Abstract
We present a review and discussion on the current state of the sciences and research on limb restoration, exploring two potential strategies: (1) regenerative capabilities from the amputated stump and (2) tissue engineering approaches to reconstruct a lost limb. Examining the possibility for a human amputated limb to be regenerated in a fashion similar to that of a salamander’s amputated limb, we discuss current scientific understanding of specific species with regenerative capabilities involving progression through a series of definable stages beginning with wound healing and culminating in the redifferentiation of a new limb. We then examine the regenerative capabilities of mammals, including humans and mice, and discuss the possibility and requirements for enhancing regenerative capabilities in complex tissues such as the finger or limb. The process for driving limb regeneration may parallel the events involved in heterotopic ossification, a condition in which bone cells develop from traumatized tissues or tissues surrounding a certain radius of the trauma. We discuss how discoveries uncovered in the regenerative processes could be used to help control unwanted bone tissue formation of amputated human limbs and thereby enable improved prosthetic fitting and minimized associated pain. Finally, we summarize the current state of tissue engineering capabilities and challenges in reconstructing the human limb.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Thanks to investments by the Department of Defense (DoD) and Department of Veterans Affairs (VA) to advance research, development, and clinical rehabilitation programs , the majority of wounded warriors with amputated limbs today lead active lifestyles that just two decades ago may not have been possible. Prior to the global war on terrorism , the majority of service-related amputations occurred mainly as the result of noncombat causes, including disease and vascular disorders, and involved older veterans [91]. Current DoD and VA investments in programs to support amputation research and care came about largely in response to recognizing that the injury patterns of service members wounded in more recent global conflicts against terrorism involved mostly young combat amputees with a desire for active lifestyles [31, 32, 56, 91, 110]. These young combat amputees desire and deserve every opportunity for functional recovery, which for some means returning to the battlefield [20, 54, 91, 123].
Some military medical investments have supported novel approaches to rehabilitating combat amputees at the Walter Reed Army Medical Center (WRAMC) Military Amputee Research Program (MARP) , initiated in 2004. The MARP closed in 2011, to be followed by the opening of the Military Advanced Training Center (MATC) at the Walter Reed National Military Medical Center (WRNMMC) . These programs have transformed amputee care from its traditional emphasis on healing and retraining basic living skills to a relatively more extensive program whose goal is to help wounded service members reclaim active and mobile lives. Through strategic partnership between MARP and the Telemedicine and Advanced Technology Research Center (TATRC) Advanced Prosthetics and Neural Engineering Research Portfolio (2004–2014), technological advancements were made possible to include motorized knees and ankles and advanced fitting that allow amputees to climb stairs, walk on uneven terrains, and in some cases even compete as Olympic athletes . Depending on patients’ injury severity scores, functional recovery, medical and physical exams, and job positions, combat amputees may also return to active duty. Though the precise numbers are difficult to pin down, a small percentage (13–16%) of combat amputees have returned to active duty, including a smaller number (57 as cited in [20] Washington Post article) have even returned to combat [8, 20, 21, 54, 106, 123].
In 2014, the Defense Advanced Research Projects Agency (DARPA) Revolutionizing Prosthetics Program , first established in 2006, produced an FDA-cleared, advanced neuro-controlled prosthetic arm that allows the user to control the arm by thought, much as if it were a native limb. Other recent significant prosthetic technical advances and improvements include interfaces that allow increased movement, mobility, and somatosensory feedback [112].
Despite dramatic technological advances, most amputees would naturally still prefer not to have lost their own natural limbs. A native limb provides a high degree of proprioception, rapid reflexes, flexible ability to walk on different terrains, energy efficiency when ambulating, dexterity to control fine finger motor movements, freedom from phantom limb pain [3, 53, 98], and the simple joy of touch. The preference to preserve these abilities is reflected in the decisions of upper limb amputees who opt for limb transplantations . Johns Hopkins surgeon Dr. W.P. Andy Lee has performed several upper limb transplants, including for military amputees, and notes that some lower limb amputees have also expressed a desire for lower limb transplants.
Much additional research and development must be explored to achieve the ideal of reestablishing natural limbs with fully preserved capabilities and without the risks currently associated with limb transplantation, which include tissue rejection, infection, and the need for immune-suppressive drugs. The next technological challenge is to accomplish limb regeneration through limb regrowth or tissue engineering. The remainder of this chapter will consider the current state of the art in recreating a lost limb, either through stump regeneration or via reattachment and reinnervation of a tissue-engineered limb.
Limb Regeneration : Past and Current Research
Limb regeneration in salamanders has been studied for centuries, but many consider it a biological exception rather than a phenomenon relevant to human limb regeneration . Newts and salamanders possess extraordinary regenerative capabilities that extend beyond limb regeneration, but these animals are generally small, aquatic or semiaquatic, and evolutionarily distant from mammals. However, the salamander limb is actually anatomically similar to the human arm, and there are large salamander species (e.g., Chinese giant salamanders grow to over 5 ft in length and weigh up to 145 lb) that retain regenerative capabilities. This suggests that the overall size of the limb does not represent an insurmountable obstacle for successful limb regeneration.
Regenerative ability has been thought an evolved trait and therefore phylogenetically specific [37]. This runs counter to a more classical view of limb regeneration as an ancestral property of all vertebrates, lost through evolution [99, 100]. If regenerative capability is an ancestral property, it may be possible to reawaken the trait in humans, as tooth formation was reawakened in birds after 75 million years of repression [16, 45]. The two views are not mutually exclusive, i.e., it is likely that regeneration is a primitive trait that has differentially evolved among specific vertebrate groups. Nevertheless, the ability to regenerate a limb in an adult vertebrate is restricted to certain organisms, so an understanding of the regeneration process can only come from studies on salamanders and newts.
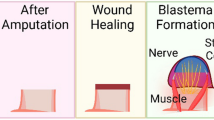
An initial and important consideration is to recognize that limb regeneration is a process , not a single event. Additionally, it is important to dismiss the misconception that the solution for human limb regeneration will be the discovery of a “magic potion” to miraculously stimulate limb regrowth. Limb regeneration involves a series of stages and steps, beginning with limb amputation and traversing through wound healing, dedifferentiation, blastema formation, growth, pattern formation, morphogenesis, and finally redifferentiation (Fig. 12.1).
Stages of limb regeneration in salamanders. Outer images represent staged drawings of newt limb regeneration. Stages include wound healing (WH), dedifferentiation (DD), early bud (EB), medium bud (MB), late bud (LB), palette (PAL), and early digit (ED) before redifferentiating into a replacement limb. Central image shows a blastema with distal mesenchyme, differentiated proximal tissue, and a gradation of differentiating tissues in between. (Modified from Iten and Bryant [48])
Key to this process is the formation of a transient structure, called a blastema , which is composed of an immature and specialized epidermis encasing a population of proliferating, undifferentiated cells. The blastema has characteristics similar to the limb bud that forms during embryogenesis [79, 80]; however, there are important differences. First, the limb bud emerges from the flank of the embryo and forms the entire limb structure, whereas the blastema forms at the level of limb amputation and only forms the limb structures that have been removed by amputation. This indicates that blastema cells need a mechanism to determine what portion of the limb structure survives the amputation injury, so the appropriate anatomy of the limb can be replaced. This characteristic of blastema cells is called positional information, an as yet poorly understood spatial map of the limb anatomy that is integral to defining what will regenerate and to establishing a functional interface with the mature tissues of the limb stump. The developing limb also needs a system of positional information to specify the various anatomical parts of the limb. Since the limb bud possesses regenerative abilities, evidence suggests a developmental map of positional information that must be amenable to the process of regeneration.
The concept of positional information lies at the heart of understanding regeneration, and the process of intercalation (see Fig. 12.2) is a unifying principle. It is generally accepted that cells must possess positional information that controls the differentiation of anatomically distinct tissues [117]. There is an abundance of indirect evidence implicating fibroblastic cells (of the interstitial tissues) as the cell type that possesses positional information [11, 15, 85, 97]. Since these cells do not differentiate into physiologically distinct parts of the body, they are proposed to act by directing cells such as myoblasts, chondroblasts, and osteoblasts to undergo spatially patterned differentiation responses. In this way, anatomically distinct components with similar physiological function can be established.
