Abstract
The xanthophylls lutein and zeaxanthin are oxygenated carotenoids that preferentially accumulate in the macular region of the retina. Lutein, zeaxanthin, and meso-zeaxanthin (a conversion product of lutein formed in the macula) are referred to as macular pigment. Lutein and zeaxanthin are also present in all other ocular structures except the vitreous, cornea, and sclera; although, their concentrations are much lower than in the macular region. Lutein and zeaxanthin protect the ocular tissues by their ability to filter damaging blue light and their antioxidant potential.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Lutein
- Zeaxanthin
- Macular pigment
- Eye disease
- Age-related macular degeneration
- Cataract
- Retinitis pigmentosa
- Visual function
-
The xanthophylls lutein and zeaxanthin are oxygenated carotenoids that preferentially accumulate in the macular region of the retina. Lutein, zeaxanthin, and meso-zeaxanthin (a conversion product of lutein formed in the macula) are referred to as macular pigment. Lutein and zeaxanthin are also present in all other ocular structures except the vitreous, cornea, and sclera; although, their concentrations are much lower than in the macular region. Lutein and zeaxanthin protect the ocular tissues by their ability to filter damaging blue light and their antioxidant potential.
-
Eye diseases that have been associated with low lutein and zeaxanthin include age-related macular degeneration (AMD)—due to the exclusive location of lutein and zeaxanthin in the macula, which is the sight of AMD insult; cataract—due to the presence of lutein and zeaxanthin in the lens, which is constantly exposed to light and oxygen; and retinitis pigmentosa—due to the presence of lutein and zeaxanthin in the rod outer segments and in the macula.
-
The etiology of each eye disease will be discussed briefly followed by a review of epidemiological evidence and findings from intervention studies with lutein and zeaxanthin, alone and in combination with other nutrients.
-
The role of lutein and zeaxanthin in improving visual function will also be examined.
-
The chapter will close with a brief summary of conclusions and future directions.
Introduction
Structure and Tissue Distribution of Lutein and Zeaxanthin
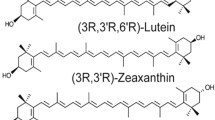
Lutein and zeaxanthin are known as oxygenated carotenoids or xanthophylls due to the presence of two hydroxyl groups, one on each of the two ionone rings (Fig. 13.1). They are 40 carbon polyisoprenoid compounds with nine conjugated double bonds in the polyene chain [1]. Lutein and zeaxanthin are stereoisomers of one another but are not interchangeable wherever they have a functional role [2]. Lutein has both β and ε type ionone rings, while zeaxanthin has two β type ionone rings [2]. The hydroxyl groups make lutein and zeaxanthin more polar in nature compared with lycopene, α-carotene, and β-carotene, which are pure hydrocarbons. The relative orientation of the hydroxyl groups dictates the observed specificity in tissue accumulation and orientation within the cell membranes. The structure is also important in the specific recognition of lutein and zeaxanthin by binding proteins [3].
Of the 700 carotenoids identified in nature, only 13 carotenoids and their 12 isomers are found in the serum and only two, lutein and zeaxanthin, exclusively accumulate in the macula of the human eye. The macula lutea is a yellowish region 5–6 mm in diameter in the posterior pole of the human retina responsible for the central 15–20° of vision [4, 5]. Lutein, zeaxanthin, and meso-zeaxanthin, a conversion product of lutein formed in the macula [6], are collectively referred to as macular pigment (MP). The macular region has a central depression, a region called the fovea (Fig. 13.2), an area so rich in cone receptors that permits maximal visual acuity [2]. The concentration of xanthophylls in the central macula is about 1 mM, which corresponds to more than 3 orders of their concentration in normal serum [7, 8]. However, in regions just a few millimeters from the central fovea the concentration of xanthophylls drops more than 100-fold [5]. The ratio of lutein to zeaxanthin increases from about 1:2.4 in the central 0–0.25 mm of the macula to greater than 2:1 in the periphery, distances exceeding 6 mm from the fovea [7, 9, 10]. Meso-zeaxanthin was shown to be present in the macular region (not 8 mm and peripheral retina) of xanthophyll-free rhesus monkeys that were fed a pure lutein diet and not those that were fed a pure zeaxanthin diet demonstrating that lutein isomerizes to meso-zeaxanthin via migration of a double bond [6]. This partially explains the disparity between the 3:1 ratio of lutein to zeaxanthin in the blood and the 1:2 in the macula and also the lower lutein to zeaxanthin ratio in the fovea compared to the periphery [5]. The lutein:zeaxanthin:meso-zeaxanthin ratio in the macula is 1:1:1 [6, 7].
An illustration of the structure of the eye showing the different regions including the macular regions where lutein and zeaxanthin exclusively accumulate. Credit for the eye image: Webvision (http://webvision.med.utah.edu/sretina.html), with permission
Knowing that these xanthophylls are present in the fovea and peripheral regions of the retina, the question arises to their precise location within the cellular and subcellular structures. Lutein and zeaxanthin are said to be asymmetrically distributed across the depth of the retina (Fig. 13.3), with highest concentrations in the inner retinal layers [7, 11, 12]. In 1984, Snodderly et al. described MP distribution patterns that hypothesized the cone axons (outer plexiform layer) to contain the highest densities of MP due to the high density of cone photoreceptors in the fovea [12]. Others have also shown the majority of the pigments to be concentrated in the outer plexiform layers of the fovea as well as the outer and inner plexiform layers of the adjacent areas [7, 12–14]. There is strong evidence showing the presence of lutein and zeaxanthin in the photoreceptor rod outer segments (ROS), where they were shown to represent 10–15% and 25% of total retinal lutein and zeaxanthin in two separate studies [15, 16]. Within the ROS, the highest density of lutein and zeaxanthin exists in the perifoveal region (Fig. 13.2), where the concentration of pigments was 2.5-times greater than in the peripheral regions of the retina [15]. Lutein and zeaxanthin were also detected in the retinal pigment epithelium (RPE), but their concentrations were less than half of that found in ROS [15]. The high selectivity and distribution of lutein and zeaxanthin in these retinal layers suggests the presence of specific binding proteins. Bernstein et al. have shown the presence of carotenoid binding proteins in the retina, which specifically bind lutein and zeaxanthin. Tubulin was shown to bind lutein and zeaxanthin in the photoreceptor axon layer, glutathione S-transferase P1 (GSTP1) concentrated in the outer and inner plexiform layers of the fovea and in the photoreceptor inner segment ellipsoid region specifically bound to zeaxanthin, and the steroidogenic acute regulatory domain protein 3 (StARD3) specifically bound to lutein (Fig. 13.3) [3, 17].
(a) Cross section of the retina showing the different neuronal cells. (b) Distribution of lutein and zeaxanthin within the cellular structures and the associated specific carotenoid binding proteins that have been detected in these regions. Credit for cross section of the retina: Purves D, Augustine GJ, Fitzpatrick D et al., editors. The retina. Neuroscience. 2nd ed. Sunderland (MA): Sinauer Associates, 2001, with permission
Lutein and zeaxanthin are present in all other ocular structures with the exception of the vitreous, cornea, and sclera [5]. Approximately 30% of the ocular lutein and zeaxanthin are present in the uveal structures (i.e., iris, ciliary body, and RPE/choroid) [5]. Long-term supplementation with high doses of lutein can increase lutein concentration in ocular tissues such as the lens and peripheral retina that generally contain low levels of lutein and zeaxanthin [18]. In the lens, 75% of the lutein and zeaxanthin are present in the epithelium and cortex [19]. Other than the ocular tissues, lutein and zeaxanthin are found in the adipose tissue, ovaries, testes, liver, skin, human milk, and more recently have been detected in the brain [20–25].
Role of Lutein and Zeaxanthin in the Eye
Unlike α-carotene, β-carotene, and β-cryptoxanthin, lutein and zeaxanthin do not have provitamin A activity [26]. The presence of hydroxyl groups in the ionone rings prevents enzymatic cleavage at the 15-15′ bond which is involved in the formation of vitamin A aldehyde [27].
Lutein and zeaxanthin are believed to function in ocular health through their ability to absorb damaging blue radiation of light and their strong antioxidant potential. The cornea and lens filter UV radiation of light allowing visible blue light to reach the retina. MP has an absorption maximum of about 450 nm and acts as an effective filter diminishing the intensity of blue light reaching the photoreceptors [28]. Lutein and zeaxanthin as MP attenuate as much as 40% of the blue light from reaching the retina [29]. Lutein has a greater filtering efficiency compared to zeaxanthin [28] due to the difference in position of the double bond within the ionone ring. Lutein is able to orient itself both parallel and perpendicular to the plane of the membrane, while zeaxanthin can only lie perpendicular to the membrane [28, 30]. The two orientations of the lutein molecule allow for blue light absorption from all directions, which is not the case with zeaxanthin [5]. Even though β-carotene and lycopene have absorption maxima around 450 nm, the absence of hydrophilic groups causes them to remain within the hydrophobic core of the membrane making them unable to efficiently filter blue light [1]. Furthermore, these carotenes are not present in significant concentrations in ocular tissue [31]. Evidence for MP providing protection from light damage comes from observations in older adults in whom an increased loss of sensitivity of the short wavelength-sensitive-cones (S-cone) was observed across the retina compared to younger adults, with more loss of sensitivity at the nonfoveal locations compared to the fovea, an area where MP is at its peak [32].
