Abstract
Vascular endothelial growth factors (VEGFs) constitute a family of polypeptides regulating blood and lymphatic vessel development. VEGFs bind to type V receptor tyrosine kinases (RTKs), VEGFR-1, VEGFR-2, and VEGFR-3, but also bind directly to neuropilins and heparan sulphate glycosaminoglycans (HSPG), or indirectly to co-receptors such integrins and semaphorins. VEGFR activation results from ligand-induced dimerisation, which is mediated by the extracellular receptor domain (ECD). Recent studies established that dimerisation is necessary, but not sufficient, for receptor activation, since it was shown that only distinct orientations of receptor monomers give rise to active receptor dimers that are capable to instigate transmembrane signalling. Additional complexity in VEGFR signalling arises from association with specific co-receptors, which is determined by ligand- and ECD-specific interaction domains.
In the following, the role of the different extracellular subdomains in VEGFR activation and signalling is discussed. We give an overview of the mechanistic concepts arising from recent structural studies that led to the development of novel allosteric receptor inhibitors and discuss their possible application in therapies aimed at pathological angiogenesis.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Biology of VEGF Family Growth Factors and Their Receptors
1.1 Introduction to VEGF
Vascular endothelial growth factor, VEGF, was originally discovered as vascular permeability factor, VPF, a tumour-secreted protein that promotes vascular leakage (Senger et al. 1983). In the meantime it became clear that VPF exerts a biological activity attributable to VEGFs which comprise a family of polypeptide growth factors encoded by five mammalian genes (VEGF-A, VEGF-B, VEGF-C, VEGF-D and placenta growth factor, PlGF). These polypeptides regulate blood and lymph vessel formation during embryonic development, in wound healing, and maintain vessel homeostasis in adult organisms by mediating endothelial cell survival, migration, proliferation and differentiation. VEGFs specifically interact with hematopoietic and endothelial precursor cells such as angioblasts, as well as with differentiating and mature endothelial cells. Excess or reduced production of VEGF leads to an imbalance in blood or lymphatic vessel homeostasis and is the cause for many human diseases.
Whilst mammalian VEGF-A and PlGF are required for blood vessel formation, VEGF-C and VEGF-D regulate the formation of lymphatic vessels (Jussila and Alitalo 2002; Takahashi and Shibuya 2005). VEGF-B, on the other hand, is important in metabolic regulation, for instance, in cellular fatty acid uptake (Hagberg et al. 2012, 2013). Additionally, the orf family parapoxviruses encode VEGF-A ortologues collectively called VEGF-E, which show a high degree of structural identity with VEGF-A (Lyttle et al. 1994; Wise et al. 2003; Mercer et al. 2002). Despite only 25–35 % amino acid sequence identity with VEGF-A, they bind with comparable affinity to VEGFR-2 (Wise et al. 1999; Ogawa et al. 1998; Meyer et al. 1999). VEGF-E family members lack the heparin-binding domain encoded by exons 6 and 7 of VEGF-A and vary in their ability to bind neuropilins (Ogawa et al. 1998; Wise et al. 1999). Several VEGF-like proteins, collectively called VEGF-F, have also been isolated from snake venoms with biological activity similar to VEGF-A (Yamazaki et al. 2003; Takahashi et al. 2004; Komori et al. 1999). Two of these VEGFs, Vammin and VR-1 isolated from Vipera ammodytes ammodytes and Daboia russelli russelli, respectively, share about 50 % amino acid sequence identity with VEGF-A and strongly stimulate proliferation of vascular endothelial cells in vitro. In vivo, these proteins induce strong hypotension similar to VEGF-A, which is why their biological function is probably aimed at paralysing prey animals upon attack by a snake.
The complexity of VEGF biology is further increased by alternative splicing and proteolytic processing which gives rise to a wide variety of isoforms with distinct biological activities (Ladomery et al. 2007; Lee et al. 2005; Cébe-Suarez et al. 2006b; Harper and Bates 2008; Perrin et al. 2005). All VEGF isoforms contain exons 1–5 and either exon 8a or 8b. A 26-amino acid signal sequence (exon 1 plus 4 amino acids of exon 2) is cleaved off during biosynthesis. The VEGFR-1 and VEGFR-2 binding domain consists of amino acids 1–109, and the VEGFR interaction sites are located at opposite poles of the dimeric molecule (Muller et al. 1997; Wiesmann et al. 1997). Exons 6–8 encode basic sequences that mediate binding to heparan sulphate glycosaminoglycans (HSPG). Exon 6a, present in VEGF-A 206, 189, 162, 145 and partially in VEGF-A183, is highly basic and confers binding to HSPG and other extracellular matrix material. Exon 6b has so far only been identified in the less well-characterised VEGF-A162 and in the longest isoform, VEGF-A206. VEGF-A 165, 183, 189 and 206 contain an additional sequence encoded by exon 7 that also confers HSPG binding due to its basic properties and thus limits ligand diffusibility and therefore allows for spatiotemporal signalling in tissues (Lundkvist et al. 2007). All VEGF-As, except VEGF-A148, end with either exon 8a or 8b. Alternative splicing of exon 8 results in the formation of two families of proteins of identical length but differing in the carboxy-terminal six amino acids (Bates et al. 2002). Members of the VEGFxxxb family identified so far include VEGF-A121b, VEGF145b, VEGF165b, VEGF-A189b, VEGF-A183b and unspecified larger isoforms. It is now clear that proteins of the VEGFxxxb family make up a major fraction of VEGF-A in most normal tissues (Bates et al. 2002; Nowak et al. 2008; Bevan et al. 2008; Perrin et al. 2005), whereas their expression is negligible in cancer cells (Woolard et al. 2004; Pritchard-Jones et al. 2007; Varey et al. 2008). At the molecular level, the anti-angiogenic effects of the VEGFxxxb proteins was ascribed to reduce signalling via VEGFR-2 (Woolard et al. 2004; Kawamura et al. 2008), in particular due to their inability to interact with neuropilin 1, a co-receptor modulating VEGFR-2 trafficking and activation (Cébe-Suarez et al. 2006a; Woolard et al. 2004; Ballmer-Hofer et al. 2011).
The complex interplay of VEGF family proteins with their receptors and co-receptors is vital for shaping and maintaining the vasculature in higher organisms. Correct spatiotemporal distribution of the individual VEGF isoforms requires not only exact temporal expression but depends also on the distinct affinities of these proteins for components of the extracellular matrix (ECM).
1.2 Structure–Function Relationship of VEGF and VEGF Receptors
1.2.1 Receptor Specificity of VEGFs
The biological activity of VEGF family polypeptides results from binding to three type V receptor tyrosine kinases (RTKs), VEGFR-1 (Flt-1), VEGFR-2 (KDR/Flk-1) and VEGFR-3 (Flt-4) (Shibuya et al. 1990; Terman et al. 1991; Pajusola et al. 1992) and to co-receptors such as neuropilin-1 and neuropilin-2 (NRP1, NRP2) (Neufeld et al. 2002b), heparan sulphate proteoglycans (Tessler et al. 1994), or indirectly via the cognate VEGF receptors to integrins (Drake et al. 1992; Senger et al. 1997) or ephrins (Sawamiphak et al. 2010).
Some VEGFs interact with multiple receptors, whilst others show very specific receptor-binding properties. PlGF and VEGF-B are specific for VEGFR-1 (Errico et al. 2004; Olofsson et al. 1998); VEGF-E binds VEGFR-2 (Wise et al. 2003; Mercer et al. 2002; Lyttle et al. 1994), and VEGF-C and VEGF-D bind VEGFR-2 and VEGFR-3 (Jussila and Alitalo 2002; Takahashi and Shibuya 2005). VEGF-A proteins bind to both VEGFR-1 and VEGFR-2 and some of their splice variants display co-receptor specificity for HSPG and neuropilins (Grünewald et al. 2010). Exons 2–5 determine binding specificity for VEGFRs 1–3, whilst exons 6 and/or 7 and 8 determine co-receptor binding. Additionally, VEGFs can simultaneously bind two distinct receptors such as, e.g. VEGFR-2 and neuropilin, even when these receptors are expressed separately on adjacent cells (Cébe-Suarez et al. 2008). This might be required for promoting endothelial cell migration and cell guidance, for instance, when vessels form along tracks defined by neural cells (Kawasaki et al. 1999; Neufeld et al. 2002a) or during endothelial tip cell guidance (Gerhardt et al. 2003, 2004).
1.2.2 Structural Analysis of VEGF Binding to VEGFR-1, VEGFR-2 and VEGFR-3
Signalling by VEGFRs is initiated upon binding of covalently linked ligand dimers to the extracellular receptor domain (Fig. 3.2). This interaction promotes receptor homo- and heterodimerisation followed by activation of the intracellular kinase domain. The receptor ECD consists of seven immunoglobulin homology domains (Ig-domains). Recent low-resolution structural analyses have identified how VEGFs promote dimerisation of the ECD (Ruch et al. 2007; Kisko et al. 2011). Furthermore, structural data revealed how ligands bind to Ig-domains 2 and 3 (D23) of VEGFR-2 (Brozzo et al. 2012; Leppänen et al. 2010) and domains 1–3 (D1-3) of VEGFR-3 (Leppänen et al. 2013), whereas domain 2 (D2) of VEGFR-1 seems to be sufficient for ligand binding (Christinger et al. 2004; Starovasnik et al. 1999; Wiesmann et al. 1997) (Fig. 3.1). Receptor dimer structure is further modified by homotypic receptor contacts in Ig-domains 4 to 7 (D4-7). These contacts increase the Gibbs free energy (ΔG) of ligand binding to VEGFR-2 by 1.5 kcal/mol. Contrary to this, the ΔG for ligand binding to VEGFR-1 and VEGFR-3 was decreased, resulting in thermodynamic stabilisation by approximately 1.5 kcal/mol (unpublished data, Ballmer-Hofer et al.). VEGFR-2 D4 and D7 are indispensable for receptor signalling, and the observed homotypic contacts were shown to allosterically regulate VEGFR-2 activity (Hyde et al. 2012). Similar structural observations were made by the Schlessinger Lab for the related type III c-Kit and PDGF receptors where homotypic contacts were observed in Ig-domain 5 (Yuzawa et al. 2007; Yang et al. 2008). It was also shown recently that the transmembrane receptor domain (TMD) plays a critical role in orienting receptor monomers in active dimers; this apparently results from intramembrane helix dimerisation (Dell’Era Dosch and Ballmer-Hofer 2009). Taken together it is clear that RTK activation entails specific orientation of receptor monomers in active dimers, which results from highly specific ligand-induced structural rearrangement of the ECD (for model see Fig. 3.2).
Model for VEGFR activation. Red arrows depict putative sites of homotypic interactions targeted by DARPins or antibodies acting as allosteric inhibitors, for details see Hyde et al. (2012)
1.2.3 Activation of VEGF Receptors
Upon ligand binding, RTK activation entails phosphorylation of specific tyrosine residues located in the intracellular juxtamembrane domain, the kinase domain, the kinase insert domain and the carboxy-terminal tail of the receptor. Subsequent interaction between VEGFRs and downstream signalling effectors is mediated through Src homology-2 (SH-2) and phosphotyrosine-binding (PTB) domains (reviewed in Schlessinger and Lemmon 2003). Since signalling by VEGF receptors has been reviewed comprehensively in recent review articles (Cébe-Suarez et al. 2006b; Shibuya and Claesson-Welsh 2005; Koch and Claesson-Welsh 2012), the following only refers to key hallmarks of VEGFR-2 activation.