Intercalation . (a) Limb cells possess positional information that identifies a cell’s position relative to the limb as a whole. Positional information is depicted here as upper arm (A–D), forearm (E–H), wrist (I–J), digits (K–N). (b) During development, cells first specify the most proximal (A/B) and the most distal (M/N) information. Intermediate positions are then established by intercalation. c During limb regeneration, positional information of the stump (A/B) and the most distal tip (M/N) are reestablished after amputation; intervening information is then formed by intercalation. (d) An accessory limb can be ectopically induced from a simple skin wound; induction requires deviation of a transected nerve to the wound and a graft of skin from the opposite side of the limb. (e, f) Stages of accessory limb formation. (d–f Modified from Endo et al. [28])
As metaphor, it may be helpful to consider the global positional system (GPS) and how we use it to navigate the planet. Targeting positional information to a specific cell type that conveys spatial information to other cell types mimics the GPS, in that positioning is controlled based on interactions with a series of satellites which in turn convey spatial information to specific geographical landmarks. In biological terms, an example to consider is the proximal-distal patterning of the vertebrate limb. The limb initially forms a chondrogenic skeletal pattern that is later replaced by more permanent osteogenic cells. The proximal-distal skeletal pattern includes a single element in the upper limb (humerus, femur), paired elements in the lower limb (radius/ulna, fibula/tibia), and multiple small elements in the hand/foot region (carpels, tarsals, and phalangeal elements) (Fig. 12.2a). Prior to differentiation, the cells of the limb bud display spatially distinct patterns of gene expression, such that some genes are specifically expressed in cells associated with the distal limb region and other genes are specifically expressed in those cells associated with the proximal limb region [111]. Studies on the mouse limb bud provide evidence that the specification of the proximal and distal limb regions occurs early in development and is followed by the specification of the intervening (intermediate) limb regions [72] (Fig. 12.2b). It has long been hypothesized that positional interactions between limb regions, as exemplified by the mouse limb studies, control cell proliferation and the formation of intervening limb regions by a process called intercalation [34].
Studies on the regenerating salamander limb help to uncover the characteristics of positional information and intercalation. Using these processes, the amount of tissue that regenerates is always linked to the level of amputation, and the pattern of the regenerate is always normal. The mature tissues of the adult salamander limb are quiescent and do not respond to spatial cues required for regeneration [13]. For this reason, it is generally thought that positional information is reacquired as a response to amputation injury and, indeed, genes that are expressed in a position-specific manner during limb development are reexpressed in the blastema during limb regeneration. One critical finding was that very early in the regeneration process, genes associated with the level of limb amputation and genes associated with the distal limb region were reexpressed at the amputation wound [36]. This creates conditions at the amputation wound that are very similar to the early developing limb: both proximal and distal cells are present in the early blastema, and as regeneration proceeds, intervening limb regions between the distal tip and the stump are proposed to regenerate by intercalation (Fig. 12.2c). The process of intercalation is presented here in a simple one-dimensional format using the proximal-distal axis of the limb to help conceptualize limb patterning in both limb development and limb regeneration. Studies using a variety of regenerating models , including insects and planaria, illustrate how universal this concept is for regeneration [1, 7, 34].
A second major difference between limb development and regeneration following amputation injury is that the latter must undergo a healing response. In salamanders, this includes a rapid wound closure response, an inflammatory response, the histolysis of existing tissues around the wound, and the release of cells with stem-like characteristics that are recruited to form the blastema . There is clear evidence that wound closure and inflammatory response are necessary for the regeneration process [39, 102], but it is not yet clear whether the two responses involve independent mechanisms. A number of progenitor cell types that are released from stump tissues and participate in blastema formation are lineage restricted, which is to say they produce in the regenerate the same cell type that they formed in the limb prior to amputation [55]. These include epidermal cells, Schwann cells, and muscle progenitor cells. The involvement of muscle progenitor cells has been the focus of considerable attention and controversy in the limb regeneration field [60]. It is now clear that muscle tissues regenerate by activating stem cells called satellite cells in the newt but that in the axolotl (also called the Mexican salamander), mature myofibers undergo a dedifferentiation response that involves cellularization and fragmentation to generate individual progenitor myoblasts [76, 101]. These differences within the urodeles , an order of amphibians that include salamanders and newts, exemplify how regenerative strategies have evolved to facilitate a successful regenerative response.
In addition to the variety of lineage restricted progenitor cells that make up the blastema, there is evidence that multipotent fibroblastic cells of the interstitial connective tissue also contribute to the blastema . The connective tissue of the dermis has been studied most extensively. Tissue grafting studies show that cells of the dermis over-contribute to the blastema [83] and that these cells participate in regenerating other limb tissues (e.g., skeletal, tendons) as well as re-forming the dermis [27, 55]. Since there are a number of distinct cell types in dermal connective tissue , e.g., fibroblasts, vascular, and perivascular cells, it remains to be demonstrated which of these cell types are multipotent. The cells of the dermis have also been implicated in controlling the process of intercalation so they represent the best candidate cell type for expressing positional information during regeneration [109]. It is unclear whether there is direct, indirect, or no relationship between dermal over-contribution to the blastema, multipotency of dermal cells, and the role that the dermis plays in regulating positional information.
Unraveling the process of blastema formation is not a simple matter. In animals that regenerate, limb amputation is always followed by blastema formation which leads to growth and morphogenesis of the regenerated limb. Traditional experimental approaches involve modulation to inhibit limb regeneration, for example, denervation of the limb inhibits blastema formation and limb regeneration. This suggests that truncated nerves produce a factor or factors necessary for limb regeneration [105]. Experiments to characterize the “neurotrophic factor ” have identified a number of factors that can rescue part or all of the denervation effect [59, 70, 75, 77]. Attempts to enhance the regenerative response of non-regenerating limbs have generally been unsuccessful [33]. The major reason for this is that limb regeneration is a process and not an event, i.e., regeneration requires an ordered series of critical steps to be successful. The clearest example of this is observed by experiments in which regeneration is stimulated not by limb amputation but by modifying a lateral wound on a limb surface to stimulate an ectopic or accessory limb to form in a salamander [28].
Skin wounds on the salamander limb undergo a rapid and perfect healing response, but deviating an intact transected nerve to the wound site stimulates blastema formation. The induced blastema eventually regresses, and the limb is not structurally modified. However, combining nerve deviation with a graft of skin from the opposite side of the limb results in the production of a blastema that undergoes growth and morphogenesis to produce an ectopic limb (Fig. 12.2d–f). One role of the nerve is to stimulate the wound epidermis to transition into a functional apical epithelial cap (AEC), which is required to initiate blastema formation [102]. This represents an important step in the regeneration process; blastema formation alone is insufficient to stimulate limb regeneration. Beyond the transformation of the wound epidermis to the AEC to stimulate blastema formation, limb regeneration requires that the blastema itself must be composed of cells from disparate parts of the limb that presumably use positional information to organize the regeneration response. Thus, a secondary requirement is to introduce cells that are derived from the opposite side of the limb to create the necessary positional disparity. This stepwise model for limb regeneration establishes a foundation for considering how limb regrowth is controlled in regeneration-competent animals [28] and provides important insight into how regeneration can potentially be stimulated in regeneration-incompetent animals such as humans .
A third difference that sets the regenerating limb apart from the developing limb is that the regenerated structure must reestablish a functional interface with the mature tissues of the limb stump. There is experimental evidence that the process of limb regeneration is autonomous, i.e., explanting a regenerating blastema to an ectopic site results in the formation of an ectopic limb [107]. Indeed, accessory limb studies show very clearly that the induced regenerated limb need not have a skeletal interface with the host limb (Fig. 12.2f). This implies that a critical aspect of regenerating a limb involves an independent integrative process of melding a developing structure with the mature tissues of the injured limb. During limb regeneration, the stump/regenerate interface appears histologically as a graded transition. For example, in the central image in Fig. 12.1, a differentiated skeletal element can be seen at the base, transitioning to the differentiating cartilaginous template of the regenerating proximal skeleton at the base of the blastema to the still undifferentiated blastema of the distal regenerate. This process has been poorly studied but likely involves the activities of matrix-eroding cells , such as osteoclasts, as well as the production of proteolytic enzymes, such as matrix metalloproteinases that are known to be upregulated in association with the regenerative response [114, 119]. This process is also likely to be important for establishing a functional interface between mature tissues and newly regenerated structures using scaffolds and/or stem cells.
Are Humans Capable of Regeneration ?
There are several factors that limit the ability of human tissues and organs to respond to injury by regenerating amputated body parts such as arms and legs. Considerable speculation is necessary to address the question of how these limitations might be overcome. One way to think about human regeneration is in developmental terms. The mature human oocyte has developmental potential – i.e., the capacity to form the human body pattern – yet cannot do so in the absence of fertilization. If the oocyte is defective, then its full developmental potential is not realized even if fertilization does occur. We can use a similar analogy to conceptualize limb regeneration. The salamander limb possesses regenerative potential because, upon amputation, a sequence of specific events temporally and spatially coordinates the replacement of amputated structures. The question of whether humans are capable of regeneration can be rephrased to ask whether human cells possess developmental (regenerative) potential. This question is easier to address because we can evaluate amputation injury in the context of regenerative mechanisms and experimentally test if structural replacement can be enhanced. Recent regeneration studies focusing on a rodent digit amputation model provide convincing evidence that the regenerative potential of mammals is quite high and that humans may indeed be capable of regeneration [120, 121].
