Abstract
Human breast milk is widely considered the optimal nutrition for the newborn infant. Aside from providing the neonate with the nutritional needs for growth and development, breast milk also contains a plethora of bioactive factors that promote health and offer protection from infections. Human milk oligosaccharides (HMO), unconjugated, complex carbohydrates, are present in human milk at 10–20 g/L, a concentration only surpassed by lactose (Lac) and lipids, and often higher than that of total protein. Bovine milk, the basis of most infant formula, is a scarce source of oligosaccharides, which are also structurally different and less complex. High abundance and structural complexity of HMO are unique to human milk, raising questions about their biological roles and potential benefits for the human infant.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
FormalPara Key Points-
Human milk oligosaccharides (HMO) are complex glycans that are highly abundant in human milk, but not in infant formula.
-
HMO are considered prebiotics as they are preferentially metabolized by specific beneficial bacteria in the infant’s intestine.
-
HMO are considered antimicrobial as they serve as soluble decoy receptors that block the attachment of microbial pathogens to the host’s mucosal surfaces in the gastrointestinal, respiratory, and urogenital tract.
-
HMO are considered immune modulators as they may interfere with leukocyte extravasation and activation.
-
HMO-derived sialic acid may be an essential nutrient for brain development and cognition.
-
HMO may protect preterm infants from necrotizing enterocolitis.
-
Most of the data on the beneficial effects of HMO stem from in vitro or ex vivo studies or are derived from animal models. Data from human intervention studies are currently not available.
-
HMO effects are often highly structure-specific and the structurally different nonhuman oligosaccharides currently added to infant formula are likely not able to mimic the full spectrum of HMO benefits.
Introduction
Human breast milk is widely considered the optimal nutrition for the newborn infant. Aside from providing the neonate with the nutritional needs for growth and development, breast milk also contains a plethora of bioactive factors that promote health and offer protection from infections. Human milk oligosaccharides (HMO), unconjugated, complex carbohydrates, are present in human milk at 10–20 g/L, a concentration only surpassed by lactose (Lac) and lipids, and often higher than that of total protein. Bovine milk, the basis of most infant formula, is a scarce source of oligosaccharides, which are also structurally different and less complex. High abundance and structural complexity of HMO are unique to human milk, raising questions about their biological roles and potential benefits for the human infant.
Research during the last decades has implicated functions for HMO in the healthy colonization of the neonatal gut, protection from infections, maturation of the immune system and neuronal development. This chapter reviews recent advances in HMO research and discusses HMO as putative prebiotics, anti-infective and anti-inflammatory agents, immune modulators, signaling molecules and nutrients for neurological development.
HMO Structures and Composition
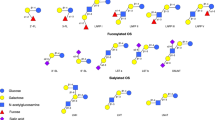
The basic structural layout of HMO shows Lac at the reducing end, which can be elongated by repeating disaccharide units of galactose (Gal) and N-acetylglucosamine (GlcNAc). Up to 15 of these Gal/GlcNAc disaccharide building blocks can be linked via β1-3 or β1-6 glycosidic bonds, forming highly complex linear or branched HMO core structures (see Fig. 14.1). Chains ending in a lacto-N-biose unit (Galβ1-3GlcNAc) are classified as type 1; chains with a terminal N-acetyllactosamine (Galβ1-4GlcNAc) are categorized as type 2. Lac or the poly-lacto-N-biose/N-acetyllactosamine core can be modified by sialic acid (Sia) and/or fucose (Fuc). Sia (in humans exclusively as N-acetylneuraminic acid, Neu5Ac) occurs in α2-3 and/or α2-6 linkages and introduces a negatively charged carboxyl-group and acidic properties, which led to the term acidic HMO. In contrast, nonsialylated HMO are named neutral HMO. Acidic or neutral HMO can be decorated with Fuc in α1-2, α1-3, and/or α1-4 linkage. Neutral HMO form the major fraction, with a high prevalence of fucosylated structures; less than 20% of HMO are sialylated. To date, the structures of more than a hundred different complex HMO have been elucidated (reviewed in Bode [1]).
Bovine milk oligosaccharides (BMO) are remarkably different in their structural layout. Disaccharides other than Lac can build the reducing end of a core BMO structure, or are found as such in bovine milk. Furthermore, the elongation of the BMO core structures is not limited to disaccharide repeats, resulting in various simple trisaccharides not found in human milk. Compared to HMO, BMO are shorter, less complex and carry more Sia. In addition to Neu5Ac, approximately 10% of the Sia on BMO is N-glycolylneuraminic acid (Neu5Gc), a specific nonhuman Sia derivative. In contrast to human milk, fucosylated oligosaccharides form only a marginal fraction in BMO (reviewed in Chichlowski et al. [2]). Major differences in the composition of human and bovine milk are listed in Table 14.1. Only a few oligosaccharides like 3′-sialyllactose (3′ SL) and 6′-sialyllactose (6′ SL) are common to both human and bovine milk.
