Abstract
Whole-body imaging plays a crucial role in the diagnosis and follow-up of pediatric malignancies, as tumor spread may involve different anatomical regions. Until recently, ultrasonography (US) and computed tomography (CT) have been the imaging technique of choice in children with cancer, but nowadays there is an increasing interest in the use of functional imaging techniques like single-photon emission computed tomography (SPECT) and positron emission tomography (PET). By combining these latter techniques with CT, it becomes possible to simultaneously acquire imaging data on the biological behavior of tumor as well as the anatomical localization and extent of tumor spread. Because of the small but not negligible risk of radiation-induced secondary cancers and the significantly improved overall survival rates of children with cancer, there is an increasing interest in the use of radiation-free imaging techniques such as magnetic resonance imaging (MRI). MRI allows for acquiring images with a high spatial resolution and excellent soft tissue contrast throughout the body. Moreover, recent technological advances have resulted in fast diagnostic sequences for whole-body MR imaging, including functional techniques such as diffusion-weighted imaging (DWI). In this chapter, the current status of the technique, major clinical applications, and future perspectives of whole-body MRI in children with cancer will be discussed.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
7.1 Introduction
Whole-body imaging is essential in pediatric oncology as tumor spread may involve different anatomical regions [1, 2]. Traditionally, ultrasonography (US) and computed tomography (CT) are used to detect tumors and to assess the extent of tumor spread before, during, and after therapy. But nowadays, there is an increasing interest in functional imaging techniques like positron emission tomography (PET) and single-photon emission computed tomography (SPECT) [3]. By combining these latter techniques with CT, it has become possible to acquire imaging data on the biological behavior of tumor as well as the anatomical localization and extent of tumor spread in one single examination throughout the body. A major disadvantage of these techniques is the use of ionizing radiation, which may be associated with induction of second cancers later during life. This small but not negligible health risk is of particular concern in children as their tissues are more radiosensitive than adults and they have more years ahead in which cancerous changes might occur. Magnetic resonance imaging (MRI) is a radiation-free imaging technique that allows for acquiring images with a high spatial resolution and excellent soft tissue contrast throughout the body. Due to recent technological advances in MRI such as moving table platforms, multichannel/multielement surface coils, and parallel imaging, it is now possible to cover the whole human body in a reasonable period of time [4,5,6,7,8]. This makes MRI an ideal imaging tool for the detection of pathology, especially in parenchymal and bone marrow locations. Moreover, with the introduction of new advanced MRI techniques like whole-body diffusion-weighted imaging (DWI), it has become possible to acquire functional information for better tumor characterization and therapy monitoring [9,10,11,12,13]. Although the feasibility of whole-body MRI in pediatric oncology has been proven in the literature over the past 10 years [14,15,16,17,18,19,20,21], several challenges remain including the frequent need for sedation or general anesthesia because of long scan times, artifacts from motion, as well as the suboptimal imaging of the lungs in the evaluation for pulmonary metastatic disease. Despite these challenges, whole-body MRI is nowadays being increasingly used in children to evaluate the extent of various oncologic entities at diagnosis and during therapy. In this chapter, the current status of the technique, major clinical applications, and future perspectives of whole-body MRI in children with cancer will be discussed.
7.2 Technique
Until now, there is no standardized technique or protocol for performing whole-body MRI. In pediatric oncology, whole-body MRI is often restricted to the skull or skull base to groin region in line with most hybrid imaging techniques, but it can involve imaging of the entire body (from vertex to toes). Most modern MRI scanners are equipped with a moving table top for sequential movement of the patient through the magnet during imaging without the need for repositioning.
In whole-body MRI, the use of phased-array surface coils is preferred over the use of the quadrature body coil integrated in the magnet bore, because of the better spatial resolution and signal-to-noise ratio (SNR) of the former over the latter. This is especially true when functional imaging techniques like whole-body DWI are planned to be included in the imaging protocol. The way these coils can be used for a whole-body image acquisition depends on the type of MRI scanner available. A very practical and easy to implement approach, the so-called “sliding table and repositioning surface coil” approach, uses tabletop spacers to place an additional table platform on the original MR table allowing manipulation of the lower part of a non-integrated phased-array surface coil without repositioning the patient (Fig. 7.1) [22]. However, nowadays, on several modern MRI scanners, dedicated multichannel surface coil systems are available allowing for whole-body imaging without the need to reposition the coil at each station (Fig. 7.2) [5].
Table preparation for whole-body MRI using a sliding surface coil approach. (a) Spacers (white arrowheads) are placed on top of the original patient table to create space for the lower part of the surface coil (black arrowhead). (b) An additional table platform is mounted on top of the spacers (black arrow). The lower part of the surface coil (black arrowhead) can be moved freely below the additional table platform (white arrows). (c, d) Patient is lying on top of the additional table platform; the surface coil can be moved freely without patient repositioning (white arrows) (adapted with permission from [22])
Until now, whole-body MRI is mainly performed on 1.5 T MRI equipment. MRI at 3 T has the intrinsic advantage of a higher signal-to-noise ratio (SNR) but remains challenging because of geometric distortion, image shearing, chemical shift, and ghosting artifacts, especially in case of larger fields of view. However, recent technical developments have improved whole-body imaging at 3 T, including the recently introduced dual-source radiofrequency transmission technology to reduce field non-homogeneities and the more robust suppression of fat by the slice-selection gradient reversal (SSGR) technique [10, 19, 23]. Furthermore, a recent study by Azzedine et al. in 23 adult patients has shown that 3 T whole-body DWI is feasible and yields accurate assessment and staging of lymphoma comparable to 1.5 T [24].
7.2.1 Sequences
The choice of sequences will depend largely on the type of tumor or clinical question, patient size, and the time available. Sequences that are typically used in whole-body MRI include short tau inversion recovery (STIR), T1-weighted fast spin-echo (FSE, TSE), and contrast material-enhanced (CE) T1-weighted three-dimensional gradient echo (VIBE, THRIVE, LAVA) sequences. Furthermore, with the introduction of diffusion-weighted whole-body imaging with background body signal suppression (DWIBS) in 2004 by Takahara et al., it became possible to perform whole-body DWI under free breathing within a clinically acceptable examination time [9].
7.2.1.1 Short Tau Inversion Recovery (STIR)
STIR is the most commonly used sequence in whole-body MRI. Due to the nulling of the fat signal and the fact that pathologic tissues are usually proton-rich (with prolonged T1 and T2 relaxation times), they will be highlighted on STIR images (Fig. 7.3) [5, 18, 19, 23, 25, 26]. Fat suppression on STIR images is more robust and homogeneous than on other types of T2-weighted fat-saturated images. Furthermore, the signal of other substances with a short T1 relaxation time (like blood, liquid protein, melanin, and gadolinium) will also be reduced. On STIR images, certain organs will have a physiological high signal, such as lymph nodes, spleen, thymus, Waldeyer’s ring, and kidneys. In these organs, pathology will be more difficult to detect or appear as low signal lesions [25]. Furthermore, as normal and pathologic lymph nodes usually show similar high signal intensity, distinction of pathologic nodes is primarily based on size criteria. Finally, STIR is a highly sensitive technique to detect osteomedullary metastases, as in particular in young children hypercellular red marrow zones persist in large parts of the bone that will obscure metastatic disease on T1-weighted imaging (Fig. 7.3). Several studies have investigated the role of STIR in whole-body MRI [14, 17, 27, 28]. Although most studies did show that whole-body STIR is a sensitive technique to detect pathologic lesions, it is not specific for malignancy as inflammatory, infectious, traumatic, necrotic, and post-therapeutic changes, as well as benign lesions such as cysts and hemangiomas, can also present with high signal intensities. In addition, possible motion artifacts from respiration or vessel pulsation may obscure parenchymal lesions.
Coronal whole-body short tau inversion recovery (STIR) (a), T1-weighted (b) and maximum intensity projection grayscale inverted diffusion-weighted (c) whole-body MRI images of a 16-year-old boy with stage IV B Hodgkin lymphoma, illustrating nodal involvement of cervical, axillary, mediastinal, hilar, para-aortic, and left para-iliac lymph nodes (arrows), as well as bone marrow involvement of vertebral body L4 (arrowhead). As illustrated in (a) and (b), bone marrow involvement is more easily detected on the STIR image when compared to the T1-weighted image, in this case due to diffuse bone marrow reconversion (asterisk in b) related to the lymphoma. On the maximum intensity projection grayscale inverted diffusion-weighted whole-body MRI image (c), an additional bone marrow lesion can be seen in the proximal femoral diaphysis (open arrowhead)
7.2.1.2 3D T2-Weighted Turbo Spin-Echo (T2-TSE)
Recent technological advances have made it possible to acquire 3D T2-weighted turbo or fast spin-echo images of large parts of the body within clinically acceptable examination times (Fig. 7.4). An important advantage of these sequences is the better anatomical delineation and higher signal-to-noise ratio when compared to STIR imaging. If fat suppression is required, the use of the (three point) Dixon technique is preferred over frequency-selective fat saturation techniques, as the former will result in a more uniform fat suppression especially in body regions such as the neck that suffer from main static magnetic field (B0) inhomogeneity [23, 29]. The Dixon technique allows generation of four sets of images with different image contrast in a single acquisition, i.e., fat-only, water-only, in-phase, and opposed-phase images. Due to its almost perfect fat suppression on the water-only images and the enhanced image contrast, this technique can be used to improve lesion detectability and characterization on pre- and post-contrast whole-body MRI [21, 23, 29,30,31]. Limitations of the Dixon technique include the fat-water separation or swapping error that sometimes occurs in one leg or around the thorax and the loss of signal around the heart due to cardiac pulsatility. However, as large-scale studies in children are still missing, it remains to be determined whether these promising new T2-weighted imaging techniques will become a mainstay in pediatric whole-body MRI beyond STIR imaging.