We start by outlining some conceptual boundaries for how mammalian regeneration studies might proceed. First, recent evolutionary studies suggest that specific molecules such as the cell surface three-finger protein Prod1 , which are critical for salamander limb regeneration, are unlikely to be present in mammals [37]. This does not mean the regenerating salamander limb model is not important; it simply raises caution that some of the molecular pathways for regeneration may be unique. Second, since the regeneration process will parallel the developmental process , we can focus on the similarities of the molecular pathways between regeneration and embryonic mammalian development. This is clearly the case for the process of differentiation during tissue turnover or replacement following injury, i.e., myogenesis during muscle repair parallels myogenesis in development [122], and the same is true for osteogenesis during bone healing [29]. Third, many studies show that relative to mammalian adult tissue, regenerative capability is enhanced in the mammalian embryo, fetus, and neonate ; this indicates that developing tissues possess an enhanced regenerative potential. In rodents, embryonic limb amputations in vitro elicit a partial regenerative response [14, 24], and in utero limb or digit amputations in mice result in clear regenerative responses [95, 116]. In humans, experiments have shown that amputated fetal digits initiate a regenerative response when maintained in long-term culture [2], and transverse limb defects in newborns are associated with small digit-like structures called “nubbins” that are hypothesized to represent a regenerative response to in utero injury [35]. Studies of skin in fetal wound healing demonstrate a scarless healing response, which in newborns transitions to scar-forming healing [62]. However, embryonic amputation studies indicate that the ability for scarless wound healing by itself is not sufficient for limb regeneration [116]. These studies point to the use of developmental models as one way to understand regenerative potential [81] and to tissue maturation as a key process that negatively impacts the ability for tissue regeneration. This process has spawned the concept that there are regeneration “barriers” that are progressively established as the limb matures [78].
Fingertip and Digit Tip Regeneration
The regeneration of fingertips in both children and adults is well documented in the clinical literature [25, 47, 65]. More recently, parallels between digit tip regeneration in mice and fingertip regeneration in humans have peaked interest in the feasibility of human limb regeneration [82]. In this regard, mouse digit regeneration has become an important experimental model to explore both the fundamental mechanisms of mammalian regeneration and potential strategies to enhance limb regenerative capabilities. The mouse digit tip regenerates following amputation from early development and through adulthood, although specific details such as regenerative rate do vary with age. Regeneration of fetal digits can also occur in vitro, making this model valuable for cell and molecular biological experimental studies. Genetic studies show that Msx1 , a transcriptional repressor important for embryonic cell differentiation and highly expressed in the forming digit tip, is required for successful fetal digit regeneration [43]. Other related transcription factors that are also expressed specifically in the developing digit tip, Msx2 and Dlx5, have been shown not to be required for regeneration [63]. The Msx1 mutant phenotype can be rescued during digit development in vitro by extrinsic treatment with BMP4, a growth factor in the bone morphogenetic protein family shown to be downstream of Msx1 activity. Both fetal and neonatal digit regeneration are inhibited by treatment with the BMP antagonist Noggin [43, 120], whereas the regeneration of normally non-regenerating digit amputations can be induced by treatment with BMP2 or BMP7 [120, 121]. Based on these studies, there has been considerable attention focused on the role of BMP signaling in both endogenous and induced regeneration (see discussion to follow).
Neonatal and adult digit tip regeneration involves the formation of a blastema [30, 44]. The mouse digit tip is structurally defined by the terminal or third phalangeal element (P3); this bone has the shape of a flattened cone with a basal bone marrow region and a pointed distal tip (Fig. 12.3a, b). The P3 element articulates proximally with the second phalangeal element (P2) forming the P2/P3 joint (Fig. 12.3b). The P3 element is unique because it is encased within the nail organ that has recently been shown to be required for the regenerative response [108]. Surrounding the P3 element and subjacent to the epidermal layer is a thin layer of loose connective tissue that consists of fibroblasts and vasculature. Digit tip amputation transects the length of the P3 element without exposing the marrow region; thus the amputation wound includes a central bony region surrounded by loose connective tissue with nail epidermis on the dorsal and lateral aspects and epidermis ventrally (Fig. 12.3c).
Digit tip regeneration. (a) External view of mouse digit tip with P3 skeletal element outlined; solid line indicates amputation plane. (b) Section of adult digit showing P3 skeletal element, bone marrow cavity (mc), connective tissue (ct), and nail organ (n). P3 element articulates proximally with P2 skeletal element. (c) Section of digit tip immediately after amputation involving bone (b), connective tissue (ct), and nail (n) but not marrow cavity (mc). (d) TRAP staining identifies osteoclasts (arrow) localized to the amputated stump. (e) Section of regenerating digit at 7 DPA showing epidermal migration (arrow) through eroded stump bone causing re-amputation and sloughing of amputated bone (b). (f) Micro-CT scan shows a re-amputated distal bone fragment (arrow) prior to being sloughed off. (g) Distal blastema (bl) and initiation of proximal skeletal differentiation (arrow) first evident by 12 DPA. (h) At 17 DPA, new trabecular bone (tb) regenerates proximally, while blastema (b) is present distally. (i, j) At 28 DPA, regenerated trabecular bone (tb) is histologically distinct from the stump bone (I) but anatomically similar (arrow) to the amputated structure (J). (k) Increased bone density of the regenerate at 128 DPA (a, c–k Modified from Fernando et al. [30])
Following amputation , the wound healing response is complicated because the wound epidermis does not heal over the amputated P3 element. Instead, there is an upregulation of osteoclasts that gradually erodes the distal bone (Fig. 12.3d), and the wound epidermis closes through the eroded bone (Fig. 12.3e). The timing of wound closure is quite variable, but results in the development of a secondary amputation plane and a distal bone fragment in the process of being discarded are often observed in micro-CT images of the regenerative response (Fig. 12.3f). This secondary amputation opens the bone marrow region to the digit tip amputation wound, and once wound closure is completed (7–10 days post-amputation), the blastema forms (Fig. 12.3g). The blastema is composed of proliferating mesenchymal cells, and cell marking studies show that cells are derived from multiple tissues of the digit stump [66, 96, 118]. Like amphibian limb regeneration, skeletal differentiation initiates in the proximal blastema and progresses distally until the complete digit tip is regenerated (Fig. 12.3h, i). The regenerated digit tip is structurally similar to the original; however, the regenerated bone forms rapidly and is trabecular rather than cortical bone (Fig. 12.3j). With time the regenerated bone becomes denser, but the trabecular nature of the bone is maintained long after the regeneration process is complete (Fig. 12.3k).
Transplantation of labeled hematopoietic stem cells and parabiosis studies show that circulating cells do not contribute to the major structural tissues of the regenerated digit [96]. Cell-specific lineage mapping studies show that a number of mesodermal and endodermal cell types are lineage restricted during digit tip regeneration. In these lineage studies, cell marking involving the induction of a cell type-specific label shows that Col2-expressing chondrocytes do not contribute to the regenerate, whereas Sp7-expressing osteoblasts and VE-cadherin-expressing endothelial cells contribute to the regenerate and are lineage restricted [66, 96]. The use of promoter-specific Cre expression to track cell lineage has also been useful in determining whether specific cell types change phenotype during regeneration. Unfortunately, it is impossible to determine whether cells of the regenerate are specifically derived from labeled stump cells or from other labeled cells. Cell labeling studies have excluded Sox9-expressing skeletal cells, Scx-expressing tendon cells, and Tie2-expressing endothelial cells, as cell types that do not undergo transdifferentiation during regeneration [96].
Epidermal cells are lineage restricted and play a critical role in the regeneration response. The major epidermal structure of the digit tip is the nail organ , which is comprised of the proximal nail matrix, the distal nail bed, and the overlying differentiated nail plate. Nail stem cells are localized in the nail matrix and give rise to proximal-distal columns of cells that extend into the nail bed and differentiate into the continuously elongating nail plate. The importance of the nail in regeneration is highlighted in a recent study showing that nail stem cell differentiation is Wnt dependent and that disrupting the canonical Wnt signaling pathway in the epidermis not only inhibits nail growth but also inhibits the skeletal regenerative response [108]. Additionally, gain-of-function studies show that activation of canonical Wnt signaling in the epidermis of proximal (non-regenerating) P3 amputations induces nail and skeletal regeneration. Since the epidermis is well known to be essential for amphibian limb regeneration, perhaps it is not surprising that mammalian regeneration is also dependent on the epidermis. It does foster confidence that parallel strategies for regeneration have been maintained between evolutionarily diverse species. Together, these cell lineage studies identify the epidermis as essential for a regenerative response and provide evidence that the regeneration blastema is composed of a heterogeneous population of multiple, lineage-restricted progenitor cell types.
BMP signaling has also been identified as a signaling pathway important for digit regeneration [120, 121]. Multiple BMPs and their receptors are prominently expressed during blastema formation, and digit tip regeneration is inhibited by treatment with the BMP antagonist, Noggin. The neonatal mouse digit has been a useful model for studying induced regenerative responses. Amputation through the P2 element is proximal to the nail organ and never elicits a regenerative response, rather always forming a truncated skeletal stump (Fig. 12.4a, b). The application of a microcarrier bead containing BMP2 induces a consistent regenerative response that involves the regrowth of the P2 skeletal element and marrow region (Fig. 12.4c, d). Like the P3 regenerative response, the newly formed bone is trabecular and smoothly integrated with the stump bone. Critical aspects of this induced regenerative response include the placement of a transient BMP2 source, and the timing of the treatment must be coincident with the completion of wound closure. This suggests that the dynamics of wound closure create conditions in which the healing response can transition to a regenerative response by modifying the microenvironment of the amputation wound.