Because of their slightly hydrophilic nature, lutein and zeaxanthin are able to quench singlet oxygen more effectively in the water phase compared to the completely hydrophobic hydrocarbon carotenoids [33]. Lutein and zeaxanthin have the ability to scavenge reactive oxygen species and limit peroxidation of membrane phospholipids. Under conditions of prolonged UV exposure, zeaxanthin is more effective than lutein in diminishing UV-induced lipid oxidation [30]. The retina is particularly susceptible to oxidative damage because of its high consumption of oxygen, its high concentration of polyunsaturated fatty acids, such as docosahexaenoic acid (DHA), and its exposure to visible light [34]. The photoreceptor cells are constantly exposed to light which could lead to generation of electrically excited species. These could react with molecular oxygen to produce reactive oxygen species and cause lipid peroxidation and cellular damage [5]. Furthermore, the presence of oxidative metabolites of lutein and zeaxanthin in the retina, such as 3-hydroxy-beta, epsilon-caroten-3′-one, and 3′-epilutein, which are not of dietary origin, is evidence for the antioxidative role of lutein and zeaxanthin in the retina [31].
In the iris, lutein and zeaxanthin most likely act as blue light filters, while in the ciliary body, an area of high metabolic activity, they most likely act as antioxidants [35]. In the lens as well, lutein and zeaxanthin most likely have an antioxidant function, with the constant exposure to oxygen and UV radiation. Due to their presence in the eye, lutein and zeaxanthin have been extensively researched in the prevention and treatment of eye diseases. The focus of this chapter is to discuss the most current findings on lutein and zeaxanthin in the treatment and prevention of age-related macular degeneration, cataract, and retinitis pigmentosa. The role of these macular carotenoids in visual function will also be discussed.
Eye Diseases
Age-Related Macular Degeneration
Age-related macular degeneration (AMD) is the leading cause of blindness in adults aged 60 years and over in industrialized countries. The prevalence of AMD increases dramatically with age. Nearly 30% of Americans >75 years have early signs of AMD and 7% have advanced AMD, whereas the respective prevalence is 8% and 0.1% in adults aged 43–54 years [36]. None of the current treatment options can reverse the damage caused by AMD.
AMD is a disease that affects the macular region of the retina causing loss of central vision (Fig. 13.4) and as the disease advances can lead to complete blindness. Posterior to the photoreceptors lies the RPE, part of the blood-ocular barrier, which has several functions including phagocytosis of the photoreceptors [37]. The clinical hallmark and usually the first clinical signs of AMD is the presence of drusen. Drusen are extracellular deposits that accumulate between the RPE and inner layer of the Bruch’s membrane and appear as pale yellow spots on the retina [38]. They are formed due to inability of the RPE to adequately perform its function of phagocytosis. Drusen can also be present in the normal aging eye. AMD can be classified into the following types based on the Age-Related Eye Disease Study (AREDS) classification system: (1) Early AMD—presence of few (<20) medium-sized drusen or retinal pigmentary abnormalities, (2) Intermediate AMD—presence of at least one large drusen, numerous medium-sized drusen, or geographic atrophy that does not extend to the center of the macula, (3) Advanced or late AMD—can either be neovascular (wet, exudative) or non-neovascular (dry, atrophic, non-exudative) [39]. Neovascular non-exudative is characterized by drusen and geographic atrophy that extends to the macula [37]. Neovascular exudative is characterized by the growth of new blood vessels under the RPE and sometimes into the sub-retinal space. The early stages of AMD are generally asymptomatic. In the later stages there may be distortion of vision or complete loss of visual function, especially central vision [40]. The pathogenesis of AMD is complex; however, the mechanisms of importance are chemical and light-induced oxidative stress, blue light induced damage to the RPE and photoreceptor rod cells, RPE dysfunction, hemodynamic processes, and also genetic factors. Age, family history, race, and female gender are considered to be non-modifiable risk factors of AMD. The modifiable risk factors include smoking, hypertension, raised serum cholesterol, excessive alcohol consumption, lifetime exposure to visible blue light, low MP levels, and possibly low intake of vitamins, minerals, and carotenoids in the diet [34, 41, 42].
Macular Pigment
Lutein and zeaxanthin are uniquely located in the fovea, which is the site of AMD insult. The association of MP density with AMD is biologically plausible given the ability of lutein and zeaxanthin to filter blue light and act as antioxidants protecting the retina from oxidative stress. Evidence shows that MP density decreases with AMD. Concentrations of lutein and zeaxanthin in the retinas from AMD donors were lower than in controls suggesting that low MP density could be a risk factor for AMD [43]. Subjects with advanced AMD in one eye had significantly lower MP density in the other eye compared to healthy eyes of subjects without AMD [44]. MP density was found to be 32% lower in the eyes of AMD patients compared to normal disease-free eyes [45]. However, in a cross-sectional study of women participating in the Carotenoids in Age-related Eye Disease Study (CAREDS) MP density was not related to intermediate AMD. After an exploratory analysis, the authors suggested that the observations may have been biased in older women, whose diets improved with age. The authors also suggested the possibility that low MP density could be the result, rather than the cause, of damage to the retina with AMD [46]. A similar observation was reported in a subset of the Rotterdam Eye Study, another cross-sectional study that examined MP density in subjects ≥55 years with or without AMD [47]. The reason for these conflicting observations may be the cross-sectional nature of the studies, as data from a prospective study found MP density to be reduced in subjects with late AMD [48]. Evidence that MP density is reduced in AMD suggests that higher dietary intake of lutein and zeaxanthin could increase their accumulation in the macula and thus protect against AMD.
Epidemiological Evidence
A number of studies have looked at the relationship between dietary intake of lutein and zeaxanthin and the risk of AMD. Three case–control studies showed high dietary intakes or plasma levels of lutein and zeaxanthin were related to reduced risk of advanced neovascular AMD [49–51]. In the Eye Disease Case–Control Study, subjects in the highest quintile of spinach consumption, a rich source of lutein and zeaxanthin had 86% lower odds of advanced AMD [50]. Case–control analysis of the AREDS population found that subjects in the highest quintile of lutein and zeaxanthin intake had lower odds of neovascular AMD and large or extensive intermediate drusen compared to the lowest quintile. Cross-sectional data from CAREDS showed that high lutein and zeaxanthin intakes (∼3 mg/day) were related to a decreased risk of intermediate AMD compared to low intakes (792 μg/day) in women <75 years, who are at risk of diet changes, but not in women ≥75 years [52]. In this population of women <75 years of age, a strong inverse relationship was observed between prevalence of intermediate AMD and high intake of green vegetables (12 servings/day of cooked greens, lettuce, spinach salad, broccoli, peas, and zucchini squash). Prospective data from the Blue Mountains Eye Study (BMES) showed that high intake of lutein/zeaxanthin (>942 μg/day) reduced the risk of incident neovascular AMD and subjects with above median intakes (>743 μg/day) had a reduced risk of indistinct soft or reticular drusen during a 5–10-year follow-up [42]. Again, subjects in the highest quintile of vegetable intake in the BMES also had reduced odds of developing any AMD. However, the observations reported by Robman et al. were exactly the opposite. In this study, higher dietary intakes of lutein and zeaxanthin were related to increased rate of progression of AMD [53]. The study included subjects who had either participated in the Melbourne Visual Impairment Project (MVIP), a population-based study that evaluated the association of cataract and dietary intake of lutein and zeaxanthin or in the Vitamin E, Cataract, and Age-related Macular Degeneration Trial (VECAT), a randomized controlled trial that showed no effect of intake of 500 IU/day of vitamin E for 4 years on cataract. One of the possible reasons for greater AMD risk could be that these subjects started eating a diet high in lutein and zeaxanthin based on the knowledge of the beneficial effects of these xanthophylls gained by participating in the MVIP and VECAT. Also, high intake may not translate to higher retinal uptake as these AMD- and cataract-affected eyes may have impaired uptake mechanisms. Intake levels of 0.88–1 mg/day of lutein and zeaxanthin combined were associated with higher odds of AMD. In other studies, the levels of 1–3 mg/day of lutein alone or up to 6 mg/day of lutein and zeaxanthin combined were associated with reduced risk of AMD. Thus, it appears that the levels may have been too low to see a beneficial effect in eyes already affected with AMD and cataract.
In a case–control study in China, serum lutein and zeaxanthin concentrations were lower in subjects with exudative AMD compared to controls [54]. In a cross-sectional study, Gale et al. found that subjects with the lowest plasma zeaxanthin concentration (<0.03 μM), not plasma lutein or lutein plus zeaxanthin, had a twofold greater risk of AMD compared to subjects with high plasma zeaxanthin (>0.05 μM) [55]. Cross-sectional examination of the baseline data from the POLA study revealed similar associations of high plasma zeaxanthin concentrations (>0.09 μM) with reduced risk of AMD compared to low plasma zeaxanthin (<0.04 μM). They also reported an association with plasma lutein and zeaxanthin combined [56]. However, in the POLA study population, the high plasma zeaxanthin concentrations were much greater than in the Gale et al. study. The authors attributed this to a higher dietary intake of these xanthophylls. Contrary to these protective inverse associations, a nested case–control study of the Beaver Dam Eye Study cohort found no difference in serum lutein and zeaxanthin concentrations between early AMD and age, sex, and smoking matched controls [57]. Serum concentrations, unlike tissue concentration, are not a good measure of long-term dietary intake, which may have caused the null findings. Indeed, high retinal levels of lutein and zeaxanthin were associated with an 82% lower risk of AMD compared with low levels [43].