Coronal 3D T2-weighted turbo spin-echo (T2-TSE) image in a 3-year-old boy with stage IV neuroblastoma, illustrating the better anatomical delineation and higher signal-to-noise ratio when compared to STIR imaging. There is a left paravertebral tumor arising from the left adrenal region with involvement of the left renal hilar region (white arrows) and extension into the inferior mediastinum (arrowhead)
7.2.1.3 T1-Weighted Fast Spin-Echo (T1-FSE)
The T1-FSE sequence is especially helpful for anatomic delineation of lesions and to increase the specificity of the detection of bone marrow involvement in older children (Fig. 7.3) [18, 32, 33]. It is usually combined with whole-body STIR imaging. In our clinical experience, STIR is very useful for assessing the extent of disease at presentation but is less suitable for assessing response during treatment because the initial involved sites often show a decrease in T2 signal during treatment. Although not commonly used in whole-body imaging, the addition of 3D contrast-enhanced (CE) T1-weighted GRE sequence can improve the diagnostic accuracy of lesion delineation and characterization [4, 5, 18, 34]. In addition, it facilitates the combination of better local tumor staging and evaluation of metastatic disease. A disadvantage of this sequence is the suboptimal fat saturation. As mentioned before, the Dixon technique might be a good alternative for this as its almost perfect fat suppression on the water-only images and the increased image contrast will improve lesion detectability and characterization on pre- and post-contrast whole-body MRI [21, 23, 29,30,31].
7.2.1.4 Diffusion-Weighted Imaging (DWI)
With the introduction of diffusion-weighted whole-body imaging with background body signal suppression (DWIBS) in 2004 by Takahara et al., it became possible to perform whole-body DWI under free breathing within a clinically acceptable examination time [9, 10, 12, 13, 23]. Single-shot echo-planar imaging (EPI) is most commonly used in whole-body DWI in order to reduce motion artifacts. To optimize background body signal suppression and avoid image degradation due to severe chemical shift in EPI sequences, either a STIR pre-pulse or a frequency-selective (chemical shift selective, CHESS) pre-pulse is used for fat suppression. Although the selection of the method for fat suppression may depend on the organ/body region under examination, STIR usually results in the most robust fat suppression over an extended field of view, in particular in the neck/shoulder region and lower extremities. In addition, for bowel signal suppression in the abdominal region, STIR is the preferred method as well. When performing whole-body DWI at higher field strengths (e.g., 3 T), the recently reintroduced slice-selection gradient reversal (SSGR) technique allows for the most robust fat suppression without prolonging scan time or increasing SAR [10, 19]. Whole-body DWI is usually acquired in the axial plane with multiplanar reconstructions in the coronal and sagittal plane, as direct coronal or sagittal acquisitions are more vulnerable to image distortion. To facilitate optimal whole-body multiplanar reconstructions from different anatomic sections, it is important to ensure that data acquisition is performed in a way that minimizes voxel shifts between imaging stations for accurate stack alignment. For visual assessment of whole-body DWI high b-values up to 1000 s/mm2 should be applied to improve lesion conspicuity. In case quantitative measurements of the diffusivity (apparent diffusion coefficient, ADC) in pathological tissues are required, the use of at least three b-values (including b0) is recommended. Besides b0 this should include two b-values at or above 100 s/mm2 to reliably separate perfusion from bulk diffusion and allow for more accurate calculation of ADC values [35, 36]. Furthermore, to minimize the chance of misregistration, and thus incorrect ADC mapping, it is better to choose high b-values that maintain some background tissue signal, typically around 500 s/mm2 up to 800 s/mm2. This background tissue visibility is also important when coregistration with conventional MRI sequences is foreseen and is especially important for free-breathing acquisitions. For a more in-depth discussion of the technique of DWI in the body, the reader is referred to Chapter 6 in this book and the recent literature [11, 13, 35,36,37].
By adding DWI to a whole-body imaging protocol, lesion detection can be improved, reading time can be decreased, and the functional information on water diffusivity can be of help to better characterize pathology and monitor therapy [10, 13, 36, 38,39,40]. For visual assessment an inverted gray scale image from the highest b-value acquisition is often used, with disease areas appearing as dark regions against a normal white background due to the restricted diffusion in these areas (Fig. 7.3). As mentioned above, the DWI images can be fused with other standard anatomic MRI sequences to further improve lesion localization (Fig. 7.5). In addition, whole-body DWI images should be reviewed in combination with conventional T1-weighted and T2-weighted MRI images to ensure accurate interpretation, as areas of diffusion restriction are not specific for malignancy and can be caused by normal red bone marrow or other disease processes, such as inflammation. Moreover, ADC maps should be used to ascertain the presence of diffusion restriction by excluding T2 shine through phenomena. Quantitative (ADC) measurements facilitate differentiation of benign versus malignant disease, although considerable overlap in ADC values does occur [18, 41, 42]. Furthermore, comparison of the mean or median ADC values of a lesion before and after treatment can be used to monitor tumor response as ADC values usually change (increase) after therapy. It should be mentioned, however, that reproducibility of ADC measurements obtained with whole-body DWI has not been established yet, particularly with regard to ADC variations across anatomic imaging stations.
Coronal whole-body short tau inversion recovery (STIR) (a), axial T2 spectral attenuated inversion recovery (SPAIR) (b), diffusion-weighted b800 (c), apparent diffusion coefficient (ADC) (d), and fused T2 SPAIR/DWI b800 images (e) in a 14-year-old girl with a stage III Hodgkin lymphoma, nicely illustrating the potential role of fusion of DWI/ADC images with other standard anatomic MRI sequences to further improve lesion localization. An additional pathologic lymph node region was detected in the splenic hilum, best appreciated on the fused T2 SPAIR/DWI b800 image (white arrow in a, white arrowhead in b–e). The corresponding PET/CT slice (f) shows increased tracer uptake in the same lymph node region suspicious of disease involvement (white arrowhead)
Several normal structures do show diffusion restriction due to its high cellularity, including red marrow, brain, salivary glands, thymus, spleen, adrenals, prostate, and gonads (Fig. 7.6) [10, 13, 18, 37]. Moreover, lymphoid tissue also shows relative restricted diffusion and long T2 values, which limits the detection of pathology in lymph nodes, which are often involved in malignancies. Therefore, current criteria for diagnosing nodal involvement often rely on a combination of signs, including nodal signal intensity (on its own or compared with the primary tumor), ADC value, and size criteria. As the apparent size of lymph nodes on DWI images is dependent on the applied window level and width, these size measurements for prediction of nodal malignancy should be performed on conventional T1-weighted and T2-weighted sequences. The use of an ultrasmall superparamagnetic iron oxide (USPIO) contrast agent allows for a better delineation of malignant nodal (and bone marrow) infiltration, even in normal sized lymph nodes. USPIO nanoparticles are retained in the normal reticuloendothelial system of the lymph nodes, liver, spleen, and bone marrow with a resulting signal drop on T2, T2*, and DWI images, whereas malignant lesions lack uptake, thereby improving the tumor-to-background contrast effectively [20, 43]. In addition, USPIO nanoparticles result in long-lasting T1 shortening in vessels, which allows acquisition of enhanced T1-weighted images for anatomical localization. Unfortunately, USPIO nanoparticles are not approved by most (inter)national drug agencies, limiting their use in daily clinical practice. Furthermore, adverse reactions after intravascular USPIO administration do occur with hypersensitivity reactions in up to 3–7% and rare events of serious anaphylactic reactions in 0–2% in the adult population [20, 43]. For this reason the FDA has issued a black box warning regarding these risks, recommending clinicians to carefully consider the potential risks and benefits of administering USPIOs including careful screening for any history of allergies, in particular regarding dextran and iron compounds.
Coronal maximum intensity projection grayscale inverted diffusion-weighted whole-body MRI images of a 9-year-old girl who underwent a whole-body MRI for the follow-up of a lymphatic malformation (not shown). The images nicely illustrate the normal diffusion restriction seen in (a) bone marrow (black arrows), kidneys (open arrow), spleen (black arrowhead), left ovary (open arrowhead), and (b) the brain and spinal cord (squared open arrow) as well as normal lymph nodes (squared black arrows)
7.2.2 Scan Plane
The choice of scan plane will depend on the region of interest, type of malignancy, and diagnostic information required for optimal treatment planning and follow-up. However, because of time efficiency (less slices needed), the coronal plane is most often acquired and displayed in whole-body MRI, in particular when STIR and T1-weighted FSE sequences are used. The 3D contrast-enhanced T1-weighted GRE and DWIBS sequences are usually acquired in the axial plane, but the obtained images can be easily post-processed for multiplanar reconstruction (MPR) and maximum intensity projection (MIP). In addition, it has been shown that the axial plane is more sensitive, especially for lesions in the ribs, scapula, and skull/brain [4,5,6, 18, 44]. If there is a specific interest in disease involvement of the spine, a complementary sagittal plane is recommended because of its physiological curves [19]. Finally, a small overlap of 3–5 cm between adjacent stations during multi-station imaging is often indicated to compensate for signal loss at the periphery of each station and facilitate seamless whole-body reconstruction.