BMP2-induced endochondral ossification center . (a) Diagram shows amputation level (blue line) and positioning of BMP2 bead when wound closure is complete 4 days after amputation. (b) Control digits show no regeneration with treatment with a BSA bead (*). (c) Digits treated with a BMP2 bead (*) show skeletal regeneration. (d) Radiographic section showing regenerated trabecular bone (tb) and skeletal irregularities (double arrow) identifying the amputation level. (e) EdU incorporation (arrow) in the BRE-Gfp transgenic reporter mouse shows that BMP2 acts as a mitogen. (f) Co-immunostaining shows that BMP2 induces endothelial cell (red) expression of the chemoattractant SDF-1α (green). (g) Section showing mesenchymal cell accumulation following BMP2 treatment (*). (h–j) In situ hybridization showing Col2a1 (arrow in h and i) and Col10a1 transcripts (arrow in j) are induced in sequence by BMP2. (k) Regenerating endochondral ossification center contains proliferating chondrocytes (PC) (arrows) apically and a quiescent proximal zone of hypertrophic chondrocytes (HC) contiguous with the stump (S). (a–e, g–k Modified from Yu et al. [121]; f Modified from Lee et al. [64])
What is the nature of the BMP2 response? The most immediate effect of BMP2 is the transient upregulation of Msx1 and Pedf, two genes that are associated with the endogenous P3 regenerative response. The role of PEDF in regeneration has not been characterized; however it is known to antagonize angiogenesis, and the digit blastema is avascular [30]. In addition, within 24 h of BMP2 treatment, there is enhanced proliferation of cells that are directly responsive to the canonical BMP signaling pathway (Fig. 12.4e). Within 48 h of BMP2 treatment at the amputation site, endothelial cells begin to express the chemokine SDF-1, which acts as a chemoattractant to recruit CXCR4-positive mesenchymal cells to the wound [64] (Fig. 12.4f). By 3 days post-BMP2 treatment, a zone of proliferating mesenchymal cells distinguishes the BMP2-treated amputation from non-regenerating controls (Fig. 12.4g). At this same time, a population of Col2a1-expressing chondrocytes initiate differentiation at the distal end of the stump. Hypertrophic chondrocytes are not present at 3 days post-BMP2 treatment. By day 5, however, the Col2a1-expressing chondrocytes begin differentiating into Col10a1-expressing hypertrophic chondrocytes in the proximal region of the regenerate (Fig. 12.4h, i). By 7 days post-BMP2 treatment, there is a distally localized zone of proliferating chondrocytes and a proximal zone of hypertrophic chondrocytes that establish an interface with the stump bone (Fig. 12.4j). In addition, osteoblasts make their first appearance at 7 days post-BMP2 treatment and initiate osteogenesis within the zone of hypertrophic chondrocytes. This BMP2-induced regenerative response only involves the regeneration of new bone tissue, and there is no evidence for the differentiation of chondrogenic tissues of the P2/P3 joint. What is novel about this response is the de novo formation of an ectopic endochondral ossification center [121]. Endochondral ossification typically occurs between two growing skeletal elements and is the mechanism for bone elongation during maturation. By understanding how to control the formation of new endochondral ossification centers, it may be possible to stimulate patterned bone regeneration from any amputated stump.
While BMP signaling is well known to play an important role in bone regrowth, studies using the digit regeneration model indicate that the cells at the amputation wound site respond to BMP2 in a position-specific manner. It is important to remember that limb regeneration requires that cells at the site of injury retain or reacquire spatial information concerning the level of amputation . Since the mammalian regenerative response is restricted to the digit tip, it has been impossible to determine whether a system of positional information is present and required for successful regeneration. BMP2 has been shown to induce a regenerative response from two distinct digit levels, and the anatomy of the responses is also distinct. Detailed analyses of the two responses show that BMP2 stimulates cells at two amputation levels to regenerate in a position-specific manner (Fig. 12.5). BMP2-induced digit regeneration, whether from a proximal P3 amputation or a mid-P2 amputation, establishes an endochondral ossification center that mediates skeletal regeneration. What is distinct between the two amputations is the proximal-distal polarity of the endochondral ossification centers. The regenerated endochondral ossification center displays an inherent polarity that can be identified based on the relationship of the proliferating and hypertrophic chondrocyte populations.
Patterning of BMP2-induced regeneration is level dependent . Distal is toward the top in all images. Microcarrrier beads are indicated by *. (a–d) Section in situ hybridization of induced regeneration from proximal P3 amputation (a, b) and P2 amputation (c, d) shows that BMP2 induces endochondral ossification centers of opposite polarity indicated by the expression domains of Col2a1 (a, c) and Co10a1 (b, d). (e) Diagrammatic summary displaying shift in polarity of the endochondral ossification centers induced by BMP2 (blue bead) from two different amputation levels (black arrows). (Reprinted from in Yu et al. [121])
During development of the distal end of P2 element, proliferating chondrocytes are distal relative to the hypertrophic chondrocytes, whereas the P3 element forms with only a proximal growth plate in which the proliferating chondrocytes are proximal to the hypertrophic chondrocytes (Fig. 12.5e). Thus, the developmental polarity of endochondral differentiation at the P2/P3 articulation is reversed. Similarly, BMP2-induced P3 regeneration forms an endochondral ossification center with proliferating chondrocytes proximal to the hypertrophic chondrocytes (Fig. 12.5a, b), whereas the BMP2 response to P2 level amputations results in an endochondral ossification center with proliferating chondrocytes distal to hypertrophic chondrocytes (Fig. 12.5c, d). These studies show that in response to the same inductive signal, cells of the P2 and P3 amputation wounds respond in a position-dependent manner to regenerate the appropriate skeletal structures. This finding establishes for the first time that a system of positional information is present in the mammalian digit and that this system is used to organize and induce regenerative responses.
The digit represents an excellent model to explore fundamental aspects of mammalian regeneration and to discover ways to enhance the regenerative response . However, by comparison to the limb, the digit is small and far less complex. For example, digits lack muscle tissue, and the amount of regenerated tissues that require reinnervation and revascularization is much smaller. To explore whether BMP2 treatment can enhance regenerative responses in adult limbs, the neonatal digit regeneration studies were used as a guide. Amputation of the hind limb shank at a level proximal to the fusion of the tibula and fibula was selected for studies, so that the distal fusion of these two skeletal elements could be used as a patterning marker for regeneration (Fig. 12.6a). Using a protocol that was appropriately modified for an adult limb amputation (i.e., proportionally enhanced dose, modification of the delivery vehicle, modification of treatment timing, etc.), a single treatment of the limb amputation wound with BMP2 was found to be effective in eliciting a patterned skeletal regenerative response in adult mice [121]. Control amputations treated with the vehicle failed to elicit a regenerative response. The BMP2 - treated amputations regenerated significantly more bone that involved bone lengthening as well as fusion of the tibia and fibula (Fig. 12.6b, c). Like the digit model, this regenerative response only involved the formation of new bone tissue, and the induced response was incomplete. Nevertheless, the data clearly show that adult limb regenerative capacity can be enhanced by the spatiotemporal targeting of BMP2 administration. This offers a proof of concept that the general strategy of tapping into unrealized regenerative potential can provide a path for future therapies in regenerative medicine.
Regeneration response to Bmp2 after adult limb amputation . Distal is toward the bottom of all images. (a) μCT image showing skeleton of the mouse hind limb shank (consisting of the tibia (t) and fibula (f) that fuse distally), and the level of amputation (arrow). Simple amputation was made through the mid-shaft of the shank to transect both tibia and fibula proximal to the point of fusion. (b, c) μCT scans of a BSA control (b) and a BMP2-treated limb (c) at 1, 3, and 8 weeks post-amputation (WPA). BMP2-treated amputations displayed organized distal bone growth resulting in skeletal elongation and distal bone fusion indicative of a properly patterned regenerative response. (Modified from Yu et al. [121])
Regeneration and Pathology
With an enhanced understanding of the regenerative potential following traumatic injury , it is instructive to reevaluate pathological conditions involving tissue overgrowth. There is growing concern about the number of modern war zone amputations displaying heterotopic ossification (HO) associated with the healing response. HO refers to the atypical formation of bone in soft tissue, joints, and muscle, which presents a significant obstacle to rehabilitation and the fitting of prosthetic devices. While HO occurs infrequently in civilian amputee populations (11%), the prevalence of HO is approximately 63% in combat-related amputations. This difference has been attributed to modern ballistics designed to maximize gross foreign contamination by the inclusion of nontraditional projectiles that maximize infection by microorganisms [26, 93, 94]. The formation of heterotopic bone is painful, creates problems with prosthetic devices, and can require multiple surgical revisions to remove the excessive bone. HO presents a major obstacle to the rehabilitation of previously healthy soldiers to high levels of activity [92]. Although HO is a significant pathological problem in combat orthopedics [19], the regeneration biologist can be encouraged by the fact that new bone is forming at the amputation wound, albeit in an inappropriate fashion. It suggests that the body is responding to the injury by attempting to regenerate, even if the conditions at the wound do not support a functional response. This represents an opportunity to use our understanding of mammalian regeneration to guide the body’s response toward controlled ossification that can be functionally integrated with the bone stump.