Intervention Studies
Epidemiological evidence listed earlier suggests that low MP density could increase the risk of AMD. The most apparent prevention and treatment strategy would thus be to increase MP density via increasing lutein and zeaxanthin intake in the diet through foods and/or supplements. Consumption of lutein- and zeaxanthin-rich foods such as spinach, corn, and eggs for periods ranging from 5 to 15 weeks has significantly increased MP density in a population aged 24–60 years and also in a population >60 years, who are at an increased risk of AMD [58–60]. However, in an ancillary study of the Women’s Health Initiative, a low-fat, high fruit and vegetable diet for about 8 years did not alter MP density in postmenopausal women [61]. The increase in fruit and vegetable intake (approximately 1.5 servings/day) was possibly of insufficient magnitude to raise MP density. Additionally, subjects in the intervention group also appeared to have similar lutein and zeaxanthin intake (2.5 mg/day) as the comparison group (2 mg/day), indicating that the increase in consumption of fruits and vegetables was not necessarily from lutein- and zeaxanthin-rich foods [61].
In addition, MP density has been shown to significantly increase through intake of supplements containing lutein and zeaxanthin in adults without and with AMD [8, 62–69]. Bone et al. were the first to show that meso-zeaxanthin is absorbed by the body. They observed increases in serum meso-zeaxanthin and also MP density following 120 days of supplementation with 15 mg meso-zeaxanthin, 5.5 mg lutein, and 1.4 mg zeaxanthin [70]. Following this, in a recent exploratory study, improvements in MP density and MP spatial profile were reported after 2 weeks of supplementation with a formulation containing 3.7 mg lutein, 0.8 mg zeaxanthin, and 7.3 mg meso-zeaxanthin [71].
Change in MP density post-supplementation appears to be related to baseline MP density levels with supplementation being most beneficial in a population with low baseline MP density [59, 60, 72]. Zeimer et al. suggested that long-term supplementation with high doses of lutein may be necessary to cause a significant improvement in MP density, but lutein and zeaxanthin levels in foods are sufficient to maintain high MP density [63]. MP enrichment can also be achieved through intake of foods that are rich in lutein and zeaxanthin, such as spinach, corn, and eggs [58–60]. All studies listed above, with the exception of one, evaluated high doses of lutein supplementation (6, 10, 12, 20, or 30 mg/day), while zeaxanthin doses were lower (1–3 mg/day). The Lutein Xanthophyll Eye Accumulation Study (LUXEA) evaluated the effects of supplementation with lutein (11 mg/day) or zeaxanthin (13 mg/day) for 6 months on MP density. Increases in MP density in the fovea were similar to the parafovea for the zeaxanthin-supplemented group. This confounded MP density measurements because parafoveal measures are used as a reference measure. Therefore, a correction was needed to determine MP increases with zeaxanthin supplementation. This was not the case with lutein supplementation, which caused MP density increase in the fovea only [67]. These findings suggest zeaxanthin is deposited more widely in the retina during supplementation, while lutein accumulates more in the fovea. Normal distribution in unsupplemented individuals is the reverse, zeaxanthin is dominant in the fovea and lutein in the periphery [9]. The authors speculated that higher amounts of zeaxanthin in the fovea compared to lutein at baseline possibly caused greater zeaxanthin deposition in the parafoveal regions during supplementation. Similarly, the normally high lutein levels in the peripheral regions of the retina compared to zeaxanthin possibly caused greater deposition of lutein in the fovea during lutein supplementation.
Supplementation with lutein and zeaxanthin as well as meso-zeaxanthin can significantly improve MP density in populations without and with AMD. The ability of supplementation with these xanthophylls to slow the progression of AMD and/or influence other visual function parameters will now be discussed. In the Lutein Antioxidant Supplementation Trial (LAST), a double-blinded, placebo-controlled, randomized trial, 10 mg/day lutein alone or in combination with antioxidants, vitamins, and minerals for 1 year improved visual function and subjective results of Amsler grid testing in patients with atrophic AMD [66]. Improvements were also reported in glare recovery and contrast sensitivity in this study. In the Age-related Maculopathy Italian Study, nonadvanced AMD patients supplemented with 10 mg lutein, 1 mg zeaxanthin, and 4 mg astaxanthin together with vitamins C and E, zinc, and copper daily for 12 months showed improvements in a selective dysfunction that affects the central retina (0°–5°) but no functional changes were reported in the more peripheral (5°–20°) regions of the retina [73]. However, supplementation with 6 mg lutein in combination with vitamins C and E, retinol, zinc, and copper for 9 months did not affect contrast sensitivity in atrophic AMD patients when compared to controls [74]. The 6 mg lutein used in this study was in an ester form, which would be equivalent to only about 3 mg of lutein [75]. Also, the authors suggested that the change in contrast sensitivity was clinically important. The authors provided plausible explanations for their counterintuitive results, which include genetic factors that affect lutein binding proteins in the retina, lower dosage of lutein, and bioavailability of lutein esters. However, free lutein and lutein esters had comparable bioavailability [75]. Supplementation with 12 mg lutein and 1 mg zeaxanthin together with vitamins C and E, zinc, and selenium for 6 months significantly increased MP density in an older adult population, 90% of whom exhibited some clinical features of AMD [76]. The AREDS intervention of vitamin C (500 mg), vitamin E (400 IU), β-carotene (15 mg), zinc (as zinc oxide, 80 mg), and copper (as copper oxide, 2 mg), but not lutein or zeaxanthin, was found to lower the risk of AMD by 25% in individuals who had intermediate or advanced AMD in one eye but not the other eye [77]. The ongoing AREDS 2 intervention is evaluating the effect of lutein, zeaxanthin, and omega-3 fatty acids on progression to advanced AMD [78].
On a slightly different note, cataract surgery, where the natural crystalline lens is replaced with a clear artificial intraocular lens, is an independent risk factor of AMD [79, 80]. There is increased transmission of short wavelength light to the retina after cataract surgery and an induced intraocular inflammatory response; either or both of these could enhance AMD risk [81, 82]. Interestingly, implantation of a blue light filtering intraocular lens, not a standard intraocular lens, during cataract surgery increased MP density 3 months post-surgery in the absence of raised serum lutein and zeaxanthin concentrations [83]. The authors did not hypothesize an increase in MP density with blue light filtering lens, but only a stabilization of MP density. They speculate that there is enhanced retinal capture of lutein and zeaxanthin following cataract surgery to offset the increased photo-oxidative damage from the increased visible light irradiation of the retina, but the photo-oxidative damage does not occur with blue light filtering lens causing an increase in MP density.
Cataract
Age-related cataract is one of the major causes of visual impairment and blindness in the aging US population. Approximately 50% of the 30–50 million cases of worldwide blindness result from unoperated cataract [84, 85]. A clinically significant cataract is present in about 5% of Caucasian Americans aged 52–64 years and rises to 46% in those aged 75–85 years [36]. In the USA, cataract extraction accompanied by ocular lens implant is the most common surgical procedure of the eye [86]. Lens implantation enables many to have reduced dependence on glasses. However, the procedure is costly, accounting for 12% of the Medicare budget and more than $3 billion in annual health expenditures [86, 87]. For these reasons, the prevention of cataract is a preferred alternative to surgery.
Three types of cataract are defined by their location in the lens. Nuclear cataract occurs in the center or nucleus of the lens and is the most common type [36], which interferes with a person’s ability to see distant objects and is usually the result of advancing age. Cortical cataract begins at the outer rim of the lens (the cortex) and progresses towards the center, and is most common in diabetics. Posterior subcapsular cataract (PSC) occurs in the central posterior cortex, just under the posterior capsule, the membrane that envelops the lens. PSC can occur in younger individuals progressing rapidly and resulting in glare and blurriness [88]. This type of cataract is usually seen in patients who use steroids, are diabetic, or have extreme nearsightedness. Figure 13.4 shows a scene viewed by a person affected with cataract.
Various factors are involved in the development of cataracts, such as long-term light exposure, diabetes, smoking, alcohol use, and advancing age [88]. Such factors can lead to aggregation of the lens proteins and osmotic damage resulting in increased scattering of light and loss of lens transparency. Protein aggregation mainly occurs in the lens nucleus and osmotic damage occurs in the lens cortex [89]. Free radicals are generated in the lens during normal metabolic activities and as a result of photo-oxidative reactions due to increased UVB exposure (related to cortical and PSC). These free radicals can also cause electrolyte imbalance and protein aggregation. Lutein and zeaxanthin are ideally located in the lens to provide protection against free radical damage. Other innate antioxidant defense mechanisms, which include enzymes such as superoxide dismutase and glutathione and non-enzymatic molecules such as vitamins C and E also protect the lens from oxidative stress.