7.2.3 Reduction of Motion Artifacts
Several methods have been developed to reduce or correct for motion artifacts, each suitable for some tasks, but not for others. The methods for reduction of motion artifacts can roughly be divided into three groups: motion prevention (e.g., feed and wrap, training, breath hold, and sedation), artifact reduction (e.g., faster imaging, triggering, and gating), and motion correction (e.g., navigators, PROPELLER technique, retrospective correction) [45]. The PROPELLER (periodically rotated overlapping parallel lines with enhanced reconstruction) technique provides substantially reduced motion sensitivity due to strong oversampling of the k-space center. This technique collects data in concentric rectangular blades that rotate around the k-space center, which (a) allows for correction of spatial inconsistencies, (b) allows rejection of data based on a correlation measure indicating through-plane motion, and (c) further decreases motion artifacts through an averaging effect for low spatial frequencies. The PROPELLER imaging method is used by all major vendors (e.g., MultiVane (Philips), BLADE (Siemens), and PROPELLER (GE)). Imaging of the thorax and upper abdomen is usually obtained using respiratory compensation techniques, whereas antiperistaltic agents can be considered if the abdomen is the site or predisposed area of the known or suspected malignancy. In children, we prefer the use of hyoscine butylbromide (scopolamine butylbromide; Buscopan™; not available in the USA) since the administration of glucagon is often associated with symptoms of nausea and vomiting and most contraindications for hyoscine butylbromide are rare in childhood.
7.2.4 Patient Preparation
As whole-body MRI scans are usually lengthy with anticipated examination times of 40–60 min, adequate patient preparation is essential to minimize movement and anxiety. Children should be warned that the scan could be noisy owing to the sequences used and vibrations of the MRI bed. The application of multiple surface coils to cover the whole body might provoke anxiety, and by leaving the head coil off (if not essential), patient tolerance can be improved. Furthermore, one or both parents should be asked to stay with their child in the MRI room, and listening to their own music or viewing a film can help to further reduce anxiety. The child should be offered the opportunity for bladder emptying before the examination to minimize discomfort during the scan. As with most lengthy MRI scans, children under the age of 6 years usually require sedation or general anesthesia.
In Tables 7.1 and 7.2, examples are given of two different whole-body MRI protocols for pediatric oncological indications used in the University Medical Center Utrecht, the Netherlands.
7.3 Current Applications
7.3.1 Bone Marrow Imaging
MRI is a sensitive method for assessing bone marrow, although it lacks specificity, especially in younger children. Normal bone marrow is composed of a combination of yellow (fatty) marrow and red (hematopoietic) marrow that together contribute to the signal seen in MRI. In children the distribution of red marrow and its cellular content varies with age. At birth, the bone marrow is entirely hematopoietic and then shortly after birth starts to become replaced by yellow marrow in an orderly and predictable sequence (Fig. 7.7) [46,47,48]. This transition from red to yellow marrow begins in the peripheral bones and progresses in a symmetrical manner to the central skeleton. Within the individual long bones, the marrow conversion occurs first in the diaphysis and then progresses to the metaphysis. The vertebral marrow remains predominantly hematopoietic during the first decade of life except for some yellow bone marrow around the central vertebral vein [49]. Good knowledge of this physiological marrow conversion as a function of age is required to be able to distinguish the areas of normal hematopoiesis from bone lesions.
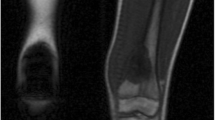
Normal age-related bone marrow conversion within the long bone. Red to yellow marrow conversion occurs in a predictable manner throughout childhood and adolescence. The adult marrow pattern is reached around 25 years of age, characterized by red marrow confined to the axial skeleton and proximal femora and humeri. During infancy there is hematopoietic marrow (red marrow) throughout the long bones. The secondary ossification centers quickly convert from red to yellow marrow. In childhood, the marrow conversion starts in the center of the long bones and progresses proximally and distally toward the metaphysis
On MRI, malignant infiltration of the bone marrow usually presents with low T1 and high T2 signal due to the combination of free water and edema in addition to replacement of the fatty content of normal marrow by malignant cells (Fig. 7.8) [33]. In children, the malignant bone marrow infiltration may be focal, such as in lymphoma (Figs. 7.3 and 7.9), but is often diffuse, e.g., in neuroblastomas and leukemias (Fig. 7.12) [18, 48, 50]. Especially in young patients it can be challenging to detect bone marrow disease, because the high cellularity of the normal red marrow can be misdiagnosed as diffuse bone marrow infiltration or mask tumor deposits, in particular on T1-weighted sequences. In these cases, the STIR sequence can be of use as the high signal intensity of the normal red marrow is frequently less distinct than that of malignant infiltration. On the other hand, it can be helpful to compare the signal intensity of the bone marrow on T1-weighted sequences with adjacent muscles or vertebral disc. Generally, after 1 year of age, the signal intensity of bone marrow should be iso- or hyperintense relative to the intervertebral disc or muscles. One should be cautious when evaluating bone marrow involvement during or after chemotherapy, as the post-therapeutic signal changes seen may be difficult to distinguish from active malignant bone lesions [46]. In addition, anemic or treatment-related medullary hyperplasia can obscure malignant infiltration. Finally, heterogeneous sharply demarcated linear areas or focal islands of red marrow can be encountered as normal variants in children [46, 51].
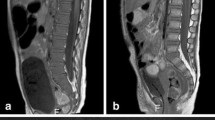
3-month-old boy presenting with inspiratory stridor due to thoracic tumor (a; white arrow) located in the posterior mediastinum with extension in the neuroforamen Th3–Th4 (open arrow). With ultrasound-guided biopsy, a neuroblastoma, standard risk was diagnosed. The bone marrow biopsy appeared normal. At presentation diffuse low T1 signal of the bone marrow is seen, isointense compared to the intervertebral disc (b with zoomed insets; white arrowhead indicating the intervertebral disc) and muscles consistent with red bone marrow. Furthermore, the signal intensity of the bone marrow at the sagittal STIR is within normal limits (c). With watchful waiting spontaneous regression of the tumor was observed (d; white arrow) and no treatment was necessary. After 6 months, there is a marked decrease in size of the tumor (d; white arrow) with increased T1 signal intensity related to internal calcifications. Furthermore, the bone marrow signal showed an increase in signal intensity (e with zoomed insets), especially centrally within the vertebral body consistent with areas of fatty bone marrow. The signal intensity at the sagittal STIR of the spine is within normal limits (f)
Coronal whole-body short tau inversion recovery (STIR) (a), T1-weighted (b) and maximum intensity projection grayscale inverted diffusion-weighted (c) whole-body MRI images in the same patient as Fig. 7.3 after two cycles of chemotherapy. Almost all initially involved lymph node stations (cervical, axillary, mediastinal, para-aortic, para-iliac) are normalized in size (white arrows in a and b), and their restricted diffusion disappeared (black arrows in c). Several bone marrow localizations still show abnormal signal intensity on both the STIR and T1-weighted images (white arrowheads in a and b) as well as signs of diffusion restriction (black arrowhead in c). Bone marrow reconversion, as seen in Fig. 7.3, has disappeared as the signal intensity of the noninvolved vertebral bodies has returned to that of normal fatty marrow (asterisk in b)
Although DWI is widely used for the assessment of bone marrow disease, it is of limited value due to the high cellular hematopoietic marrow that will exhibit impeded diffusion. This is especially true for young children. Ording-Müller et al. demonstrated that areas of restricted diffusion in the pelvic skeleton and lumbar spine are a normal finding in 48% of healthy children [52]. Therefore, DWI should not be used in isolation but in conjunction with T1- and T2-weighted images. Another technique that has been proposed as a complementary tool for the assessment of bone marrow disease in adults is chemical shift imaging (opposed-phase imaging) [53]. In chemical shift imaging, the differentiation of malignant bone marrow infiltration from normal red marrow is based on the detection of small quantities of fat.
Meyer et al. tried to identify the best MRI sequence or imaging criteria for the diagnosis of bone marrow metastases in children with neuroblastoma [54]. They found that homogeneous low T1 signal had the highest sensitivity (88%), whereas a heterogeneous pattern on the post-gadolinium was highly specific (97%) but relatively insensitive (65%) for detecting metastasis.
7.3.2 Malignant Lymphoma
A pediatric malignancy for which whole-body MRI has been widely used already for many years is lymphoma. It is the third most common malignancy in children and includes Hodgkin lymphoma (HL) and non-Hodgkin lymphoma (NHL) [55]. NHL is most frequent in children younger than 15 years, whereas HL is predominantly diagnosed in teenagers. Pediatric lymphomas are staged using the modified Ann Arbor (Lugano) and Murphy classifications for HL and NHL, respectively [56, 57]. Recently, a new revised International Pediatric Non-Hodgkin Lymphoma Staging System (IPNHLSS) has been developed incorporating new histologic entities, extranodal dissemination, improved diagnostic methods, and advanced imaging technology [58]. Accurate assessment of disease extent at diagnosis and response to treatment are essential to optimize therapy and prevent disease relapse while minimizing late therapy-related side effects.
