Abstract
A subfamily of orphan receptors, estrogen receptor-related receptors (ERRs), has been demonstrated to modulate the transcription of some estrogen responsive genes via variant estrogen response elements (EREs). This study was conducted to determine whether human ERRα, ERRβ, and ERRγ might be involved in the tumorigenesis of ovarian cancer. RT-PCR was performed to analyze the expression of hERRα, hERRβ, hERRβ-2, and hERRγ mRNA in five ovarian cancer cell lines as well as 33 samples of ovarian cancer and 12 samples of normal ovary. Serum CA-125 levels were also analyzed in all samples by ELISA. Progression-free survival and overall survival of patients with different expression of ERRs were analyzed by the Kaplan–Meier method. To analyze the subcellular localization of ERRα, a green fluorescent protein (GFP)-reporter plasmid of hERRα was constructed and transfected into the ovarian cancer cell line OVCAR-3. Expression of hERRα-GFP fusion protein was observed in the nucleus of OVCAR-3 ovarian cancer cell lines. We observed increased expression of hERRα mRNA (P=0.020) and hERRγ mRNA (P=0.045) in ovarian cancers compared to normal ovaries. In contrast, hERRβ was only observed in 9.1% of ovarian cancers. We found a positive correlation between the serum CA-125 levels and hERRα expression (P=0.012), but not hERRβ and hERRγ expression. Survival analysis showed that the hERRα-positive group has a reduced overall survival (P=0.015), and the ERRγ-positive group has a longer progression-free survival (P=0.020). In multivariate analysis, expression of hERRα was an independent prognostic factor for poor survival (relative risk, 3.032; 95% CI, 1.27–6.06). Based on our results, ERRs may play an important role in ovarian cancer. hERRα may represent a biomarker of poor prognosis, and hERRγ may be a new therapeutic target in ovarian cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Epithelial ovarian cancer is the leading cause of death from gynecological malignancies in Western countries [1]. One of the reasons for the poor prognosis is the high rate of advanced tumors at the time of diagnosis: about 75% of all patients are diagnosed in FIGO stage III or IV [2]. High serum levels of estrogen have been implicated as a risk factor for ovarian carcinoma, but the cellular signal pathways involved are not completely clear so far [3, 4]. Estrogen acts via two classical nuclear receptors, estrogen receptor alpha and estrogen receptor beta (ERα and ERβ, American Nomenclature Committee-named as NR3A1 and NR3A2). ERα and ERβ are highly expressed in normal human ovaries, benign ovarian tumors, borderline and malignant ovarian tumors, as well as in primary cultures of normal human ovarian surface epithelial cells and established ovarian cancer cell lines [3, 4]. However, only low response rates have been observed to anti-estrogen treatment based on the blocking of estrogen–ER binding [4–6]. Furthermore, expressions of ER mRNA have small prognostic value in the hormone-related ovarian cancer [5, 6].
Recently, several studies described a family of the so-called orphan nuclear receptors. In contrast to the ligand-dependent classic receptors, orphan receptors were found be activated in a constitutive manner without any defined ligand [7–11]. There is a subfamily of orphan nuclear receptors closely related to the ERs, which is named estrogen receptor-related receptors (ERRs) [7, 10, 11]. The ERR family includes three subtypes: ERRα, ERRβ, and ERRγ (American Nomenclature Committee 1999 meeting-named as NR3B1, NR3B2, and NR3B3), and each member has several different isoforms [11]. They were originally isolated on the basis of sequence similarity in their DNA-binding domain with ERα, but they are not activated by natural estrogens [9–11]. ERRs can activate some estrogen responsive genes such as pS2 and the aromatase genes in breast cancer cell lines [12–14] and serve as biomarkers independent of the estrogen–ER signal pathway [11, 14]. Estrogen–ER complexes exert their function and drive transcription following binding to estrogen response elements (EREs) in the promoter of target genes [4, 9–11]. ERRs share target genes, co-regulatory proteins, and DNA-binding sites with the ERs [7–11]. Moreover, ERRs have a high-affinity binding to sites containing 5′-TCA-AGGTCA-3′ as a monomer. This sequence was not only observed in the ERE, but also observed in the estrogen receptor related-receptor response element (ERRE), steroid factor-1 receptor response element, and thyroid response element [10–17]. It has been suggested that there is a key role of ERRs to regulate the estrogen signal pathway in tumors, though the mechanisms of this crosstalk are still unclear [9–11].
Do ERRs play a critical role in the etiology of ovarian cancers? To determine whether this subfamily of orphan nuclear receptors might be associated with ovarian cancer, we studied the expression of the major isoforms of the ERR family, hERRα, hERRβ, and hERRγ, in ovarian cancer cell lines as well as in malignant and normal ovaries. Furthermore, we investigated the clinicopathological relevance of these orphan receptors.
