Abstract
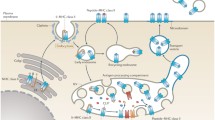
Proteases generate peptides that bind to MHC class II molecules to interact with a wide diversity of CD4+ T cells. They are expressed in dedicated organelles: endosomes and lysosomes of professional antigen-presenting cells (pAPCs) such as B cells, macrophages, and dendritic cells. The identification of endosomal proteases which produce antigenic peptides is important for example for better vaccination and to prevent autoimmune diseases. Here, we describe a panel of techniques (in vitro digestion assays of protein with recombinant proteases or purified endosomes/lysosomes, T cell stimulation) to monitor the production of MHC class II ligands.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Kvist S, Wiman K, Claesson L, Peterson PA, Dobberstein B (1982) Membrane insertion and oligomeric assembly of HLA-DR histocompatibility antigens. Cell 29:61–69
Lotteau V, Teyton L, Peleraux A, Nilsson T, Karlsson L, Schmid SL, Quaranta V, Peterson PA (1990) Intracellular transport of class II MHC molecules directed by invariant chain. Nature 348:600–605
Bikoff EK, Huang LY, Episkopou V, van Meerwijk J, Germain RN, Robertson EJ (1993) Defective major histocompatibility complex class II assembly, transport, peptide acquisition, and CD4+ T cell selection in mice lacking invariant chain expression. J Exp Med 177:1699–1712
Denzin LK, Cresswell P (1995) HLA-DM induces CLIP dissociation from MHC class II alpha beta dimers and facilitates peptide loading. Cell 82:155–165
Rawlings ND, Barret AJ (1993) Evolutionary families of peptidases. Biochem J 290:205–218
Nakagawa T, Brissette WH, Lira PD, Griffiths RJ, Petrushova N, Stock J, McNeish JD, Eastman SE, Howard ED, Clarke SRM et al (1999) Impaired invariant chain degradation and antigen presentation and diminished collagen induced arthritis in cathepsin S null mice. Immunity 10:207–217
Riese RJ, Wolf PR, Bromme D, Natkin LR, Villadangos JA, Ploegh HL, Chapman HA (1996) Essential role for cathepsin S in MHC class II-associated invariant chain processing and peptide loading. Immunity 4:357–366
Nakagawa T, Roth W, Wong P, Nelson A, Farr A, Deussing J, Villadangos JA, Ploegh H, Peters C, Rudensky AY (1998) Cathepsin L: critical role in Ii degradation and CD4 T cell selection in the thymus. Science 280:450–453
Colbert JD, Matthews SP, Miller G, Watts C (2009) Diverse regulatory roles for lysosomal proteases in the immune response. Eur J Immunol 39:2955–2965
Watts C (2012) The endosome-lysosomes pathway and information generation in the immune system. Biochim Biophys Acta 8:4–6
Manoury B, Hewitt EW, Morrice N, Dando PM, Barrett AJ, Watts C (1998) An asparagynil endopeptidase processes a microbial antigen for class II MHC presentation. Nature 396:695–699
Manoury B, Mazzeo D, Fugger L, Viner N, Ponsford M, Streeter H, Mazza G, Wraith DC, Watts C (2002) Destructive processing by asparagine endopeptidase limits presentation of a dominant T cell epitope in MBP. Nat Immunol 3:169–174
Moss CX, Villadangos JA, Watts C (2005) Destructive potential of the aspartyl protease cathepsin D in MHC class-restricted antigen processing. Eur J Immunol 35:3442–3451
Schein CH (1989) Production of soluble recombinant proteins in bacteria. Nat Biotechnol 7:1141–1149
Mitraki A, King J (1989) Protein folding intermediates and inclusion bodies formation. Nat Biotechnol 7:690–697
Lai MZ, Ross DT, Guillet JG, Briner TJ, Gedter ML, Smith JA (1987) T lymphocyte response to bacteriophage l repressor cI protein recognition of the same peptide presented by Ia molecules of different halotypes. J Immunol 139:3973–3977
Matthews SP, Werber I, Deussing C, Peters C, Reinheckel T, Watts C (2010) Distinct proteases requirement for antigen presentation in vitro and in vivo. J Immunol 184:2423–2431
Edgington-Mitchell LE, Bogyo M, Verdoes M (2017) Live cell imaging and profiling of cysteine cathepsin activity using a quenched activity-based probe. Methods Mol Biol 1491:145–149
Acknowledgments
This work was supported by INSERM (ANR 2010 MIDI 008 01), an ARC Ph.D. fellowship to S.M. and an Institut Curie Ph.D. fellowship to M.T.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Science+Business Media, LLC, part of Springer Nature
About this protocol
Cite this protocol
Tohmé, M., Maschalidi, S., Manoury, B. (2019). In Vitro Digestion with Proteases Producing MHC Class II Ligands. In: van Endert, P. (eds) Antigen Processing. Methods in Molecular Biology, vol 1988. Humana, New York, NY. https://doi.org/10.1007/978-1-4939-9450-2_21
Download citation
DOI: https://doi.org/10.1007/978-1-4939-9450-2_21
Published:
Publisher Name: Humana, New York, NY
Print ISBN: 978-1-4939-9449-6
Online ISBN: 978-1-4939-9450-2
eBook Packages: Springer Protocols