Y951, Y1054, Y1059, Y1175 and Y1214 were identified as the most prominent phosphorylation sites of VEGFR-2 (Matsumoto et al. 2005). Y1054 and Y1059 are located in the activation loop and were classified as autophosphorylation sites important for the catalytic activity of the receptor kinase (Kendall et al. 1999). Site-directed mutagenesis led to the identification of Y801 and Y1175 as binding sites of phospholipase C-γ (PLC-γ) (Cunningham et al. 1997). Phosphorylation and activation of PLC-γ gives rise to diacylglycerol and inositol trisphosphate which stimulate protein kinase C (PKC) (Nishizuka 1984). Mitogenic signalling by VEGFR-2 is Ras independent and mediated by PKC via ERK kinases (Takahashi et al. 1999). VEGF-induced endothelial cell migration is mediated by the adaptor protein ‘T cell-specific adaptor’ (TSAd) also called VRAP (Matsumoto et al. 2005; Wu et al. 2000). Upon binding to Y951 of VEGFR-2, this adapter is phosphorylated and recruits and activates Src tyrosine kinase followed by actin reorganisation and cell migration (Matsumoto et al. 2005). The adapter protein Shb also binds to phosphorylated Y1175 and leads to phosphoinositide-3-kinase (PI 3-kinase)-mediated cytoskeleton reorganisation as well as activation of focal adhesion kinase (FAK) (Holmqvist et al. 2004). Cell migration and capillary formation are regulated by VEGF through Gab1, which acts as an adaptor for Grb2, PI 3-kinase and the tyrosine phosphatase SHP-2 (Laramee et al. 2007; Dance et al. 2006). VEGF-induced actin remodelling is also triggered through sequential activation of the small GTPase Cdc42 and stress-activated protein kinase (SAPK/p38) resulting from phosphorylation of VEGFR-2 at Y1214 (Lamalice et al. 2004). This leads to phosphorylation and release of heat-shock protein (HSP) 27. Early molecular events in cytoskeleton reorganisation include recruitment of the adaptor protein Nck and the Src family kinase Fyn to VEGFR-2 and trigger phosphorylation of p21-activated protein kinase-2 (PAK2) and activation of Cdc42 and p38 MAPK (Lamalice et al. 2006). An additional important function of VEGF is cell survival signalling via activation of PI 3-kinase and phosphorylation of Akt (Gerber et al. 1998). Finally, signalling by VEGFR-2 is important for endothelial cell specification, a process that requires activation of the Ras-ERK pathway (Kawasaki et al. 2008).
2 VEGFR-2 as Part of a Signalling Platform
As described above, the polypeptides encoded by the three VEGFRs and five VEGF genes, including all isoforms, form the VEGF signalling network. In addition, VEGFRs require additional co-receptors to fulfil their diverse functions as, for example, sprouting angiogenesis during development or regulation of vessel permeability. These co-receptors interact either directly or indirectly with the receptor or are associated together with VEGFRs in specific membrane subdomains such as adherens junctions. Here we summarise the features of the most prominent of these co-receptors and describe their influence on VEGFR signalling and angiogenesis.
2.1 Neuropilins (NRPs)
Neuropilins are type I transmembrane proteins that consists of two CUB (a1a2), two discoidin (b1b2), one MAM domain, a transmembrane helix and a short cytoplasmic tail with a PDZ-binding motif. They were originally identified as receptors for the axonal chemorepellent semaphoring III (He and Tessier-Lavigne 1997; Kolodkin et al. 1997). Subsequently, it was shown that a specific binding motif of NRP1 interacts with some isoforms of VEGF-A (Whitaker et al. 2001). NRPs are expressed in various tissues but especially in axons and endothelial cells where they play a similar role in axon and vessel guidance (Adams and Eichmann 2010). VEGF-A165a binds simultaneously to VEGFR-2 and NRP1 thereby forming multimeric VEGFR-2/NRP1/VEGF-A complexes. These complexes are formed either in cis (VEGFR-2 and NRP1 expressed in the same cell) or in trans (VEGFR-2 and NRP1 expressed in neighbouring cells) configuration. VEGF-A165a binds to NRP1 via its C-terminal tail which is encoded by exon 8a. VEGF isoforms with a C-terminal tail encoded by exon 8b do not bind to NRP1 (Cébe-Suarez et al. 2006a). Interestingly, VEGF-A121 also binds directly to NRP1; however, this does not lead to complex formation between NRP1 and VEGFR-2, presumably due to steric reasons (Pan et al. 2007b). Recruitment of NRP1 as a co-receptor in VEGFR-2/NRP1 expressing endothelial cells by VEGF-A165a leads to increased p38 activation as compared to VEGF-A165b (Kawamura et al. 2008). This effect is mediated by the C-terminal PDZ domain of NRP1 (Ballmer-Hofer et al. 2011). So far, synectin/GIPC is the only known interaction partner of this PDZ-binding motif (Cai and Reed 1999). GIPC is a small protein with a single PDZ domain that exists as monomer or trimer in the cytoplasm (Kedlaya et al. 2011). Early after internalisation, GIPC binds, together with myosin VI, to activated receptors (Naccache et al. 2006). Myosin VI is an atypical minus-end-directed myosin that uses the F-actin network underneath the plasma membrane to drive cargo-loaded vesicles towards the cell body. The PDZ-binding motif of NRP1 promotes the association with Rab5, Rab4 and Rab11 vesicles, which together form the so-called slow recycling pathway. VEGFR-2, which is normally not recycled through Rab11 vesicles, uses this recycling pathway when bound to NRP1 via VEGF-A165a (Ballmer-Hofer et al. 2011). Many receptors use GIPC as adaptor protein for internalisation and recycling, but the modes of interaction are different. VEGFR-2 and TrkA (Varsano et al. 2006) bind GIPC via their co-receptors NRP1 and APPL1, respectively, whilst the beta 2 adrenergic receptor binds GIPC directly (Hu et al. 2003).
The in vivo function of NRP1 was studied in mice and zebrafish. Interestingly, complete knockout or truncation of the C-terminal tail of NRP1 gave rise to different phenotypes. Complete knockout mice died at E12.5 with a severely disorganised vascular network (Kawasaki et al. 1999). In contrast, mice lacking only the cytoplasmic tail of NRP1 were viable with normal developmental angiogenesis, but impaired arteriogenesis and increased frequency of artery-vein crossing in the retina (Fantin et al. 2011; Lanahan et al. 2013). A possible explanation for this discrepancy might be that cytoplasmic tail-truncated NRP1 is still capable to fulfil its trans signalling function. On the other hand, if NRP1, GIPC or myosin VI were ablated in zebrafish, fish clear vascular defects were observed suggesting that the VEGFR-2/NRP1/GIPC/myosin VI complex plays an important role in arteriogenesis in vivo (Chittenden et al. 2006; Wang et al. 2006; Lanahan et al. 2010). In addition, blocking NRP1 function with antibodies enhanced the anti-angiogenic activity of anti-VEGF antibodies in blocking tumour growth; identifying NRP1 is a potential target in cancer treatment (Pan et al. 2007a).
2.2 Ephrin-B2
Ephrin-B2 is a cell surface transmembrane ligand for Eph receptors, a large family of RTKs. The binding occurs between adjacent cells in trans leading to contact-dependent bidirectional signalling. Mice carrying inactive ephrin-B2 showed compromised vasculogenesis and angiogenesis (Adams et al. 1999). Ephrin-B2 binds to EPHA4, EPHA3 and EPHB4. In the vasculature, ephrin-B2 is expressed in arterial endothelial cells, whereas EphB4, one of the cognate receptors, is predominantly expressed in the venous endothelium. Together with VEGFR-2 and VEGFR-3, they regulate sprouting of new vessels. Similar to NRP1, ephrin-B2 carries a carboxy-terminal PDZ-binding motif. Mutant mice lacking this motif show a reduction in the number of tip cells with fewer filopodial extensions at the vascular front in the developing mouse retina. In addition, they show decreased tumour vascularisation and tumour growth (Wang et al. 2010; Sawamiphak et al. 2010). VEGFR-2 and VEGFR-3 do not directly interact with ephrin-B2, but rather, they bind via the cytoplasmic proteins Dab2 and PAR-3. The PDZ-binding motif of ephrin-B2 thus binds to PAR-3, which associates with Dab2 and VEGFR-2 and VEGFR-3. PAR-3 and DAB2 are indispensable for rapid VEGFR-2 turnover at the angiogenic front, a process that is further negatively regulated by atypical protein kinase C (aPKC). aPKC activity is typically low in developing sprouts but high in more mature vessels (Nakayama et al. 2013). Interestingly, similar to GIPC, Dab2 binds to myosin VI (Spudich et al. 2007), and it is therefore likely that the ephrin-B2/PAR3/Dab2/VEGFR complex relies on the same internalisation mechanism as the VEGFR-2/NRP1/GIPC complex. Ephrin-B2 and NRP1 might therefore be functionally redundant in vivo, explaining the mild phenotype observed in mice expressing PDZ-binding motif deleted NRP1.
2.3 VE-Cadherin
VE-cadherin is the main driver of adherens junction formation in endothelial cells. Adherens junctions appear in more mature vessels where they regulate vascular growth and permeability. VE-cadherin connects endothelial cells in vessels by a calcium-dependent homophilic interaction, and β-catenin, p120-catenin and plakoglobin are the direct link to the cytoskeleton. Many additional proteins that form a molecular or functional interaction with VE-cadherin are known (reviewed by Giannotta et al. 2013). Targeted deletion or C-terminal truncation of the VE-cadherin gene in mice leads to embryonic death at E9.5 due to increased endothelial apoptosis. In these mice VEGF-A is not able to activate Akt, an important kinase involved in survival signalling, and this apparently results from the fact that VEGFR-2 was unable to form a complex with VE-cadherin (Carmeliet et al. 1999). Association of VEGFR-2 with VE-cadherin depends on β-catenin. Activation of VEGFR-2 by VEGF is blocked in this complex as a consequence of the presence of the phosphatase DEP-1 that dephosphorylates VEGFR-2 (Grazia Lampugnani et al. 2003). In addition, VE-cadherin also interferes with VEGFR-2 internalisation and thereby inhibits signalling from internal cellular compartments (Lampugnani et al. 2006). VE-PTP is a second phosphatase which is localised at and therefore stabilises adherens junctions by dephosphorylating VEGFR-2 and VE-cadherin. VE-PTP is also expressed in stalk cells during angiogenesis, maintaining VEGFR-2 at adherens junctions in a quiescent state (Hayashi et al. 2013). In contrast, low expression of VE-PTP in tip cells results in high receptor turnover and signal output by VEGFR-2. The effect of these adherens junction phosphatases thus leads to VE-cadherin dephosphorylation and the stabilisation of adherens junctions, which can be reverted by high amounts of VEGF (Esser et al. 1998). In vivo, the dissociation of VE-PTP from VE-cadherin is induced by both leukocyte binding or by VEGF; in both cases this results in the opening of endothelial cell contacts and leukocyte extravasation (Broermann et al. 2011).
2.4 Dopamine Receptor D2
Dopamine is well known as a neurotransmitter in the nervous system. Nevertheless, a significant amount of dopamine circulates in the bloodstream where it exerts a variety of effects on the cardiovascular system. Dopamine receptors are also expressed on endothelial cells (Ricci et al. 1994; Bacic et al. 1991), and Basu et al. showed that dopamine thereby inhibits VEGF-induced vascular hyperpermeability and angiogenesis. Dopamine blocks autophosphorylation of VEGFR-2 following VEGF administration and leads to increased internalisation of VEGFR-2 (Basu et al. 2001). Consequently, high doses of dopamine or related agonists interfere with malignant tumour growth by blocking tumour vascularisation whereas antagonists accelerate wound healing (Shome et al. 2011). Dopamine receptor D2 and VEGFR-2 colocalise at the plasma membrane of endothelial cells, and dopamine recruits the cytoplasmic phosphatase SHP-2 to this complex. VEGF promotes activation of VEGFR-2 and thereby leads to SHP-2 activation resulting in dephosphorylation of VEGFR-2 at Y951, Y996 and Y1059, but not at Y1175. Decreased phosphorylation of VEGFR-2 at Y951 was shown to block VEGF-induced cell migration (Sinha et al. 2009).
2.5 CD146
CD146 (MUC18) is a cell adhesion molecule of the immunoglobulin superfamily consisting of five extracellular Ig-domains, a transmembrane helix and a cytoplasmic tail. It was originally identified as a marker for melanomas with poor prognosis (Lehmann et al. 1989). Later, it was shown that it is expressed on endothelial cells and that it promotes tumour growth, angiogenesis and metastasis. In zebrafish, knockdown of CD146 resulted in angiogenic sprouting defects in intersegmental vessels and reduced tumour angiogenesis (So et al. 2010). In addition, CD146 plays an important role in a variety of biological and pathological processes (reviewed by Wang and Yan 2013). It was shown recently that VEGFR-2 directly interacts with CD146 in endothelial cells and that activation with VEGF leads to increased p38/IKK/NF-κB signalling and high Akt activity. Enhanced activation of p38 was also described for the VEGFR-2/NRP1 complex (Kawamura et al. 2008). In vitro, the VEGFR-2/CD146 complex mediates increased cell migration and tube formation, and mice lacking CD146 in endothelial cells showed reduced vascular density in Matrigel implanted plugs (Jiang et al. 2012). CD146 is therefore an attractive target for antibody-based cancer therapy. ABX-MA1, a fully humanised anti-CD146 antibody, reduced tumour growth in an in vivo model and disrupted spheroid formation of CD146-expressing melanoma cells and their ability to attach to primary endothelial cells such as HUVEC in vitro (Mills et al. 2002). Jiang et al. used an antibody (AA98) that blocks dimer formation, thereby inhibiting downstream signalling pathways. This antibody also reduces tumour growth in vivo, and in combination with bevacizumab, a VEGF-neutralising antibody, this activity is further enhanced (Jiang et al. 2012). The anti-CD146 antibody may be a promising therapeutic agent for treating other diseases such as multiple sclerosis. As a matter of fact, treating mice with the anti-CD146 antibody AA98 attenuated neuroinflammation by limiting lymphocyte extravasation to the CNS in a mouse model of multiple sclerosis. Endothelial CD146 therefore plays also an important role in the maintenance of the blood–brain barrier (Duan et al. 2013).
