Abstract
Arsenic is a semimetal that forms a part of more than 200 minerals. In many places of the world concentrations of arsenic in water are high, which is an issue of high importance in connection with human health. It has three allotrope forms; the gray one is the most common. Among numerous arsenic isotopes only 75As is stable. The element is produced mainly in the form of a trioxide. Arsenic is used in electronic, metallurgy, pesticides, and defoliants. The most common use is in the production of wood preservatives (which, along with fossil fuel combustion, represents the largest anthropogenic arsenic source in the environment). In some parts of the world arsenic compounds are used as a supplement in poultry farming. Recent research also shows its potential use in medicine. Arsenic toxicity depends on its form (organic and inorganic), as well as on its oxidation state, solubility, and species exposed. In the body, the methylation of its inorganic form takes place mainly in the liver. Following exposure to arsenic, it can be found in various tissues, organs and materials, as kidneys, blood, lungs, feathers, hair, and fur, but mainly in the liver. Arsenic bioaccumulation is low, and biomagnification is still questioned in terrestrial ecosystems. Some biomarkers of exposure, apart from concentration measurements (especially in urine, blood, hair, fur, and feathers) may be used. Among internal tissues, the liver is the most commonly studied.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
1 Introduction
Arsenic (chemical symbol As) is a metalloid (semimetal), but it is commonly included in the list of “heavy metals” based upon its toxicity (IUPAC 1971; Duffus 2002). As a semimetal it presents some properties of metals and nonmetals. It is a component of numerous minerals and reaches a concentration of 2 mg kg−1 in rocks (Mandal and Suzuki 2002). Arsenic’s main toxicity combines with its inorganic forms occurring in groundwater in many places around the world, including more than 70 countries (Ravenscroft et al. 2009). This is the main reason for the global interest in As occurrence, availability, and exposure.
Arsenic, along with its compounds, has been widely used in industry and agriculture. It has found primary application in wood preservatives, insecticides, and poisons (Rahman et al. 2004). It has been also used in medical treatment, pharmaceuticals, and even in supplementation in animals (Jones 2007). Other applications include alloy production, glass processing, and the production of semiconductors, ammunition, batteries, pigments, paper, and metal adhesives. Elevated As concentrations in organisms cause poisoning and stimulate cancer development (IARC 2012). However, numerous studies also reveal that As deficiency in the diet of birds and mammals causes physiological disorders, especially with respect to methionine metabolism (Uthus 2003).
2 General Properties
Arsenic (Lat. Arsenicum) lies between germanium and selenium in the nitrogen group (pnictogen) of the periodic table. Its atomic number is 33 and atomic weight is 74.9 Da (Haynes 2014). Among the three As allotropes, the most stable is the gray form (also called α-crystalline). A yellow cubic form is less stable and after warming reverts to the basic form. The black β form is also stable. The density of the gray form is 5.73 g cm−3, and its melting temperature is 817 °C; sublimation occurs at 616 °C (Norman 1998). Generally, 29 As isotopes have been identified (64As–92As). However, some scientists include an additional four (60As–63As). The only isotope considered stable and naturally occurring is 75As and due to that fact As is often treated a monoisotopic element.
Arsenic occurs in four oxidation states: −3, 0, +3, and +5 (Adriano 2001). In nature, two major groups of As compounds occur, inorganic and organic (Lunde 1977; Andreae 1978). Compounds with the element on +3 (arsenite) and +5 (arsenate) oxidation levels dominate (Andreae 1978; Morita and Edmonds 1992; Rosen 2002). Apart from those, compounds as arsines and methylarsines with As on the −3 level also occur, but they are unstable in the air. Free arsenic As(0) is rarely encountered in nature (Eisler 1988). In terms of As use and application, the most important form is As trioxide (As2O3).
3 Arsenic Minerals, Production, and Uses
Arsenic constitutes a part of more than 200 minerals, of which 60% are treated as mainly As ones (Kabata-Pendias 2011). The most common are arsenopyrite (FeAsS), arsenolite (As4O6), loellingite (FeAs2), orpiment (As2S3), and realgar (AsS). Arsenic also occurs in ores of other metals (such as iron, nickel, cobalt, and copper) and reaches high concentrations in sulfide deposits: arsenides (27 minerals), sulfides (13 minerals), and sulfosalts (65 minerals) (Adriano 2001; Hammond 2004).
Arsenic is produced mainly in the form of As2O3 (most reports present production data expressed in terms of this compound’s values). Main global As production in 2015 was estimated at 36,000 metric tons (Fig. 13.1). For many years, largest quantities of As2O3 were produced (expressed in metric tons) in China (25,000), Chile (10,000), Morocco (7500), and Russia (1500) (USGS 2015, 2016). The USA has not produced As since 1985 (USGS 2011). At present, As2O3 is produced mainly by volatilization during the mining and production of other elements. Probably only China still mines As ores intentionally (Grund et al. 2005). Metallic As is produced in significantly smaller quantities, but detailed data are not available (USGS 2015). Of the total As imported by the USA, no more than 4% is in metallic form, which is usually produced by the reduction of ores or As2O3 with coal monoxide (Mandal and Suzuki 2002; Solo-Gabriele et al. 2003; USGS 2006).
The metallic form is used in electronics and nonferrous alloys. As2O3 has been used mainly in agriculture and forestry as an ingredient of pesticides and defoliants. The most common use is in wood preservatives (most often chromated copper arsenate, CCA) (USGS 2006). Since the 1980s among various As pesticides only CCA was still approved for use. This is why, in the 1990s, more than 80% of total As in the USA was used as a wood preservative (Solo-Gabriele et al. 2003). In 2004 the US Environmental Protection Agency (EPA) introduced a ban on CCA use for residences, led to a drastic decrease in CCA consumption (Jones 2007). Arsenic compounds have also been used as feed additives for poultry, which resulted in increased growth rates, improved feed spent, and better pigmentation. However, because of As’s toxicity, As compounds were withdrawn from use in the European Union (EU) in 1998. In the USA they are still in use (Nachman et al. 2005, 2013).
Arsenic is known in history as one of the most commonly used poisons for homicidal and suicidal purposes (Mandal and Suzuki 2002). However, previously, around the world but still in many countries today, As and its compounds were widely used in medicine, especially in treatments of syphilis, various parasitic infections, amoebic dysentery, and trypanosomiasis. In the second half of the twentieth century it was also used in stomatology, where As2O3 was applied to devitalize the dental pulp (Aso and Abiko 1978). Now recent research has shown the efficiency of As compounds in the treatment of relapsed or refractory acute promyelocytic leukemia (Shen et al. 1997; Antman 2001; Firkin 2014).
4 Arsenic in Nature: Geogenic and Anthropogenic Sources
Arsenic is the 20th most abundant element in the Earth’s crust, with an average concentration of 0.00005% (Mandal and Suzuki 2002). A natural source of As in the environment is volcanic activity (USGS 2011). Its concentrations in rocks vary significantly around the world and in some geographical regions reach high values (Duker et al. 2005). Arsenic’s highest concentrations are found in sedimentary rocks, especially clayey ones (Fig. 13.2). In some offshore areas, claystone concentrations run as high as 490 mg kg−1.