Four milk groups. The HMO composition of a woman’s milk depends largely on her genetic constitution. Based on the Lewis Secretor blood group system, four milk groups can be distinguished, depending on the activity of two gene loci encoding for two fucosyltransferases [3]. Individuals with an active Se locus, which encodes for the α1-2-fucoslyltransferase FUT2, are classified as Secretors. These women express FUT2 in secretory tissues and generate α1-2-linked epitopes in their milk, which is characterized by 2′-fucosyllactose (2′ FL) and lacto-N-fucopentaose 1 (LNFP1). Nonsecretors are deficient in a functional FUT2 enzyme and therefore do not produce these specific HMO. The Lewis blood group status of an individual reflects the activity of the α1-3/4-fucosyltransferase FUT3, encoded by the Le gene. Lewis-negative women produce milk that lacks α1-4-fucosylated HMO. Based on the expression of FUT2 and FUT3, breast milk can be assigned to one of four groups: Lewis-positive Secretor, Lewis-negative Secretor, Lewis-positive Nonsecretor and Lewis-negative Nonsecretor [3].
Intrapersonal variations in HMO composition. The HMO composition of a woman’s milk also varies during the course of lactation. In early stages of lactation, the total HMO concentration is generally higher and declines within the first 3 months [4, 5]. In colostrum, total HMO concentrations peak at over 20 g/L and drop to 5–12 g/L in transitional and mature milk. The relative abundance of sialylated HMO is highest in colostrum, and concentrations decrease during the transition to mature milk [6].
In cow milk, the BMO concentration peaks at parturition at approximately 20 times lower amounts compared to human colostrum, with 3′ SL being the most prominent BMO. Abundance and composition of BMO change rapidly, and mature bovine milk contains only trace amounts of oligosaccharides [6].
HMO Metabolism
To fully appreciate the possible health benefits for the infant, it is important to understand the fate of HMO in the breast-fed infant. In vivo data on the extent of HMO degradation, absorption, and fermentation are however limited. Studies on mother–infant pairs reported that the oligosaccharide profiles in the infant’s feces and urine closely resemble the oligosaccharides in the mother’s milk. These results suggested that HMO are mostly excreted unaltered with the feces, while a small percentage is absorbed intact into the circulation and excreted with the urine. Formula-fed infants present entirely different fecal glycan profiles [7]. Furthermore, ex vivo and in vitro studies demonstrated that HMO are resistant to the low pH in the stomach and to pancreas and brush border enzymes, further strengthening the general idea that HMO are nondigestible to the host [8, 9].
Nevertheless, the view of HMO as “inert” to intestinal breakdown is currently being challenged. It is now becoming evident that partial HMO degradation and bioconversion take place in the infant’s intestine. In recent studies, sialylated HMO or other specific HMO structures as well as blood type-specific bioconversion products of HMO accumulated in the feces of breast-fed infants, resulting in profiles remarkably different to the HMO profiles in their mother’s milk [10]. Early post-partum, fecal profiles match those in the respective milk, indicating minimal HMO utilization in the gut lumen. At 2 months of age, HMO metabolism products appear in the feces, suggesting a higher level of degradation and personalized re-modeling of HMO. Once solid food is introduced in addition to breast milk, oligosaccharides derived from these new carbohydrate sources can be found in the infant’s feces [11]. Together, these findings indicate an active gastrointestinal metabolism of HMO depending on the age of the breast-fed infant, and the individual adaption and maturation of the gastrointestinal system [11]. Overall, HMO utilization might occur to a higher degree as previously anticipated. The resulting changes in HMO composition and the occurrence of degradation products might be of biological significance. Also, it remains unclear to which extent the infant’s own intestinal enzymes or the microbiota contribute to HMO degradation and remodeling in the intestine.
While efforts to directly measure HMO in infant blood have not been successful, HMO are regularly found in urine of breast-fed, but not formula-fed infants [12], providing indirect evidence for intestinal absorption. Metabolic labeling studies [13] estimate a 1% intestinal absorbance rate, but the exact kinetics remain poorly understood. The hypothesis that HMO are absorbed is further supported by in vitro studies demonstrating that intestinal epithelial cells mediate active and passive HMO transport [14]. How these translocations contribute to intestinal absorption in vivo has yet to be investigated.