Epidemiological Studies
A number of studies have investigated the relationship between dietary intake of lutein and zeaxanthin and the incidence of cataract. In prospective data from the Nurses’ Health Study population, a significant reduction in the incidence of cataract extraction was observed with high dietary intake of dark green vegetables such as spinach, which are rich in lutein, but not with vegetables such as carrots, sweet potatoes, and squash, which are rich in carotenes [90]. The risk of cataract extraction was 19% lower in the US male health professionals who had the highest lutein and zeaxanthin intake compared to those with the lowest intake [91]. Again, broccoli and spinach were more consistently associated with reduced risk compared to iceberg and romaine lettuce and corn indicating a protective effect of lutein-rich foods. A similar inverse association was observed in a case–control study conducted in Northern Italy. In this study, reduced odds of cataract extraction were reported in subjects with the highest intake of cruciferous vegetables and spinach [92]. The evidence of a beneficial association between lutein and zeaxanthin intake and nuclear cataract seems promising. Higher dietary intake of lutein and zeaxanthin was associated with a reduced risk of nuclear cataract compared to low intakes [93, 94]. When examining specific foods, Christen et al. found a borderline significant inverse relation with green leafy vegetables and raw spinach. The MVIP found an inverse association between high dietary intake of lutein and zeaxanthin and prevalence of nuclear cataract, but not cortical and PSC in an Australian population aged 40 years and older [95]. Also, CAREDS found that women in the highest quintile of dietary intake or serum lutein and zeaxanthin were 32% less likely to have nuclear cataract compared to those in the lowest quintile [96]. In the prospective POLA study, plasma zeaxanthin, and not lutein, was associated with a 75% reduced risk of nuclear cataract in residents of Southern France [56]. Similarly, high zeaxanthin intake was protective against cataract in institutionalized men aged 65 years and older [97]. In the same study, men and women who consumed >3.29 mg/day of lutein were less likely to have cataracts than those whose consumption was <0.26 mg/day. Neither dietary intake nor plasma lutein and zeaxanthin were found to be related to cortical cataract risk [98–100]. Risk of PSC was 50% lower in women with the highest vs. lowest lutein/zeaxanthin intake and also in individuals with high plasma lutein/zeaxanthin concentrations [99, 101]. Among the epidemiological studies mentioned above, lutein and zeaxanthin intake in only one study included intake from both diet and supplements [98]; in the other studies, dietary intake represents lutein and zeaxanthin from food sources alone. Unlike the protective effects reported by other studies, the Beaver Dam Eye study reported an increased risk of nuclear cataract in women with high serum lutein levels [102]. This inconsistent finding may be due to the cross-sectional nature of the study or the fact that serum lutein may not reflect lutein levels in the lens [103].
Intervention Studies
Two intervention studies to date have evaluated the effect of lutein supplementation on subjects diagnosed with cataract. In these two separate long-term intervention studies, supplementation with 7 mg/day of lutein (equivalent to lutein in ∼100 g of spinach [104]) and 12 mg of lutein three times a week was shown to improve visual acuity and glare sensitivity in cataract patients [105, 106]. Several randomized controlled trials have evaluated a combination of vitamins and micronutrients; the most frequently studied were vitamins C and E and β-carotene. The AREDS, the Antioxidants in the Prevention of Cataract (APC) study done in southern India, the Roche European American Cataract Trial (REACT), and the Alpha-tocopherol Beta-carotene (ATBC) study did not demonstrate beneficial effects of supplementation [107–110]. Reduction in lens opacity or nuclear cataract incidence with supplementation was observed in the US population of the REACT study, the Linxian study done in rural China, and the Italian Clinical Trial of Nutritional Supplements and Age-related cataract study [109, 111, 112]. However, the interventions used in these studies were a combination of multivitamins and minerals, which included β-carotene but not lutein and zeaxanthin. The ability of lutein and zeaxanthin to retard the development or progression of cataract or lens opacity has not been evaluated.
In conclusion, epidemiological studies provide strong evidence to suggest that long-term intake of dietary lutein and zeaxanthin may provide protection against the incidence of nuclear cataract, to a lesser extent PSC, but not cortical cataract. Most noteworthy is the fact that pharmacological doses are not necessary, because an adequate amount is found in foods such as spinach, kale, and broccoli.
Retinitis Pigmentosa
Retinitis pigmentosa (RP) is the leading cause of inherited blindness in the developed world, affecting 50,000–100,000 people in the USA and an estimated 1.5 million people worldwide [113, 114]. RP refers to a group of inherited retinal disorders that result in the degeneration of rod and cone photoreceptors [115]. Clinically, rod cell death translates to night blindness which generally precedes defects in peripheral visual field, i.e., tunnel vision (Fig. 13.4), by years or even decades. Cones are seldom directly affected by identified mutations. They degenerate secondarily to the rods as the disease progresses, which results in loss of central vision and complete blindness [113]. Many individuals with RP are not legally blind until their 40s or 50s and some RP patients even retain partial sight throughout life. On the other hand, some RP patients go completely blind during childhood.
RP can be inherited as an autosomal-dominant (30–40% of cases), autosomal-recessive (50–60%), or X-linked (5–15%) trait [115]. Mutations in the rhodopsin gene account for ∼25% of dominant RP. Mutations in the USH2A gene may be the cause of ∼20% of the recessive RP [116]. The USH2A gene encodes for the protein usherin and may be important for retinal development and maintaining homeostasis. Mutations in the GTPase regulator gene may account for ∼70% of X-linked RP [115]. These mutations cause ∼30% of all cases of RP. Mutations identified in other genes include: enzymes of the phototransduction cascade [transducin α-subunit, guanylate cyclase, cGMP-dependent phosphodiesterase, and arrestin]; structural or trafficking proteins [peripherin/RD, ABCR]; more rarely genes encoding proteins involved in vitamin A metabolism [CRALBP, RPE 65] and phagocytosis of photoreceptor outer segments [117–125]. Mutations affecting proteins involved in specific biochemical pathways that transduce light can cause hyperpolarization and apoptosis of the rod photoreceptor cells.
The high concentration of lutein and zeaxanthin in the ROS and also in the macula warrants their investigation in the treatment of RP. In a small, uncontrolled study of 13 RP patients and 3 patients with other retinal diseases, supplementation with 40 mg lutein/day for 9 weeks improved visual acuity and visual field area [126]. Lutein supplementation (10 mg/day for 12 weeks followed by 30 mg/day for 12 weeks) also improved central visual field in 34 RP patients in a randomized, cross-over, placebo-controlled, double-blinded study [127]. In another double-blinded, randomized control trial (RCT), nonsmoking RP patients supplemented with 12 mg/day of lutein along with 15,000 IU/day of vitamin A for 4 years had a slower decline in midperipheral visual field sensitivity compared to controls who were taking 15,000 IU/day of vitamin A [128]. Maximum slowing of midperipheral sensitivity also occurred among those with the highest serum lutein concentration and greatest increase in MP density and was not limited to those with milder disease. Unlike the previous two studies, no significant effect of this intervention was observed on central field sensitivity. The detectable benefit of lutein supplementation in preserving midperipheral function but not central function may reflect an increased requirement for antioxidants in the photoreceptor outer segments, where cells are most impaired. No toxic effects of lutein supplementation were observed in this study. No beneficial effect on central vision was observed in a small group of patients (n = 23) with RP and Usher’s syndrome who were supplemented with 20 mg lutein/day for 6 months [129]. MP density profile was not significantly different between controls and RP patients. MP density was related to foveal structure in RP patients compared with controls. MP density was lower in patients with reduced inner retinal thickness, suggesting that loss of inner retinal tissue is known to occur with outer retinal degenerations. Similar observations were made in a cross-sectional study that showed decreased MP density in eyes with more photoreceptor cell loss and in eyes with cystoid macular edema, which occurs in more than 25% of RP patients [130]. MP density increased in only half the subjects post-lutein supplementation of 20 mg/day for 6 months despite increases in serum lutein concentration [129]. The nonresponse in MP density may have been due to loss of photoreceptor cells or disruption of lutein uptake mechanisms in the macula [130]. Supplementation may have also caused increases in lutein levels in peripheral regions of the retina, which cannot be detected by heterochromatic flicker photometry. This is a noninvasive, psychophysical technique that can measure pigment density in the macular region only.
The evidence to date indicates that short-term lutein supplementation at doses of ≥30 mg/day or long-term supplementation at doses of 10–20 mg/day in combination with 15,000 IU/day of vitamin A may benefit central or midperipheral visual fields, respectively. Larger-scale placebo-controlled dose–response clinical studies are needed to derive more conclusive evidence of the benefits of lutein supplementation in specific types of RP. Preservation of central vision is necessary for preventing complete blindness in RP patients. Vitamins A and E and omega-3 fatty acids benefit RP patients [131–136]. More studies are needed to determine if lutein in combination with vitamin A and omega-3 fatty acids can help preserve central vision and also improve peripheral vision in RP patients.
Visual Function
The role of lutein and zeaxanthin as MP and also their importance in the retina and lens is now well-established. Data are also accumulating that suggest lutein and zeaxanthin as MP can improve visual function. There are two hypotheses that explain the mechanism by which MP improves visual function [137]. The acuity hypothesis states that MP reduces the effect of chromatic aberration. The visibility hypothesis states that MP may improve vision through the atmosphere by preferentially absorbing blue haze (short-wave dominant air light that produces a veiling luminance when viewing objects at a distance). MP may improve glare disability and photostress recovery because of its light filtering properties [138]. Some biological mechanisms, such as improvement in neuronal signaling efficiency in the eye, have also been suggested by which lutein and zeaxanthin may improve visual function [139].
High lutein and zeaxanthin intake improves MP density; therefore, it may improve visual function measures. Subjects from the LUXEA study, described in the AMD section, showed improved contrast acuity thresholds at high mesopic levels, which means better visual performance when ambient illumination is low [140]. Although increases in MP density were not related to contrast acuity thresholds, the results of this study suggest that lutein and zeaxanthin supplementation may benefit activities such as driving at night. Six months of supplementation with 12 mg/day of lutein was shown to increase MP density in healthy subjects with a mean age of 23 years and improve visual performance in glare function tests [141]. Lutein has been reported to protect against the detrimental effects of long-term computer display light exposure in healthy subjects aged 22–30 years of age [142]. In this study, 12 weeks of supplementation with 12 mg/day of lutein also improved contrast sensitivity. Lutein supplementation (5 mg) in combination with zeaxanthin (1 mg) and blackcurrant extract (200 mg) was also shown to reduce symptoms of visual fatigue associated with visual proof-reading tasks in healthy subjects aged 22–45 years [143].