Average arsenic concentrations in fossil fuels and rocks (data from Kabata-Pendias and Pendias 1999)
In river sediments, As concentrations are even higher—up to 4000 mg kg−1 (Mandal and Suzuki 2002). The element occurs in almost all soil types and other environmental matrixes, but its major repositories are aquatic systems (Adriano 2001; Smedley and Kinniburgh 2002; Nordstrom 2002; Kabata-Pendias 2011; Magellan et al. 2014). Arsenic occurrence in soils is strictly connected with the initial material from which soils were formed, and As’s form depends on many factors, including oxidation, pH, and microbial activity (Xu et al. 1991). Background As levels in various soils usually do not exceed 10 mg kg−1 (Kabata-Pendias 2011). However, owing to the common use of pesticides and other As products, concentrations found in agricultural soils are much higher, even up to 2500 mg kg−1 in Japan, the UK, and the USA (Adriano 2001; Kabata-Pendias 2011).
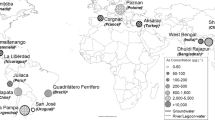
Arsenic is released from soils and rocks into the atmosphere by high-temperature processes and erosion. Later these forms are dispersed with the air on land and in water. However, the most dangerous geogenic exposure to inorganic As (the most toxic form) of humans and animals is through drinking groundwater in a number of places around the world, such as Mexico, the USA, Argentina, Chile, Bangladesh, India, and China (Welch et al. 2000; WHO 2010). The biggest problem is in the Bengal Basin (in Bangladesh and partially in India), where almost 60 million people drink water that contains elevated As levels. One million people have already developed strong symptoms of arsenicosis (Henke 2009).
Among fossil fuels, coal has relatively high As concentrations, within a range of 5–15 mg kg−1. Petroleum’s concentrations are lower, 0.005–0.14 ppm, with an average value of 0.07 ppm (Fig. 13.2). Concentrations usually found in gasoline fall within a range of 0.02–2 ppm (Kabata-Pendias and Pendias 1999). Fossil fuel combustion and metal smelters are the main anthropogenic As sources next to pesticides and wood-preservative run-off (USGS 2015). In the EU a recent decrease in such activities resulted in a 68% reduction of atmospheric As emissions in the period 1990–2013 (EEA 2016). However, a substantial part of industry still depends on coal combustion, which is linked with As emissions, mainly through particulate matter. Arsenic is observed mainly in the air in the form of arsenites and arsenates. The exceptions are areas where pesticides based on other As forms are sprayed (Davidson et al. 1985). The lowest air As concentration was observed over the South Pole (0.007 ng m−3) and Spitsbergen (0.01–1.5 ng m−3) (Kabata-Pendias and Pendias 1999). Arsenic concentrations in certain American cities average 2 ng m−3 (Chen and Lippmann 2009). Average concentrations in remote areas in the USA were estimated to fall within a range of 1–3 ng m−3, in urban areas 20–100 ng m−3, and in industrial areas 70–770 ng m−3 (ATSDR 2007a; Geiger and Cooper 2010). Arsenic concentrations in Europe are generally low, and the EU As target value in ambient air was established at a level of 6 ng m−3 (EU 2005, 2008; Strincone et al. 2013; Guerreiro et al. 2014).
Arsenic compounds are used as feed additives in animal farming, so the possibility of its deposition in manure arises. Simulations show that using manure to enrich agriculture soils in nutrients may lead to pollution of groundwater and the creation of another pathway of exposure, but environmental studies have not confirmed this problem (Nachman et al. 2005; Jones 2007).
5 Biological Status of Arsenic
Resistance to and metabolism of As is generally known in bacteria that exert an influence on the global As geocycle (Mukhopadhyay et al. 2002; Stolz et al. 2006). Even As’s physiological role is suspected in some types of microbial photosynthesis in biofilms, but it has not been fully proven (Kulp et al. 2008; Schoepp-Cothenet et al. 2009). Arsenic is known as a nonessential and toxic element for plants, but some specimens evolved to metabolize it efficiently (Finnegan and Chen 2012). The main mechanism of its detoxification is the reduction process of arsenate into arsenite, controlled by the arsenate reductase enzyme (Chao et al. 2014). Some fern species are even As hyperaccumulators, but still the reaction of most plants to As compounds makes it possible to use them as ingredients in herbicides and defoliants (NAS 1977; Zhao et al. 2009).
Arsenic essentiality in insects is not known either, and the use of herbicides has demonstrated the sensitivity of insects and other invertebrates to this element (Eisler 1988). The sensitivity of different species may vary significantly, and some of them may play an important role in the retention and cycle of As (Riedel et al. 1989; Schaller et al. 2010).
In birds and mammals, the problem of As essentiality is still disputed. Some observations suggest that inorganic As may be an essential nutrient for goats, chicks, minipigs, and rats (EPA 1998; Adriano 2001). The positive influence of As on animal growth has been long observed and resulted in the use of its compounds in animal breeding as food additives. Studies on birds revealed increased body weight and immune organs of chickens after As supplementation (Ai-zhi and Zhen-yong 2007). However, the main mechanism remained unknown for a long time (Anke 1986). Probably the increased growth of animals bred with the aforementioned feed additives is connected with intestinal health. Organoarsenic additives (the most common being Roxarsone) are very toxic to parasites and significantly decrease their number, which results in a better general condition of animals (Lasky et al. 2004; Jones 2007; FDA 2011). Bearing this in mind, such an influence cannot be treated as a positive function in physiology, but rather as a drug treatment.
5.1 Toxicity of Various Arsenic Forms in Homeothermic Animals
Toxic As’s effect is undisputed and significantly depends on its form. Arsenic compounds that are still used in medicine showed adverse effects on the body, including lethal cardiac dysfunctions (Ohnishi et al. 2000; Lin et al. 2005). In spite of the fact that various forms stimulate different levels of toxicity, signs of poisoning are similar (Woolson 1975; NRCC 1978). Generally for all organisms, inorganic As forms are more toxic than organic ones (Tamaki and Frankenberger Jr 1992). However, some observations dispute this statement. The positive relationship between the toxicity of As compounds and solubility in water has been noted (Eisler 1988). The solution showed a toxicity that was as much as ten times higher than that of the undissolved form (Schwartze 1922; Harrisson et al. 1958). Two oxidation states of arsenic are usually discussed in connection with effects on animals: As(iii) and As(v). As(iii) forms strong bonds with the thiolates of cysteine residues and are regarded as more toxic than As(v)—by as much as 60 times (Rosen 2002; Ventura-Lima et al. 2011). The level of As toxicity also depends on other factors, for example, the species. Comparison of the resistance to As influence between rats and humans revealed that humans are more sensitive than rats (NAS 1977).
The species affects not only the toxicity but also accumulation and distribution of As in the body (Ducoff et al. 1948). The oral LD50 of As2O3 is on the level of 31.5 mg kg−1 in mouse and 14.6 mg kg−1 in rat. The LD50 of As given intraperitoneally is 46.2 mg for mouse and 13.4 for rat. Adequate values of oral intoxication were consecutively 145 mg kg−1 and 763 mg kg−1, respectively (TOXNET 2015). The acute minimum As lethal dose in humans fell in the 70–200 mg range, or 1 mg kg−1 per day (Dart 2004).
In addition to toxicity through ingestion, inhalation of As compounds is also harmful. Lethal cases, diarrhea, respiratory distress, and decreased body weight have been observed in rodents exposed to As pesticide fumes (Stevens et al. 1979). The penetration of organic As in fetuses is negligible, but inorganic As compounds may cross the placental barrier and even cause death of newborns (Lugo et al. 1969).
