Abstract
Retinal, a precursor of vitamin A, has been used in foods, cosmetics, pharmaceuticals, nutraceuticals, and animal feed additives. Carotenoid 15,15′-oxygenases, including β-carotene 15,15′-oxygenases from mammalians, chickens, fruit flies, zebrafishes, the uncultured marine bacterium, and the fungus Fusarium fujikuroi, and apo-carotenoid 15,15′-oxygenases from cyanobacteria produce retinal from carotenoids. In this article, the biochemical properties, reaction mechanism, and substrate specificity of carotenoid oxygenases are reviewed, along with a description of the enzymatic biotransformation of carotenoids to retinal. Retinal producing methods using metabolically engineered cells and uncharacterized proteins are suggested.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The retinoids are a class of compounds that includes retinal, retinol, and retinoic acid. Retinal is the aldehyde form of vitamin A, retinol is the alcohol form of vitamin A, and retinoic acid is the oxidized form of vitamin A. Retinal, a precursor of retinol, is a necessary structural component of the light-sensitive pigment, rhodopsin, which is found within the rod and cone cells of the retina (Bates 1995); retinal is known to be crucially important for development, cell differentiation, cancer prevention, and membrane and skin protection (De Luca 1991; Fisher and Voorhees 1996; Semba 1999). Animals cannot synthesize retinoids de novo. Thus, animals generate retinoids from β-carotene obtained from plants or photosynthetic organisms, via the use of retinoid producing enzymes. Retinoids and their derivatives have been utilized in foods, cosmetics, pharmaceuticals, nutraceuticals, and animal feed additives, owing primarily to their anti-infective, anti-cancer, anti-oxidant, and anti-wrinkle functions (De Luca 1991; Fisher and Voorhees 1996; Semba 1999).
Commercial retinal production is currently conducted via chemical synthesis through the acid or base reduction of a pentadiene derivative followed by the acidification/hydrolysis of the isomeric mixture to generate retinol (Mercier and Chabardes 1994). However, this chemical process has some disadvantages, including complex purification steps, the formation of chemical wastes, and the formation of undesired by-products. Thus, the biological manufacture of retinal from carotenoids using carotenoid 15,15′-oxygenase (CO), has been the focus of intensive study in recent years.
Carotenoids, as substrates of CO, are naturally occurring pigments in plants and microorganisms and exist in their hydrocarbon forms as carotenes, including lycopene, α-carotene, and β-carotene, as well as in oxygenated derivative forms as xanthophylls, including lutein, α-cryptoxanthin, zeaxanthin, canthaxanthin, and astaxanthin. Apo-carotenoids consist of apo-carotenals, apo-carotenols, and apo-lycopenal (Scherzinger et al. 2006). Carotenoids and apo-carotenoids are converted to retinal by β-carotene 15,15′-dioxygenase (BCDO), β-carotene 15,15′-monooxygenase (BCMO), and apo-carotenoid 15,15′-oxygenase (ACO), and their chemical structures with the cleavage site are provided in Table 1.
The principal objective of this work is to elucidate the enzymatic production of retinal using CO. The biochemical properties, reaction mechanism, and substrate specificity of retinal producing CO are reviewed. The biotransformation of carotenoids to retinal by COs derived from various sources and the enhanced retinal production resulting from the optimization of detergent micelles of carotenoids under aqueous conditions are introduced in this study. Moreover, the production of retinal by metabolically engineered cells and uncharacterized proteins, which have been proposed as retinal producing BCDOs such as bacterioopsin-related protein (Brp) and bacterioopsin-related protein-like homolog protein (Blh), is also discussed.
Retinal producing enzymes
The retinal producing enzyme, CO, which cleaves carotenoids into retinal, includes β-carotene 15,15′-oxygenase (BCO) and ACO. CO is classified into BCDO, BCMO, and apo-carotenoid 15,15′-dioxygenase by the reaction mechanism.
BCOs catalyze the formation of retinal by the central cleavage of 15,15′ C–C double bond in β-carotene (Nagao and Olson 1994). Vertebrate BCMO, formally known as BCDO, is an important enzyme in the vitamin A synthesis pathway (von Lintig and Wyss 2001). This enzyme was isolated from rats in the 1960s (Olson and Hayaishi 1965), and its activity has been previously noted in the intestinal mucosa (Lakshman et al. 1989), jejunum enterocytes, liver, lung, kidney, and brain (During et al. 1996; van Vliet et al. 1996; Wyss et al. 2001) in vertebrata such as humans (Yan et al. 2001; Lindqvist and Andersson 2002), mice (Paik et al. 2001; Redmond et al. 2001; Boulanger et al. 2003; Poliakov et al. 2005), chickens (Wyss et al. 2000), and rats (Grolier et al. 1997). Other BCOs have also been reported in the fungus Fusarium fujikuroi (Prado-Cabrero et al. 2007), the fruit fly (von Lintig and Vogt 2000), and the zebrafish (Lampert et al. 2003). Recently, BCDO (Blh protein) from the uncultured marine bacterium has been identified and characterized (Kim et al. 2009).