HMO as Prebiotics
Historically, HMO have been mainly thought of as prebiotics that stimulate the colonization of beneficial microorganisms in the intestine of the breast-fed neonate. Already in 1900, Bifidobacteria were found enriched in the stool of breast-fed compared to formula-fed infants [15], an observation that initiated the search for the “bifidogenic factor” and eventually led to the discovery of the first HMO in 1954 [16]. Today, the differences in the intestinal microbial composition seem less apparent and not entirely due to a predominance of Bifidobacteria or Lactobacilli. Nevertheless, the intestinal community of breast-fed infants seems to be less complex and more stable than that of formula-fed infants (reviewed in Morelli [17]). Species in the genus Bifidobacteria are frequently isolated from breast-fed infants and have been shown to grow on HMO in vitro [18]. Several bifidobacterial strains employ a dedicated metabolic pathway, the Galacto-N-biose/Lacto-N-biose (GNB/LNB) pathway, for degrading type 1 core structures after the extracellular enzymatic breakdown to the disaccharides lacto-N-biose 1 and Lac. Furthermore, various bifidobacterial strains can also degrade the type 2 chain Lacto-N-neotetraose (LNnT), or possess extracellular fucosidases and sialidases, releasing unsubstituted HMO backbones for further degradation (reviewed in Fushinobu [19]).
B. longum infantis (short: B. infantis) seems to be the single most adapted species to comprehensively utilize HMO and is able to use HMO as sole carbon source [20]. The genome of B. infantis contains a unique cluster of ABC transporters and intracellular glycosylhydrolases (HMO-1 cluster) allowing intracellular HMO uptake, degradation and fermentation via catabolic pathways [21]. In addition, B. infantis employs enzymes to selectively digest type 1 or type 2 chains after intracellular uptake [22] and is able to utilize certain sialylated HMO.
HMO utilization does not seem to be limited to the genus Bifidobacteria. Bacteroides strains, for example, are also capable of metabolizing HMO [23]. Other genera like Lactobacilli cannot degrade HMO themselves but are able to utilize intermediates and metabolites released by Bifidobacteria [24]. Due to different degrees of adaptation to HMO usage, structures unique to HMO might select for a specific composition of the bacterial community [23]. However, there is no evidence from human studies that different HMO compositions shape the assembly of microbiota in breast-fed infants. In fact, a recent study found no significant differences in the infant’s intestinal microbiota composition between the Secretor- and Lewis-dependent milk groups, contradicting an association of genera to specific HMO fucosylation patterns [25].
HMO as Antimicrobials
Aside from reducing the risk for infections by promoting a healthy gut-colonization, HMO might also serve as a direct line of defense against bacterial, viral and protozoan pathogens (see Fig. 14.2). It was noticed early on that breast-fed infants have lower incidences of infectious diseases of the intestinal, urinary, and respiratory tract [26]. Many pathogens such as Escherichia coli, Campylobacter jejuni, Shigella strains, Vibrio cholera and Salmonella employ ligand–receptor interactions to attach to the host’s mucosal surfaces and initiate infection [1, 27]. Pathogens can either express lectins, proteins that bind to host epithelial glycan receptors, or they can mimic host glycan structures to dock to the cognate lectins expressed on the side of the host. Some HMO structurally resemble the glycan epitopes on the host surface and could therefore function as soluble decoy receptors, resulting in reduced adhesion and enhanced pathogen clearance [27]. For example, α1-2-fucosylated HMO like 2′ FL have been shown to interfere with the binding of several enteric pathogens in vitro, including C. jejuni [27, 28]. Ex vivo studies showed that 2′ FL inhibited Campylobacter colonization of human intestinal mucosa [28]. As outlined above, α1-2-fucosylated oligosaccharides are only present in the milk of Secretor women, and a study in Mexico showed that the expression of α1-2-fucosylated HMO in the mother’s milk is correlated to a lower incidence of infant Campylobacter and Calicivirus diarrhea [29]. Other specific HMO have been shown to effectively prevent and attenuate pneumococcal pneumonia in rabbits and rat pups in vivo [30].
Antiadhesive effects of HMO. HMO structurally resemble host glycans and function as soluble decoy receptors that block the attachment of toxins, bacteria, viruses and protozoan parasites to mucosal surfaces. Listed are examples of microbial pathogens with reported in vitro and ex vivo antiadhesive effects
In vitro, antiadhesive effects of HMO have also been described against viruses such as Calicivirus strains [31], or HIV-gp120, the glycoprotein critically involved in HIV entry upon binding to DC-SIGN [32]. Similarly, protozoan parasites such as Entamoeba (E.) histolytica also employ lectins to bind to human cells and might be inhibited by certain HMO epitopes. Our lab recently showed that physiological concentrations of specific HMO can block E. histolytica adhesion to and cytotoxicity against human intestinal cells in vitro [33].