Medical studies that reveal positive As impacts in leukemia treatment point out also observations regarding the further development of thyroid cancer in patients, probably because of As’s carcinogenicity (Firkin 2014). Co-occurrence of lung cancer among people chronically exposed to airborne As compounds has also been observed (Nordberg et al. 2007). The carcinogenic properties of inorganic forms have been confirmed, but the main mechanism is not fully understood (Sakurai 2003). Arsenic and inorganic As compounds have been classified in human carcinogen group 1 based on consistent evidence of associations mainly with lung, skin, and bladder cancers. Arsenobetaine and other organic As compounds have not been classified as carcinogens (IARC 2012). The interactions between As and other elements, such as zinc, selenium, and antimony, are suspected in the etiology of carcinogenicity (Gebel 2000).
Most of the organisms already studied show evolved mechanisms of defense against As toxicity (Rosen 2002; Cullen 2014). Arsenic methylation, which leads to the transfer of inorganic forms into less toxic methylarsenic(v) [MMA(v)], was long treated as a very efficient detoxification process. However, further research showed that methylation may lead to the production of other organic compounds such as methylarsenic(iii) (Cullen 2014) (Fig. 13.3). Some methylated organic compounds [such as monomethylarsonate MMA(iii)] are more toxic to plants and animals (including humans) than inorganic forms and certain organic forms containing As(v) like dimethyloarsenic [DMA(v)] (Meharg and Hartley-Whitaker 2002; Rahman et al. 2012). Research carried out on human liver cells revealed that the toxicity of various As forms can be presented in the order: MMA(iii) > arsenite > arsenate > MMA(v) = DMA(v) (Petrick et al. 2000). MMA and DMA may negatively influence enzymes that work in the energetic cycles in cells. Inorganic As interacts with sulfhydryl groups; the toxicity of MMA is mainly associated with the thiol groups reaction, and the toxicity of DMA with decreasing oxidative phosphorylation (Khan et al. 2014). Some evidence also suggest that MMA(v) is a carcinogen in rodents. There are also suspicions that in humans, DMA may be methylated further into arsenobetaine, which is characterized by a low toxicity for animals (Kaise et al. 1985; Newcombe et al. 2010).
5.2 Toxicokinetics and Effects of Arsenic in Wildlife
Both main types of inorganic As—arsenite and arsenate—are well absorbed by ingestion and inhalation. Significantly lower absorption occurs through the skin (ATSDR 2007b). Experiments with As2O3 in rats showed elevated As concentration in kidneys, liver, lungs, skin, spleen, and blood 24 h following administration. A similar dynamic was observed in humans (Graeme and Pollack 1998). Arsenic accumulates in blood cells, so concentrations in blood and spleen 2 months after subcutaneous implantation remained high. Interestingly, accumulation was not observed in hair and brain (Vallee et al. 1960; Aso and Abiko 1978), but studies on patients during leukemia treatment revealed elevated concentrations in hair, nails, and urine following intravenous infusion of As drugs (Shen et al. 1997).
In higher animals and humans, following various administrations (including oral, fume, and injection exposure), arsenates are partially reduced to arsenites (Vahter and Marafante 1983; Buchet et al. 1998; ATSDR 2007a). This occurs because of the activity of glutathione, which is an electron donor for the reduction (Styblo et al. 2000). Following parental administration of As2O3 to rabbits, As(iii) was the major form of the element detected in blood, lungs, and liver (Vahter and Marafante 1983; Lin et al. 2005). However, the main organ containing As following exposure is the liver (Vahidnia et al. 2007b). Inorganic forms of the element in humans are methylated into MMA and DMA, and partially further to trimethylarsenic (TMA) compounds in liver (Yamauchi and Yamamura 1985; Styblo et al. 2000). The process takes several steps, mainly in the liver, but other organs also showed methylation activity (Fig. 13.3) (Khan et al. 2014). Methylation is catalyzed by methyltransferase, which uses S-adenosylmethionine as the methyl group donor (Zakharyan et al. 1995). First, inorganic As(iii) is converted into MMA(v). Then MMA(v) is reduced to MMA(iii), which is further methylated into DMA(v). Next methylation step (into TMA) is also proceeded by the reduction into DMA(iii) (Styblo et al. 2000; Cullen 2014). However, TMA metabolism is still disputed (Kaise et al. 1985; Newcombe et al. 2010) (Fig. 13.3).
The speed of methylation varies among species and is higher, for example, in mice than in rabbits. The excretion of organic forms is faster than that of inorganic forms (Vahter and Marafante 1983). Following absorption, more than 90% of inorganic As is cleared from the blood in 2–4 h and as much as 70% of the intake is excreted in 48 h (Jones 2007).
Arsenic significantly affects the central and peripheral nervous systems. Its effects had already been observed in chronic and acute exposure, but the main mechanisms remain unclear. There are some observations that, not inorganic forms, but organic forms of As are responsible for the impact of the element on nerve cells (Vahidnia et al. 2007a). The time after exposure in single-dose studies varied between 10 days and 3 weeks, but the initial effects may be observed even after a couple of hours (Winship 1984; Vahidnia et al. 2007b). The most characteristic clinical signs of As-induced neuropathy are numbness, paresthesias, and pain (especially in feet soles). They are connected with axonal degeneration and disorganization of the cytoskeletal framework (Vahidnia et al. 2007b).
Studies strictly examining the toxicokinetics and effects of As in birds mainly concern farm species that may be intentionally exposed via feedstuff and unintentionally in some cases through drinking water (Khan et al. 2014). Wild living birds are rarely studied in this respect. Generally, there are numerous observations of lower appetite, weight loss, deterioration of blood parameters, depression, ataxia, dullness, and other neurological disorders among poultry exposed through drinking water or food (Halder et al. 2007; Islam et al. 2009; Sharaf et al. 2013; Khan et al. 2014). Weight loss and even death were observed in mallard (Anas platyrhynchos) ducklings (Hoffman et al. 1992). The negative influence of As on the heart (ecchymotic hemorrhages), liver (congestion and hemorrhages), spleen (regression and hemorrhages), kidneys (swelling), and intestinal mucosa (congestion) were already observed in broiler chicks after administration of sodium arsenite (Kalavathi et al. 2011). Studies on bird histopathology showed atrophy of bursa of Fabricius stimulated by a mixture of chemicals including As (also cadmium, lead, benzene, and trichloroethylene), as well as liver lesions (Hoffman et al. 1992; Vodela et al. 1997). Separate analysis of sodium arsenite revealed the substantial negative impact on among others cardiac muscle (disruption of bundles), kidneys (infiltration of mononuclear cells), and spleen (depletion of lymphocytes). The toxic effects of As in these chicks were partially counteracted by supplementation with ascorbic acid and vitamin E (Kalavathi et al. 2011). Increased concentrations of plasma calcium and decreases in plasma glutathione activity are also linked with exposure to As in birds. All toxic effects are more common in birds with limited access to food (Hoffman et al. 1992).
5.3 Bioaccumulation of Arsenic
There is a discrepancy in the scientific literature regarding the occurrence and efficiency of As bioaccumulation. Human studies suggest that the element does not bioaccumulate on a large scale, even over time (Jones 2007). Similar observations were made among aquatic organisms, in which the bioconcentration factor (except for algae) is relatively low (Eisler 1988). However, phytoplankton that bioaccumulate As compounds are a major food source for animals of higher levels, so they might be exposed to the element. For this reason, some species of fish are being used in biomonitoring of arsenicals (Rahman et al. 2012).