ACOs from cyanobacteria, such as Nostoc sp. PCC7120 (Scherzinger et al. 2006) and Synechocystis sp. PCC6803 (Ruch et al. 2005), cleave 15,15′ C–C double bond in β-apo-8′-carotenal to form retinal.
Biochemical properties of CO
Vertebrata BCMOs and cyanobacteria ACOs exist as tetramers composed of 54–60 kDa subunits (Lindqvist and Andersson 2002; Ruch et al. 2005; Kim and Oh 2009), whereas BCDO from the uncultured marine bacterium exists as a dimer of 32 kDa subunits (Kim et al. 2009). The calculated subunit molecular masses of BCO from F. fujikuroi and the fruit fly are 78 and 70 kDa, respectively (von Lintig and Vogt 2000; Prado-Cabrero et al. 2007).
The optimal pH values of BCOs are 8.0 for the uncultured marine bacterium, humans, chickens, rabbits, and F. fujikuroi, 7.7 for the rat, 8.5 for the guinea pig, and 9.0 for the mouse. The optimal temperature ranges of BCOs from the uncultured marine bacterium and vertebrata are 37–40°C (Goodman et al. 1967; Fidge et al. 1969; Lakshmanan et al. 1972; Singh and Cama 1974; Nagao et al. 1996; Paik et al. 2001; Redmond et al. 2001; Poliakov et al. 2005; Kim et al. 2007, 2009), whereas those from F. fujikuroi and the fruit fly are 27–30°C (von Lintig and Vogt 2000; Prado-Cabrero et al. 2007). The optimal pH and temperature for ACOs are 6.8–7.0°C and 27°C, respectively (Ruch et al. 2005; Scherzinger et al. 2006).
Fe2+ is essential cofactor for CO family and is inhibited by iron-chelating agents (Goodman et al. 1967; Lakshmanan et al. 1972; Kim et al. 2009). According to the crystal structure of Synechocystis sp. ACO, Fe2+ is bound by four histidine residues (His183, His238, His304, and His484) in the active site (Kloer et al. 2005). These histidine residues are absolutely conserved across all of COs including BCMO, BCDO, ACO, and uncharacterized Blh and Brp proteins, based on amino acid sequence alignment. Four histidine residues (H172, H237, H308, and H514 in mouse and human BCMOs and H21, H78, H188, and H192 in BCDO from the uncultured marine bacterium) are determined as the iron binding residues by side-directed mutagenesis, molecular modeling, and ICP-AES analysis (Poliakov et al. 2005; Kim et al. 2009, 2010a).
Human BCMO exhibits above 70% amino acid sequence identity to mouse, chicken, and rat BCMOs. Synechocystis sp. ACO shows 57% and 28% amino acid sequence identity to Nostoc sp. ACO and human BCMO, respectively. These results suggest that BCMO is genetically related to ACO with a low similarity. BCDO from the uncultured marine bacterium exhibits no extensive homology with BCMO and ACO, which is genetically different with other COs. However, the metal-binding residues (four histidine residues) of BMDO, BCMO, and ACO are completely conserved, which were involved in the catalytic center of active pocket. Thus, the homology models of the active site residues in human BCMO and BCDO from the uncultured marine bacterium based on the determined structure of Synechocystis sp. ACO have been used in docking studies (Kim et al. 2009, 2010a).
Reaction mechanism of CO
The reaction mechanism for the conversion of β-carotene to retinal can be divided into mono- and dioxygenases on the basis of oxygen provision (Fig. 1). The oxygen atom of the product from the monooxygenase is provided by molecular oxygen and water via an epoxide intermediate, whereas that from the dioxygenase is provided by molecular oxygen rather than from water via a dioxetane intermediate (Leuenberger et al. 2001; Borowski et al. 2008). The reactions of vertebrata BCOs follow the monooxygenase mechanism (Leuenberger et al. 2001), whereas the reaction of BCO from the uncultured marine bacterium follows the dioxygenase mechanism (Kim et al. 2009). Although BCO from the uncultured marine bacterium and vertebrata BCMOs catalyze the same biochemical reaction of β-carotene to retinal, the two enzymes are unrelated; this conclusion is supported by observed differences in the properties of these two enzymes, including the DNA and amino acid sequences, molecular masses, forms of association, reaction mechanisms, and substrate specificities. The reactions of ACOs follow the dioxygenase mechanism exploited in the cleavage of apo-carotenoids (Kloer et al. 2005; Borowski et al. 2008).
Two different mechanisms for β-carotene cleavage. a Dioxygenase mechanism. The oxygen atom of the product is provided by molecular oxygen rather than water via a dioxetane intermediate. The reactions of ACOs and BCO from the uncultured marine bacterium follow the dioxygenase mechanism. b Monooxygenase mechanism. The oxygen atom of the product is provided by molecular oxygen and water via an epoxide intermediate. The reaction of vertebrate BCO follows the monooxygenase mechanism
Synechocystis sp. ACO contains Fe2+–four His arrangement at the axis of a seven-bladed beta-propeller chain fold covered by a dome formed by six large loops (Fig. 2). Fe2+ is accessible through a long nonpolar tunnel that holds the substrate carotenoid (Kloer et al. 2005). In the reaction mechanism of the dioxygenase, the active site exhibits coordination of Fe2+ with four conserved histidine residues. According to the cleaving mechanism of apo-carotenoid oxygenase (Borowski et al. 2008), the O2 molecule in the active site of the dioxygenase binds to the coordination shell of Fe2+ in a side-on fashion. The side-on complex of Fe–O2 then attacks and cleaves between the 15,15′ C–C atoms when bound to the dioxygenase. The three-dimensional structure of BCMO has not been reported yet, but four histidine residues in iron coordination are absolutely conserved and its active sites are similar to ACO-based sequence alignment and homology modeling. Thus, the reaction mechanism of the monooxygenase seems to be similar except the oxygen source for intermediate.