Materials and methods
Cell culture
Ovarian cancer cell lines SKOV-3, OVCAR-3, OAW-42, ES-2, and Mdah-2774 were from the American Typical Culture Collection (Rockville, Md., USA). All cell lines were cultured in 90% Dulbecco’s modified Eagle’s medium [(DMEM) Gibco, Carlsbad, Calif., USA], supplemented with 10% fetal bovine serum (PAN Biotech, Aldenbach, Germany), 4 mM L-glutamine (Sigma, St. Louis, Mo., USA) adjusted to contain 1.5 g/l sodium bicarbonate, 4.5 g/l glucose, 1.0 mM sodium pyruvate, 1% penicillin (100 IU/ml), and 1% streptomycin (100 IU/ml), in a 37°C, 5% CO2 incubator.
Study population
A total of 33 ovarian cancer samples and 12 normal unmatched ovary samples were included in this study. After pathological review, the parts of ovarian surface epithelium (OSE) from the normal ovaries were used as controls. All samples and the related clinical data were obtained from the Tumor Bank Ovarian Cancer (Charité Medical University, Berlin, Germany). Samples were collected during 1999–2001 and diagnosed by pathological review (Institute of Pathology, Charité Medical University). Approval from the local ethics board was gained and written. Informed consent was obtained from each patient. All ovarian cancer patients enrolled in this study received the primary surgery with the attempt of maximal tumor reduction and postoperative systemic chemotherapy with carboplatin and paclitaxel. The samples were snap-shock frozen in liquid nitrogen as soon as they were separated from the body and stored at −80°C until analysis. The clinicopathological characteristics of ovarian cancer patients are summarized in Table 1.
Plasmids and plasmid construction
The full-length cDNA plasmids of pSG-hERRα, pSG-mERRβ, and pSG-mERRγ were generous gifts from Prof. J.M. Vanacker (LBMC, Lyon, France) and used as RT-PCR positive controls [15, 16]. The green fluorescent protein (GFP) reporter plasmid was a generous gift from Dr. Eckardt Treuter (Karolinska Institute Hospital, Sweden). The hERRα-GFP reporter plasmid was constructed as follows: The pSG-hERRα plasmid and pCN3-GFP reporter plasmid were digested by the restricted enzymes EcoRI 1.03 and BamHI. The full-length hERRα-1 cDNA (1–2,221 bp) was recovered and purified with QIAEX II kits (Qiagen, Hilden, Germany). Full-length hERRα-1 cDNA was directionally inserted into the EcoRI 1.03 and BamHI sites in the pCN3-GFP report plasmid with a PCR Cloning Kit (Qiagen) and named the hERRα-GFP reporter plasmid. The plasmid pSG-HA-tag-hERRγ was a generous gift from Dr. Michel R. Stallcup (University of Southern California, USA) [14]. A nucleotide sequence containing hemagglutinin [(HA) amino acids sequence, YPYDVPDYA] epitope tag-coding codons and a new EcoRI site was inserted between the original EcoRI and BamHI sites of the pSG5 basic plasmid. All plasmids were subcloned in TOP-10 EcoRI bacilli (Invitrogen, Karlsruhe, Germany) and harvested with a QIA Spinprep kit (Qiagen).
Subcellular localization of ERRα
0.6 μl FuGENE6 (Roche, Mannheim, Germany) and 0.1 μg hERRα-GFP-reporter plasmids were mixed in 19 μl serum-free medium and incubated for 15 min. Five-microliter mixtures per well were added to the normal cultured medium in a four-chamber-cultured-slide (Nunc, Rochester, N.Y., USA). Serum-free DMEM medium was used as a negative control. After 48 h of culture, the medium was discarded. Ovarian cancer cells line OVCAR-3 was washed two times with PBS for 5 min and fixed by methanol at −20°C for 10 min. Cell nuclei were stained with 4′,6-diamidino-2phenylindole [(DAPI) 1:1,000]. The cells were analyzed using a confocal scanning microscope (Leica, Solms, Germany).
Western blot of HA-tag-ERRγ fusion protein
Ovarian cancer cell lines ES-2, OVCAR-3, and SKOV-3 were plated at a density of 3×105 cells/well in 35-mm plates. After 24 h culture, 0.5 µg or 1 μg pSG-HA-tag-hERRγ plasmid was mixed with 3 μl FuGENE6 and added to the cells in the 35-mm plates. A control group was set up by treatment with serum-free DMEM. After transfection for 72 h, nuclear protein and whole-cell protein were extracted according to the protocol provided by the kits (Clontech, Palo Alto, Calif., USA). Protein content was determined by the ELISA method (Pierce). In all, 10 μg nuclear protein, 50 μg cytoplasmic protein, or 100 μg whole-cell protein per lane was loaded on an 8% polyacrylamide gel. Proteins were blotted onto nitrocellulose membranes (Schleicher and Schuell, Dassel, Germany). The blot was washed in PBS and incubated in blocking buffer [1× PBS, 0.1% Tween-20, 5% I-Block (Tropix, Bedford, Mass., USA)] at 18°C for 1 h. Membranes were incubated overnight at 18°C with a monoclonal anti-HA antibody (1:1,500, Roche) in blocking buffer, followed by incubation with alkaline phosphatase-conjugated-anti-mouse secondary antibody (1:4,000, Santa Cruz, Canada). Bands were visualized using the CDP star RTU luminescence system (Tropix).
