Abstract
Surveillance imaging has become an increasingly important topic in pediatric oncology. More children than ever before are now alive at 5 years from the time of diagnosis.
With the number of cancer survivors growing each year, we are faced with the unique new challenge of determining how best to monitor these patients for disease recurrence. There has been increasing discussion related to how frequently surveillance imaging should be performed, with which imaging modalities, and whether aggressive surveillance imaging recommendations are justifiable in terms of the risks associated with frequent imaging and whether early relapse detection results in improved clinical outcomes.
This chapter will focus on the growing body of literature related to the role surveillance imaging plays in the care of patients with a variety of common pediatric malignancies. Evidence-based recommendations, where available, will be presented along with considerations for the appropriate use of imaging as an essential element of a comprehensive end-of-therapy monitoring plan.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
21.1 Introduction
Over the past 60 years, improvements in the diagnosis and treatment of pediatric malignancy have led to increases in both event-free survival (EFS) and overall survival (OS) for the majority of childhood cancers. More than 80% of the children diagnosed with cancer today will be 5-year survivors, and many of these will be cured of their disease [1]. This presents unique new challenges for both the clinician and the imaging specialist in determining how best to monitor patients for disease recurrence. It would seem sensible and intuitive that diseases that historically had universally poor outcomes should now require some type of post-therapy imaging surveillance. Surveillance imaging, by definition, commences when patients have completed all of their intended therapy and are either free of demonstrable disease or have reached a point of disease stability for which further aggressive treatment is no longer indicated. For the purposes of this review, surveillance imaging will refer primarily to those patients who are free of disease at completion of therapy. For surveillance imaging to be useful, of course, it should lead to an improved survival benefit. To date that is largely unproven, but the value of surveillance is being evaluated in many current studies.
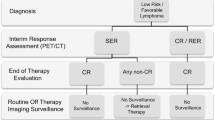
Developing an algorithm for both clinical and imaging surveillance is an essential aspect of these patients’ follow-up care and demands knowledge of the patient’s primary disease, which includes an understanding of the initial stage of disease, tumor location, presence of metastatic disease, and response to initial therapy. Also important are historical data that inform us on the likelihood and typical time course of disease recurrence based on the above characteristics of the tumor. For example, low-stage, low-grade tumors that have been completely resected (e.g., stage 1 Wilms tumor) or that have enjoyed a complete response to therapy (e.g., non-bulky stage 2a Hodgkin disease) may require a less intensive post-therapy surveillance regimen. In contrast, a patient with relapsed high-risk stage 4 neuroblastoma who is now free of disease following aggressive relapse therapy has a much greater likelihood of early disease recurrence and may require more intensive off-treatment imaging surveillance. This approach assumes that detection of relapse will lead to a better outcome, but that remains unproven for many pediatric tumors. What imaging modalities should be used, how frequently imaging should be performed, and the relative risks and benefits of different surveillance imaging strategies will be the focus of this review.
A related topic is the approach to imaging surveillance for patients with cancer predisposition syndromes. The technologic advances in whole genomic screening techniques, coupled with a rapidly expanding list of tumor-specific molecular genetic markers, have led to the increasing use of genetic testing to identify inherited gene mutations that might put a person at higher risk of developing certain types of cancer. The imaging approaches used to screen patients with known cancer predisposition syndromes share many features with the off-therapy surveillance imaging used to monitor patients who have already been treated for cancer. In both instances patients are free of disease but have a finite risk of developing either new or recurrent cancer, and the imaging modalities used and frequency with which imaging is performed must be tailored to the specific disease or syndrome [2]. Because of the increasing number of recognized cancer predisposition syndromes, and the unique features that characterize many of these syndromes, features that in turn dictate the approach to radiologic screening, a separate chapter in this textbook has been dedicated to the imaging of cancer predisposition syndromes; the reader is referred to this section (Chap. 20) for a more comprehensive treatise on this important subject.
In order to decide on appropriate methods of surveillance, it is necessary to study both the contribution and usefulness of radiological and clinical findings. The effects on event-free survival (EFS) and overall survival (OS) need to be taken into consideration, and these must be weighed against the potential disadvantages of the available radiological techniques. For example, the more frequent use of imaging surveillance in low-risk/low-stage patients with a very good prognosis, for whom treatment intensity is being reduced and who may thus experience increased rates of relapse, may be justifiable, despite the likelihood of a good overall outcome that may not be affected by earlier detection of recurrence. In contrast, there are certain high-risk patients for whom risk of relapse remains high after completion of therapy and for whom there are no good alternative treatment options. In these patients, identifying recurrent disease earlier with frequent surveillance imaging will likely not impact outcome, and the approach to imaging surveillance may require additional consideration. In both populations of patients, the decision to undertake a specific surveillance imaging strategy should follow from a discussion between the treating clinician and the radiologist, so as to balance the risks and benefits of the various techniques.
In recent years, with improvement in outcome for children with cancer, the aim has shifted to reducing treatment-related toxicity and general long-term comorbidity associated with many of the therapies used to treat pediatric patients [3, 4]. The result has been a judicious reduction in the intensity of cytotoxic therapies and a shift toward use of molecularly targeted agents. At the same time, radiologists and oncologists have become increasingly aware of the high cumulative radiation doses children may receive from CT scans and nuclear medicine studies obtained during and after their treatment, doses which—when added to the toxicity of chemotherapy and radiotherapy—have caused many to express concern that these heavily treated and intensively imaged children may be receiving unnecessarily high doses of ionizing radiation as a result of surveillance imaging [5,6,7,8].
For children whose cancers have been successfully treated, imaging surveillance—when indicated—is only one aspect of comprehensive end-of-therapy monitoring. The need for regular clinical follow-up is essential, despite relatively low overall rates of tumor recurrence detected during routine clinic visits. Indeed, one study reported that 804 clinic visits were needed to detect one tumor recurrence [9]. Nonetheless, clinic visits are useful for patient and family reassurance, and in many instances, the findings on physical examination, when coupled with other laboratory tests, complement the results obtained from surveillance imaging, and together each plays an important role in pediatric oncology surveillance.
This chapter describes the basic principles of disease surveillance in medicine and places them in the context of pediatric oncology. Posttreatment surveillance is discussed with a focus on imaging and the potential risks related to anesthesia and exposure to ionizing radiation versus the benefits that accrue from early detection of disease. Available literature on specific types of malignancy is presented with the aim of elucidating the usefulness of surveillance with different tumors. In those situations where radiological surveillance is needed, we suggest to replacing CT with MRI when appropriate, with the addition of diffusion-weighted imaging (DWI) [10, 11], and routine ultrasound examinations wherever possible.
21.2 Problems with Surveillance
We all know the mantra: early detection is the best protection. Intuitively, short-term imaging surveillance should lead to earlier detection of disease recurrence in posttreatment oncological patients as compared to clinical surveillance alone, with a resultant improvement in overall outcome. This, however, depends on two factors: firstly that the imaging modality being used for surveillance (e.g., MRI, ultrasound, CT) has sufficient sensitivity and specificity in detecting disease before clinical signs or symptoms appear and secondly that early radiological detection of disease recurrences improves outcome as compared to clinical detection [12,13,14,15].
In evaluating the efficacy of surveillance imaging for detecting disease and improving outcome, an understanding of lead-time and length-time bias is fundamental. Lead-time bias relates to the time between detection of cancer by imaging and the time point of clinical detection. When determining the impact of early detection on survival, the modality that leads to earlier detection will always appear to result in a longer survival time. Therefore, even if early detection by imaging surveillance does not actually improve overall survival, the earlier diagnosis by imaging and the resultant increase in time to progression will appear favorable when compared to clinical surveillance [13,14,15] (Fig. 21.1). Diagnosing a disease earlier does not automatically make patients live longer; instead they merely live for a longer time with the disease label. Put another way, survival appears longer because the disease clock starts earlier [16]. Such lead-time bias can be overcome, however, by analyzing the results by date of birth instead of age at diagnosis.