The effect of lutein supplementation on visual performance was evaluated in patients with eye disease. In a double-blind, placebo-controlled study involving cataract patients (n = 17), supplementation with 15 mg lutein three times a week resulted in improved visual acuity and glare sensitivity [105]. In patients with retinal degeneration, lutein supplementation (20–40 mg/day, 26 weeks) improved visual acuity and mean visual field area, which began 2–4 weeks after the intervention but plateaued at 6–14 weeks [126]. RCTs involving AMD patients have shown that lutein supplementation, ranging in dose from 8 to 15 mg, improved dark adaptation, visual acuity, foveal sensitivity, contrast sensitivity, and glare recovery [66, 144, 145]. Evidence suggests that high MP density can improve visual function measures.
Conclusions
Epidemiological evidence suggests that high dietary intake of lutein and zeaxanthin is related to reduced risk of AMD. The majority of these studies evaluated neovascular or advanced AMD risk. Lower AMD risk was associated with higher consumption of lutein-rich dark green vegetables, such as spinach, broccoli, and lettuce, indicating supraphysiological doses may not be necessary for prevention. The lutein amounts in the high intake groups of epidemiological studies were 1–3 mg/day, while the lutein and zeaxanthin combined amount was ∼6 mg/day. MP density can be augmented by consumption of lutein- and zeaxanthin-rich foods, such as spinach, eggs, and corn. Also, intake of high doses of lutein and meso-zeaxanthin supplements increases MP density. High dose zeaxanthin supplementation, however, may increase pigment levels in the peripheral regions of the retina and not necessarily in the macular region. Evidence suggests that increased MP density can be maintained by consumption of lutein- and zeaxanthin-rich foods. MP density can also be increased in AMD affected eyes. Long-term lutein supplementation at doses of 10 mg/day with or without supplementation with other antioxidants may improve visual function measures in AMD patients. Lutein and zeaxanthin have potential impact in the prevention and treatment of AMD.
Despite the limitations associated with evaluating dietary intakes, epidemiological studies have consistently shown high dietary intake of lutein and zeaxanthin to be protective against the incidence of nuclear cataract and cataract extraction. The most noteworthy is that among the different food groups evaluated, high intake of lutein-rich dark green vegetables, such as spinach and broccoli, was related to decreased cataract risk [90–94]. In the reviewed studies, high lutein and zeaxanthin amounts were 6 mg/day [91, 94], >3.2 mg/day [96, 97], and >1 mg/day [93, 95] and the corresponding low doses were 1 mg/day and 250–600 μg/day. The evidence of a beneficial effect of high lutein and zeaxanthin intake on PSC is very scarce and no associations have been observed with cortical cataract. More lutein and zeaxanthin intervention studies are necessary to provide convincing evidence that lutein and zeaxanthin can improve visual function in cataract patients.
Supplementation with high doses of lutein preserved central visual acuity in small RCTs on RP patients. Lutein in combination with 15,000 IU/day of vitamin A preserved midperipheral visual function. Both these findings indicate that lutein may play a role in preventing complete blindness in RP patients.
In 2006, the Food and Drug Administration reviewed all the epidemiological and clinical intervention studies on AMD and cataract and concluded that there is no credible evidence for a health claim about the intake of lutein or zeaxanthin (or both) on the risk of AMD and cataract [146]. In observational studies, lutein and zeaxanthin intake was calculated using food frequency questionnaires, diet recalls, or diet records, in which the type and amount of foods consumed was recorded. Lutein and zeaxanthin intake was then estimated from the USDA’s database on carotenoid composition of foods. The limitations of these dietary assessment tools include subject’s poor memory, overestimation or underestimation of portion sizes, the variation in lutein and zeaxanthin levels in the foods, the effect of consumption method (i.e., cooked vs. raw), type and amount of fat or fiber consumed with the lutein and zeaxanthin source, and other factors that affect bioavailability. Dietary assessments do not allow nutrients or food components to be studied in isolation, which may not yield the same findings. Thus, the findings of observational studies presented in this chapter must be interpreted with caution.
Future Directions
The evidence to date suggests that lutein and zeaxanthin can have a significant impact on AMD and cataract risk. Of note is that these are the only two carotenoids that are preferentially taken up into the retina and the lens. More randomized, controlled clinical trials are necessary to determine the dosage at which lutein and zeaxanthin interventions are most effective. In the case of AMD, it is evident that low MP density is one of the modifiable pre-disposing risk factors. MP density can be measured with ease using techniques such as heterochromatic flicker photometry, motion detection photometry, fundus reflectance spectroscopy, Raman spectrometry, and autofluorescence spectrometry [147].
Clinical models of these MP measurement devices are now available that could be potentially used by ophthalmologists to assess an individual’s MP density as part of a routine eye examination. If low MP density is detected early in life, consumption of lutein- and zeaxanthin-rich foods can increase pigment levels and possibly prevent AMD development later in life. Future studies should focus on standardizing the clinical devices used for MP assessment and comparing measurements to other established techniques. Thus, there is scope of prevention of AMD through early identification of depleted MP levels in the retina. The majority of studies have focused on effects of lutein supplementation on MP enrichment. Supplementation with lutein or zeaxanthin alone seems to increase pigment density in different regions of the macula. One study showed supplementation with a high dose of zeaxanthin increased pigment levels more in the peripheral regions, which normally contain higher lutein levels. Future studies need to delve deeper into the differential effects of lutein and zeaxanthin supplementation on pigment enrichment and also functional effects. Studies should also be designed to evaluate different doses of lutein and zeaxanthin and also the impact of supplementation on different stages of AMD.
There is an urgent need to evaluate the effect of lutein and zeaxanthin intervention on progression of cataract, especially because epidemiological evidence showed a positive effect of high lutein and zeaxanthin intake on nuclear cataract. Nuclear cataract is the result of advancing age; thus, consuming a lutein- and zeaxanthin-rich diet early in life may possibly prevent incidence. Large-scale studies are required to evaluate the effect of lutein intervention in the preservation of vision in RP patients to prevent the incidence of blindness.
Other than their role in eye disease prevention and visual function, lutein and zeaxanthin may influence neural functions within the retina and enhance gap junctional communication, which in the retina is crucial for light processing and may be important for the development of neural circuitry within the visual system [148]. High MP density has been associated with improved visual processing speed, which is measured as critical flicker fusion threshold [149, 150]. MP density was shown to be inversely related to scotopic noise; lutein and zeaxanthin may thus improve efficiency of rod cell functioning [151, 152]. The exclusive accumulation of lutein and zeaxanthin in the neural retina warrants further investigation of their role in modifying neural functions.
References
Britton G. Structure and properties of carotenoids in relation to function. FASEB J. 1995;9(15):1551–8.
Krinsky NI, Landrum JT, Bone RA. Biologic mechanism of the protective role of lutein and zeaxanthin in the eye. Annu Rev Nutr. 2003;23:171–201.
Li B, Vachali P, Bernstein PS. Human ocular carotenoid-binding proteins. Photochem Photobiol Sci. 2010;9(11):1418–25.
Frederick K. Distribution and metabolism of dietary carotenoids in humans as a criterion for development of nutritional supplements. Pure Appl Chem. 2006;78(8):7.
Ahmed SS, Lott MN, Marcus DM. The macular xanthophylls. Surv Ophthalmol. 2005;50(2):183–93.
Johnson EJ, Neuringer M, Russell RM, Schalch W, Snodderly DM. Nutritional manipulation of primate retinas. III: Effects of lutein or zeaxanthin supplementation on adipose tissue and retina of xanthophyll-free monkeys. Invest Ophthalmol Vis Sci. 2005;46(2):692–702.
Bone RA, Landrum JT, Friedes LM, et al. Distribution of lutein and zeaxanthin stereoisomers in the human retina. Exp Eye Res. 1997;64(2):211–8.
Landrum JT, Bone RA, Sprague K, Moore L. A one-year study of supplementation with lutein on the macular pigment. Exp Eye Res. 1997;65:57–62.
Bone RA, Landrum JT, Fernandez L, Tarsis SL. Analysis of the macular pigment by HPLC: retinal distribution and age study. Invest Ophthalmol Vis Sci. 1988;29:843–9.
Handelman GJ, Dratz EA, Reay CC, van Kuijk JG. Carotenoids in the human macula and whole retina. Invest Ophthalmol Vis Sci. 1988;29(6):850–5.
Algvere PV, Marshall J, Seregard S. Age-related maculopathy and the impact of blue light hazard. Acta Ophthalmol Scand. 2006;84(1):4–15.
Snodderly DM, Auran JD, Delori FC. The macular pigment. II. Spatial distribution in primate retinas. Invest Ophthalmol Vis Sci. 1984;25:12.
Landrum JT, Bone RA. Lutein, zeaxanthin, and the macular pigment. Arch Biochem Biophys. 2001;385(1):28–40.
Bone RA, Landrum JT. Macular pigment in henle fiber membranes: a model for Haidinger’s brushes. Vision Res. 1984;24(2):103–8.
Rapp LM, Maple SS, Choi JH. Lutein and zeaxanthin concentrations in rod outer segment membranes from perifoveal and peripheral human retina. Invest Ophthalmol Vis Sci. 2000;41(5):1200–9.
Sommerburg LG, Siems WG, Hurst JS, Lewis JW, van Kuijk FJGM. Lutein and zeaxanthin are associated with photoreceptors in the human retina. Curr Eye Res. 1999;19:491–5.
Li B, Vachali P, Frederick JM, Bernstein PS. Identification of StARD3 as a lutein-binding protein in the macula of the primate retina. Biochemistry. 2011;50(13):2541–9.