RNA isolation and RT-PCR
Total RNA was isolated according to the protocol provided by a MiniRNA kit (Qiagen). The quality and content of the mRNA was assessed by DNA Counter (Bio-Rad, Munich, Germany). Only samples with the OD260/280 ratio above 1.6 were used in the experiments. Special primer sets of ERRs were designed by Oliog6.0 and confirmed by BLAST analysis (National Center for Biotechnology Information). The following primers were synthesized by the TIB syntheselabor (TIB Molbiol, Berlin, Germany): hERRα sense 5′-Tgg TCC AgC TCC CAC TCg CT-3′ and anti-sense 5′-TgA gAC ACC AgT gCA TTC ACT g-3′ (482 bp); hERRβ-1 sense 5′-TCA AgT gCg AgT ACA TgC TC-3′ and anti-sense 5′-gAA ATT TgT AAg CTC Agg TA-3′ (340 bp); hERRβ-2 sense 5′CAT TCC ACg gAg gCA TCC TC-3′, anti-sense 5′-TgC AAg CCT CgC Agg Agg CC-3′ (537 bp); hERRγ sense 5′-CTC gCC ACC TCT CTA CCC TT-3′, anti-sense 5′-gCT TgT ACT TCT gCC gAC CTC-3′ (395 bp); and GADPH was used as an internal control by the primer set: sense 5′-ACg CAT TTg gTC gTA TTg gg-3′ and anti-sense 5′-TgA TTT Tgg Agg gAT CTC gC-3′(230 bp). The Access RT-PCR system (Promega, Mannheim, Germany) was used according to the manufacturer’s instructions. Briefly, in a total 50-μl reaction system were included 1 μg total RNA, 5 U reverse transcriptase, 5 U Tfh polymerase, 10 nm dNTP, random hexamers, 1 U Rnase inhibitor, 1 nm each sense and anti-sense primers, and 15 nm MgCl2. For amplification, we used the same protocol: reverse transcript, 22°C for 10 min, 42°C for 45 min, and 95°C 5 min; PCR, 94°C for 3 min, 94°C for 30 s, 60°C for 1 min, 72°C for 1 min, 35 cycles; and 72°C 10 min.
CA-125 assay
The patient sera were stored at −20°C until analysis. The commercially available ELISA-Kit (MEDAC, Hamburg, Germany) was used. According to the instructions of the manufacturer, the serum CA-125 levels were determined by another technician, who did not have any clinical information about the patients.
Statistical analysis
The statistical analysis was performed via SPSS, version 11.0, software (SPSS, Chicago, Ill., USA). ANOVA and the independent sample t-test were used to compare the parametric data, and the chi-square test was used to analyze non-parametric data. The correlations between expression of ERRs and clinicopathological parameters were analyzed by the bivariate correlation analysis. To analyze the prognosis of ovarian cancer patients according to the different expression of ERRs, the primary outcome measure of this non-randomized study was overall survival; secondary outcome was progression-free survival. Overall survival was defined as the time from first surgery to death from any cause. Progression-free survival was defined as the time from first surgery to first clinical or pathological evidence of recurrence. The Kaplan–Meier method was used to calculate overall survival time or progression-free survival time, and survival curves were compared by the log-rank test. Multivariate survival analysis was performed using the Cox regression model. Generally, a P-value less than 0.05 was considered as significant.
Results
Expression of ERR fusion protein
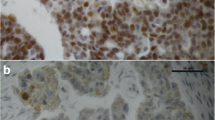
Derived by the thymidine kinase promoter, the hERR-αGFP reporter plasmid can produce a fusion protein of the GFP and hERRα protein. Excited by the 480-nm illuminations, part of GFP can produce a high-level, green autofluorescent signal. Excited by the UV illuminations, the cell nucleus stained with DAPI can give a blue fluorescent signal. The hERRα-GFP fusion protein was mainly observed in the nucleus of ovarian cancer cell line OVCAR-3 as judged by using confocal scanning microscopy (Fig. 1). Compared to the high expression of hERRα in the nucleus, almost no green signal could be observed in the cytoplasm. Some reports had shown the expression of ERR protein by Western blot detection of the recombined fusion tag-ERR protein in an in vitro transcription and translate system [17, 18]. In our studies, after transfection with 1.0 μg plasmid, the HA-ERRγ recombined fusion protein could be detected by antibodies anti to the HA-tag epitope in the nuclear protein extraction of ovarian cancer cell lines SKOV-3, OVCAR-3, and ES-2 (Fig. 2). In contrast to the transfected group, there was no visible hERRγ special band in the control group.
