Abstract
The use of anorectal physiological assessment in the management of patients undergoing reoperative surgery has diminished over the years; it has become clear that functional outcome does not directly correlate with preoperative manometry. Vector volume manometry and neurophysiologic testing are outlined in this chapter along with the interpretation techniques and limitations in their determination and assessment particularly as they pertain to the reoperative case.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Vector volume manometry
- Neurophysiologic testing (NPT)
- Reoperative
- Reconstructive
- Cortical somatosensory evoked potentials (CSSEPs)
Vector Volume Manometry
Longitudinal pressure profilometry is part of standard urodynamic practice [1] and forms the physiological basis of assessment of the lower esophageal sphincter before and after antireflux surgery [2]. However, it has been used only in a limited way in anorectal disease [3]. Longitudinal pressure asymmetry across the anal canal was first recorded by Taylor and colleagues [4] in patients with anal canals of varying lengths, in an effort to standardize anal pressure recordings in both men and women. They utilized a sliding double-lumen catheter, which resulted in the simultaneous measurement of longitudinal and radial pressure profiles. Traditional estimates of the high-pressure zone (HPZ) (at rest or during sustained squeeze) – the HPZ length represents that part of the sphincter where pressures exceed 50 % of the maximal pressure – are, at best, a crude reflection of the median physiological sphincter extent, which is not representative of the asymmetry expected between sexes or in patients with known anterior, postobstetric external anal sphincter (EAS) defects.
The recent introduction of an automated rapid pull-through method for conducting conventional anorectal manometry (ARM) assisted in minimizing these expected variations in functional anal sphincter length, which normally would compromise measurements [5, 6]. To conduct this new longitudinal pressure profilometry technique, called vector volume manometry (VVM), the polyethylene catheter used must be specialized at 4.8 mm in diameter (Arndorfer, Inc., Greenvale, WI) with at least eight radially disposed port holes for sectorial (anterior, right lateral, posterior, and left lateral) averaging; the more lumina that are used, the more data points will be obtained during catheter withdrawal and consequently the less mathematical interpolation required by the computer software (Polygram Lower GI edition software 5.05, C4 version, Synectics Medical, Inc., Irving, TX) to create a three-dimensional (3D) conformation of the anal sphincter zone, known as a vectorgram. This computer software transfers the data into a 3D triplet of coordinates, which is dependent upon the axial position of the probe, using specified vector calculation. Here, the x and y coordinates are recorded at an angulated point away from the central point of the anal canal, with the z axis determined vertically so that for any given 3D pressure point, a sector pressure polygon is created corresponding to the vector volume such that:
where 1 is the level above the anal verge with recordable pressures, d is the distance between station measurements, and P doublets of pressure are the pressure vectors for each sector wedge. The sine is 45 given the eight different channels used, but this would vary if more channels were employed. As such, the volume has no parameter representing the total integration of vector polygons at a given speed of catheter withdrawal. The PC is interfaced with a high-resolution color monitor, which has a graded palette to create a color-coded vectorgram that can be rotated to assess graphic indentations representing increases in overall sector pressure [7, 8]. For an average sphincter length at a withdrawal speed of 1 cm/s, there are an average of 15,000 individual data points with smoothing vectography provided by spline-curve interpolation to create the vectorgram. For this reason, an increase in the number of channels would result in less data interpolation and a smoother image. The vectorgram may be obtained either as an open mesh-net or as a solid-state design. Figure 7.1 shows the physical principles of vector volume construction.
Physical principles of vector volume construction/manometry. An eight-channel, radially disposed polyethylene catheter is used. At a constant rate of withdrawal, this provides a sector polygon of summated pressures at 45° angles that are interpreted to create a vector volume measurement and vectorgram. L left, LA left anterior, LP left posterior, A anterior, RA right anterior, R right, RP right posterior, P posterior (Reproduced with permission from Zbar et al. [9])
There are few comparisons of conventional ARM and VVM in either health [9] or disease [10]. Work comparing conventional ARM with VVM has been conducted in normal patients and those with passive fecal incontinence, full-thickness rectal prolapse, and chronic anal fissure, measuring the mean resting vector volume (MRVV), mean squeeze vector volume (MSVV), HPZ lengths (at rest and during squeeze), maximal averaged pressure at rest (MR), maximal averaged pressure during sustained squeeze (MPS), and the percentage asymmetry as the percent deviation of the integrated cross-sections from a perfect circle. Sectorial pressures have been pooled for analysis, creating right, left, anterior, and posterior mean pressures. As expected, significant differences at rest and during squeeze are demonstrable for MRVV, MSVV, MPR, and MPS that mirror those obtained with conventional ARM, whereas, in general, MPR values tend to exceed mean resting anal pressure, and MPS values tend to be lower, on average, than mean squeeze pressure values. It may be that during automated catheter withdrawal, there is some voluntary sphincter contraction that triggers the MPR recording to be higher than the mean resting anal pressure value obtained with conventional ARM. This also may result from perfusate leakage. The lower value of MPS over mean squeeze pressure (in conventional ARM) may reflect a greater difficulty in sustaining an adequate squeeze contraction during the vector volume technique (Table 7.1) [11].