Length-time bias refers to the amount of time needed for a malignancy to manifest clinically, versus being detected by imaging. If, as typically is the case for aggressive tumors, this length of time is less than the interval between surveillance imaging exams, the cancer will present clinically and surveillance imaging will have little apparent impact. Because of this, there will be a bias toward surveillance imaging detecting malignancies that are inherently less aggressive and slower-growing (Fig. 21.2). Length-time bias can therefore result in recurrences detected by surveillance imaging correlating with longer survival, when in fact surveillance imaging may simply be detecting less aggressive, more indolent tumors [13,14,15].
21.3 Lessons from Neuroblastoma Screening
Experience with historical screening for neuroblastoma is instructive in several ways. Survival rates for affected children depend on factors such as the age of the child, which part of the body is affected, how widely disseminated the tumor is when diagnosed, and biological parameters. The overall 5-year survival rate of children aged 1–4 years at diagnosis is around 50–60%. In addition, it is a well-known idiosyncrasy of neuroblastoma, particularly when diagnosed in infancy, that the tumor can undergo complete spontaneous regression without treatment.
Neuroblastoma was therefore a tempting target for screening for four reasons: (1) children who are diagnosed before the age of 1 year are known to have a better outlook than those who are diagnosed later; (2) children with advanced disease fare much worse than those with early disease; (3) there was a simple and cheap screening test that can be carried out by blotting wet diapers and measuring catecholamines in the urine; and (4) the test detects nine out of ten children with neuroblastoma.
Mass screening of infants for neuroblastoma at 6 months of age was first introduced in Japan in 1985 without the benefit of any evidence from clinical trials. Based on the above considerations, it seemed a sensible and logical approach to screen for a tumor which historically often had a poor prognosis. During the first 3 years of nationwide screening, over 337 infants were diagnosed, 97% of whom were alive in 1990 following treatment. But 20 years later, there was no evidence that neuroblastoma screening had reduced the number of children dying from this cancer [17]. How could that be?
When the evidence on which screening had been introduced and promoted in Japan was scrutinized, it turned out that there were serious flaws but a ready explanation. The impressive 97% survival rate illustrates the effect of length-time bias—meaning that screening works best at picking up slowly developing conditions (slow-growing tumors in this case). By contrast, fast-growing tumors are, of course, less likely to be picked up by screening but will lead to clinical signs in the infant such as abdominal distension or a palpable mass, either of which will rapidly be brought to a doctor’s attention. These fast-growing tumors are potentially much more serious than slow-growing ones. Slow-growing neuroblastomas usually have a good outcome, and spontaneous regression is observed in many patients up to 18 months of age.
So the 337 cases diagnosed by screening would mostly have had a good outcome anyway and would not have included infants with the worst potential outcomes. Furthermore screening would have detected some neuroblastomas that would have disappeared spontaneously. Without screening no one would ever have known that these tumors existed; with screening, this overdiagnosis turned the affected babies into patients, who then went on to be exposed to unnecessary harms associated with treatment and management.
In addition, the encouraging results from small studies that had led to the nationwide screening in Japan had initially been analyzed by looking at length of survival from the date of diagnosis of neuroblastoma, not at length of survival from date of birth. This is important because diagnosing a disease earlier does not automatically make patients live longer—they merely live for a longer time with the disease. Put another way, survival appears longer because the disease happened to be picked up earlier. As mentioned above this is lead-time bias, and it can be overcome by analyzing results by date of birth instead of age at diagnosis.
By contrast, when unbiased evidence was obtained from clinical trials done in Canada and Germany, involving about three million children in all, researchers were unable to detect any benefit from screening [17]. But, there were apparent potential harms that resulted from screening, including unjustified surgery and chemotherapy, both of which can have serious unwanted side effects. In light of this evidence, infant screening for neuroblastoma in Japan was stopped in 2004.
At the same time, infants in New South Wales in Australia were fortunately spared from neuroblastoma screening, which had been planned in the 1980s after the encouraging early Japanese studies. When an Australian expert reanalyzed the Japanese results from dates of birth of the infants rather than from dates of diagnosis, this analysis did not detect any difference in the survival rates between the screened and the unscreened infants. That study convinced the New South Wales authorities to abandon their proposed screening program, thereby saving infants from unnecessary treatments and the health service from unnecessary expense [16].
The lesson here is we should not assume early detection is always worthwhile. Screening for neuroblastoma illustrates how easily one can fall into the trap of assuming that because a disease can be detected early, screening must be beneficial. The studies above demonstrate not only how neuroblastoma screening provided no value in terms of patient outcome but also how a well-intentioned but ill-conceived screening program led to overdiagnosis and in many cases identified tumors that would have spontaneously regressed.
Going forward the significance of lead-time bias and length-time bias on the interpretation of the various other retrospective observational case-based and small population studies available in the pediatric oncology literature is uncertain. Prospective randomized studies that seek to minimize lead-time and length-time bias are needed to assess the true sensitivity and specificity of the various surveillance imaging approaches. The degree to which imaging surveillance may improve overall survival will be similarly challenging to unequivocally demonstrate, and caution is needed when interpreting such studies. With these considerations in mind, the available literature will be presented with a focus on determining the necessity and usefulness of surveillance imaging in pediatric oncology.
21.4 Risks and Benefits of Imaging Surveillance
21.4.1 Ionizing Radiation Risks
Exposure to low doses of ionizing radiation for diagnostic imaging (in particular CT and nuclear medicine) can amount to significant cumulative radiation doses in childhood malignancy [18,19,20]. The cumulative effective dose (CED) varies depending on diagnosis, local protocols, individual disease stage, and clinical course. One study [18] reported the CEDs in a population consisting of 150 patients with five cancer groups (leukemia, lymphoma, brain tumors, neuroblastoma, and assorted tumors). They found a median CED of 61 mSv with a range of <1 mSv to 642 mSv. The leukemia subgroup had the lowest median CED (5 mSv), while the neuroblastoma and lymphoma groups—groups that typically undergo intensive multimodality imaging throughout their treatment course—were highest (median 213 mSv and 191 mSv, respectively) [18].
Over the past two decades, radiologists have become increasingly aware of the potential risks associated with cumulative exposure to ionizing radiation related to diagnostic imaging examinations. It should be noted at the outset that the doses associated with diagnostic imaging are orders of magnitude less than the doses used for radiation therapy, the latter being doses that are delivered with therapeutic and potentially curative intent. This caveat aside, radiologists and clinicians are increasingly mindful of the ALARA (As Low As Reasonably Achievable) principle, which asserts that the use of radiation-based imaging techniques should be justified, the imaging protocols should be optimized, and the doses should be kept to the minimum needed to achieve the necessary diagnostic goals. This principle is based on extrapolation from epidemiologic studies that investigated the potential danger of relatively “low-dose” radiation exposure. The first study was the continuing Life Span Study (LSS) of the survivors of the atomic bombings in 1945 in Japan. This cohort consisted of approximately 100,000 people, in which 30,000 were exposed to doses ranging from 5 to 125 mSv (average 34 mSv) [6, 21]. This lowest-dose subgroup demonstrates a statistically significant ERR (excess relative risk) of developing cancer following exposure to doses of ionizing radiation that may overlap with the CED related to diagnostic imaging. While the validity of extrapolating population-based epidemiologic data in order to estimate lifetime cancer mortality risk attributable to radiation exposures encountered in diagnostic imaging has been challenged and is vigorously debated, the notion that cumulative exposures to low doses of ionizing radiation may have untoward health effects is supported by a recent study that showed an increased relative risk (RR) for leukemia and brain tumors in children with a CED of 30 mGy and 50–74 mGy, respectively [8]. Another study followed 680,211 patients and found an increase in incidence of cancer after CT exposure, where the average effective radiation dose was estimated at 4.5 mSv (5). Interestingly, reanalysis of one population-based cohort revealed that underlying unreported conditions might have introduced bias into the cancer risk assessments for CT, resulting in overestimates of relative risk [22]. Furthermore, not all cancers have an increased incidence in patients exposed to low doses of ionizing radiation: a recent epidemiologic study of 178,601 patients showed no association between radiation dose from pediatric CT scans and risk of developing Hodgkin lymphoma [23].