Subcellular localization of the isoform estrogen receptor-related receptor alpha (hERRα) protein: The full-length hERRα (2,221 bp)–green fluorescent protein (GFP) reporter plasmid was constructed and transfected into ovarian cancer cell line OVCAR-3. After 48 h of culture, the cells were fixed and stained with 4′,6-diamidino-2phenylindole (DAPI). Excited by 480-nm illuminations, a strong, green autofluorescent signal was observed in the OVCAR-3 cells transfected with hERRα-GFP; excited by UV, the cell nucleus stained with DAPI can produce a blue fluorescent signal (a). A single cell (a, c) was magnified (b, d). Compared with the strong signal observed in the cell nucleus, no signal could be observed in the cytoplasm (white arrow). In the negative-control group (c, d), the cell lines were treated only with serum-free Dulbecco’s modified Eagle’s medium. The cells did not show any endogenous signal
Expression of hemagglutinin (HA)-tag-ERRγ protein and its subcellular distribution. a Whole-cell protein: ovarian cancer cell lines SKOV-3 (Sk) and OVCAR-3 (Ov) were transfected with 1.0 μg or 0.5 μg pSGHA-tag-hERR γ plasmids. HA-tag-hERRγ fusion protein can be detected in the whole-cell protein. b Nuclear protein and cytoplasmic protein extraction: transfected with 1.0 μg pSG-HA-tag-hERRγ plasmids. A special band was detected in the nuclear protein extraction of ovarian cancer cell lines ES-2 (Es), SKOV-3, and OVCAR-3. In contrast, there were no visible bands of hERRγ fusion protein in the nuclear extraction of control cell lines. Compared with the cytoplasmic extraction, the HA-tag-hERRγ protein was chiefly expressed in the nuclear extraction
Expression of ERR mRNA in ovarian cancer cell lines
We compared the mRNA expression of human ERRα, ERRβ, and ERRγ in ovarian cancer cell lines by RT-PCR (Fig. 3). There are at least two major isoforms of ERRα, human ERRα (full-length cDNA, 2,421 bp) and human ERRα-1 (full-length cDNA, 2,221 bp) [11, 19, 20]. We used a special primer set to amplify an identified 482-bp fragment of hERRα and hERRα-1. This product was observed in all five ovarian cancer cell lines and named as hERRα in our study. In contrast to the high expression of hERRα, expression of hERRβ-1 and its isoform hERRβ-2 seemed minimal in the ovarian cancer cell lines. hERRβ-1 was detected in ovarian cancer cell lines Mdah-2774 and SKOV-3, and hERRβ-2 only could be detected in cell line SKOV-3. By applying an ERRγ special primer set, an amplified product of a 395-bp ERRγ fragment was detected in ovarian cancer cell lines Mdah-2774, OVCAR-3, and SKOV-3.
Sera CA-125 levels in ovarian cancer patients with different expression of ERRs. The mean serum CA-125 level in the hERRα-positive group was 1,954.8±494.8 (31.0–15,489.0) and in the hERRα-negative group was 448.6±176.4 (5.0–3,461.0). In the hERRβ-positive group, the mean serum CA-125 level was 1,675.8±933.6 (5.0–4,858.0) and in the hERRβ-negative group was 1,454.5±869.8 (14.0–15,489.0). The mean serum CA-125 level in the ERRγ-positive group was 1622.0±961.1 (5.0–15489.0) and in the hERRγ-negative group was 1201.1±788.8 (14.0–4842.0). Independent sample t-tests were used to analyze the parametric data. In the hERRα-positive group, two cases were outside the sera CA-125 levels of the hERRα-negative group. There was still a significant difference between the hERRα-positive group without these two cases and the negative group (P=0.016)
Expression of ERR mRNA in ovarian cancer
Overall, 33 samples of ovarian cancer and 12 samples of normal ovary were included in this study (Table 2). The differences of average ages were not significant (between groups, P=0.350; within groups P=0.563, ANOVA). The mRNA of hERRα was detected in 19 of 33 ovarian cancers (57.6%) and 2 of 12 normal ovaries (16.7%). Compared to the normal ovarian tissues, ovarian cancer showed a higher expression of hERRα (P=0.020). Only two (6.1%) ovarian cancers expressed the hERRβ-1, and one (3.0%) ovarian cancer expressed hERRβ-2. We did not detect any expression of either hERRβ-1 or hERRβ-2 in normal ovaries. There were no different expression of hERRβ between the ovarian cancers and normal ovarian tissues (P=0.543). Expression of hERRγ mRNA was observed in 16 of 33 ovarian cancers (48.5%) and 4 of 12 normal ovaries (33.3%). Similar to hERRα, expression of hERRγ was also significantly increased in ovarian cancer patients (P=0.045). We also analyzed the correlation between the expression of ERRs and clinicopathological parameters such as FIGO stage, grading, ascites, and histological types. Bivariate correlation analysis showed that the expression of hERRα mRNA had significant correlation with the FIGO stage (P=0.017) and histological grading (P=0.022). Expression of hERRα mRNA was associated with more advanced FIGO stage and grading. A positive correlation was also observed between the FIGO stage and expression of hERRγ (P=0.040). In comparison to hERRα and hERRγ, the numbers of hERRβ-positive samples were not high enough to perform an analysis. Of all 33 ovarian cancers, two (6.1%) samples showed expression of all the three members of ERRs, four (12.1%) samples co-expression of ERRα and ERRγ, one (3.0%) sample co-expression of ERRα and ERRβ, 12 (36.4%) samples only expression of ERRα, and ten (30.3%) samples only expression of ERRγ. The hERRα-positive samples were usually associated with a negative expression of hERRγ. However, the survival analysis was not possible for the ERRα/ERRβ/ERRγ all-negative ovarian cancer patients, because there were only four (12.1%) samples.