2.6 CD44
CD44 is a cell adhesion molecule with an extracellular domain, a single transmembrane helix and a short cytoplasmic tail. It binds to hyaluronan and other components of the ECM (Naor et al. 1997). CD44 knockout mice are viable but the vascularisation of Matrigel implants as well as tumour and wound angiogenesis were reduced (Cao et al. 2006). Most interestingly, alternative splicing of exons 6 to 15 leads to many different isoforms (CD44vx). CD44v6 was shown to play an important role in cancer metastasis (Gunthert et al. 1991). A peptide derived from this CD44 isoform blocked c-Met in several cancer cells indicating that CD44v6 acts as a co-receptor of this RTK (Orian-Rousseau et al. 2002). In addition, VEGFR-2 forms a constitutive complex with CD44v6 thereby blocking downstream signalling. This inhibitory activity is ablated by the CD44v6-derived peptide described above. VEGF-dependent migration and tube formation of endothelial cells, as well as the formation of new spheroidal vasculature in vivo, were all blocked by this peptide showing that CD44v6 has a similar biological function as CD146 in VEGFR-2 signalling (Tremmel et al. 2009).
3 Extracellular Components of the VEGF/VEGFR Signalling Cascade as Targets for Therapy and Functional Inhibition
3.1 VEGF/VEGFRs in Disease
Owing to its key role in vascular homeostasis, neo-angiogenesis and lymphangiogenesis, VEGF and its receptors have presented themselves as attractive targets for the therapeutic management of several pathologies. These include soluble VEGFR-1 (sVEGFR-1) for the pregnancy-associated condition of pre-eclampsia and VEGFR-2 for coronary diseases, neural injuries and retinopathies such as age-related macular degeneration as well as chronic inflammatory conditions such as rheumatoid arthritis. Last but not least, all three VEGF receptors have been implicated in the progression of cancer for their respective involvement in the process of neo-angiogenesis, lymphangiogenesis or metastasis.
To date, a number of studies have identified sVEGFR-1 as a critical component of the pathological manifestation of pre-eclampsia. sVEGFR-1 is a soluble form of the cell membrane-bound VEGFR-1 lacking the transmembrane and cytoplasmic domains (Kendall and Thomas 1993). Similar to the membrane-bound variant, sVEGFR-1 binds to both VEGF-A and PlGF, thereby acting as a decoy receptor for these two endogenous ligands (Park et al. 1994; Kendall and Thomas 1993). Recently, it has been shown that rats injected with sVEGFR-1 present with hypertension and proteinuria (Maynard et al. 2003), both hallmarks of pre-eclampsia. Furthermore, the excess of sVEGFR-1 was found to be accompanied by a concomitant decrease in concentrations of VEGF-A and PlGF, making sVEGFR-1 an interesting target for treatment and prevention of pre-eclampsia.
In ischaemic heart disease or peripheral artery disease, insufficient blood vasculature leads to tissue ischaemia. Several lines of research have shown that VEGF-mediated therapy stimulates localised angiogenesis and thereby limits necrotic tissue arising from ischaemic heart disease. Recently, a gene therapy approach using adenovirus-carrying human VEGF-A165-transfected mesenchymal stem cells was able to produce effective myogenesis and host-derived angiogenesis, resulting in the prevention of progressive heart dysfunction after myocardial infarction (Gao et al. 2007). In atherosclerosis, however, the role of angiogenesis remains a highly contentious issue, and no consensus exists as to whether angiogenesis is either a key causative factor in the pathogenesis of atherosclerotic plaque formation or one of its consequences (Khurana et al. 2005). Indeed, a number of recent investigations using animal models correlate the presence of VEGF and other angiogenic factors with atherosclerosis. It is hypothesised that this promotion of intralesion angiogenesis leads to the destabilisation of coronary plaques (Moulton et al. 1999; Celletti et al. 2001), highlighting the importance of further site-directed investigations benefitting from highly specific VEGF/VEGFR-targeted inhibitory molecules.
In age-related macular degeneration, the loss of homeostasis between pro-angiogenic VEGF and the anti-angiogenic pigment epithelium-derived factor (PEDF) may lead to choroidal neovascularisation (Nowak 2006; Smith et al. 2001). Furthermore, in response to localised inflammation, infiltrating leukocytes via their own secretion further contribute to a circulating pool of VEGF, whereby VEGF-A164 has been shown to selectively induce inflammation and cellular immunity, thus contributing to pathological ocular neovascularisation (Ishida et al. 2003). Overall, the VEGF/VEGFR-2 pathway presents itself as an attractive target for the treatment of neovascular and ischaemic eye diseases such as choroidal neovascularisation, macular oedema secondary to diabetic retinopathy or retinal vein occlusion and neovascularisation.
Another area where VEGF is abundantly produced is in the brain. Here, VEGF is typically expressed by neurons and vascular cells, thereby mediating neuronal survival and angiogenesis in an autocrine fashion (Sun et al. 2003). Not surprisingly, it has been shown that VEGF plays an important role in adult neurogenesis after traumatic brain injury and that the process involves VEGFR-2 and the Raf/MEK/ERK signalling cascade (Lu et al. 2011). Consequently, it has been hypothesised that VEGF also exerts beneficial effects in ischaemic stroke. Indeed, elevated serum levels of VEGF have been reported for human stroke patients (Slevin et al. 2000; Lee et al. 2010). This is in line with findings from experimental ischaemic stroke in rats, which model cardioembolic stroke in humans, where the expression of VEGF and VEGFR-1 were both found upregulated in neurons and vascular cells in peri-infarct areas (Lennmyr et al. 1998). Interestingly, however, in this context suppression rather than stimulation of VEGF/VEGFR-1 signalling seems to correlate with beneficial effects on the brain (Storkebaum et al. 2004), making highly VEGFR-specific agents all the more critical for research and therapeutic applications with the goal to block VEGF signalling in this field.
In rheumatoid arthritis (RA), the progressive destruction of cartilage and bone typically results from chronic inflammation of the joints. This symptomatic arises due to progressive infiltration of the inflammatory sites by plasma cells, lymphocytes and macrophages together with hyperplasia of synovial cells, resulting in the overgrowth of a fibrovascular granulation tissue, known as pannus (Paleolog 2002). Indeed, the perpetuation of neovascularisation in inflammatory diseases, such as rheumatoid arthritis, spondyloarthropathies and some systemic autoimmune diseases, might facilitate the ingress of inflammatory cells into the synovium and, therefore, stimulate pannus formation (Szekanecz and Koch 2007). Indeed, the importance of VEGF signalling in the pathogenesis of RA has only recently been further underlined by identifying significantly elevated levels of pro-angiogenic factors in the synovium tissue of RA patients (Schroeder et al. 2013). As such, the development of novel angiostatic treatments for chronic inflammatory joint disease may lead to a new therapeutic approach in controlling disease progression (Colville-Nash and Scott 1992).
With regard to cancer it seems that all three VEGF receptors play a role in the progression of the disease. Although VEGFR-1 is functionally expressed in various normal cell types, VEGFR-1 is also detected in cancer cells of a wide variety of tumour types, including leukaemia, lymphoma, multiple myeloma, melanoma and carcinomas of breast, colon, lung, pancreas and prostate (Wu et al. 2006). VEGFR-1 has been implicated in carcinogenesis by mediating cellular functions in tumour vascular endothelium as well as cancer cells. In particular, it seems that VEGFR-1 plays a role in the establishment of metastasis and premetastatic niches (Hiratsuka et al. 2002; Kaplan et al. 2005). Blockade of VEGFR-1 activation has been shown to inhibit pathological angiogenesis and tumour growth (Wu et al. 2006). VEGFR-2 and VEGFR-3, on the other hand, have been implicated in carcinogenesis for their involvement in the processes of neo-angiogenesis and lymphangiogenesis, respectively, preceding progression, invasion and metastasis spread (Cao et al. 2012; Martins et al. 2013; Chatterjee et al. 2013; Matsumoto et al. 2013).
3.2 VEGF/VEGFRs as Targets in Therapeutic Inhibition
Given the aforementioned range of effects of VEGF and its receptors in neo-angiogenesis and carcinogenesis, the implications for using angiogenic signals as therapeutic targets are well established. In addition and as the aforementioned paragraph has outlined, the role of the receptor ECD in signalling has been increasingly recognised. As such, it is not surprising that this knowledge has served as basis for the development of novel inhibitory agents. The following aims at giving an overview of the current approaches to date.
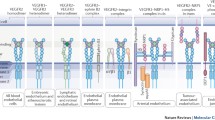
Of the biologically active binding proteins, antibodies probably represent the best-studied group. Although they are widely used in biological research and clinical therapeutics, their applications are somewhat limited due to their size, poor stability, production costs and batch-to-batch variation. However, unlike small molecule inhibitors (SMIs), antibodies usually have the benefit of excellent biocompatibility and high target specificity, which is why they represent favourable agents for extracellular targeting approaches. Surprisingly, there are only a few good immunotargets shared by human and mouse vasculature known. In targeting the VEGFR ECD, three main mechanisms of action have been identified: VEGF-neutralising antibodies, VEGFR D23-targeting agents that promote competitive ligand-binding inhibition or VEGFR D4-7-targeting agents leading to allosteric inhibition of receptor activity.
3.2.1 VEGF-Neutralising Agents
The approach of using VEGF-neutralising antibodies is aimed at inhibiting the interaction between VEGF and its binding domain D23 on the ECD of VEGFRs. This mechanism of action is being pursued by several pharmaceutical companies including Genentech’s ranibizumab (Lucentis®) for intravitreal treatment of neovascular retinopathies by injection. Ranibizumab is a 48 kDa recombinant humanised monovalent antibody fragment and is licensed by the US Food and Drug Administration (FDA) for the treatment of AMD and macular oedema following retinal vein occlusion as well as diabetic macular oedema. Ranibizumab binds to all biologically active isoforms of VEGF-A and presents with a half-life of 2–4 days (Kinnunen and Yla-Herttuala 2012; Ciulla and Rosenfeld 2009). Similarly, Genentech’s bevacizumab (Avastin®) is a full-length anti-VEGF recombinant humanised antibody of 149 kDa, comprising approximately 93 % human and 7 % murine sequence lineage which binds to all VEGF-A isoforms with an affinity of approximately 500 pM (Presta et al. 1997; Ferrara et al. 2004). In humans, the terminal half-life of bevacizumab has been reported to be 17–21 days (Ferrara et al. 2004). However, bevacizumab differs from ranibizumab in that it undergoes two binding interactions with VEGF rather than just one. Bevacizumab is currently FDA-approved for the treatment of metastatic renal cell carcinoma, as well as the first- and second-line treatment of metastatic colorectal cancer, the first-line treatment of NSCLC and second-line treatment of glioblastoma multiforme. In multinational clinical trials, this agent has been shown to improve efficacy outcomes over platinum-based chemotherapy alone in the treatment of advanced NSCLC in two phase III randomised trials (Dy and Adjei 2006; Gridelli et al. 2007) as well as in the treatment of fluoropyrimidine-based chemotherapy of colorectal cancer that has progressed (Khurana et al. 2005). In addition to its application in tumour therapy, bevacizumab has been used off-label in neovascular ocular disease, where it has been shown to penetrate the inner limiting membrane and reach the subretinal space less effectively, but with a longer in vitro half-life of 8–10 days than ranibizumab (Fung et al. 2006; Shahar et al. 2006). Along similar lines, Regeneron Pharmaceuticals’ aflibercept (Eylea®) acts as VEGF trap, but displays binding affinities to VEGF that exceed bevacizumab by a factor 10. Aflibercept is a 110 kDa soluble receptor fusion protein consisting of the extracellular receptor domains of VEGFR-1 and VEGFR-2 linked to a human Fc domain of immunoglobulin G1 (IgG1). This protein acts as a soluble decoy receptor and has been found to bind with high affinity to all VEGF-A isoforms as well as VEGF-B, but not to VEGF-C or VEGF-D (Kinnunen and Yla-Herttuala 2012). Furthermore, the murine IgG2a,κ mAb 2C3, licensed by Peregrine Pharmaceuticals to Affitech AS, is another example of an anti-VEGF agent that has been shown to reduce vascular permeability and decreases endothelial cell and tumour growth in mice bearing human tumour xenografts (Brekken et al. 1998). Additionally, 2C3 was associated with a reduction in tumour microvessel density and macrophage infiltration (Dineen et al. 2008) and downregulation of VEGFR-2 expression on the tumour vasculature (Zhang et al. 2002). The desirable anti-angiogenic effects of 2C3 led to the development of a human antibody that retains 2C3 specificity. r84 (AT001, Affitech AS) is a fully human mAb that was generated by screening a human anti-VEGF single-chain variable fragment (scFv) library for 2C3-like properties. r84 has been shown to bind to both human and mouse VEGF-A and selectively blocks VEGF-A from interacting with VEGFR-2 whilst reportedly not interfering with its interaction with VEGFR-1 (Sullivan et al. 2010) and has recently entered phase I clinical trial stage in Russia. As a new member of anti-VEGF agents, aflibercept has recently been investigated as vascular-directed therapy in tumour management. Data from a phase III trial (VELOUR), which included aflibercept with irinotecan/5-FU as second-line chemotherapy, has shown extended progression-free survival and overall survival of metastatic colorectal cancer patients (He et al. 2012). Last but not least, pegaptanib, an aptamer inhibitor specific for the VEGF-A165 isoform and distributed by Macugen(R), Eyetech Pharmaceuticals and Pfizer has also entered phases II–III clinical trials for the treatment of AMD (Ng et al. 2006). Aptamers are macromolecules composed of chemically synthesised single-stranded nucleic acids (either RNA or DNA) that bind with a high degree of selectivity and affinity to target proteins. Pegaptanib is a PEGylated modified oligonucleotide that antagonistically binds to extracellular VEGF by adopting a specific three-dimensional configuration (Fraunfelder 2005).