Current guidelines encourage the use of FDG-PET/CT in staging and response assessment of FDG-avid lymphomas [56]. As both PET and CT involve substantial radiation exposure and children often undergo several PET/CT examinations during the course of treatment, there is an increasing interest in the use of whole-body MRI as a good radiation-free alternative for staging and follow-up. Several studies have shown that whole-body MRI is feasible in children (Fig. 7.3) [14, 28, 59]. In the study by Punwani et al., a very good agreement for nodal and extranodal disease involvement between whole-body MRI compared to an FDG-PET/CT reference standard was reported, despite only using STIR for whole-body MRI [28]. Due to the clear visualization of lymphoid tissue, DWI is a potentially interesting technique for the evaluation of lymphoma. Lin et al. showed an excellent agreement between whole-body DWI and FDG-PET/CT in 15 adult patients with diffuse large B-cell lymphomas [60]. Similar results have been reported by Gu et al. and Stephane et al. based on studies performed in adult patients with varying types of lymphoma [38, 61]. However, in other studies the additional value of DWI to conventional MRI sequences could not be demonstrated [59, 62]. This could be related to the fact that both benign and malignant nodes demonstrate restricted diffusion. Furthermore, there are no validated ADC values yet for discriminating involved from noninvolved sites [41]. Therefore, the determination of pathologic lymph node involvement in whole-body DWI is still mainly based on size criteria. An additional issue with DWI is that several normal extranodal structures that can be involved in lymphoma (including the brain, Waldeyer’s ring, thymus, spleen, testes, ovaries, spinal cord, peripheral nerves, and bone marrow) may demonstrate restricted diffusion (Fig. 7.6). Consequently, pathology in any of these areas may be obscured.
Klenck et al. prospectively compared ferumoxytol-enhanced whole-body diffusion-weighted MRI to FDG-PET/CT in 22 children and young adults for staging lymphoma and sarcoma [20]. This ultrasmall superparamagnetic iron oxide (USPIO) contrast agent is thought to overcome the limitations of conventional whole-body MRI regarding detection of nodal, bone marrow, and splenic involvement. Ferumoxytol has been shown to discriminate normal lymphoid tissues, which take up the USPIO agent, from lymphoid malignancies, which do not. Thus the better detection of lymphomatous involvement in the reticuloendothelial system (RES) depends on the uptake of USPIO in the noninvolved reticuloendothelial system (RES) and the non-uptake by (metastatic) tumor deposits.
Assessment of response to therapy is important for determining treatment effectiveness and predicting clinical outcome. The concept of early response assessment with FDG-PET/CT in lymphoma has received considerable attention in the past few years, although it is still not officially recommended outside clinical trials [56]. In addition, the value of relying solely on interim and end-of-treatment FDG-PET/CT in predicting outcome was demonstrated to be unsatisfactory by recent meta-analyses [63, 64]. The role of whole-body MRI in response assessment in children with lymphoma is still under investigation (Fig. 7.9). Mayerhoefer et al. recently published the results of their prospective study in 64 adult lymphoma patients that showed that whole-body MRI with DWI could serve as a feasible alternative for FDG-PET/CT during follow-up and treatment response assessment [65]. Several, mostly pilot, studies compared the quantitative data from FDG-PET/CT (SUV) with DWI (ADC values) for interim response assessment with inconclusive results. They reported presence or absence of an inverse correlation between ADC and SUV [66,67,68,69,70].
7.3.3 Histiocytosis
Langerhans cell histiocytosis (LCH) is a rare disease with an incidence of approximately 5 cases per 1 million children under age 15 years (median age at presentation 30 months) and an equal distribution among boys and girls [71]. LCH can involve almost any organ, but the most common presentation includes skin rashes and/or painful bone lesions. Less frequently, children present with diabetes insipidus due to pituitary involvement or back pain caused by vertebra plana. Adequate staging at diagnosis is essential, first of all because the presence or absence of liver, spleen, and/or bone marrow involvement determines whether the patient belongs to the clinical “high-risk” or “low-risk” group. “High-risk” LCH patients have a more than 85% long-term survival rate, whereas the survival rate of “low-risk” LCH patients approaches 100%. Furthermore, the number and locations of lesions at diagnosis influence the choice of (chemo)therapy.
The standard approach to staging usually consists of a combination of laboratory tests and imaging [71, 72]. Traditionally, the imaging evaluation of patients with LCH includes chest radiography and a complete skeletal survey. More recently an abdominal ultrasound has been added to rule out/demonstrate intra-abdominal organ involvement. Skeletal involvement is the most common radiographic abnormality with the skull, ribs, spine (vertebra plana), pelvis, and scapula as the most common sites of involvement. In case of long bone involvement, the femora are the most commonly affected site. Extension of the primary bony lesion into the surrounding soft tissues and epidural space can be seen, especially with bone lesions in the skull, ribs, and spine. Bone scintigraphy has been used for the evaluation of LCH with a lesion detection rate compared to the skeletal survey. However, the scintigraphic appearance of LCH may vary especially when the lesions are small or fail to incite a significant osteoblastic response [72, 73]. The use of 18F-FDG-PET for the evaluation of pediatric LCH has been reported, with high sensitivity and specificity of this imaging technique that is superior to the skeletal survey and bone scintigraphy [74,75,76]. An advantage of 18F-FDG-PET over the traditional techniques is the possibility of identifying extra-osseous localizations of LCH. Furthermore, 18F-FDG-PET shows lesion response to therapy earlier than conventional radiography and CT.
Interestingly, a recent study by Mueller et al. also showed that the overall sensitivity of 18F-FDG-PET was lower than whole-body MRI, especially for small bony infiltrates (mean diameter 12 mm) and central nervous system involvement [76]. As stated before, whole-body MRI is in particular well suited for the evaluation of bone marrow involvement, accompanying soft tissue masses, as well as other extra-osseous manifestations. In LCH, most bony lesions will show an intermediate to hypointense signal intensity on T1-weighted images and a hyperintense signal intensity on T2-weighted and STIR images (Fig. 7.10) [77, 78]. On post-contrast T1-weighted images, the LCH lesions usually show marked enhancement, although predominantly peripheral enhancement can be seen (Fig. 7.10e). Early-stage lesions usually show edema in the adjacent bone marrow, periosteum, and soft tissues. During treatment, healing lesions will show decrease in signal intensity on STIR imaging.
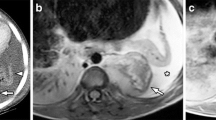
Sagittal short tau inversion recovery (STIR) (a) and T1-weighted (b) images of the vertebral column in a 1.5-year-old boy with Langerhans cell histiocytosis (LCH), illustrating the typical vertebral plana at the level of Th12 (white arrow) as well as involvement of vertebral body L4 and S2 (white arrowheads). Furthermore, a heterogeneous enlarged thymus is present (open white arrow), suspicious of LCH involvement. Coronal 3D T2-weighted turbo spin-echo (T2-TSE) image (c) acquired to demonstrate/rule out intrathoracic and intra-abdominal organ involvement, once again showing the thymic involvement in LCH (white arrow). Coronal short tau inversion recovery (STIR) (d) and contrast-enhanced T1-weighted (e) images of the skull, showing two LCH lesions in the skull with epidural extension (white arrows)
Unfortunately, large prospective studies on the diagnostic accuracy of whole-body MRI in pediatric LCH are missing. A small study in only nine children with LCH by Goo et al. compared the use of whole-body MRI to plain radiography and bone scintigraphy [77]. Additional skeletal lesions were identified by whole-body MRI in 38% of patients when compared with plain radiography and in 25% when compared with scintigraphy. Furthermore, whole-body MRI detected extra-osseous lesions in five of nine patients exclusively (56%). In a study of six children with LCH at diagnosis and during follow-up, Steinborn et al. showed that whole-body MRI also detected additional lesions in two patients when compared to the skeletal survey, which resulted in a change of therapy in both children [78]. On the other hand, Mueller et al. showed that, although whole-body MRI was more sensitive for the detection of LCH lesions when compared to 18F-FDG-PET, specificity was significantly lower (sensitivity and specificity: 67% and 76% for PET vs. 81% and 47% for whole-body MRI, respectively) [76]. This lower specificity was mainly due to the detection of false-positive bone lesions by whole-body MRI. In addition, residual T2 hyperintense signal and contrast enhancement after treatment on whole-body MRI resulted in false-positive findings during follow-up. Similar findings have been described by Goo et al. in 2006 [77]. Interestingly, Mueller at al. also described that a combined analysis of 18F-FDG-PET and whole-body MRI decreased the number of false-negative findings at primary staging, whereas no advantage over 18F-FDG-PET alone was seen in terms of false-positive or false-negative results during follow-up [76]. In view of the limited available literature, it seems justified to conclude that the skeletal survey and skeletal scintigram can be replaced by whole-body MRI (preferably in combination with PET), although further research is needed to replicate the initial findings of Goo and Mueller in larger patient cohorts.
7.3.4 Neuroblastoma
Neuroblastoma is the most common solid extracranial tumor in children and infants, representing approximately 6% of all cases of childhood cancer [55]. The peak incidence of presentation of neuroblastoma is around 2–3 years of age, and 90% of patients are diagnosed before 6 years. It is an embryonic tumor arising from primordial neural crest cells that are the precursors of the sympathetic nervous system. The most common site of the primary tumor is within the abdomen (the adrenal medulla in 35%), but it can occur anywhere along the sympathetic chain from neck to pelvis [79]. Almost 70% of children with neuroblastoma will have metastatic disease at diagnosis in the bone marrow, lymph nodes, liver, and/or skin. The prognosis is variable with some tumors behaving aggressively, while others, usually in the younger age group, regress spontaneously. Overall, neuroblastoma accounts for 15% of cancer deaths in children.
