Abstract
The interaction of breast epithelial cells with the surrounding extracellular matrix (ECM) is known to play a pivotal role during normal mammary gland development and function. It is also critical during the pathological changes that lead to breast cancer initiation and progression. A bidirectional crosstalk emerges upon interactions of epithelial cells with the ECM, which eventually dictates the genotypic and phenotypic programs that define normal gland function. Consequently, disruption of this communication contributes to the development of malignant phenotypes, which illustrate the process of breast cancer progression. The Discoidin Domain Receptors (DDRs) are collagen-binding receptor tyrosine kinases that are emerging as key mediators of cell−collagen interactions in breast tissues. DDRs signal in response to both basement membrane and interstitial collagens and thus they are well positioned to activate matrix-induced cellular programs during normal mammary gland development and function, and during dissemination of breast cancer cells. This chapter summarizes the current knowledge on the expression and function of DDRs in breast epithelial cells and their potential involvement in physiological and malignant processes. We also discuss the current challenges in understanding DDR expression and function in breast cancer tissues and experimental models and their potential as therapeutic targets.
Rodrigo Fernandez-Valdivia and Rafael Fridman are cosenior authors
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
The DDRs are the only receptor tyrosine kinases (RTKs) that signal in response to collagen. This characteristic of DDRs places these receptors at the center of the signaling networks that drive cell–matrix interactions in physiological and pathological conditions. DDRs bind and are activated by both basement membrane and interstitial collagens, and therefore they can transduce collagen-initiated signals in a variety of epithelial and mesenchymal cells. The DDR family comprises two receptors, DDR1 and DDR2 [1]. DDR1 undergoes phosphorylation in response to both fibrillar (connective tissue) and nonfibrillar (basement membrane) collagens, whereas DDR2 is activated only by fibrillar collagens. DDR1 is mostly expressed by epithelial cells and DDR2 is found in cells of mesenchymal origin. Alternative splicing generates five DDR1 isoforms: DDR1a, DDR1b, and DDR1c are full-length functional receptors, whereas DDR1d and DDR1e are truncated or kinase-inactive receptors. DDR1b and DDR1c contain an additional 37 residues (including an extra tyrosine residue) within the intracellular juxtamembrane region, suggesting that these receptors activate distinct signaling pathways in response to collagen and may be differentially expressed in malignant cells. In contrast, a single protein has been identified for DDR2. Upon collagen-binding DDRs undergo tyrosine autophosphorylation. DDRs mediate cell−collagen interactions and regulate diverse biological function including cell adhesion, migration, and invasion in a DDR type- and cell type-dependent manners (details on structure−function relationship are discussed in various chapters). Accumulating evidence from DDR-deficient mice point to a critical role for these receptors in developmental and pathological processes including cancer, fibrosis, and inflammation, which are the topics of several chapters in this book. Because cell−collagen interactions are central to normal mammary gland development and function, and breast cancer progression [2–5], a significant effort was invested in defining the relative contribution of DDRs in these processes. DDRs are kinases, and as such they can be targeted by specific kinase inhibitors. They are therefore potential therapeutic targets in disease conditions with pathological DDR-mediated signaling. Emerging evidence support the use of inhibitors to target DDRs in breast cancer but these data although promising awaits a better understanding of how DDRs contribute to disease progression. This chapter attempts to provide a comprehensive overview on the expression and function of DDRs in breast tissues, from the early stages of mammary gland development to the ultimate dissemination of metastatic breast cancer cells.
2 Role of DDRs in Mammary Gland Development
The highly regenerative mammary gland tissue begins its developmental journey during embryogenesis, goes through allometric pubertal ductal morphogenesis and alveologenesis, and undergoes, potentially recurrent, pregnancy-regulated alveolar functional differentiation, lactation, and regression/involution. This morphogenetic odyssey is tightly and precisely controlled by the orchestrated action of intracellular factors, endocrine cues, intercellular signals, and microenvironmental and stromal entities [2, 3, 6–9]. Although most of the attention in mammary gland biology has been focused on mammary epithelial cell–cell interactions and hormonal regulation, likely because of their evident involvement in breast development and cancer, multiple evidence highlighted the importance of the crosstalk between mammary epithelium and mammary stroma for mammary development, homeostasis, and tumorigenesis [10]. The function of the stroma in breast biology regulation relies on the dynamic interaction between mammary epithelial cells, mammary stroma cells, and the extracellular matrix (ECM) and is, in great extent, determined by factors regulating the ECM. To date, these factors include known regulators of cell–matrix interactions such as members of the α and β integrins family [11], laminins [5], collagens [12–14], matrix metalloproteinases [15], and DDRs [16, 17].
DDR1 : Insight into the function of DDR1 in breast development has come from mouse genetic studies. DDR1-deficient mice were generated by Vogel and colleagues by deleting the first 12 exons of the DDR1 gene [17]. Pups from homozygous females appeared malnourished 1 day after birth, had small amounts of milk in their stomachs, and, if kept with the DDR1-deficient females, they eventually died. However, transfer of the pups to wild-type foster mothers shortly after birth prevented their death, suggesting a lactating defect in the mammary gland of DDR1-deficient females, consistent with a role for DDR1 in mammary gland function [17]. Histological analyses of mammary gland tissue derived from the DDR1 knockout mice at various stages confirmed these findings and revealed that lack of DDR1 was associated with disruption of glandular organization, and cell proliferation and differentiation [17]. Interestingly, however, whereas the 3-week-old DDR1-deficient mammary gland appeared delayed in its development and displayed a significant impairment in ductal growth compared to wild-type mammary glands, a marked increase in the number and diameter of mammary ducts was observed in the mammary gland of DDR1-deficient adult virgin female mice compared to their wild-type counterparts [17]. This marked increase in ductal growth in DDR1-deficient mammary glands was caused by an augmented epithelial cell proliferation rate (between fourfold and sixfold increase) and accompanied by a significant increase in collagen deposition within the stroma, which was observed around the mammary epithelial tissue and in the adjacent adipose tissue [17]. Interestingly, the terminal end buds (TEB) in the prepubertal DDR1-deficient mammary gland were enlarged compared to the ones present in a wild-type mammary gland. Together, these observations supported the notion that DDR1 exerts a cell proliferation suppression function in the developing (pubertal) and adult nulliparous mammary epithelium. To further strengthen this idea, it has been shown that compound deficiency for c-Jun kinases (JNK) JNK1 and JNK2 in the mammary epithelium causes an increase in mammary branching morphogenesis and a significant downregulation in the expression of DDR1 and also of integrins α1, α5, α6, and β1 mRNA in mammary epithelial cells [18]. Furthermore, inhibition of transforming growth factor (TGF)-β (TGF-β) signaling, which results in accelerated ductal elongation and side branching development [19, 20] and decreased levels of Wnt5a mRNA and protein levels in the mammary epithelium, causes a significant decrease in DDR1 phosphorylation that is mediated by Wnt5a downregulation [21].
Transplantation studies have also shed light on DDR1 function and have uncovered a dual role of DDR1 in the mammary epithelium. Faraci-Orf et al. [16] conducted a study to determine whether the mammary phenotypes displayed by DDR1-deficient mice were intrinsic to the mammary epithelium or a result of altered endocrine and/or paracrine/juxtacrine function. They found that even though the transplanted DDR1-null and wild-type nulliparous mammary epithelium comparably populated and filled the fat pad of the recipient mice, DDR1-deficient ducts showed a significant reduced number of branch points and lessen branching growth, and the TBEs remained larger when compared to the ones present in wild-type transplants [16]. These observations suggested a dual role of DDR1 in the mammary epithelium, acting as a suppressor of cell proliferation in the developing TEBs and, contrastingly, supporting cell proliferation in the mammary ducts during branching morphogenesis. Interestingly, DDR1 expression was found to be upregulated by progesterone treatment in the nulliparous murine mammary gland, and this upregulation occurred at a time where strong progesterone-induced cell proliferation was observed and when the expression of known key mediators of progesterone’s action, including RANKL, Wnt4, and inhibitor of differentiation 4 (ID4), was also upregulated [22]. Whether DDR1 is a direct progesterone receptor (PR) target and whether it is a mediator in the robust proliferative response of the mammary epithelium to progesterone signal remains to be determined.
Attributed DDR1 function in the late-pregnant and lactating mammary epithelium seems to be less compounded and more in line with a cell differentiation and a milk protein synthesis promoting role. Histological analysis of DDR1-null late pregnant mammary glands revealed that a more condensed alveolar structure, and with small number of lipid vesicles, was present in DDR1-null mice compared to wild-type animals [17]. Moreover, the histological examination and molecular analysis of postpartum mammary glands revealed that—albeit expressing normal levels of mRNA transcripts for milk proteins—DDR1-deficient mammary glands failed to produce various milk proteins [17]. Interestingly, the mammary glands from postpartum DDR1-deficient animals were largely composed of adipocytes, and the alveoli were condensed, had very little milk on them, and, eventually, collapsed [17]. Remarkably, the cell differentiation defects in late-pregnant DDR1-null mammary glands are not accompanied by premature cell death [17]. Importantly, the lactating defects conferred by DDR1 deficiency [16, 17] are somewhat similar to those observed upon loss of prolactin receptor (PrlR) or its downstream effector Signal transducer and activator of transcription 5(Stat5) [23–25]. Moreover, it has been shown that DDR1-expressing mammary epithelial cells participate, upon contact with a collagen-rich matrix, in PrlR signaling by sustaining Stat5 phosphorylation and transcriptional activity [16]. However, if DDR1 function in the late-pregnant and lactating mammary epithelium is positioned upstream of Stat5, DDR1 must also be controlling other signaling networks, as it is shown that the lactation defects in targeted WAP-Cre-mediated genetic inactivation of Stat5 is accompanied by increased cell apoptosis [24]. Moreover, the lactation defect observed in mice deficient for RANKL, which has been shown to be a downstream target of Stat5 and PR [6, 22, 26, 27], is, in contrast to the one caused by absence of DDR1, accompanied by enhanced apoptosis [28]. It should be noted that DDR1-null epithelial transplants fail to undergo lobuloalveolar outgrowth upon induction of pregnancy [16], suggesting that DDR1 also acts as a mediator of pregnancy-induced cell proliferation, which could also be in concordance with the fact that DDR1 is a downstream target of progesterone signal [22]. Interestingly, it has been shown that the WW domain-containing protein 1 (WWC1) KIBRA, a transactivator of estrogen receptor (ER) [29] that is positively regulated by progesterone and is downregulated in PrlR-deficient mammary tissue [30, 31], physically interacts with DDR1 and protein kinase Cζ (pKCζ), and that this interaction is negatively regulated by collagen-triggered DDR1 phosphorylation [31].