3.2.2 Anti-VEGFR-1 Agents
Owing to the implications of VEGFR-1 as a potential therapeutic target, a rat anti-mouse VEGFR-1 IgG1 mAb designated as MF-1 was produced by ImClone Systems. MF-1 targets D23 of VEGFR-1, thereby effectively interfering with ligand binding and was first shown to suppress angiogenesis and inflammation in prototypic angiogenic disorders such as cancer, retinal ischemia, arthritis and atherosclerosis (Luttun et al. 2002). Subsequent development of this antibody led to the production of the fully human mAb icrucumab (IMC-18F1, ImClone Systems) with an affinity for VEGFR-1 of 54 pM. Icrucumab has been particularly produced for the inhibition of cancer growth (Schwartz et al. 2010) and has been successfully tested for the treatment of human breast cancer xenografts, where it was shown to inhibit VEGFR-1 from interacting with VEGF-A and VEGF-B as well as PlGF (Wu et al. 2006). Icrucumab has demonstrated a favourable safety profile in phase I trials (Schwartz et al. 2010) and is currently being tested in phase II clinical trials, in particular for combination therapy together with conventional chemotherapy in the treatment of colorectal cancer. In addition, and unlike antibodies against VEGFR-1, Korean researchers have identified a readily synthesisable hexapeptide (Gly-Asn-Gln-Trp-Phe-Ile or GNQWFI) using peptide libraries. This anti-VEGFR-1 peptide was shown to be receptor-specific and interfere with ligand binding to D23 of VEGFR-1 of VEGF and PlGF as well as VEGF/PlGF heterodimers (Bae et al. 2005). Most recently, this agent has been tested as hyaluronate conjugate for the treatment of retinal neovascularisation and diabetic retinopathy (Oh et al. 2011).
3.2.3 Anti-VEGFR-2 D23 Agents
In contrast to anti-VEGF approaches, targeted treatment against the ligand-binding site D23 of VEGFR-2 has the benefit of specificity for VEGF/VEGFR-2 signalling. Over a decade ago, proof-of-concept was established with the development of a monoclonal rat anti-mouse antibody termed DC101 (ImClone Systems) (Rockwell et al. 1995). DC-101 has been shown to inhibit tumour-induced neovascularisation and growth of several tumours using syngenic and xenograft tumour models (Prewett et al. 1999). However, since DC101 does not cross-react with human VEGFR-2, it could not be further evaluated for in vivo efficacy in humans (Witte et al. 1998). Indeed, it seems that despite the high sequence similarity between mouse Flk-1 and human VEGFR-2, finding a human/mouse cross-reactive antibody is challenging. Finally, in 2004, a chimeric rabbit/human antigen fragment antibody (Fab) selected from an immune b9 allotype rabbit antibody library which demonstrated human/mouse cross-reactivity was successfully identified (Popkov et al. 2004), although no further follow-up studies using these compounds as extracellular targeting agents have been reported. Another compound is CDP-971 (UCB Group), a PEGylated humanised Fab2 that binds VEGFR-2 with a dissociation constant of 49 pM which was shown to inhibit VEGF-A and VEGF-C signalling in vitro and was anti-angiogenic in vivo (Ton et al. 2007). Subsequent data from clinical phase I and II trials were encouraging and support further clinical development of CDP791 in first line non-small cell lung cancer treatment. Additionally, and building on DC101’s preclinical success, ImClone Systems Corporation has further pursued the production of anti-hVEGFR-2 D23 mAbs. This has led to the identification of several lead compounds. IMC-1C11 (ImClone Systems) is a chimeric mouse/human IgG1 mAb derived from an scFv with an affinity of 300 pM (Zhu et al. 1999), which unfortunately was cross-reactive for dog and monkey VEGFR-2, but not for rat or mouse. Nonetheless, IMC-1C11 was entered into phase I clinical trials in order to determine its efficacy in the treatment of metastatic colorectal carcinoma (Hunt 2001; Posey et al. 2003). Its follow-up compound, ramucirumab (IMC-1121B, ImClone Systems), is a fully human IgG1 mAb derived from affinity maturation of a Fab fragment isolated from a large naïve human phage display library with a target affinity of 50 pM (Spratlin et al. 2010). Ramucirumab was shown to significantly increase the survival of mice in a model of leukaemia, owing to its drastic inhibitory potential (Zhu et al. 2003; Miao et al. 2006) and is currently in phase II and III clinical trials for various combination therapies of several solid forms of cancer (Krupitskaya and Wakelee 2009; Spratlin 2011). In terms of most recent developments, the following compounds should be noted. These are the production of two specific scFvs (KDR1.3 and KDR2.6) selected from a V-gene phage display library which were only monospecific for hVEGFR-2 (Erdag et al. 2011), as well as the identification and characterisation of an antibody-like designed ankyrin repeat protein (DARPin) which displayed profound inhibitory properties of functional output in endothelial cell model systems (Hyde et al. 2012). Additionally, DARPin D23b displayed high receptor specificity and affinity in the picomolar range as well as partial cross-reactivity for mouse Flk-1 and human VEGFR-2 (Hyde et al. 2012). Last but not least, the generation of a humanised rabbit mAb by Mutational Lineage Guided (MLG) Humanisation technology recently yielded a human/mouse VEGFR-2 cross-reactive antibody with proven tumour growth inhibition in mouse xenograft models (Yu et al. 2013).
3.2.4 Anti-VEGFR-2 D4-7 Agents
Whilst the concept of targeting the ligand-binding site on VEGFR-2 may seem intuitive, novel insights have revealed a key functional role for the extracellular Ig-domains D4-7 of VEGFR-2. Not only have these domains proven an attractive target for the generation of novel detection antibodies which may be used in clinical applications as specific marker for VEGFR-2-mediated angiogenesis (Böldicke et al. 2001), they have also shown to play a significant part in the downstream signal propagation upon VEGFR-2 dimerisation. This was first indicated by 33C3 (AstraZeneca), a fully human antibody generated by XenoMouse technology, which was selected for its binding to recombinant VEGFR-2 ECD D4-7 with an affinity below 1 nM (Kendrew et al. 2011). Using endothelial cell lines overexpressing VEGFR-2, as well as human xenograft models in immunocompromised mice, 33C3 was shown to inhibit VEGFR-2 phosphorylation as well as VEGFR-2-dependent angiogenesis. However, to date the exact binding specificity of 33C3 has not been further specified. More recently, the implications of D4–7 targeted inhibition were further unravelled and fully characterised for their role in the activation and functional inhibition of angiogenic output in endothelial cell lines. Building on low-resolution structural data (Ruch et al. 2007; Kisko et al. 2011), which suggested the presence of homotypic contacts in membrane-proximal Ig-domains D4 and D7, the essential role of these domains as allosteric regulators was further described. Using DARPins that interact specifically with D4 or D7, it was shown that these domains are indispensable for full receptor signalling and that, whilst DARPins specific for D4 or D7 did not prevent ligand binding nor receptor dimerisation, they effectively blocked receptor signalling and functional output with affinities in the picomolar range (Hyde et al. 2012). Unfortunately, none of the here mentioned D4–7 targeting peptides cross-react with mouse Flk-1.
3.3 Limitations to VEGF/VEGFR Targeted Therapy
One of the concerns of working with antibodies is the potential of an allergic reaction to the drug. For instance, as a recombinant mAb, ranibizumab contains both mouse- and human-derived segments against which patients were found to develop systemic antibodies (Ciulla and Rosenfeld 2009; Brown et al. 2009). Clearly, fully human antibodies have several potential advantages over murine, chimeric or humanised antibodies in terms of both safety and efficacy. First, their lack of nonhuman residues makes fully human antibodies less likely to generate a host immune response following administration. Second, fully human antibodies generally exhibit lower clearance rates than other antibody types. This decreased clearance rate allows for the use of lower dosage and treatment frequencies. Much more importantly, however, especially in terms of the preclinical evaluation of potential therapeutic agents is the lack of cross-reactivity of humanised mouse mAbs with the mouse antigen. This fact still presents itself as a major obstacle in the quest for novel inhibitors, not only for those directed to VEGFR-2 but also to VEGF and integrin αvβ3 (Klohs and Hamby 1999). Last but not least, the principal toxicities associated with therapeutics targeting the VEGFR-2/VEGF-A signalling cascade, including hypertension, vascular thrombotic events and proteinuria (Gordon et al. 2001; Faivre et al. 2006; Holden et al. 2005; Gressett and Shah 2009), still largely remain to be addressed.
3.4 Outlook and Conclusions
For the first time it seems possible to develop reagents which are highly specific for VEGFR-2, without showing cross-reactivity with other related receptor family members. Clearly, the major benefit of targeting essential membrane-proximal homotypic interactions in the ECD over VEGF-neutralising or VEGFR-2 D23-targeting approaches, as well as SMIs targeting the tyrosine kinase domain, is that VEGFR-2 D4-7 inhibitory compounds do not compete with VEGF binding but still provide an extracellular binding site with high receptor specificity and therefore hopefully also an improved safety profile. As such, these agents could represent a generation of novel receptor-inhibitory compounds for in vivo applications such as targeting of VEGFRs in medical diagnostics and for the treatment of vascular pathologies. Considering that it has been possible to generate specific reagents that robustly inhibit activity at concentrations below 1 nM, it is essential that these reagents are tested in preclinical disease, in order to validate VEGFR-2 ECD D4–7 as a therapeutic target in angiogenic pathologies.
