehensive analysis of EOLIA provides valuable insights into the evolving role of ECMO and its future use in ARDS. This chapter will summarize the rationale for the trial, provide an in-depth interpretation of the results, and explore their implications on the role of ECMO in the management of ARDS.
2 Background
ECMO provides oxygenation and carbon dioxide removal in respiratory failure and both gas exchange and circulatory support for patients in cardiac failure. Much like dialysis for renal failure, this form of extracorporeal organ support (ECOS) was developed as an adjunct to mechanical ventilation in refractory respiratory failure [3]. In most approaches to ECMO, a cannula is placed in a central vein from which venous blood is removed by an external pump, passes through an oxygenator that removes carbon dioxide and directly oxygenates the blood, and is then reinfused back into the patient. When the drainage and reinfusion cannulae are both located in central veins, the circuit is referred to as VV-ECMO. This is in contrast to venoarterial ECMO (VA-ECMO), whereby blood is drained from a vein and reinfused into an artery to provide hemodynamic support.
The most common indication for VV-ECMO in respiratory failure is severe ARDS [4]. ARDS is characterized by an acute, diffuse inflammatory lung injury causing increased alveolar permeability and impaired gas exchange, resulting in hypoxemia, decreased respiratory system compliance and increased physiologic deadspace [5]. Clinically, it is defined by the presence of bilateral infiltrates on chest imaging within seven days of an inciting event, impaired oxygenation, and cannot entirely be explained by cardiogenic pulmonary edema. The grading of severity depends on the extent of hypoxemia, with severe ARDS characterized by the ratio of the partial pressure of arterial oxygen to the fraction of inspired oxygen (PaO2/FiO2) ≤100 mmHg in the presence of at least 5 cmH2O of positive end-expiratory pressure (PEEP) [6].
Globally, ARDS accounts for over 10% of all ICU admissions and 24% of patients receiving mechanical ventilation [7]. This translates into approximately three million patients developing ARDS annually, with hospital mortality ranging from 35% to 46% for the categories of mild to severe ARDS. Importantly, survivors may have significant quality of life impairment long after recovery [8].
Although invasive mechanical ventilation remains the standard of care in the most severe forms of ARDS, patients in whom gas exchange is refractory to conventional ventilation, or who have especially low respiratory system compliance with excessively high airway pressures despite optimal ventilator management, may benefit from the addition of ECMO.
The incorporation of ECMO into the management of respiratory failure traces its roots to the 1970s, with the first reported use by Hill et al. in 1972 [9]. However, a subsequent randomized, controlled trial failed to demonstrate a survival benefit with ECMO [10]. Another negative trial, often included as part of the early experience with ECMO, was actually performed with a related technique - extracorporeal carbon dioxide removal (ECCO2R) - and it, too, demonstrated no survival benefit over conventional management [11].
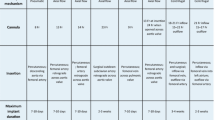
Until 2018, the only randomized, controlled trial that incorporated relatively modern ECMO technology in adults with respiratory failure was the Conventional ventilation or ECMO for Severe Adult Respiratory failure (CESAR) trial [12]. A summary of the randomized controlled ECMO trials can be found in Table 7.1. Although there was a reduction in death or severe disability at 6 months in the CESAR ECMO referral group (37% vs. 53%, relative risk [RR] 0.69, 95% CI 0.05–0.97, p = 0.03), there were important aspects to the trial that limit the interpretation of the results. With only 76% of patients in the ECMO referral arm actually receiving ECMO, and a lack of mandated lung-protective ventilation in the control arm (only 70% of control subjects received lung-protective ventilation at any time during the trial), this was not truly a randomized trial of ECMO versus standard-of-care mechanical ventilation. One conclusion that can be drawn from CESAR, however, is that patients with severe acute respiratory failure, including ARDS, may benefit from referral to expert centers that adhere to standard-of-care lung-protective ventilatory strategies, and are also capable of providing ECMO as part of a defined management algorithm.