Heterotopic bone formation is attributed to a number of distinct healing responses including inflammation, vascularization, and an ectopic BMP signaling source that initiates endochondral ossification [67, 94]. Chondrocytes initially form a chondrogenic template that is subsequently invaded by the vasculature and osteoprogenitor cells that form the new bone. Our understanding of HO is enhanced from research on the genetic disorders fibrodysplasia ossificans progressiva (FOP) [51] and progressive osseous heteroplasia [50], both of which present with heterotopic bone formation. Studies on patient-derived cell lines suggest that overexpression of both BMP4 and BMPR1A , as well as underexpression of BMP antagonists, is required for HO development [23, 103]. There is evidence that a number of different cell types can be recruited and act as progenitors for HO, including adipocytes, mesenchymal stem cells, and perivascular cells [67, 86, 90]. The pathology of HO has clear parallels to the BMP-induced regenerative response described above, with the BMP signaling cascade acting as an initiating center that stimulates proliferation, recruitment, and chondrogenesis to establish an endochondral ossification center. There is also a temporal component to the regenerative response in that BMP2 treatment prior to wound closure or late in the wound healing process induces ectopic bone formation rather than regeneration (Yu and Muneoka unpublished data). Thus, there is a clear relationship between the spatiotemporal positioning of a BMP source and whether or not a regenerative response is induced. It is significant that the non-regenerative BMP2-induced response is the formation of ectopic bone similar to the pathological condition of heterotopic bone. Obvious parallels between this model of regenerated/ectopic bone and the pathology of HO suggest that one approach to controlling HO is to focus on the BMP signaling pathway during wound healing. Since HO initiates by forming an endochondral ossification center, then one obvious therapeutic approach would be to inhibit this process by modulating the recruitment, proliferation, or differentiation of chondroblast progenitor cells. Alternatively, it may be possible to manipulate the presentation of HO to engineer a functional ossification response and control skeletal regeneration of the amputated bone stump. In this way, broadening our understanding of regenerative potential in mammals and humans in particular has dual benefits. On the one hand, this understanding can be applied to treating specific pathological conditions such as HO. On the other hand, the pathological response to traumatic injury can be instructive for gaining insight into human regenerative potential.
Limb Regeneration Through Tissue Engineering Strategies
In this section, we explore the concept of tissue engineering, including regenerative medicine-based strategies and bioprinting technology currently being explored for the development of replacement tissues and organs (see also [71]). Tissue engineering involves the use of living cells and other materials living and/or nonliving to form a scaffold structure that can support tissue formation. Since tissue engineering was first conceived in 1987, its potential has been advanced by more recent developments and significant achievements in regenerative medicine, including autologous engineered bladder constructs for cystoplasty [4], tissue-engineered cartilage for knee repair [46], and tissue-engineered airway for replacement and transplantation [69]. Some tissue-engineered products have been implanted into patients with favorable outcomes [40, 42, 89]. Given these accomplishments, it seems possible that the same principles of tissue engineering and regenerative medicine could be applied to reconstruct other human body parts such as digits and limbs. Certainly we recognize that the tissues successfully engineered to date are significantly less complex than entire human extremities characterized by multiple tissue types and layers, intricate micro-architectural structures, elaborate microvasculature, and an integrated peripheral nervous system. Formidable challenges must be overcome to make possible in practice what we can, for now, imagine only in principle. Here we provide an overview of potential near-term strategies and applications, with reference to supporting literature. We encourage the interested reader to learn more from other more comprehensive works in the field of tissue engineering (e.g., [61, 115]).
We begin with a focus on the current possibility of using tissue engineering and regenerative medicine strategies to reconstruct functional tissue of the amputated limb itself, as may be useful to improve prosthetic socket fit, regenerate stronger bone and muscle tissues for osseointegration , or implant tissue-engineered constructs to strengthen peripheral nerve interfaces for electromyogram (EMG ) control of a prosthetic device. Where there is insufficient tissue mass or functional tissue to don and control a prosthetic device, for example, tissue engineering could be applied to create a larger tissue mass. Direct implantation of an experimental acellular biologic scaffold material that received an investigational device exemption (IDE) from the US Food and Drug Administration (FDA) has been clinically tested for the treatment of volumetric muscle loss in a small number of patients with varying success to increase strength and function [38, 73, 104]; however, a different research group has tried to duplicate the study in small preclinical animal model but failed to observe notable functional improvements in the treated animals [5]. Also in development is an in vitro tissue-engineered muscle repair construct combined with bladder acellular matrices for treating volumetric muscle loss injury; this approach was evaluated in a small preclinical animal model and shown to improve functional outcome compared to the untreated leg of a control animal [18]. Scaffold-free approaches have also been considered [113]. These strategies could be applied to address lost muscle volume and to strengthen the surrounding muscle tissue of an amputated limb for improving fit and control of the prosthetic device along with a prescriptive rehabilitation program [38].
Recent papers offer comparison of tissue engineering and regenerative medicine strategies for regeneration of volumetric muscle loss and identify key challenges [41, 74]. TATRC has also invested and explored development of tissue-engineered muscle constructs as biomimetic peripheral nerve interfaces to act as a signal amplifier for improving the control of an EMG-based prosthetic device . For upper limb amputees who lack sufficient musculature to control motorized neural prosthetics, this approach could offer an alternative to surgical targeted reinnervation without having to sacrifice healthy tissues [57, 58].
In theory, tissue engineering and regenerative medicine-based strategies could be applied together to engineer tissue constructs for the replacement of a human limb , in whole or in part. A scaffold would first be needed to create the desired tissue architecture. Scaffolds can be engineered to provide geometry, porosity, mechanical compliance, and microstructure similar to the tissues or organs needed. Cells are then placed on the scaffold to form the desired tissues. This can be done using a variety of cell sources and types, including autologous cells, stem cells, or induced pluripotent cells (iPS). Biologically active molecules such as growth factors may be added to encourage neovascularization . Formation of the tissue and its microstructures occurs largely through self-assembly; a combination of forces including diffusion and intramolecular forces drives the placement of cells and biologically active molecules on the scaffold. Uniform cell distribution may not always be achieved; placement and orientation of the cells may not result as desired. Surfaces of the scaffold may need to be engineered to provide directional guidance for the cells to align in desired configuration.
More recently, bioprinting has been explored as a technique to create tissue structures and organs [12, 49, 84]. Bioprinting enables creation of complex, three-dimensional tissue-engineered structures by dispensing live cells (in liquid or gel form) in a specific programmed pattern without the need for a three-dimensional scaffold [87]. Bioprinters work by depositing one or several cells at a time through the nozzle of each print head, in the same way an inkjet printer dispenses ink onto a piece of paper. This cell printing action can generate one layer of cells at a time using one or multiple print heads and cartridges. By printing multiple tissue layers, one over the next, the resulting multiple ultrathin layers of living cells produce a three-dimensional cellular object such as a living tissue. Printing can be programmed and designed to dispense multiple ultrathin layers composed of one or more types of cells and to include other biological factors or biocompatible components (e.g., growth factors and nutrients) in various patterns to create specific micro-architectural details and cell orientations. Thus, placement of cells through bioprinting is more precise than by tissue engineering. When combined with three-dimensional design software (e.g., computer-assisted design, commonly known as CAD) that translates medical images of an organ or other body part, a personalized tissue structure can be replicated, including the intricate micro-architectural details of replacement tissue or an organ capillary network. Advantages of this approach include greater personalization, improved precision, and the capability to more closely mimic natural tissue structure.
Although bioprinting offers distinct capabilities and flexibilities for design and creation of living tissue structures, this technology also faces the same formidable engineering challenges associated with traditional tissue engineering. These challenges include (1) the need to create a vast, complex microvascular system to transport nutrients through the tissues and to the cells, export cellular debris, and exchange gases to keep engineered tissues viable; (2) technical difficulties associated with culturing neurons, which are required to engineer nerve pathways; and (3) the need to coax innervations from the host system to enable implanted muscle tissue to function. In addition, it is an enormous hurdle to integrate multiple and different tissue types and structures such that when combined, they can function together as a whole to include muscle tissue innervation and neurosensory pathway regeneration. Here we will focus on the essential challenge to engineer a vast microvascular network that is capable of sustaining viable tissue-engineered constructs.
Researchers working in tissue engineering and bioprinting have explored ways to create a microvascular networks. Tissue engineering strategies have evolved beyond the inclusion and controlled delivery of active biological molecules, such as angiogenic growth factors, to activate development of neovascularization networks [88]. Combination approaches now include novel scaffold design with incorporation of a perfusion system [10] and use of protein or cell therapy with endothelial and endothelial progenitor cells [17]. Nonetheless, the ability to engineer a sophisticated microvascular network that can reach every cell and tissue layer remains an elusive achievement [6, 52]. Advances in bioprinting technologies now provide the capability to create a microvascular system that can mimic original tissue through the use of biodegradable hydrogel or biopolymer to create the channel space [9]. Channels are lined by dispensing and printing the appropriate cells [22] to mimic microvascular system properties (e.g., network, flexibility, pathways, diameters, etc.). However, this approach is a slow process. Depending on channel diameter size and network complexity, the bioprinting process could take several days or weeks; it is driven largely by the biology and time scale of cell and tissue development. Delay is problematic because in the absence of a finished and functional microvascular network, earlier layers of printed cells may lose viability. Without a microvascular network in place, nutrient supply is driven mainly by diffusion, which is a slow delivery process that approaches a zero gradient once a critical distance is reached. Thus, the layers of cells and tissues that are located farthest from oxygen and nutrient sources will die. To date, it has not been possible to construct a microvascular system beyond a few millimeters in thickness [68]. This is a significant limiting factor for the engineering of large and complex human organs and tissue structures that are multiple layers of tissues thick. Still, hope remains that a functional engineered limb may one day be realized.