References
Adams RH, Eichmann A (2010) Axon guidance molecules in vascular patterning. Cold Spring Harb Perspect Biol 2(5):a001875
Adams RH, Wilkinson GA, Weiss C, Diella F, Gale NW, Deutsch U, Risau W, Klein R (1999) Roles of ephrinB ligands and EphB receptors in cardiovascular development: demarcation of arterial/venous domains, vascular morphogenesis, and sprouting angiogenesis. Genes Dev 13(3):295–306
Bacic F, Uematsu S, McCarron RM, Spatz M (1991) Dopaminergic receptors linked to adenylate cyclase in human cerebromicrovascular endothelium. J Neurochem 57(5):1774–1780
Bae DG, Kim TD, Li G, Yoon WH, Chae CB (2005) Anti-flt1 peptide, a vascular endothelial growth factor receptor 1-specific hexapeptide, inhibits tumor growth and metastasis. Clin Cancer Res 11(7):2651–2661
Ballmer-Hofer K, Andersson AE, Ratcliffe LE, Berger P (2011) Neuropilin-1 promotes VEGFR-2 trafficking through Rab11 vesicles thereby specifying signal output. Blood 118(3):816–826
Basu S, Nagy JA, Pal S, Vasile E, Eckelhoefer IA, Bliss VS, Manseau EJ, Dasgupta PS, Dvorak HF, Mukhopadhyay D (2001) The neurotransmitter dopamine inhibits angiogenesis induced by vascular permeability factor/vascular endothelial growth factor. Nat Med 7(5):569–574
Bates DO, Cui TG, Doughty JM, Winkler M, Sugiono M, Shields JD, Peat D, Gillatt D, Harper SJ (2002) VEGF165b, an inhibitory splice variant of vascular endothelial growth factor, is down-regulated in renal cell carcinoma. Cancer Res 62(14):4123–4131
Bevan HS, van den Akker NM, Qiu Y, Polman JA, Foster RR, Yem J, Nishikawa A, Satchell SC, Harper SJ, Gittenberger-de Groot AC, Bates DO (2008) The alternatively spliced anti-angiogenic family of VEGF isoforms VEGFxxxb in human kidney development. Nephron Physiol 110(4):57–67
Böldicke T, Tesar M, Griesel C, Rohde M, Gröne HJ, Waltenberger J, Kollet O, Lapidot T, Yayon A, Weich H (2001) Anti-VEGFR-2 scFvs for cell isolation. Single-chain antibodies recognizing the human vascular endothelial growth factor receptor-2 (VEGFR-2/flk-1) on the surface of primary endothelial cells and preselected CD34+ cells from cord blood. Stem Cells 19(1):24–36
Brekken RA, Huang X, King SW, Thorpe PE (1998) Vascular endothelial growth factor as a marker of tumor endothelium. Cancer Res 58(9):1952–1959
Broermann A, Winderlich M, Block H, Frye M, Rossaint J, Zarbock A, Cagna G, Linnepe R, Schulte D, Nottebaum AF, Vestweber D (2011) Dissociation of VE-PTP from VE-cadherin is required for leukocyte extravasation and for VEGF-induced vascular permeability in vivo. J Exp Med 208(12):2393–2401
Brown DM, Michels M, Kaiser PK, Heier JS, Sy JP, Ianchulev T (2009) Ranibizumab versus verteporfin photodynamic therapy for neovascular age-related macular degeneration: two-year results of the ANCHOR study. Ophthalmology 116(1):57–65
Brozzo MS, Bjelic S, Kisko K, Schleier T, Leppanen VM, Alitalo K, Winkler FK, Ballmer-Hofer K (2012) Thermodynamic and structural description of allosterically regulated VEGF receptor 2 dimerization. Blood 119(7):1781–1788
Cai H, Reed RR (1999) Cloning and characterization of neuropilin-1-interacting protein: a PSD-95/Dlg/ZO-1 domain-containing protein that interacts with the cytoplasmic domain of neuropilin-1. J Neurosci 19(15):6519–6527
Cao G, Savani RC, Fehrenbach M, Lyons C, Zhang L, Coukos G, DeLisser HM (2006) Involvement of endothelial CD44 during in vivo angiogenesis. Am J Pathol 169(1):325–336
Cao R, Ji H, Feng N, Zhang Y, Yang X, Andersson P, Sun Y, Tritsaris K, Hansen AJ, Dissing S, Cao Y (2012) Collaborative interplay between FGF-2 and VEGF-C promotes lymphangiogenesis and metastasis. Proc Natl Acad Sci U S A 109(39):15894–15899
Carmeliet P, Lampugnani MG, Moons L, Breviario F, Compernolle V, Bono F, Balconi G, Spagnuolo R, Oostuyse B, Dewerchin M, Zanetti A, Angellilo A, Mattot V, Nuyens D, Lutgens E, Clotman F, de Ruiter MC, Gittenberger dG, Poelmann R, Lupu F, Herbert JM, Collen D, DeJana E (1999) Targeted deficiency or cytosolic truncation of the VE-cadherin gene in mice impairs VEGF-mediated endothelial survival and angiogenesis. Cell 98(2):147–157
Cébe-Suarez S, Pieren M, Cariolato L, Arn S, Hoffmann U, Bogucki A, Manlius C, Wood J, Ballmer-Hofer K (2006a) A VEGF-A splice variant defective for heparan sulfate and neuropilin-1 binding shows attenuated signaling through VEGFR-2. Cell Mol Life Sci 63(17):2067–2077
Cébe-Suarez S, Zehnder-Fjällman AH, Ballmer-Hofer K (2006b) The role of VEGF receptors in angiogenesis; complex partnerships. Cell Mol Life Sci 63(5):601–615
Cébe-Suarez S, Grünewald FS, Jaussi R, Li X, Claesson-Welsh L, Spillmann D, Mercer AA, Prota AE, Ballmer-Hofer K (2008) Orf virus VEGF-E NZ2 promotes paracellular NRP-1/VEGFR-2 coreceptor assembly via the peptide RPPR. FASEB J 22(8):3078–3086
Celletti FL, Waugh JM, Amabile PG, Brendolan A, Hilfiker PR, Dake MD (2001) Vascular endothelial growth factor enhances atherosclerotic plaque progression. Nat Med 7:425–429
Chatterjee S, Heukamp LC, Siobal M, Schöttle J, Wieczorek C, Peifer M, Frasca D, Koker M, König K, Meder L, Rauh D, Buettner R, Wolf J, Brekken RA, Neumaier B, Christofori G, Thomas RK, Ullrich RT (2013) Tumor VEGF:VEGFR2 autocrine feed-forward loop triggers angiogenesis in lung cancer. J Clin Invest 123(7):3183
Chittenden TW, Claes F, Lanahan AA, Autiero M, Palac RT, Tkachenko EV, Elfenbein A, de Ruiz AC, Dedkov E, Tomanek R, Li W, Westmore M, Singh JP, Horowitz A, Mulligan-Kehoe MJ, Moodie KL, Zhuang ZW, Carmeliet P, Simons M (2006) Selective regulation of arterial branching morphogenesis by synectin. Dev Cell 10(6):783–795
Christinger HW, Fuh G, de Vos AM, Wiesmann C (2004) The crystal structure of PlGF in complex with domain 2 of VEGFR1. J Biol Chem 279(11):10382–10388
Ciulla TA, Rosenfeld PJ (2009) Antivascular endothelial growth factor therapy for neovascular age-related macular degeneration. Curr Opin Ophthalmol 20(3):158–165
Colville-Nash PR, Scott DL (1992) Angiogenesis and rheumatoid arthritis: pathogenic and therapeutic implications. Ann Rheumat Dis 51(7):919–925
Cunningham SA, Arrate MP, Brock TA, Waxham MN (1997) Interactions of FLT-1 and KDR with phospholipase C γ: identification of the phosphotyrosine binding sites. Biochem Biophys Res Commun 240(3):635–639
Dance M, Montagner A, Yart A, Masri B, Audigier Y, Perret B, Salles JP, Raynal P (2006) The adaptor protein Gab1 couples the stimulation of vascular endothelial growth factor receptor-2 to the activation of phosphoinositide 3-kinase. J Biol Chem 281(32):23285–23295
Dell’Era Dosch D, Ballmer-Hofer K (2009) Transmembrane domain-mediated orientation of receptor monomers in active VEGFR-2 dimers. FASEB J 24(1):32–38
Dineen SP, Lynn KD, Holloway SE, Miller AF, Sullivan JP, Shames DS, Beck AW, Barnett CC, Fleming JB, Brekken RA (2008) Vascular endothelial growth factor receptor 2 mediates macrophage infiltration into orthotopic pancreatic tumors in mice. Cancer Res 68(11):4340–4346
Drake CJ, Davis LA, Little CD (1992) Antibodies to beta 1-integrins cause alterations of aortic vasculogenesis, in vivo. Dev Dyn 193(1):83–91
Duan H, Xing S, Luo Y, Feng L, Gramaglia I, Zhang Y, Lu D, Zeng Q, Fan K, Feng J, Yang D, Qin Z, Couraud PO, Romero IA, Weksler B, Yan X (2013) Targeting endothelial CD146 attenuates neuroinflammation by limiting lymphocyte extravasation to the CNS. Sci Rep 3:1687
Dy GK, Adjei AA (2006) Angiogenesis inhibitors in lung cancer: a promise fulfilled. Clin Lung Cancer 7(4):S145–S149
Erdag B, Koray Balcioglu B, Ozdemir Bahadir A, Serhatli M, Kacar O, Bahar A, Seker UOS, Akgun E, Ozkan A, Kilic T, Tamerler C, Baysal K (2011) Identification of novel neutralizing single-chain antibodies against vascular endothelial growth factor receptor 2. Biotechnol Appl Biochem 58(6):412–422
Errico M, Riccioni T, Iyer S, Pisano C, Acharya KR, Persico GM, De FS (2004) Identification of placental growth factor determinants for binding and activation of Flt-1 receptor. J Biol Chem 279(42):43929–43939
Esser S, Lampugnani MG, Corada M, DeJana E, Risau W (1998) Vascular endothelial growth factor induces VE-cadherin tyrosine phosphorylation in endothelial cells. J Cell Sci 111(Pt13):1853–1865
Faivre S, Delbaldo C, Vera K, Robert C, Lozahic S, Lassau N, Bello C, Deprimo S, Brega N, Massimini G, Armand JP, Scigalla P, Raymond E (2006) Safety, pharmacokinetic, and antitumor activity of SU11248, a novel oral multitarget tyrosine kinase inhibitor, in patients with cancer. J Clin Oncol 24(1):25–35
Fantin A, Schwarz Q, Davidson K, Normando EM, Denti L, Ruhrberg C (2011) The cytoplasmic domain of neuropilin 1 is dispensable for angiogenesis, but promotes the spatial separation of retinal arteries and veins. Development 138(19):4185–4191
Ferrara N, Hillan KJ, Gerber HP, Novotny W (2004) Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat Rev Drug Discov 3(5):391–400
Fraunfelder FW (2005) Pegaptanib for wet macular degeneration. Drugs Today 41(11):703–709
Fung AE, Rosenfeld PJ, Reichel E (2006) The International Intravitreal Bevacizumab Safety Survey: using the internet to assess drug safety worldwide. Br J Ophtalmol 90(11):1344–1349
Gao F, He T, Wang HB, Yu SQ, Yi DH, Liu WY, Cai ZJ (2007) A promising strategy for the treatment of ischemic heart disease: mesenchymal stem cell-mediated vascular endothelial growth factor gene transfer in rats. Can J Cardiol 23(11):891–898
Gerber HP, McMurtrey A, Kowalski J, Yan M, Keyt BA, Dixit V, Ferrara N (1998) Vascular endothelial growth factor regulates endothelial cell survival through the phosphatidylinositol 3′-kinase/Akt signal transduction pathway. Requirement for Flk-1/KDR activation. J Biol Chem 273(46):30336–30343
Gerhardt H, Golding M, Fruttiger M, Ruhrberg C, Lundkvist A, Abramsson A, Jeltsch M, Mitchell C, Alitalo K, Shima D, Betsholtz C (2003) VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J Cell Biol 161(6):1163–1177
Gerhardt H, Ruhrberg C, Abramsson A, Fujisawa H, Shima D, Betsholtz C (2004) Neuropilin-1 is required for endothelial tip cell guidance in the developing central nervous system. Dev Dyn 231(3):503–509
Giannotta M, Trani M, DeJana E (2013) VE-cadherin and endothelial adherens junctions: active guardians of vascular integrity. Dev Cell 26(5):441–454
Gordon MS, Margolin K, Talpaz M, Sledge GW, Holmgren E, Benjamin R, Stalter S, Shak S, Adelman D (2001) Phase I safety and pharmacokinetic study of recombinant human anti-vascular endothelial growth factor in patients with advanced cancer. J Clin Oncol 19:843–850
Grazia Lampugnani M, Zanetti A, Corada M, Takahashi T, Balconi G, Breviario F, Orsenigo F, Cattelino A, Kemler R, Daniel TO, DeJana E (2003) Contact inhibition of VEGF-induced proliferation requires vascular endothelial cadherin, beta-catenin, and the phosphatase DEP-1/CD148. J Cell Biol 161(4):793–804