Bhosale P, Zhao DY, Bernstein PS. HPLC measurement of ocular carotenoid levels in human donor eyes in the lutein supplementation era. Invest Ophthalmol Vis Sci. 2007;48(2):543–9.
Yeum K-J, Shang F, Schalch W, Russell RM, Taylor A. Fat-soluble nutrient concentrations in different layers of human cataractous lens. Curr Eye Res. 1999;19(6):502–5.
Yeum KJ, Ahn SH, Rupp de Paiva SA, Lee Kim YC, Krinsky NI, Russell RM. Correlation between carotenoid concentrations in serum and normal breast adipose tissue of women with benign breast tumor or cancer. J Nutr. 1998;128:1920–6.
Khachik F, Spangler CJ, Smith Jr JC, Canfield LM, Steck A, Pfander H. Identification, quantificantion, and relative concentrations of carotenoids and their metabolites in human milk and serum. Anal Chem. 1997;69(10):7.
Leo MA, Ahmed S, Aleynik SI, Siegel JH, Kasmin F, Lieber CS. Carotenoids and tocopherols in various hepatobiliary conditions. J Hepatol. 1995;23(5):550–6.
Peng YM, Peng YS, Lin Y. A nonsaponification method for the determination of carotenoids, retinoids, and tocopherols in solid human tissues. Cancer Epidemiol Biomarkers Prev. 1993;2(2):6.
Furr HC, Clark RM. Intestinal absorption and tissue distribution of carotenoids. J Nutr Biochem. 1997;8(7):364–77.
Craft NE, Haitema TB, Garnett KM, Fitch KA, Dorey CK. Carotenoid, tocopherol, and retinol concentrations in elderly human brain. J Nutr Health Aging. 2004;8:156–62.
Demmig-Adams B, Adams WW. Antioxidants in photosynthesis and human nutrition. Science. 2002;298(5601):2149–53.
Kim Y-S, Oh D-K. Biotransformation of carotenoids to retinal by carotenoid 15,15′-oxygenase. Appl Microbiol Biotechnol. 2010;88(4):807–16.
Junghans A, Sies H, Stahl W. Macular pigments lutein and zeaxanthin as blue light filters studied in liposomes. Arch Biochem Biophys. 2001;391:160–4.
Snodderly DM, Auran JD, Delori FC. The macular pigment. II. Spatial distribution in primate retinas. Invest Ophthalmol Vis Sci. 1984;25(6):674–85.
Sujak A, Gabrielska J, Grudzinski W, Borc R, Mazurek P, Gruszecki WI. Lutein and zeaxanthin as protectors of lipid membranes against oxidative damage: the structual evidence. Arch Biochem Biophys. 1999;371(2):301–7.
Khachik F, Bernstein PS, Garland DL. Identification of lutein and zeaxanthin oxidation products in human and monkey retinas. Invest Ophthalmol Vis Sci. 1997;38(9):1802–11.
Haegerstrom-Portney G. Short-wavelength-sensitive-cone sensitivity loss with aging: a protective role for macular pigment? J Opt Soc Am A. 1988;5:2140–4.
Ojima F, Sakamoto H, Ishiguro Y, Terao J. Consumption of carotenoids in photosensitized oxidation of human plasma and plasma low-density lipoprotein. Free Radic Biol Med. 1993;15(4):377–84.
Nolan J, O’Donovan O, Beatty S. The role of macular pigment in the defence against AMD. AMD. 2003;39–41.
Bernstein PS, Khachik F, Carvalho LS, et al. Identification and quantitation of carotenoids and their metabolites in the tissues of the human eye. Exp Eye Res. 2001;72:215–23.
Congdon N, O’Colmain B, Klaver CCW, et al. Causes and prevalence of visual impairment among adults in the United States. Arch Ophthalmol. 2004;122(4):477–85.
Jager RD, Mieler WF, Miller JW. Age-related macular degeneration. N Engl J Med. 2008;358(24):2606–17.
Abdelsalam A, Del Priore L, Zarbin MA. Drusen in age-related macular degeneration: pathogenesis, natural course, and laser photocoagulation-induced regression. Surv Ophthalmol. 1999;44(1):1–29.
Age-related Eye Disease Study Research Group. A simplified severity scale for age-related macular degeneration. AREDS report No. 18. Arch Ophthalmol. 2005;123(11):5.
Snodderly DM. Evidence for protection against age-related macular degeneration by carotenoids and antioxidant vitamins. Am J Clin Nutr. 1995;62(6 Suppl):1448S–61.
Ambati J, Ambati BK, Yoo SH, Ianchulev S, Adamis AP. Age-related macular degeneration: etiology, pathogenesis, and therapeutic strategies. Surv Ophthalmol. 2003;48(3):257–93.
Tan JSL, Wang JJ, Flood V, Rochtchina E, Smith W, Mitchell P. Dietary antioxidants and the long-term incidence of age-related macular degeneration: the Blue Mountains Eye Study. Ophthalmology. 2008;115(2):334–41.
Bone RA, Landrum JT, Mayne ST, Gomez CM, Tibor SE, Twaroska EE. Macular pigment in donor eyes with and without AMD: a case-control study. Invest Ophthalmol Vis Sci. 2001;42(1):235–40 [Erratum appears in Invest Ophthalmol Vis Sci 2001 Mar;42(3):548].
Beatty S, Murray IJ, Henson DB, Carden D, Koh H, Boulton ME. Macular pigment and risk for age-related macular degeneration in subjects from a Northern European population. Invest Ophthalmol Vis Sci. 2001;42(2):439–46.
Bernstein PS, Shao D-Y, Wintch SW. Resonance Raman measurement of macular carotenoids in normal subjects and in age-related macular degeneration patients. Ophthalmology. 2002;109:1780–7.
LaRowe TL, Mares JA, Snodderly DM, et al. Macular pigment density and age-related maculopathy in the Carotenoids in Age-Related Eye Disease Study. An ancillary study of the women’s health initiative. Ophthalmology. 2008;115(5):876–83. e871.
Berendschot TTJM, Willemse-Assink JJM, Bastiaanse M, de Jong PTVM, van Norren D. Macular pigment and melanin in age-related maculopathy in a general population. Invest Ophthalmol Vis Sci. 2002;43(6):1928–32.
Schweitzer D, Lang GE, Remsch H, et al. Age-related maculopathy. Comparative studies of patients, their children and healthy controls. (German). Ophthalmologe. 2000;97:84–90.
Eye Disease Case-Control Study Group (EDCCSG). Antioxidant status and neovascular age-related macular degeneration. Arch Ophthalmol. 1993;111:104–9.
Seddon JM, Ajani UA, Sperduto RD, et al. Dietary carotenoids, vitamins A, C, and E, and advanced age-related macular degeneration. Eye Disease Case-Control Study Group. JAMA. 1994;272(18):1413–20.
Snellen EL, Verbeek AL, Van Den Hoogen GW, Cruysberg JR, Hoyng CB. Neovascular age-related macular degeneration and its relationship to antioxidant intake. Acta Ophthalmol Scand. 2002;80(4):368–71.
Moeller SM, Parekh N, Tinker L, et al. Associations between intermediate age-related macular degeneration and lutein and zeaxanthin in the Carotenoids in Age-related Eye Disease Study (CAREDS): ancillary study of the Women’s Health Initiative. Arch Ophthalmol. 2006;124(8):1151–62.
Robman L, Vu H, Hodge A, et al. Dietary lutein, zeaxanthin, and fats and the progression of age-related macular degeneration. Can J Ophthalmol. 2007;42(5):7.
Zhou H, Zhao X, Johnson EJ, et al. Serum carotenoids and risk of age-related macular degeneration in a Chinese population sample. Invest Ophthalmol Vis Sci. 2011;52:4338–44.
Gale CR, Hall NF, Phillips DIK, Martyn CN. Lutein and zeaxanthin status and risk of age-related macular degeneration. Invest Ophthalmol Vis Sci. 2003;44:2461–5.
Delcourt C, Carrière I, Delage M, Barberger-Gateau P, Schalch W, POLA Study Group. Plasma lutein and zeaxanthin and other carotenoids as modifiable risk factors for age-related maculopathy and cataract: the POLA study. Invest Ophthalmol Vis Sci. 2006;47(6):2329–35.
Mares-Perlman JA, Brady WE, Klein R, et al. Serum antioxidants and age-related macular degeneration in a population-based case-control study. Arch Ophthalmol. 1995;113(12):1518–23.
Hammond Jr BR, Johnson EJ, Russell RM, et al. Dietary modification of human macular pigment density. Invest Ophthalmol Vis Sci. 1997;38:1795–801.
Wenzel AJ, Gerweck C, Barbato D, Nicolosi RJ, Handelman GJ, Curran-Celentano J. A 12-wk egg intervention increases serum zeaxanthin and macular pigment optical density in women. J Nutr. 2006;136(10):2568–73.
Vishwanathan R, Goodrow-Kotyla EF, Wooten BR, Wilson TA, Nicolosi RJ. Consumption of 2 and 4 egg yolks/d for 5 wk increases macular pigment concentrations in older adults with low macular pigment taking cholesterol-lowering statins. Am J Clin Nutr. 2009;90(5):1272–9.
Moeller SM, Voland R, Sarto GE, Gobel VL, Streicher SL, Mares JA. Women’s health initiative diet intervention did not increase macular pigment optical density in an ancillary study of a subsample of the women’s health initiative. J Nutr. 2009;139(9):1692–9.
Bone RA, Landrum JT. Dose-dependent response of serum lutein and macular pigment optical density to supplementation with lutein esters. Arch Biochem Biophys. 2010;504(1):50–5.