While applying the principles of ALARA seems appropriate and supports the accepted ethical and moral imperatives to do what is best for the patient, the discussion around whether low-dose radiation is truly harmful is still under discussion [21, 24]. Proponents of the linear no-threshold (LNTH) model argue that biologic injury from ionizing radiation is directly proportional to dose, that risk increases with cumulative exposure, and that there is no dose threshold below which risk is absent. Others have argued that the risks of ionizing radiation cannot be evaluated by traditional epidemiologic methods because the data are imprecise and contain methodological errors. Such studies refute the validity of the LNTH model at effective doses below 100 mSv, submitting that a finite, although not universally agreed upon, radiation threshold must be achieved for risk to be present. Supporters of this so-called threshold model also point to evidence that biological repair mechanisms exist to correct radiation-induced DNA damage [25, 26].
While the acceptance of a single unifying hypothesis for carcinogenesis risk from exposure to low doses of radiation inducing cancer seems unlikely in the near future, given the ongoing debate in the literature, adherence to the ALARA principle needs to be upheld as long as the effects of low-dose radiation are not fully understood. CT remains a very effective modality for diagnosing and characterizing many pediatric malignancies. With the introduction of improved CT detector technology and advanced iterative reconstruction techniques, we can now push the ALARA principles toward routinely achieving high-quality diagnostic CT scans at sub-mSv effective doses. This is particularly important for situations where CT is the best modality for optimal imaging (e.g., for lung nodule detection) or analysis of comorbidity of treatment (e.g., chest fungal infection). These are situations when we should not refrain from performing CT but should rather strive toward optimizing our CT technique. With regard to surveillance imaging, the question is not only whether surveillance imaging should be performed, but also how. Whenever imaging is necessary, the modality that is the most effective at identifying and characterizing disease with the least toxicity should be used [3]. For example, initial and follow-up CT—when appropriate—can often be replaced by ultrasound and MRI. Particularly with the increasing use of ultrasound contrast agents, diffusion-weighted MR imaging (DWI), and development of new fast, sensitive, and tissue-specific MR imaging techniques, other imaging modalities frequently provide diagnostic information that is similar or superior to CT with no associated radiation burden [10, 11, 20].
A comprehensive discussion of second malignant neoplasms (SMNs) is outside of the scope of this review but bears mention since they can occur in survivors of pediatric cancer and are most likely related to the cumulative toxicities that accompany treatment with cytotoxic chemotherapy and radiation therapy. The degree to which the relatively low cumulative effective doses of ionizing radiation derived from repeated diagnostic imaging examinations contribute to increases in the attributable risk of developing a SMN is unknown, but should not be discounted. SMNs are not tumor recurrence per se. These malignancies are unrelated histologically to the first cancer that was treated and may occur many years after the first tumor treatment. Survivors of childhood cancer are reported to have a life expectancy which is 4–18 years shorter compared to the normal population, with 50% of non-recurrence mortality caused by another cancer (20). Chemotherapy causes less treatment-related risks for SMN than radiotherapy but appears to potentiate the effect of irradiation (21). One paper studying Hodgkin lymphoma (HL) posttreatment SMN reported breast carcinoma as most common, followed by thyroid carcinoma, bone, colorectal, lung, and stomach tumors. Younger age and use of radiation therapy were important risk factors; the risk of an SMN was 10% at 20 years and 26% at 30 years (22). Abdominopelvic radiation therapy has been shown to increase the risk of gastrointestinal carcinoma and colorectal carcinoma in patients who as children had Wilms tumor or HL (23). Despite the importance of SMNs in the overall long-term health and well-being of pediatric cancer survivors, because of the relatively low incidence of SMNs and their long latency period for development, routine imaging surveillance is not advocated for early detection of SMN, with reliance rather on regular thorough clinical examination for follow-up.
21.4.2 Sedation Risks
Risks associated with diagnostic imaging are not limited to ionizing radiation. There is increasing awareness that repeated exposures to sedation and anesthesia are associated with potential risks in the pediatric population. These risks include the immediate or short-term risk of anesthesia exposure and long-term neurocognitive effects related to repeated exposures to sedation/anesthesia. The immediate, or short-term, risks relate primarily to medical management issues that accompany an episode of sedation or anesthesia and include airway management, hemodynamic instability, allergic reactions, as well as managing the coexisting medical issues that are commonplace in children being treated for cancer. Long-term neurocognitive deficits related to exposure to sedation/anesthesia medications have been increasingly recognized as leading to potential adverse health outcomes. While these concerns are based primarily on preclinical data, there are some human epidemiologic studies that suggest there may be detrimental effects in patients who have had anesthesia early in life. The FDA, acknowledging these concerns, has warned that “repeated or lengthy use of general anesthetic and sedation drugs during surgeries or procedures in children younger than 3 years or in pregnant women during their third trimester may affect the development of children’s brains,” although they do note that further research is needed to fully characterize how early life anesthetic exposure affects children’s brain development.
These concerns are particularly important for surveillance imaging [27]. Weighing the relative risks of carcinogenesis from ionizing radiation against the potential risks of anesthesia is challenging and demands a thoughtful approach from both the clinician and the radiologist. It has been our tendency to simply replace CT imaging, whenever possible, with MRI, an approach that may not be best for every patient. This is particularly true when CT and MRI exams are diagnostically equivalent and an ultrafast sub-mSv CT scan can be performed without sedation versus an MRI that requires 30–60 min of sedation and/or general anesthesia to obtain a high-quality motion-free exam. In addition, a contrast-enhanced MRI scan carries with it another complicating factor, namely gadolinium deposition in the brain, with its unknown long-term consequences [28].
With these considerations in mind, when developing a surveillance strategy, the modalities chosen should reflect an understanding of the underlying disease, the sensitivity and specificity of the different imaging modalities for the disease being imaged, and the potential risks and benefits of the options available for surveillance imaging.
21.5 Surveillance for Relapse in Children with Malignancy
Advances in treatment have led to prolonged EFS and in many cases improvements in OS for many pediatric malignancies. This has resulted in a heretofore unprecedented need to re-evaluate the strategies we employ when monitoring for tumor recurrence [29]. For surveillance to be useful and justifiable, there should be evidence not only that the chosen imaging modality has sufficient sensitivity and specificity for detecting disease but also that early detection of recurrence leads to improved outcome. The most recent and relevant literature on surveillance imaging are reviewed below, with a focus on those pediatric malignancies for which posttreatment surveillance imaging either has been, or is becoming, an important consideration.
21.5.1 Neurological/CNS Tumors
Surveillance imaging for neurological tumors is most effectively performed with MRI [15]; however when to initiate imaging surveillance and with which frequency remain the subject of investigation. One study [15] reported medulloblastoma relapse detection by MRI in 17 out of 24 patients, with prolonged median survival from the time of relapse to 44 months, as compared to the seven patients whose relapse was identified based on clinical symptoms and who died between scans. They found that the relapses detected by surveillance imaging were less advanced, and more amenable to salvage therapy, accounting for the improvements in outcome. Another study [30] had similar results, showing longer time to recurrence in those patients for whom initial relapse was detected by imaging surveillance compared to clinical relapse. These patients included children with malignant glioma (7.8 months versus 4.3 months, p = 0.041) and medulloblastoma (23.6 months versus 8.9 months, p = 0.0006) but not ependymoma (19.5 months versus 13.3 months, p = 0.19). This was confirmed in a study that reported longer post-treatment EFS and longer OS for the cohort of medulloblastoma patients whose disease recurrence was detected by surveillance imaging as compared to those who presented with clinical symptoms. The mean EFS for patients whose asymptomatic recurrence was detected by imaging was 26.1 months, with a mean OS from the time of recurrence of 8.0 months. This contrasted with mean EFS of 19.1 months and OS of 3.6 months for patients with symptomatic recurrence [31].