At around the same time the CESAR trial was published, there happened to be a high rate of severe ARDS associated with influenza A(H1N1), presenting a unique opportunity to study the adjunctive benefits of ECMO in severe respiratory failure seemingly refractory to conventional ventilator management. A multicenter observational study in Australia and New Zealand reported a 75% rate of survival to discharge among 68 patients treated with ECMO for influenza A(H1N1)-associated severe ARDS [13, 14]. Subsequent matched-pairs analyses of distinct European influenza A(H1N1)-associated ARDS cohorts generated conflicting data about the benefit of ECMO [15, 16].
A meta-analysis of eight studies involving 266 influenza A(H1N1)-associated ARDS patients who received ECMO identified the benefit of a short duration between the start of mechanical ventilation and the initiation of ECMO (median 2 days), and highlighted the potential importance of referral to specialized ECMO centers [17].
3 Resurgence of ECMO and the Need for More Data
With the publication of the CESAR trial and the H1N1 studies, rates of adult ECMO usage for respiratory failure surged, as evidenced by a 433% increase in the United States from 2006 to 2011 [18], and a threefold increase from 2007 to 2012 in Germany [19]. Despite growing enthusiasm for ECMO, overall in-hospital mortality in these studies reached nearly 60%, considerably higher than reports from specialized ECMO centers worldwide. These data suggested that the rate of increased use of ECMO may not have been entirely justified based on the available evidence at the time [20] or that ECMO was not being uniformly applied. An inconclusive body of evidence supporting the use of ECMO in patients with ARDS largely relegated ECMO to a last-resort, salvage therapy in ARDS management [21].
4 Lung-Protective Ventilation
A major limitation in interpreting prior ECMO trials was a lack of strict adherence to modern standards of lung-protective ventilation in all patients, which represents the current standard of care for invasive mechanical ventilation in ARDS [22, 23]. However, the hypercapnia and respiratory acidosis that may arise from low-volume, low-pressure ventilation strategies have been cited as reasons for nonadherence with lung-protective ventilation [24].
Recent literature has suggested that tidal volumes less than the standard-of-care 4–8 mL/kg predicted body weight (PBW), may offer even greater lung protection, particularly given the correlation between lower tidal volumes and airway pressures and reductions in inflammatory cytokines associated with ventilator-induced lung injury (VILI) [25,26,27]. Typically, achieving very low tidal volumes or very low airway pressures would be limited by unacceptable levels of hypercapnia and respiratory acidosis. However, ECMO (or ECCO2R) mitigates this problem by directly removing carbon dioxide from the blood. A comparison between very low tidal volume ventilation (3 mL/kg PBW) with ECCO2R and low tidal volume ventilation (6 mL/kg PBW) without ECCO2R in overall less severe ARDS patients revealed a significant reduction in ventilator-free days in the ECCO2R patients [28]. This so-called “ultra-lung-protective” ventilation strategy could also be achieved with the aid of ECMO in the most severe forms of ARDS. In fact, it has become common practice at ECMO centers to lower tidal volumes and airway pressures beyond traditional lung-protective ventilation goals when using ECMO for the management of severe ARDS [29].
Adjunctive strategies, including prone positioning (for which there is very strong data) and neuromuscular blocking agents (NMBAs), when used in conjunction with lung-protective ventilation, have been found to have survival benefits in randomized, controlled trials [30, 31]. High-frequency oscillatory ventilation (HFOV), thought to limit volutrauma while recruiting atelectatic lung regions, failed to demonstrate a benefit in two large randomized, controlled trials in moderate to severe ARDS and is no longer routinely recommended [32, 33]. The data supporting the use of recruitment maneuvers and inhaled vasodilators remains controversial [34,35,36]. Modern ARDS management strategies defined the standard of care against which ECMO would ideally be compared in the EOLIA trial in order to best assess the true efficacy of ECMO over optimal conventional management. Although enrollment in EOLIA preceded the establishment of some of this standard of care, especially prone positioning, the trial anticipated the use of what is now considered optimal management of patients with severe forms of ARDS.