In conclusion, breast-feeding is known to reduce the risk of infections caused by bacteria, viruses, or protozoan parasites. Evidently, HMO have antiadhesive effects on these infectious agents in vitro, and HMO functions seem to be highly structure-specific. If different HMO protect from different pathogens, it follows that a diverse mixture of structurally distinct HMO in breast milk offers the infant a greater level of defense than a single HMO could do. Accordingly, individual differences in HMO composition in milk due to genetic and other factors might explain the different degree of protection in breast-fed infants. However, to date, clinical intervention studies to link specific HMO to protection from certain infectious diseases are not available.
HMO as Signaling Molecules
To keep pathogens in check, HMO may not only interact with the microbial side, but might also act on the host cells. Exposure of enterocytes to 3′ SL, one of the predominant sialylated HMO, changed cell surface glycan profiles in vitro [34]. These HMO-induced glycome modifications led to a dramatic reduction in binding of enteropathogenic E. coli (EPEC) to CaCo-2 cells. HMO-mediated alteration of surface glycans on host cells might be an alternative defense strategy against pathogen binding. It is not known whether 3′ SL also regulates other glycan-related genes, or whether different HMO have similar or differential effects.
If HMO can act as signaling molecules, one can speculate that their roles might go beyond direct defense mechanisms against pathogens. In vitro studies reporting that HMO affect proliferation, differentiation and apoptosis in intestinal cell lines supported this notion and raised speculations on whether HMO are involved in the regulation of intestinal growth and maturation in the breast-fed infant [35]. How HMO signal and influence gene expression or possible other downstream effects remains unknown. Also, whether these in vitro observations have implications for the breast-fed infant has to be investigated.
HMO as Immune Modulators
Cell–cell interactions of the innate and adapted immune system are largely mediated by lectins, and HMO are potential interaction partners for several human lectins, such as selectins, siglecs or galectins [31]. Estimated concentrations of HMO in the circulation range from 100 to 200 μg/mL, which make systemic effects seem plausible.
The physiological binding determinants of selectins are sialyl-Lex and sialyl-Ley, epitopes present also in sialylated and fucosylated HMO. P- and L-selectins are critically involved in leukocyte deceleration and adhesion to endothelial cells, leading to extravasation at sites of inflammation [36]. Additionally, P-selectins regulate formation and activation of platelet-neutrophil complexes (PNC), highly active neutrophils primed for adhesion, phagocytosis and production of reactive oxygen species [37]. In vitro and ex vivo models showed that physiological concentrations of sialylated HMO reduce neutrophil rolling and adhesion to activated endothelial cells [38]. It was further demonstrated that sialylated HMO impede the formation of PNC [39], suggesting that HMO function as anti-inflammatory agents.
There is substantial evidence that breastfeeding obviates allergies in infants and the development of autoimmune diseases later in life. Furthermore, it is acknowledged that HMO contribute to the maturation of the naive immune system [40]. Beneficial effects of HMO on the immune response have mainly been accounted to the growth and metabolism of microbial commensals in the gut leading to oral tolerance and protection against pathogens. Nevertheless, an increasing number of studies suggests that HMO can affect immune cells in a direct, microbiota-independent way [41–44].
In neonates, the immune system is immature and biased toward a Th2 profile to avoid adverse inflammatory conditions. Sialylated HMO stimulated cytokine production and activated cord blood-derived T cells from neonates ex vivo [44], indicating that HMO could have direct immune modulatory effects, promoting a shift in T cells response toward a more balanced Th1/Th2-cytokine production and low-level immunity. These findings suggest a role for HMO in guiding the postnatal maturation of the immune system and in preventing allergies.
HMO as Protective Agents Against Necrotizing Enterocolitis
Breast-fed infants have a lower risk to develop necrotizing enterocolitis (NEC) than formula-fed infants. Hallmarks of this life-threatening disease that affects almost 10% of very-low-birth-weight preterm infants are excessive inflammation, bacterial colonization, and impaired barrier function, which can lead to intestinal necrosis and bacterial sepsis. Our lab recently showed that pooled HMO prevent NEC in a neonatal rat model. Out of the pool of HMO, a single HMO, disialyllacto-N-tetraose (DSLNT), could be identified as the protective agent, and its function was highly structure-specific. Galactooligosaccharides (GOS), currently added to infant formula had no effect [45]. How DSLNT prevents NEC and whether these results translate to human infants has yet to be determined.