Surveillance imaging for CNS tumors, therefore, appears to detect at least a portion of the asymptomatic relapses. These comprise approximately one-third of relapses, which in turn may create opportunities for alternative therapies or investigational agent trials [30]. All studies showed a longer time to relapse and longer survival when relapses were detected by imaging. Two studies found that detection rates for asymptomatic tumor recurrences were 1.59–2.1% for surveillance imaging [31, 32]; the degree to which this reflects early detection of less aggressive disease is uncertain. This has led some to speculate that longer survival might in part be a reflection of lead-time and length-time bias [31], with more indolent tumors presenting later than more aggressive tumors, and thus some question whether first year imaging surveillance is truly effective [30].
21.5.2 Hodgkin Lymphoma
Young patients diagnosed with Hodgkin lymphoma (HL) enjoy early event-free and overall survival rates that often exceed 90%. As such, the usefulness and cost-effectiveness of surveillance imaging for HL have been reassessed. One study [33] reported a relapse rate of 11.6% in 216 children with HL, with a median time to relapse of 7.6 months. Detection of disease relapse was based on clinical examination in 76% and by surveillance imaging in 24% of patients. Surprisingly, the primary determinant of overall survival was time to relapse, not how the relapse was detected. Deaths only occurred in children whose relapses occurred during the first year following completion of therapy. Patients whose relapses occurred beyond 1 year, regardless how those relapses were detected, responded to salvage therapy with no impact on OS. Another study [34] found that only 9% of relapses were detected with CT in a young adult age group (median age 33 years); however this accounted for 29% of the total cost of follow-up. A study of non-HL patients found that CT also had a low relapse detection rate of 5.7% in asymptomatic patients [35], and a similarly low rate has been reported in adult HL surveillance studies [36]. Rathore et al. [37] reported relapses in 13 of 99 children (13%), 11 of which occurred within 5 months of treatment. They also reported a low detection rate of relapse based on surveillance imaging of 1.3% (17 out of 1358 scans).
The mean radiation dosages reported in a pediatric HL surveillance study ranged from 31.97 to 51.35 mSv depending on disease stage and treatment protocol [37]. As described above, these exposures, while relatively low when compared to the doses delivery for radiotherapy, may still be contributing to an excess relative risk of developing delayed secondary tumors and a decreased lifetime expectancy [29, 38]. This was reaffirmed by an earlier study [39] which reported an 18.5-fold increased risk of developing SMNs compared with the general population in 1380 children with HL during long-term follow-up (median 17.0 years). The degree to which the additional exposure to ionizing radiation from multiple CT scans and nuclear medicine studies during the off-therapy surveillance period contributed to the SMNs is uncertain.
A study comparing MRI and PET/CT showed that there was very good agreement between MRI and the enhanced PET/CT for nodal and extranodal staging (k = 0.96 and 0.86, respectively). They found that sensitivity and specificity of MR were 98% and 99% for nodal disease and 91% and 99% for extranodal disease [40]. Although not widely employed for HL imaging or surveillance, with improvements in MR imaging techniques in the thorax (which is the primary site of disease in the majority of patients with HL), MRI is increasingly being used in place of CT whenever possible. Fortunately, HL patients tend to be adolescents and older teenagers and thus do not require anesthesia for MRI.
Because the majority of pediatric HL relapses occur within the first year after therapy, this time period should be the focus of end-of-therapy imaging surveillance. Based on the principle that surveillance imaging should be cost-effective and sensitive for disease detection and that early detection of relapse should improve overall outcome, the evidence that has emerged over the past 5 years indicates that CT scanning has been overutilized in the management of HL, with little impact on outcome. With the majority of HL relapses occurring clinically and with radiation doses employed for lymphoma being among the highest in pediatric oncology, an alternative strategy for imaging surveillance in HL has been proposed [33]. This surveillance scheme for routine post-therapy surveillance in HL has been incorporated into ongoing Children’s Oncology Group HL protocols, with a substantial reduction in post-therapy CT scanning being embraced by both clinical oncologists and radiologists [4].
21.5.3 Neuroblastoma
Posttreatment survival and risk of recurrence in neuroblastoma (NBL) vary greatly, depending on stage and risk classification at the time of diagnosis, with a reported range of 27.3–58.9% [41, 42]. For example, stage 4S (MS) represents a subset of low-risk disease—occurring in children under 1 year with a localized primary site of disease and metastases limited to the liver, skin, and up to 10% of bone marrow. These children were recognized as having an excellent overall outcome, often without the need for chemotherapy or surgical resection of the primary mass [43]. These children have relatively frequent monitoring early on in the course of treatment/observation but then require only minimal disease surveillance once response has been established. In contrast, for higher-stage, high-risk patients (risk stratification being determined based on age, disease stage, histology, and molecular pathology), certain end-of-therapy surveillance imaging protocols require children to receive numerous CT or MR scans and MIBG evaluations, as often as every 3 months for the first year, followed by biannual and eventually annual surveillance for up to 5 years [1]. One study reported a CED of 214 mSv [18], which is higher than many children received during the atomic bombings in Japan in 1945 [6, 21], potentially increasing the risk of SMN and a resulting shortened life span [39].
For neuroblastoma patients with higher-risk disease, the value of early relapse detection is uncertain. In one study, median progression-free survival following relapse was reported as 8.4 months for high-risk patients and 11.8 months for those with intermediate-risk disease [44]. In the high-risk group, 80% of the relapses occurred within 2 years, compared to 50% for the intermediate-risk group. Given results such as these for difficult to treat cancers such as neuroblastoma, where salvage regimen options are limited and are often not curative [1], the impact on survival following early detection of recurrence is unclear.
As with many pediatric malignancies, relapse in neuroblastoma often presents with symptoms or is identified by laboratory or non-CT imaging techniques such as routine outpatient ultrasound. In one study of patients with non-thoracic primary neuroblastoma, thoracic relapses were rare, and the majority presented with symptoms or were identified by other non-CT imaging modalities; only 14% of the thoracic recurrences were detected in asymptomatic children [41], indicating that elimination of routine surveillance chest CT imaging can substantially decrease radiation exposure without compromising disease detection. Another study [42] noted that 74% of patients had clinically evident recurrence or that relapse was detected by X-ray, ultrasound, or urinary catecholamines. Sixteen percent of relapses were detected by MIBG scintigraphy, with only 10% using cross-sectional imaging (CT/MRI), although it should be noted that monitoring tumor markers alone (VMA and HVA) is not sufficiently sensitive to serve as the only means of monitoring for relapse [45]. Patients with neuroblastoma are heavily treated and are at increased risk of developing treatment-related morbidities, including development of second malignancies. As such, efforts have been made to understand the impact of reducing the intensity of routine CT imaging surveillance. One study reported a CED from imaging in neuroblastoma of 214 mSv [18], while other studies have shown that reducing or eliminating routine CTs from surveillance imaging protocols could lead to dose savings of 30–40% [41].
Because the extent and frequency of surveillance imaging for children with neuroblastoma are so variable, it is difficult to present a single evidence-based recommendation for disease surveillance. It seems reasonable, following the principles outlined in this review, that for low-risk abdominal NBL and stage 4S disease, the potential for recurrence can be reliably monitored in most cases with ultrasound, combined with clinical observation and tumor markers. Monitoring children with high-risk disease is more challenging. Patients who achieve early complete response to chemotherapy and surgical resection based on MIBG and CT/MRI have better outcomes as compared to those with residual MIBG avid disease. A response-based algorithm could therefore be considered, reserving more intensive surveillance for those patients with greater likelihood of relapse. Whether this could be accomplished with a single comprehensive whole-body examination remains to be determined [43, 46].