5 ECMO to rescue Lung Injury in severe ARDS (EOLIA) Trial
The primary objective of the EOLIA trial was to compare the effect of early initiation of ECMO to optimal conventional management on 60-day mortality in patients with the most severe forms of ARDS within seven days of starting invasive mechanical ventilation. Several important considerations went into the design of EOLIA, informed by limitations from previous studies. Recruitment was limited to centers with extensive experience with ARDS management and the ability to either initiate ECMO soon after enrollment or promptly transport patients to ECMO-capable centers. In order to optimize safe and timely transfer, a mobile ECMO team would be deployed to the non-ECMO center where patients would be initiated on ECMO and transported back to an ECMO center [37]. Additionally, it was mandated that centers strictly adhere to pre-specified invasive mechanical ventilation strategies, which included standard of care lung-protective ventilation and adjunctive therapies (especially prone positioning and NMBAs) in the control arm and an ultra-lung-protective ventilation strategy in the ECMO arm.
Patients included in the study met one of the following three criteria: (1) PaO2/FiO2 ratio <50 mmHg for >3 h despite optimization of mechanical ventilation and the potential use of adjunctive therapies (inhaled nitric oxide or prostacyclin, recruitment maneuvers, HFOV, or almitrine infusion); (2) PaO2/FiO2 <80 mmHg for >6 h (otherwise, as above); or (3) pH <7.25 with a PaCO2 ≥60 mmHg for >6 h (with respiratory rate increased to 35 breaths per minute) resulting from mechanical ventilation settings adjusted to keep plateau airway pressure (Pplat) ≤32 cmH2O. Physicians were strongly encouraged to use NMBAs and prone positioning prior to randomization in all patients. A full list of exclusion criteria can be found in the EOLIA supplementary appendix [2].
Patients randomized to the ECMO arm underwent percutaneous venovenous cannulation. To limit VILI, mechanical ventilation was set to: volume-assist control, FiO2 0.3–0.6, PEEP ≥10 cmH2O, tidal volume adjusted for Pplat ≤24 cmH2O, with a respiratory rate of 10–30 breaths per minute, or a version of airway pressure release ventilation (APRV) mode with a high pressure level ≤24 cmH2O and a low pressure level ≥10 cmH2O.
Patients in the control arm were managed according to the settings outlined by the “pulmonary recruitment” group of the Express trial with volume-assist control, tidal volumes of 6 mL/kg PBW and PEEP set so as not to exceed a Pplat of 28–30 cmH2O. Crossover to ECMO was permitted in patients with refractory hypoxemia defined as a saturation of arterial oxygen (SaO2) <80% for >6 h despite the use of prone positioning, NMBAs and other adjunctive therapies as feasible, and only in the absence of irreversible multiple organ failure and when the treating clinician felt that ECMO could change the outcome of the patient.
Controversially, at the recommendation of the Data Safety Monitoring Board (DSMB), enrollment was stopped in April 2017 after continuation was determined to be futile in achieving the primary endpoint based on pre-specified criteria. Of the 249 patients who had been randomized, 124 had been assigned to the ECMO group and 125 to the control group. Among the control patients, 35 (28%) crossed over to ECMO for refractory hypoxemia.
At 60 days, 44 patients (35%) in the ECMO group and 57 (46%) in the control group had died (RR 0.76, 95% CI: 0.55–1.04, p = 0.09). The Kaplan-Meier survival estimates for the primary outcome are presented in Fig. 7.1.