Substrate specificity of CO
The specificity for carotenoids as substrates of CO, which produce retinal, is summarized in Table 1. Vertebrate BCDO and BCMO from the uncultured marine bacterium convert the β-carotene substrate into two molecules of retinal and convert some carotenoids and apo-carotenoids such as α-carotene, γ-carotene, β-cryptoxanthin, and β-apo-4′-carotenal into one molecule of retinal (Lakshmanan et al. 1968; Singh and Cama 1974; Kim et al. 2009; Kim and Oh 2009). However, the enzymes show no activity for β-apo-12′-carotenal, lutein, zeaxanthin, and lycopene. Vertebrate BCMO exhibits activity for β-apo-8′-carotenal and β-apo-10′-carotenal (a C27 compound), whereas BCDO from the uncultured marine bacterium exhibits no such activity. The difference in the substrate specificity between BCMO and BCDO does not be explained because the structures of these enzymes have not been determined.
β-Carotene, α-carotene, γ-carotene, β-cryptoxanthin, and β-apo-carotenals harbor one or two β-ionone rings. Zeaxanthin contains hydroxyl ε-ionone and hydroxyl β-ionone rings, lutein contains two hydroxyl β-ionone rings, and lycopene contains no β-ionone ring. The presence of one unsubstituted β-ionone ring in the substrate with a molecular mass of greater than C27 for BCMO or C35 for BCDO is critically important for enzyme activity. The substrate specificity of carotenoid oxygenase (CarX) from F. fujikuroi is similar to vertebrate BCMO (Prado-Cabrero et al. 2007). The enzyme exhibits activity for β-carotene, β-apo-8′-carotenal, and γ-carotene, but no activity for lycopene, thereby demonstrating that the presence of one β-ionone ring in the substrate is also required for enzyme activity.
Apo-carotenoids are formed from carotenoids by carotenoid cleavage dioxygenases from Arabidopsis thaliana and Nostoc sp. (Marasco et al. 2006; Schmidt et al. 2006). ACO from Nostoc sp. converts apo-carotenoids, such as β-apo-8′-carotenal, β-apo-10′-carotenal, and β-apo-8′-carotenol, into one retinal molecule (Scherzinger et al. 2006). ACO from Synechocystis sp. shows cleavage activity for β-apo-4′-carotenal, β-apo-8′-carotenal, β-apo-10′-carotenal, β-apo-12′-carotenal, β-apo-8′-carotenol, and β-apo-12′-carotenol (Kloer et al. 2005; Ruch et al. 2005). However, both ACOs cannot cleave β-carotene. The substrate specificity of ACO from Synechocystis sp. is broader than that from Nostoc sp. It was previously reported that the undetermined structure of Nostoc sp. ACO did not allow explaining the two different cleavage patterns (Scherzinger and Al-Babili 2008).
The kinetic parameters of carotenoid 15,15′-oxygenase for carotenoids as substrates including α-carotene, β-carotene, γ-carotene, β-cryptoxanthin, β-apo-4′-carotenal, β-apo-8′-carotenal, β-apo-10′-carotenal, β-apo-12′-carotenal, and β-apo-8′-carotenol are presented in Table 2. The affinity and maximum velocity of BCO for β-carotene are the highest, thereby indicating that β-carotene is an authentic substrate for the enzyme. ACO exhibits the highest affinity and maximum velocity for β-apo-8′-carotenal among the tested β-apo-carotenals and shows a higher maximum velocity for β-apo-8′-carotenol than for β-apo-8′-carotenal.
Retinal production by CO
The reaction catalyzed by BCO requires water (Leuenberger et al. 2001; Woggon 2002); however, in vitro, β-carotene is largely insoluble because of its hydrophobic nature (During and Harrison 2004). Thus, low levels of retinal are produced from β-carotene by BCO under aqueous conditions. To dissolve β-carotene in an aqueous reaction solution, it must be formed into detergent micelles using an organic solvent. The enzymatic production of retinal from β-carotene by BCO has been previously evaluated in detergent micelles under aqueous conditions, and the increased production of retinal has been achieved by selecting solvent and by optimizing the detergent, substrate, and enzyme concentrations (El-Gorab 1973; Devery and Milborrow 1994; During et al. 1996; Lindqvist and Andersson 2002; Kim et al. 2007, 2008, 2010b).