In terrestrial trophic chains, the situation is different. Generally, inorganic As forms dominate in soil. Soil microbiota may change them into organic ones, methylate and demethylate them, and carry out these processes in opposite ways (Turpeinen et al. 1999). They may get into plants or invertebrates, exposing the animals at higher levels of the trophic pyramid, including birds and mammals, that metabolize the compounds into organic species (Tamaki and Frankenberger Jr 1992; Vahter 2000; Meharg and Hartley-Whitaker 2002).
Despite the fact that As is a known xenobiotic and carcinogen, few studies have been conducted on its concentration and influence in birds. This knowledge gap is especially significant in passerines among which around 75% of studies were conducted only on the great tit (Parus major) and pied flycatcher (Ficedula hypoleuca). The most often internal tissues have been studied (32.5%), followed by feces (27.5%) and blood (15%) (Sánchez-Virosta et al. 2015). The values considered normal were estimated on levels (mg kg−1 dw) 0.01–0.25 for liver and 0.01–0.2 for kidneys (WVDL 2015). Concentrations in internal tissues exceeding 10 mg kg−1 wet weight (ww) (~41.6 mg kg−1 dry weight, dw, recalculated according to Binkowski 2012) are treated as symptoms of As poisoning (Goede 1985). Such high concentrations are not very common, and in most cases accumulation does not exceed background levels—for liver on average 1.5 mg kg−1 dw at pristine sites and 5.8 mg kg−1 dw at polluted sites (Berglund et al. 2012; Sánchez-Virosta et al. 2015). However, particular specimens may reach significantly higher concentrations, as high as around 13 mg kg−1 dw (pied flycatcher) (Berglund et al. 2012). It is worth mentioning that such high concentrations are harmful to animals since significantly lower concentrations were the cause of disturbances in their biochemistry and growth (Sánchez-Virosta et al. 2015). Among other bird groups, studies on dunlins (Calidris alpina) across Europe (including the Netherlands, Norway, and Sweden) revealed that in many cases concentrations were lower than the detection limit. The highest mean concentration was noted for juvenile dunlin and reached 6.2 mg kg−1 dw, but the mean value for all studied specimens was lower than 3 mg kg−1 dw. Additionally, birds from pristine areas (Scandinavia vs. Western Europe) accumulated significantly lower amounts of the element (Goede et al. 1989). Arsenic concentrations found in the liver of dabbling ducks are similar. A study carried out on common species, including the mallard, blue-winged teal (Anas discors), and shoveler (Anas clypeata), revealed a mean value of 4.76 mg kg−1 dw (Pereda-Solis et al. 2012). Higher As concentrations were suspected among birds exposed to spent lead shot that contained detectable As levels (Hall and Fisher 1985). Studies verifying this hypothesis in nature did not show high As concentrations in lead-poisoned birds. However, the conclusions are not very obvious because a strong correlation was observed between lead and arsenic in exposed birds (Mateo et al. 2003), so this issue requires further investigation.
Concentrations in feathers are generally lower than in internal organs, and they are used in in vivo biomonitoring. Molting stage and age of the feather are very important here (Burger 1993). The interesting question is whether the contamination of feathers comes mainly from internal distribution or external deposition. A strong argument for the second route is the correlation of As concentrations in preen glands and in feathers (Goede and De Bruin 1984; Goede 1985; Goede et al. 1989). Concentrations of As in feathers of various species of Anseriformes are similar, but a slightly higher one was noted locally for diving duck (pochard Aythya ferina), where the mean reached 0.50 μg g−1 dw (Karimi et al. 2016). These observations rank Anseriformes in the middle of the common range of species from different trophic levels (e.g., raven, condor, and red knot), whose mean was 0.96 μg g−1 dw (Burger 1993). Karimi et al. (2016) also noted an interesting positive but weak correlation between lead and arsenic concentrations in primary feathers of Anseriformes. A similar but stronger relationship was observed in great tit feathers (Janssens et al. 2001). Some studies have also been conducted on As concentrations in bones of birds. The reason for this is that arsenate is structurally very similar to phosphate, which builds bones, so the possibility of phosphate substitution by arsenate may occur (Adriano 2001; WHO 2001; Mateo et al. 2003).
Arsenic is not as widely studied an element in mammals as, for example, lead or cadmium, but because of the potential harm to residential wildlife, studies are being carried out (Saunders et al. 2011). However, among such studies, laboratory acute toxicity issues dominate, and As chronic toxicity studies among wild living animals remain scarce (Drouhot et al. 2014). Normal values, for example in deer, do not exceed 0.5 mg kg−1 dw in liver and kidneys. Normal concentrations in other mammals are even lower (WVDL 2015). An interesting procedure for monitoring As levels in shrews was developed by Moriarty et al. (2012). It entails using for analysis the entire torso of an individual. This study revealed that shrews are efficient at processing and excreting As. Animals from heavily contaminated areas may accumulate as much as twice the As body burden as animals from uncontaminated sites. However, not only environmental contamination but also other factors, such as habitat, diet preferences, and animal mobility, play a significant role in exposure and accumulation. Arsenic concentrations in the stomach contents of various small rodent species observed in southern France fell within a very wide range, from below the detection limit to 1669 mg kg−1 dw (but in most cases the upper limit did not exceed 50 mg kg−1 dw). These values did not correspond clearly to concentrations in soil from different emission zones, and animals from cleaner zones sometimes had higher concentrations in their stomach content. Internal concentrations (mg kg−1 dw) fell within a range of 0.05–90.4 for liver, 0.24–50.9 for kidneys, and 0.31–37.7 for lungs (Drouhot et al. 2014). Studies on As accumulation in small mammals led to varying conclusions (Erry et al. 2000). Some revealed that in polluted areas As is accumulated by animals, some revealed no accumulation, and others only in some organs (Sharma and Shupe 1977; Smith and Rongstad 1982; Ismail and Roberts 1992; Peles and Barrett 1997). Studies on large animals, like cattle, showed that particular tissues, such as blood, kidneys, liver, and muscles, accumulate As at statistically different rates of efficiency. What is more, these animals accumulate As only in areas of higher soil concentrations. Maximum values (mg kg−1 dw) may reach 122.6 in liver, 135.6 in kidneys, and 8.55 in muscles [values recalculated from ww according to Binkowski (2012)] (López Alonso et al. 2002).
5.4 Ecological Effects of Arsenic
The main processes under dispute in terms of the ecological aspects of a given element or compound are bioaccumulation and biomagnification. They are usually separately evaluated for aquatic and terrestrial environments, but generally they are more efficient in aquatic ones. In aquatic environments (both marine and freshwater), inorganic As species dominate, but they are methylated into organic species by aquatic organisms. Because bioaccumulation of total As in fish reaches as high as 22.1, the exposure of predators through fish is likely (Kar et al. 2011). These may include aquatic birds and mammals. In some areas, to limit exposure, As bioremediation with algae is proposed (Magellan et al. 2014).