In the past, staging of neuroblastoma was based on a postsurgical staging system, the International Neuroblastoma Staging System (INSS). However, in 2009, a new staging system was introduced, the International Neuroblastoma Risk Group Staging System (INRGSS), shifting focus to pre-treatment staging with identification of imaging defined risk factors (IDRF) [80, 81]. These IDRFs describe the relationship between the tumor and adjacent structures that ideally should not be injured during surgery (e.g., major vascular encasement, airway compression, nerve, plexus, or CNS infiltration). The evaluation of local and distant extent of disease at staging and during follow-up is usually based on a combination of cross-sectional imaging (either CT or MRI), I-123 MIBG scintigraphy, and bone marrow biopsy, although whole-body MRI is increasingly used for anatomic imaging instead of CT (Fig. 7.4) [79, 81, 82]. At presentation, neuroblastomas (and ganglioneuromas) usually occur as relatively hyperintense solid masses at T2-weighted imaging in the adrenal or paravertebral region from neck to pelvis (Fig. 7.11). They can be multifocal and present with areas of hemorrhage and/or necrosis, especially in case of extensive disease at diagnosis. Vascular encasement in the abdomen is common, and small calcifications are often seen, although the latter is usually difficult to appreciate on MRI. Another IDRF that is not infrequently seen in neuroblastoma is intraspinal extension of the primary tumor. Lymphadenopathy is a frequent finding at diagnosis with similar imaging appearances as the primary tumor. On DWI, neuroblastomas show diffusion restriction in the solid parts of the lesions. In addition, DWI can guide biopsy as more differentiated parts of lesions (ganglioneuroma/ganglioneuroblastoma) show relative higher ADC values [83]. Bone marrow involvement can be focal or diffuse with relatively low signal intensity on T1-weighted imaging compared to muscle or the intervertebral disc and increased signal intensity on STIR imaging (Fig. 7.12). Liver metastases usually present as T2 hyperintense lesions (Fig. 7.13).
Whole-body MRI in a 3-year-old boy diagnosed with stage IV neuroblastoma (same patient as Fig. 7.4). Coronal 3D T2-weighted turbo spin-echo (T2-TSE) image (a) with sagittal reconstruction (b) demonstrates the primary tumor arising from the left adrenal gland with retroperitoneal extension (white arrows in a), vascular encasement (arrowheads in a and b), and lifting of the aorta (open arrow in b). The contrast-enhanced T1-weighted image (c) illustrates heterogeneous enhancement of the retroperitoneal tumor (white arrows) and the inferior mediastinal extension (white arrowhead). There is diffusion restriction seen as high signal at the b1000 image (d) with corresponding low signal at the ADC map (e)
Sagittal T1-weighted (a) and short tau inversion recovery (STIR) (b) in the same patient as Fig. 7.11, illustrating the bone marrow metastases in several thoracic and lumbar vertebral bodies (white arrows)
Coronal whole-body 3D T2-weighted turbo spin-echo (T2-TSE) image in a 6-month-old boy diagnosed with a stage IVs neuroblastoma, illustrating the diffuse involvement of the liver (white arrows) as well as the retroperitoneal primary tumor mass (white arrowhead) which did arise from the left adrenal gland
As in many other pediatric oncological entities, the role of whole-body MRI in neuroblastoma has not yet been thoroughly evaluated. Pfluger et al. performed a retrospective study of 50 MRI and I-123 MIBG examinations in 28 patients acquired for the assessment of neuroblastoma lesions at presentation and follow-up [84]. They concluded that integrated imaging with I-123 MIBG scintigraphy and MRI increased the diagnostic accuracy. Goo et al. showed that in a group of 13 children with neuroblastoma, whole-body MRI had a higher sensitivity for detection of bone metastases than MIBG and CT (100%, 25%, and 10%, respectively) but poor positive predictive values for detection of skeletal and extraskeletal metastases in comparison with MIBG [15]. On the other hand, MIBG also has its limitations including failure to detect bone marrow involvement, lack of MIBG avidity in up to 10% of patients, loss of MIBG avidity at relapse, and suboptimal visualization of small lesions in the liver [18]. In the study by Gahr et al., the role of DWI in differentiating neuroblastoma, ganglioneuroblastoma, and ganglioneuroma was investigated in 15 patients with 16 histologically classified tumors [83]. They found that there was a significant difference in ADC between neuroblastoma and ganglioneuroblastoma/ganglioneuroma, although there was a considerable overlap between the two groups, and inter- and intraobserver variability of their applied method was not tested. Based on the above, it is suggested that a combination of nuclear medicine techniques (I-123 MIBG scintigraphy) and anatomical imaging will increase the diagnostic accuracy, both at staging and during follow-up, with whole-body MRI increasingly being preferred over CT.
7.3.5 Other Tumors
There is an increasing use of whole-body MRI for the staging (and follow-up) of other pediatric malignancies such as Ewing sarcoma, osteosarcoma, and rhabdomyosarcoma (Fig. 7.14) [17, 20, 27, 32, 85, 86]. Most studies focus on the detection of metastatic bone disease, with comparable or often increased sensitivity when compared to traditional imaging techniques. As the bony metastases can be seen anywhere in the skeleton, even the hands and feet, it is suggested that whole-body MRI should include the entire body from vertex to toe. The main limitation of whole-body MRI in the context of staging is still its limited ability to detect small pulmonary nodules, although there is some recent literature on the detection of small pulmonary nodules of ≥4 mm in size, in particular when using ultrashort echo time MRI sequences [87, 88].
A 3-year-old girl presenting with abdominal complaints was diagnosed with nephroblastoma stage IV, intermediate risk. Coronal 3D T2-weighted image shows a large tumor arising from the right kidney with few cystic areas (a; open arrows). Several metastases in the lungs are seen (a; white arrow). There is shrinkage of the tumor after preoperative treatment (b). The axial fat-suppressed T1-weighted image (c) after intravenous contrast shows a claw sign (arrowheads) indicating this lesion is arising from and not compressing upon the renal parenchyma. Axial T2-weighted image (d) shows the low T2 signal pseudo capsule (open arrowhead)
Several studies have investigated the role of whole-body MRI in patients with neurofibromatosis I (NF-1), as these patients have an increased risk of developing malignant peripheral nerve sheath tumors (MPNSTs) [89,90,91]. Both Mautner et al. and Nguyen et al. found a strong association between the benign whole-body tumor volume and the occurrence of MPNSTs in NF-1 patients younger than 30 years [89, 91]. Furthermore, MRI features like rapid growth, intratumoral lobulation, ill-defined margins, and irregular enhancement on post-contrast images can help to differentiate benign from malignant nerve sheath tumors. However, in the study by Derlin et al. evaluating the accuracy of whole-body MRI and 18F-FDG PET/CT for detection of MPNSTs in 31 people with NF-1, PET/CT had a sensitivity of 100%, whereas whole-body MRI had a sensitivity of 66.7% for identifying malignant tumors [90]. From these studies, it can be concluded that whole-body MRI cannot replace FDG PET/CT for the detection of MPNSTs in this patient population but that the combination of PET and MRI might improve the diagnostic accuracy thereby diminishing the need for unnecessary biopsies or closer clinical follow-up.
7.3.6 Cancer Predisposition Syndromes
Whole-body MRI is a promising imaging tool for cancer surveillance and evaluation of genetic cancer predisposition syndromes (CPS), especially because of its lack of ionizing radiation [6, 92,93,94,95,96]. Children with CPS (including NF-1, Beckwith-Wiedemann, multiple endocrine neoplasia, Li-Fraumeni, von Hippel-Lindau, DICER1 syndrome, and rhabdoid tumor syndrome) are at a significantly increased risk of developing cancer. Whole-body screening on a regular basis is usually recommended in these children as it is difficult to predict in which organs tumors will develop. The frequency of imaging in this population depends on the specific syndrome and risk stratification. Although several publications have presented guidelines for the use of whole-body MRI as a screening method in CPS, studies on the performance of this technique in children are scarce. Friedman et al. retrospectively investigated the role of whole-body MRI as a screening tool in the follow-up of children and adolescents with hereditary retinoblastoma [92]. Whole-body MRI detected suspicious lesions in 5 of 25 patients during follow-up of which only two appeared to be malignant (osteosarcoma). One additional patient in this study was diagnosed with osteosarcoma 3 months after a normal MRI. They calculated that the sensitivity of whole-body MRI to detect subsequent malignant lesions in this specific patient group was 66.7% and the specificity 92.1%.
In another study by Jasperson et al., the role of screening whole-body MRI in a patient population with succinate dehydrogenase (SDH) mutation was investigated because of the increased risk of developing paragangliomas and pheochromocytomas related to this mutation [93]. They evaluated 37 SDH mutation carriers as young as 10 years of age during a 4-year follow-up period using annual biochemical testing as well as biennial whole-body MRI including non-contrast axial and coronal half-Fourier acquisition single-shot fast spin-echo (HASTE) sequences performed with a 5-mm slice thickness from the skull base to pelvis. A total of six tumors were detected in this cohort, one by biochemical testing and five by whole-body MRI, the latter of which were negative by biochemical testing. In this study, the sensitivity of whole-body MRI was 87.5%, and the specificity was 94.7%, while the sensitivity of biochemical testing was 37.5% and the specificity was 94.9%.