HMO as Nutrients for Brain Development
Breastfeeding has long been associated with higher intelligence in children [46], although it is challenging to provide direct evidence for a nutritional factor. Recently, sialylated HMO have obtained great interest as a potential source of nutrients to improve neurological development. Postmortem analysis of Sia concentrations in brain gangliosides and glycoproteins revealed higher amounts in breast-fed than in formula-fed infants [47], suggesting differences in synaptogenesis and neuronal development. Sia concentrations in brain gangliosides and glycoproteins have been associated with learning ability. Administration of free or conjugated Sia enhanced cognitive and behavioral performance in rats and piglets [48, 49]. In suckling rat pups, the occurrence of maximal Sia concentration in milk in early lactation is concurrent with the up-regulation of enzymes involved in Sia catabolism in the colon. At weaning, when Sia levels in rat milk were the lowest, a change in colonic enzyme expression seems to expedite de novo synthesis [50]. The differential expression of intestinal enzymes associated with Sia utilization and anabolism suggests adaption of the intestinal system to the transient high dietary source of Sia early in lactation. These observations imply that the high 3′ SL concentration in mother’s milk meets the enormous demand of the growing brain for Sia. Whether supplementation of formulas with Sia is effective in stimulating brain development in infants has yet to be investigated.
Alternatives for HMO in Formula
Bovine milk-based infant formula contains marginal levels of complex oligosaccharides. Addition of HMO to infant formula is currently not feasible due to the limited availability and extremely high costs. At present, infant formula manufacturers are supplementing their products with nonhuman oligosaccharides in an attempt to mimic the effects of HMO. Commonly added oligosaccharides are GOS and fructooligosaccharides (FOS). GOS consist of one to seven Gal units linked to a reducing end Lac. FOS are short-chain oligomers build of β1-2-linked fructose (Fruc) residues bound to a reducing end glucose (Glc). Apart from the common building block Gal in GOS, these nondigestible dietary oligosaccharides do not share structural similarities with HMO. Nevertheless, GOS and FOS have reportedly shown prebiotic [51] and immunomodulatory [52] effects similar to HMO. Whereas some HMO effects can potentially be mimicked by GOS/FOS, there are most certainly limitations to their potential use as functionally equivalent alternatives to HMO. For example, a single specific HMO, DSLNT, could prevent NEC in neonatal rats whereas GOS had no effect [45]. In addition, although considered save for the use in infants, studies on long-term effects of feeding nonhuman oligosaccharides during the neonatal period are not available.
Concluding Remarks
HMO seem to have a multitude of benefits for the breast-fed infant that go beyond the prebiotic aspects (see Fig. 14.3). The potential benefits of HMO in infections and allergies, gut maturation or brain development remain to be confirmed in vivo. However, accumulating evidence from in vitro data warrants further research directed toward possible applications of these bioactive components.
Schematic overview of the potential health benefits of HMO in breast-fed infants. HMO are indigestible and reach the colon intact where they serve as prebiotics and promote healthy gut colonization. HMO block adhesion of microbial pathogens to mucosal surfaces, preventing infections of the respiratory, gastrointestinal and urogenital tract. HMO could serve as signaling molecules and guide growth and maturation of the intestine and affect mucosal immunity. Systemic HMO effects might include attenuation of selectin-mediated inflammatory events. HMO-derived sialic acid may be incorporated in the rapidly growing brain and support neuronal development. HMO might reduce the risk of necrotizing enterocolitis, but the underlying mechanisms remain unknown
It is becoming evident that the specific structure of individual HMO determines their function. This structure–function relationship might have implications on the supplementation of infant formula with non-HMO. Structurally different oligosaccharides are likely not able to imitate all HMO effects, and potential side effects have to be considered and further investigated.
The individual mix of oligosaccharides in breast milk seems to be optimized for the changing needs of the developing infant. The fact that certain HMO in breast milk are age-dependently expressed, and that infants process HMO very differently depending on their age and maturity suggests that the qualitative and quantitative oligosaccharide composition in milk is fine-tuned to the demands of the infant. Whether these observations have implications, e.g. for the nutrition of preterm infants with nonage-matched donor milk, has yet to be investigated. In the end, the beneficial effects of HMO add yet another reason to encourage mothers to breastfeed their infants.