Gressett SM, Shah SR (2009) Intricacies of bevacizumab-induced toxicities and their management. Ann Pharmacother 43(3):490–501
Gridelli C, Maione P, Rossi A, De Marinis F (2007) The role of bevacizumab in the treatment of non-small cell lung cancer: current indications and future developments. Oncologist 12(10):1183–1193
Grünewald FS, Prota AE, Giese A, Ballmer-Hofer K (2010) Structure-function analysis of VEGF receptor activation and the role of coreceptors in angiogenic signaling. Biochim Biophys Acta 1804(3):567–580
Gunthert U, Hofmann M, Rudy W, Reber S, Zoller M, Haussmann I, Matzku S, Wenzel A, Ponta H, Herrlich P (1991) A new variant of glycoprotein CD44 confers metastatic potential to rat carcinoma cells. Cell 65(1):13–24
Hagberg CE, Mehlem A, Falkevall A, Muhl L, Fam BC, Ortsater H, Scotney P, Nyqvist D, Samen E, Lu L, Stone-Elander S, Proietto J, Andrikopoulos S, Sjoholm A, Nash A, Eriksson U (2012) Targeting VEGF-B as a novel treatment for insulin resistance and type 2 diabetes. Nature 490(7420):426–430
Hagberg C, Mehlem A, Falkevall A, Muhl L, Eriksson U (2013) Endothelial fatty acid transport: role of vascular endothelial growth factor B. Physiology 28(2):125–134
Harper SJ, Bates DO (2008) VEGF-A splicing: the key to anti-angiogenic therapeutics? Nat Rev Cancer 8(11):880–887
Hayashi M, Majumdar A, Li X, Adler J, Sun Z, Vertuani S, Hellberg C, Mellberg S, Koch S, Dimberg A, Koh GY, DeJana E, Belting HG, Affolter M, Thurston G, Holmgren L, Vestweber D, Claesson-Welsh L (2013) VE-PTP regulates VEGFR2 activity in stalk cells to establish endothelial cell polarity and lumen formation. Nat Commun 4:1672
He Z, Tessier-Lavigne M (1997) Neuropilin is a receptor for the axonal chemorepellent Semaphorin III. Cell 90(4):739–751
He K, Cui B, Li G, Wang H, Jin K, Teng L (2012) The effect of anti-VEGF drugs (bevacizumab and aflibercept) on the survival of patients with metastatic colorectal cancer (mCRC). OncoTargets Ther 5:59–65
Hiratsuka S, Nakamura K, Iwai S, Murakami M, Itoh T, Kijima H, Shipley JM, Senior RM, Shibuya M (2002) MMP9 induction by vascular endothelial growth factor receptor-1 is involved in lung-specific metastasis. Cancer Cell 2(4):289–300
Holden SN, Eckhardt SG, Basser R, de Boer R, Rischin D, Green M, Rosenthal MA, Wheeler C, Barge A, Hurwitz HI (2005) Clinical evaluation of ZD6474, an orally active inhibitor of VEGF and EGF receptor signaling, in patients with solid, malignant tumors. Ann Oncol 16(8):1391–1397
Holmqvist K, Cross MJ, Rolny C, Hägerkvist R, Rahimi N, Matsumoto T, Claesson-Welsh L, Welsh M (2004) The adaptor protein Shb binds to tyrosine 1175 in the vascular endothelial growth factor (VEGF) receptor-2 and regulates VEGF-dependent cellular migration. J Biol Chem 279(21):22267–22275
Hu LA, Chen W, Martin NP, Whalen EJ, Premont RT, Lefkowitz RJ (2003) GIPC interacts with the beta1-adrenergic receptor and regulates beta1-adrenergic receptor-mediated ERK activation. J Biol Chem 278(28):26295–26301
Hunt S (2001) Technology evaluation: IMC-1C11, ImClone systems. Curr Opin Mol Ther 3(4):418–424
Hyde CA, Giese A, Stuttfeld E, Abram SJ, Villemagne D, Schleier T, Binz HK, Ballmer-Hofer K (2012) Targeting the extracellular domains D4 and D7 of VEGFR-2 reveals allosteric receptor regulatory sites. Mol Cell Biol 32(19):3802–3813
Ishida S, Usui T, Yamashiro K, Kaji Y, Amano S, Ogura Y, Hida T, Oguchi Y, Ambati J, Miller JW, Gragoudas ES, Ng YS, D’Amore PA, Shima DT, Adamis AP (2003) VEGF164-mediated inflammation is required for pathological, but not physiological, ischemia-induced retinal neovascularization. J Exp Med 198(3):483–489
Jiang T, Zhuang J, Duan H, Luo Y, Zeng Q, Fan K, Yan H, Lu D, Ye Z, Hao J, Feng J, Yang D, Yan X (2012) CD146 is a co-receptor for VEGFR-2 in tumor angiogenesis. Blood 120(11):2330–2339
Jussila L, Alitalo K (2002) Vascular growth factors and lymphangiogenesis. Physiol Rev 82(3):673–700
Kaplan RN, Riba RD, Zacharoulis S, Bramley AH, Vincent L, Costa C, MacDonald DD, Jin DK, Shido K, Kerns SA, Zhu Z, Hicklin D, Wu Y, Port JL, Altorki N, Port ER, Ruggero D, Shmelkov SV, Jensen KK, Rafii S, Lyden D (2005) VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature 438(7069):820–827
Kawamura H, Li X, Goishi K, van Meeteren LA, Jakobsson L, Cébe-Suarez S, Shimizu A, Edholm D, Ballmer-Hofer K, Kjellen L, Klagsbrun M, Claesson-Welsh L (2008) Neuropilin-1 in regulation of VEGF-induced activation of p38MAPK and endothelial cell organization. Blood 112(9):3638–3649
Kawasaki T, Kitsukawa T, Bekku Y, Matsuda Y, Sanbo M, Yagi T, Fujisawa H (1999) A requirement for neuropilin-1 in embryonic vessel formation. Development 126(21):4895–4902
Kawasaki K, Watabe T, Sase H, Hirashima M, Koide H, Morishita Y, Yuki K, Sasaoka T, Suda T, Katsuki M, Miyazono K, Miyazawa K (2008) Ras signaling directs endothelial specification of VEGFR2+ vascular progenitor cells. J Cell Biol 181(1):131–141
Kedlaya R, Kandala G, Liu TF, Maddodi N, Devi S, Setaluri V (2011) Interactions between GIPC-APPL and GIPC-TRP1 regulate melanosomal protein trafficking and melanogenesis in human melanocytes. Arch Biochem Biophys 508(2):227–233
Kendall RL, Thomas KA (1993) Inhibition of vascular endothelial cell growth factor activity by an endogenously encoded soluble receptor. Proc Natl Acad Sci U S A 90(22):10705–10709
Kendall RL, Rutledge RZ, Mao X, Tebben AJ, Hungate RW, Thomas K (1999) Vascular endothelial growth factor receptor KDR tyrosine kinase activity is increased by autophosphorylation of two activation loop tyrosine residues. J Biol Chem 274(10):6453–6460
Kendrew J, Eberlein C, Hedberg B, McDaid K, Smith NR, Weir HM, Wedge SR, Blakey DC, Foltz IN, Zhou J, Kang JS, Barry ST (2011) An antibody targeted to VEGFR-2 Ig domains 4–7 inhibits VEGFR-2 activation and VEGFR-2 dependent angiogenesis without affecting ligand binding. Mol Cancer Ther 10(5):770–783
Khurana R, Simons M, Martin JF, Zachary IC (2005) Role of angiogenesis in cardiovascular disease: a critical appraisal. Circulation 112(12):1813–1824
Kinnunen K, Yla-Herttuala S (2012) Vascular endothelial growth factors in retinal and choroidal neovascular diseases. Ann Med 44(1):1–17
Kisko K, Brozzo MS, Missimer J, Schleier T, Menzel A, Leppänen VM, Alitalo K, Walzthoeni T, Aebersold R, Ballmer-Hofer K (2011) Structural analysis of vascular endothelial growth factor receptor-2/ligand complexes by small-angle X-ray solution scattering. FASEB J 25(9):2980–2986
Klohs WD, Hamby JM (1999) Antiangiogenic agents. Curr Opin Biotechnol 10(6):544–549
Koch S, Claesson-Welsh L (2012) Signal transduction by vascular endothelial growth factor receptors. Cold Spring Harb Perspect Med 2(7):a006502
Kolodkin AL, Levengood DV, Rowe EG, Tai YT, Giger RJ, Ginty DD (1997) Neuropilin is a semaphorin III receptor. Cell 90(4):753–762
Komori Y, Nikai T, Taniguchi K, Masuda K, Sugihara H (1999) Vascular endothelial growth factor VEGF-like heparin-binding protein from the venom of Vipera aspis aspis (Aspic viper). Biochemistry 38(36):11796–11803
Krupitskaya Y, Wakelee HA (2009) Ramucirumab, a fully human mAb to the transmembrane signaling tyrosine kinase VEGFR-2 for the potential treatment of cancer. Curr Opin Investig Drugs 10(6):597–605
Ladomery MR, Harper SJ, Bates DO (2007) Alternative splicing in angiogenesis: the vascular endothelial growth factor paradigm. Cancer Lett 249(2):133–142
Lamalice L, Houle F, Jourdan G, Huot J (2004) Phosphorylation of tyrosine 1214 on VEGFR2 is required for VEGF-induced activation of Cdc42 upstream of SAPK2/p38. Oncogene 23(2):434–445
Lamalice L, Houle F, Huot J (2006) Phosphorylation of Tyr1214 within VEGFR-2 triggers the recruitment of Nck and activation of Fyn leading to SAPK2/p38 activation and endothelial cell migration in response to VEGF. J Biol Chem 281(45):34009–34020
Lampugnani MG, Orsenigo F, Gagliani MC, Tacchetti C, DeJana E (2006) Vascular endothelial cadherin controls VEGFR-2 internalization and signaling from intracellular compartments. J Cell Biol 174(4):593–604
Lanahan AA, Hermans K, Claes F, Kerley-Hamilton JS, Zhuang ZW, Giordano FJ, Carmeliet P, Simons M (2010) VEGF receptor 2 endocytic trafficking regulates arterial morphogenesis. Dev Cell 18(5):713–724
Lanahan A, Zhang X, Fantin A, Zhuang Z, Rivera-Molina F, Speichinger K, Prahst C, Zhang J, Wang Y, Davis G, Toomre D, Ruhrberg C, Simons M (2013) The Neuropilin 1 cytoplasmic domain is required for VEGF-A-dependent arteriogenesis. Dev Cell 25(2):156–168
Laramee M, Chabot C, Cloutier M, Stenne R, Holgado-Madruga M, Wong AJ, Royal I (2007) The scaffolding adapter Gab1 mediates VEGF signaling and is required for endothelial cell migration and capillary formation. J Biol Chem 282:7758–7769
Lee S, Jilani SM, Nikolova GV, Carpizo D, Iruela-Arispe ML (2005) Processing of VEGF-A by matrix metalloproteinases regulates bioavailability and vascular patterning in tumors. J Cell Biol 169(4):681–691
Lee SC, Lee KY, Kim YJ, Kim SH, Koh SH, Lee YJ (2010) Serum VEGF levels in acute ischaemic strokes are correlated with long-term prognosis. Eur J Neurologyj Biol 17(1):45–51
Lehmann JM, Riethmuller G, Johnson JP (1989) MUC18, a marker of tumor progression in human melanoma, shows sequence similarity to the neural cell adhesion molecules of the immunoglobulin superfamily. Proc Natl Acad Sci U S A 86(24):9891–9895
Lennmyr F, Ata KA, Funa K, Olsson Y, Terent A (1998) Expression of vascular endothelial growth factor (VEGF) and its receptors (Flt-1 and Flk-1) following permanent and transient occlusion of the middle cerebral artery in the rat. J Neuropathol Exp Neurol 57(9):874–882
Leppänen VM, Prota AE, Jeltsch M, Anisimov A, Kalkkinen N, Strandin T, Lankinen H, Goldman A, Ballmer-Hofer K, Alitalo K (2010) Structural determinants of growth factor binding and specificity by VEGF receptor 2. Proc Natl Acad Sci U S A 107(6):2425–2430
Leppänen VM, Tvorogov D, Kisko K, Prota AE, Jeltsch M, Anisimov A, Markovic-Mueller S, Stuttfeld E, Goldie KN, Ballmer-Hofer K, Alitalo K (2013) Structural and mechanistic insights into VEGF receptor 3 ligand binding and activation. Proc Natl Acad Sci U S A 110(32):12960–12965
Lu KT, Sun CL, Wo PYY, Yen HH, Tang TH, Ng MC, Huang ML, Yang YL (2011) Hippocampal neurogenesis after traumatic brain injury is mediated by vascular endothelial growth factor receptor-2 and the Raf/MEK/ERK cascade. J Neurotrauma 28(3):441–450
Lundkvist A, Lee S, Iruela-Arispe L, Betsholtz C, Gerhardt H (2007) Growth factor gradients in vascular patterning. Novartis Found Symp 283:194–201