Finally, in a recent retrospective study by Anupindi et al., which included 50 WB-MRI examinations in 24 children with genetic CPS, they found that whole-body MRI is a valuable screening tool with a high sensitivity of 100% (95% CI, 6–100%), specificity of 94% (82–98%), and negative predictive value of 94% (90–100%) [94]. Suspicious lesions were detected in 9 out of 50 whole-body MRI examinations, including two high-risk, two moderate-risk, and five low-risk lesions. However, of the four high- to moderate-risk lesions, only one lesion appeared to be malignant resulting in a positive predictive value of only 25% (95% CI, 1–78%). The other lesions and all low-risk lesions had a benign origin. Of interest, this study also showed that incidental findings were detected in 23 of 24 patients, most of which did not require imaging follow-up. All abnormalities were best detected on the fluid-sensitive STIR images. Based on their results, Anupindi et al. recommend that interpretation of these studies should be reserved to radiologists who are familiar with whole-body MRI to appropriately risk stratify abnormalities and minimize unnecessary interventions. None of the studies cited investigated the role of DWI as a screening technique. Based on the above, it can be concluded that, although whole-body MRI can be regarded as a valuable screening tool, larger cohort studies are needed to validate its role and cost-effectiveness in this specific group of patients.
7.4 Future Perspectives
As illustrated in this chapter, whole-body MRI is already widely used in pediatric oncology for diagnosis and follow-up, despite the fact that validation through large prospective cohort studies is still lacking. Although there are obvious technical advantages of whole-body MRI over other imaging modalities (including its high spatial resolution, excellent soft tissue contrast, and ability to acquire functional information), the main reason for this is our primary incentive to reduce radiation dose to children as much as possible.
Nevertheless, there is a strong need for large prospective cohort studies to better validate the role and cost-effectiveness of whole-body MRI in children with cancer, both for diagnosis and therapy response assessment and post-treatment surveillance. These studies should focus on further optimization of anatomic sequences (including reduction of scan duration), as well as on the introduction and validation of functional techniques such as DWI (including quantitative ADC measurements). In addition to the potential additional role of DWI in increasing sensitivity and establishing the extent of disease during staging, DWI could be of special interest in assessing response to treatment before size change can occur. Response to treatment is generally based on shrinkage of lesions. However, most often, this is a rather late marker of treatment-related changes, and sometimes volume change does not correspond to histopathological response; tumors that shrink can still contain considerable amounts of aggressive tumor cells. On the other hand, responding tumors can increase in size due to hemorrhage and necrosis or during differentiation as seen in nephroblastomas (Wilms tumor) and neuroblastomas. This increase in differentiation could be detected with an increase in ADC values [83, 97].
Other functional techniques that can play a role are dynamic contrast-enhanced MRI (DCE-MRI) and arterial spin labelling (ASL). Both techniques focus on tumor vascularization, with the major advantage of ASL over DCE-MRI that it does not need intravenous contrast material injection. In line with this, the potential role of low b-value (<100 mm/s2) perfusion fraction as a measure of tumor vascularization also needs further exploration. Magnetic resonance spectroscopy (MRS) allows for a noninvasive separation of the MRI signal from a given tissue into its different chemical components, which may further improve lesion characterization and prediction of clinical outcome. Although ASL and MRS are already established techniques in neuroradiology, their possible role in (pediatric) malignancies outside the brain has yet to be validated, in particular in a whole-body imaging setting. In this context, the recent development of integrated PET/MRI systems is very interesting, combining the superior structural imaging of whole-body MRI with the functional (molecular) information of both imaging techniques while decreasing the radiation dose. Although the first publications in children show promising results, it currently remains a technical challenge to construct and use these hybrid systems [98,99,100].
The major challenge will be to acquire enough data in children that will allow for definitive and statistically significant results. As most institutions will not have sufficient patient volume for this, due to the comparatively low incidence of most pediatric malignancies, an increasing international multi-institutional collaboration and establishment of multicenter clinical trials is needed [101].
7.5 Conclusion
Whole-body MRI is a very promising and already widely used imaging technique in pediatric oncology. The main reasons for this are the lack of ionizing radiation, its superior spatial resolution and soft tissue contrast, as well as its potential to noninvasively generate functional information on tumor biology. Whole-body MRI provides complementary information to the increasingly used molecular imaging techniques like PET/CT and SPECT/CT and can be a useful alternative to these techniques in patients in whom ionizing radiation is contraindicated and in regions with less availability. However, large-scale prospective cohort studies are still needed to better validate the role and cost-effectiveness of whole-body MRI in pediatric oncology.
References
Carty F, Shortt CP, Shelly MJ, Eustace SJ, O’Connell MJ. Whole-body imaging modalities in oncology. Semin Musculoskelet Radiol. 2010;14(1):68–85.
Goo HW. Regional and whole-body imaging in pediatric oncology. Pediatr Radiol. 2011;41(Suppl 1):S186–94.
Uslu L, Donig J, Link M, Rosenberg J, Quon A, Daldrup-Link HE. Value of 18F-FDG PET and PET/CT for evaluation of pediatric malignancies. J Nucl Med. 2015;56(2):274–86.
Darge K, Jaramillo D, Siegel MJ. Whole-body MRI in children: current status and future applications. Eur J Radiol. 2008;68:289–98.
Chavhan GB, Babyn PS. Whole body MR imaging in children: principles, technique, current applications, and future directions. Radiographics. 2011;31:1757–72.
Eutsler EP, Khanna G. Whole-body magnetic resonance imaging in children: technique and clinical applications. Pediatr Radiol. 2016;46:858–72.
Nievelstein RA, Littooij AS. Whole-body MRI in paediatric oncology. Radiol Med. 2016;121(5):442–53.
Smith EA, Dillman JR. Current role of body MRI in pediatric oncology. Pediatr Radiol. 2016;46(6):873–80.
Takahara T, Imai Y, Yamashita T, Yasuda S, Nasu S, Van Cauteren M. Diffusion weighted whole body imaging with background body signal suppression (DWIBS): technical improvement using free breathing, STIR and high resolution 3D display. Radiat Med. 2004;22(4):275–82.
Kwee TC, Takahara T, Vermoolen MA, Bierings MB, Mali WP, Nievelstein RA. Whole-body diffusion-weighted imaging for staging malignant lymphoma in children. Pediatr Radiol. 2010;40(10):1592–602.
Malayeri AA, El Khouli RH, Zaheer A, Jacobs MA, Corona-Villalobos CP, Kamel IR, Macura KJ. Principles and applications of diffusion-weighted imaging in cancer detection, staging, and treatment follow-up. Radiographics. 2011;31(6):1773–91.
Padhani AR, Koh DM, Collins DJ. Whole-body diffusion-weighted MR imaging in cancer: current status and research directions. Radiology. 2011;261:700–18.
Koh DM, Blackledge M, Padhani AR, Takahara T, Kwee TC, Leach MO, Collins DJ. Whole-body diffusion-weighted MRI: tips, tricks, and pitfalls. AJR Am J Roentgenol. 2012;199(2):252–62.
Kellenberger CJ, Miller SF, Khan M, Gilday DL, Weitzman S, Babyn PS. Initial experience with FSE STIR whole-body MR imaging for staging lymphoma in children. Eur Radiol. 2004;14(10):1829–41.
Goo HW, Choi SH, Ghim T, Moon HN, Seo JJ. Whole-body MRI of paediatric malignant tumours: comparison with conventional oncological imaging methods. Pediatr Radiol. 2005;35:766–73.
Ley S, Ley-Zaporozhan J, Schenk JP. Whole-body MRI in the pediatric patient. Eur J Radiol. 2009;70:442–51.
Siegel MJ, Acharyya S, Hoffer FA, Wyly JB, Friedmann AM, Snyder BS, et al. Whole-body MR imaging for staging of malignant tumours in pediatric patients: results of the American College of Radiology Imaging Network 6660 trial. Radiology. 2013;266:599–609.
Atkin KL, Ditchfield MR. The role of whole-body MRI in pediatric oncology. J Pediatr Hematol Oncol. 2014;36:342–52.
Canale S, Vilcot L, Ammari S, Lemery M, Bidault F, Balleyguier C, et al. Whole body MRI in paediatric oncology. Diagn Interv Imaging. 2014;95:541–50.
Klenck C, Gawande R, Uslu L, Khurana A, Qiu D, Quon A, et al. Ionising radiation-free whole-body MRI versus (18)F-fluorodeoxyglucose PET/CT scans for children and young adults with cancer: a prospective, non-randomised, single-centre study. Lancet Oncol. 2014;15(3):275–85.
Guimarães MD, Noschang J, Teixeira SR, Santos MK, Lederman HM, Tostes V, et al. Whole-body MRI in pediatric patients with cancer. Cancer Imaging. 2017;17:6–12.
Takahara T, Kwee TC, Kifune S, Ochiai R, Sakamoto T, Niwa T, et al. Whole-body MRI using a sliding table and repositioning surface coil approach. Eur Radiol. 2010;20:1366–73.
Goo HW. Whole-body MRI in children: current imaging techniques and clinical applications. Korean J Radiol. 2015;16(5):973–85.
Azzedine B, Kahina MB, Dimitri P, Christophe P, Alain D, Claude M. Whole-body diffusion-weighted MRI for staging lymphoma at 3.0T: comparative study with MR imaging at 1.5T. Clin Imaging. 2015;39:104–9.