CA-125 in patients with different ERR expression
CA-125 is the most important well-established tumor marker in the clinical management of ovarian cancer [2]. We also analyzed the association of the serum CA-125 levels with the expression of ERRs (Fig. 4). In the total population, the average value of CA-125 was 1,303.5±466.8 U/ml (5.0–15,489.0 U/ml). The mean of the CA-125 level in the hERRα-positive group (1,954.8 U/ml) was higher than the hERRα-negative group (448.6 U/ml, P=0.012). In the hERRα-positive group, two cases were detected with very high levels of CA-125. To exclude the impact made by these two cases, we performed a new analysis excluding them. The results showed that there still was a significant difference (P=0.016). Thus, we think the significant difference of CA-125 levels was not due to a few outliers, but due to the different expression of ERRα. In contrast to hERRα, CA-125 levels in the hERRβ-positive group (1,675.8 U/ml) showed no difference from the hERRβ-negative group (1,454.5 U/ml, P=0.795). Similar to hERRβ, the hERRγ-positive group and -negative group did not show a significant difference in the serum CA-125 levels (1,622.0 U/ml vs 1,201.1 U/ml, P=0.515).
RT-PCR result of ERRs expression in ovarian cancer cell lines, normal ovary, and ovarian cancer. hERRα (482 bp) was highly expressed in ovarian cancer cell lines and samples; hERRβ (including hERRβ-1, 340 bp and hERRβ-2, 537 bp) was poorly expressed in ovarian cancer cell lines and tumor samples; hERRγ (395 bp) was expressed on in both ovarian cell lines and cancer samples. a Positive control (PCR result of the respectively plasmid), b ES-2, c Mdah-2774, d OAW-42, e SKOV-3, f OVCAR-3, g normal ovary, h, i ovarian cancers. A 100-bp Ladder (Gibco) was used as a DNA marker.
Survival analysis
All patients were enrolled in a follow-up program. The median follow-up time was 31.54 months (2.0–76 months). Valid follow-up data were available for 29 cases (87.8%) of 33 ovarian cancer patients. The median overall survival (n=29) was 26.8 months (2.0–65.0 months), and the median progression-free survival time was 13.8 months (1.0–40.0 months; other details can be seen in Table 3 and Fig. 5). The median overall survival time of ovarian cancer patients with hERRα-positive expression was 19.0 months; compared to the hERRα-negative group, the overall survival time was significantly reduced (log-rank test, P=0.015). The median progression-free survival of the hERRα-positive group and -negative group was 12.6 months and 14.5 months, respectively (P=0.820). The median overall survival showed no significant difference between the ERRγ-positive group (23.4 months) and -negative group (19.6 months, P=0.092). However, the hERRγ-positive ovarian cancer patients had a longer progression-free survival time (18.0 months) than the hERRγ-negative group (13.5 months, log-rank test, P=0.020). We used a multivariate analysis to test the independent value of each parameter predicting overall survival and progression-free survival. Expression of hERRα was an independent prognostic factor for poor survival (relative risk, 3.032; 95% CI, 1.27–6.06). Other independent prognostic factors associated with poor prognosis were histological grade and FIGO stage (Table 4). Volume of ascites and expression of hERRγ were not independent prognostic factors for poor survival.
Overall survival and progression-free survival curve, analyzed by the Kaplan–Meier method. a The overall survival time of ovarian cancer patients with hERRα expression (n=15,19.0 months, 95% CI: 6.9-27.4) was significantly reduced compared with the hERRα-negative group (n=14, 31.5 months, 95% CI: 13.1-54.2) (log rank test, P=0.015). b The progression-free survival time was not difference between the hERRα-positive and -negative groups. c The overall survival time of ovarian cancer patients was not different between the hERRγ-positive and -negative groups. d hERRγ-positive ovarian cancer patients has a longer progression-free survival time (n=14, 18.0 months, 95% CI: 14.5-21.5) than hERRγ-negative patients (n=15, 13.5 months, 95% CI: 9.8-17.0) (log rank test, P=0.020)
Discussion
Because we are just beginning to understand ERR function, the present study is, to our knowledge, the first investigating the role of ERR mRNA in ovarian cancer. Ovarian cancer can be considered as a hormone-dependent cancer [3, 4]; however, it is not sensitive to hormonal therapy [5, 6]. Consensus ERE was reported in several esorogen responsive genes such as, pS2, c-fos, c-myc, epidermal growth factor (EGF), EGF-receptor, cyclin D1, breast cancer-1 (BRCA-1), which were rate-limit with the development of estrogen related cancers [21, 22]. Zhang and Teng [23] reported that ERRα-1 could activate transcription of some estrogen responsive genes and exert ER-like function via binding to ERE or ERRE sites. To explore the potential role of ERRs in ovarian cancer cells, we studied the subcellular localization of hERRα. Comparing the different green autofluorescent signals produced in the cell nucleus and cytoplasm, we concluded that hERRα was mainly expressed in the cell nucleus. Some studies also reported that the ERRα was a kind of nuclear receptor [7, 15]. Furthermore, the expression of recombinant hERRγ protein could be detected in the nuclear protein extraction but not in the cytoplasmic protein extraction by Western blot analysis. However, when we performed this study, there were no anti-ERRs antibodies commercially available. The antibodies used in our studies are directed against the HA-tag epitope but not against the ERR protein, so it could not be used for the detection endogenous ERR protein in clinical samples.