While the utility of surveillance imaging for neuroblastoma patients continues to be the subject of debate [42, 47], there is currently no compelling data to support the elimination of imaging surveillance in low-/intermediate-risk patients, for whom treatment intensity is being reduced and who may experience increased rates of relapse, or in high-risk patients, for whom risk of relapse after completion of therapy remains high. In both populations of patients, however, it is unclear which poses a greater risk to the patient: the cumulative radiation dose from surveillance CT scans performed on modern equipment with low-dose techniques or—as was noted earlier—the multiple exposures to anesthetics for serial MRI examinations. Further work is clearly needed.
21.5.4 Wilms Tumor
Survival of Wilms tumor patients is one of the best in pediatric oncology. Overall 10-year survival rates for stages 1–3 range from 96% to 89%, decreasing to 81% for stage 4 (hematogenous metastases to the lungs and liver) and 78% for stage 5 (bilateral renal tumor) [48]. Differences in treatment between Europe and America may impact local recurrence rates and strategies for end-of-therapy surveillance. The International Society of Pediatric Oncology (SIOP), based in Europe, prefers preoperative chemotherapy prior to surgery, resulting in downstaging of some patients, which in turn makes surgical resection easier with less risk of tumor spillage. Children who are downstaged from stage 3 will avoid radiotherapy and thus avoid the potential long-term sequelae related to radiotherapy [47]. When compared to COG studies, in which up-front surgical resection, followed by chemotherapy and radiation, is the preferred approach, SIOP studies report slightly higher recurrence rates, although these radiotherapy-naïve patients do appear to have high salvage rates [49].
Recurrence in Wilms tumor occurs most frequently in the lungs [50,51,52], with abdominal and pelvic recurrences seen in only ~10% of patients [50]. Survival after relapse of Wilms tumor is 50–80% depending on therapy [50, 52]. One study [53] reported recurrence in 10/53 patients receiving 210 CT examinations. Eight of the recurrences were apparent through clinical symptoms, radiographs, or ultrasound. Only two patients with recurrence in the chest were found by CT and not by plain radiograph, although the relationship between early detection of pulmonary recurrence by CT and survival in these patients was not established. Another study [54] reported on 80 patients who underwent a total of 605 routine pelvic surveillance CTs. Sixteen children (15%) relapsed after a median of 11.3 months (range 5 months to 7.3 years) after diagnosis, of whom four died. Three of the 16 patients had relapses detected on pelvic CT; however these patients also had clinical evidence of recurrence and all survived. These authors concluded that pelvic CT could be omitted from routine Wilms tumor follow-up, a conclusion supported by another more recent study [55]. Omitting pelvic CT would have saved on 30.5–44.9% of radiation dose (depending on age). In this study two additional patients died of SMN, and one developed a desmoid tumor of the abdominal wall.
Despite the low incidence of asymptomatic pelvic and chest recurrences, routine chest and abdominal CT or MRI has historically been an integral part of Wilms tumor trials in the Children’s Oncology Group [56]. However, in a recent COG study of 281 patients with relapsed unilateral favorable histology Wilms tumor treated on the fifth National Wilms Tumor Study (NWTS-5) protocol, detection of relapse with CT was not associated with improved survival compared to detection with CXR/US, and the authors concluded that eliminating CT scans from surveillance programs would result in substantial reduction in radiation exposure and health-care costs without compromising overall survival [57]. Whether these recommendations can be extended to higher-risk patients or incorporated into risk-adapted off-treatment monitoring programs remains to be studied.
Based on available data, therefore, it seems appropriate to refrain from routine chest CTs and abdomen/pelvic CTs in low risk, low stage Wilms tumor, with abdominal US or MRI as a reasonable surveillance imaging alternative if indeed surveillance imaging is indicated. In agreement with this approach, the SIOP recommendations for nonmetastatic Wilms tumor surveillance are to follow up with chest X-rays and abdominal ultrasound. We are not aware of any evidence to suggest that patients with Wilms tumors in SIOP trials relapse with more advanced disease [56], based on delays in detection.
21.5.5 Hepatoblastoma
As with many other pediatric solid tumors, children with hepatoblastoma often undergo frequent and long-term surveillance imaging, which can contribute to significant cumulative exposures to ionizing radiation, in addition to repeated exposures to sedation and anesthesia agents. One study [58] reported a median recurrence time after initial diagnosis of 12 months (range 4–115 months) in 12% of patients after complete remission, resulting in 59 patients with recurrence. Twenty-seven of these children had recurrences in the lung, 21 in the liver, and five in both the liver and lung. Fifty out of 59 patients had increased alpha fetoprotein (AFP) at the time of recurrence. Thirty-one out of 59 patients achieved secondary complete remission, and 3-year OS was 43%. Another study [59] found increased AFP in the 5/26 patients in whom recurrence was identified; none of the patients, despite intensive surveillance imaging, had their recurrences detected by imaging alone, prior to AFP elevation. They reported two false-positive AFP levels and 15 false-positive imaging exams, and when compared to either FDG PET/CT or CT, AFP elevation was significantly more accurate in detecting recurrence. Another small study [60] in which no recurrences were detected by pelvic CT further supports the recommendations to reduce the use of routine CT for hepatoblastoma surveillance. Rather, serum AFP appears to be the preferred method of surveillance in children with hepatoblastoma. It is important to note, however, that imaging surveillance, ideally with MRI for liver tumors, must still be tailored to the individual patient and should be considered in high-risk patients for whom early detection of relapse may affect surgical resectability and impact survival [59, 61].
21.5.6 Bone and Soft Tissue Sarcomas
Surveillance imaging recommendations following treatment of osteosarcoma, Ewing sarcoma, rhabdomyosarcoma, and other more rare pediatric sarcomas are based mainly on results from both adult and pediatric trials. The only prospective randomized controlled trial that included children and examined the impact of surveillance imaging in sarcoma patients was published by Puri et al. [62]. The purpose of this study, which included 500 nonmetastatic extremity sarcoma (bone and soft tissue) patients (median age 20 years, range 3–65 years), was to determine the impact of a less intense post-therapy follow-up regimen on 3-year OS. This study showed no difference in outcome for patients undergoing surveillance with chest radiographs at 3 or 6 month intervals versus those receiving a more intensive CT surveillance regimen at similar 3 or 6 month intervals; local control/primary site imaging was similar for all groups. Furthermore, almost 90% of the local recurrences were identified clinically. Although this study included both children and adults, their findings provide a high level of evidence to justify eliminating chest CT from routine follow-up in nonmetastatic sarcoma patients and provide further evidence to support the use of clinical exam findings and symptoms for initial detection of local disease recurrence.
Another study [63] of adult sarcoma patients found a 47% relapse rate in patients with a soft tissue extremity sarcoma (mean age 51, range 13–88). Local recurrences were detected clinically in 30/31 patients; MRI identified one local recurrence. Twenty-eight patients developed isolated lung metastases; these were detected by CXR in 19 patients, chest CT in three patients, and clinically in 11 patients. More than 80% of the relapses occurred in the first 2 years of follow-up. In some settings (i.e., nonmetastatic localized disease), therefore, clinical examination appears to be an important means of detecting relapse. However, in a study of mixed soft tissue or bone sarcoma patients, only half (14/29) of the relapses that occurred were detected clinically [64], although this study did not find that regular imaging surveillance improved OS.
Körholz et al. [65] looked specifically at a population of pediatric osteosarcoma patients. Twenty-eight of their 72 patients demonstrated recurrence, of which 90% occurred within 3 years following primary therapy. Recurrence was detected primarily by clinical examination, CXR, and chest CTs. Bone scintigraphy was important for detection of distant metastases; routine radiographs of the primary tumor site did not detect any recurrence. While they recommended routine CXR, chest CT, and clinical examination for at least the first 3 years, they noted that the impact of early recurrence detection on overall survival was uncertain. The COG bone tumor committee recommendations for imaging osteosarcoma and Ewing sarcoma call for a fairly intensive, albeit decreasing frequency of chest CT scans, performed every 3 months in the first year, every 6 months in the second and third years, and yearly in the fourth and fifth years, followed by yearly chest radiographs during post-chemotherapy surveillance [66]. The committee acknowledged, however, that there was a “lack of evidence to support this guideline,” responding to concerns raised by Dauer et al. [12] who stated that frequent and repeated chest CTs during post-chemotherapy surveillance for osteosarcoma are not adequately justified.