The solvents employed in the detergent micelles were acetone (Devery and Milborrow 1994; During et al. 1996; Paik et al. 2001), hexane (Lindqvist and Andersson 2002), chloroform (Kim et al. 2007), and toluene (Kim et al. 2008, 2010b). The detergents used in the detergent micelles were Tween 20 (Kim et al. 2010b), Tween 80 (Paik et al. 2001; Kim et al. 2008), and 1-S-octyl-β-d-thioglucopyranoside (Lindqvist and Andersson 2002).
Among the tested solvents, toluene was identified as the optimum solvent for retinal production by BCO (Kim et al. 2008, 2010b). The optimum detergent for retinal production is Tween 80 for human BCMO and Tween 20 for BCDO from the uncultured marine bacterium. The optimum concentrations of detergent, enzyme, and substrate by human BCMO were 2.4%, 0.2 U ml−1, and 200 mg l−1, respectively. Under optimal conditions, human BCMO produces 98 mg l−1 retinal with a conversion yield of 49% (w/w) (Kim et al. 2008; Table 3). The optimal concentrations of detergent, enzyme, and substrate for retinal production by BCDO from the uncultured marine bacterium are 2.4%, 0.15 U ml−1 enzyme, and 350 mg l−1 β-carotene. Under optimal conditions, the enzyme produces 181 mg l−1, which is the highest reported concentration, with a conversion yield of 52% (w/w) (Kim et al. 2010b). The highest reported conversion yield, 60% (w/w), was noted with the chicken BCMO (Kim and Oh 2009). Although ACO converts apo-carotenoids into retinal, the detailed quantitative data for the enzymatic production of retinal have not been reported.
The low solubility of the hydrophobic substrate β-carotene is a limitation to high-level production of retinal under aqueous conditions. To overcome the limitation, the enzyme reaction systems, such as two-phase and co-solvent systems, should be developed, the immobilization methods of BCO should be investigated for continuous retinal production, and the structure analysis and mutation studies should be performed to increase the specific activity of BCO.
Further research
Microbial production of retinal
The production of carotenoids has previously been conducted using metabolically engineered Escherichia coli, which was prepared from the introduction of the foreign carotenoid synthesizing genes into non-carotenogenic E. coli (Misawa et al. 1990). E. coli was identified as an appropriate host for the production of carotenoids such as lycopene, β-carotene, canthaxanthin, zeaxanthin, and astaxanthin because it has a powerful genetic tool system for metabolic engineering (Cunningham et al. 1993; Ruther et al. 1997; Ye et al. 2006). However, the production of retinal using metabolically engineered cells has never been reported. Retinal producing metabolically engineered E. coli can be made by introducing the foreign CO gene into carotenogenic E. coli. Thus, we introduced the BCDO gene from the uncultured marine bacterium into β-carotene-producing E. coli (Yoon et al. 2007b). The resultant E. coli produced 137 mg l−1 of retinal on 2YT medium containing glycerol in a flask. Alternative retinal production may be a possible use of metabolically engineered E. coli.
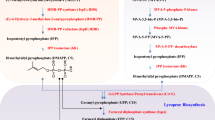
The proposed biosynthesis pathway of retinoids from glucose or glycerol is shown in Fig. 3. The 2-C-methyl-d-erythritol 4-phosphate and mevalonate pathways generate isopentenyl diphosphate (IPP) and its isomer of dimethylallyl diphosphate (DMAPP), the building blocks for carotenoid synthesis (Rohmer et al. 1993; Maury et al. 2005). Farnesyl diphosphate (FPP) synthase (IspA) synthesizes geranyl diphosphate (C10) and FPP (C15) and geranylgeranyl diphosphate (GGPP) synthase (CrtE) synthesize GGPP (C20) via the consecutive condensation reaction of IPP to DMAPP. Phytoene synthase (CrtB) catalyzes two molecules of GGPP to produce phytoene, which is subsequently converted into lycopene by phytoene desaturase (CrtI; Yoon et al. 2007a). A lycopene β-cyclase (CrtY) catalyzes the cyclization of lycopene ends to generate β-carotene (Yoon et al. 2007b), which is converted into two molecules of retinal by BCO. Retinal is converted to retinol and retinoic acid by retinol dehydrogenase (Liden and Eriksson 2006) and retinal dehydrogenase (Lin et al. 2003), respectively.