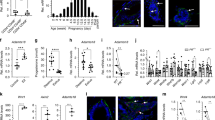
It is also important to highlight that among the known collagen receptors, DDR1 is the one that appears to have a more prominent role in mammary gland function. For instance, mice lacking integrin α2 show modest alterations in mammary epithelial duct branching and no lactation defect [32], and mice deficient for integrin α1 display a normal lactation phenotype [33]. Finally, another important aspect of DDR1 involvement in breast development that has been much less explored is its potential role in mammary gland stem cell function. In this regard, DDR1 was recently found, along with collagen 1α1 (Col1α1), Col9α1, several Notch signaling downstream targets, and laminins α1, α2, and α4, to be significantly upregulated in fetal mammary gland stem cells [34, 35]. Given that mammary gland stem cells are preferentially located in the TEBs [36, 37] of the developing gland, the above observations could, therefore, be in line with the enlarged TEBs phenotype displayed in pubertal DDR1-deficient mice [17]. Taken together, these data are consistent with (1) a role of DDR1 in suppression of cell proliferation within the mammary epithelium, (2) a cell differentiation promoting function during pregnancy, and (3) a role in lactation-associated lobuloalveolar functional differentiation. Thus, DDR1 may function in a convergent point for several signaling pathways governing breast development, function, homeostasis, and neoplastic conversion (Fig 7.1).
Proposed roles of DDR1 in mammary gland development and function. (a) DDR1 exerts an inhibitory function during pubertal development of the mammary gland as revealed by the increased cell proliferation observed in DDR1-deficient mice (arrow in blue). Similarly, the marked increased in Ki-67 positive cells in the adult virgin mammary glands from DDR1-deficient mice indicates that DDR1 has cell proliferation suppression function (arrow in blue). During pregnancy, DDR1 is required for proper cell proliferation of the mammary epithelium as evidenced by the increased cell proliferation, excessive filling of the fat pad, and the over condensed alveolar structures exhibited by DDR1-null pregnant female mice (arrow in blue). Interestingly, transplantation studies in which DDR1-deficient mammary tissue was transplanted into cleared fat pads of wild-type female mice have shown that DDR1 may have an inductive role in mammary branching morphogenesis and pregnancy-induced alveologenesis (dashed arrows). Remarkably, the terminal end buds generated by the transplanted DDR1-null epithelium displays an increased cell proliferation phenotype, corroborating the cell proliferation suppression function of DDR1. During lactation, DDR1 promotes alveolar differentiation and cell survival (arrow in magenta) as demonstrated by the failure to produce milk and the premature regression and collapse of the mammary alveoli seen in female mice lacking DDR1. (b) Schematics depicting DDR1 at the convergence of several signaling pathways governing important molecular and cellular processes in breast biology. Upon contact with collagen, DDR1 participates in prolactin (Prl)/prolactin receptor (PrlR) signaling by sustaining Stat5 phosphorylation and transcriptional activity. DDR1 expression has been found to be upregulated by progesterone (P4) progesterone receptor (PR) signal in the mammary gland. Importantly, KIBRA, a downstream PrlR target that acts as a transactivator of estrogen (E2)/estrogen receptor α (ER) complex and that is positively regulated by progesterone, has been shown to physically interact with DDR1 and form a complex that dissociates upon collagen-triggered DDR1 phosphorylation. DDR1 has been found to be a downstream target of JNK and TGF-β in the mammary epithelium. DDR1 has been demonstrated to have a cell proliferation suppression function in the mammary epithelium during pubertal, adult virgin and pregnancy-induced mammary development, and a cell differentiation promoting role in the lactating mammary epithelium. Extracellular matrix deposition has been shown to be negatively regulated by DDR1. Similarly, DDR1 has been found to negatively regulate cell proliferation in the terminal end buds, a mammary structure rich in epithelial stem cells
DDR2: The role of DDR2 in normal mammary gland development and homeostasis remains largely unexplored. Three types of mice carrying targeted and spontaneous inactivating mutations in the DDR2 gene have shown DDR2’s central role in cellular growth and endocrine and gonadal function [38–40]. Thus, whereas female mice homozygous for the slie mutation, which encompasses a large ~150 kb spontaneous deletion that removes most of DDR2 gene, display a failure in the formation of the corpus luteum [38], females homozygous for a DDR2-null allele progressively become infertile with age [40]. However, a recent study from Corsa et al. found that genetic deletion of DDR2 in mice had no evident effect on mammary gland development [118]. Interestingly, previous work from the Longmore laboratory in Snail1 regulation of epithelial to mesenchymal transition (EMT) shed light into DDR2 function in the human breast. Specifically, Zhang et al. [41] showed that DDR2 expression was induced along with Snail1 in normal human mammary epithelial cells MCF-10A during TGF-β-triggered EMT. Intriguingly, it was observed that although Snail1 levels were reduced in DDR2-depleted, nonmalignant human, breast epithelial MCF10A cells, which is consistent with the Snail1-stabilizing properties of DDR2, the TGF-β-triggered EMT induction was not affected [41]. These observations, however, are in manifest contrast with the fact that Snail1 alone was able to induce EMT in MCF-10A cells [41] and indicate that DDR2 could be functionally relevant in Snail1-triggered, TGF-β-independent EMT. Remarkably, DDR2 has been found differentially expressed in human mammary stem/progenitor cells compared to differentiated mammary epithelial cells [42], and it is upregulated in hyperplastic mammary glands of MMTV-Wnt1 mice [43], which have been shown to harbor an aberrantly increased mammary stem cell pool [37], and preferentially develop mammary tumors from progenitor cells [44]. This evidence is consistent with DDR2 being a critical player in Snail1-triggered EMT in mammary epithelial cells, and plausibly, a relevant player in mammary stem cell function, and in Wnt1-triggered mammary tumorigenesis. Further studies using front-end mouse genetics and patient-derived xenografts along with cell culture and biochemical and structural assays will be required to delineate DDRs’ function in the mammary epithelium and stroma as well as in breast cancer initiation, progression, and metastasis. Figure 7.1 summarizes the current known roles of and molecular pathways associated with DDR1 during mammary gland development.
3 Expression and Role of DDRs in Breast Cancer Progression
Invasive breast carcinomas are a heterogeneous group of malignant epithelial tumors that arise in the breast parenchyma and are characterized by their invasion of adjacent tissue and metastatic ability. Invasive breast carcinomas comprise various histological morphologies and biological features, and exhibit different clinical behaviors and treatment responses. The morphological classification of invasive breast carcinomas depends on their degree of differentiation, which reflects how closely a tumor resembles normal breast glandular epithelium in structural organization, cytological features, and growth pattern [45]. Most invasive tumors in the breast are invasive ductal carcinomas, accounting for approximately 80 % of invasive breast cancer. Invasive lobular carcinomas comprise 10–15 % of all breast cancers, and the remainder constitutes special histological types including mucinous, tubular, micropapillary, and others. The morphologic features and cell proliferation status are robust surrogates for biological variables that determine and relate to their natural behaviors. Importantly, hormone receptor and human epidermal growth factor receptor 2 (HER-2/neu) status was shown to have prognostic value and predict clinical responses [46–48]. However, it has become increasingly evident that breast cancer heterogeneity extends beyond the classic immunohistochemistry-based divisions of ER, PR, and HER-2/neu. The development of the molecular classification of breast cancers [49] was recently supported by the Cancer Genome Atlas (TCGA) Program through mRNA, miRNA, DNA, and epigenetic analyses [50]. Invasive carcinomas may belong to the luminal A, luminal B, HER2, and triple negative subtypes. Of note, these subtypes are also heterogeneous and maybe further refined. For example, recent studies defined four subgroups of triple negative breast cancers (TNBCs): luminal androgen receptor, mesenchymal, basal-like immunosuppressed, and basal-like immune-activated groups [51].
In addition to the intrinsic characteristics of breast cancer cells, the interactions with the cellular and structural components of the microenvironment are crucial for breast cancer development and progression. Studies have shown that high mammographic density is associated with a twofold risk of breast cancer development [52–54]. While in the normal adult breast there are several types of collagen, breast density is mainly due to increased deposition of type I collagen [54–56]. Pathologists have noticed the presence of a desmoplastic stroma associated with invasive carcinomas decades ago, which results from increased deposition of extracellular matrix proteins [45]. It is now recognized that stromal desmoplasia alters the chemical composition and the mechanical properties of the ECM [57, 58]. Breast cancer cells respond to these changes in the ECM through deregulated signaling pathways, which promote neoplastic functions and result in loss of normal architecture, invasion, and increased proliferation [57, 58]. The tumor-associated desmoplastic stroma is particularly enriched in fibrillar collagens , which have been shown to provide a path for breast cancer cell invasion [59]. Because DDRs are part of the arsenal of cell surface receptors that mediate tumor cell−collagen interactions [60, 61], they may play an important role in breast cancer progression [41, 60, 62, 63].