Another challenge is when and how to incorporate new imaging modalities into existing surveillance regimens and whether to eliminate historical modalities. For example, FDG-PET imaging has been required along with a bone scan for metastatic disease evaluation in several trials of pediatric bone and soft tissue sarcoma. However, a study that compared FDG PET/CT and bone scintigraphy in 29 children (mean age 12 ± 5 years), of whom 72% had bone sarcoma and 28% a soft tissue sarcoma, found an accuracy of 100% for FDG PET/CT, compared to 82–90% for bone scintigraphy, providing compelling evidence for eliminating bone scintigraphy from surveillance regimens [67].
The soft tissue sarcomas present a separate challenge when developing evidence-based surveillance imaging recommendations, given the different histologic subtypes, age at diagnosis, location of primary disease, and presence of both locoregional nodal and metastatic spread. Children with rhabdomyosarcoma were specifically studied by Lin et al. [68]. OS was compared between recurrence detected clinically (28/47 children) and by imaging (15/47 children). Three-year survival rates in this cohort of patients were poor, and did not differ significantly (p = 0.38) between the groups, with 20% 3-year OS for imaging-based detection and 11% for clinical detection.
21.6 Conclusion
Surveillance imaging plays an important role in the end-of-treatment care of patients with cancer. As more patients become long-term survivors of their childhood malignancies, we must continually work to improve our approach to imaging these patients, being mindful of the risks and benefits that accompany any radiologic examination. Although disease surveillance has received much recent attention, the available evidence on how to best perform routine surveillance imaging is limited and largely based on small retrospective observational studies; more prospective studies are clearly needed. Going forward it would be ideal, albeit difficult to implement, if posttreatment surveillance studies were established whereby children are randomized to clinical and imaging surveillance versus clinical surveillance alone (omitting repeated radiologic imaging) [47]. While some families find a normal follow-up surveillance study to be reassuring, this needs to be balanced with the need for and hazards of ionizing radiation, potential for follow-on testing related to false-positive results, and, particularly in young children, risks of repeated anesthesia.
There are some unfortunate children who present with high-risk metastatic disease for which, if relapse occurs after completion of treatment, there is no further salvage therapy available. While this represents a minority of pediatric oncology patients, it may be appropriate to consider sparing these families the additional burden of repeated surveillance imaging that will have little impact on outcome.
As we have emphasized here, evidence has begun to emerge showing little to no benefit from routinely performed surveillance imaging in patients who are otherwise asymptomatic, particularly when viewed against the yardstick of disease-free survival. For many reasons, it can still be argued that early detection of disease when it is likely to occur is important, both for individual patients and in certain diseases where retrieval therapies exist. However, the choice of imaging modality, surveillance imaging frequency, and duration of surveillance should be part of a thoughtful consideration between the patient, the radiologist, and the treating oncologist, to ensure the most appropriate use of imaging for a given patient’s disease.
References
Weiser DA, Kaste SC, Siegel MJ, Adamson PC. Imaging in childhood cancer: a Society for Pediatric Radiology and Children’s Oncology Group Joint Task Force report. Pediatr Blood Cancer. 2013;60(8):1253–60. https://doi.org/10.1002/pbc.24533.
Greer MC, Voss SD, States LJ. Pediatric cancer predisposition imaging: focus on whole-body MRI. Clin Cancer Res. 2017;23(11):e6–e13. https://doi.org/10.1158/1078-0432.CCR-17-0515.
Kaste SC. Oncological imaging: tumor surveillance in children. Pediatr Radiol. 2011;41(Suppl 2):505–8. https://doi.org/10.1007/s00247-011-2108-1.
Voss SD. Surveillance imaging in pediatric Hodgkin Lymphoma. Curr Hematol Malig Rep. 2013;8(3):218–25. https://doi.org/10.1007/s11899-013-0168-z.
Goske MJ, Applegate KE, Bulas D, Butler PF, Callahan MJ, Coley BD, Don S, Frush DP, Hernanz-Schulman M, Kaste SC, Morrison G, Sidhu M, Strauss KJ, Treves ST, Alliance for Radiation Safety in Pediatric I. Image gently: progress and challenges in CT education and advocacy. Pediatr Radiol. 2011;41(Suppl 2):461–6. https://doi.org/10.1007/s00247-011-2133-0.
Hall EJ, Brenner DJ. Cancer risks from diagnostic radiology. Br J Radiol. 2008;81(965):362–78. https://doi.org/10.1259/bjr/01948454.
Mathews JD, Forsythe AV, Brady Z, Butler MW, Goergen SK, Byrnes GB, Giles GG, Wallace AB, Anderson PR, Guiver TA, McGale P, Cain TM, Dowty JG, Bickerstaffe AC, Darby SC. Cancer risk in 680,000 people exposed to computed tomography scans in childhood or adolescence: data linkage study of 11 million Australians. BMJ. 2013;346:f2360. https://doi.org/10.1136/bmj.f2360.
Pearce MS, Salotti JA, Little MP, McHugh K, Lee C, Kim KP, Howe NL, Ronckers CM, Rajaraman P, Sir Craft AW, Parker L, Berrington de Gonzalez A. Radiation exposure from CT scans in childhood and subsequent risk of leukaemia and brain tumours: a retrospective cohort study. Lancet. 2012;380(9840):499–505. https://doi.org/10.1016/S0140-6736(12)60815-0.
Howell L, Mensah A, Brennan B, Makin G. Detection of recurrence in childhood solid tumors. Cancer. 2005;103(6):1274–9. https://doi.org/10.1002/cncr.20896.
Gawande RS, Gonzalez G, Messing S, Khurana A, Daldrup-Link HE. Role of diffusion-weighted imaging in differentiating benign and malignant pediatric abdominal tumors. Pediatr Radiol. 2013;43(7):836–45. https://doi.org/10.1007/s00247-013-2626-0.
Humphries PD, Sebire NJ, Siegel MJ, Olsen OE. Tumors in pediatric patients at diffusion-weighted MR imaging: apparent diffusion coefficient and tumor cellularity. Radiology. 2007;245(3):848–54. https://doi.org/10.1148/radiol.2452061535.
Dauer LT, St Germain J, Meyers PA. Let’s image gently: reducing excessive reliance on CT scans. Pediatr Blood Cancer. 2008;51(6):838.; author reply 839-840. https://doi.org/10.1002/pbc.21725.
Fletcher RH, Fletcher SW, Wagner EH. Clinical epidemiology, the essentials. 5th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2012.
Mckenzie K, Powell H. Health screening. Learn Disab Pract. 2004;7(10):34–8.
Roebuck DJ, Villablanca JG, Maher K, Nelson MD Jr. Surveillance imaging in children with medulloblastoma (posterior fossa PNET). Pediatr Radiol. 2000;30(7):447–50. https://doi.org/10.1007/s002470000235.
Evans I, Thornton H, Chalmers I, Glasziou P. Earlier is not necessarily better. In: Testing treatments. 2nd ed. London: Pinter & Martin; 2011.
Marcus PM, Prorok PC, Miller AB, DeVoto EJ, Kramer BS. Conceptualizing overdiagnosis in cancer screening. J Natl Cancer Inst. 2015;107(4) https://doi.org/10.1093/jnci/djv014.
Ahmed BA, Connolly BL, Shroff P, Chong AL, Gordon C, Grant R, Greenberg ML, Thomas KE. Cumulative effective doses from radiologic procedures for pediatric oncology patients. Pediatrics. 2010;126(4):e851–8. https://doi.org/10.1542/peds.2009-2675.
Chong AL, Grant RM, Ahmed BA, Thomas KE, Connolly BL, Greenberg M. Imaging in pediatric patients: time to think again about surveillance. Pediatr Blood Cancer. 2010;55(3):407–13. https://doi.org/10.1002/pbc.22575.