Proposed biosynthesis pathway of retinoids in the metabolically engineered E. coli. dxs 1-deoxy-d-xylulose-5-phosphate synthase, dxr 1-deoxy-d-xylulose-5-phosphate reductoisomerase, cms 4-diphosphocytidyl-2C-methyl-d-erythritol synthase, cmk 4-diphosphocytidyl-2-C-methyl-d-erythritol kinase, mecps 2C-methyl-d-erythriol-2,4-cyclodiphosphate synthase, atoB acetyl-CoA acetyltransferase, mvaA HMG-CoA reductase, mvaS HMG-CoA synthase, mvak1 mevalonate kinase, mvak2 phosphomevalonate kinase, mvaD diphosphomevalonate decarboxylase, idi IPP isomerase, ispA famesyl diphosphate synthase, crtE geranylgeranyl diphosphate synthase, crtB phytoene synthase, crtI phytoene desaturase, crtY lycopene β-cyclases, BCO β-carotene oxygenase, G3P glyceraldehydes-3-phosphate, HMG-CoA 3-hydroxy-3-methylglutaryl-CoA, IPP isopentenyl diphosphate, DMAPP dimethylallyl diphosphate, FPP farnesyl diphosphate, GGPP geranylgeranyl diphosphate
Construction of CO library is important for enhanced retinal production by metabolic engineered cells. An effective retinal producing enzyme can be selected from CO library. The gene encoding the effective retinal producing enzyme can be introduced into carotenogenic E. coli. The culture conditions such as media, temperature, pH, and culture time in a fed-batch culture of the metabolically engineered E. coli should be optimized for enhanced retinal production. Moreover, carotenoids and retinoids decomposed over time in the reaction solution because they are unstable compounds (Osuna-Garcia et al. 1997; Han et al. 2003), which are sensitive to oxygen and light. The supplementation of additives, such as fatty acids and detergents, in culture medium should be performed for preventing the degradation of carotenoids and retinoids.
Sources of bacterial retinal producing enzymes
We suggest the uncharacterized microbial Brp and Blh proteins, which have been proposed as good sources for catalyzing the conversion of β-carotene to retinal (Peck et al. 2001; McCarren and DeLong 2007). The sources of brp genes have been previously reported in Haloarcula marismortui (Baliga et al. 2004), Halobacterium sp. NRC-1 (Ng et al. 2000), Halobacterium halobium (Shand and Betlach 1994), Haloquadratum walsbyi, and Salinibacter ruber (Mongodin et al. 2005), and the sources of blh genes have been previously reported as Halobacterium sp. NRC-1 (Ng et al. 2000), H. marismortui (Baliga et al. 2004), Halobacterium salinarum (Pfeiffer et al. 2008), uncultured marine bacterium 66A03 (Sabehi et al. 2005), and the uncultured marine bacterium HF10 19E08 (Martinez et al. 2007). However, the Brp and Blh proteins have not been characterized except only one Blh protein from the uncultured marine bacterium 66A03 (Kim et al. 2009). The cloning of brp and blh genes and characterization of their expressed BCO such as the Brp and Blh proteins should be conducted in order to produce retinal from β-carotene.
Application of retinal producing enzymes
Golden rice (provitamin A rice) is produced via genetic engineering to biosynthesize β-carotene, a retinal precursor, in the rice plant (Ye et al. 2000). Golden rice 2 was developed via the introduction of a phytoene synthase from maize, which has been previously derived from different sources and was the limiting protein in β-carotene accumulation. The β-carotene content of golden rice 2 (37 μg/g) was 23-fold that of the original golden rice (Paine et al. 2005). If the CO gene should be introduced in crops such as rice, the vitamin A crops will be obtained. Vitamin A crops are expected to help prevent vitamin A deficiency and to promote human health.
In conclusion, we described the enzymatic biotransformation of carotenoids to retinal, which has been used in foods, cosmetics, pharmaceuticals, nutraceuticals, and animal feed additives. Retinal producing methods using metabolically engineered cells and uncharacterized proteins are suggested. However, these biological methods are still in early stage. In the future, CO will be able to produce industrially retinal because the biotransformation has some advantage over the chemical process, such as mild reaction conditions and retinal production without by-products.
References
Baliga NS, Bonneau R, Facciotti MT, Pan M, Glusman G, Deutsch EW, Shannon P, Chiu Y, Weng RS, Gan RR, Hung P, Date SV, Marcotte E, Hood L, Ng WV (2004) Genome sequence of Haloarcula marismortui: a halophilic archaeon from the Dead Sea. Genome Res 14:2221–2234
Bates CJ (1995) Vitamin A. Lancet 345:31–35