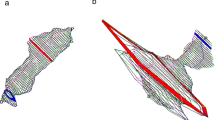
Although there is an expected sectorial ordering from patients with incontinence through to hypertonic anal fissure, there is no evidence of inherent sectorial differences for the different anorectal conditions, although there is a trend toward higher anterior sector pressures in patients with fissure. This sectorial variation has been identified in some other studies [12–14] and is shown in Fig. 7.2a, b. The correlation coefficients between conventional and vector volumetric variables for rest and squeeze confirm a strong correlation for HPZ length measurement with both techniques (see Table 7.1). There is, however, no correlation between sectorial asymmetry and demonstrable EAS defects [15] or in patients after internal anal sphincter (IAS) division for chronic anal fissure [16], although recent data has suggested that VVM may assist in defining those cases with an EAS defect in the last centimeter of the anal canal [17, 18]. It would seem, however, that when initial ultrasound is inconclusive in the diagnosis of a reparable sphincter defect, an ultrasonographically defined use of VVM would somewhat defeat its purpose [19, 20]. Further recent data have shown significant differences in all vector resting parameters, HPZ length at rest, and percentage asymmetry at rest, along with changes in most squeeze variables, after IAS division for topically resistant chronic anal fissure [16], with marked differences between postoperative continent and incontinent cohorts, particularly in resting HPZ length. This latter finding may suggest an overly zealous internal anal sphincterotomy that has been previously endosonographically recorded [21] and during which the extent of IAS division often can be far greater than intended. In continent postoperative patients, percentage resting asymmetry tends to increase (by about 6.7 %), whereas in incontinent postoperative cases it tends to fall (by about 3.1 %). The explanations for these changes in squeeze parameters are not understood, but it is conceivable that there is excessive voluntary sphincter fatigue after surgery between groups (even in continent cohorts), where impending leakage occurs because contents entering the anal canal after rectal motor activity are poorly discriminated. This latter phenomenon, known as “anorectal sampling,” permitting the distinction between flatus and feces, is discussed in the section in Chap. 6 on the rectoanal inhibitory reflex and is believed to represent one of the functions of the IAS [22]. It also may be that in some patients there is a constitutively deficient subcutaneous, overlapping segment of the EAS (as has been shown using endoanal magnetic resonance imaging preoperatively in some patients with fissure [23]) so that distal IAS division will render the distal anal canal unsupported and lead to incontinence and possible attendant weakness in postoperative squeeze function [24]. At this time, the role of VVM must still be regarded as experimental, although it has provided an interesting tool for the study of sectorial sphincter asymmetry. The equipment and software is expensive and not widely available, but as a manometric instrument, VVM has been validated sufficiently [25]. It is conceivable that, with its use in prospective trials of patients with fissure, it could identify those patients who are likely to function poorly after IAS division and assist in the decision making for sphincter-sparing surgical alternatives [26, 27]. Further specific parameter assessment may delineate subtle dysfunctions that may predictably respond to directed biofeedback therapies in some forms of incontinence after anorectal surgery and that may better identify patients more suited to neorectal reservoir reconstruction or who are precluded from perineal rectosigmoidectomy for rectal prolapse [28–30].
(a) Sectorial pressures at rest (means and 95 % confidence intervals) as derived from vector volumetry for different anorectal conditions. (b) Sectorial pressures during sustained squeeze (means plus 95 % confidence intrervals) as derived from vectorvoumetry for different anorectal conditions. Prol prolapse, CAF chronic anal fissure, (Reprinted with permission from Zbar et al. [9])
Neurophysiologic Testing
Traditional neurophysiologic testing (NPT) in the anal canal consisted of the use of painful concentric needle electromyography (CNEMG) and single-fiber electromyography (SFEMG); techniques that were designed to aid operative decision making for EAS sphincter repair. This was coupled with the use of the endoanal of pudendal nerve terminal motor latency (PNTML) assessment using contact electromyography (EMG) where it had been deemed that extensive (particularly bilateral) pudendal neuropathy was a negative prognostic variable for the prolonged successful outcome of sphincteroplasty in incontinence. In the first case, the advent of validated endoanal ultrasonography has obviated the need for CNEMG and SFEMG [31, 32], whereas PNTML assessment has not precluded the successful use of sphincter repair [33]. NPT has taken on a resurgence, initially with an improved understanding of the mechanism of action of dynamic graciloplasty in fecal incontinence (covered elsewhere in this book), where there was a need to correlate the changes in muscle physiology predictive of a successful outcome. There also has been an increase in the use of sacral neuromodulation and peripheral nerve stimulation techniques (largely for incontinence but also in some forms of slow- and normal-transit severe constipation), where the assessment of the central mechanisms using somatosensory evoked potentials has proven to be of value in the prediction of longer-term success. Much of this work has come about as a translation of neurophysiologic and neuroanatomic understanding in urodynamics. Standard testing using NPT technology that was part of routine anorectal practice today has only a specialized place in the treatment of those with incontinence and in the assessment of reoperative cases [34].