Robbins E. Radiation risks from imaging studies in children with cancer. Pediatr Blood Cancer. 2008;51(4):453–7. https://doi.org/10.1002/pbc.21599.
Hendee WR, O’Connor MK. Radiation risks of medical imaging: separating fact from fantasy. Radiology. 2012;264(2):312–21. https://doi.org/10.1148/radiol.12112678.
Berrington de Gonzalez A, Salotti JA, McHugh K, Little MP, Harbron RW, Lee C, Ntowe E, Braganza MZ, Parker L, Rajaraman P, Stiller C, Stewart DR, Craft AW, Pearce MS. Relationship between paediatric CT scans and subsequent risk of leukaemia and brain tumours: assessment of the impact of underlying conditions. Br J Cancer. 2016;114(4):388–94. https://doi.org/10.1038/bjc.2015.415.
Berrington de Gonzalez A, Journy N, Lee C, Morton LM, Harbron RW, Stewart DR, Parker L, Craft AW, McHugh K, Little MP, Pearce MS. No association between radiation dose from pediatric CT scans and risk of subsequent Hodgkin lymphoma. Cancer Epidemiol Biomarkers Prev. 2017;26(5):804–6. https://doi.org/10.1158/1055-9965.EPI-16-1011.
Andronikou S. Letting go of what we believe about radiation and the risk of cancer in children. Pediatr Radiol. 2017;47(1):113–5. https://doi.org/10.1007/s00247-016-3697-5.
Ulsh BA. Checking the foundation: recent radiobiology and the linear no-threshold theory. Health Phys. 2010;99(6):747–58. https://doi.org/10.1097/HP.0b013e3181e32477.
Guillerman RP. From ‘Image Gently’ to image intelligently: a personalized perspective on diagnostic radiation risk. Pediatr Radiol. 2014;44(Suppl 3):444–9. https://doi.org/10.1007/s00247-014-3037-6.
Callahan MJ, MacDougall RD, Bixby SD, Voss SD, Robertson RL, Cravero JP. Ionizing radiation from computed tomography versus anesthesia for magnetic resonance imaging in infants and children: patient safety considerations. Pediatr Radiol. 2017;48:21. https://doi.org/10.1007/s00247-017-4023-6.
Soares BP, Lequin MH, Huisman T. Safety of contrast material use in children. Magn Reson Imaging Clin N Am. 2017;25(4):779–85. https://doi.org/10.1016/j.mric.2017.06.009.
Yeh JM, Nekhlyudov L, Goldie SJ, Mertens AC, Diller L. A model-based estimate of cumulative excess mortality in survivors of childhood cancer. Ann Intern Med. 2010;152(7):409–17., W131–408. https://doi.org/10.7326/0003-4819-152-7-201004060-00005.
Minn AY, Pollock BH, Garzarella L, Dahl GV, Kun LE, Ducore JM, Shibata A, Kepner J, Fisher PG. Surveillance neuroimaging to detect relapse in childhood brain tumors: a Pediatric Oncology Group study. J Clin Oncol. 2001;19(21):4135–40. https://doi.org/10.1200/JCO.2001.19.21.4135.
Yalcin B, Buyukpamukcu M, Akalan N, Cila A, Kutluk MT, Akyuz C. Value of surveillance imaging in the management of medulloblastoma. Med Pediatr Oncol. 2002;38(2):91–7.
Kovanlikaya A, Karabay N, Cakmakci H, Uysal K, Olgun N, Ergor G. Surveillance imaging and cost effectivity in pediatric brain tumors. Eur J Radiol. 2003;47(3):188–92.
Voss SD, Chen L, Constine LS, Chauvenet A, Fitzgerald TJ, Kaste SC, Slovis T, Schwartz CL. Surveillance computed tomography imaging and detection of relapse in intermediate- and advanced-stage pediatric Hodgkin’s lymphoma: a report from the Children’s Oncology Group. J Clin Oncol. 2012;30(21):2635–40. https://doi.org/10.1200/JCO.2011.40.7841.
Dryver ET, Jernstrom H, Tompkins K, Buckstein R, Imrie KR. Follow-up of patients with Hodgkin’s disease following curative treatment: the routine CT scan is of little value. Br J Cancer. 2003;89(3):482–6. https://doi.org/10.1038/sj.bjc.6601052.
Guppy AE, Tebbutt NC, Norman A, Cunningham D. The role of surveillance CT scans in patients with diffuse large B-cell non-Hodgkin’s lymphoma. Leuk Lymphoma. 2003;44(1):123–5. https://doi.org/10.1080/1042819021000040323.
Torrey MJ, Poen JC, Hoppe RT. Detection of relapse in early-stage Hodgkin’s disease: role of routine follow-up studies. J Clin Oncol. 1997;15(3):1123–30. https://doi.org/10.1200/JCO.1997.15.3.1123.
Rathore N, Eissa HM, Margolin JF, Liu H, Wu MF, Horton T, Kamdar K, Dreyer Z, Steuber P, Rabin KR, Redell M, Allen CE, McClain KL, Guillerman RP, Bollard CM. Pediatric Hodgkin lymphoma: are we over-scanning our patients? Pediatr Hematol Oncol. 2012;29(5):415–23. https://doi.org/10.3109/08880018.2012.684198.
Meadows AT, Friedman DL, Neglia JP, Mertens AC, Donaldson SS, Stovall M, Hammond S, Yasui Y, Inskip PD. Second neoplasms in survivors of childhood cancer: findings from the Childhood Cancer Survivor Study cohort. J Clin Oncol. 2009;27(14):2356–62. https://doi.org/10.1200/JCO.2008.21.1920.
Bhatia S, Yasui Y, Robison LL, Birch JM, Bogue MK, Diller L, DeLaat C, Fossati-Bellani F, Morgan E, Oberlin O, Reaman G, Ruymann FB, Tersak J, Meadows AT, Late Effects Study G. High risk of subsequent neoplasms continues with extended follow-up of childhood Hodgkin’s disease: report from the Late Effects Study Group. J Clin Oncol. 2003;21(23):4386–94. https://doi.org/10.1200/JCO.2003.11.059.
Punwani S, Taylor SA, Bainbridge A, Prakash V, Bandula S, De Vita E, Olsen OE, Hain SF, Stevens N, Daw S, Shankar A, Bomanji JB, Humphries PD. Pediatric and adolescent lymphoma: comparison of whole-body STIR half-Fourier RARE MR imaging with an enhanced PET/CT reference for initial staging. Radiology. 2010;255(1):182–90.
Federico SM, Brady SL, Pappo A, Wu J, Mao S, McPherson VJ, Young A, Furman WL, Kaufman R, Kaste S. The role of chest computed tomography (CT) as a surveillance tool in children with high-risk neuroblastoma. Pediatr Blood Cancer. 2015;62(6):976–81. https://doi.org/10.1002/pbc.25400.
Owens C, Li BK, Thomas KE, Irwin MS. Surveillance imaging and radiation exposure in the detection of relapsed neuroblastoma. Pediatr Blood Cancer. 2016;63(10):1786–93. https://doi.org/10.1002/pbc.26099.
Papaioannou G, McHugh K. Neuroblastoma in childhood: review and radiological findings. Cancer Imaging. 2005;5:116–27. https://doi.org/10.1102/1470-7330.2005.0104.
Basta NO, Halliday GC, Makin G, Birch J, Feltbower R, Bown N, Elliott M, Moreno L, Barone G, Pearson AD, James PW, Tweddle DA, McNally RJ. Factors associated with recurrence and survival length following relapse in patients with neuroblastoma. Br J Cancer. 2016;115(9):1048–57. https://doi.org/10.1038/bjc.2016.302.
Simon T, Hero B, Hunneman DH, Berthold F. Tumour markers are poor predictors for relapse or progression in neuroblastoma. Eur J Cancer. 2003;39(13):1899–903.