3.1 Expression of DDRs in Normal and Cancerous Breast Tissues
DDR1 : Immunohistochemical studies showed that in normal human mammary gland DDR1 protein is highly expressed in the epithelium throughout the gland, but it is not detected in the stroma [62]. Antibodies directed to the extracellular domain revealed clear membranous DDR1 localization in normal epithelial cells (our data). We also found DDR1 to be highly expressed in the acinar-like structures of MCF10A cells cultured within Matrigel, where it preferentially localizes at cell–cell contacts with a pattern similar to that displayed by E-cadherin. DDR1 was also highly expressed in tumors produced by MCF10A.DCIS.COM cells in mice (our unpublished data). In specimens of human ductal in situ carcinomas (DCIS), DDR1 protein was also expressed in the epithelial cells [62]. Thus, DDR1 expression in breast epithelial cells does not appear to be significantly altered in the early stages of breast cancer suggesting that neoplastic transformation does not involve changes in DDRs. In agreement with these studies, mRNA analyses showed that DDR1 was readily detected in normal breast tissues and in benign tumors (cystic hyperplasia and fibroadenoma). However, the benign tumors showed a trend to express higher levels of DDR1 mRNA [64]. In breast tumors, the profile of DDR1 expression appears to be complex. For instance, the studies of Ren et al. [64] showed significantly downregulation of DDR1 mRNA expression in normal vs. cancerous breast tissues. Analyses of protein expression by immunohistochemistry, however, showed a complex pattern of expression. Dejmek et al. found DDR1 to be heterogeneously expressed in invasive breast carcinomas [65]. Turashvili et al. reported that DDR1 is expressed by most ductal but not lobular carcinomas, and thus these investigators proposed that DDR1 could be an additional marker to distinguish between these two cancer subtypes [66]. In this regard, the reported association of DDR1 with ductal carcinomas appears to resemble that of E-cadherin, which is highly expressed in ductal but not in lobular carcinomas. Interestingly, DDR1 has been found to interact with E-cadherin at cell–cell contacts [67–69], and in some cancer types, absence of E-cadherin correlates with lack of DDR1 expression [69]. Thus, loss of E-cadherin may occur concomitantly with loss of DDR1. The study of Ameli et al., however, found positive DDR1 expression in both ductal and lobular carcinomas, and a lack of correlation between DDR1 levels with tumor type, grade, or receptor status [70]. Another study, however, reported that the majority of ductal carcinomas exhibited a reduced or a lack of DDR1 mRNA expression [71]. However, a strong correlation between reduced DDR1 mRNA expression and malignancy could not be established [64]. In the study of Toy et al. [62], invasive breast carcinomas displayed a heterogeneous expression of DDR1 protein, with half of the tumors expressing high levels, whereas the other half showing reduced levels of DDR1 protein [62]. Moreover, DDR1 expression was not associated with clinicopathological features in this set of tumors, and both luminal and TNBC subtypes exhibited an equal distribution of high and low DDR1-expressing tumors . Another study focusing on TNBCs found low DDR1 expression, which correlated with poor disease-free survival [72]. Yet, a significant number of invasive breast cancer tumors, including TNBC, express DDR1 [62, 66, 73], and at levels similar to those found in normal breast tissue and DCIS [62]. Since TNBCs show significant heterogeneity [74–76], differences in DDR1 expression profile may reflect this fact, and the complex role of DDR1 in various TNBC contexts. For instance, based on experimental findings, high DDR1 expression in certain TNBCs may be associated with activation of prosurvival signals, under stress conditions [77] (discussed below). On the other hand, reduced DDR1 expression in a subset of TNBCs may reflect the reported antimigratory and anti-invasive effects of DDR1 in breast cancer cell lines [72]. Interestingly, clinical association studies showed that among the various combinations of DDR expression profiles, TNBC patients displaying a DDR1Low/DDR2High profile exhibited a significantly worse survival when compared to patients that were negative for this DDR profile [62]. This observation suggests that in certain aggressive TNBCs, concomitant expression of both DDRs may be counterproductive for disease progression. Both DDR1 and DDR2 recognize similar fibrillar collagen ligands, yet they exhibit significant structural and regulatory differences, and consequently activate distinct signaling pathways [1]. It is possible that in the course of tumor evolution there is preferential expansion of tumor cell subpopulations with a DDR profile that is compatible with cancer survival (DDR1 high?) and/or dissemination (DDR2 high?), within a defined collagen microenvironment. Understanding the signaling dynamics (cooperating or antagonizing) of DDRs within a cellular background will shed light on how breast cancer cells sort the collagen-initiated signals in conditions of single or dual DDR receptor expression, and how the activated networks may impact outcome in TNBC subtypes. Another important aspect that needs considerations, when analyzing the association of DDR1 expression with disease progression by immunohistochemical methods, is the expression and role of the DDR1 different isoforms. However, at present, there are no antibodies that can specifically distinguish between the various DDR1 isoforms in breast cancer tissues, and therefore the association between clinicopathological features and DDR1 isoforms remains unknown. This current limitation may be important because there are five structurally different DDR1 isoforms, from which DDR1a and DDR1b are the most common. DDR1a and DDR1b display structural differences within their intracellular juxtamembrane region including the presence of two additional tyrosine residues in DDR1b. Because tyrosine phosphorylation is critical in RTK-mediated signal transduction , these DDR1 isoforms may activate distinct signaling networks in response to collagen binding, and consequently may also elicit isoform-specific effects (promoter, suppressive) on cancer progression. So far the data show that a significant proportion of invasive breast tumors express DDR1 at levels similar to those found in normal breast epithelium [62, 66, 73], raising the question about its role(s) in malignant tissues. Evidence suggests that de novo expression DDR1 is part of a kinome reprograming process that takes place in TNBC cells when exposed to MEK inhibitors [77]. Thus, expression of DDR1 may be a part of the genetic program that confers survival in TNBC cells exposed to stress. However, there is no reported evidence that high expression of DDR1 in invasive cancers predicts outcome. In contrast, TNBC patients with tumors displaying low DDR1 expression were reported to have a poorer survival [62, 72].
In recent years, significant genomic data have been obtained from sequencing breast tumors from multiple patients. Analyses of these data sets revealed little evidence for the occurrence of DDR1 somatic gene aberrations in breast cancer. Moreover, the lack of evidence for DDR1 mutations in the literature is reinforced by information extracted from functional genomics databases. According to data published by The Cancer Genome Atlas (TCGA) and accessed via cBioPortal a single breast cancer sample out of 825 cases showed a somatic DDR1 mutation—a missense mutation at codon E618Q that is, however, predicted to have no functional consequence [50, 78]. Also according to TCGA data, DDR1 gene amplification was identified in one other sample; and there were no instances of DDR1 gene copy number loss [50, 78]. Furthermore, by query of a variety of breast cancer gene expression databases, results were either mixed or demonstrated no significant association for survival or recurrence outcomes and DDR1 mRNA expression levels (tested on complete datasets and subtyped datasets) [79–81]. Thus, the findings so far suggest that DDR1 plays complex roles in breast cancer, with possibly pro- and antimalignant effects depending on the tumor subtype and genetic background. Whether these potential opposite roles of DDR1 in breast cancer are isoform-specific warrant further studies.
DDR2: In nonmalignant (carcinoma-associated) breast tissue, DDR2 protein and mRNA are undetectable both in the epithelial and the stromal compartments [41, 62, 64]. Consistently, DDR2 is not expressed in acinar-like structures generated by cultured MCF10A cells (our unpublished data). The lack of DDR2 expression in normal breast epithelial cells is consistent with the fact that in normal breast tissues the epithelial cells are separated from the interstitial collagen matrix by an underlying basement membrane, which contains collagen IV, a ligand of DDR1 but not of DDR2. However, in the course of disease progression, breast cancer cells begin to interact with fibrillar collagen s, which areDDR2 ligands, particularly during the process of degradation and invasion through the basement membrane. Consistent with this scenario, Toy et al. [62] found that whereas the majority of the DCIS lesions were negative for DDR2 protein, small groups of DCIS cells facing the tumor−stromal interface displayed specific DDR2 immunoreactivity. Possibly, de novo expression of DDR2 may aid the proinvasive phenotype by allowing invading breast cancer cells to confront the interstitial matrix. Indeed, invasive breast cancers express high levels of DDR2 mRNA [64] and protein [41, 62]. Ren et al. found elevated expression of DDR2 mRNA in 122 samples of invasive breast cancer (ductal and lobular) when compared to the adjacent nonneoplastic breast tissue or nonmalignant (cystic hyperplasia and fibroadenoma) [64]. In the invasive breast cancers, the majority of the ductal carcinomas displayed high DDR2 mRNA levels. Moreover, DDR2 expression was associated with presence of lymph node and distant metastasis, and advanced stage [64]. Importantly, breast cancer patients with high DDR2 mRNA showed worse overall survival and high risk to relapse [64]. Ren et al. also found a strong association between DDR2 levels and expression of hypoxia-inducible factor-1α (HIF-1α), a marker of hypoxia, and its target vascular endothelial growth factor (VEGF), in samples of invasive breast cancer tissues [71]. In these samples, high expression of DDR2 and low levels of E-cadherin correlated with lymph node metastases, whereas tumors with no detectable DDR2 expression, regardless of the levels of E-cadherin, displayed low incidence of lymph node metastases [71]. Based on these studies, Ren et al. suggested that the combined analyses of DDR2 and E-cadherin could distinguish metastatic from nonmetastatic breast cancer [71]. Immunohistochemical studies by Zhang et al. in invasive breast cancer found that 71 % of invasive ductal carcinomas and 21 % of invasive lobular carcinomas expressed DDR2 [41], in agreement with the studies of Ren et al. [64]. However, contrary to their studies, Zhang et al. found no association between DDR2 expression and lymph node involvement [41]. DDR2 expression was significantly associated with Snail1 positivity in the tumor cells, consistent with the experimental findings showing stabilization of Snail1 by DDR2 [41], and previous studies showing that induction of EMT in human breast epithelial cells leads to de novo expression of DDR2 and downregulation of DDR1 [82]. Indeed, Zeb1, an EMT transcription factor, was shown to be a negative transcriptional regulator of DDR1 expression [72]. In a set of 198 tumor specimens, Toy et al. found that high DDR2 protein expression was significantly associated with higher histological grade, negative ER and PR status, negative HER2/neu overexpression, and with the TNBC subtype [62]. Moreover, patients with tumors expressing high DDR2 had a significantly worse overall survival than those expressing low DDR2 after initial surgical treatment. When examined in combination with DDR1, patients with tumors displaying a specific profile of DDR1Low/DDR2High protein expression had a shorter overall survival compared to other DDR expression profiles. Importantly, this specific DDR profile predicted survival independently of tumor size, TNBC phenotype, and lymphovascular invasion [62].