Kaplan-meier survival estimates in the intention-to-treat population during the first 60 days of EOLIA. From [2] with permission. Copyright © (2018) Massachusetts Medical Society
Of the three inclusion criteria described above, one potentially important signal occurred among patients meeting Criteria #3—those with an arterial pH <7.25 with a PaCO2 ≥60 mmHg for >6 h. In this group, mortality was 24% (6/25 patients) for ECMO-supported patients compared with 55% for control (11/20) patients. Although clearly underpowered to detect a statistically significant difference, this observation suggests that patients with severely reduced respiratory system compliance may receive the greatest benefit from ECMO through a ventilation strategy that is beyond standard of care low-volume, low-pressure ventilation; a hypothesis that warrants further investigation.
The predicted mortalities in the conventional treatment and ECMO groups were 60% and 40%, respectively. With observed mortalities of 46% and 35%, the study was underpowered to achieve significance based on prespecified calculations. The high prevalence of proning (90%) in the control arm, even prior to the adoption of prone positioning as the ideal standard of care [30], likely contributed to these lower than predicted mortality rates, further diminishing the ability to detect a statistically significant difference between the groups. One limiting factor in conducting a larger study than in the past was the anticipated low rate of enrollment in EOLIA (less than 1 patient/unit/year). It has been estimated that 624 patients would have been required to have enough power to achieve statistical significance based on the actual mortality rates in EOLIA [38]. Even if 100 units had participated, such a study would take approximately 9 years to complete, which may be considered an impractical amount of time, especially given rapidly evolving changes in technology and practice.
Another controversial feature of EOLIA was the high rate of crossover from the control group to the ECMO group (28%), much higher than the anticipated 5% rate of crossover. These patients were noted, after the fact, to have markers of more severe ARDS at baseline, with higher plateau and driving pressures, lower respiratory system compliance, and more extensive infiltrates on chest radiography. Before crossing over, 25% of these patients had cardiac arrests, 20% had severe right heart failure, and 17% received ECMO while undergoing cardiopulmonary resuscitation (CPR). Sixty-day mortality was 57% for the crossover patients, compared with 41% for control patients who did not cross over and 35% for the ECMO group.
Allowing patients to cross over from control to intervention dilutes the estimated treatment effect, if any, when analyzing the data as intention-to-treat. However, at the time EOLIA was designed, there was insufficient clinical equipoise at most ECMO centers to conduct a trial of ECMO versus conventional management without the option for crossover, the very centers where EOLIA would need to be conducted. This then begs the question, how would the effect estimate have changed if these crossover patients had not received ECMO? Of the 35 patients who crossed over from conventional therapy to salvage ECMO, 15 (43%) survived. It is impossible to know what would have happened to those patients had they not received ECMO. At the very least, among those who received ECMO during cardiac arrest, the likelihood of survival would assuredly have been very low. In order to account for this uncertainty, post hoc sensitivity analysis of treatment failure at 60 days was conducted for different hypothetical survival rates in the crossover group, ranging from 0 to 33%. A survival rate of 33% or less in the crossover group had they not received ECMO (rather than the observed rate of 43% with crossover to ECMO) would have led to a statistically significant relative risk of death favoring the ECMO arm. Moreover, a rank-preserving structural failure time model, used to adjust for the effect of crossover, estimated a hazard ratio for death within 60 days that approached statistical significance (0.51 [95% CI: 0.24–1.02, p = 0.055]), further suggesting that there was a true effect of ECMO in reducing mortality, contrary to what the p value alone would traditionally indicate.
6 Lessons Learned from EOLIA
Overall, the EOLIA trial was a negative study, having failed to achieve a statistically significant improvement in survival with ECMO compared with conventional mechanical ventilation. This result, in combination with low enrollment rates and an unexpectedly high rate of crossover, may lead clinicians to conclude that ECMO has no advantage over optimal conventional management in very severe forms of ARDS. However, the results of the EOLIA trial are informative in how ECMO may be paired with lung-protective ventilation strategies beyond the current standard of care to improve outcomes (Box 7.1).