Most ovarian cancers are mainly derived from OSE [5]. However, the normal OSE is only a single layer of cells. Similar to various work groups [5, 24, 25], we compared the expression of ERR mRNA in ovarian cancers and normal ovaries that included the OSE and stroma. We cannot exclude that the small amount of ovarian stroma in these normal ovaries may have influenced the result of the PCR. To further investigate the expression pattern of ERRs in ovarian epithelium and stromal tissue, a study should be performed when the anti-sera are commercially available. In our study, 57.6% of ovarian cancers were observed with expression of hERRα and 48.5% ovarian cancers with expression of hERRγ, which indicates that high levels of hERRα and hERRγ might be associated with cancer of the ovary. Our results suggest that the human ERR family might also be involved in the tumorigenesis via binding to the ERRE of oncogenes in ovarian cancer. However, this hypothesis needs to be validated by additional studies on the function of ERRs in ovarian cancer. High expression of hERRα may provide another pathway to stimulate cell overgrowth in ovarian cancers. Other studies showed that ERRα could not be activated nor repressed by natural estrogen, agonists, or antagonists of estrogen [26–28]. Because hERRα is resistant to the classical inhibitor of estrogen, this may explain why ovarian cancers poorly respond to anti-estrogen therapy based on only blocking the estrogen–ER signaling pathway. Moreover, hERRα-positive ovarian cancer patients were associated with more aggressive disease: ERRα-positive ovarian cancers showed higher CA-125 levels than ERRα negative cancers (P<0.05), which was associated with poor prognosis, short median overall survival time (P<0.05), and more advanced FIGO stage (P<0.05). These data strongly suggest an important role for hERRα in ovarian cancer. However, expression of hERRα was found in both the anti-ER therapy-sensitive cell line OVCAR-3 and -insensitive cell line SKOV-3. Both of them were reported to express ERα [5, 6]. Results from other studies showed ERα and ERRα were competitively bound to the DNA-response elements in a cell-specific, promoter-specific manner [23, 28]. A quantitative analysis of ERs and ERRs in these cell lines, especially the relationship between mRNA and protein levels of ERα and ERRα, should be performed in further studies. The design of new selective estrogen receptor modulators (SERMs) that could specially block ERRα activity, or both ER and ERRα activity should be explored [9, 11].
In contrast to ERRα, ovarian cancer and normal ovary showed high expression of ERRγ. The CA-125 levels in the hERRγ-positive and the hERRγ-negative group were not different (P>0.05). hERRγ-positive ovarian cancer patients have a significantly longer progression-free survival (P<0.05). Moreover, the activity of hERRγ can be inhibited by 4-hydroxytamoxifen (4-OHT), which is a SERM used in the therapy of breast cancer [29, 30]. This could suggest that ovarian cancer patients with hERRγ expression may be more sensitive to the treatment with SERMs. Indeed, the key discovery that 4-OHT is an inhibitor or probably ligand for ERRγ predicts novel and unexpected use for current SERMs [11]. Ariazi et al. [31] were the first to apply real-time Q-PCR to assess the importance of ERRs in human breast cancer by comparing their mRNA profiles with established clinicopathological indicators. They also observed that increased hERRα levels were associated with more aggressive breast tumor behavior and increased hERRγ levels with preferred clinical outcomes; hERRγ was over-expressed in 75% of breast cancer [31]. In the present study, 45% of ovarian cancers were positive for hERRγ. This may reflect tissue-specific expression of hERRγ. In contrast to the abundant expression of hERRα and hERRγ, two hERRβ isoforms (hERRβ-1 and hERRβ-2) seem to be poorly expressed in both the ovarian cancer and normal ovaries. ERRβ is present in the early developing placenta in a subset of cells in extra-embryonic ectoderm destined to develop the chorion and only found in very low amounts in specific rat tissues [32, 33]. In mice lacking ERRβ, trophoblast stem cell differentiation is impaired, and the placenta fails to develop normally [34]. The knowledge about hERRβ is limited due to low and restricted expression.