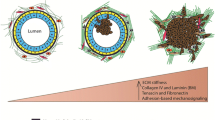
The evidence for somatic DDR2 aberrations in breast cancer is somewhat stronger than the data for DDR1, where according to TCGA data 4 % of breast cancer samples showed copy number amplification [50, 78], but for these same samples, gene expression did not appear to concomitantly increase with copy number amplification. Of the 825 patient samples in the study, single nucleotide variants were identified in six (at codons S123I, E361D, A407P, K616N, S674Y, and R752H) [50, 78]. Among these, codon DDR2 S123I introduces a missense mutation in the Discoidin domain and is strongly predicted to have an impact on function [83]. Queries of breast cancer gene expression databases indicated that for luminal and ERα-positive breast cancers there were associations for high DDR2 expression and increased overall patient survival [81]. However, other datasets demonstrated no significant association for survival or recurrence outcomes and DDR2 mRNA expression levels [79, 80]. These findings in current databases are in conflict with the expression data showing a strong association between high levels of DDR2 and poor outcome in women with invasive carcinomas, particularly of the TNBC subtype [41, 62, 64]. These differences highlight the need to continue our effort to unveil whether a specific profile of DDR expression (and activation) is associated with a particular breast cancer subtype, histopathological features, and/or patient outcome. Figure 7.2 illustrates the profile of DDRs in normal and cancerous breast epithelial tissues, and its association with disease progression.
Expression of DDRs in normal and cancerous breast tissues. (a) Normal human breast tissues express DDR1 in the epithelial compartment, whereas DDR2 is not detected [41, 62]. In situ carcinomas are rich in DDR1 but lack substantial DDR2 expression [62]. In normal and in situ carcinoma tissues, the epithelial cells are within the confines of a collagen-IV-containing basement membrane. Thus, in those sites, DDR1, which is activated in response to collagen IV, may be the operative kinase. (b) To metastasize, invasive breast cancer cells penetrate basement membranes and subsequently transmigrate within interstitial matrices, which are enriched in fibrillar collagens. Both DDRs are therefore likely to mediate collagen signaling in disseminated breast cancer cells. Evidence suggests a complex prolife of DDR expression in invasive carcinomas [62]. These tumors appear to display a heterogeneous DDR1 profile with some tumors being highly positive and others negative for DDR1 expression. However, the biological and pathological reasons and the functional consequences for this heterogeneity remain unknown. In contrast, most invasive tumors, particularly TNBC, appear to be consistently associated with higher levels of DDR2 [41, 62, 64], which may become a worthwhile target in this tumor subtype
3.2 Role of DDRs in Experimental Models of Breast Cancer
As discussed above, both IHC and gene expression studies have implicated DDRs in breast cancer progression. In addition, large cancer genome studies from breast cancer tissues have shown alterations in DDR genes, involving mostly copy number aberrations than point mutations or rearrangements. However, by their nature, these types of studies cannot reveal how DDRs contribute to breast cancer development and progression. In the last decade, a number of studies began to dissect the functions of DDRs in breast epithelium and in breast cancer cells in cell culture and animal models. The emerging picture however is rather complex and far from being complete. For instance, in the case of DDR1 there is a significant lack of consensus on whether or not this receptor supports malignant activities, which is also compounded by inconsistencies on the profile of basal DDR1 expression in various breast cancer cell lines, among different laboratories [84–87]. The following section summarizes current information on DDR function in experimental models of breast cancer. A special effort was taken to provide for the specific conditions (cell line, assay type, and in vitro vs. in vivo) of the findings, which we hope aid in data interpretation and a more rigorous assessment of the status of the field.
3.2.1 Roles in Cell Proliferation, Survival, and Apoptosis
DDR1 : DDR1 is expressed in cell lines of human [69] and mouse [88] mammary epithelial cells, where it colocalizes with E-cadherin at cell–cell junctions. DDR1 was reported to stabilize E-cadherin in cell–cell contacts, and consequently DDR1 may play a role in maintenance of epithelial cell organization in cultured cells [88]. There are conflict data regarding the role of DDR1 in proliferation of breast cancer cells. Gao et al. reported significant inhibition of cell proliferation in various breast cancer cell lines (MCF7, T47D, and MDA-MB-435S) when treated with a specific DDR1 type I kinase inhibitor [89]. However, a selective DDR1 type II kinase inhibitor (DDR1-IN-1) that inhibited collagen-induced activation showed no antiproliferative effects in T47D and SKBR3 breast cancer cells at concentrations below 10 μM, which effectively and selectively blocked receptor activation over other kinases including DDR2 [90]. Therefore, any effect of DDR1-IN-1 on cell proliferation at concentrations above 10 μM was attributed to inhibition of other kinases, possibly in working in conjunction with DDR1 [90]. Consistent with the lack of effect of DDR1 inhibition on cell proliferation, collagen stimulation of adherent T47D cells had modest effect on phosphorylation of ERK1/2, a known effector of proliferation stimuli mediated by the MAPK pathway [91]. Moreover, robust DDR1 collagen-induced activation in suspended T47D cells did not result in ERK1/2 phosphorylation [91]. Thus, a clear relationship between the extent and kinetics of DDR1 activation and the MAPK pathway could not be established in T47D cells. Surprisingly, using the same DDR1-IN-1 inhibitor in MCF7 cells, Malaguarnera et al. found partial inhibition (~20 %) of cell proliferation at doses of 0.4 and 1 μM [87]. The reasons for these conflicting results are unclear but may include issues of inhibitor selectivity and affinity, differences among cell lines, and experimental conditions, just to mention a few. It should also be noted that these studies were conducted without collagen stimulation. Therefore, in addition to the above-mentioned issues, the effect of DDR1 kinase inhibitors on collagen-stimulated cell proliferation, including in 2D and 3D collagen microenvironments, remains unclear .
Other studies utilized RNA interference (RNAi) to address the function of DDR1 in cell proliferation. Experiments aimed at identifying genes essential for cancer cell survival, using a library of shRNAs to screen 72 cancer cell lines, 29 of which were breast cancer cell lines, identified DDR1 as one the genes to be critical for cell proliferation [92]. In these studies, RNA silencing of DDR1 in Cal51, MCF7, Sk-Br-3, BT-20, HCC1954, and HCC38 breast cancer cells inhibited cell proliferation, suggesting that DDR1 is critical for cell growth in breast cancer lines belonging to different breast cancer subtypes [92]. Similar results were reported in the studies of Malaguarnera et al. [87], which showed that transient silencing of DDR1 expression reduced cell proliferation and colony formation in MCF-7, BT-474, and MDA-MB-231 breast cancer cells. In contrast, overexpression of wild-type or kinase dead (K618A) DDR1 enhanced these activities, suggesting a role for DDR1 in in vitro cell growth and survival, independently of receptor phosphorylation. Although the effects of collagen under those conditions were not tested, the investigators found that DDR1 was also required for insulin growth factor (IGF)-1-stimulated cell proliferation [87]. Interestingly, IGF-1 stimulation of MCF7 cells resulted in collagen-independent DDR1 phosphorylation, which was ascribed to the formation of a complex between DDR1 and IGF-1R, leading to rapid DDR1 phosphorylation and internalization, in the absence of collagen. Therefore, the enhancement of proliferation and survival in these breast cancer cells by IGF-1 was mediated in part by activation of DDR1 independently of collagen but required IGF-1R.
Large-scale functional gene screen approaches identified DDR1 as one of the genes required for cell viability in several breast epithelial cell lines. For instance, shRNA screens found that DDR1 downregulation inhibits the viability of human mammary epithelial cells (HMECs) isolated from a reduction mammoplasty and immortalized with human telomerase [93]. Another study aimed at identifying essential genes in human breast epithelial cells by RNAi screening found DDR1 to be one of the genes required for the survival of MCF10A cells and breast cancer MDA-MB-435 cells [94]. Thus, according to these studies, DDR1 plays a role in support of cell survival.
DDR1 and DDR2 are among the RTKs that have been implicated in the resistance of TNBC cell lines (SUM159 and MDA-MB-231) and genetically engineered C3tag mice harboring mammary tumors to MEK inhibitors [77]. It was found that transcriptional induction of DDR1 and DDR2 expression were part of a kinome reprograming mechanism in TNBC cells, which allowed the tumor cells to escape the growth arrest induced in response to MEK inhibition. Under these conditions, kinome reprograming was mediated by the proteolytic degradation of Myc as a result of MEK−ERK inhibition. The role of DDRs in this resistance mechanism was demonstrated by siRNA knockdown of DDR1 or DDR2 (among other kinases), which restored growth inhibition in SUM159 and MDA-MB-231 cells treated with two MEK inhibitors. Thus, DDRs appear to play a key role in supporting the proliferation of TNBC cells in the presence of MEK inhibitors. Interestingly, siRNAs to DDR1 in untreated SUM159 cells had inconsistent effects on cell proliferation, in spite of knockdown of DDR1 expression of 70–90 %. Likewise, knockdown of DDR2 in untreated SUM159 and MDA-MB-231 cells had modest effect on cell growth [77]. These studies suggest that in these TNBC cell lines, DDRs are not critical for cell proliferation under basal conditions. However, DDRs appear to be critical in supporting proliferation of TNBC cells that become resistant to therapies targeting the RAF−MEK−ERK pathway. These results suggest that DDRs may constitute promising therapeutic targets in TNBC patients who fail to respond to MEK-targeted therapies. However, more studies are required to determine whether DDRs are also involved in resistance to other therapies in breast cancer patients.