The implementation of ECMO permitted considerable reductions in mechanical ventilatory parameters. Patients receiving ECMO were able to have their tidal volumes, plateau airway pressures, driving pressures, and respiratory rates decreased well below those in the control groups. Specifically, after one day on ECMO, tidal volumes were reduced by more than 40%, driving pressures by 25%, plateau pressures by nearly 20%, and respiratory rates by nearly 25%. These changes inevitably translated into a marked reduction in the mechanical forces applied to the lungs, compared with the conventional arm, very likely with an associated decrease in the risk or degree of VILI.
The design and results of EOLIA point to the importance of ARDS management by experienced centers. Volume-outcome relationships in healthcare have been well established, including in the use of mechanical ventilation for respiratory failure [39]. This association seemed to hold true in the CESAR trial in which patients with severe acute respiratory failure transferred to a regional ECMO referral center had better outcomes and were more likely to receive lung-protective ventilation, regardless of whether or not they received ECMO, compared to non-transferred, control patients. Poor adherence to lung-protective ventilation, as well as an inability to perform more advanced maneuvers, such as prone positioning, at less experienced centers, suggests that transferring patients with ARDS to more experienced respiratory failure centers would optimize outcomes [3]. A proposed algorithm for ARDS management is outlined in Fig. 7.2.
Suggested algorithm for management of acute respiratory distress syndrome (ARDS). Adapted from [5] with permission. CPAP continuous positive airway pressure, FiO 2 fraction of inspired oxygen, PEEP positive end-expiratory pressure, Pplat plateau pressure measured after a 0.5 s end-inspiratory pause when there is no flow, SpO 2 oxygen saturation measured by pulse oximetry, VV-ECMO venovenous extracorporeal membrane oxygenation. aAdjunctive therapies, in addition to prone positioning and the use of neuromuscular blocking agents, as used in the EOLIA trial, including: inhaled nitric oxide or prostacyclin, recruitment maneuvers, high-frequency oscillatory ventilation, or almitrine infusion
Given the complex, resource-intensive nature of ECMO, it is not surprising that a favorable volume-outcome relationship has been suggested both by data from the influenza A(H1N1) pandemic and more recently by an international registry-based study of over 50,000 patients [15, 40]. These findings, along with the results of EOLIA, further support the regionalization of ECMO programs in many settings for patients with respiratory failure to ensure the safe use of ECMO and adherence to the highest standards of care [41]. The reassuringly low rate of complications in the EOLIA trial is likely to be, at least in part, a reflection of the level of experience with ECMO at participating sites. If a patient warrants ECMO support but ECMO is unavailable at that hospital, referral to a center with ECMO transport capabilities should be considered [42].
7 Future Directions and Areas of Uncertainty
7.1 Optimal Ventilatory Parameters During ECMO
The EOLIA trial, while in and of itself informative, opens the door to multiple future avenues of research. The purpose of ECMO in the EOLIA trial was not to replace conventional mechanical ventilation, but rather to demonstrate how the two could be used synergistically to improve outcomes, mostly through the use of a very-lung-protective ventilation strategy to minimize VILI. EOLIA used a mechanical ventilation approach in the ECMO group that limited Pplat to 24 cmH2O, with moderate levels of PEEP and what may be considered by some to be only a modest reduction in the respiratory rate (compared to what may be achievable with ECCO2R). It remains to be determined what the optimal ventilator settings are during ECMO support for severe ARDS in order to maximally reduce VILI, and whether reductions in parameters beyond those used in EOLIA could offer additional benefit. Questions remain as to whether the use of ECCO2R to achieve similar reductions in mechanical ventilation in patients with less severe forms of ARDS can likewise improve outcomes. The feasibility and effects of ECCO2R-facilitated ultra-lung-protective ventilation in less severe forms of ARDS are currently being evaluated by a large prospective randomized trial (pRotective vEntilation with veno-venouS lung assisT in respiratory failure [REST]; ClinicalTrials.gov identifier: NCT02654327). Additionally, an international, multicenter pilot study (Strategy of UltraProtective lung ventilation with Extracorporeal CO2 Removal for New-Onset moderate to seVere ARDS [SUPERNOVA]; ClinicalTrials.gov Identifier: NCT02282657T) assessing the safety and feasibility of 4 mL/kg tidal volumes with the use of ECCO2R was recently completed and has helped inform the design of an upcoming randomized control trial. There have also been consensus statements by groups of ECMO experts calling for an ECMO research agenda to address transfusion policies, anticoagulation strategies, and the role of early mobilization during ECMO support, among other areas of uncertainty [1].