Guimaraes MD, Noschang J, Teixeira SR, Santos MK, Lederman HM, Tostes V, Kundra V, Oliveira AD, Hochhegger B, Marchiori E. Whole-body MRI in pediatric patients with cancer. Cancer Imaging. 2017;17(1):6. https://doi.org/10.1186/s40644-017-0107-7.
McHugh K, Roebuck DJ. Pediatric oncology surveillance imaging: two recommendations. Abandon CT scanning, and randomize to imaging or solely clinical follow-up. Pediatr Blood Cancer. 2014;61(1):3–6. https://doi.org/10.1002/pbc.24757.
Davidoff AM. Wilms tumor. Adv Pediatr. 2012;59(1):247–67. https://doi.org/10.1016/j.yapd.2012.04.001.
Kembhavi SA, Qureshi S, Vora T, Chinnaswamy G, Laskar S, Ramadwar M, Arora B. Understanding the principles in management of Wilms’ tumour: can imaging assist in patient selection? Clin Radiol. 2013;68(7):646–53. https://doi.org/10.1016/j.crad.2012.11.012.
Green DM. Considerations in the diagnosis and management of pediatric patients with favorable histology wilms tumor who present with only pulmonary nodules. Pediatr Blood Cancer. 2016;63(4):589–92. https://doi.org/10.1002/pbc.25840.
Malogolowkin M, Cotton CA, Green DM, Breslow NE, Perlman E, Miser J, Ritchey ML, Thomas PR, Grundy PE, D’Angio GJ, Beckwith JB, Shamberger RC, Haase GM, Donaldson M, Weetman R, Coppes MJ, Shearer P, Coccia P, Kletzel M, Macklis R, Tomlinson G, Huff V, Newbury R, Weeks D, National Wilms Tumor Study G. Treatment of Wilms tumor relapsing after initial treatment with vincristine, actinomycin D, and doxorubicin. A report from the National Wilms Tumor Study Group. Pediatr Blood Cancer. 2008;50(2):236–41. https://doi.org/10.1002/pbc.21267.
Breslow N, Sharples K, Beckwith JB, Takashima J, Kelalis PP, Green DM, D’Angio GJ. Prognostic factors in nonmetastatic, favorable histology Wilms’ tumor. Results of the Third National Wilms’ Tumor Study. Cancer. 1991;68(11):2345–53.
Wilimas JA, Hammond E, Douglass EC, Champion J, Parvey L, Coburn T. The value of computerized tomography as a routine follow-up procedure for patients with Wilms’ tumor. Med Pediatr Oncol. 1984;12(3):221–3.
Kaste SC, Brady SL, Yee B, McPherson VJ, Kaufman RA, Billups CA, Daw NC, Pappo AS. Is routine pelvic surveillance imaging necessary in patients with Wilms tumor? Cancer. 2013;119(1):182–8. https://doi.org/10.1002/cncr.27687.
Mirza W, McHugh K, Aslam M, Sajjad Z, Abid W, Youssef T, Ali A, Fadoo Z. CT pelvis in children; should we routinely scan pelvis for wilms tumor and hepatoblastoma? Implications for imaging protocol development. J Coll Physicians Surg Pak. 2015;25(10):768770–695. https://doi.org/08.2014/JCPSP.768770
Dumba M, Jawad N, McHugh K. Neuroblastoma and nephroblastoma: a radiological review. Cancer Imaging. 2015;15:5. https://doi.org/10.1186/s40644-015-0040-6.
Mullen EA, Chi YY, Hibbitts E, Anderson JR, Steacy KJ, Geller JI, Green DM, Khanna G, Malogolowkin MH, Grundy PE, Fernandez CV, Dome JS. Impact of surveillance imaging modality on survival after recurrence in patients with favorable-histology wilms tumor: a report from the Children’s Oncology Group. J Clin Oncol. 2018;18:JCO1800076. https://doi.org/10.1200/JCO.18.00076.
Semeraro M, Branchereau S, Maibach R, Zsiros J, Casanova M, Brock P, Domerg C, Aronson DC, Zimmermann A, Laithier V, Childs M, Roebuck D, Perilongo G, Czauderna P, Brugieres L. Relapses in hepatoblastoma patients: clinical characteristics and outcome--experience of the International Childhood Liver Tumour Strategy Group (SIOPEL). Eur J Cancer. 2013;49(4):915–22. https://doi.org/10.1016/j.ejca.2012.10.003.
Rojas Y, Guillerman RP, Zhang W, Vasudevan SA, Nuchtern JG, Thompson PA. Relapse surveillance in AFP-positive hepatoblastoma: re-evaluating the role of imaging. Pediatr Radiol. 2014;44(10):1275–80. https://doi.org/10.1007/s00247-014-3000-6.
Kan JH, Hwang M, Lowas SR, Hernanz-Schulman M. Impact of pelvic CT on staging, surveillance, and survival of pediatric patients with Wilms tumor and hepatoblastoma. AJR Am J Roentgenol. 2011;196(5):W515–8. https://doi.org/10.2214/AJR.10.5179.
Shelmerdine SC, Roebuck DJ, Towbin AJ, McHugh K. MRI of paediatric liver tumours: how we review and report. Cancer Imaging. 2016;16(1):21. https://doi.org/10.1186/s40644-016-0083-3.
Puri A, Gulia A, Hawaldar R, Ranganathan P, Badwe RA. Does intensity of surveillance affect survival after surgery for sarcomas? Results of a randomized noninferiority trial. Clin Orthop Relat Res. 2014;472(5):1568–75. https://doi.org/10.1007/s11999-013-3385-9.
Rothermundt C, Whelan JS, Dileo P, Strauss SJ, Coleman J, Briggs TW, Haile SR, Seddon BM. What is the role of routine follow-up for localised limb soft tissue sarcomas? A retrospective analysis of 174 patients. Br J Cancer. 2014;110(10):2420–6. https://doi.org/10.1038/bjc.2014.200.
Postovsky S, Barzilai M, Meller I, Kollander Y, Futerman B, Ben Arush MW. Does regular follow-up influence the survival of patients with sarcoma after recurrence? The Miri Shitrit pediatric oncology department experience. J Pediatr Hematol Oncol. 2008;30(3):189–95. https://doi.org/10.1097/MPH.0b013e31815d88fa.
Korholz D, Verheyen J, Kemperdick HF, Gobel U. Evaluation of follow-up investigations in osteosarcoma patients: suggestions for an effective follow-up program. Med Pediatr Oncol. 1998;30(1):52–8.
Meyer JS, Nadel HR, Marina N, Womer RB, Brown KL, Eary JF, Gorlick R, Grier HE, Randall RL, Lawlor ER, Lessnick SL, Schomberg PJ, Kailo MD. Imaging guidelines for children with Ewing sarcoma and osteosarcoma: a report from the Children’s Oncology Group Bone Tumor Committee. Pediatr Blood Cancer. 2008;51(2):163–70. https://doi.org/10.1002/pbc.21596.
Walter F, Czernin J, Hall T, Allen-Auerbach M, Walter MA, Dunkelmann S, Federman N. Is there a need for dedicated bone imaging in addition to 18F-FDG PET/CT imaging in pediatric sarcoma patients? J Pediatr Hematol Oncol. 2012;34(2):131–6. https://doi.org/10.1097/MPH.0b013e3182282825.
Lin JL, Guillerman RP, Russell HV, Lupo PJ, Nicholls L, Okcu MF. Does routine imaging of patients for progression or relapse improve survival in rhabdomyosarcoma? Pediatr Blood Cancer. 2016;63(2):202–5. https://doi.org/10.1002/pbc.25750.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Verhagen, M.V., McHugh, K., Voss, S.D. (2019). Surveillance Imaging in Pediatric Oncology. In: Voss, S., McHugh, K. (eds) Imaging in Pediatric Oncology. Pediatric Oncology. Springer, Cham. https://doi.org/10.1007/978-3-030-03777-2_21
Download citation
DOI: https://doi.org/10.1007/978-3-030-03777-2_21
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-03776-5
Online ISBN: 978-3-030-03777-2
eBook Packages: MedicineMedicine (R0)