Kellenberger CJ, Epelman M, Miller SF, Babyn PS. Fast STIR whole-body MR imaging in children. Radiographics. 2004;24:1317–30.
Merlini L, Carpentier M, Ferrey S, Anooshiravani M, Poletti PA, Hanquinet S. Whole-body MRI in children: would a 3D STIR sequence alone be sufficient for investigating common paediatric conditions? A comparative study. Eur J Radiol. 2017;88:155–62.
Mazumdar A, Siegel MJ, Narra V, Luchtman-Jones L. Whole-body fast inversion recovery MR imaging of small cell neoplasms in pediatric patients: a pilot study. AJR Am J Roentgenol. 2002;179(5):1261–6.
Punwani S, Taylor SA, Bainbridge A, Prakash V, Bandula S, De Vita E, et al. Pediatric and adolescent lymphoma: comparison of whole-body STIR half-Fourier RARE MR imaging with an enhanced PET/CT reference for initial staging. Radiology. 2010;255(1):182–90.
Costelloe CM, Madewell JE, Kundra V, Harrell RK, Bassett RL Jr, Ma J. Conspicuity of bone metastases on fast Dixon-based multisequence whole-body MRI: clinical utility per sequence. Magn Reson Imaging. 2013;31:669–75.
Ma J, Costelloe CM, Madewell JE, Hortobagyi GN, Green MC, Cao G, et al. Fast dixon-based multisequence and multiplanar MRI for whole-body detection of cancer metastases. J Magn Reson Imaging. 2009;29(5):1154–62.
Vasanawala SS, Madhuranthakam AJ, Venkatesan R, Sonik A, Lai P, Brau AC. Volumetric fat-water separated T2-weighted MRI. Pediatr Radiol. 2011;41(7):875–83.
Daldrup-Link HE, Franzius C, Link TM, Laukamp D, Sciuk J, Jürgens H, et al. Whole-body MR imaging for detection of bone metastases in children and young adults: comparison with skeletal scintigraphy and FDG PET. AJR Am J Roentgenol. 2001;177(1):229–36.
Schmidt GP, Schoenberg SO, Reiser MF, Baur-Melnyk A. Whole-body MR imaging of bone marrow. Eur J Radiol. 2005;55(1):33–40.
Manenti G, Cicciò C, Squillaci E, Strigari L, Calabria F, Danieli R, et al. Role of combined DWIBS/3D-CE-T1w whole-body MRI in tumor staging: comparison with PET-CT. Eur J Radiol. 2012;81(8):1917–25.
Kwee TC, Takahara T, Ochiai R, Nievelstein RAJ, Luijten PR. Diffusion-weighted whole-body imaging with background body signal suppression (DWIBS): features and potential applications in oncology. Eur Radiol. 2008;18:1937–52.
Attariwala R, Picker W. Whole body MRI: improved lesion detection and characterization with diffusion weighted techniques. J Magn Reson Imaging. 2013;38:253–68.
Parameswaran BK, Lau E, Ferris NJ. Recognising pitfalls in assessment of tumours by diffusion-weighted MRI: a pictorial essay. J Med Imaging Radiat Oncol. 2015;59(2):188–94.
Gu J, Chan T, Zhang J, Leung AYH, Kwong YL, Khong PL. Whole-body diffusion-weighted imaging: the added value to whole-body MRI at initial diagnosis of lymphoma. AJR Am J Roentgenol. 2011;197:W384–91.
Li B, Li Q, Nie W, Liu S. Diagnostic value of whole-body diffusion-weighted magnetic resonance imaging for detection of primary and metastatic malignancies: a meta-analysis. Eur J Radiol. 2014;83(2):338–44.
Toledano-Massiah S, Luciani A, Itti E, Zerbib P, Vignaud A, Belhadj K, et al. Whole-body diffusion-weighted imaging in Hodgkin lymphoma and diffuse large B-cell lymphoma. Radiographics. 2015;35:747–64.
Kwee TC, Takahara T, Luijten PR, Nievelstein RA. ADC measurements of lymph nodes: inter- and intra-observer reproducibility study and an overview of the literature. Eur J Radiol. 2010;75(2):215–20.
Vermoolen MA, Kwee TC, Nievelstein RAJ. Apparent diffusion coefficient measurements in the differentiation between benign and malignant lesions: a systematic review. Insights Imaging. 2012;3:395–409.
Daldrup-Link HE. Ten things you might not know about iron oxide nanoparticles. Radiology. 2017;284(3):616–29.
Hirsch W, Kluge R, et al. Preliminary results in whole body MRI in children - a prospective study [abstract]. Pediatr Radiol. 2005;35:S89.
Zaitsev M, Maclaren J, Herbst M. Motion artifacts in MRI: a complex problem with many partial solutions. J Magn Reson Imaging. 2015;42(4):887–901.
Burdiles A, Babyn PS. Pediatric bone marrow MR imaging. Magn Reson Imaging Clin N Am. 2009;17:391–409.
Murphy DT, Moynagh MR, Eustace SJ, Kavanagh EC. Bone marrow. Magn Reson Imaging Clin N Am. 2010;18:727–35.
Guillerman RP. Marrow: red, yellow and bad. Pediatr Radiol. 2013;43:S181–92.
Laor T, Jaramillo D. MR imaging insights into skeletal maturation: what is normal? Radiology. 2009;250(1):28–38.
Ryan SP, Weinberger E, White KS, Shaw DWW, Patterson K, Nazar-Stewart V, Miser J. MR imaging of bone marrow in children with osteosarcoma: effect of granulocyte colony-stimulating factor. AJR Am J Roentgenol. 1995;165:915–20.
Shabshin N, Schweitzer ME, Morrison WB, Carrino JA, Keller MS, Grissom LE. High-signal T2 changes of the bone marrow of the foot and ankle in children: red marrow or traumatic changes? Pediatr Radiol. 2006;36:670–6.
Ording-Müller LS, Avenarius D, Olsen OE. High signal in bone marrow at diffusion-weighted imaging with body background suppression (DWIBS) in healthy children. Pediatr Radiol. 2011;41(2):221–6.
Zajick DC, Morrison WB, Schweitzer ME, Parellada JA, Carrino JA. Benign and malignant processes: normal values and differentiation with chemical shift MR imaging in vertebral marrow. Radiology. 2005;237:590–6.
Meyer JS, Siegel MJ, Farooqui SO, Jaramillo D, Fletcher BD, Hoffer FA. Which MRI sequence of the spine best reveals bone-marrow metastases of neuroblastoma? Pediatr Radiol. 2005;35(8):778–85.
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5–29.
Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32(27):3059–68.
Johnson SA, Kumar A, Matasar MJ, Schöder H, Rademaker J. Imaging for staging and response assessment in lymphoma. Radiology. 2015;276(2):323–38.
Rosolen A, Perkins SL, Pinkerton CR, Guillerman RP, Sandlund JT, Patte C, et al. Revised international pediatric non-Hodgkin lymphoma staging system. J Clin Oncol. 2015;33(18):2112–8.
Littooij AS, Kwee TC, Barber I, Vermoolen MA, Enriquez G, Zsiros J, et al. Whole-body MRI for initial staging of paediatric lymphoma: prospective comparison to an FDG-PET/CT-based reference standard. Eur Radiol. 2014;24(5):1153–65.
Lin C, Luciani A, Itti E, El-Gnaoui T, Vignaud A, Beaussart P, et al. Whole-body diffusion-weighted magnetic resonance imaging with apparent diffusion coefficient mapping for staging patients with diffuse large B-cell lymphoma. Eur Radiol. 2010;20(8):2027–38.
Stéphane V, Samuel B, Vincent D, Joelle G, Remy P, Francois GG, Jean-Pierre T. Comparison of PET-CT and magnetic resonance diffusion weighted imaging with body suppression (DWIBS) for initial staging of malignant lymphomas. Eur J Radiol. 2013;82(11):2011–7.
Kwee TC, van Ufford HM, Beek FJ, Takahara T, Uiterwaal CS, Bierings MB, et al. Whole-body MRI, including diffusion-weighted imaging, for the initial staging of malignant lymphoma: comparison to computed tomography. Invest Radiol. 2009;44(10):683–90.
Sun N, Zhao J, Qiao W, Wang T. Predictive value of interim PET/CT in DLBCL treated with R-CHOP: meta-analysis. Biomed Res Int. 2015;2015:648572. https://doi.org/10.1155/2015/648572.
Adams HJ, Nievelstein RA, Kwee TC. Prognostic value of interim FDG-PET in Hodgkin lymphoma: systematic review and meta-analysis. Br J Haematol. 2015;170(3):356–66.
Mayerhoefer ME, Karanikas G, Kletter K, Prosch H, Kiesewetter B, Skrabs C, et al. Evaluation of diffusion-weighted magnetic resonance imaging for follow-up and treatment response assessment of lymphoma: results of an 18F-FDG-PET/CT-controlled prospective study in 64 patients. Clin Cancer Res. 2015;21(11):2506–13.
Punwani S, Prakash V, Bainbridge A, Taylor SA, Bandula S, Olsen OE, et al. Quantitative diffusion weighted MRI: a functional biomarker of nodal disease in Hodgkin lymphoma? Cancer Biomark. 2010;7(4):249–59.