Contrary to the findings suggesting a role for DDR1 in cell proliferation and survival, studies with MCF7 and ZR-75-1 breast cancer cells cultured within a 3D collagen matrix suggested a role for DDR1 in 3D collagen-induced apoptosis, in a process that was proposed to involve the induction of Bcl-2-interacting killer (BIK) protein, a proapoptotic member of the Bcl-2 family [95]. Consistently, inhibition of DDR1 phosphorylation with inhibitor DDR1-IN-1 [90] reduced apoptosis and induced BIK in cells cultured within 3D collagen [95]. Interestingly, MT1-MMP, a potent membrane-anchored, was shown to prevent apoptosis of breast cancer cells in 3D collagen [96] and to negatively regulate DDR1 activation by initiating receptor cleavage [95, 97]. Therefore, it was proposed that an MT1−MMP/DDR1 axis regulates apoptosis of breast cancer cells embedded in 3D collagen in which collagenolytic activity of the protease and DDR1 cleavage support survival of the tumor cells [95]. Although these studies were limited to two ERα-positive luminal breast cancer lines, the proposed model of DDR1 action in cell survival takes into consideration that invasive breast cancer cells thrive within a stroma enriched in collagen fibers, which elicits profound effects on cell behavior, likely to be mediated in part by DDR1 signaling. More studies are needed to address the pro- and antiapoptotic effects of DDR1 in a broad spectrum of breast cancer cell lines in various matrix conditions. In summary, the accumulating data points to a role for DDR1 in supporting cell proliferation and survival in breast cancer cells. However, there are still important inconsistences, which appear to be related to the assay conditions (plastic vs. 3D collagen) and approaches to stimulate or inhibit DDR1 expression/activity.
DDR2 : Silencing of DDR2 expression in MDA-MB-231 and 4T1 breast cancer cells had no effect on cell proliferation in vitro [41]. However, studies on kinome reprograming showed that resistance of MDA-MB-231 and SUM159 breast cancer cells to a MEK1/2 inhibitor resulted in de novo expression of DDR2 [77], suggesting a prosurvival for DDR2. Consistently, downregulation of DDR2 resulted in strong synthetic lethality in the presence of the MEK1/2 inhibitor. These studies suggest that DDR2 may be required for cell proliferation and survival under stress conditions.
3.2.2 Roles in Cell Migration , Invasion, and Metastasis
A key aspect of malignancy is the ability of cancer cells to migrate and invade through extracellular matrices, including basement membranes and interstitial stroma, both of which are rich in collagen proteins. Because DDRs are collagen receptors, many studies addressed the contribution of DDRs to breast cancer migration and invasion in various established assays utilizing Matrigel, a reconstituted basement membrane enriched in collagen IV and laminin, and collagen I, a component of the breast stromal matrix, as protein barriers. Matrigel is used to mimic the step of basement membrane invasion and is expected to induce the activation of DDR1 but not of DDR2 [1]. In contrast, collagen I is an activator of both DDR1 and DDR2 [1]. Therefore, if ligand-dependent DDR activation were required for tumor cell dissemination (migration, invasion, and metastasis), DDR1 would be expected to play a role during penetration of basement membranes, whereas DDR1 and DDR2 would play a role during invasion of the interstitial matrix. Thus, a breast cancer cell with a defined set of DDRs may or may not activate these receptors in migration/invasion depending on the nature of the matrix environment utilized in the assay. On the other hand, effects of DDRs may not necessary be mediated via their kinase activity, which would indicate ligand-independent effects. However, a clear understanding how DDRs elicit biological actions without activating their kinase function is still missing.
DDR1 : The role of DDR1 in experimental models of breast cancer metastasis in mice remains to be determined. However, there are multiple reports on its role migration and invasion of breast cancer cells in in vitro assays. Castro-Sanchez et al. reported that collagen-IV-induced migration [98] and Matrigel invasion [84] of MDA-MB-231 breast cancer cells required expression of DDR1. This effect of collagen IV involved induction of the tetraspanin CD9 through a DDR1-dependent pathway, which in turn induced the expression of MMP-2 and MMP-9, two MMPs associated with tumor cell invasion [84, 98]. In agreement with a promigratory role for DDR1, Neuhaus et al. found that silencing of DDR1 mRNA in human nonmalignant HB2 breast epithelial cells and T47D and MDA-MB-468 breast cancer cells reduced cell migration, as determined using Boyden chambers assembled with uncoated filters, and fibroblast conditioned media as chemoattractant [99]. These investigators also found that DDR1 restored the antimigratory activity induced by Syk (spleen tyrosine kinase), a nonreceptor tyrosine kinase shown to elicit antimalignant effects in breast carcinoma cells [100], and shown to form a complex with DDR1 in nonmalignant HC11 mammary epithelial cells [99, 101]. Malaguarnera et al. [87] also reported promigratory effects of DDR1 in MCF-7, BT-474, and MDA-MB-231 breast cancer cells in response to IGF-1 using fibronectin- or collagen-IV-coated filters. Indeed, knockdown of DDR1 expression by siRNA reduced cell migration, whereas wild type but to a lesser extent kinase dead DDR1 overexpression enhanced migration. As with the findings with cell proliferation, DDR1 also regulated IGF-1-stimulated cell migration. Interestingly, because the ability of DDR1 to support cell migration was also observed on fibronectin substrates, the investigators concluded that DDR1 regulation of cell migration is independent of its function as a collagen receptor [87]. Studies in MDA-MB-231 cells with depleted DDR1 expression by siRNA showed that DDR1 was required for in vitro invasion of a 3D collagen I matrix [85]. This process appeared to involve DDR1-mediated formation of invadosomes, an F-actin-rich structure that was shown to focus proteolytic activity in invasive cancer cells and thus facilitates matrix invasion [102]. Moreover, the proinvasive effects of DDR1 required metalloproteinase activity [85]. Interestingly, Juin et al. showed that the involvement of DDR1 in invadosome formation in response to collagen I was independent of its kinase activity [85]. The proposed mechanism for collagen I-induced invadosome formation involved the activation of the Rho GTPase cell division control protein 42 homolog (Cdc42), which required DDR1 expression. DDR1’s role in Cdc42 activation involved the action of the guanine nucleotide-exchange factor, Tuba, which colocalized with DDR1 in invadosomes [85]. However, how DDR1 mediates these effects, independently of its kinase activity, when invasive cells confront the fibrillar collagen matrix, which is known to induce receptor phosphorylation, remains unclear.
Contrary to the reports ascribing a promigratory effect of DDR1 in breast cancer cells, other studies found DDR1 to be a negative regulator of cell migration in breast epithelial cells regardless of malignancy status. Using the yeast two-hybrid system to identify DDR1 interacting proteins, Hansen et al. identified DARPP-32 (dopamine and cAMP-regulated phosphoprotein of 32 kDa), a protein initially known to play a role in regulation of neurotransmission, as a DDR1-binding protein. Functional assays revealed that coexpression of DARPP-32 and DDR1 in breast cancer cells inhibited in vitro cell migration [86]. This effect required a Wnt5a-cAMP-PKA-mediated phosphorylation of DARPP-32 at threonine 34 [103]. Interestingly, Wnt5a, a noncanonical Wnt signaling protein, was shown to enhance cell adhesion and consequently to inhibit cell migration of breast cancer cells [104, 105]. Importantly, downregulation of Wnt5a impaired collagen-dependent DDR1 activation suggesting that the antimigratory effects of Wnt5a, and possibly its tumor suppressive roles in breast cancer, are mediated in part by DDR1 activation [65, 104–106]. In breast tumor specimens, loss of Wnt5a correlated with disease recurrence and was an independent predictor of poor prognosis [101, 107], consistent with a tumor suppressive role for Wnt5a in breast cancer progression [106–109]. If so, it will be interesting to examine whether loss of Wnt5a in aggressive breast tumors is also associated with reduced levels of phosphorylated DDR1 . Nevertheless, the potential relationship between Wnt5a and DDR1 activation suggest a tumor suppressive role for DDR1 in certain breast cancers, possibly as a negative regulator of cell migration. The studies of Koh et al. also showed an antimigratory and anti-invasive effect of DDR1 in both nonmalignant and malignant breast epithelial cells [72]. In MCF10A cells, silencing of DDR1 expression enhanced in vitro cell migration and invasion, whereas overexpression of DDR1 in triple negative Hs587T and MDA-MB-231 breast cancer cells inhibited in vitro and in vivo cell invasion [72], consistent with DDR1 being a suppressor of promalignant cellular activities. Consistently, expression of mutated H-Ras in MCF10A cells, which induced EMT via Zeb1 upregulation, resulted in acquisition of a migratory and invasive phenotype with a concomitant transcriptional downregulation of DDR1 [72]. Likewise, ectopic expression of Zeb1 in MCF10A cells inhibited DDR1 expression [72]. DDR1 downregulation was also observed after treatment of MCF10A cells with TGF-β, which upregulated Snail1 and Twist1 [41]. Interestingly, earlier studies showed that TGF-β treatment induces the in vitro migration and invasion of MCF10A cells [110], raising the possibility that the promigratory effects of TGF-β were mediated in part by DDR1 downregulation. Although this possibility remains to be proven, the emerging evidence suggests that inhibition of DDR1 expression is part of the EMT program that promotes malignancy [82]. However, loss of DDR1 expression alone was not sufficient to induce EMT in parental MCF10A cells but, interestingly, it was sufficient to enhance migratory and invasive activities [72]. These results suggest that although DDR1 is not a direct regulator of EMT in breast epithelial cells, it can directly influence cell migratory activities. The molecular mechanism(s) by which DDR1 regulates cell migration in breast epithelial cells remains to be defined.