7.2 The Economics of ECMO
In the current era of value-based healthcare, the costs of ECMO will be under scrutiny. Unadjusted cost-analysis of the 2009 influenza A(H1N1) pandemic in Australia and New Zealand found the use of ECMO to be associated with a five-fold increase in costs compared with those who did not receive ECMO [43]. Moreover, an economic evaluation of the CESAR trial in the United Kingdom found that the average cost per ECMO-referred patient was more than double the average cost of non-referred patients. However, when assessed as a lifetime prediction of cost per quality-adjusted life year, the costs were within the values regarded as affordable by many healthcare systems [12]. A later Brazilian study reported similarly appropriate cost-utility ratios [44]. While regionalization of ECMO at select centers may reduce costs, future studies across a variety of countries and healthcare systems are needed to assess the true global economic impact of ECMO to better guide policymaking in healthcare.
8 Conclusion
The EOLIA trial compared the use of ECMO to optimal conventional management in the most severe forms of ARDS. While considered a traditionally negative study statistically, owing in part to a high rate of crossover to ECMO, and a mortality rate in the control arm that was less than anticipated in the setting of high rates of prone positioning, it nonetheless remains highly informative [45]. The effect size and confidence intervals, along with post hoc analyses and secondary outcomes favoring the ECMO arm, all suggest a clinical benefit to the use of ECMO in this setting. EOLIA demonstrated relatively low complication rates with ECMO, identified a subset of patients (i.e., those with more severe reductions in respiratory system compliance) who may receive greater benefit from extracorporeal support, and highlighted the importance of ARDS (and ECMO) management at expert centers.
Research networks, such as the international ECMO Network (ECMONet; www.internationalecmonetwork.org), have been established to better define the role of ECMO in respiratory and cardiac failure by facilitating high-quality, collaborative research, along with the Extracorporeal Life Support Organization (ELSO; www.elso.org) and others. With the results of EOLIA and growing momentum for additional ECMO and ECCO2R trials, there will likely be greater acceptance of including ECMO in the management algorithm of ARDS.
References
Combes A, Brodie D, Chen YS, et al. The ICM research agenda on extracorporeal life support. Intensive Care Med. 2017;43:1306–18.
Combes A, Hajage D, Capellier G, et al. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. N Engl J Med. 2018;378:1965–75.
Ranieri VM, Brodie D, Vincent JL. Extracorporeal organ support: from technological tool to clinical strategy supporting severe organ failure. JAMA. 2017;318:1105–6.
Brodie D, Bacchetta M. Extracorporeal membrane oxygenation for ARDS in adults. N Engl J Med. 2011;365:1905–14.
Fan E, Brodie D, Slutsky AS. Acute respiratory distress syndrome: advances in diagnosis and treatment. JAMA. 2018;319:698–710.
Ranieri VM, Rubenfeld GD, Thompson BT, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307:2526–33.
Bellani G, Laffey JG, Pham T, et al. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA. 2016;315:788–800.
Herridge MS, Tansey CM, Matte A, et al. Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med. 2011;364:1293–304.
Hill JD, O’Brien TG, Murray JJ, et al. Prolonged extracorporeal oxygenation for acute post-traumatic respiratory failure (shock-lung syndrome). Use of the Bramson membrane lung. N Engl J Med. 1972;286:629–34.