Borowski T, Blomberg MR, Siegbahn PE (2008) Reaction mechanism of apocarotenoid oxygenase (ACO): a DFT study. Chemistry 14:2264–2276
Boulanger A, McLemore P, Copeland N, Gilbert D, Jenkins N, Yu S, Gentleman S, Redmond T (2003) Identification of beta-carotene 15, 15′-monooxygenase as a peroxisome proliferator-activated receptor target gene. FASEB J 17:1304–1306
Cunningham FX Jr, Chamovitz D, Misawa N, Gantt E, Hirschberg J (1993) Cloning and functional expression in Escherichia coli of a cyanobacterial gene for lycopene cyclase, the enzyme that catalyzes the biosynthesis of beta-carotene. FEBS Lett 328:130–138
De Luca LM (1991) Retinoids and their receptors in differentiation, embryogenesis, and neoplasia. FASEB J 5:2924–2933
Devery J, Milborrow BV (1994) beta-Carotene-15, 15′-dioxygenase (EC 1.13.11.21) isolation reaction mechanism and an improved assay procedure. Br J Nutr 72:397–414
During A, Harrison EH (2004) Intestinal absorption and metabolism of carotenoids: insights from cell culture. Arch Biochem Biophys 430:77–88
During A, Nagao A, Hoshino C, Terao J (1996) Assay of beta-carotene 15, 15′-dioxygenase activity by reverse-phase high-pressure liquid chromatography. Anal Biochem 241:199–205
El-Gorab M (1973) Solubilization of beta-carotene and retinol into aqueous solutions of mixed micelles. Biochim Biophys Acta 306:58–66
Ershov Iu V, Dmitrovskii AA, Bykhovskii V (1993) Properties of beta-carotene-15, 15′-dioxygenase, stabilized during purification with lutein and dithiothreitol. Biokhimiia 58:416–423
Fidge N, Smith F, Goodman D (1969) Vitamin A and carotenoids. The enzymic conversion of beta-carotene into retinal in hog intestinal mucosa. Biochem J 114:689–694
Fisher GJ, Voorhees JJ (1996) Molecular mechanisms of retinoid actions in skin. FASEB J 10:1002–1013
Goodman D, Huang H, Kanai M, Shiratori T (1967) The enzymatic conversion of all-trans beta-carotene into retinal. J Biol Chem 242:3543–3554
Grolier P, Duszka C, Borel P, Alexandre-Gouabau M, Azais-Braesco V (1997) In vitro and in vivo inhibition of beta-carotene dioxygenase activity by canthaxanthin in rat intestine. Arch Biochem Biophys 348:233–238
Han HS, Kwon YJ, Park MS, Park SH, Cho SM, Rho YS, Kim JW, Sin HS, Um SJ (2003) Efficacy validation of synthesized retinol derivatives in vitro: stability, toxicity, and activity. Bioorg Med Chem 11:3839–3845
Kim YS, Oh DK (2009) Substrate specificity of a recombinant chicken beta-carotene 15, 15′-monooxygenase that converts beta-carotene into retinal. Biotechnol Lett 31:403–408
Kim YS, Kim NH, Kim HJ, Lee JK, Kim SW, Oh DK (2007) Effective production of retinal from beta-carotene using recombinant mouse beta-carotene 15, 15′-monooxygenase. Appl Microbiol Biotechnol 76:1339–1345
Kim NH, Kim YS, Kim HJ, Oh DK (2008) Optimized formation of detergent micelles of beta-carotene and retinal production using recombinant human beta, beta-carotene 15, 15′-monooxygenase. Biotechnol Prog 24:227–231
Kim YS, Kim NH, Yeom SJ, Kim SW, Oh DK (2009) In vitro characterization of a recombinant Blh protein from an uncultured marine bacterium as a beta-carotene 15, 15′-dioxygenase. J Biol Chem 284:15781–15793
Kim YS, Park CS, Oh DK (2010a) Hydrophobicity of residue 108 specifically affects the affinity of human beta-carotene 15, 15′-monooxygenase for substrates with two ionone rings. Biotechnol Lett 32:847–853
Kim YS, Park CS, Oh DK (2010b) Retinal production from beta-carotene by beta-carotene 15, 15′-dioxygenase from an unculturable marine bacterium. Biotechnol Lett 32:957–961
Kloer DP, Ruch S, Al-Babili S, Beyer P, Schulz GE (2005) The structure of a retinal-forming carotenoid oxygenase. Science 308:267–269
Lakshman MR, Mychkovsky I, Attlesey M (1989) Enzymatic conversion of all-trans-beta-carotene to retinal by a cytosolic enzyme from rabbit and rat intestinal mucosa. Proc Natl Acad Sci U S A 86:9124–9128
Lakshmanan MR, Pope JL, Olson JA (1968) The specificity of a partially purified carotenoid cleavage enzyme of rabbit intestine. Biochem Biophys Res Commun 33:347–352
Lakshmanan M, Chansang H, Olson J (1972) Purification and properties of carotene 15, 15′-dioxygenase of rabbit intestine. J Lipid Res 13:477–482
Lampert JM, Holzschuh J, Hessel S, Driever W, Vogt K, von Lintig J (2003) Provitamin A conversion to retinal via the beta, beta-carotene-15, 15′-oxygenase (bcox) is essential for pattern formation and differentiation during zebrafish embryogenesis. Development 130:2173–2186
Leuenberger MG, Engeloch-Jarret C, Woggon WD (2001) The reaction mechanism of the enzyme-catalyzed central cleavage of beta-carotene to retinal. Angew Chem Int Ed Engl 40:2613–2617