An association between DDR1 and EMT is also suggested by the findings with KIBRA, a protein that was shown to bind DDR1 in MCF10A and T47-D cells [31] and to regulate EMT via the Hippo pathway in MCF10A cells [111]. Early studies showed that DDR1 forms a stable complex with KIBRA that dissociates upon stimulation with collagen [31]. It was also shown that knockdown of KIBRA in MCF10A cells reduced collagen IV-induced ERK phosphorylation, suggesting that KIBRA, upon dissociation from the DDR1/KIBRA complex, plays a permissive role in collagen IV-induced MAPK activation [31]. However, whether the role of KIBRA in regulation of collagen IV-induced ERK phosphorylation is mediated by DDR1 activation remains to be determined. This missing information is critical because collagen may activate the MAPK pathway via other receptors (i.e., integrins) [112, 113]. Interestingly, Moleirinho et al. reported that silencing of KIBRA expression in MCF10A cells induced expression of EMT markers and enhanced cell migration, a process that was related to decreased LATS and YAP phosphorylation [111], two kinases involved in the Hippo pathway [114]. However, the role of DDR1 in EMT induced by downregulation of KIBRA remains to be determined. Regardless, these data suggest the existence of a crosstalk between DDR1, KIBRA, and the Hippo pathway in regulation of EMT in breast epithelial cells. Notably, accumulating evidence shows a key role for the Hippo pathway in dictating the cellular phenotype in response to cell–matrix interactions, in particular in response to matrix stiffness [115, 116]. Because DDRs are collagen receptors, which may play a role in mechanosensing [117], it will be interesting to investigate the role of DDRs in regulation of Hippo pathway in breast development and breast cancer progression.
Taken together, the emerging evidence points to a complex role for DDR1 in breast cancer cell behavior, and therefore at this junction there is no clear consensus as to whether DDR1 plays suppressive or promoting effects or both on disease progression. This complexity is further highlighted by the finding of a heterogeneous expression of DDR1 in breast tissues showing that approximately 50 % of invasive breast carcinomas display high levels of DDR1, whereas the other half show reduced or no expression of receptor [58]. Thus, it is reasonable to speculate that the function of DDR1 in disease progression may be dictated by the unique genomic and proteomic makeup of each particular breast tumor. Identifying the molecular and environmental factors that influence DDR1 action in breast cancer will help selecting those patients who will potentially benefit from anti-DDR1 therapies.
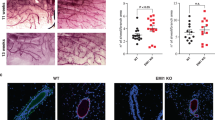
DDR2 : The accumulating data point to a role for DDR2 in supporting the invasive and metastatic activities of breast cancer cells both in in vitro and in vivo assays. Using human MDA-MB 231 and mouse 4T1 breast cancer cells, and RNA silencing, Zhang et al. [41] demonstrated a key role for DDR2 in supporting in vitro tumor cell migration and invasion through a collagen matrix. Importantly, DDR2-silenced 4T1 cells inoculated into the mammary fat pad developed fewer lung metastases [41]. In agreement with these studies, Ren et al. [71] found that DDR2 supported the metastatic ability of MDA-MB-231 cells inoculated into the mammary fat pad. The effects of DDR2 on metastases were specific to the process of tumor cell dissemination because DDR2 had no effect on the growth of the primary tumors [41, 71]. The prometastatic role of DDR2 was associated with the development of EMT and the ability of DDR2 to support Snail1 protein stabilization via an ERK-mediated pathway [41]. Ren et al. also showed that hypoxic conditions enhanced DDR2 expression in SK-BR3, MDA-MB-231 MDA-MD-468, and MCF-7 cells, concomitant with a promigratory and proinvasive in vitro activity, which dependent on DDR2 expression [71]. The association between EMT and DDR2 was also shown in nonmalignant MCF10A cells, which upon treatment with TFG-β display induction of the EMT transcription factors Snail1 and Twist1, with the concomitant increase in DDR2 expression [41]. Thus, DDR2 is an EMT upregulated gene in both nonmalignant and malignant breast epithelial cells, which supports cell migration. A recent study in genetically modified mice harboring a specific deletion of DDR2 and mammary tumor virus-polyoma middle T antigen (MMTV-PyMT) tumors demonstrated the importance of this receptor in the development of breast cancer metastases, but not in the formation of primary tumors [118]. Importantly, these studies highlighted the contribution of stromal DDR2, rather than tumor-derived DDR2, in formation of lung metastases. Specifically, transplantation of MMTV-PyMT breast cancer cells into syngeneic mice with global DDR2 deletion significantly inhibited lung metastasis formation, regardless of the expression of DDR2 in the tumor cells. Further analyses showed that the primary tumors growing in DDR2-deficient mice displayed a significant reduction in fibrillar collagen and reduced vascularization. Disruption in collagen deposition and organization was ascribed to ineffective function of the cancer-associated fibroblasts, a major collagen-producing cells, present in the DDR2-null mice. These cells also exhibited a reduced fibrogenic gene expression profile suggesting that DDR2 is a pro-fibrotic gene in tumor stroma. Collectively, these studies demonstrated a key role for tumor and stromal DDR2 in breast cancer metastases, and provided strong support for developing DDR2 inhibitors for the treatment of metastatic breast cancer [118]. Figure 7.3 provides a schematic representation of the reported functions of DDRs in experimental models of breast cancer.
Potential roles of DDRs in breast cancer cells. DDR1 : (a) DDR1 promotes or supports cell proliferation and survival in response IGF-1 or MEK1/2 inhibitors. Knockdown of DDR1 expression inhibits cell proliferation in MEK-resistant cells [77], unstimulated or IGF1-stimulated cells [87], or in genetic RNAi screen assays [92–94]. (b) Tyrosine kinase inhibitors (TKi) of DDR1 activity provided conflicting results (magenta box), with some inhibiting [87, 89] and others having no effect [90]. Likewise, downregulation of DDR1 by RNAi, under basal conditions, had no effects on cell proliferation [77]. (c) DDR1 mediates the induction of apoptosis in cells grown within 3D-collagen I [95]. This effect is blocked upon cleavage of DDR1 by MMP14 [96]. (d) DDR1 supports cell migration in response to IGF-1 [87], 3D-collagen I [85], and collagen IV [87, 98], and supports invasion through Matrigel [84]. Inhibition of DDR1 expression by RNAi blocks migration [85, 87, 99]. Syk kinase elicits its an-migratory activity by blocking DDR1 [99]. (e) DDR1 is an inhibitor of cell migration and invasion: H-Ras/Zeb1 induces migration and invasion by downregulating DDR1 expression [72], and Wnt5a blocks migration by impairing collagen-dependent DDR1 activation [65, 105]. Inhibition of DDR1 expression by RNAi enhances migration and invasion [72]. Coexpression of DARPP-32 and DDR1 inhibits cell migration [103]. DDR2: (a) DDR2 is induced in response to resistance to MEK1/2 inhibition in TNBC cells, as a part of a kinome reprograming process designed to promote cell survival. DDR2 RNAi elicits synthetic lethality when administered with MEK inhibitors [77]. (b) DDR2 is not required for in vitro and in vivo proliferation of breast cancer cell lines [41, 71], as determined by RNAi. (c) TGF-β and hypoxia induce DDR2 expression and consequently enhance in vitro migration and invasion [41, 71]. (d) DDR2 is required for metastatic dissemination of human breast cancer cells when inoculated into mammary fat pads [41, 71] and in the mouse MMTV-PyMT model of mammary cancer [118]. Solid arrows show a positive or negative effect, dotted arrows indicate conflicting results; green boxes indicate a positive effect, and red boxes indicate an inhibitory effect
4 Conclusions: DDRs in Breast Tissues—an Evolving Field
The importance of the ECM in mammary gland development and breast cancer progression, the pivotal role that RTKs play in cancer, and the fact that DDRs are the only kinases that recognize collagen as their ligands provide a strong impetus to decipher the action of DDRs in normal and malignant breast tissues. Overall, the accumulated evidence strongly suggests a central role for DDRs in both normal and malignant breast tissues. However, in spite of the encouraging findings, so far the overall picture remains incomplete and in many cases the evidence is limited and contradictory. Certain DDR-mediated functions have yet to be confirmed or reproduced. Issues of kinase or ligand dependency for the biological effects observed remain unclear. Significant inconsistencies exist in the experimental conditions; for instance, the contribution of collagen (2D, 3D) when analyzing effects on cell proliferation/survival are not clearly delineated. Likewise, the nature and status of the substrate and/or presence of collagen ligands in assays of cell migration and invasion are usually not addressed. Analyses of receptor activation status and kinetics in functional assays are not always examined. These uncertainties are further compounded by the fact that cell lines may express both DDRs, which may complicate data interpretation in conditions that use common ligands. Moreover, discrepancies exist in the basal levels of DDRs expressed by breast cancer cell lines. We also lack a thorough understanding of the signaling networks and downstream effectors regulated by DDRs in breast epithelial cells in response to collagen stimulation. Importantly, how these DDR signaling outcomes are affected, by the biophysical status of the collagen matrix, which may be key in conditions in which disease progression is associated with tissue stiffness. In this regard, the role of DDRs in mechanosensing remains practically unknown. In mice studies, cell-specific deletion of DDRs in the mammary gland need to be performed to clearly define the role of DDRs in development and differentiation. In human tissues, more studies are required to better define the relative levels of expression and receptor activation in various breast cancer subtypes and in primary vs. metastatic sites, utilizing highly specific antibodies and probes. Finally, the assessment of DDRs as therapeutic targets in breast cancer awaits the development of specific kinase inhibitors, a major challenge, but one that will help to elucidate their contribution to disease progression in more rigorous ways. These are challenges that are constantly met and thus we are hopeful that new and exciting findings will illuminate the DDR field and elucidate their role and therapeutic value in normal and diseased breast tissues .