Abbreviations
- 2′ FL:
-
2′-Fucosyllactose
- 3′ SL:
-
3′-Sialyllactose
- 3FL:
-
3-Fucosyllactose
- 6′ SL:
-
6′-Sialyllactose
- BMO:
-
Bovine milk oligosaccharides
- DSLNT:
-
Disialyllacto-N-tetraose
- FOS:
-
Fructooligosaccharides
- Fruc:
-
Fructose
- Fuc:
-
Fucose
- Gal:
-
Galactose
- Glc:
-
Glucose
- GlcNAc:
-
N-acetylglucosamine
- GOS:
-
Galactooligosaccharides
- HMO:
-
Human milk oligosaccharides
- Lac:
-
Lactose
- LNFP1,2:
-
Lacto-N-fucopentaose 1,2
- LNnT:
-
Lacto-N-neotetraose
- LNT:
-
Lacto-N-tetraose
- NEC:
-
Necrotizing enterocolitis
- Neu5Ac:
-
N-actetylneuraminic acid
- Neu5Gc:
-
N-glycolylneuraminic acid
- PNC:
-
Platelet-neutrophil complex
- Sia:
-
Sialic acid
References
Bode L. Human milk oligosaccharides: prebiotics and beyond. Nutr Rev. 2009;67 Suppl 2:S183–91.
Chichlowski M, German JB, Lebrilla CB, Mills DA. The influence of milk oligosaccharides on microbiota of infants: opportunities for formulas. Annu Rev Food Sci Technol. 2011;2:331–51.
Thurl S, Henker J, Siegel M, Tovar K, Sawatzki G. Detection of four human milk groups with respect to Lewis blood group dependent oligosaccharides. Glycoconj J. 1997;14:795–9.
Chaturvedi P, Warren CD, Altaye M, Morrow AL, Ruiz-Palacios G, Pickering LK, et al. Fucosylated human milk oligosaccharides vary between individuals and over the course of lactation. Glycobiology. 2001;11:365–72.
Thurl S, Munzert M, Henker J, Boehm G, Muller-Werner B, Jelinek J, et al. Variation of human milk oligosaccharides in relation to milk groups and lactational periods. Br J Nutr. 2010;104:1261–71.
Martin-Sosa S, Martin MJ, Garcia-Pardo LA, Hueso P. Sialyloligosaccharides in human and bovine milk and in infant formulas: variations with the progression of lactation. J Dairy Sci. 2003;86:52–9.
Chaturvedi P, Warren CD, Buescher CR, Pickering LK, Newburg DS. Survival of human milk oligosaccharides in the intestine of infants. Adv Exp Med Biol. 2001;501:315–23.
Engfer MB, Stahl B, Finke B, Sawatzki G, Daniel H. Human milk oligosaccharides are resistant to enzymatic hydrolysis in the upper gastrointestinal tract. Am J Clin Nutr. 2000;71:1589–96.
Gnoth MJ, Kunz C, Kinne-Saffran E, Rudloff S. Human milk oligosaccharides are minimally digested in vitro. J Nutr. 2000;130:3014–20.
Albrecht S, Schols HA, van Zoeren D, van Lingen RA, Groot Jebbink LJ, van den Heuvel EG, et al. Oligosaccharides in feces of breast- and formula-fed babies. Carbohydr Res. 2011;346:2173–81.
Albrecht S, Schols HA, van den Heuvel EG, Voragen AG, Gruppen H. Occurrence of oligosaccharides in feces of breast-fed babies in their first six months of life and the corresponding breast milk. Carbohydr Res. 2011;29:2540–50.
Rudloff S, Pohlentz G, Diekmann L, Egge H, Kunz C. Urinary excretion of lactose and oligosaccharides in preterm infants fed human milk or infant formula. Acta Paediatr. 1996;85:598–603.
Rudloff S, Pohlentz G, Borsch C, Lentze MJ, Kunz C. Urinary excretion of in vivo 13C-labelled milk oligosaccharides in breastfed infants. Br J Nutr. 2012;107(7):1–7.
Gnoth MJ, Rudloff S, Kunz C, Kinne RK. Investigations of the in vitro transport of human milk oligosaccharides by a Caco-2 monolayer using a novel high performance liquid chromatography-mass spectrometry technique. J Biol Chem. 2001;276:34363–70.
Moro E. Morphologische und bakteriologische Untersuchungen über die Darmbakterien des Säuglings: die Bakterienflora des normalen Frauenmilchstuhls. Jahrbuch Kinderh. 1900;61:686–734.
György PNR, Rose CS. Bifidus factor I. A variant of Lactobacillus bifidus requiring a special growth factor. Arch Biochem Biophys. 1954;48:193–201.