Luttun A, Tjwa M, Moons L, Wu Y, Angelillo-Scherrer A, Liao F, Nagy JA, Hooper A, Priller J, De Klerck B, Compernolle V, Daci E, Bohlen P, Dewerchin M, Herbert JM, Fava R, Matthys P, Carmeliet G, Collen D, Dvorak HF, Hicklin DJ, Carmeliet P (2002) Revascularization of ischemic tissues by PlGF treatment, and inhibition of tumor angiogenesis, arthritis and atherosclerosis by anti-Flt1. Nat Med 8:831–840
Lyttle DJ, Fraser KM, Fleming SB, Mercer AA, Robinson AJ (1994) Homologs of vascular endothelial growth factor are encoded by the poxvirus orf virus. J Virol 68(1):84–92
Martins SF, Garcia EA, Luz MAM, Pardal F, Rodrigues M, Filho AL (2013) Clinicopathological correlation and prognostic significance of VEGF-A, VEGF-C, VEGFR-2 and VEGFR-3 expression in colorectal cancer. Can Gen Proteomics 10(2):55–68
Matsumoto T, Bohman S, Dixelius J, Berge T, Dimberg A, Magnusson P, Wang L, Wikner C, Qi JH, Wernstedt C, Wu J, Bruheim S, Mugishima H, Mukhopadhyay D, Spurkland A, Claesson-Welsh L (2005) VEGF receptor-2 Y951 signaling and a role for the adapter molecule TSAd in tumor angiogenesis. EMBO J 24(13):2342–2353
Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, Libermann TA, Morgan JP, Sellke FW, Stillman IE, Epstein FH, Sukhatme VP, Karumanchi SA (2003) Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest 111(5):649–658
Mercer AA, Wise LM, Scagliarini A, McInnes CJ, Buttner M, Rziha HJ, McCaughan CA, Fleming SB, Ueda N, Nettleton PF (2002) Vascular endothelial growth factors encoded by Orf virus show surprising sequence variation but have a conserved, functionally relevant structure. J Gen Virol 83(Pt 11):2845–2855
Meyer M, Clauss M, Lepple WA, Waltenberger J, Augustin HG, Ziche M, Lanz C, Buttner M, Rziha HJ, Dehio C (1999) A novel vascular endothelial growth factor encoded by Orf virus, VEGF-E, mediates angiogenesis via signalling through VEGFR-2 (KDR) but not VEGFR-1 (Flt-1) receptor tyrosine kinases. EMBO J 18(2):363–374
Miao HQ, Hu K, Jimenez X, Navarro E, Zhang H, Lu D, Ludwig DL, Balderes P, Zhu Z (2006) Potent neutralization of VEGF biological activities with a fully human antibody Fab fragment directed against VEGF receptor 2. Biochem Biophys Res Commun 345(1):438–445
Mills L, Tellez C, Huang S, Baker C, McCarty M, Green L, Gudas JM, Feng X, Bar-Eli M (2002) Fully human antibodies to MCAM/MUC18 inhibit tumor growth and metastasis of human melanoma. Cancer Res 62(17):5106–5114
Matsumoto M, Roufail S, Inder R, Caesar C, Karnezis T, Shayan R, Farnsworth RH, Sato T, Achen MG, Mann GB, Stacker SA (2013) Signaling for lymphangiogenesis via VEGFR-3 is required for the early events of metastasis. Clinical and Experimental Metastasis 30(6):819–832
Moulton KS, Heller E, Konerding MA, Flynn E, Palinski W, Folkman J (1999) Angiogenesis inhibitors endostatin or TNP-470 reduce intimal neovascularization and plaque growth in apolipoprotein E-deficient mice. Circulation 99(13):1726–1732
Muller YA, Li B, Christinger HW, Wells JA, Cunningham BC, de Vos AM (1997) Vascular endothelial growth factor: crystal structure and functional mapping of the kinase domain receptor binding site. Proc Natl Acad Sci U S A 94(14):7192–7197
Naccache SN, Hasson T, Horowitz A (2006) Binding of internalized receptors to the PDZ domain of GIPC/synectin recruits myosin VI to endocytic vesicles. Proc Natl Acad Sci U S A 103(34):12735–12740
Nakayama M, Nakayama A, van Lessen M, Yamamoto H, Hoffmann S, Drexler HC, Itoh N, Hirose T, Breier G, Vestweber D, Cooper JA, Ohno S, Kaibuchi K, Adams RH (2013) Spatial regulation of VEGF receptor endocytosis in angiogenesis. Nat Cell Biol 15(3):249–260
Naor D, Sionov RV, Ish-Shalom D (1997) CD44: structure, function, and association with the malignant process. Adv Cancer Res 71:241–319
Neufeld G, Cohen T, Shraga N, Lange T, Kessler O, Herzog Y (2002a) The neuropilins: multifunctional semaphorin and VEGF receptors that modulate axon guidance and angiogenesis. Trends Cardiovasc Med 12(1):13–19
Neufeld G, Kessler O, Herzog Y (2002b) The interaction of Neuropilin-1 and Neuropilin-2 with tyrosine-kinase receptors for VEGF. Adv Exp Med Biol 515:81–90
Ng EWM, Shima DT, Calias P, Cunningham J, Guyer DR, Adamis AP (2006) Pegaptanib, a targeted anti-VEGF aptamer for ocular vascular disease. Nat Rev Drug Discov 5(2):123–132
Nishizuka Y (1984) The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature 308:693–698
Nowak JZ (2006) Age-related macular degeneration (AMD): pathogenesis and therapy. Pharmacol Rep 58(3):353–363
Nowak DG, Woolard J, Amin EM, Konopatskaya O, Saleem MA, Churchill AJ, Ladomery MR, Harper SJ, Bates DO (2008) Expression of pro- and anti-angiogenic isoforms of VEGF is differentially regulated by splicing and growth factors. J Cell Sci 121(Pt 20):3487–3495
Ogawa S, Oku A, Sawano A, Yamaguchi S, Yazaki Y, Shibuya M (1998) A novel type of vascular endothelial growth factor, VEGF-E (NZ-7 VEGF), preferentially utilizes KDR/Flk-1 receptor and carries a potent mitotic activity without heparin-binding domain. J Biol Chem 273(47):31273–31282
Oh EJ, Choi JS, Kim H, Joo CK, Hahn SK (2011) Anti-Flt1 peptide – hyaluronate conjugate for the treatment of retinal neovascularization and diabetic retinopathy. Biomaterials 32(11):3115–3123
Olofsson B, Korpelainen E, Pepper MS, Mandriota SJ, Aase K, Kumar GY, Jeltsch MM, Shibuya M, Alitalo K, Eriksson U (1998) Vascular endothelial growth factor B (VEGF-B) binds to VEGF receptor-1 and regulates plasminogen activator activity in endothelial cells. Proc Natl Acad Sci U S A 95(20):11709–11714
Orian-Rousseau V, Chen L, Sleeman JP, Herrlich P, Ponta H (2002) CD44 is required for two consecutive steps in HGF/c-Met signaling. Genes Dev 16(23):3074–3086
Pajusola K, Aprelikova O, Korhonen J, Kaipainen A, Pertovaara L, Alitalo R, Alitalo K (1992) FLT4 receptor tyrosine kinase contains seven immunoglobulin-like loops and is expressed in multiple human tissues and cell lines. Cancer Res 52(20):5738–5743
Paleolog EM (2002) Angiogenesis in rheumatoid arthritis. Arthritis Res 4(Suppl 3):S81–S90
Pan Q, Chanthery Y, Liang WC, Stawicki S, Mak J, Rathore N, Tong RK, Kowalski J, Yee SF, Pacheco G, Ross S, Cheng Z, Le CJ, Plowman G, Peale F, Koch AW, Wu Y, Bagri A, Tessier-Lavigne M, Watts RJ (2007a) Blocking neuropilin-1 function has an additive effect with anti-VEGF to inhibit tumor growth. Cancer Cell 11(1):53–67
Pan Q, Chanthery Y, Wu Y, Rahtore N, Tong RK, Peale F, Bagri A, Tessier-Lavigne M, Koch AW, Watts RJ (2007b) Neuropilin-1 binds to VEGF121 and regulates endothelial cell migration and sprouting. J Biol Chem 282(33):24049–24056
Park JE, Chen HH, Winer J, Houck KA, Ferrara N (1994) Placenta growth factor. Potentiation of vascular endothelial growth factor bioactivity, in vitro and in vivo, and high affinity binding to Flt-1 but not to Flk-1/KDR. J Biol Chem 269(41):25646–25654
Perrin R, Konopatskaya O, Qiu Y, Harper S, Bates D, Churchill A (2005) Diabetic retinopathy is associated with a switch in splicing from anti- to pro-angiogenic isoforms of vascular endothelial growth factor. Diabetologia 48(11):2422–2427
Popkov M, Jendreyko N, Gonzalez-Sapienza G, Mage RG, Rader C, Barbas CF III (2004) Human/mouse cross-reactive anti-VEGF receptor 2 recombinant antibodies selected from an immune b9 allotype rabbit antibody library. J Immunol Methods 288(1–2):149–164
Posey JA, Ng TC, Yang B, Khazaeli MB, Carpenter MD, Fox F, Needle M, Waksal H, LoBuglio AF (2003) A phase I study of anti-kinase insert domain-containing receptor antibody, IMC-1C11, in patients with liver metastases from colorectal carcinoma. Clin Cancer Res 9(4):1323–1332
Presta LG, Chen H, Connor SJ, Chisholm V, Meng YG, Krummen L, Winkler M, Ferrara N (1997) Humanization of an anti-vascular endothelial growth factor monoclonal antibody for the therapy of solid tumors and other disorders. Cancer Res 57(20):4593–4599
Prewett M, Huber J, Li Y, Santiago A, Connor W, King K, Overholser- J, Hooper A, Pytowski B, Witte L, Bohlen P, Hicklin DJ (1999) Antivascular endothelial growth factor receptor (fetal liver kinase 1) monoclonal antibody inhibits tumor angiogenesis and growth of several mouse and human tumors. Cancer Res 59(20):5209–5218
Pritchard-Jones RO, Dunn DB, Qiu Y, Varey AH, Orlando A, Rigby H, Harper SJ, Bates DO (2007) Expression of VEGFxxxb, the inhibitory isoforms of VEGF, in malignant melanoma. Br J Cancer 97(2):223–230
Ricci A, Collier WL, Amenta F (1994) Pharmacological characterization and autoradiographic localization of dopamine receptors in the portal vein. J Auton Pharmacol 14(1):61–68
Rockwell P, Neufeld G, Glassman A, Caron D, Goldstein N (1995) In vitro neutralization of vascular endothelial growth factor activation of Flk-1 by a monoclonal antibody. Mol Cell Differ 3(1):91–109
Ruch C, Skiniotis G, Steinmetz MO, Walz T, Ballmer-Hofer K (2007) Structure of a VEGF-VEGF receptor complex determined by electron microscopy. Nat Struct Mol Biol 14(3):249–250
Sawamiphak S, Seidel S, Essmann CL, Wilkinson GA, Pitulescu ME, Acker T, Acker-Palmer A (2010) Ephrin-B2 regulates VEGFR2 function in developmental and tumour angiogenesis. Nature 465(7297):487–491
Schlessinger J, Lemmon MA (2003) SH2 and PTB domains in tyrosine kinase signaling. Sci STKE 2003(191):RE12
Schroeder M, Viezens L, Fuhrhop I, Rüther W, Schaefer C, Schwarzloh B, Algenstaedt P, Fink B, Hansen-Algenstaedt N (2013) Angiogenic growth factors in rheumatoid arthritis. Rheumatol Int 33(2):523–527
Schwartz JD, Rowinsky EK, Youssoufian H, Pytowski B, Wu Y (2010) Vascular endothelial growth factor receptor-1 in human cancer: concise review and rationale for development of IMC-18F1 (Human antibody targeting vascular endothelial growth factor receptor-1). Cancer 116(4):1027–1032
Senger DR, Galli SJ, Dvorak AM, Perruzzi CA, Harvey VS, Dvorak HF (1983) Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science 219(4587):983–985
Senger DR, Claffey KP, Benes JE, Perruzzi CA, Sergiou AP, Detmar M (1997) Angiogenesis promoted by vascular endothelial growth factor: regulation through alpha1beta1 and alpha2beta1 integrins. Proc Natl Acad Sci U S A 94(25):13612–13617
Shahar J, Avery RL, Heilweil G, Barak A, Zemel E, Lewis GP, Johnson PT, Fisher SK, Perlman I, Loewenstein A (2006) Electrophysiologic and retinal penetration studies following intravitreal injection of bevacizumab (Avastin). Retina 26(3):262–269
Shibuya M, Claesson-Welsh L (2005) Signal transduction by VEGF receptors in regulation of angiogenesis and lymphangiogenesis. Exp Cell Res 312(5):549–560
Shibuya M, Yamaguchi S, Yamane A, Ikeda T, Tojo A, Matsushime H, Sato M (1990) Nucleotide sequence and expression of a novel human receptor- type tyrosine kinase gene (flt) closely related to the fms family. Oncogene 5(4):519–524