Zapol WM, Snider MT, Hill JD, et al. Extracorporeal membrane oxygenation in severe acute respiratory failure. A randomized prospective study. JAMA. 1979;242:2193–6.
Morris AH, Wallace CJ, Menlove RL, et al. Randomized clinical trial of pressure-controlled inverse ratio ventilation and extracorporeal CO2 removal for adult respiratory distress syndrome. Am J Respir Crit Care Med. 1994;149:295–305.
Peek GJ, Mugford M, Tiruvoipati R, et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet. 2009;374:1351–63.
Davies A, Jones D, Bailey M, et al. Extracorporeal membrane oxygenation for 2009 influenza A(H1N1) acute respiratory distress syndrome. JAMA. 2009;302:1888–95.
Freebairn R, McHugh G, Hickling K. Extracorporeal membrane oxygenation for ARDS due to 2009 influenza A(H1N1). JAMA. 2010;303:941–2; author Reply 2
Noah MA, Peek GJ, Finney SJ, et al. Referral to an extracorporeal membrane oxygenation center and mortality among patients with severe 2009 influenza A(H1N1). JAMA. 2011;306:1659–68.
Pham T, Combes A, Roze H, et al. Extracorporeal membrane oxygenation for pandemic influenza A(H1N1)-induced acute respiratory distress syndrome: a cohort study and propensity-matched analysis. Am J Respir Crit Care Med. 2013;187:276–85.
Zangrillo A, Biondi-Zoccai G, Landoni G, et al. Extracorporeal membrane oxygenation (ECMO) in patients with H1N1 influenza infection: a systematic review and meta-analysis including 8 studies and 266 patients receiving ECMO. Crit Care. 2013;17:R30.
Sauer CM, Yuh DD, Bonde P. Extracorporeal membrane oxygenation use has increased by 433% in adults in the United States from 2006 to 2011. ASAIO J. 2015;61:31–6.
Karagiannidis C, Brodie D, Strassmann S, et al. Extracorporeal membrane oxygenation: evolving epidemiology and mortality. Intensive Care Med. 2016;42:889–96.
Tramm R, Ilic D, Davies AR, Pellegrino VA, Romero L, Hodgson C. Extracorporeal membrane oxygenation for critically ill adults. Cochrane Database Syst Rev. 2015;1:CD010381.
Abrams D, Brodie D. Extracorporeal circulatory approaches to treat acute respiratory distress syndrome. Clin Chest Med. 2014;35:765–79.
Brower RG, Matthay MA, Morris A, Schoenfeld D, Thompson BT, Wheeler A. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342:1301–8.
Mercat A, Richard JC, Vielle B, et al. Positive end-expiratory pressure setting in adults with acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA. 2008;299:646–55.
Rubenfeld GD, Cooper C, Carter G, Thompson BT, Hudson LD. Barriers to providing lung-protective ventilation to patients with acute lung injury. Crit Care Med. 2004;32:1289–93.
Hager DN, Krishnan JA, Hayden DL, Brower RG. Tidal volume reduction in patients with acute lung injury when plateau pressures are not high. Am J Respir Crit Care Med. 2005;172:1241–5.
Terragni PP, Del Sorbo L, Mascia L, et al. Tidal volume lower than 6 ml/kg enhances lung protection: role of extracorporeal carbon dioxide removal. Anesthesiology. 2009;111:826–35.
Needham DM, Colantuoni E, Mendez-Tellez PA, et al. Lung protective mechanical ventilation and two year survival in patients with acute lung injury: prospective cohort study. BMJ. 2012;344:e2124.
Bein T, Weber-Carstens S, Goldmann A, et al. Lower tidal volume strategy (approximately 3 mL/kg) combined with extracorporeal CO2 removal versus ‘conventional’ protective ventilation (6 mL/kg) in severe ARDS: the prospective randomized Xtravent-study. Intensive Care Med. 2013;39:847–56.