References
Fu HL et al (2013) Discoidin domain receptors: unique receptor tyrosine kinases in collagen-mediated signaling. J Biol Chem 288(11):7430–7437
Inman JL et al (2015) Mammary gland development: cell fate specification, stem cells and the microenvironment. Development 142(6):1028–1042
Sternlicht MD (2006) Key stages in mammary gland development: the cues that regulate ductal branching morphogenesis. Breast Cancer Res 8(1):201
Oskarsson T (2013) Extracellular matrix components in breast cancer progression and metastasis. Breast 22(Suppl 2):S66–S72
Muschler J, Streuli CH (2010) Cell-matrix interactions in mammary gland development and breast cancer. Cold Spring Harb Perspect Biol 2(10):a003202
Obr AE et al (2013) Progesterone receptor and Stat5 signaling cross talk through RANKL in mammary epithelial cells. Mol Endocrinol 27(11):1808–1824
Fernandez-Valdivia R, Lydon JP (2012) From the ranks of mammary progesterone mediators, RANKL takes the spotlight. Mol Cell Endocrinol 357(1-2):91–100
Fernandez-Valdivia R et al (2005) Revealing progesterone’s role in uterine and mammary gland biology: insights from the mouse. Semin Reprod Med 23(1):22–37
Wiseman BS, Werb Z (2002) Stromal effects on mammary gland development and breast cancer. Science 296(5570):1046–1049
Sakakura T, Suzuki Y, Shiurba R (2013) Mammary stroma in development and carcinogenesis. J Mammary Gland Biol Neoplasia 18(2):189–197
Lambert AW, Ozturk S, Thiagalingam S (2012) Integrin signaling in mammary epithelial cells and breast cancer. ISRN Oncol 2012:493283
Schedin P, Keely PJ (2011) Mammary gland ECM remodeling, stiffness, and mechanosignaling in normal development and tumor progression. Cold Spring Harb Perspect Biol 3(1):a003228
Brownfield DG et al (2013) Patterned collagen fibers orient branching mammary epithelium through distinct signaling modules. Curr Biol 23(8):703–709
Ingman WV et al (2006) Macrophages promote collagen fibrillogenesis around terminal end buds of the developing mammary gland. Dev Dyn 235(12):3222–3229
Rodriguez D, Morrison CJ, Overall CM (2010) Matrix metalloproteinases: what do they not do? New substrates and biological roles identified by murine models and proteomics. Biochim Biophys Acta 1803(1):39–54
Faraci-Orf E, McFadden C, Vogel WF (2006) DDR1 signaling is essential to sustain Stat5 function during lactogenesis. J Cell Biochem 97(1):109–121
Vogel WF et al (2001) Discoidin domain receptor 1 tyrosine kinase has an essential role in mammary gland development. Mol Cell Biol 21(8):2906–2917
Cellurale C et al (2012) Role of JNK in mammary gland development and breast cancer. Cancer Res 72(2):472–481
Crowley MR, Bowtell D, Serra R (2005) TGF-beta, c-Cbl, and PDGFR-alpha the in mammary stroma. Dev Biol 279(1):58–72
Joseph H et al (1999) Overexpression of a kinase-deficient transforming growth factor-beta type II receptor in mouse mammary stroma results in increased epithelial branching. Mol Biol Cell 10(4):1221–1234
Roarty K, Serra R (2007) Wnt5a is required for proper mammary gland development and TGF-beta-mediated inhibition of ductal growth. Development 134(21):3929–3939
Fernandez-Valdivia R et al (2008) Transcriptional response of the murine mammary gland to acute progesterone exposure. Endocrinology 149(12):6236–6250
Brisken C et al (1999) Prolactin controls mammary gland development via direct and indirect mechanisms. Dev Biol 210(1):96–106
Cui Y et al (2004) Inactivation of Stat5 in mouse mammary epithelium during pregnancy reveals distinct functions in cell proliferation, survival, and differentiation. Mol Cell Biol 24(18):8037–8047
Miyoshi K et al (2001) Signal transducer and activator of transcription (Stat) 5 controls the proliferation and differentiation of mammary alveolar epithelium. J Cell Biol 155(4):531–542
Mukherjee A et al (2010) Targeting RANKL to a specific subset of murine mammary epithelial cells induces ordered branching morphogenesis and alveologenesis in the absence of progesterone receptor expression. FASEB J 24(11):4408–4419
Santos SJ, Haslam SZ, Conrad SE (2010) Signal transducer and activator of transcription 5a mediates mammary ductal branching and proliferation in the nulliparous mouse. Endocrinology 151(6):2876–2885
Fata JE et al (2000) The osteoclast differentiation factor osteoprotegerin-ligand is essential for mammary gland development. Cell 103(1):41–50
Rayala SK et al (2006) Essential role of KIBRA in co-activator function of dynein light chain 1 in mammalian cells. J Biol Chem 281(28):19092–19099
Harris J et al (2006) Socs2 and elf5 mediate prolactin-induced mammary gland development. Mol Endocrinol 20(5):1177–1187
Hilton HN et al (2008) KIBRA interacts with discoidin domain receptor 1 to modulate collagen-induced signalling. Biochim Biophys Acta 1783(3):383–393
Chen J et al (2002) The alpha(2) integrin subunit-deficient mouse: a multifaceted phenotype including defects of branching morphogenesis and hemostasis. Am J Pathol 161(1):337–344
Gardner H et al (1996) Deletion of integrin alpha 1 by homologous recombination permits normal murine development but gives rise to a specific deficit in cell adhesion. Dev Biol 175(2):301–313
Spike BT et al (2012) A mammary stem cell population identified and characterized in late embryogenesis reveals similarities to human breast cancer. Cell Stem Cell 10(2):183–197
Wansbury O et al (2011) Transcriptome analysis of embryonic mammary cells reveals insights into mammary lineage establishment. Breast Cancer Res 13(4):R79
Bai L, Rohrschneider LR (2010) s-SHIP promoter expression marks activated stem cells in developing mouse mammary tissue. Genes Dev 24(17):1882–1892
Shackleton M et al (2006) Generation of a functional mammary gland from a single stem cell. Nature 439(7072):84–88
Kano K et al (2008) A novel dwarfism with gonadal dysfunction due to loss-of-function allele of the collagen receptor gene, Ddr2, in the mouse. Mol Endocrinol 22(8):1866–1880
Labrador JP et al (2001) The collagen receptor DDR2 regulates proliferation and its elimination leads to dwarfism. EMBO Rep 2(5):446–452
Cowling RT et al (2014) Discoidin domain receptor 2 germline gene deletion leads to altered heart structure and function in the mouse. Am J Physiol Heart Circ Physiol 307(5):H773–H781
Zhang K et al (2013) The collagen receptor discoidin domain receptor 2 stabilizes SNAIL1 to facilitate breast cancer metastasis. Nat Cell Biol 15(6):677–687
Dontu G et al (2003) In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes Dev 17(10):1253–1270
Huang S et al (2005) Changes in gene expression during the development of mammary tumors in MMTV-Wnt-1 transgenic mice. Genome Biol 6(10):R84
Li Y et al (2003) Evidence that transgenes encoding components of the Wnt signaling pathway preferentially induce mammary cancers from progenitor cells. Proc Natl Acad Sci U S A 100(26):15853–15858
Rosen PP (2009) Rosen’s breast pathology, 3rd edn. Wolters Kluwer/Lippincott Williams & Wilkins, Philadelphia, p 1116
Elledge RM et al (1998) HER-2 expression and response to tamoxifen in estrogen receptor-positive breast cancer: a Southwest Oncology Group Study. Clin Cancer Res 4(1):7–12
Osborne CK (1998) Tamoxifen in the treatment of breast cancer. N Engl J Med 339(22):1609–1618
Slamon D, Pegram M (2001) Rationale for trastuzumab (Herceptin) in adjuvant breast cancer trials. Semin Oncol 28(1 Suppl 3):13–19
Perou CM et al (2000) Molecular portraits of human breast tumours. Nature 406(6797):747–752
Cancer Genome Atlas Network (2012) Comprehensive molecular portraits of human breast tumours. Nature 490(7418):61–70
Burstein MD et al (2015) Comprehensive genomic analysis identifies novel subtypes and targets of triple-negative breast cancer. Clin Cancer Res 21(7):1688–1698
Maskarinec G et al (2013) Mammographic density as a predictor of breast cancer survival: the Multiethnic Cohort. Breast Cancer Res 15(1):R7
Tice JA et al (2013) Benign breast disease, mammographic breast density, and the risk of breast cancer. J Natl Cancer Inst 105(14):1043–1049
Ursin G et al (2005) Greatly increased occurrence of breast cancers in areas of mammographically dense tissue. Breast Cancer Res 7(5):R605–R608
Boyd NF et al (2001) Mammographic density as a marker of susceptibility to breast cancer: a hypothesis. IARC Sci Publ 154:163–169
Martin LJ, Boyd NF (2008) Mammographic density. Potential mechanisms of breast cancer risk associated with mammographic density: hypotheses based on epidemiological evidence. Breast Cancer Res 10(1):201
Paszek MJ et al (2005) Tensional homeostasis and the malignant phenotype. Cancer Cell 8(3):241–254
Provenzano PP, Eliceiri KW, Keely PJ (2009) Shining new light on 3D cell motility and the metastatic process. Trends Cell Biol 19(11):638–648
Provenzano PP et al (2006) Collagen reorganization at the tumor-stromal interface facilitates local invasion. BMC Med 4(1):38
Valiathan RR et al (2012) Discoidin domain receptor tyrosine kinases: new players in cancer progression. Cancer Metastasis Rev 31(1-2):295–321
Vogel W et al (1997) The discoidin domain receptor tyrosine kinases are activated by collagen. Mol Cell 1(1):13–23
Toy KA et al (2015) Tyrosine kinase discoidin domain receptors DDR1 and DDR2 are coordinately deregulated in triple-negative breast cancer. Breast Cancer Res Treat 150(1):9–18