Shome S, Rana T, Ganguly S, Basu B, Chaki CS, Sarkar C, Chakroborty D, Dasgupta PS, Basu S (2011) Dopamine regulates angiogenesis in normal dermal wound tissues. PLoS One 6(9):e25215
Sinha S, Vohra PK, Bhattacharya R, Dutta S, Sinha S, Mukhopadhyay D (2009) Dopamine regulates phosphorylation of VEGF receptor 2 by engaging Src-homology-2-domain-containing protein tyrosine phosphatase 2. J Cell Sci 122(Pt 18):3385–3392
Slevin M, Krupinski J, Slowik A, Kumar P, Szczudlik A, Gaffney J (2000) Serial measurement of vascular endothelial growth factor and transforming growth factor−+¦1 in serum of patients with acute ischemic stroke. Stroke 31(8):1863–1870
Smith W, Assink J, Klein R, Mitchell P, Klaver CCW, Klein BEK, Hofman A, Jensen S, Wang JJ, De Jong PTVM (2001) Risk factors for age-related macular degeneration: pooled findings from three continents. Ophthalmology 108(4):697–704
So JH, Hong SK, Kim HT, Jung SH, Lee MS, Choi JH, Bae YK, Kudoh T, Kim JH, Kim CH (2010) Gicerin/Cd146 is involved in zebrafish cardiovascular development and tumor angiogenesis. Genes Cells 15(11):1099–1110
Spratlin J (2011) Ramucirumab (IMC-1121B): monoclonal antibody inhibition of vascular endothelial growth factor receptor-2. Curr Oncol Rep 13(2):97–102
Spratlin JL, Cohen RB, Eadens M, Gore L, Camidge DR, Diab S, Leong S, O’Bryant C, Chow LQ, Serkova NJ, Meropol NJ, Lewis NL, Chiorean EG, Fox F, Youssoufian H, Rowinsky EK, Eckhardt SG (2010) Phase I pharmacologic and biologic study of ramucirumab (IMC-1121B), a fully human immunoglobulin G1 monoclonal antibody targeting the vascular endothelial growth factor receptor-2. J Clin Oncol 28(5):780–787
Spudich G, Chibalina MV, Au JS, Arden SD, Buss F, Kendrick-Jones J (2007) Myosin VI targeting to clathrin-coated structures and dimerization is mediated by binding to Disabled-2 and PtdIns(4,5)P2. Nat Cell Biol 9(2):176–183
Starovasnik MA, Christinger HW, Wiesmann C, Champe MA, de Vos AM, Skelton NJ (1999) Solution structure of the VEGF-binding domain of Flt-1: comparison of its free and bound states. J Mol Biol 293(3):531–544
Storkebaum E, Lambrechts D, Carmeliet P (2004) VEGF: once regarded as a specific angiogenic factor, now implicated in neuroprotection. Bioessays 26(9):943–954
Sullivan LA, Carbon JG, Roland CL, Toombs JE, Nyquist-Andersen M, Kavlie A, Schlunegger K, Richardson JA, Brekken RA (2010) r84, a novel therapeutic antibody against mouse and human vegf with potent anti-tumor activity and limited toxicity induction. PLoS One 5(8):e12031
Sun Y, Jin K, Xie L, Childs J, Mao XO, Logvinova A, Greenberg DA (2003) VEGF-induced neuroprotection, neurogenesis, and angiogenesis after focal cerebral ischemia. J Clin Invest 111(12):1843–1851
Szekanecz Z, Koch AE (2007) Mechanisms of disease: angiogenesis in inflammatory diseases. Nat Clin Pract Rheumatol 3(11):635–643
Takahashi H, Shibuya M (2005) The vascular endothelial growth factor (VEGF)/VEGF receptor system and its role under physiological and pathological conditions. Clin Sci (Lond) 109(3):227–241
Takahashi T, Ueno H, Shibuya M (1999) VEGF activates protein kinase C-dependent, but Ras-independent Raf-MEK-MAP kinase pathway for DNA synthesis in primary endothelial cells. Oncogene 18(13):2221–2230
Takahashi H, Hattori S, Iwamatsu A, Takizawa H, Shibuya M (2004) A novel snake venom vascular endothelial growth factor (VEGF) predominantly induces vascular permeability through preferential signaling via VEGF receptor-1. J Biol Chem 279(44):46304–46314
Terman BI, Carrion ME, Kovacs E, Rasmussen BA, Eddy RL, Shows TB (1991) Identification of a new endothelial cell growth factor receptor tyrosine kinase. Oncogene 6(9):1677–1683
Tessler S, Rockwell P, Hicklin D, Cohen T, Levi BZ, Witte L, Lemischka IR, Neufeld G (1994) Heparin modulates the interaction of VEGF165 with soluble and cell associated flk-1 receptors. J Biol Chem 269(17):12456–12461
Ton NC, Parker GJM, Jackson A, Mullamitha S, Buonaccorsi GA, Roberts C, Watson Y, Davies K, Cheung S, Hope L, Power F, Lawrance J, Valle J, Saunders M, Felix R, Soranson JA, Rolfe L, Zinkewich-Peotti K, Jayson GC (2007) Phase I evaluation of CDP791, a PEGylated Di-Fab conjugate that binds vascular endothelial growth factor receptor 2. Clin Cancer Res 13(23):7113–7118
Tremmel M, Matzke A, Albrecht I, Laib AM, Olaku V, Ballmer-Hofer K, Christofori G, Heroult M, Augustin HG, Ponta H, Orian-Rousseau V (2009) A CD44v6 peptide reveals a role of CD44 in VEGFR-2 signaling and angiogenesis. Blood 114(25):5236–5244
Varey AH, Rennel ES, Qiu Y, Bevan HS, Perrin RM, Raffy S, Dixon AR, Paraskeva C, Zaccheo O, Hassan AB, Harper SJ, Bates DO (2008) VEGF165b, an antiangiogenic VEGF-A isoform, binds and inhibits bevacizumab treatment in experimental colorectal carcinoma: balance of pro- and antiangiogenic VEGF-A isoforms has implications for therapy. Br J Cancer 98:1366–1379
Varsano T, Dong MQ, Niesman I, Gacula H, Lou X, Ma T, Testa JR, Yates JR III, Farquhar MG (2006) GIPC is recruited by APPL to peripheral TrkA endosomes and regulates TrkA trafficking and signaling. Mol Cell Biol 26(23):8942–8952
Wang Z, Yan X (2013) CD146, a multi-functional molecule beyond adhesion. Cancer Lett 330(2):150–162
Wang L, Mukhopadhyay D, Xu X (2006) C terminus of RGS-GAIP-interacting protein conveys neuropilin-1-mediated signaling during angiogenesis. FASEB J 20(9):1513–1515
Wang Y, Nakayama M, Pitulescu ME, Schmidt TS, Bochenek ML, Sakakibara A, Adams S, Davy A, Deutsch U, Luthi U, Barberis A, Benjamin LE, Makinen T, Nobes CD, Adams RH (2010) Ephrin-B2 controls VEGF-induced angiogenesis and lymphangiogenesis. Nature 465(7297):483–486
Whitaker GB, Limberg BJ, Rosenbaum JS (2001) Vascular endothelial growth factor receptor-2 and neuropilin-1 form a receptor complex that is responsible for the differential signaling potency of VEGF(165) and VEGF(121). J Biol Chem 276(27):25520–25531
Wiesmann C, Fuh G, Christinger HW, Eigenbrot C, Wells JA, de Vos AM (1997) Crystal structure at 1.7 Å resolution of VEGF in complex with domain 2 of the Flt-1 receptor. Cell 91(5):695–704
Wise LM, Veikkola T, Mercer AA, Savory LJ, Fleming SB, Caesar C, Vitali A, Makinen T, Alitalo K, Stacker SA (1999) Vascular endothelial growth factor (VEGF)-like protein from orf virus NZ2 binds to VEGFR2 and neuropilin-1. Proc Natl Acad Sci U S A 96(6):3071–3076
Wise LM, Ueda N, Dryden NH, Fleming SB, Caesar C, Roufail S, Achen MG, Stacker SA, Mercer AA (2003) Viral vascular endothelial growth factors vary extensively in amino acid sequence, receptor-binding specificities, and the ability to induce vascular permeability yet are uniformly active mitogens. J Biol Chem 278(39):38004–38014
Witte L, Hicklin DJ, Zhu Z, Pytowski B, Kotanides H, Rockwell P, Bohlen P (1998) Monoclonal antibodies targeting the VEGF receptor-2 (Flk1/KDR) as an anti-angiogenic therapeutic strategy. Cancer Metastasis Rev 17(2):155–161
Woolard J, Wang WY, Bevan HS, Qiu Y, Morbidelli L, Pritchard-Jones RO, Cui TG, Sugiono M, Waine E, Perrin R, Foster R, Digby-Bell J, Shields JD, Whittles CE, Mushens RE, Gillatt DA, Ziche M, Harper SJ, Bates DO (2004) VEGF165b, an inhibitory vascular endothelial growth factor splice variant: mechanism of action, in vivo effect on angiogenesis and endogenous protein expression. Cancer Res 64(21):7822–7835
Wu LW, Mayo LD, Dunbar JD, Kessler KM, Ozes ON, Warren RS, Donner DB (2000) VRAP is an adaptor protein that binds KDR, a receptor for vascular endothelial cell growth factor. J Biol Chem 275(9):6059–6062
Wu Y, Zhong Z, Huber J, Bassi R, Finnerty B, Corcoran E, Li H, Navarro E, Balderes P, Jimenez X, Koo H, Mangalampalli VR, Ludwig DL, Tonra JR, Hicklin DJ (2006) Anti-vascular endothelial growth factor receptor-1 antagonist antibody as a therapeutic agent for cancer. Clin Cancer Res 12(21):6573–6584
Yamazaki Y, Takani K, Atoda H, Morita T (2003) Snake venom vascular endothelial growth factors (VEGFs) exhibit potent activity through their specific recognition of KDR (VEGF receptor 2). J Biol Chem 278(52):51985–51988
Yang Y, Yuzawa S, Schlessinger J (2008) Contacts between membrane proximal regions of the PDGF receptor ectodomain are required for receptor activation but not for receptor dimerization. Proc Natl Acad Sci U S A 105(22):7681–7686
Yu Y, Lee P, Ke Y, Zhang Y, Chen J, Dai J, Li M, Zhu W, Yu GL (2013) Development of humanized rabbit monoclonal antibodies against vascular endothelial growth factor receptor 2 with potential antitumor effects. Biochem Biophys Res Commun 436(3):543–550
Yuzawa S, Opatowsky Y, Zhang Z, Mandiyan V, Lax I, Schlessinger J (2007) Structural basis for activation of the receptor tyrosine kinase KIT by stem cell factor. Cell 130(2):323–334
Zhang W, Ran S, Sambade M, Huang X, Thorpe PE (2002) A monoclonal antibody that blocks VEGF binding to VEGFR2 (KDR/Flk-1) inhibits vascular expression of Flk-1 and tumor growth in an orthotopic human breast cancer model. Angiogenesis 5(1–2):35–44
Zhu Z, Lu D, Kotanides H, Santiago A, Jimenez X, Simcox T, Hicklin D, Bohlen P, Witte L (1999) Inhibition of vascular endothelial growth factor induced mitogenesis of human endothelial cells by a chimeric anti-kinase insert domain- containing receptor antibody. Cancer Lett 136(2):203–213
Zhu Z, Hattori K, Zhang H, Jimenez X, Ludwig DL, Dias S, Kussie P, Koo H, Kim HJ, Lu D, Liu M, Tejada R, Friedrich M, Bohlen P, Witte L, Rafii S (2003) Inhibition of human leukemia in an animal model with human antibodies directed against vascular endothelial growth factor receptor 2. Correlation between antibody affinity and biological activity. Leukemia 17(3):604–611
Acknowledgements
The authors thank Swiss National Science Foundation (grant 31003A-130463 issued to K.B.–H. and PMCDP3-134208/1 issued to C.A.C.H.), Oncosuisse (grant OC2 01200-08-2007 issued to K.B.–H.) and NOVARTIS Stiftung für medizinisch-biologische Forschung (grant 10C61 issued to K.B.–H.).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer-Verlag France
About this chapter
Cite this chapter
Hyde, C.A.C., Berger, P., Ballmer-Hofer, K. (2014). Finding New Partnerships: The Function of Individual Extracellular Receptor Domains in Angiogenic Signalling by VEGF Receptors. In: Feige, JJ., Pagès, G., Soncin, F. (eds) Molecular Mechanisms of Angiogenesis. Springer, Paris. https://doi.org/10.1007/978-2-8178-0466-8_3
Download citation
DOI: https://doi.org/10.1007/978-2-8178-0466-8_3
Published:
Publisher Name: Springer, Paris
Print ISBN: 978-2-8178-0465-1
Online ISBN: 978-2-8178-0466-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)