Liden M, Eriksson U (2006) Understanding retinol metabolism: structure and function of retinol dehydrogenases. J Biol Chem 281:13001–13004
Lin M, Zhang M, Abraham M, Smith SM, Napoli JL (2003) Mouse retinal dehydrogenase 4 (RALDH4), molecular cloning, cellular expression, and activity in 9-cis-retinoic acid biosynthesis in intact cells. J Biol Chem 278:9856–9861
Lindqvist A, Andersson S (2002) Biochemical properties of purified recombinant human beta-carotene 15, 15′-monooxygenase. J Biol Chem 277:23942–23948
Marasco EK, Vay K, Schmidt-Dannert C (2006) Identification of carotenoid cleavage dioxygenases from Nostoc sp. PCC 7120 with different cleavage activities. J Biol Chem 281:31583–31593
Martinez A, Bradley AS, Waldbauer JR, Summons RE, DeLong EF (2007) Proteorhodopsin photosystem gene expression enables photophosphorylation in a heterologous host. Proc Natl Acad Sci U S A 104:5590–5595
Maury J, Asadollahi MA, Moller K, Clark A, Nielsen J (2005) Microbial isoprenoid production: an example of green chemistry through metabolic engineering. Adv Biochem Eng Biotechnol 100:19–51
McCarren J, DeLong EF (2007) Proteorhodopsin photosystem gene clusters exhibit co-evolutionary trends and shared ancestry among diverse marine microbial phyla. Environ Microbiol 9:846–858
Mercier C, Chabardes P (1994) Organometallic chemistry in industrial vitamin A and vitamin E synthesis. Pure Appl Chem 66:1509–1518
Misawa N, Nakagawa M, Kobayashi K, Yamano S, Izawa Y, Nakamura K, Harashima K (1990) Elucidation of the Erwinia uredovora carotenoid biosynthetic pathway by functional analysis of gene products expressed in Escherichia coli. J Bacteriol 172:6704–6712
Mongodin EF, Nelson KE, Daugherty S, Deboy RT, Wister J, Khouri H, Weidman J, Walsh DA, Papke RT, Sanchez Perez G, Sharma AK, Nesbo CL, MacLeod D, Bapteste E, Doolittle WF, Charlebois RL, Legault B, Rodriguez-Valera F (2005) The genome of Salinibacter ruber: convergence and gene exchange among hyperhalophilic bacteria and archaea. Proc Natl Acad Sci U S A 102:18147–18152
Nagao A, Olson JA (1994) Enzymatic formation of 9-cis, 13-cis, and all-trans retinals from isomers of beta-carotene. FASEB J 8:968–973
Nagao A, During A, Hoshino C, Terao J, Olson JA (1996) Stoichiometric conversion of all trans-beta-carotene to retinal by pig intestinal extract. Arch Biochem Biophys 328:57–63
Ng WV, Kennedy SP, Mahairas GG, Berquist B, Pan M, Shukla HD, Lasky SR, Baliga NS, Thorsson V, Sbrogna J, Swartzell S, Weir D, Hall J, Dahl TA, Welti R, Goo YA, Leithauser B, Keller K, Cruz R, Danson MJ, Hough DW, Maddocks DG, Jablonski PE, Krebs MP, Angevine CM, Dale H, Isenbarger TA, Peck RF, Pohlschroder M, Spudich JL, Jung KW, Alam M, Freitas T, Hou S, Daniels CJ, Dennis PP, Omer AD, Ebhardt H, Lowe TM, Liang P, Riley M, Hood L, DasSarma S (2000) Genome sequence of Halobacterium species NRC-1. Proc Natl Acad Sci U S A 97:12176–12181
Olson J, Hayaishi O (1965) The enzymatic cleavage of beta-carotene into vitamin A by soluble enzymes of rat liver and intestine. Proc Natl Acad Sci U S A 54:1364–1370
Osuna-Garcia JA, Wall MM, Wadell CA (1997) Natural antioxidants for preventing color loss in stored paprika. J Food Sci 62:1017–1021
Paik J, During A, Harrison E, Mendelsohn C, Lai K, Blaner W (2001) Expression and characterization of a murine enzyme able to cleave beta-carotene. The formation of retinoids. J Biol Chem 276:32160–32168
Paine JA, Shipton CA, Chaggar S, Howells RM, Kennedy MJ, Vernon G, Wright SY, Hinchliffe E, Adams JL, Silverstone AL, Drake R (2005) Improving the nutritional value of Golden Rice through increased pro-vitamin A content. Nat Biotechnol 23:482–487
Peck RF, Echavarri-Erasun C, Johnson EA, Ng WV, Kennedy SP, Hood L, DasSarma S, Krebs MP (2001) brp and blh are required for synthesis of the retinal cofactor of bacteriorhodopsin in Halobacterium salinarum. J Biol Chem 276:5739–5744
Pfeiffer F, Schuster SC, Broicher A, Falb M, Palm P, Rodewald K, Ruepp A, Soppa J, Tittor J, Oesterhelt D (2008) Evolution in the laboratory: the genome of Halobacterium salinarum strain R1 compared to that of strain NRC-1. Genomics 91:335–346
Poliakov E, Gentleman S, Cunningham FJ, Miller-Ihli N, Redmond T (2005) Key role of conserved histidines in recombinant mouse beta-carotene 15, 15′-monooxygenase-1 activity. J Biol Chem 280:29217–29223
Prado-Cabrero A, Scherzinger D, Avalos J, Al-Babili S (2007) Retinal biosynthesis in fungi: characterization of the carotenoid oxygenase CarX from Fusarium fujikuroi. Eukaryot Cell 6:650–657