The data of our study suggest that hERRα and hERRγ may be important in ovarian cancer and may contribute to the development and progression of ovarian cancer. In our study, hERRα is a tumor marker associated with poor prognosis, and hERRγ seems to be a tumor marker for favorable prognosis in ovarian cancer. Results from in vitro research showed that ERRs are potential therapy targets for endocrinopathic cancer [35–37]. Hormonal therapy for recurrent epithelial ovarian cancer has resulted in uneven but consistent responses [38]. A systematic Cochrane review of 623 patients has documented a moderate activity of tamoxifen in relapsed ovarian cancer; the response rate varied from 0% to 56% [39]. The complicating different therapeutic results using SERMs in the treatment of ovarian cancer might be explained by a very complicated ERs and ERRs status, which is still unclear. The determination of the status of human ERRs expression in ovarian cancer may improve hormonal therapy and the prognostic evaluation of ovarian cancers. However, large-scale prospective and retrospective studies are needed to establish whether ERRs expression is indeed of practical utility as a prognostic predictor. Further studies should focus on the relationship between the hERRs in vivo and in vitro therapy trials of ovarian cancers target on hERRs.
References
Landis SH, Murray T, Bolden S, Wingo PA (1999) Cancer statistics. CA Cancer J Clin 49:8–31
Sehouli J, Akdogan Z, Heinze T, Konsgen D, Stengel D, Mustea A, Lichtenegger W (2003) Preoperative determination of CASA and CA-125 for the discrimination between benign and malignant pelvic tumor mass: a prospective study. Anticancer Res 23:1115–1118
Muramatsu M, Inoue S (2000) Estrogens receptors: how do they control reproductive and nonreproductive functions? Biochem Biophys Res Commun 270:1–10
Clinton GM, Hua W (1997) Estrogen action in human ovarian cancer. Crit Rev Oncol Hematol 25:1–9
Brandenberger AW, Tee MK, Jaffe RB (1998) Estrogen receptor alpha and beta mRNAs in normal ovary, ovarian serous cystadenocarcinoma and ovarian cancer cell lines: down-regulation of ER-β in neoplastic tissues. J Clin Endocrinol Metab 83:1025–1028
Lau KM, Mok SC, Ho SM (1999) Expression of human estrogen receptor-α and -β, progesterone receptor and androgen receptor mRNA in normal and malignant ovarian epithelial cells. Proc Natl Acad Sci USA 96:5722–5727
Giguere V, Yang N, Segui P, Evans RM (1988) Identification of a new class of steroid hormone receptors. Nature 331:91–94
Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schutz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P (1995) The nuclear receptor superfamily: the second decade. Cell 83:835–839
Giguere V (1999) Orphan nuclear receptors: from gene to function. Endocr Rev 20:689–725
Horard B, Vanacker JM (2003) Estrogen receptor-related receptors: orphan receptors desperately seeking a ligand. J Mol Endocrinol 31:349–357
Giguere V (2002) To ERR in the estrogen pathway. Trends Endocrinol Metab 13:220–225
Chen S, Itoh T, Wu K, Zhou D, Yang C (2002) Transcriptional regulation of aromatase expression in human breast tissue. J Steroid Biochem Mol Biol 83:93–99
Lu D, Kiriyama Y, Lee KY, Giguere V (2001) Transcriptional regulation of the estrogen-inducible pS2 breast cancer marker gene by the ERR family of orphan nuclear receptors. Cancer Res 61: 6755–6761
Hong H, Yang L, Stallcup MR (1999) Hormone-independent transcriptional activation and coactivator binding by novel orphan nuclear receptor ERR3. J Biol Chem 274:22618–22626
Vanacker JM, Bonnelye E, Chopin-Delannoy S, Delmarre C, Cavailles V, Laudet V (1999) Transcriptional activities of the orphan nuclear receptor ERR alpha (estrogen receptor-related receptor-α). Mol Endocrinol 13:764–773
Vanacker JM, Pettersson K, Gustafsson JA, Laudet V (1999) Transcriptional targets shared by estrogen receptor-related receptors (ERRs) and estrogen receptor (ER) alpha but not by ER beta. EMBO J 18:4270–4279
Zhang Z, Teng CT (2000) Estrogen receptor-related receptor alpha-1 interacts with coactivator and constitutively activates the estrogen response elements of the human lactoferrin gene. J Biol Chem 275:20837–20846
Bonnelye E, Merdad L, Kung V, Aubin JE (2001) The orphan nuclear estrogen receptor-related receptor alpha (ERR alpha) is expressed throughout osteoblast differentiation and regulates bone formation in vitro. J Cell Biol 153:971–984
Yang N, Shigeta H, Shi H, Teng CT (1996) Estrogen-related receptor, hERR1, modulates estrogen receptor-mediated response of human lactoferrin gene promoter. J Biol Chem 271:5795–5804
Yang C, Zhou D, Chen S (1998) Modulation of aromatase expression in the breast tissue by ERR alpha-1 orphan nuclear receptor. Cancer Res 58:5695–5700