Summary
Humans have an innate ability to heal following traumatic injury, but we have no innate ability to regenerate critical parts of ourselves lost to injury or disease. We have discovered methods to clone complete animals’ single adult cells, and we have established the sequence of our entire genome. We can defy aging by reprogramming an adult cell back to its embryonic state. These are enormous achievements, to be sure. More profound still would be the ability to regenerate lost or injured arms, legs, spinal cords, hearts, jaws, eyes, and other organs and structures. This objective is not yet easily within reach, but it is well within the realm of the possible. It is no longer merely the stuff of science fiction.
Recent success demonstrating induced regenerative responses in adult mammals [121] provides proof of concept to validate a vision of human regenerative potential. Commitment to the vision and additional research will be necessary to advance medical science and technology in support. Tissue engineering and bioprinting strategies may make it possible eventually to engineer, modify, and/or replace lost limbs with functionally equivalent extremities. Artificially regenerated and tissue-engineered limbs are now at least conceivable in theory. In practice, engineering challenges must be overcome through continued advancement of scientific discovery and technology development.
References
Agata K, Tanaka T, Kobayashi C, Kato K, Saitoh Y. Intercalary regeneration in planarians. Dev Dyn. 2003;226:308–16.
Allan CH, Fleckman P, Fernandes RJ, Hager B, James J, Wisecarver Z, Satterstrom FK, Gutierrez A, Norman A, Pirrone A, et al. Tissue response and Msx1 expression after human fetal digit tip amputation in vitro. Wound Repair Regen. 2006;14:398–404.
Alphonso AL, Monson BT, Zeher MJ, Armiger RS, Weeks SR, Burck JM, Moran C, Davoodie R, Loeb G, Pasquina PF, Tsao JW. Use of a virtual integrated environment in prosthetic limb development and phantom limb pain. Stud Health Technol Inform. 2012;181:305–9.
Atala A, Bauer SB, Soker S, Yoo JJ, Retik AB. Tissue-engineered autologous bladders for patients needing cystoplasty. Lancet. 2006;367:1241–6.
Aurora A, Roe JL, Corona BT, Walters TJ. An acellular biologic scaffold does not regenerate appreciable de novo muscle tissue in rat models of volumetric muscle loss injury. Biomaterials. 2015;67:393–407.
Baiguera S, Ribatti D. Endothelialization approaches for viable engineered tissues. Angiogenesis. 2013;16:1–14.
Bando T, Mito T, Maeda Y, Nakamura T, Ito F, Watanabe T, Ohuchi H, Noji S. Regulation of leg size and shape by the Dachsous/Fat signalling pathway during regeneration. Development. 2009;136:2235–45.
Belisle JG, Wenke JC, Krueger CA. Return-to-duty rates among US military combat-related amputees in the global war on terror: job description matters. J Trauma Acute Care Surg. 2013;75:279–86.
Bertassoni LE, Cecconi M, Manoharan V, Nikkhah M, Hjortnaes J, Cristino AL, Barabaschi G, Demarchi D, Dokmeci MR, Yang Y, Khademhosseini A. Hydrogel bioprinted microchannel networks for vascularization of tissue engineering constructs. Lab Chip. 2014;14:2202–11.
Bettahalli NM, Vicente J, Moroni L, Higuera GA, van Blitterswijk CA, Wessling M, Stamatialis DF. Integration of hollow fiber membranes improves nutrient supply in three-dimensional tissue constructs. Acta Biomater. 2011;7:3312–24.
Bryant SV, Endo T, Gardiner DM. Vertebrate limb regeneration and the origin of limb stem cells. Int J Dev Biol. 2002;46:887–96.
Campbell PG, Weiss LE. Tissue engineering with the aid of inkjet printers. Expert Opin Bio Ther. 2007;7:1123–7.
Carlson BM. Morphogenetic interactions between rotated skin cuffs and underlying stump tissues in regenerating axolotl forelimbs. Dev Biol. 1974;39:263–85.
Chan WY, Lee KK, Tam PP. Regenerative capacity of forelimb buds after amputation in mouse embryos at the early-organogenesis stage. J Exp Zool. 1991;260:74–83.
Chang HY, Chi JT, Dudoit S, Bondre C, van de Rijn M, Botstein D, Brown PO. Diversity, topographic differentiation, and positional memory in human fibroblasts. Proc Natl Acad Sci U S A. 2002;99:12877–82.
Chen Y, Zhang Y, Jiang TX, Barlow AJ, St Amand TR, Hu Y, Heaney S, Francis-West P, Chuong CM, Maas R. Conservation of early odontogenic signaling pathways in Aves. Proc Natl Acad Sci U S A. 2000;97:10044–9.
Chung J, Shum-Tim D. Neovascularization in tissue engineering. Cell. 2012;1:1246–60.
Corona BT, Ward CL, Baker HB, Walters TJ, Christ GJ. Implantation of in vitro tissue engineered muscle repair constructs and bladder acellular matrices partially restore in vivo skeletal muscle function in a rat model of volumetric muscle loss injury. Tissue Eng Part A. 2014;20:705–15.
Covey DC. Combat orthopaedics: a view from the trenches. J Am Acad Orthop Surg. 2006;14:S10–7.
Craig T. Soldier who lost leg in Afghanistan vowed ‘I will return.’ This is what it took to get back [Internet]. The Washington Post; 2014. Available from: https://www.washingtonpost.com/world/soldier-who-lost-leg-in-afghanistan-vowed-i-will-return-this-is-what-it-took-to-get-back/2014/05/01/36af6e3c-b3ae-11e3-8cb6-284052554d74_story.html/.
Cronk TM. Power prosthetics propel service members to better lives [Internet]. American Forces Press Service; 2012. Available from: http://archive.defense.gov/news/newsarticle.aspx?id=116120/.
Cui X, Boland T. Human microvasculature fabrication using thermal inkjet printing technology. Biomaterials. 2009;30:6221–7.
de la Pena LS, Billings PC, Fiori JL, Ahn J, Kaplan FS, Shore EM. Fibrodysplasia ossificans progressiva (FOP), a disorder of ectopic osteogenesis, misregulates cell surface expression and trafficking of BMPRIA. J Bone Miner Res. 2005;20:1168–76.
Deuchar E. Regeneration of amputated limb-buds in early rat embryos. J Embryol Exp Morpholog. 1976;35:345–54.
Douglas BS. Conservative management of guillotine amputation of the finger in children. Aust Paediatr J. 1972;8:86–9.
Dudek NL, DeHaan MN, Marks MB. Bone overgrowth in the adult traumatic amputee. Am J Phys Med Rehabil. 2003;82:897–900.
Dunis DA, Namenwirth M. The role of grafted skin in the regeneration of x-irradiated axolotl limbs. Dev Biol. 1977;56:97–109.
Endo T, Bryant SV, Gardiner DM. A stepwise model system for limb regeneration. Dev Biol. 2004;270:135–45.
Ferguson C, Alpern E, Miclau T, Helms JA. Does adult fracture repair recapitulate embryonic skeletal formation? Mech Dev. 1999;87:57–66.
Fernando WA, Leininger E, Simkin J, Li N, Malcom CA, Sathyamoorthi S, Han M, Muneoka K. Wound healing and blastema formation in regenerating digit tips of adult mice. Dev Biol. 2011;350:301–10.
Fisher H.. A guide to US military casualty statistics: Operation Freedom’s Sentinel, Operation Inherent Resolve, Operation New Dawn, Operation Iraqi Freedom, and Operation Enduring Freedom [Internet]. Washington: Congressional Research Service Report for Congress; 2015. Available from: https://www.fas.org/sgp/crs/natsec/RS22452.pdf/.
Fisher H. US military casualty statistics: Operation New Dawn, Operation Iraqi Freedom, and Operation Enduring Freedom [Internet]. Washington: Congressional Research Service Report for Congress; 2013. Available from: http://journalistsresource.org/wp-content/uploads/2013/02/RS22452.pdf/.
Fleming MW, Tassava RA. Preamputation and postamputation histology of the neonatal opossum hindlimb: implications for regeneration experiments. J Exp Zool. 1981;215:143–9.
French V, Bryant PJ, Bryant SV. Pattern regulation in epimorphic fields. Science. 1976;193:969–81.
Gardiner DM, Holmes LB. Hypothesis: terminal transverse limb defects with “nubbins” represent a regenerative process during limb development in human fetuses. Birth Defects Res A Clin Mol Teratol. 2012;94:129–33.
Gardiner DM, Blumberg B, Komine Y, Bryant SV. Regulation of HoxA expression in developing and regenerating axolotl limbs. Development. 1995;121:1731–41.
Garza-Garcia AA, Driscoll PC, Brockes JP. Evidence for the local evolution of mechanisms underlying limb regeneration in salamanders. Integr Comp Biol. 2010;50:528–35.