Nerve conduction and EMG studies measure the efferent (motor) innervations; afferent fiber injury is more difficult to quantify. Traditional EMG was first described by Beck [35] in 1930, [35] with the design of a concentric needle by Adrian and Bronck [36] in 1929 adapted for its use; the basic technique differs little from these initial descriptions. Within this estimation, a motor unit consists of a single anterior horn cell, all its peripheral nerve fibers, motor end plates, and the muscle fibers it innervates. One muscle fiber (MF) is innervated by a single motor neuron (MN), but one MN can innervate many MFs. The composition of an MF depends on its functional demands, where striated MFs typically are divided into two main types, namely, type I and type II. Type I muscle units are slow tonic fibers, whereas type II fibers are fast phasic fibers [37]. In this context, the MFs of the levator ani are mostly type I maintaining constant tone and type II MFs are more widely distributed in perianal and periurethral sites [38]. The use of dynamic graciloplasty as a stimulated technique of the anal sphincter aims to convert predominantly type II into type I fibers by conditioning, an effect that has been shown to occur in successful cases using immunohistopathology.
For the purposes of recording, during voluntary contraction of individual units within a given muscle, these units combine into a motor unit potential (MUP), where the amplitude of the signal obtained contains each single fiber potential, and the shape of the signal depends upon the number of fibers discharging simultaneously. The duration of the signal is the time between the first recorded deflection and its return to baseline. For the anus, EMG can be performed by surface, concentric needle (CN), single fiber (SF) and wire electrodes. EMG mapping of the anal sphincters has largely become unnecessarily invasive, and although puborectalis EMG may be more accurate, particularly during provocative maneuvers, dynamic magnetic resonance imaging (MRI) has largely replaced its use. If EMG is to be used at all, it has a specialized role in an ever-diminishing number of laboratories where the overall experience of its use is now restricted. It will define MF denervation and reinnervation and some cases of sphincter integrity where there is doubt based on ultrasonography or MRI doubt. The latter is of little consequence in some cases of failed sphincteroplasty because many of these patients will be considered for sacral nerve stimulation, regardless of the status of their sphincter integrity. The medium-term data in this area, however, are still not available and often includes an eclectic group of patients that may not be strictly comparable [39]. It is potentially possible that EMG recordings may have clinical benefit in some cases of poor functional outcome after the repair of anorectal malformations [40].
CNEMG of the EAS (Fig. 7.3) was the first technique used, and it evaluates spontaneous activity, recruitment patterns, and MUP waveforms. The concentric needle consists of a fine (0.7 mm) platinum wire mounted inside a metal cannula with a larger diameter, about 65 mm wide, so that the inner wire is fully insulated. Optimal recording occurs with a frequency range of 10 Hz–10 KHz and a sensitivity of 100–500 mV, with a sweep speed of 20 ms per record at rest and during squeeze, strain, and cough. The introduction of the CN electrode on to the left and right sides of the sphincter is accompanied by a reactive MUP discharge that is separable from an initial insertion discharge, an effect that disappears rapidly when the patient relaxes during the procedure. In those cases of denervation, normal MUP activity is replaced by fibrillation denervation potentials with separable EAS and puborectalis recordings. Each of these activities are separated during provocative maneuvers as the needle is both advanced and withdrawn. Abnormal waveforms will be evident with a reduction in the number of MFs or in denervation. During the reinnervation process that occurs from preserved axons, motor units tend to have larger amplitudes, have longer durations, and become polyphasic in character. In myopathic states, the motor units tend to have lower overall amplitudes and be low duration waveforms. The technique also can better define paradoxical puborectalis syndrome with increased activity during strain [41]. Overall decreased amplitudes, and activity is seen in postobstetric or traumatic EAS damage. In some cases, spontaneous fibrillation and myoclonia in underlying neurological disorders [42].
Concentric needle electromyographic recording of the external anal sphincter (Reprinted with permission from Rosato and Lumi [46])
SFEMG was originally described by Stälberg and Trontelj [43] to record individual MF action potentials as a complement to CNEMG [43]. An electrode with a smaller needle and a surface of only 25 μm is used and is capable of detecting electrical signals over a recording surface of only 0.0003 mm2. Single fiber potentials (SFPs) have a shorter duration, higher amplitude, and more rapid rise time than those obtained with CNEMG, providing specific information about fiber density (the mean number obtained from an analysis of MF potentials in multiple different positions within the same muscle). An increase in recordable SFPs (>100 μV) reflect reinnervation as a result of nerve sprouting, showing that local axons are innervating new MFs, with an increase in fiber density preceding CNEMG changes. This data must be correlated with age where there is a trend towards an increase in fiber density (over the normal of 1.5 ± 0.16) over the age of 60 years [44–46]. Recently, anal sphincter EMG has been reintroduced in specialized circumstances for the diagnosis of atypical parkinsonism where there are subtle changes in motor evoked potentials of the EAS in the absence of demonstrable peripheral neuropathy [47]. It has been used intraoperatively during spinal surgery [48] and in autonomic disorders [49]. High-density surface EMG has been used in a diagnostic setting to define zones of EAS innervation, although its clinical applications are as yet untested [50]. In newer modeling of conventional EMG, Bayesian analysis seems to better characterize EMG MUPs than the visual separation into “normal” and “abnormal” [51] through the use of newer probe electrodes that are more selective and provide faster decays for different depths of deployment and smaller detectable signals [52].