In the case of terrestrial ecosystems, As bioaccumulation may be observed, as mentioned earlier (Sect. 5.3). However, studies on mammals also reveal that the bioaccumulation factor is lower than 1 (0.69), which means that As bioaccumulation does not occur in these animals (Erry et al. 2000). In both types of environment, a further step in bioaccumulation—As biomagnification—has been widely questioned (Woolson 1975; NRCC 1978; Eisler 1988; Jones 2007). However, the lack of biomagnification does not mean that As does not affect the ecosystem as a whole. It does affect the ecosystem in areas of polluted water or massive amounts of poultry feces deposition, where animals are supplemented with As compounds (Eisler 1988).
5.5 Bioindicators and Biomarkers of Arsenic in Ecotoxicological Studies
Metabolomic studies on As bioindicators and biomarkers are mainly done on rodents. Only a few studies have been carried out on humans. It is worth emphasizing that As, after cadmium, is the most frequently studied element in these aspects (García-Sevillano et al. 2015). Despite the fact that the main mechanisms of its toxicity remain unclear, its connection with enzymatic inhibition and oxidative stress is widely observed, which may be employed in biomarker studies. However, there is still a need for As-sensitive and appropriate biomarkers in environmental studies (Marchiset-Ferlay et al. 2012).
It is known that As affects certain enzymes in heme biosynthesis, such as aminolevulinate synthase, porphobilingoen deaminase, and heme oxygenase (Garcia-Vargas and Hernandez-Zavala 1996). The exposure to a mixture of elements (arsenic, cadmium, lead) causes perturbations in lipid and amino acid metabolism in blood serum (Dudka et al. 2014). Additionally, the connection between As exposure and blood porphyrins and their urinary excretion has been observed (Garcia-Vargas and Hernandez-Zavala 1996; Marchiset-Ferlay et al. 2012). All these relationships may be implied to some extent as biomarkers, but their usefulness, especially in animal studies, awaits confirmation. In the range of genotoxicity, DNA damage, chromosomal aberrations, sister chromatic exchange and micronuclei formation are linked with As exposure. Based on these effects, genotoxicity biomarkers may be used (Liou et al. 1999; Chen et al. 2005; Marchiset-Ferlay et al. 2012).
Blood and urine are the most frequently used biomarkers of As exposure. Arsenic is purged from the blood, so the concentration in blood reveals only present and recent exposures (Andrade et al. 2015). A better biomarker is probably concentration in urine, because it reflects chronic exposure. Other very good biomarkers of chronic exposure are hair and nails (thus feathers and claws in animals), but the concentrations here cannot be recalculated as the dose ingested (Marchiset-Ferlay et al. 2012). Arsenic exposure may also be evaluated on the basis of its concentration and distribution in internal tissues. Probably the most commonly studied and useful tissue in this connection is liver tissue.
6 Conclusions
-
Arsenic is a semimetal that is toxic for birds and mammals at elevated concentrations. Inorganic forms of arsenic possess carcinogenic properties. Additionally, the occurrence of arsenic in water in many parts of the world is an issue of high importance because of the high incidence of arsenicosis in people. Despite its toxicity, As has been used in medicine and as a supplement for farm poultry in some parts of the world (e.g., USA).
-
Arsenic forms a part of more than 200 minerals, and its natural sources are rocks, soils, and volcanic activity. In industry, arsenic is used and produced mainly in the form of trioxide. The most prevalent uses of As are in the production of wood preservatives (such as chromated copper arsenate), pesticides, nonferrous alloys, and electronics.
-
Bioaccumulation of As in birds and mammals is not high, and biomagnification is still disputed. Concentrations of up to 0.25 mg kg−1 dw in bird liver and kidneys are treated as normal. In mammals such values do not exceed 0.5 mg kg−1 dw.
-
A potential relationship between exposure to arsenic and enzyme activities has been observed, but the precise biomarker needs to be found. Exposure may be evaluated on the basis of internal concentrations in hair, fur, nails, claws, urine, and organs, preferably in liver.
References
Adriano DC (2001) Arsenic. In: Trace elements in the terrestrial environments: biogeochemistry, bioavailability, and risks of metals. Springer, New York, pp 219–261
Ai-zhi C, Zhen-yong W (2007) Effect of different supplemented arsenic preparation on growth of body weight and main immune organs in chickens. J Domest Anim Ecol 1:63–65
Andrade VM, Mateus ML, Batoreu MC, Aschner M, Marreilha dos Santos AP (2015) Lead, arsenic, and manganese metal mixture exposures: focus on biomarkers of effect. Biol Trace Elem Res 166:13–23
Andreae MO (1978) Distribution and speciation of arsenic in natural waters and some marine algae. Deep Sea Res 25:391–402
Anke M (1986) Arsenic. In: Mertz W (ed) Trace elements in human and animal nutrition, vol 2. Academic Press, New York, pp 347–372
Antman KH (2001) Introduction: the history of arsenic trioxide in cancer therapy. Oncologist 6(Suppl 2):1–2
Aso T, Abiko Y (1978) Tissue distribution of arsenic after subcutaneous implantation of arsenic trioxide pellet in rats. J Toxicol Sci 3:109–116
ATSDR (2007a) ToxGuide for Arsenic. CAS # 7440-38-2. U.S Public Health Service, Agency for Toxic Substances and Disease Registry, Atlanta
ATSDR (2007b) Toxicological Profile for Arsenic. Agency for Toxic Substances and Disease, Atlanta
Berglund ÅMM, Rainio MJ, Eeva T (2012) Decreased metal accumulation in passerines as a result of reduced emissions. Environ Toxicol Chem 31:1317–1323
Binkowski ŁJ (2012) The effect of material preparation on the dry weight used in trace elements determination in biological samples. Fresenius Environ Bull 21:1956–1960
Buchet JP, Apostoli P, Lison D (1998) Arsenobetaine is not a major metabolite of arsine gas in the rat. Arch Toxicol 72:706–710
Burger J (1993) Metals in avian feathers: bioindicators of environmental pollution. Rev Environ Toxicol 5:203–311
Chao D-Y, Chen Y, Chen J, Shi S, Chen Z, Wang C, Danku JM, Zhao F-J, Salt DE (2014) Genome-wide association mapping identifies a new arsenate reductase enzyme critical for limiting arsenic accumulation in plants. PLoS Biol 12:e1002009
Chen LC, Lippmann M (2009) Effects of metals within ambient air particulate matter (PM) on human health. Inhal Toxicol 21:1–31
Chen CJ, Hsu LI, Wang CH, Shih WL, Hsu YH, Tseng MP et al (2005) Biomarkers of exposure, effect, and susceptibility of arsenic-induced health hazards in Taiwan. Toxicol Appl Pharmacol 206:198–206