Morelli L. Postnatal development of intestinal microflora as influenced by infant nutrition. J Nutr. 2008;138:1791S–5.
LoCascio RG, Ninonuevo MR, Freeman SL, Sela DA, Grimm R, Lebrilla CB, et al. Glycoprofiling of bifidobacterial consumption of human milk oligosaccharides demonstrates strain specific, preferential consumption of small chain glycans secreted in early human lactation. J Agric Food Chem. 2007;55:8914–9.
Fushinobu S. Unique sugar metabolic pathways of bifidobacteria. Biosci Biotechnol Biochem. 2010;74:2374–84.
Ward RE, Ninonuevo M, Mills DA, Lebrilla CB, German JB. In vitro fermentation of breast milk oligosaccharides by Bifidobacterium infantis and Lactobacillus gasseri. Appl Environ Microbiol. 2006;72:4497–9.
Sela DA, Chapman J, Adeuya A, Kim JH, Chen F, Whitehead TR, et al. The genome sequence of Bifidobacterium longum subsp. infantis reveals adaptations for milk utilization within the infant microbiome. Proc Natl Acad Sci U S A. 2008;105:18964–9.
Yoshida E, Sakurama H, Kiyohara M, Nakajima M, Kitaoka M, Ashida H, et al. Bifidobacterium longum subsp. infantis uses two different {beta}-galactosidases for selectively degrading type-1 and type-2 human milk oligosaccharides. Glycobiology. 2012;22(3):361–8.
Marcobal A, Barboza M, Sonnenburg ED, Pudlo N, Martens EC, Desai P, et al. Bacteroides in the infant gut consume milk oligosaccharides via mucus-utilization pathways. Cell Host Microbe. 2011;10(5):507–14.
Schwab C, Ganzle M. Lactic acid bacteria fermentation of human milk oligosaccharide components, human milk oligosaccharides and galactooligosaccharides. FEMS Microbiol Lett. 2011;315:141–8.
Coppa GV, Gabrielli O, Zampini L, Galeazzi T, Ficcadenti A, Padella L, et al. Oligosaccharides in 4 different milk groups, Bifidobacteria, and Ruminococcus obeum. J Pediatr Gastroenterol Nutr. 2011;53:80–7.
Hanson LA. Session 1: feeding and infant development breast-feeding and immune function. Proc Nutr Soc. 2007;66:384–96.
Coppa GV, Zampini L, Galeazzi T, Facinelli B, Ferrante L, Capretti R, et al. Human milk oligosaccharides inhibit the adhesion to Caco-2 cells of diarrheal pathogens: Escherichia coli, Vibrio cholerae, and Salmonella fyris. Pediatr Res. 2006;59:377–82.
Ruiz-Palacios GM, Cervantes LE, Ramos P, Chavez-Munguia B, Newburg DS. Campylobacter jejuni binds intestinal H(O) antigen (Fuc alpha 1, 2Gal beta 1, 4GlcNAc), and fucosyloligosaccharides of human milk inhibit its binding and infection. J Biol Chem. 2003;278:14112–20.
Morrow AL, Ruiz-Palacios GM, Altaye M, Jiang X, Guerrero ML, Meinzen-Derr JK, et al. Human milk oligosaccharide blood group epitopes and innate immune protection against campylobacter and calicivirus diarrhea in breastfed infants. Adv Exp Med Biol. 2004;554:443–6.
Idanpaan-Heikkila I, Simon PM, Zopf D, Vullo T, Cahill P, Sokol K, et al. Oligosaccharides interfere with the establishment and progression of experimental pneumococcal pneumonia. J Infect Dis. 1997;176:704–12.
Bode L. Recent advances on structure, metabolism, and function of human milk oligosaccharides. J Nutr. 2006;136:2127–30.
Hong P, Ninonuevo MR, Lee B, Lebrilla C, Bode L. Human milk oligosaccharides reduce HIV-1-gp120 binding to dendritic cell-specific ICAM3-grabbing non-integrin (DC-SIGN). Br J Nutr. 2009;101:482–6.
Jantscher-Krenn E, Lauwaet T, Bliss LA, Reed SL, Gillin FD, Bode L. Human milk oligosaccharides reduce Entamoeba histolytica attachment and cytotoxicity in vitro. Br J Nutr. 2012;23:1–8.
Angeloni S, Ridet JL, Kusy N, Gao H, Crevoisier F, Guinchard S, et al. Glycoprofiling with micro-arrays of glycoconjugates and lectins. Glycobiology. 2005;15:31–41.