The use of pudendal nerve stimulation (the pudendal nerve terminal motor latency test [PNTML]) was originally devised by Kiff and colleagues [53] and assesses the distal motor innervations of the pelvic floor musculature, measuring the time interval between the neural stimulus and the muscle response (Fig. 7.4). For this purpose, a patented, disposable, self-adhesive electrode has been developed (Dantec Electronic Tonsbakken 16–18 DK-2740, Skovlunde Denmark and St. Mark’s Hospital). This is pre-attached to the volar aspect of the index finger of a glove and consists of a bipolar stimulating electrode with the recording electrode placed a standardized distance away at the base of the finger. Direction of the recording is made by moving the index finger toward the ischial spine until a signal is obtained, with stimulation providing a square stimulus of 0.1–0.2 ms at 1-s intervals up to but not exceeding 15 mA. The maneuver is performed for the left pudendal nerve by turning the examining finger 180 degrees, resulting in an inverted wave form. Normal PNTML values are designated as 2.0 ± 0.2 ms per side; prolongation of this value has been shown after vaginal delivery, with rectal prolapse, and during aging [54]. The presence of pudendal neuropathy (defined on the basis of slowed PNTML), particularly if bilateral neuropathy was thought to preclude successful sphincter repair, but it is currently regarded as only a relative contraindication and merely as part of the counseling of incontinent patients against the longer-term success of the procedure [33, 45, 55]. There is, however, a clear correlation between incontinence scores and MUP recruitment and measurable PNTML values at both the puborectalis and EAS levels for both right and left sides [56]. These correlations are complex: A prolonged PNTML and an abnormal SFEMG do not always correspond to the incontinence score. In constipation, the recruitment MUPs may be assessed by asking patients to relax their sphincter, and CNEMG can assist in the diagnosis of paradoxical puborectalis contraction or incomplete relaxation. In this setting, EMG may have a role in this specific diagnosis. Its value in the IAS in states such as fissure, which has been reported in the past, seems to have largely been abandoned in the anorectal laboratory [56]. Nowadays, multichannel surface EMG for endoanal insertion and signal recording at different depths within the canal have been developed for less invasive use and can provide accurate information about the innervation zones, fiber length, amplitude, repetitive firing frequency of individual MUPs, and conduction velocity using innovative circumferential signaling technology, which may prove to be of value in those cases with doubtful sphincter integrity based on endoanal ultrasonography and in the diagnosis of anismus in cases of evacuatory difficulty. This technology may also provide more objective information regarding rehabilitative therapies in functional disease [57, 58].
Technique of pudendal nerve terminal motor latency measurement (Reprinted with permission from Rosato and Lumi [46])
Cortical Somatosensory Evoked Potentials
In some laboratories, the measurement of cortical somatosensory evoked potentials (CSSEPs) has been introduced to determine some cases of successful sacral neuromodulation in patients, particularly those presenting with intractable fecal incontinence. This topic is covered in greater detail in this book in Chap. 35, but the mechanisms of sacral nerve stimulation (SNS) are currently poorly understood [59]. CSSEPs have been used extensively in voiding dysfunction including symptoms of frequency, urgency, urge incontinence, urinary retention, and painful bladder syndrome [60], where positron emission tomography parameter mapping analysis in regions of interest between responders and nonresponders has been examined with stimulation off/ and anticipation of neurostimulation/threshold stimulation, which have shown silenced premotor, somatosensory cycles, and supplementary motor cortices during the on mode, with secondary stimulation of the amygdala and frontal cortices and stronger deactivation of the right premotor cortex in responders [61]. Equally, there is a significant but poorly understood central effect of both sacral and peripheral nerve stimulation, wherein SNS produces a significant decrease in pudendal somatosensory evoked potentials latency at its first deflection at both the ipsilateral and contralateral sites. In this sense, SNS modulates cortical activity at the afferent level to the cortical sensory area, and this finding is believed to function as a prognostic factor for successful SNS outcomes [62]. Similar effects have been noted with the use of surface sacral electrical stimulation [60].