Cullen WR (2014) Chemical mechanism of arsenic biomethylation. Chem Res Toxicol 27:457–461
Dart RC (2004) Medical toxicology. Lippincott Williams & Wilkins, Philadelphia
Davidson CI, Wiersma GB, Brown KW, Goold WD, Mathison TP, Reilly MT (1985) Airborne trace elements in Great Smoky Mountains, Olympic, and Glacier National Parks. Environ Sci Technol 19:27–35
Drouhot S, Raoul F, Crini N, Tougard C, Prudent AS, Druart C, Rieffel D, Lambert JC, Tête N, Giraudoux P, Scheifler R (2014) Responses of wild small mammals to arsenic pollution at a partially remediated mining site in Southern France. Sci Total Environ 470–471:1012–1022
Ducoff HS, Neal WB, Straube R, Jacobson L, Brues A (1948) Biological studies with arsenic; excretion and tissue localization. Proc Soc Exp Biol Med 69:548–554
Dudka I, Kossowska B, Senhadri H, Latajka R, Hajek J, Andrzejak R, Antonowicz-Juchniewicz J, Gancarz R (2014) Metabonomic analysis of serum of workers occupationally exposed to arsenic, cadmium and lead for biomarker research: a preliminary study. Environ Int 68:71–81
Duffus JH (2002) “Heavy metals” – a meaningless term? Pure Appl Chem 74:793–807
Duker AA, Carranza EJM, Hale M (2005) Arsenic geochemistry and health. Environ Int 31:631–641
EEA (2016) European Union emission inventory report 1990–2014 under the UNECE Convention on Long-range Transboundary Air Pollution (LRTAP). Europaen Environment Agency
Eisler R (1988) Arsenic hazards to fish, wildlife, and invertabrates: a synoptic review. Patuxent Wildlife Research Center, Laurel
EPA (1998) Integrated risk information system – arsenic, inorganic (CASRN 7440-38-2). Europaen Environment Agency
Erry BV, MacNair MR, Meharg AA, Shore RF (2000) Arsenic contamination in wood mice (Apodemus sylvaticus) and bank voles (Clethrionomys glareolus) on abandoned mine sites in southwest Britain. Environ Pollut 110:179–187
EU (2005) Directive 2004/107/EC of the European Parliament and of the Council of 15/12/2004 relating to arsenic, cadmium, mercury, nickel and polycyclic aromatic hydrocarbons in ambient air. Off J Eur Union 23:3–16
EU (2008) Directive 2008/50/EC of the European Parliament and of the Council of 21 May 2008 on ambient air quality and cleaner air for Europe. Off J Eur Communities 152:1–43
FDA (2011) Questions and answers regarding 3-nitro (roxarsone). In: U.S. Food Drug Administration website http://www.fda.gov/AnimalVeterinary/SafetyHealth/ProductSafetyInformation/ucm258313.htm. Accessed 30 Sep 2015
Finnegan PM, Chen W (2012) Arsenic toxicity: the effects on plant metabolism. Front Physiol 3:1–18
Firkin F (2014) Carcinogenic risk of retained arsenic after successful treatment of acute promyelocytic leukemia with arsenic trioxide: a cause for concern? Leuk Lymphoma 55:977–978
García-Sevillano MÁ, García-Barrera T, Gómez-Ariza JL (2015) Environmental metabolomics: biological markers for metal toxicity. Electrophoresis 36:2348–2365
Garcia-Vargas GG, Hernandez-Zavala A (1996) Urinary porphyrins and heme biosynthetic enzyme activities measured by HPLC in arsenic toxicity. Biomed Chromatogr 10:278–284
Gebel T (2000) Confounding variables in the environmental toxicology of arsenic. Toxicology 144:155–162
Geiger A, Cooper J (2010) Overview of airborne metal regulations, exposure limits, health effects and contemporary research. Cooper Environmental Services, Portland
Goede AA (1985) Mercury, selenium, arsenic and zinc in waders from the Dutch Wadden Sea. Environ Pollut 37:287–309
Goede AA, De Bruin M (1984) The use of bird feather parts as a monitor for metal pollution. Environ Pollut 8:281–298
Goede AA, Nygard T, de Bruin M, Steinnes E (1989) Selenium, mercury, arsenic and cadmium in the lifecycle of the dunlin, Calidris alpina, a migrant wader. Sci Total Environ 78:205–218
Graeme KA, Pollack CV (1998) Heavy metal toxicity, part I: arsenic and mercury. J Emerg Med 16:45–56
Grund SC, Hanusch K, Wolf HU (2005) Arsenic and arsenic compounds. In: Ullmann’s encyclopedia of industrial chemistry. Wiley, Weinheim, pp 31–34
Guerreiro CBB, Foltescu V, De Leeuw F (2014) Air quality status and trends in Europe. Atmos Environ 98:376–384. https://doi.org/10.1016/j.atmosenv.2014.09.017
Halder G, Mondal S, Paul SK, Roy B, Samanta G (2007) Chronic arsenic toxicity with and without excess supplementation of methionine on the performance and metabolizability of nutrients in layer chicken. Asian J Anim Sci 1:18–25
Hall SL, Fisher FM (1985) Lead concentrations in tissues of marsh birds: relationship of feeding habits and grit preference to spent shot ingestion. Bull Environ Contam Toxicol 35:1–8
Hammond CR (2004) The elements. In: Lide DR (ed) CRC Handbook of chemistry and physics, 86th edn. CRC Press, Boca Raton, pp 1–34
Harrisson JW, Packman EW, Abbott DD (1958) Acute oral toxicity and chemical and physical properties of arsenic trioxides. AMA Arch Ind Health 17:118–123
Haynes WM (2014) Handbook of chemistry & physics, 95th edn. CRC Press, Boca Raton
Henke KR (2009) Arsenic: environmental chemistry, health threats and waste treatment. John Wiley & Sons, Chichester
Hoffman DJ, Sanderson CJ, LeCaptain LJ, Cromartie E, Pendleton GW (1992) Interactive effects of arsenate, selenium, and dietary protein on survival, growth, and physiology in mallard ducklings. Arch Environ Contam Toxicol 22:55–62
IARC (2012) IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. A review of human carcinogens. Part C: arsenic, metals, fibres, and dusts. International Agency for Research on Cancer, Lyon
Islam MS, Awal MA, Mostofa M, Begum F, Khair A, Myenuddin M (2009) Effect of spirulina on biochemical parameters and reduction of tissue arsenic concentration in arsenic induced toxicities in ducks. Int J Poult Sci 8:69–74
Ismail A, Roberts RD (1992) Arsenic in small mammals. Environ Technol 13:1091–1095
IUPAC (1971) Nomenclature of inorganic chemistry. International Union of Pure and Applied Chemistry, London
Janssens E, Dauwe T, Bervoets L, Eens M (2001) Heavy metals and selenium in feathers of great tits (Parus major) along a pollution gradient. Environ Toxicol Chem 20:2815–2820
Jones FT (2007) A broad view of arsenic. Poult Sci 86:2–14
Kabata-Pendias A (2011) Trace elements in soils and plants, 4th edn. CRC Press, Boca Raton
Kabata-Pendias A, Pendias H (1999) Biogeochemistry of trace elements (in Polish, Biogeochemia pierwiastków śladowych), 2nd edn. Wydawnictwo Naukowe PWN, Warszawa
Kaise T, Watanabe S, Itoh K (1985) The acute toxicity of arsenobetaine. Chemosphere 14:1327–1332
Kalavathi S, Kumar AA, Reddy AG, Srilatha C, Reddy AR (2011) Sodium arsenite toxicity in broiler chicks and its amelioration: haemato-biochemical and pathological studies. Indian J Vet Pathol 35:171–176