Zeimer M, Hense HW, Heimes B, Austermann U, Fobker M, Pauleikhoff D. The macular pigment: short- and intermediate-term changes of macular pigment optical density following supplementation with lutein and zeaxanthin and co-antioxidants. The LUNA Study. Ophthalmologe. 2009;106(1):29–36.
Johnson EJ, Chung H-Y, Caldarella SM, Snodderly DM. The influence of supplemental lutein and docosahexaenoic acid on serum, lipoproteins, and macular pigmentation. Am J Clin Nutr. 2008;87(5):1521–9.
Wenzel AJ, Sheehan JP, Gerweck C, Stringham JM, Fuld K, Curran-Celentano J. Macular pigment optical density at four retinal loci during 120 days of lutein supplementation. Ophthalmic Physiol Opt. 2007;27(4):329–35.
Richer S, Stiles W, Statkute L, et al. Double masked, placebo-controlled, randomized trial of lutein and antioxidant supplementation in the intervention of atrophic age-related macular degeneration: the Veteran’s LAST study (Lutein Antioxidant Supplementation Trial). Optometry. 2004;75:216–30.
Schalch W, Cohn W, Barker FM, et al. Xanthophyll accumulation in the human retina during supplementation with lutein or zeaxanthin—the LUXEA (LUtein Xanthophyll Eye Accumulation) study. Arch Biochem Biophys. 2007;458:128–35.
Koh H-H, Murray IJ, Nolan D, Carden D, Feather J, Beatty S. Plasma and macular responses to lutein supplement in subjects with and without age-related maculopathy: a pilot study. Exp Eye Res. 2004;79(1):21–7.
Bone RA, Landrum JT, Guerra LH, Ruiz CA. Lutein and zeaxanthin dietary supplements raise macular pigment density and serum concentrations of these carotenoids in humans. J Nutr. 2003;133:992–8.
Bone RA, Landrum JT, Cao Y, Howard AN, Alvarez-Calderon F. Macular pigment response to a supplement containing meso-zeaxanthin, lutein and zeaxanthin. Nutr Metab (Lond). 2007;4:12.
Connolly EE, Beatty S, Thurnham DI, et al. Augmentation of macular pigment following supplementation with all three macular carotenoids: an exploratory study. Curr Eye Res. 2010;35(4):335–51.
Richer S, Devenport J, Lang JC. LAST II: differential temporal responses of macular pigment optical density in patients with atrophic age-related macular degeneration to dietary supplementation with xanthophylls. Optometry. 2007;78(5):213–9.
Parisi V, Tedeschi M, Gallinaro G, Varano M, Saviano S, Piermarocchi S. Carotenoids and antioxidants in age-related maculopathy Italian study: multifocal electroretinogram modifications after 1 year. Ophthalmology. 2008;115(2):324–33. e322.
Bartlett HE, Eperjesi F. Effect of lutein and antioxidant dietary supplementation on contrast sensitivity in age-related macular disease: a randomized controlled trial. Eur J Clin Nutr. 2007;61(9):1121–7.
Chung H-Y, Rasmussen HM, Johnson EJ. Lutein bioavailability is higher from lutein-enriched eggs than from supplements and spinach in men. J Nutr. 2004;134(8):1887–93.
Trieschmann M, Beatty S, Nolan JM, et al. Changes in macular pigment optical density and serum concentrations of its constituent carotenoids following supplemental lutein and zeaxanthin: The LUNA study. Exp Eye Res. 2007;84(4):718–28.
Age-Related Eye Disease Study Research Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol. 2001;119(10):1417–36 [Erratum appears in Arch Ophthalmol. 2008;126(9):1251].
Age-related Eye Disease Study. AREDS 2. www.areds2.org. Accessed 10 June 2011.
Cugati S, Mitchell P, Rochtchina E, Tan AG, Smith W, Wang JJ. Cataract surgery and the 10-year incidence of age-related maculopathy: the Blue Mountains Eye study. Ophthalmology. 2006;113(11):2020–5.
Wang JJ, Klein R, Smith W, Klein BEK, Tomany S, Mitchell P. Cataract surgery and the 5-year incidence of late-stage age-related maculopathy: pooled findings from the Beaver Dam and Blue Mountains Eye Studies. Ophthalmology. 2003;110(10):1960–7.
Brockmann C, Schulz M, Laube T. Transmittance characteristics of ultraviolet and blue-light-filtering intraocular lenses. J Cataract Refract Surg. 2008;34(7):1161–6.
Gaillard ER, Zheng L, Merriam JC, Dillon J. Age-related changes in the absorption characteristics of the primate lens. Invest Ophthalmol Vis Sci. 2000;41(6):1454–9.
Nolan JM, O’Reilly P, Loughman J, et al. Augmentation of macular pigment following implantation of blue light-filtering intraocular lenses at the time of cataract surgery. Invest Ophthalmol Vis Sci. 2009;50(10):4777–85.
World Health Organization. Use of intraocular lenses in cataract surgery in developing countries. Bull World Health Organ. 1991;69:657–66.
Thylefors B, Negrel AD, Pararajasegaram R, Dadzie KY. Global data on blindness. Bull World Health Organ. 1995;69:115–21.
Javitt JC. Who does cataract surgery in the United States? Arch Ophthalmol. 1993;111:1329.
Steinberg EP, Javitt JC, Sharkey PD, et al. The content and cost of cataract surgery. Arch Ophthalmol. 1993;111:1041–9.
Asbell PA, Dualan I, Mindel J, Brocks D, Ahmad M, Epstein S. Age-related cataract. Lancet. 2005;365(9459):599–609.
Bunce GE, Kinoshita J, Horwitz J. Nutritional factors in cataract. Annu Rev Nutr. 1990;10(1):233–54.
Hankinson SE, Stampfer MJ, Seddon JM, et al. Nutrient intake and cataract extraction in women: a prospective study. BMJ. 1992;305:244–51.
Brown L, Rimm EB, Seddon JM, et al. A prospective study of carotenoid intake and risk of cataract extraction in US men. Am J Clin Nutr. 1999;70:517–24.
Tavani A, Negri E, LaVeccia C. Food and nutrient intake and risk of cataract. Ann Epidemiol. 1996;6:41–6.
Lyle B, Mares-Perlman JA, Klein BEK, Klein R, Gregor JL. Antioxidant intake and risk of incident of age-related nuclear cataracts in the Beaver Dam Eye Study. Am J Epidemiol. 1999;149:801–9.
Christen WG, Liu S, Glynn RJ, Gaziano JM, Buring JE. Dietary carotenoids, vitamins C and E, and risk of cataract in women: a prospective study. Arch Ophthalmol. 2008;126(1):102–9.
Vu HTV, Robman L, Hodge A, McCarty CA, Taylor HR. Lutein and zeaxanthin and the risk of cataract: the Melbourne visual impairment project. Invest Ophthalmol Vis Sci. 2006;47(9):3783–6.
Moeller SM, Voland R, Tinker L, et al. Associations between age-related nuclear cataract and lutein and zeaxanthin in the diet and serum in the carotenoids in the age-related eye disease study (CAREDS), an ancillary study of the women’s health initiative. Arch Ophthalmol. 2008;126(3):354–64.
Rodriguez RE, Ortega RM, Lopez-Sobaler AM, Aparicio A, Bermejo LM, Marin-Arias LI. The relationship between antioxidant nutrient intake and cataracts in older people. Int J Vitam Nutr Res. 2006;76(6):8.
Taylor A, Jacques PF, Chylack Jr LT, et al. Long-term intake of vitamins and carotenoids and odds of early age-related cortical and posterior subcapsular lens opacities. Am J Clin Nutr. 2002;75(3):540–9.
Gale CR, Hall NF, Phillips DIK, Martyn CN. Plasma antioxidant vitamins and carotenoids and age-related cataract. Ophthalmology. 2001;108:1992–8.
Vitale S, West S, Hallfrisch J, et al. Plasma antioxidants and risk of cortical and nuclear cataract. Epidemiology. 1993;4:195–203.
Chasen-Taber L, Willett WC, Seddon JM, et al. A prospective study of carotenoid and vitamin A intakes and risk of cataract extraction in US women. Am J Clin Nutr. 1999;70:517–24.
Mares-Perlman JA, Brady WE, Klein BE, et al. Serum carotenoids and tocopherols and severity of nuclear and cortical opacities. Invest Ophthalmol Vis Sci. 1995;36:276–88.
Moeller SM, Jacques PF, Blumberg JB. The potential role of dietary xanthophylls in cataract and age-related macular degeneration. J Am Coll Nutr. 2000;19(5 Suppl):522S–7.
USDA, ARS. National Nutrient Database for Standard Reference, Release 19. Nutrient Data Laboratory Home Page. 2006.
Olmedilla B, Granado F, Blanco I, Vaquero M. Lutein, but not alpha-tocopherol, supplementation improves visual function in patients with age-related cataracts: a 2-y double-blind, placebo-controlled pilot study. Nutrition. 2003;19(1):21–4.
Olmedilla B, Granado F, Blanco I, Vaquero M, Cajigal C. Lutein in patients with cataracts and age-related macular degeneration: a long-term supplementation study. J Sci Food Agric. 2001;81:904–9.
Age-Related Eye Disease Study Research Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E and beta carotene for age-related cataract and vision loss: AREDS report no. 9. Arch Ophthalmol. 2001;119(10):1439–52 [Erratum appears in Arch Ophthalmol. 2008 Sep;126(9):1251].
Gritz DC, Srinivasan M, Smith SD, et al. The antioxidants in prevention of cataracts study: effects of antioxidant supplements on cataract progression in south India. Br J Ophthalmol. 2006;90(7):847–51.