The chemical basis of this action also is unknown, although experimental studies of spinally transected rats have shown that urinary hyperreflexia associated with spinal transection is abolished by percutaneous SNS and is associated with an attenuated rise in various neuropeptides such as substance P, neurokinin A, and calcitonin gene--related peptide released from the dorsal root ganglia, suggesting that blockade of C-afferent fiber activity is one of the mechanisms of SNS action [63]. The effects here are complex; they have been associated with an impaired perfusion on single photon emission computed tomography analysis of the frontal brain areas of the right anterior cingulated gyrus and right inferior frontal gyrus, which are involved in voluntary voiding in elderly men, so that there is a peripheral and central neuropeptide-brain axis in response to stimuli [64, 65].
These effects have been assessed in women with implanted neurostimulators presenting with urge incontinence. The effects on peripheral sensory, spinal, and cortical areas are unknown, and because the optimum result is not achieved initially, there is some plasticity centrally. During SNS assessment by positron emission tomography, there are significant decreases in cerebral blood flow in the middle part of the cingulate gyrus, the ventromedial orbitofrontal cortex, the midbrain, and the adjacent midline thalamus, with an increase in flow in the dorsolateral prefrontal cortex. There are differences in the early part of SNS, when during initial stimulation there is a decrease in the cerebral blood flow in the middle cerebellum and increases in the right postcentral gyrus, insular region, and ventromedial orbitofrontal cortex. In those with chronic implantation, however, this shifts so that there are differences in group analysis of the sensory cortex, premotor cortex, and the cerebellum, all of which are areas involved in learning behavior. This effect is probably influenced by the spinal cord to re-educate the brain in areas implicated in learning behavior so that acute SNS modulates areas involved in sensorimotor learning, presumably become less active during the chronic course of SNS stimulation [66]. At this stage, the central functional equivalents in anal sphincter control are less well known than they are in urinary incontinence, although positron emission tomography differences recently have been shown in the premotor/anterior cingulate gyrus and the putamen/claustrum/insula region in patients with frontotemporal degenerative disease and incontinence [67]. In a recent study of inferior rectal nerve crush to induce an animal model of fecal incontinence for study, Pierce and colleagues [68] have shown reduced CSSEPs in the absence of morphologic effects on the nerve, suggesting an alteration in cortical awareness as a form of process modification that is central and not peripheral. This alters second-order neuronal excitation and central input. There is some corroborative evidence to show (using magnetic encephalography) that CSSEPs after sacral stimulation with surface electrodes occurs in healthy subjects; Sheldon et al. [69] showed an alteration by peripheral nerve injury of sensory afferent activity, which may occur in the absence of peripheral nerve myelination [69].
Electrophysiological activation of the motor pathways also can be stimulated by magnetic as well as electrical stimulation [70], an effect that is cortically enhanced by prior lumbosacral nerve stimulation, pudendal nerve stimulation, or both [71]. This effect is asymmetric with dominant pudendal stimulation, exerting a greater effect so that the central pathway is affected by the pre-existing state of pudendal nerve conditioning [72]. These results have to be interpreted with caution because they are dependent upon the site of sacral magnetic stimulation and the limited magnetic field strength used [73]. This type of stimulation physiologically reduces rectal volume/capacity, with differential central effects on the anorectal musculature when compared with peripheral muscles when assessing motor threshold, intracortical facilitation, and cortical silent periods during repeated stimulation [74]. These issues are complex and dependent upon the frequency and chronicity of stimulation; in successful cases of SNS, cortical mapping shows a decrease in representation and overall excitability immediately after SNS commences, but this decrease disappears after SNS removal. Anal EMG is corepresented with this cortical stimulation over multiple points using a scalp grid covering the bilateral medial cortex [75], although this does not actually correlate with any demonstrable improvement in anorectal manometry [76].
Conclusion
The use of CSSEPs is, of course, experimental and specialized, but future work probably will show a coordination and differentiation with SNS and peripheral nerve stimulation for indicated use in coloproctology, particularly in patients presenting with intractable fecal incontinence for whom other conventional approaches have failed.
References
Klopper PJ, de Haas F, Dijkhuis T. Computerized experimental multichannel urethral pressure profilometry. World J Urol. 1990;8:159–62.
Bombeck CT, Vaz O, DeSalvo J, Donahue PE, Nyhus LM. Computerized axial manometry of the esophagus: a new method for the assessment of antireflux operations. Ann Surg. 1987;206:465–72.
Perry RE, Blatchford GJ, Christensen MA, Thorson AG, Attwood SEA. Manometric diagnosis of anal sphincter injuries. Am J Surg. 1990;159:112–7.
Taylor BM, Beart RW, Phillips SF. Longitudinal and radial variations of pressure in \the human anal sphincter. Gastroenterology. 1984;86:693–7.
Coller JA. Clinical application of anorectal manometry. Surg Clin N Am. 1987;16:17–33.
Coller JA, Sangwan Y. Computerized anal sphincter manometry performance and analysis. In: Smith LE, editor. Practical guide to anorectal testing. 2nd ed. New York: Igaku-Shoin; 1995. p. 51–100.