Gentile NE, Stearns KM, Brown EH, Rubin JP, Boninger ML, Dearth CL, Ambrosio F, Badylak SF. Targeted rehabilitation after extracellular matrix scaffold transplantation for the treatment of volumetric muscle loss. Am J Phys Med Rehabil. 2014;93:S79–87.
Godwin JW, Pinto AR, Rosenthal NA. Macrophages are required for adult salamander limb regeneration. Proc Natl Acad Sci U S A. 2013;110:9415–20.
Gonflotti A, Janus MO, Barale D, Baiguera S, Comin C, Lavorini F, Fontana G, Sibila O, Rombola G, Jungebluth P, Macchiarini P. The first tissue-engineered airway transplantation: 5-year follow-up results. Lancet. 2014;383:238–44.
Grasman JM, Zayas MJ, Page RL, Pins GD. Biomimetic scaffolds for regeneration of volumetric muscle loss in skeletal muscle injuries. Acta Biomater. 2015;25:2–15.
Hamilton NJ, Kanani M, Roebuck DJ, Hewitt RJ, Cetto R, Culme-Seymour EJ, Toll E, Bates AJ, Comerford AP, McLaren CA, Butler CR, Crowley C, McIntyre D, Sebire NJ, Janes SM, O’Callaghan C, Mason C, De Coppi P, Lowdell MW, Elliott MJ, Birchall MA. Tissue-engineered tracheal replacement in a child: a 4-year follow-up study. Am J Transplant. 2015;15:2750–7.
Han M, Yang X, Farrington JE, Muneoka K. Digit regeneration is regulated by Msx1 and BMP4 in fetal mice. Development. 2003;130:5123–32.
Han M, Yang X, Lee J, Allan CH, Muneoka K. Development and regeneration of the neonatal digit tip in mice. Dev Biol. 2008;315:125–35.
Harris MP, Hasso SM, Ferguson MW, Fallon JF. The development of archosaurian first-generation teeth in a chicken mutant. Curr Biol. 2006;16:371–7.
Hollander AP, Dickinson SC, Sims TJ, Brun P, Cortivo R, Kon E, Marcacci M, Zanasi S, Borrione A, De Luca C, Pavesio A, Soranzo C, Abatangelo G. Maturation of tissue engineered cartilage implanted in injured and osteoarthritic human knees. Tissue Eng. 2006;12:1787–98.
Illingworth CM. Trapped fingers and amputated finger tips in children. J Pediatr Surg. 1974;9:853–8.
Iten LE, Bryant SV. Forelimb regeneration from different levels of amputation in the newt, Notophthalmus virisdescens: length, rate, and stages. Wilhelm Roux’ Archiv. 1973;173:263–82.
Jakab K, Norotte C, Marga F, Murphy K, Vunjak-Novakovic G, Forgacs G. Tissue engineering by self-assembly and bioprinting of living cells. Biofabrication. 2010;2:022001. Epub 2010 Jun 2.
Kaplan FS, Shore EM. Progressive osseous heteroplasia. J Bone Miner Res. 2000;15:2084–94.
Kaplan FS, McCluskey W, Hahn G, Taba JA, Muenke M, Zasloff MA. Genetic transmission of fibrodysplasia ossificans progressiva. Report of a family. J Bone Joint Surg Am. 1993;75:1214–20.
Kaully T, Kaufman-Francis K, Lesman A, Levenberg S. Vascularization – the conduit to viable engineered tissues. Tissue Eng Part B Rev. 2009;15:159–69.
Ketz AK. The experience of phantom limb pain in patients with combat-related traumatic amputations. Arch Phys Med Rehabil. 2008;89:1127–32.
Koebler J. New prosthetics keep amputee soldiers on active duty [Internet]. US News; 2012. Available from: http://www.usnews.com/news/articles/2012/05/25/new-prosthetics-keep-amputee-soldiers-on-active-duty/.
Kragl M, Knapp D, Nacu E, Khattak S, Maden M, Epperlein HH, Tanaka EM. Cells keep a memory of their tissue origin during axolotl limb regeneration. Nature. 2009;460:60–5.
Krueger CA, Wenke JC, Ficke JR. Ten years at war: comprehensive analysis of amputation trends. J Trauma Acute Care Surg. 2012;73:S438–44.
Kuiken T. Targeted reinnervation for improved prosthetic function. Phys Med Rehabil Clin N Am. 2006;17:1–13.
Kuiken TA, Miller LA, Lipschutz RD, Lock BA, Stubblefield K, Marasco PD, Zhou P, Dumanian GA. Targeted reinnervation for enhanced prosthetic arm function in a woman with a proximal amputation: a case study. Lancet. 2007;369:371–80.
Kumar A, Godwin JW, Gates PB, Garza-Garcia AA, Brockes JP. Molecular basis for the nerve dependence of limb regeneration in an adult vertebrate. Science. 2007;318:772–7.
Kumar A, Velloso CP, Imokawa Y, Brockes JP. The regenerative plasticity of isolated urodele myofibers and its dependence on MSX1. PLoS Biol. 2004;2:E218.
Lanza R, Langer R, Vacanti J, editors. Principles of tissue engineering. 4th ed. New York: Academic; 2013.
Larson BJ, Longaker MT, Lorenz HP. Scarless fetal wound healing: a basic science review. Plast Reconstr Surg. 2010;126:1172–80.
Lee J, Corcoran A, Han M, Gardiner DM, Muneoka K. Dlx5 and Msx2 regulate mouse anterior neural tube closure through ephrinA5-EphA7. Develop Growth Differ. 2013a;55:341–9.
Lee J, Marrero L, Yu L, Dawson LA, Muneoka K, Han M. SDF-1alpha/CXCR4 signaling mediates digit tip regeneration promoted by BMP-2. Dev Biol. 2013b;382:98–109.
Lee LP, Lau PY, Chan CW. A simple and efficient treatment for fingertip injuries. J Hand Surg (Br). 1995;20:63–71.
Lehoczky JA, Robert B, Tabin CJ. Mouse digit tip regeneration is mediated by fate-restricted progenitor cells. Proc Natl Acad Sci U S A. 2011;108:20609–14.
Lounev VY, Ramachandran R, Wosczyna MN, Yamamoto M, Maidment AD, Shore EM, Glaser DL, Goldhamer DJ, Kaplan FS. Identification of progenitor cells that contribute to heterotopic skeletogenesis. J Bone Joint Surg Am. 2009;91:652–63.
Lovett M, Lee K, Edwards A, Kaplan DL. Vascularization strategies for tissue engineering. Tissue Eng Part B Rev. 2009;15:353–70.
Macchiarini P, Jungebluth P, Go T, Asnaghi MA, Rees LE, Cogan TA, Dodson A, Martorell J, Bellini S, Parnigotto PP, Dickinson SC, Hollander AP, Mantero S, Conconi MT, Birchall MA. Clinical transplantation of a tissue-engineered airway. Lancet. 2008;372:2023–30.
Makanae A, Hirata A, Honjo Y, Mitogawa K, Satoh A. Nerve independent limb induction in axolotls. Dev Biol. 2013;381:213–26.
Mao AS, Mooney DJ. Regenerative medicine: current therapies and future directions. Proc Natl Acad Sci U S A. 2015;112:14452–9.
Mariani FV, Ahn CP, Martin GR. Genetic evidence that FGFs have an instructive role in limb proximal-distal patterning. Nature. 2008;453:401–5.
Mase VJ Jr, Hsu JR, Wolf SE, Wenke JC, Baer DG, Owens J, Badylak SF, Walters TJ. Clinical application of an acellular biologic scaffold for surgical repair of a large, traumatic quadriceps femoris muscle defect. Orthopedics. 2010;33:511.
Mertens JP, Sugg KB, Lee JD, Larkin LM. Engineering muscle constructs for the creation of functional engineered musculoskeletal tissue. Regen Med. 2014;9:89–100.
Mescher AL, Connell E, Hsu C, Patel C, Overton B. Transferrin is necessary and sufficient for the neural effect on growth in amphibian limb regeneration blastemas. Develop Growth Differ. 1997;39:677–84.
Morrison JI, Loof S, He P, Simon A. Salamander limb regeneration involves the activation of a multipotent skeletal muscle satellite cell population. J Cell Biol. 2006;172:433–40.
Mullen LM, Bryant SV, Torok MA, Blumberg B, Gardiner DM. Nerve dependency of regeneration: the role of Distal-less and FGF signaling in amphibian limb regeneration. Development. 1996;122:3487–97.
Muller TL, Ngo-Muller V, Reginelli A, Taylor G, Anderson R, Muneoka K. Regeneration in higher vertebrates: limb buds and digit tips. Semin Cell Dev Biol. 1999;10:405–13.
Muneoka K, Bryant SV. Evidence that patterning mechanisms in developing and regenerating limbs are the same. Nature. 1982;298:369–71.
Muneoka K, Bryant SV. Cellular contribution to supernumerary limbs resulting from the interaction between developing and regenerating tissues in the axolotl. Dev Biol. 1984;105:179–87.