Chylack LTJ, Brown NB, Bron A, et al. The Roche European American Cataract Trial (REACT): a randomized clinical trial to investigate the efficiency of a antioxidant micronutrient mixture to slow progression of age-related cataract. Ophthalmic Epidemiol. 2002;9:49–80.
Teikari JM, Virtamo J, Rautalahti M, Palmgren J, Liesto K, Heinonen OP. Long-term supplementation with alpha-tocopherol and beta-carotene and age-related cataract. Acta Ophthalmol Scand. 1997;75(6):634–40.
Sperduto RD, Hu TS, Milton RC, et al. The Linxian cataract studies. Two nutrition intervention trials. Arch Ophthalmol. 1993;111(9):1246–53.
Clinical Trial of Nutritional Supplements and Age-Related Cataract Study Group, Maraini G, Sperduto RD, et al. A randomized, double-masked, placebo-controlled clinical trial of multivitamin supplementation for age-related lens opacities. Clinical trial of nutritional supplements and age-related cataract report no. 3. Ophthalmology. 2008;115(4):599–607. e591.
Delyfer M-N, Léveillard T, Mohand-Saïd S, Hicks D, Picaud S, Sahel J-A. Inherited retinal degenerations: therapeutic prospects. Biol Cell. 2004;96(4):261–9.
Berson EL. Nutrition and retinal degenerations. Int Ophthalmol Clin. 2000;40(4):93–111.
Hartong DT, Berson EL, Dryja TP. Retinitis pigmentosa. Lancet. 2006;368:1795–809.
Liu X, Bulgakov OV, Darrow KN, et al. Usherin is required for maintenance of retinal photoreceptors and normal development of cochlear hair cells. Proc Natl Acad Sci U S A. 2007;104(11):4413–8.
Dryja TP, Hahn LB, Reboul T, Arnaud B. Missense mutation in the gene encoding the alpha subunit of rod transducin in the Nougaret form of congenital stationary night blindness. Nat Genet. 1996;13(3):358–60.
Perrault I, Rozet JM, Calvas P, et al. Retinal-specific guanylate cyclase gene mutations in Leber’s congenital amaurosis. Nat Genet. 1996;14(4):461–4.
McLaughlin ME, Sandberg MA, Berson EL, Dryja TP. Recessive mutations in the gene encoding the beta-subunit of rod phosphodiesterase in patients with retinitis pigmentosa. Nat Genet. 1993;4(2):130–4.
Fuchs S, Nakazawa M, Maw M, Tamai M, Oguchi Y, Gal A. A homozygous 1-base pair deletion in the arrestin gene is a frequent cause of Oguchi disease in Japanese. Nat Genet. 1995;10(3):360–2.
Dryja TP, Hahn LB, Kajiwara K, Berson EL. Dominant and digenic mutations in the peripherin/RDS and ROM1 genes in retinitis pigmentosa. Invest Ophthalmol Vis Sci. 1997;38(10):1972–82.
Allikmets R. Simple and complex ABCR: Genetic predisposition to retinal disease. Am J Hum Genet. 2000;67(4):793–9.
Maw MA, Kennedy B, Knight A, et al. Mutation of the gene encoding cellular retinaldehyde-binding protein in autosomal recessive retinitis pigmentosa. Nat Genet. 1997;17(2):198–200.
Marlhens F, Bareil C, Griffoin JM, et al. Mutations in RPE65 cause Leber’s congenital amaurosis. Nat Genet. 1997;17(2):139–41.
D’Cruz PM, Yasumura D, Weir J, et al. Mutation of the receptor tyrosine kinase gene Mertk in the retinal dystrophic RCS rat. Hum Mol Genet. 2000;9(4):645–51.
Dagnelie G, Zorge IS, McDonald TM. Lutein improves visual function in some patients with retinal regeneration: a pilot study via the internet. Optom Vis Sci. 2000;71:147–64.
Bahrami H, Melia M, Dagnelie G. Lutein supplementation in retinitis pigmentosa: PC-based vision assessment in a randomized double-masked placebo-controlled clinical trial [NCT00029289]. BMC Ophthalmol. 2006;6(1):23.
Berson EL, Rosner B, Sandberg MA, et al. Clinical trial of lutein in patients with retinitis pigmentosa receiving vitamin A. Arch Ophthalmol. 2010;128(4):403–11.
Aleman TS, Duncan JL, Bieber ML, et al. Macular pigment and lutein supplementation in retinitis pigmentosa and usher syndrome. Invest Ophthalmol Vis Sci. 2001;42(8):1873–81.
Sandberg MA, Johnson EJ, Berson EL. The relationship of macular pigment optical density to serum lutein in retinitis pigmentosa. Invest Ophthalmol Vis Sci. 2010;51(2):1086–91.
Berson EL, Rosner B, Sandberg MA, et al. A randomized trial of vitamin A and vitamin E supplementation for retinitis pigmentosa. Arch Ophthalmol. 1993;111(6):761–72.
Berson EL, Rosner B, Sandberg MA, et al. Vitamin A supplementation for retinitis pigmentosa. Arch Ophthalmol. 1993;111(11):1456–9.
Bishara S, Merin S, Cooper M, Azizi E, Delpre G, Deckelbaum RJ. Combined vitamin A and E therapy prevents retinal electrophysiological deterioration in abetalipoproteinaemia. Br J Ophthalmol. 1982;66(12):767–70.
Yokota T, Shiojiri T, Gotoda T, Arai H. Retinitis pigmentosa and ataxia caused by a mutation in the gene for the α-tocopherol-transfer protein. N Engl J Med. 1996;335(23):1770–1.
Berson EL, Rosner B, Sandberg MA, et al. Clinical trial of docosahexaenoic acid in patients with retinitis pigmentosa receiving vitamin A treatment. Arch Ophthalmol. 2004;122(9):1297–305.
Berson EL, Rosner B, Sandberg MA, et al. Further evaluation of docosahexaenoic acid in patients with retinitis pigmentosa receiving vitamin A treatment: subgroup analyses. Arch Ophthalmol. 2004;122(9):1306–14.
Wooten BR, Hammond BR. Macular pigment: influences on visual acuity and visibility. Prog Retin Eye Res. 2002;21(2):225–40.
Stringham JM, Hammond BR. The glare hypothesis for macular pigment function. Optom Vis Sci. 2007;84(9):859–64.
Stringham JM, Hammond Jr BR. Dietary lutein and zeaxanthin: possible effects on visual function. Nutr Rev. 2005;63(2):59–64.
Kvansakul J, Rodriguex-Carmona M, Edgar D, Barker FM, Kopcke W, Barbur JL. Supplementation with the carotenoids lutein or zeaxanthin improves human visual performance. Ophthalmic Physiol Opt. 2006;26:362–71.
Stringham JM, Hammond BR. Macular pigment and visual performance under glare conditions. Optom Vis Sci. 2008;85:82–8.
Ma L, Lin X-M, Zou Z-Y, Xu X-R, Li Y, Xu R. A 12-week lutein supplementation improves visual function in Chinese people with long-term computer display light exposure. Br J Nutr. 2009;102(2):186–90.
Yagi A, Fujimoto K, Michihiro K, Goh B, Tsi D, Nagai H. The effect of lutein supplementation on visual fatigue: a psychophysiological analysis. Appl Ergon. 2009;40:1047–54.
Falsini B, Piccardi M, Iarossi G, Fadda A, Merendino E, Valentini P. Influence of short-term antioxidant supplementation on macular function in age-related maculopathy: a pilot study including electrophysiologic assessment. Ophthalmology. 2003;110(1):51–60. discussion 61.
Cangemi FE. TOZAL study: an open case-control study of an oral antioxidant and omega-3 supplement for dry AMD. BMC Ophthalmol. 2007;7:3.
Trumbo PR, Ellwood KC. Lutein and zeaxanthin intakes and risk of age-related macular degeneration and cataracts: an evaluation using the Food and Drug Administration’s evidence-based review system for health claims. Am J Clin Nutr. 2006;84(5):971–4.
Leung IYF. Macular pigment: new clinical methods of detection and the role of carotenoids in age-related macular degeneration. Optometry. 2008;79(5):266–72.
Stahl W, Seis H. Effects of carotenoids and retinoids on gap junctional communication. Biofactors. 2001;15:95–8.
Hammond BR, Wooten BR. CFF thresholds: relation to macular pigment optical density. Ophthalmic Physiol Opt. 2005;25(4):315–9.
Renzi LM, Hammond BR. The relation between the macular carotenoids, lutein and zeaxanthin, and temporal vision. Ophthalmic Physiol Opt. 2010;30(4):351–7.
Zimmer JP, Hammond BR. Possible influences of lutein and zeaxanthin on the developing retina. Clin Ophthalmol. 2007;1(1):11.
Gutherie AH, Hammond BR. Macular pigment and scotopic noise. ARVO Abstracts [abstract]. Invest Ophthalmol Vis Sci. 2005;E1784.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer Science+Business Media New York
About this chapter
Cite this chapter
Vishwanathan, R., Johnson, E.J. (2013). Lutein and Zeaxanthin and Eye Disease. In: Tanumihardjo, S. (eds) Carotenoids and Human Health. Nutrition and Health. Humana Press, Totowa, NJ. https://doi.org/10.1007/978-1-62703-203-2_13
Download citation
DOI: https://doi.org/10.1007/978-1-62703-203-2_13
Published:
Publisher Name: Humana Press, Totowa, NJ
Print ISBN: 978-1-62703-202-5
Online ISBN: 978-1-62703-203-2
eBook Packages: MedicineMedicine (R0)