Dijkhuis T, Bendiman WA, van der Hulst VPM, Klopper PJ. Graphical representation of eight-channel pressure profiles on a personal computer. Med Biol Eng Comput. 1990;28:502–4.
Waegermaekers CTBJ. Profilemeting van de distal oesophagus sphincter [thesis]. University of Amsterdam, Amsterdam, 1980
Zbar AP, Aslam M, Hider A, Toomey P, Kmiot WA. Comparison of vector volume manometry and conventional manometry in anorectal dysfunction. Tech Coloproctol. 1998;2:84–90.
Zbar A. Vectorvolume manometry. In: Wexner SD, Zbar AP, Pescatori M, editors. Complex anorectal disorders: investigation and management. New York: Springer; 2005. p. 48–62.
Braun JC, Trentner KH, Drew B, Klimaszewski M, Schumpelick V. Vector manometry for differential diagnosis of fecal incontinence. Dis Colon Rectum. 1994;37:989–96.
Keck JO, Staniunas RJ, Coller JA, Barrett RC, Oster ME. Computer-generated profiles of the anal canal in patients with anal fissure. Dis Colon Rectum. 1995;38:72–9.
Williams N, Barlow J, Hobson A, Scott N, Irving M. Manometric asymmetry in the anal canal in controls and patients with faecal incontinence. Dis Colon Rectum. 1995;38:1275–80.
Williams N, Scott A, Irving M. Effect of lateral sphincterotomy on internal anal sphincter function: a computerized vector manometric study. Dis Colon Rectum. 1995;38:700–4.
Sultan AH, Kamm MA, Nicholls RJ, Bartram CI. Prospective study of the extent of internal anal sphincter division during lateral sphincterotomy. Dis Colon Rectum. 1994;37:1031–3.
Zbar AP, Aslam M, Allgar V. Faecal incontinence after internal sphincterotomy for anal fissure. Tech Coloproctol. 2000;4:25–8.
Grande M, Caddedu F, Sileri P, Ciano P, Attina GM, Selvaggio I, et al. Anal vector volume analysis: an effective tool in the management of pelvic floor disorders. Tech Coloproctol. 2011;15:31–7. Epub 2010 Dec 14.
Schizas AMP, Emmanuel AV, Williams AB. Anal canal vector volume manometry. Dis Colon Rectum. 2011;54:759–68.
Zbar AP. Reading too much into anal vectorvolumetric parameters: correspondence for “anal vector volume analysis: an effective tool in the management of pelvic floor disorders”. Tech Coloproctol. 2011;15(4):473–4. Epub 2011 Nov 9.
Zbar AP. Anal vectorvolumetry: a bridge too far. Dis Colon Rectum. 2011;54(10):e258. author reply e258.
Sultan AH, Kamm MA, Nicholls RJ, Bartram CI. Prospective study of the extent of internal anal sphincter division during lateral sphincterotomy. Dis Colon Rectum. 1994 Oct;37(10):1031–3.
Miller R, Bartolo DCC, Cervero F, Mortensen NJ. Anorectal sampling: a comparison of normal and incontinent patients. Br J Surg. 1988;75:44.
Zbar AP, Kmiot WA, Aslam M, Williams A, Hider A, Audisio RA, et al. Use of vector volume manometry and endoanal magnetic resonance imaging in the adult female for assessment of anal sphincter dysfunction. Dis Colon Rectum. 1999;42:928–33.
Zbar AP, Khaikin M. For debate: should we care about the internal anal sphincter? Dis Colon Rectum. 2012;55(1):105–8.
Yang Y-K, Wexner SD. Anal pressure vectography is of no apparent benefit for sphincter evaluation. Int J Colorectal Dis. 1994;9:92–5.
Hussein AM, Shehata MAS, Zaki Y, El Seweify MEA. Computerized anal vector manometric analysis in patients with tailored lateral sphincterotomy for anal fissure. Tech Coloproctol. 2000;4:143–9.
Giordano P, Gravante G, Grondona P, Ruggiero B, Porrett T, Lunniss PJ. Simple cutaneous advancement flap anoplasty for resistant chronic anal fissure: a prospective study. World J Surg. 2009;33:1058–63.
Pucciani F, Rottoli ML, Bologna A, Cianchi F, Forconi S, Cutelle M, et al. Pelvic floor dyssynergia and bimodal rehabilitation: results of combined pelviperineal kinesitherapy and biofeedback training. Int J Colorectal Dis. 1998;13:124–30.
Glasgow SC, Birnbaum EH, Kodner IJ, et al. Preoperative anal manometry predicts continence after perineal proctectomy for rectal prolapse. Dis Colon Rectum. 2006;49:1052–8.
Zbar AP, Nguyen H. Management guidelines in the treatment of full-thickness prolapse. In: Altomare D, Pucciani F, editors. Rectal Prolapse: Diagnosis and Clinical Management. Rome: Springer; 2008. p. 201–6.