Kar S, Maity JP, Jean J-S, Liu C-C, Liu C-W, Bundschuh J, Lu H-Y (2011) Health risks for human intake of aquacultural fish: arsenic bioaccumulation and contamination. J Environ Sci Health Part A 46:1266–1273
Karimi M-HS, Hassanpour M, Pourkhabbaz A-R, Błaszczyk M, Paluch J, Binkowski ŁJ (2016) Trace element concentrations in feathers of five Anseriformes in the south of the Caspian Sea. Iran Environ Monit Assess 188:1–7
Khan A, Hussain HI, Sattar A, Khan MZ, Abbas RZ (2014) Toxico-pathological aspects of arsenic in birds and mammals: a review. Int J Agric Biol 16:1213–1224
Kulp TR, Hoeft SE, Asao M, Madigan MT, Hollibaugh JT, Fisher JC et al (2008) Arsenic(iii) fuels anoxygenic photosynthesis in hot spring biofilms from Mono Lake, California. Science 321:967–970
Lasky T, Sun W, Kadry A, Hoffman MK (2004) Mean total arsenic concentrations in chicken 1989-2000 and estimated exposures for consumers of chicken. Environ Health Perspect 112:18–21
Lin C-J, Wu M-H, Hsueh Y-M, Sun SS-M, Cheng A-L (2005) Tissue distribution of arsenic species in rabbits after single and multiple parenteral administration of arsenic trioxide: tissue accumulation and the reversibility after washout are tissue-selective. Cancer Chemother Pharmacol 55:170–178
Liou S, Lung J, Chen Y, Yang T, Hsieh L, Chen C (1999) Increased chromosome-type chromosome aberration frequencies as biomarkers of cancer risk in a blackfoot endemic area increased chromosome-type chromosome aberration frequencies as biomarkers of cancer risk in a blackfoot endemic area. Cancer Res 59:1481–1484
López Alonso M, Benedito JL, Miranda M, Castillo C, Hernández J, Shore RF (2002) Cattle as biomonitors of soil arsenic, copper, and zinc concentrations in Galicia (NW Spain). Arch Environ Contam Toxicol 43:103–108
Lugo G, Cassady G, Palmisano P, Birmingham A (1969) Acute maternal arsenic. Am J Dis Child 117:328
Lunde G (1977) Occurrence and transformation of arsenic in the marine environment. Environ Health Perspect 19:47–52
Magellan K, Barral-Fraga L, Rovira M, Srean P, Urrea G, García-Berthou E, Guasch H (2014) Behavioural and physical effects of arsenic exposure in fish are aggravated by aquatic algae. Aquat Toxicol 156:116–124
Mandal BK, Suzuki KT (2002) Arsenic round the world: a review. Talanta 58:201–235
Marchiset-Ferlay N, Savanovitch C, Sauvant-Rochat MP (2012) What is the best biomarker to assess arsenic exposure via drinking water? Environ Int 39:150–171
Mateo R, Taggart MA, Meharg AA (2003) Lead and arsenic in bones of birds of prey from Spain. Environ Pollut 126:107–114
Meharg AA, Hartley-Whitaker J (2002) Arsenic uptake and metabolism in arsenic resistant and nonresistant plant species. New Phytol 154:29–43
Moriarty MM, Koch I, Reimer KJ (2012) Arsenic speciation, distribution, and bioaccessibility in shrews and their food. Arch Environ Contam Toxicol 62:529–538
Morita M, Edmonds JS (1992) Determination of arsenic species in biological and environmental samples (Technical Report). Pure Appl Chem 64:575–590
Mukhopadhyay R, Rosen BP, Phung LT, Silver S (2002) Microbial arsenic: from geocycles to genes and enzymes. FEMS Microbiol Rev 26:311–325
Nachman KE, Graham JP, Price LB, Silbergeld EK (2005) Arsenic: a roadblock to potential animal waste management solutions. Environ Health Perspect 113:1123–1124
Nachman KE, Baron PA, Raber G, Francesconi KA, Love DC (2013) Arsenic levels in chicken. Environ Health Perspect 121:A267
NAS (1977) Biologic effects of arsenic on plants and animals. In: Arsenic: medical and biological effects of environmental pollutants. National Academy of Sciences, Washington, pp 117–172
Newcombe C, Raab A, Williams PN, Deacon C, Haris PI, Meharg AA, Feldmann J (2010) Accumulation or production of arsenobetaine in humans? J Environ Monit 12:832–837
Nordberg GF, Fowler BA, Nordberg M, Friberg LT (2007) Handbook on the toxicology of metals. Elsevier, London
Nordstrom DK (2002) Public health: worldwide occurrences of arsenic in ground water. Science 296:2143–2145
Norman NC (1998) Chemistry of arsenic, antimony and bismuth. Thomson Science, London
NRCC (1978) Effects of arsenic in the Canadian environment. National Research Council Canada, Ottawa
Ohnishi K, Yoshida H, Shigeno K, Nakamura S, Fujisawa S, Naito K, Shinjo K, Fujita Y, Matsui H, Takeshita A, Sugiyama S, Satoh H, Terada H, Ohno R (2000) Prolongation of the QT interval and ventricular tachycardia in patients treated with arsenic trioxide for acute promyelocytic leukemia. Ann Intern Med 133:881–885
Peles JD, Barrett GW (1997) Assessment of metal uptake and genetic damage in small mammals inhabiting a Fly Ash Basin. Bull Environ Contam Toxicol 59:279–284
Pereda-Solis ME, Martinez-Guerrero JH, Toca-Ramirez JA (2012) Detection of zinc, lead, cadmium and arsenic in dabbling ducks from Durango, Mexico. Asian J Anim Vet Adv 7:761–766
Petrick JS, Ayala-Fierro F, Cullen WR, Carter DE, Vasken Aposhian H (2000) Monomethylarsonous acid (MMA(iii)) is more toxic than arsenite in Chang human hepatocytes. Toxicol Appl Pharmacol 163:203–207
Rahman FA, Allan DL, Sadowsky MJ (2004) Arsenic availability from Chromated Copper Arsenate (CCA)–treated wood. J Environ Qual 33:173–180
Rahman MA, Hasegawa H, Lim RP (2012) Bioaccumulation, biotransformation and trophic transfer of arsenic in the aquatic food chain. Environ Res 116:118–135
Ravenscroft P, Brammer H, Richards K (2009) Arsenic pollution: a global synthesis. Willey-Blackwell, Chichester
Riedel GF, Sanders JG, Osman RW (1989) The role of three species of benthic invertebrates in the transport of arsenic from contaminated estuarine sediment. J Exp Mar Bio Ecol 134:143–155
Rosen BP (2002) Biochemistry of arsenic detoxification. FEBS Lett 529:86–92
Sakurai T (2003) Biomethylation of arsenic is essentially detoxicating event. J Health Sci 49:171–178
Sánchez-Virosta P, Espín S, García-Fernández AJ, Eeva T (2015) A review on exposure and effects of arsenic in passerine birds. Sci Total Environ 512–513:506–525
Saunders JR, Hough C, Knopper LD, Koch I, Reimer KJ (2011) Arsenic transformations in terrestrial small mammal food chains from contaminated sites in Canada. J Environ Monit 13:1784–1792
Schaller J, Weiske A, Mkandawire M, Dudel EG (2010) Invertebrates control metals and arsenic sequestration as ecosystem engineers. Chemosphere 79:169–173
Schoepp-Cothenet B, Duval S, Santini JM, Nitschke W (2009) Comment on “Arsenic(iii) fuels anoxygenic photosynthesis in hot spring biofilms from Mono Lake, California”. Science 323:583
Schwartze EW (1922) The so-called habituation to “arsenic:” variation in the toxicity of arsenious oxide. J Pharmacol Exp Ther 20:181–203