Yeh YC, Wang CZ, Tang MJ (2009) Discoidin domain receptor 1 activation suppresses alpha2beta1 integrin-dependent cell spreading through inhibition of Cdc42 activity. J Cell Physiol 218(1):146–156
Ren T et al (2013) Increased expression of discoidin domain receptor 2 (DDR2): a novel independent prognostic marker of worse outcome in breast cancer patients. Med Oncol 30(1):397
Dejmek J et al (2003) Wnt-5a and G-protein signaling are required for collagen-induced DDR1 receptor activation and normal mammary cell adhesion. Int J Cancer 103(3):344–351
Turashvili G et al (2007) Novel markers for differentiation of lobular and ductal invasive breast carcinomas by laser microdissection and microarray analysis. BMC Cancer 7:55
Wang CZ, Yeh YC, Tang MJ (2009) DDR1/E-cadherin complex regulates the activation of DDR1 and cell spreading. Am J Physiol Cell Physiol 297(2):C419–C429
Eswaramoorthy R et al (2010) DDR1 regulates the stabilization of cell surface E-cadherin and E-cadherin-mediated cell aggregation. J Cell Physiol 224(2):387–397
Hidalgo-Carcedo C et al (2011) Collective cell migration requires suppression of actomyosin at cell-cell contacts mediated by DDR1 and the cell polarity regulators Par3 and Par6. Nat Cell Biol 13(1):49–58
Ameli F, Rose IM, Masir N (2015) Expression of DDR1 and DVL1 in invasive ductal and lobular breast carcinoma does not correlate with histological type, grade and hormone receptor status. Asian Pac J Cancer Prev 16(6):2385–2390
Ren T et al (2014) Discoidin domain receptor 2 (DDR2) promotes breast cancer cell metastasis and the mechanism implicates epithelial-mesenchymal transition programme under hypoxia. J Pathol 234(4):526–537
Koh M et al (2015) Discoidin domain receptor 1 is a novel transcriptional target of ZEB1 in breast epithelial cells undergoing H-Ras-induced epithelial to mesenchymal transition. Int J Cancer 136(6):E508–E520
Morikawa A et al (2015) Expression of beclin-1 in the microenvironment of invasive ductal carcinoma of the breast: correlation with prognosis and the cancer-stromal interaction. PLoS One 10(5):e0125762
Lehmann BD et al (2011) Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest 121(7):2750–2767
Al-Ejeh F et al (2014) Kinome profiling reveals breast cancer heterogeneity and identifies targeted therapeutic opportunities for triple negative breast cancer. Oncotarget 5(10):3145–3158
Mayer IA et al (2014) New strategies for triple-negative breast cancer—deciphering the heterogeneity. Clin Cancer Res 20(4):782–790
Duncan JS et al (2012) Dynamic reprogramming of the kinome in response to targeted MEK inhibition in triple-negative breast cancer. Cell 149(2):307–321
Cerami E et al (2012) The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov 2(5):401–404
Gentles AJ et al (2015) The prognostic landscape of genes and infiltrating immune cells across human cancers. Nat Med 21(8):938–945
Gyorffy B et al (2013) Online survival analysis software to assess the prognostic value of biomarkers using transcriptomic data in non-small-cell lung cancer. PLoS One 8(12):e82241
Jezequel P et al (2013) bc-GenExMiner 3.0: new mining module computes breast cancer gene expression correlation analyses. Database (Oxford) 2013:bas060
Taube JH et al (2010) Core epithelial-to-mesenchymal transition interactome gene-expression signature is associated with claudin-low and metaplastic breast cancer subtypes. Proc Natl Acad Sci U S A 107(35):15449–15454
Reva B, Antipin Y, Sander C (2011) Predicting the functional impact of protein mutations: application to cancer genomics. Nucleic Acids Res 39(17):e118
Castro-Sanchez L et al (2011) Role of DDR1 in the gelatinases secretion induced by native type IV collagen in MDA-MB-231 breast cancer cells. Clin Exp Metastasis 28(5):463–477
Juin A et al (2014) Discoidin domain receptor 1 controls linear invadosome formation via a Cdc42-Tuba pathway. J Cell Biol 207(4):517–533
Hansen C et al (2006) Phosphorylation of DARPP-32 regulates breast cancer cell migration downstream of the receptor tyrosine kinase DDR1. Exp Cell Res 312(20):4011–4018
Malaguarnera R et al (2015) Novel cross talk between IGF-IR and DDR1 regulates IGF-IR trafficking, signaling and biological responses. Oncotarget 6(18):16084–16105
Yeh YC et al (2011) DDR1 triggers epithelial cell differentiation by promoting cell adhesion through stabilization of E-cadherin. Mol Biol Cell 22(7):940–953
Gao M et al (2013) Discovery and optimization of 3-(2-(Pyrazolo[1,5-a]pyrimidin-6-yl)ethynyl)benzamides as novel selective and orally bioavailable discoidin domain receptor 1 (DDR1) inhibitors. J Med Chem 56(8):3281–3295
Kim HG et al (2013) Discovery of a potent and selective DDR1 receptor tyrosine kinase inhibitor. ACS Chem Biol 8(10):2145–2150
L’Hote CG, Thomas PH, Ganesan TS (2002) Functional analysis of discoidin domain receptor 1: effect of adhesion on DDR1 phosphorylation. FASEB J 16(2):234–236
Marcotte R et al (2012) Essential gene profiles in breast, pancreatic, and ovarian cancer cells. Cancer Discov 2(2):172–189
Schlabach MR et al (2008) Cancer proliferation gene discovery through functional genomics. Science 319(5863):620–624
Silva JM et al (2008) Profiling essential genes in human mammary cells by multiplex RNAi screening. Science 319(5863):617–620
Assent D et al (2015) A membrane-type-1 matrix metalloproteinase (MT1-MMP)-discoidin domain receptor 1 axis regulates collagen-induced apoptosis in breast cancer cells. PLoS One 10(3):e0116006
Maquoi E et al (2012) MT1-MMP protects breast carcinoma cells against type I collagen-induced apoptosis. Oncogene 31(4):480–493
Fu HL et al (2013) Shedding of discoidin domain receptor 1 by membrane-type matrix metalloproteinases. J Biol Chem 288(17):12114–12129
Castro-Sanchez L et al (2010) Native type IV collagen induces cell migration through a CD9 and DDR1-dependent pathway in MDA-MB-231 breast cancer cells. Eur J Cell Biol 89(11):843–852
Neuhaus B et al (2011) Migration inhibition of mammary epithelial cells by Syk is blocked in the presence of DDR1 receptors. Cell Mol Life Sci 68(22):3757–3770
Krisenko MO, Geahlen RL (2015) Calling in SYK: SYK’s dual role as a tumor promoter and tumor suppressor in cancer. Biochim Biophys Acta 1853(1):254–263
Dejmek J et al (2005) Expression and signaling activity of Wnt-5a/discoidin domain receptor-1 and Syk plays distinct but decisive roles in breast cancer patient survival. Clin Cancer Res 11(2 Pt 1):520–528
Paz H, Pathak N, Yang J (2014) Invading one step at a time: the role of invadopodia in tumor metastasis. Oncogene 33(33):4193–4202
Hansen C et al (2009) Wnt-5a-induced phosphorylation of DARPP-32 inhibits breast cancer cell migration in a CREB-dependent manner. J Biol Chem 284(40):27533–27543
Safholm A et al (2006) A formylated hexapeptide ligand mimics the ability of Wnt-5a to impair migration of human breast epithelial cells. J Biol Chem 281(5):2740–2749
Jonsson M, Andersson T (2001) Repression of Wnt-5a impairs DDR1 phosphorylation and modifies adhesion and migration of mammary cells. J Cell Sci 114(Pt 11):2043–2053
Serra R et al (2011) Wnt5a as an effector of TGFbeta in mammary development and cancer. J Mammary Gland Biol Neoplasia 16(2):157–167
Jonsson M et al (2002) Loss of Wnt-5a protein is associated with early relapse in invasive ductal breast carcinomas. Cancer Res 62(2):409–416
McDonald SL, Silver A (2009) The opposing roles of Wnt-5a in cancer. Br J Cancer 101(2):209–214
Zhu N et al (2014) Challenging role of Wnt5a and its signaling pathway in cancer metastasis (Review). Exp Ther Med 8(1):3–8
Kim ES, Kim MS, Moon A (2004) TGF-beta-induced upregulation of MMP-2 and MMP-9 depends on p38 MAPK, but not ERK signaling in MCF10A human breast epithelial cells. Int J Oncol 25(5):1375–1382
Moleirinho S et al (2013) KIBRA exhibits MST-independent functional regulation of the Hippo signaling pathway in mammals. Oncogene 32(14):1821–1830
Barczyk M, Carracedo S, Gullberg D (2010) Integrins. Cell Tissue Res 339(1):269–280
Leitinger B (2011) Transmembrane collagen receptors. Annu Rev Cell Dev Biol 27:265–290
Yu FX, Zhao B, Guan KL (2015) Hippo pathway in organ size control, tissue homeostasis, and cancer. Cell 163(4):811–828
Dupont S (2015) Role of YAP/TAZ in cell-matrix adhesion-mediated signalling and mechanotransduction. Exp Cell Res 343(1):42–53
Dupont S et al (2011) Role of YAP/TAZ in mechanotransduction. Nature 474(7350):179–183
Ghosh S et al (2013) Regulation of adipose oestrogen output by mechanical stress. Nat Commun 4:1821
Corsa CAS et al (2016) The action of Discoidin Domain Receptor 2 in basal tumor cells and stromal cancer-associated fibroblasts is critical for breast cancer metastases. Cell Rep 15(11):2510–2523
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer Science+Business Media New York
About this chapter
Cite this chapter
Ekanayaka, S.A., Kleer, C.G., Bollig-Fischer, A., Fernandez-Valdivia, R., Fridman, R. (2016). Discoidin Domain Receptors in Normal Mammary Development and Breast Cancer Progression. In: Fridman, R., Huang, P. (eds) Discoidin Domain Receptors in Health and Disease. Springer, New York, NY. https://doi.org/10.1007/978-1-4939-6383-6_7
Download citation
DOI: https://doi.org/10.1007/978-1-4939-6383-6_7
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4939-6381-2
Online ISBN: 978-1-4939-6383-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)