Tjandra JJ, Milsom JW, Schroeder T, Fazio VW. Endoluminal ultrasound is preferable to electromyography in mapping anal sphincteric defects. Dis Colon Rectum. 1993;36:689–92.
Sultan AH, Kamm MA, Talbot IC, Nicholls RJ, Bartram CI. Anal endosonography for identifying external anal sphincter defects confirmed histologically. Br J Surg. 1994;81:463–5.
Chen AS, Luchtefeld MA, Senagore AJ, MacKeigan JM, Hoyt C. Pudendal nerve latency: does it predict outcome after of anal sphincter repair? Dis Colon Rectum. 1998;41:1005–9.
Rosato GO, Lumi CM, Miguel AA. Anal sphincter electromyography and pudendal nerve terminal motor latency assessment. Semin Colon Rectal Surg. 1992;3:68–74.
Beck A. Electromyographische untersuchungen am sphincter ani. Arch Physiol. 1930;224:278–92.
Adrian ED, Bronk DW. The discharge of impulsesd in motor nerve fibers. II. The frequency of discharge in reflex and voluntary contractions. J Physiol. 1929;67:119–51.
Buchtal F. The general concept of the motor unit: neuromuscular disorders. Res Publ Assoc Res Nerv Ment Dis. 1961;38:3–30.
Pette D. Fiber transformation and fiber replacement in chronically stimulated muscle. J Heart Lung Transplant. 1992;11:S299–305.
Chan MK, Tjandra JJ. Sacral nerve stimulation for fecal incontinence: external anal sphincter defect vs. intact anal sphincter. Dis Colon Rectum. 2008;51:1015–25.
Iwai N, Kanida H, Taniguchi H, Tsuto T, Yanigahara J, Takahashi T. Postoperative continence assessed by electromyography of the external sphincterin anorectal malformations. Z Kinderchir. 1985;40:87–90.
Wexner SD, Cheape JD, Jorge JMN, Heymen S, Jagelman DG. A prospective assessment of biofeedback for the treatment of paradoxical puborectalis contraction. Dis Colon Rectum. 1992;35:145–50.
Fowler CJ. Pelvic floor neurophysiology. Meth Clin Neurophysiol. 1991;2:1–20.
Stälberg E, Trontelj J. Single fiber electromyography. Surrey: Mirvalle; 1979.
Snooks SJ, Swash M. Nerve stimulation techniques. In: Henry MM, Swash M, editors. Coloproctology and the pelvic floor: pathophysiology and management. London: Butterworths; 1985. p. 184–206.
Lauerberg S, Swash M. Effects of ageing on anorectal sphincters and their innervations. Dis Colon Rectum. 1989;32:737–42.
Rosato O, Lumi CM. Neurophysiology in pelvic floor disorders. In: Wexner SD, Zbar AP, Pescatori M, editors. Complex anorectal disorders: investigation and management. London: Springer; 2005. p. 153–69.
Winge K, Jennum P, Lokkegaard A, Werdelin L. Anal sphincter EMG in the diagnosis of Parkinsonian syndromes. Acta Neurol Scand. 2010;121:198–203. Epub 2009 Sept 24.
Khealanai B, Husain AM. Neuwophysiologic intraoperative monitoring during surgery for tethered cord syndrome. J Clin Neurophysiol. 2009;26:76–81.
Sakakibara R, Uchiyama T, Yamanishi T, Kishi M. Sphincter EMG as a diagnostic tool in autonomic disorders. Clin Auton Res. 2009;19:20–31.
Mesin L, Gazzoni M, Merletti R. Automatic localisation of innervations zones: a simulation study of the external anal sphincter. J Electromyogr Kinesiol. 2009;19:e413–21.
Pino LJ, Stashuk DW, Podnar S. Bayesian characterization of external anal sphincter muscles using quantitative electromyography. Clin Neurophysiol. 2008;119:2266–73.
Mesin L, Gervasio R. Detection volume of simulated electrode systems for recording sphincter muscle electromyogram. Med Eng Phys. 2008;30:896–904.
Kiff ES, Swash M. Normal proximal and delayed distal conduction in the pudendal nerves of patients with idiopathic (neurogenic) faecal incontinence. J Neurol Neurosurg Psychiatry. 1984;47:820–3.
Ryhammer AM, Lauerberg S, Hermann P. Long-term effect of vaginal deliveries on anorectal function in normal perimenopausal women. Dis Colon Rectum. 1996;39:852–9.
Sangwan YP, Coller JA, Barrett RC, Roberts PL, Murray JJ, Rusin L. Unilateral pudendal neuropathy. Impact on outcome of anal sphincter repair. Dis Colon Rectum. 1996;39:686–9.
Sorensen M, Nielsen MB, Pedersen JF, Christiansen J. Electromyography of the internal anal sphincter performed under endosonographic guidance. Description of a new method. Dis Colon Rectum. 1994;37:138–43.