Rollerova E, Urbancikova M (2000) Intracellular estrogen receptors, their characterization and function. Endocr Regul 34:203–218
Sanchez R, Nguyen D, Rocha W, White JH, Mader S (2002) Diversity in the mechanisms of gene regulation by estrogen receptors. BioEssays 24:244–254
Zhang Z, Teng CT (2001) Estrogen receptor α and estrogen receptor-related receptor alpha1 compete for binding and coactivator. Mol Cell Endocrinol 172:223–233
Akahira J, Suzuki T, Ito K, Kaneko C, Darnel AD, Moriya T, Okamura K, Yaegashi N, Sasano H (2002) Differential expression of progesterone receptor isoforms A and B in the normal ovary, and in benign, borderline, and malignant ovarian tumors. Jpn J Cancer Res 93:807–815
Kyo S, Kanaya T, Takakura M, Tanaka M, Yamashita A, Inoue H, Inoue M (1999) Expression of human telomerase subunits in ovarian malignant, borderline and benign tumors. Int J Cancer 80:804–809
Johnston SD, Liu X, Zuo F, Eisenbraun TL, Wiley SR, Kraus RJ, Mertz JE (1997) Estrogen-related receptor alpha1 functionally binds as a monomer to extended half-site sequence including ones contained within estrogen-response elements. Mol Endocrinol 11:342–352
Xie W, Hong H, Yang NN, Lin RJ, Simon CM, Stallcup MR, Evans RM (1999) Constitutive activation of transcription and binding of coactivator by estrogen-related receptors 1 and 2. Mol Endocrinol 13:2151–2162
Kraus RJ, Ariazi EA, Farrell ML, Mertz JE (2002) Estrogen-related receptor α-1 actively antagonizes estrogen receptor-regulated transcription in MCF-7 mammary cells. J Biol Chem 277:24826–24834
Coward P, Lee D, Hull MV, Lehmann JM (2001) 4-Hydroxytamoxifen binds to and deactivates the estrogen-related receptor gamma. Proc Natl Acad Sci USA 98:8880–8884
Tremblay GB, Bergeron D, Giguere V (2001) 4-Hydroxytamoxifen is an isoform-specific inhibitor of orphan estrogen-receptor-related (ERR) nuclear receptors beta and gamma. Endocrinology 142:4572–4575
Ariazi EA, Clark GM, Mertz JE (2002) Estrogen-related receptor alpha and estrogen-relate receptor gamma associated with unfavorable and favorable biomarkers, respectively, in human breast cancer. Cancer Res 62:6510–6518
Pettersson K, Svensson K, Mattsson R, Carlsson B, Ohlsson B, Berkenstam A (1996) Expression of a novel member of estrogen response element-binding nuclear receptors is restricted to the early stages of chorion formation during mouse embryogenesis. Mech Dev 54:211–223
Tremblay GB, Kunath T, Bergeron D, Lapointe L, Champigny C, Bader JA, Rossant J, Giguere V (2001) Diethylstilbestrol regulates trophoblast stem cell differentiation as a ligand of orphan nuclear receptor ERR beta. Genes Dev 15:833–838
Luo J, Sladek R, Bader JA, Matthyssen A, Rossant J, Giguere V (1997) Placental abnormalities in mouse embryos lacking the orphan nuclear receptor ERR-beta. Nature 388:778–782
Russo J, Hu YF, Yang X, Russo IH (2000) Developmental, cellular and molecular basis of human breast cancer. J Natl Cancer Inst Monogr 27:17–37
Chen S, Zhou D, Yang C, Sherman M (2001) Molecular basis for the constitutive activity of estrogen-related receptor alpha-1. J Biol Chem 276:28465–28470
Yang C, Chen S (1999) Two organochlorine pesticides, toxaphene and chlordane, are antagonists for estrogen-related receptor alpha-1 orphan receptor. Cancer Res 59:4519–4524
Papadimitriou CA, Markaki S, Siapkaras J, Vlachos G, Efstathiou E, Grimani I, Hamilos G, Zorzou M, Dimopoulos MA (2004) Hormonal therapy with letrozole for relapsed epithelial ovarian cancer long-term results of a phase II study. Oncology 66:112–117
Williams CJ, Simera I (2004) Tamoxifen for relapse of ovarian cancer. The Cochrane Library, 3rd edn. Wiley, Chichester
Acknowledgements
Pengming Sun was a scholarship holder of the Gottlieb–Daimler Benz Foundation (Serial No.17/05-03). This research was partly supported by the National Natural Science Foundation of China (Serial No. 30371477). We thank Prof. Jean Marc Vanacker for plasmids pSG-hERRα, pSG-mERRβ, pSG-mERRγ, and comments on the manuscript. We thank Dr. Eckardt Treuter for the GFP-reporter plasmid. We thank Dr. Michel R. Stallcup for the HA-tag-hERRγ plasmid. We thank Miss Cristina Pirvulescu for her excellent follow-up work. We also thank Dr. Wilko Weichert, Mrs. Elisabeth Glanz, and Mrs. Beate Pesche for their great support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sun, P., Sehouli, J., Denkert, C. et al. Expression of estrogen receptor-related receptors, a subfamily of orphan nuclear receptors, as new tumor biomarkers in ovarian cancer cells. J Mol Med 83, 457–467 (2005). https://doi.org/10.1007/s00109-005-0639-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-005-0639-3