Sharaf R, Khan A, Khan MZ, Hussain I, Abbas RZ, Gul ST et al (2013) Arsenic induced toxicity in broiler chicks and its amelioration with ascorbic acid: clinical, hematological and pathological study. Pak Vet J 33:277–281
Sharma RP, Shupe JL (1977) Lead, cadmium, and arsenic residues in animal tissues in relation to those in their surrounding habitat. Sci Total Environ 7:53–62
Shen ZX, Chen GQ, Ni JH, Li XS, Xiong SM, Qiu QY et al (1997) Use of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia (APL) II. Clinical efficacy and pharmacokinetics in relapsed patients. Blood 89:3354–3360
Smedley P, Kinniburgh D (2002) A review of the source, behaviour and distribution of arsenic in natural waters. Appl Geochem 17:517–568
Smith GJ, Rongstad OJ (1982) Small mammal heavy metal concentrations from mined and control sites. Environ Pollut 28:121–134
Solo-Gabriele H, Sakura-Lemessy DM, Townsend T, Du-bey B, Jambeck J (2003) Quantities of arsenic within the state of Florida. Report #03–06. Florida Center for Solid and Hazardous Waste Management, Gainesville
Stevens JT, DiPasquale LC, Farmer JD (1979) The acute inhalation toxicology of the technical grade organoarsenical herbicides, cacodylic acid and disodium methanearsonic acid; a route comparison. Bull Environ Contam Toxicol 21:304–311
Stolz JF, Basu P, Santini JM, Oremland RS (2006) Arsenic and selenium in microbial metabolism. Annu Rev Microbiol 60:107–130
Strincone M, Fino A, Cattani G, Catrambone M, Pirrone N (2013) Emissions, air concentrations and atmospheric depositions of arsenic, cadmium, lead and nickel in Italy in the last two decades: a review of recent trends in relation to policy strategies adopted locally, regionally and globally. E3S Web Conf 1:38003
Styblo M, Del Razo LM, Vega L, Germolec DR, LeCluyse EL, Hamilton GA et al (2000) Comparative toxicity of trivalent and pentavalent inorganic and methylated arsenicals in rat and human cells. Arch Toxicol 74:289–299
Tamaki S, Frankenberger WT Jr (1992) Environmental biochemistry of arsenic. In: Ware GW (ed) Reviews of environmental contamination and toxicology. Springer, New York, pp 79–110
TOXNET (2015) Toxicology Data Network. NIH U.S. National Library of Medicine. http://toxnet.nlm.nih.gov/. Accessed 4 Oct 2015
Turpeinen R, Pantsar-Kallio M, Häggblom M, Kairesalo T (1999) Influence of microbes on the mobilization, toxicity and biomethylation of arsenic in soil. Sci Total Environ 236:173–180
USGS (1950) 1950 Minerals yearbook arsenic. U.S. Geological Survey
USGS (1955) 1955 Minerals yearbook arsenic. U.S. Geological Survey
USGS (1960) 1960 Minerals yearbook arsenic. U.S. Geological Survey
USGS (1965) 1965 Minerals yearbook minor metals. U.S. Geological Survey
USGS (1970) 1970 Minerals yearbook minor metals. U.S. Geological Survey
USGS (1975) 1975 Minerals yearbook minor metals. U.S. Geological Survey
USGS (1980) 1980 Minerals yearbook minor metals. U.S. Geological Survey
USGS (1985) 1985 Minerals yearbook other metals. U.S. Geological Survey
USGS (1990) 1990 Minerals yearbook arsenic. U.S. Geological Survey
USGS (1995) 1995 Minerals yearbook arsenic. U.S. Geological Survey
USGS (2000) 2000 Minerals yearbook arsenic. U.S. Geological Survey
USGS (2006) 2005 Minerals yearbook arsenic. U.S. Geological Survey
USGS (2011) 2010 Minerals yearbook arsenic. U.S. Geological Survey
USGS (2015) 2013 Minerals yearbook arsenic. U.S. Geological Survey
USGS (2016) Mineral commodity summary arsenic. U.S. Geological Survey
Uthus AO (2003) Arsenic essentially: a role affecting methionine metabolism. J Trace Elem Exp Med:345–355
Vahidnia A, van der Straaten RJHM, Romijn F, van Pelt J, van der Voet GB, de Wolff FA (2007a) Arsenic metabolites affect expression of the neurofilament and tau genes: an in-vitro study into the mechanism of arsenic neurotoxicity. Toxicol In Vitro 21:1104–1112
Vahidnia A, van der Voet GB, de Wolff FA (2007b) Arsenic neurotoxicity – a review. Hum Exp Toxicol 26:823–832
Vahter M (2000) Genetic polymorphism in the biotransformation of inorganic arsenic and its role in toxicity. Toxicol Lett 112–113:209–217
Vahter M, Marafante E (1983) Intracellular interaction and metabolic fate of arsenite and arsenate in mice and rabbits. Chem Biol Interact 47:29–44
Vallee BL, Ulmer DD, Wacker WEC (1960) Arsenic toxicology and biochemistry. Arch Ind Health 21:132–151
Ventura-Lima J, Bogo MR, Monserrat JM (2011) Arsenic toxicity in mammals and aquatic animals: a comparative biochemical approach. Ecotoxicol Environ Saf 74:211–218
Vodela JK, Renden JA, Lenz SD, McElhenney WH, Kemppainen BW (1997) Drinking water contaminants (arsenic, cadmium, lead, benzene, and trichloroethylene). 1. Interaction of contaminants with nutritional status on general performance and immune function in broiler chickens. Poult Sci 76:1474–1492
Welch AH, Westjohn DB, Helsel DR, Wanty RB (2000) Arsenic in ground water of the United States: occurrence and geochemistry. Ground Water 38:589–604
WHO (2001) Environmental health criteria 224: arsenic and arsenic compunds. World Health Organization, Geneva
WHO (2010) Exposure to arsenic: a major public health concern. World Health Organization, Geneva
Winship KA (1984) Toxicity of inorganic arsenic salts. Adverse Drug React Acute Poisoning Rev 3:129–160
Woolson EA (1975) Arsenical pesticides. ACS Symp Ser 7:126–136
WVDL (2015) Normal range values for WVDL toxicology. https://www.yumpu.com/en/document/view/52919318/normal-range-values-for-wvdl-toxicology. Accessed 28 April 2015
Xu H, Allard B, Grimvall A (1991) Effects of acidification and natural organic materials on the mobility of arsenic in the environment. Water Air Soil Pollut 57–58:269–278
Yamauchi H, Yamamura Y (1985) Metabolism and excretion of orally administrated arsenic trioxide in the hamster. Toxicology 34:113–121
Zakharyan R, Wu Y, Bogdan GM, Aposhian HV (1995) Enzymatic methylation of arsenic compounds: assay, partial purification, and properties of arsenite methyltransferase and monomethylarsonic acid methyltransferase of rabbit liver. Chem Res Toxicol 8:1029–1038
Zhao FJ, Ma JF, Meharg AA, McGrath SP (2009) Arsenic uptake and metabolism in plants. New Phytol 181:777–794
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Binkowski, Ł.J. (2019). Arsenic, As. In: Kalisińska, E. (eds) Mammals and Birds as Bioindicators of Trace Element Contaminations in Terrestrial Environments. Springer, Cham. https://doi.org/10.1007/978-3-030-00121-6_13
Download citation
DOI: https://doi.org/10.1007/978-3-030-00121-6_13
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-00119-3
Online ISBN: 978-3-030-00121-6
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)