Merletti R, Bottin A, Cescon C, Farina D, Gazzoni M, Martina S, et al. Multichannel surface EMG for the non-invasive assessment of the anal sphincter muscle. Digestion. 2004;69:112–22.
Enck P, Hinninghofen H, Merletti R, Azpiroz F. The external anal sphincter and the role of surface electromyography. Neurogastroenterol Motil. 2005;17 Suppl 1:60–7.
Matzel K, Matzel KE. Sacral nerve stimulation for fecal disorders: evolution, current status, and future directions. Acta Neurochir. 2007;97(Pt 1):351–7. Review.
Matsushita M, Nakasato N, Nakagawa H, Kanno A, Kaiho Y, Arai Y. Primary somatosensory evoked magnetic fields elicited by sacral surface electrical stimulation. Neurosci Lett. 2008;431:77–80.
Malaguti S, Spinelli M, Giardello G, Lazzeri M, Van Den Hombergh U. Neurophysiological evidence may predict the outcome e of sacral neuromodulation. J Urol. 2003;170(6 Pt 1):2323–6.
Govaert B, Melenhorst J, Nieman FH, Bols EM, van Gemert WG, Baeten CG. Factors associated with percutaneous nerve evaluation and permanent sacral nerve modulation outcome in patients with fecal incontinence. Dis Colon Rectum. 2009;52:1688–94.
Shaker H, Wang Y, Loung D, Balbaa L, Fehlings MG, Hassouna MM. Role of C-afferent fibres in the mechanism of action of sacral root neuromodulation in chronic spinal cord injury. BJU Int. 2000;85:905–10.
Griffiths D. Clinical studies of cerebral and urinary tract function in elderly people with urinary incontinence. Behav Brain Res. 1998;92:151–5.
Blok BF, Stums LM, Holstege G. Brain activation during micturition in women. Brain. 1998;121(Pt 11):2033–42.
Blok BF, Groen J, Bosch JL, Veltman DJ, Lammertsmaa AA. Different brain effects during chronic and acute sacral neuromodulation in urge incontinent patients with implanted neurostimulators. BJU Int. 2006;98:1238–43.
Perneczky R, Diehl-Schmid J, Forstl H, Drzezga A, May F, Kurz A. Urinary incontinence and its functional anatomy in frontotemporal lobar degenerations. Eur J Nucl Med Mol Imaging. 2008;35:605–10.
Pierce C, Healy CF, O’Herlihy C, O’Connell PR, Jones JFX. Reduced somatosensory cortical activation in experimental models of neuropathic fecal incontinence. Dis Colon Rectum. 2009;52:1417–22.
Sheldon R, Kiff ES, Clarke A, Harris ML, Hamdy S. Sacral nerve stimulation reduces corticoanal excitability in patients with faecal incontinence. Br J Surg. 2005;92:1423–31.
Pellicione G, Scarpinoi O, Piloni V. Motor evoked potentials recorded from external anal sphincter by cortical and lumbo-sacral magnetic stimulation: normative data. J Neurol Sci. 1997;149:69–72.
Hamdy S, Enck P, Aziz Q, Rothwell JC, Uengdergil S, Hobson A, et al. Spinal and pudendal nerve modulation of human corticoanal motor pathways. Am J Physiol. 1998;274(2 Pt 1):G419–23.
Hamdy S, Enck P, Aziz Q, Unergoergil S, Hobson A, Thompson DG. Laterality effects of human pudendal nerve stimulation on corticoanal pathways: evidence for functional asymmetry. Gut. 1999;45:58–63.
Morren GL, Walter S, Lindehammar H, Halböök O, Sjödahl R. Evaluation of the sacroanal motor pathway by magnetic and electrical stimulation in patients with fecal incontinence. Dis Colon Rectum. 2001;44:167–72.
Morren GL, Walter S, Halböök O, Sjödahl R. Effects of magnetic sacral root stimulation on anorectal pressure and volume. Dis Colon Rectum. 2001;44:1827–33.
Lefaucheur JP. Excitability of the motor cortical representation of the external anal sphincter. Exp Brain Res. 2005;160:268–72.
Harris ML, Singh S, Rothwell J, Thompson DG, Hamdy S. Rapid rate magnetic stimulation of human sacral nerve roots alters excitability within the cortico-anal pathway. Neurogastroenterol Motil. 2008;20:1132–9.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer-Verlag London
About this chapter
Cite this chapter
Zbar, A.P. (2013). Vector Volume Manometry and Neurophysiologic Assessment in the Reoperative Case: Recommendations. In: Zbar, A., Madoff, R., Wexner, S. (eds) Reconstructive Surgery of the Rectum, Anus and Perineum. Springer, London. https://doi.org/10.1007/978-1-84882-413-3_7
Download citation
DOI: https://doi.org/10.1007/978-1-84882-413-3_7
Published:
Publisher Name: Springer, London
Print ISBN: 978-1-84882-412-6
Online ISBN: 978-1-84882-413-3
eBook Packages: MedicineMedicine (R0)