Marhong JD, Munshi L, Detsky M, Telesnicki T, Fan E. Mechanical ventilation during extracorporeal life support (ECLS): a systematic review. Intensive Care Med. 2015;41:994–1003.
Guerin C, Reignier J, Richard JC, et al. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med. 2013;368:2159–68.
Papazian L, Forel JM, Gacouin A, et al. Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med. 2010;363:1107–16.
Young D, Lamb SE, Shah S, et al. High-frequency oscillation for acute respiratory distress syndrome. N Engl J Med. 2013;368:806–13.
Ferguson ND, Cook DJ, Guyatt GH, et al. High-frequency oscillation in early acute respiratory distress syndrome. N Engl J Med. 2013;368:795–805.
Hodgson CL, Tuxen DV, Davies AR, et al. A randomised controlled trial of an open lung strategy with staircase recruitment, titrated PEEP and targeted low airway pressures in patients with acute respiratory distress syndrome. Crit Care. 2011;15:R133.
Cavalcanti AB, Suzumura EA, Laranjeira LN, et al. Effect of lung recruitment and titrated positive end-expiratory pressure (PEEP) vs. low PEEP on mortality in patients with acute respiratory distress syndrome: a randomized clinical trial. JAMA. 2017;318:1335–45.
Artigas A, Camprubi-Rimblas M, Tantinya N, Bringue J, Guillamat-Prats R, Matthay MA. Inhalation therapies in acute respiratory distress syndrome. Ann Transl Med. 2017;5:293.
Combes A, Brechot N, Luyt CE, Schmidt M. Indications for extracorporeal support: why do we need the results of the EOLIA trial? Med Klin Intensivmed Notfmed. 2018;113:21–5.
Gattinoni L, Vasques F, Quintel M. Use of ECMO in ARDS: does the EOLIA trial really help? Crit Care. 2018;22:171.
Kahn JM, Goss CH, Heagerty PJ, Kramer AA, O’Brien CR, Rubenfeld GD. Hospital volume and the outcomes of mechanical ventilation. N Engl J Med. 2006;355:41–50.
Barbaro RP, Odetola FO, Kidwell KM, et al. Association of hospital-level volume of extracorporeal membrane oxygenation cases and mortality. Analysis of the extracorporeal life support organization registry. Am J Respir Crit Care Med. 2015;191:894–901.
Combes A, Brodie D, Bartlett R, et al. Position paper for the organization of extracorporeal membrane oxygenation programs for acute respiratory failure in adult patients. Am J Respir Crit Care Med. 2014;190:488–96.
Salna M, Chicotka S, Biscotti M 3rd, et al. Management of surge in extracorporeal membrane oxygenation transport. Ann Thorac Surg. 2018;105:528–34.
Higgins AM, Pettila V, Harris AH, et al. The critical care costs of the influenza A/H1N1 2009 pandemic in Australia and New Zealand. Anaesth Intensive Care. 2011;39:384–91.
Park M, Mendes PV, Zampieri FG, et al. The economic effect of extracorporeal membrane oxygenation to support adults with severe respiratory failure in Brazil: a hypothetical analysis. Rev Bras Ter Intensiva. 2014;26:253–62.
Harrington D, Drazen JM. Learning from a trial stopped by a data and safety monitoring board. N Engl J Med. 2018;378:2031–2.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Salna, M., Abrams, D., Brodie, D. (2019). ECMO After EOLIA: The Evolving Role of Extracorporeal Support in ARDS. In: Vincent, JL. (eds) Annual Update in Intensive Care and Emergency Medicine 2019. Annual Update in Intensive Care and Emergency Medicine. Springer, Cham. https://doi.org/10.1007/978-3-030-06067-1_7
Download citation
DOI: https://doi.org/10.1007/978-3-030-06067-1_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-06066-4
Online ISBN: 978-3-030-06067-1
eBook Packages: MedicineMedicine (R0)