Redmond T, Gentleman S, Duncan T, Yu S, Wiggert B, Gantt E, Cunningham FJ (2001) Identification, expression, and substrate specificity of a mammalian beta-carotene 15, 15′-dioxygenase. J Biol Chem 276:6560–6565
Rohmer M, Knani M, Simonin P, Sutter B, Sahm H (1993) Isoprenoid biosynthesis in bacteria: a novel pathway for the early steps leading to isopentenyl diphosphate. Biochem J 295:517–524
Ruch S, Beyer P, Ernst H, Al-Babili S (2005) Retinal biosynthesis in Eubacteria: in vitro characterization of a novel carotenoid oxygenase from Synechocystis sp. PCC 6803. Mol Microbiol 55:1015–1024
Ruther A, Misawa N, Boger P, Sandmann G (1997) Production of zeaxanthin in Escherichia coli transformed with different carotenogenic plasmids. Appl Microbiol Biotechnol 48:162–167
Sabehi G, Loy A, Jung KH, Partha R, Spudich JL, Isaacson T, Hirschberg J, Wagner M, Beja O (2005) New insights into metabolic properties of marine bacteria encoding proteorhodopsins. PLOS Biol 3:1409–1417
Scherzinger D, Al-Babili S (2008) In vitro characterization of a carotenoid cleavage dioxygenase from Nostoc sp. PCC 7120 reveals a novel cleavage pattern, cytosolic localization and induction by highlight. Mol Microbiol 69:231–244
Scherzinger D, Ruch S, Kloer DP, Wilde A, Al-Babili S (2006) Retinal is formed from apo-carotenoids in Nostoc sp. PCC7120: in vitro characterization of an apo-carotenoid oxygenase. Biochem J 398:361–369
Schmidt H, Kurtzer R, Eisenreich W, Schwab W (2006) The carotenase AtCCD1 from Arabidopsis thaliana is a dioxygenase. J Biol Chem 281:9845–9851
Semba RD (1999) Vitamin A as “anti-infective” therapy, 1920–1940. J Nutr 129:783–791
Shand RF, Betlach MC (1994) bop gene cluster expression in bacteriorhodopsin-overproducing mutants of Halobacterium halobium. J Bacteriol 176:1655–1660
Singh H, Cama HR (1974) Enzymatic cleavage of carotenoids. Biochim Biophys Acta 370:49–61
van Vliet T, van Vlissingen MF, van Schaik F, van den Berg H (1996) beta-Carotene absorption and cleavage in rats is affected by the vitamin A concentration of the diet. J Nutr 126:499–508
von Lintig J, Vogt K (2000) Filling the gap in vitamin A research. Molecular identification of an enzyme cleaving beta-carotene to retinal. J Biol Chem 275:11915–11920
von Lintig J, Wyss A (2001) Molecular analysis of vitamin A formation: cloning and characterization of beta-carotene 15, 15′-dioxygenases. Arch Biochem Biophys 385:47–52
Woggon WD (2002) Oxidative cleavage of carotenoids catalyzed by enzyme models and beta-carotene 15, 15′-monooxygenase. Pure Appl Chem 74:1397–1408
Wyss A, Wirtz G, Woggon W, Brugger R, Wyss M, Friedlein A, Bachmann H, Hunziker W (2000) Cloning and expression of beta, beta-carotene 15, 15′-dioxygenase. Biochem Biophys Res Commun 271:334–336
Wyss A, Wirtz GM, Woggon WD, Brugger R, Wyss M, Friedlein A, Riss G, Bachmann H, Hunziker W (2001) Expression pattern and localization of beta, beta-carotene 15, 15′-dioxygenase in different tissues. Biochem J 354:521–529
Yan W, Jang G, Haeseleer F, Esumi N, Chang J, Kerrigan M, Campochiaro M, Campochiaro P, Palczewski K, Zack D (2001) Cloning and characterization of a human beta, beta-carotene-15, 15′-dioxygenase that is highly expressed in the retinal pigment epithelium. Genomics 72:193–202
Ye X, Al-Babili S, Kloti A, Zhang J, Lucca P, Beyer P, Potrykus I (2000) Engineering the provitamin A (beta-carotene) biosynthetic pathway into (carotenoid-free) rice endosperm. Science 287:303–305
Ye RW, Stead KJ, Yao H, He H (2006) Mutational and functional analysis of the beta-carotene ketolase involved in the production of canthaxanthin and astaxanthin. Appl Environ Microbiol 72:5829–5837
Yoon SH, Kim JE, Lee SH, Park HM, Choi MS, Kim JY, Shin YC, Keasling JD, Kim SW (2007a) Engineering the lycopene synthetic pathway in E. coli by comparison of the carotenoid genes of Pantoea agglomerans and Pantoea ananatis. Appl Microbiol Biotechnol 74:131–139
Yoon SH, Park HM, Kim JE, Lee SH, Choi MS, Kim JY, Oh DK, Keasling JD, Kim SW (2007b) Increased beta-carotene production in recombinant Escherichia coli harboring an engineered isoprenoid precursor pathway with mevalonate addition. Biotechnol Prog 23:599–605
Acknowledgment
This study was supported by a grant (2009-0084490) from the Basic Research Program, Ministry of Education, Science, and Technology, Republic of Korea.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, YS., Oh, DK. Biotransformation of carotenoids to retinal by carotenoid 15,15′-oxygenase. Appl Microbiol Biotechnol 88, 807–816 (2010). https://doi.org/10.1007/s00253-010-2823-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-010-2823-9