Kuntz S, Rudloff S, Kunz C. Oligosaccharides from human milk influence growth-related characteristics of intestinally transformed and non-transformed intestinal cells. Br J Nutr. 2008;99:462–71.
Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–14.
Peters MJ, Dixon G, Kotowicz KT, Hatch DJ, Heyderman RS, Klein NJ. Circulating platelet-neutrophil complexes represent a subpopulation of activated neutrophils primed for adhesion, phagocytosis and intracellular killing. Br J Haematol. 1999;106:391–9.
Bode L, Kunz C, Muhly-Reinholz M, Mayer K, Seeger W, Rudloff S. Inhibition of monocyte, lymphocyte, and neutrophil adhesion to endothelial cells by human milk oligosaccharides. Thromb Haemost. 2004;92:1402–10.
Bode L, Rudloff S, Kunz C, Strobel S, Klein N. Human milk oligosaccharides reduce platelet-neutrophil complex formation leading to a decrease in neutrophil beta 2 integrin expression. J Leukoc Biol. 2004;76:820–6.
de Kivit SKA, Garssen J, Willemsen LE. Glycan recognition at the interface of the intestinal immune system: target for immune modulation via dietary components. Eur J Pharmacol. 2011;668:S124–32.
Atochina O, Da’dara AA, Walker M, Harn DA. The immunomodulatory glycan LNFPIII initiates alternative activation of murine macrophages in vivo. Immunology. 2008;125:111–21.
Atochina O, Harn D. Prevention of psoriasis-like lesions development in fsn/fsn mice by helminth glycans. Exp Dermatol. 2006;15:461–8.
Eiwegger T, Stahl B, Haidl P, Schmitt J, Boehm G, Dehlink E, et al. Prebiotic oligosaccharides: in vitro evidence for gastrointestinal epithelial transfer and immunomodulatory properties. Pediatr Allergy Immunol. 2010;21:1179–88.
Eiwegger T, Stahl B, Schmitt J, Boehm G, Gerstmayr M, Pichler J, et al. Human milk-derived oligosaccharides and plant-derived oligosaccharides stimulate cytokine production of cord blood T-cells in vitro. Pediatr Res. 2004;56:536–40.
Jantscher-Krenn E, Zherebtsov M, Nissan C, Goth K, Guner YS, Naidu N, et al. The human milk oligosaccharide disialyllacto-N-tetraose prevents necrotising enterocolitis in neonatal rats. Gut. 2012 Oct;61(10):1417–25. Epub 2011 Dec 3.
Anderson JW, Johnstone BM, Remley DT. Breast-feeding and cognitive development: a meta-analysis. Am J Clin Nutr. 1999;70:525–35.
Wang B, McVeagh P, Petocz P, Brand-Miller J. Brain ganglioside and glycoprotein sialic acid in breastfed compared with formula-fed infants. Am J Clin Nutr. 2003;78:1024–9.
Morgan BL, Winick M. Effects of administration of N-acetylneuraminic acid (NANA) on brain NANA content and behavior. J Nutr. 1980;110:416–24.
Wang B, Yu B, Karim M, Hu H, Sun Y, McGreevy P, et al. Dietary sialic acid supplementation improves learning and memory in piglets. Am J Clin Nutr. 2007;85:561–9.
Duncan PI, Raymond F, Fuerholz A, Sprenger N. Sialic acid utilisation and synthesis in the neonatal rat revisited. PLoS One. 2009;4:e8241.
Knol J, Scholtens P, Kafka C, Steenbakkers J, Gro S, Helm K, et al. Colon microflora in infants fed formula with galacto- and fructo-oligosaccharides: more like breast-fed infants. J Pediatr Gastroenterol Nutr. 2005;40:36–42.
Rijnierse A, Jeurink PV, van Esch BC, Garssen J, Knippels LM. Food-derived oligosaccharides exhibit pharmaceutical properties. Eur J Pharmacol. 2011;668 Suppl 1:S117–23.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer Science+Business Media New York
About this chapter
Cite this chapter
Jantscher-Krenn, E., Bode, L. (2013). Human Milk Oligosaccharides: Role in Infant Health. In: Watson, R., Grimble, G., Preedy, V., Zibadi, S. (eds) Nutrition in Infancy. Nutrition and Health. Humana Press, Totowa, NJ. https://doi.org/10.1007/978-1-62703-224-7_14
Download citation
DOI: https://doi.org/10.1007/978-1-62703-224-7_14
Published:
Publisher Name: Humana Press, Totowa, NJ
Print ISBN: 978-1-62703-223-0
Online ISBN: 978-1-62703-224-7
eBook Packages: MedicineMedicine (R0)