Muneoka K, Sassoon D. Molecular aspects of regeneration in developing vertebrate limbs. Dev Biol. 1992;152:37–49.
Muneoka K, Allan CH, Yang X, Lee J, Han M. Mammalian regeneration and regenerative medicine. Birth Defects Res C Embryo Today. 2008;84:265–80.
Muneoka K, Fox WF, Bryant SV. Cellular contribution from dermis and cartilage to the regenerating limb blastema in axolotls. Dev Biol. 1986;116:256–60.
Murphy SV, Atala A. 3D bioprinting of tissues and organs. Nat Biotechnol. 2014;32:773–85.
Nacu E, Glausch M, Le HQ, Damanik FF, Schuez M, Knapp D, Khattak S, Richter T, Tanaka EM. Connective tissue cells, but not muscle cells, are involved in establishing the proximo-distal outcome of limb regeneration in the axolotl. Development. 2013;140:513–8.
Nesti LJ, Jackson WM, Shanti RM, Koehler SM, Aragon AB, Bailey JR, Sracic MK, Freedman BA, Giuliani JR, Tuan RS. Differentiation potential of multipotent progenitor cells derived from war-traumatized muscle tissue. J Bone Joint Surg Am. 2008;90:2390–8.
Norotte C, Marga FS, Niklason LE, Forgacs G. Scaffold-free vascular tissue engineering using bioprinting. Biomaterials. 2009;30:5910–7.
Novosel EC, Kleinhans C, Kluger PJ. Vascularization is the key challenge in tissue engineering. Adv Drug Deliv Rev. 2011;63:300–11.
Nyame TT, Chiang HA, Leavitt T, Ozambela M, Orgill DP. Tissue-engineered skin substitutes. Plast Reconstr Surg. 2015;136:1379–88.
Olmsted-Davis E, Gannon FH, Ozen M, Ittmann MM, Gugala Z, Hipp JA, Moran KM, Fouletier-Dilling CM, Schumara-Martin S, Lindsey RW, et al. Hypoxic adipocytes pattern early heterotopic bone formation. Am J Pathol. 2007;170:620–32.
Pasquina PF, Cooper RA, editors. Care of the combat amputee. Washington, DC: The Office of the Surgeon General, Borden Institute, Walter Reed Army Medical Center; 2009.
Pierce RO Jr, Kernek CB, Ambrose TA 2nd. The plight of the traumatic amputee. Orthopedics. 1993;16:793–7.
Potter BK, Burns TC, Lacap AP, Granville RR, Gajewski DA. Heterotopic ossification following traumatic and combat-related amputations. Prevalence, risk factors, and preliminary results of excision. J Bone Joint Surg Am. 2007;89:476–86.
Potter BK, Forsberg JA, Davis TA, Evans KN, Hawksworth JS, Tadaki D, Brown TS, Crane NJ, Burns TC, O’Brien FP, et al. Heterotopic ossification following combat-related trauma. J Bone Joint Surg Am. 2010;92(Suppl 2):74–89.
Reginelli AD, Wang YQ, Sassoon D, Muneoka K. Digit tip regeneration correlates with regions of Msx1 (Hox 7) expression in fetal and newborn mice. Development. 1995;121:1065–76.
Rinkevich Y, Lindau P, Ueno H, Longaker MT, Weissman IL. Germ-layer and lineage-restricted stem/progenitors regenerate the mouse digit tip. Nature. 2011;476:409–13.
Rinn JL, Bondre C, Gladstone HB, Brown PO, Chang HY. Anatomic demarcation by positional variation in fibroblast gene expression programs. PLoS Genet. 2006;2:e119.
Robbins CB, Vreeman DJ, Sothmann MS, Wilson SL, Oldridge NB. A review of the long-term health outcome associated with war-related amputation. Mil Med. 2009;174:588–92.
Sanchez AA. Regeneration in the metazoans: why does it happen? BioEssays. 2000;22:578–90.
Sanchez Alvarado A, Tsonis PA. Bridging the regeneration gap: genetic insights from diverse animal models. Nat Rev Genet. 2006;7:873–84.
Sandoval-Guzmán T, Wang H, Khattak S, Schuez M, Roensch K, Nacu E, Tazaki A, Joven A, Tanaka EM, Simon A. Fundamental differences in dedifferentiation and stem cell recruitment during skeletal muscle regeneration in two salamander species. Cell Stem Cell. 2014;14(2):174–87.
Satoh A, Graham GM, Bryant SV, Gardiner DM. Neurotrophic regulation of epidermal dedifferentiation during wound healing and limb regeneration in the axolotl (Ambystoma mexicanum). Dev Biol. 2008;319:321–35.
Shafritz AB, Shore EM, Gannon FH, Zasloff MA, Taub R, Muenke M, Kaplan FS. Overexpression of an osteogenic morphogen in fibrodysplasia ossificans progressiva. N Engl J Med. 1996;335:555–61.
Sicari BM, Dziki JL, Badylak SF. Strategies for functional bioscaffold-based skeletal muscle reconstruction. Ann Transl Med. 2015;3:256.
Singer M. The influence of the nerve in regeneration of the amphibian extremity. Q Rev Biol. 1952;27:169–200.
Stinner DJ, Burns TC, Kirk KL, Ficke JR. Return to duty rate of amputee soldiers in the current conflicts in Afghanistan and Iraq. J Trauma. 2010;68:1476–9.
Stocum DL. The urodele limb regeneration blastema: a self-organizing system. I. Differentiation in vitro. Dev Biol. 1968;18:441–56.
Takeo M, Chou WC, Sun Q, Lee W, Rabbani P, Loomis C, Taketo MM, Ito M. Wnt activation in nail epithelium couples nail growth to digit regeneration. Nature. 2013;499:228–32.
Tank PW. The ability of localized implants of whole or minced dermis to disrupt pattern formation in the regenerating forelimb of the axolotl. Am J Anat. 1981;162:315–26.
Thompson M. Wars’ amputees: 4 of 5 say “life is full” [Internet]. Time; 2012. Available from: http://nation.time.com/2012/03/12/wars-amputees-4-of-5-say-life-is-full/.
Towers M, Wolpert L, Tickle C. Gradients of signalling in the developing limb. Curr Opin Cell Biol. 2012;24:181–7.
Tyler DJ. Neural interfaces for somatosensory feedback: bringing life to a prosthesis. Curr Opin Neurol. 2015;28:574–81.
VanDusen KW, Syverud BC, Williams ML, Lee JD, Larkin LM. Engineered skeletal muscle units for repair of volumetric muscle loss in the tibialis anterior muscle of a rat. Tissue Eng Part A. 2014;20:2920–30.
Vinarsky V, Atkinson DL, Stevenson TJ, Keating MT, Odelberg S. J. Normal newt limb regeneration requires matrix metalloproteinase function. Dev Biol. 2005;279:86–98.
Viola J, Lal B, Grad O. The emergence of tissue engineering as a research field [Internet]. Arlington: National Science Foundation; 2003. Available from http://www.nsf.gov/pubs/2004/nsf0450/.
Wanek N, Muneoka K, Bryant SV. Evidence for regulation following amputation and tissue grafting in the developing mouse limb. J Exp Zool. 1989;249:55–61.
Wolpert L. Positional information and patterning revisited. J Theor Biol. 2011;269:359–65.
Wu Y, Wang K, Karapetyan A, Fernando WA, Simkin J, Han M, Rugg EL, Muneoka K. Connective tissue fibroblast properties are position-dependent during mouse digit tip regeneration. PLoS One. 2013;8:e54764.
Yang EV, Gardiner DM, Carlson MR, Nugas CA, Bryant SV. Expression of Mmp-9 and related matrix metalloproteinase genes during axolotl limb regeneration. Dev Dyn. 1999;216:2–9.
Yu L, Han M, Yan M, Lee EC, Lee J, Muneoka K. BMP signaling induces digit regeneration in neonatal mice. Development. 2010;137:551–9.
Yu L, Han M, Yan M, Lee J, Muneoka K. BMP2 induces segment-specific skeletal regeneration from digit and limb amputations by establishing a new endochondral ossification center. Dev Biol. 2012;372:263–73.
Zhao P, Hoffman EP. Embryonic myogenesis pathways in muscle regeneration. Dev Dyn. 2004;229:380–92.
Zoroya G. Despite the loss of a leg, Army Ranger is back in the fight [Internet]. USA Today; 2011. Available from: http://usatoday30.usatoday.com/news/military/2011-06-19-Afghanistan-Iraq-Army-Rangers-amputee-Purple-Heart_n.htm/.
Disclaimer
The views expressed in this presentation are those of the author(s) and do not reflect the official policy or position of the US Army, Department of Defense, or the US Government.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer Science+Business Media LLC
About this chapter
Cite this chapter
Muneoka, K., Lai, E., Christy, R.J., Mogford, J.E. (2017). Limb Regrowth and Tissue Engineering Alternatives. In: Tepe, V., Peterson, C. (eds) Full Stride. Springer, New York, NY. https://doi.org/10.1007/978-1-4939-7247-0_12
Download citation
DOI: https://doi.org/10.1007/978-1-4939-7247-0_12
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4939-7245-6
Online ISBN: 978-1-4939-7247-0
eBook Packages: MedicineMedicine (R0)