Lin C, Itti E, Luciani A, Zegai B, Lin SJ, Kuhnowski F, et al. Whole-body diffusion-weighted imaging with apparent diffusion coefficient mapping for treatment response assessment in patients with diffuse large B-cell lymphoma: pilot study. Invest Radiol. 2011;46(5):341–9.
Punwani S, Taylor SA, Saad ZZ, Bainbridge A, Groves A, Daw S, et al. Diffusion-weighted MRI of lymphoma: prognostic utility and implications for PET/MRI? Eur J Nucl Med Mol Imaging. 2013;240(3):373–85.
Wu X, Pertovaara H, Dastidar P, Vornanen M, Paavolainen L, Järvenpää R, et al. ADC measurements in diffuse large B-cell lymphoma and follicular lymphoma: a DWI and cellularity study. Eur J Radiol. 2013;82:e158–64.
Siegel MJ, Jokerst CE, Rajderkar D, Hildebolt CF, Goyal S, Dehdashti F, et al. Diffusion weighted MRI for staging and evaluating response in diffuse large B-cell lymphoma: a pilot study. NMR Biomed. 2014;27(6):681–91.
Allen CE, Kelly KM, Bollard CM. Pediatric lymphomas and histiocytic disorders of childhood. Pediatr Clin North Am. 2015;62:139–65.
Azouz EM, Saigal G, Rodriguez MM, Podda A. Langerhans’ cell histiocytosis: pathology, imaging and treatment of skeletal involvement. Pediatr Radiol. 2005;35:103–15.
Van Nieuwenhuyse JP, Clapuyt P, Malghem J, Everarts P, Melin J, Pauwels S, et al. Radiographic skeletal survey and radionuclide bone scan in Langerhans cell histiocytosis of bone. Pediatr Radiol. 1996;26(10):734–8.
Binkovitz LA, Olshefski RS, Adler BH. Coincidence FDG-PET in the evaluation of Langerhans’ cell histiocytosis: preliminary findings. Pediatr Radiol. 2003;33:598–602.
Phillips M, Allen C, Gerson P, McClain K. Comparison of FDG-PET scans to conventional radiography and bone scans in management of Langerhans cell histiocytosis. Pediatr Blood Cancer. 2009;52:97–101.
Mueller WP, Melzer HI, Schmid I, Coppenrath E, Bartenstein P, Pfluger T. The diagnostic value of 18F-FDG PET and MRI in paediatric histiocytosis. Eur J Nucl Med Mol Imaging. 2013;40:356–63.
Goo HW, Yang DH, Ra YS, Song JS, Im HJ, Seo JJ, et al. Whole-body MRI of Langerhans cell histiocytosis: comparison with radiography and bone scintigraphy. Pediatr Radiol. 2006;36:1019–31.
Steinborn M, Wörtler K, Nathrath M, Schöniger M, Hahn H, Rummeny EJ. Whole-body MRI in children with langerhans cell histiocytosis for the evaluation of the skeletal system. RoFo. 2008;180:646–53.
Papaioannou G, McHugh K. Neuroblastoma in childhood: review and radiological findings. Cancer Imaging. 2005;5:116–27.
Monclair T, Brodeur GM, Ambros PF, Brisse HJ, Cecchetto G, Holmes K, et al. The International Neuroblastoma Risk Group (INRG) staging system: an INRG Task Force report. J Clin Oncol. 2009;27(2):298–303.
Brisse HJ, McCarville MB, Granata C, Krug KB, Wootton-Gorges SL, Kanegawa K, et al. Guidelines for imaging and staging of neuroblastic tumors: consensus report from the International Neuroblastoma Risk Group Project. Radiology. 2011;261(1):243–57.
Goo HW. Whole-body MRI of neuroblastoma. Eur J Radiol. 2010;75:306–14.
Gahr N, Darge K, Hahn G, Kreher BW, von Buiren M, Uhl M. Diffusion-weighted MRI for differentiation of neuroblastoma and ganglioneuroblastoma/ganglioneuroma. Eur J Radiol. 2011;79(3):443–6.
Pfluger T, Schmied C, Porn U, Leinsinger G, Vollmar C, Dresel S, et al. Integrated imaging using MRI and 123I metaiodobenzylguanidine scintigraphy to improve sensitivity and specificity in the diagnosis of pediatric neuroblastoma. AJR Am J Roentgenol. 2003;181(4):1115–24.
Furth C, Amthauer H, Denecke T, Ruf J, Henze G, Gutberlet M. Impact of whole-body MRI and FDG-PET on staging and assessment of therapy response in a patient with Ewing sarcoma. Pediatr Blood Cancer. 2006;47:607–11.
Krohmer S, Sorge I, Krausse A, Kluge R, Bierbach U, Marwede D, et al. Whole-body MRI for primary evaluation of malignant disease in children. Eur J Radiol. 2010;74:256–61.
Gorkem SB, Coskun A, Yikilmaz A, Zurakowski D, Mulkern RV, Lee EY. Evaluation of pediatric thoracic disorders: a comparison of unenhanced fast imaging sequence 1.5T MRI and contrast-enhanced MDCT. AJR Am J Roentgenol. 2013;200:1352–7.
Burris NS, Johnson KM, Larson PE, Hope MD, Nagle SK, Behr SC, Hope TA. Detection of small pulmonary nodules with ultrashort echo time sequences in oncology patients by using a PET/MR system. Radiology. 2016;278:239–46.
Mautner VF, Asuagbor FA, Dombi E, Fünsterer C, Kluwe L, Wenzel R, et al. (2008) Assessment of benign tumor burden by whole-body MRI in patients with neurofibromatosis 1. Neuro Oncol. 2008;10:593–8.
Derlin T, Tornquist K, Munster S, Apostolova I, Hagel C, Friedrich RE, et al. Comparative effectiveness of 18F-FDG PET/CT versus whole-body MRI for detection of malignant peripheral nerve sheath tumors in neurofibromatosis type 1. Clin Nucl Med. 2013;38:e19–25.
Nguyen R, Jett K, Harris GJ, Cai W, Friedman JM, Mautner VF. Benign whole body tumor volume is a risk factor for malignant peripheral nerve sheath tumors in neurofibromatosis type 1. J Neurooncol. 2014;116:307–13.
Friedman DN, Lis E, Sklar CA, Oeffinger KC, Reppucci M, Fleischut MH, et al. Whole-body magnetic resonance imaging (WB-MRI) as surveillance for subsequent malignancies in survivors of hereditary retinoblastoma: a pilot study. Pediatr Blood Cancer. 2014;61(8):1440–4.
Jasperson KW, Kohlmann W, Gammon A, Slack H, Buchmann L, Hunt J, et al. Role of rapid sequence whole-body MRI screening in SDH-associated hereditary paraganglioma families. Fam Cancer. 2014;13:257–65.
Anupindi SA, Bedoya MA, Lindell RB, Rambhatla SJ, Zelley K, Nichols KE, Chauvin NA. (2015) Diagnostic performance of whole-body MRI as a tool for cancer screening in children with genetic cancer-predisposing conditions. AJR Am J Roentgenol. 2015;205:400–8.
Bueno MT, Martínez-Ríos C, la Puente GA, Ahyad RA, Villani A, Druker H, et al. Pediatric imaging in DICER1 syndrome. Pediatr Radiol. 2017;47(10):1292–301.
Van Engelen K, Villani A, Wasserman JD, Aronoff L, Greer MC, Tijerin Bueno M, et al. DICER1 syndrome: approach to testing and management at a large pediatric tertiary care center. Pediatr Blood Cancer. 2018;65(1) https://doi.org/10.1002/pbc.26720.
Littooij AS, Nikkels PG, Hulsbergen-van Kaa CA, van de Ven CP, van den Heuvel-Eibrink MM, Olsen ØE. Apparent diffusion coefficient as it relates to histopathology findings in postchemotherapy nephroblastoma: a feasibility study. Pediatr Radiol. 2017;47(12):1608–14.
Hirsch FW, Sattler B, Sorge I, Kurch L, Viehweger A, Ritter L, et al. PET/MR in children. Initial clinical experience in paediatric oncology using an integrated PET/MR scanner. Pediatr Radiol. 2013;43:860–75.
Eiber M, Takei T, Souvatzoglou M, Mayerhoefer ME, Fürst S, Gaertner FC, et al. Performance of whole-body integrated 18F-FDG PET/MR in comparison to PET/CT for evaluation of malignant bone lesions. J Nucl Med. 2014;55:191–7.
Schäfer JF, Gatidis S, Schmidt H, Gückel B, Bezrukov I, Pfannenberg CA, et al. Simultaneous whole-body PET/MR imaging in comparison to PET/CT in pediatric oncology: initial results. Radiology. 2014;273:220–31.
Offiah AC, Andronikou S, Avni F, Daltro P, Donnelly LF, Jaramillo D, et al. Expert opinion: what are the greatest challenges and barriers to applying evidence-based and practical approaches to preclinical and clinical research in the field of pediatric radiology? Pediatr Radiol. 2014;44(10):1209–12.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Nievelstein, R.A.J., Littooij, A.S. (2019). Whole-Body MRI in Pediatric Oncology. In: Voss, S., McHugh, K. (eds) Imaging in Pediatric Oncology. Pediatric Oncology. Springer, Cham. https://doi.org/10.1007/978-3-030-03777-2_7
Download citation
DOI: https://doi.org/10.1007/978-3-030-03777-2_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-03776-5
Online ISBN: 978-3-030-03777-2
eBook Packages: MedicineMedicine (R0)