Abstract
Rationale
Cigarette smoking is one of the most serious health problems worldwide and people trying to stop smoking have high rates of relapse. Zebrafish (Danio rerio), by combining pharmacological and behavioral assays, is a promising animal model for rapidly screening new compounds to induce smoking cessation.
Objectives
This study aims to identify possible acetylcholine nicotinic receptors (nAChRs) involved in mediating nicotine (NIC)-induced conditioned place preference (CPP) in zebrafish and investigate the effect of the CC4 and CC26 cytisine derivatives in reducing NIC-induced CPP.
Methods
CPP was evaluated using a two-compartment chamber, and the zebrafish were given CC4 (0.001–5 mg/kg), CC26 (0.001–1 mg/kg), cytisine (0.1–2.5 mg/kg), and varenicline (1–10 mg/kg) alone or with NIC (0.001 mg/kg). Swimming activity was evaluated using a square observational chamber. The affinity of the nicotinic ligands for native zebrafish brain nAChRs was evaluated by binding studies using [3H]-Epibatidine (Epi) and [125I]-αBungarotoxin (αBgtx) radioligands, and their subtype specificity was determined by means of electrophysiological assay of oocyte-expressed α4β2 and α7 subtypes.
Results
CC4 and CC26 induced CPP with an inverted U-shaped dose–response curve similar to that of NIC. However, when co-administered with NIC, they blocked its reinforcing or slightly aversive effect. Binding and electrophysiological studies showed that this effect was due to binding to high-affinity heteromeric but not α7-containing receptors.
Conclusions
We have further characterized CC4 and identified a new compound (CC26) that may be active in inducing smoking cessation. Zebrafish is a very useful model for screening new compounds that can affect the rewarding properties of NIC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Smoking is one of the most difficult addictions to overcome because of the reinforcing effects of nicotine (NIC) and possibly other substances contained in tobacco and the presence of withdrawal symptoms, which is why tobacco dependence remains a major health problem worldwide.
NIC is the main psychoactive ingredient in cigarettes, and its binding to neuronal nicotinic acetylcholine receptors (nAChRs) evokes physiological effects by stimulating the mesolimbic brain reward system (Picciotto and Kenny 2013). Receptors consisting of five subunits encoded by at least nine different genes (α2 − α7; β2 − β4) and having specific pharmacological and functional properties have been found in mammals. Two main classes of nAChR subtypes have been identified: homomeric α7 or α9 or heteromeric α9-α10 αBungarotoxin (αBgtx)-sensitive receptors, and heteromeric α(α2-α6) and β(β2-β4) αBgtx-insensitive receptors (Gotti et al. 2009).
Current first-line pharmacotherapies aimed at encouraging people to stop smoking include NIC replacement therapy, bupropion hydrochloride, and varenicline (VAR) tartrate. NIC replacement therapy slightly decreases relapse rates in smokers by interfering with the action of NIC (Raupach and van Schayck 2011). The atypical antidepressant bupropion reduces the severity of NIC craving during withdrawal, but its long-term beneficial effects on relapse are not clear (Hughes et al. 2007). Cytisine (CYT)-related VAR tartrate (Chantix or Champix) is a partial agonist of α4β2* and α6β2 * nAChR subtypes and a full agonist of α7 and α3β4 nAChRs. It acts on β2-containing receptors and induces the release of mesolimbic dopamine, which counteracts withdrawal symptoms and reduces smoking satisfaction (Foulds 2006; Mihalak et al. 2006; Rollema et al. 2007). Clinical trials indicate that VAR is effective in decreasing relapse to smoking in humans (Cahill et al. 2011; Gonzales et al. 2006; Jorenby et al. 2006; Tonstad et al. 2006; Zierler-Brown and Kyle 2007), but adverse cardiovascular effects and/or neuropsychiatric events have recently been reported including (but not limited to) depression, suicidal ideation, suicide attempts, and completed suicide (Freedman 2007; Moore et al. 2011; Singh et al. 2011). It is possible that at least some of these are related to VAR acting on subtypes other than these β2-containing subtypes.
In our search for new drugs that act on dopamine release, we have recently identified the compound 1,2-bis(cytisin-12-yl)ethane (CC4), a dimer of CYT that mediates striatal dopamine release by partially activating the α4β2 and α6β2 nAChRs and, when co-incubated with NIC, reduces NIC-induced dopamine release (Sala et al. 2013). It also has low affinity for the α3β4 and α7 nAChR subtypes, and its effects on these subtypes are less efficacious than those of NIC or CYT. In line with the partial agonist selectivity of CC4 for β2-nAChRs, our in vitro and in vivo findings in rat have shown that, like VAR, it reduces some behaviors associated with NIC addiction such as conditioned place preference (CPP) and NIC self-administration.
Rodent studies have been important in the field of NIC addiction, but they are extremely time-consuming, expensive, and difficult to develop for the screening of many compounds. We therefore decided to test the CYT derivatives using zebrafish (Danio rerio), a recently emerging alternative preclinical vertebrate model for behavioral studies of NIC exposure, particularly for studying the complex processes involved in the development of NIC addiction (Mathur and Guo 2010). In particular, zebrafish can be used to screen for chemical compounds that can modulate NIC-induced CPP, a behavioral model used to study the rewarding properties of NIC (Kedikian et al. 2013).
Zebrafish express the same set of neuronal nAChR genes as those present in other vertebrates (α2, α3, α4, α6, α7, α8, β2, β3, β4) (Ackerman et al. 2009; Zirger et al. 2003), and there is a high degree of protein sequence conservation between human and zebrafish orthologues. Transcripts for the α2, α7, and β3 subunits are expressed very early (3 h post-fertilization) and the α4 and α6 subunits later on, although still early in development, consistent with their possible role in signaling events early during nervous system development. The β2 subunit has been localized by means of immunocytochemistry in various areas of the CNS, and it seems that nAChR development and subtype expression are similar between zebrafish and humans, but little work has been done on the pharmacological and biochemical characterization of native adult brain zebrafish subtypes. Moreover, functional studies on heterologously expressed α4β2 and α7 subtypes have shown that in zebrafish only some nicotinic drugs have maintained the subtype specificity determined in mammals (Papke et al. 2012).
The aim of this study was to assess the ability of NIC, CYT, and CYT derivatives to induce CPP in zebrafish and the ability of CYT derivatives to reverse the rewarding effect of NIC-induced CPP. As very little is known about the pharmacology of the native nicotinic subtypes expressed in zebrafish, we used binding and pharmacological experiments to identify the native subtypes expressed in adult zebrafish brain by means of subtype-selective antagonists. We also characterized the neuropharmacology of CC4 and the new CYT derivative 1,4-Bis(cytisin-12-yl)-2-butyne (CC26), by means of behavioral and binding studies on native zebrafish subtypes and by electrophysiological experiments on oocyte-expressed zebrafish α4β2 and α7 subtypes.
Materials and methods
Animals
Adult wild-type (WT) zebrafish (D. rerio) (0.4–1 g) of heterogeneous genetic background were obtained by a local aquarium supply store (Aquarium Center, Milan, Italy). Zebrafish were aged between 6 and 12 months, and were between 3 and 4 cm long. In all of the experiments, the sex ratio of zebrafish was 50–50 %. Males and females were identified according to Streisinger (2000). The animals were kept at approximately 28.5 °C on a 14/10-h light/dark cycle (lights on at 08.00 hours). In order to minimize stress, the experiments were began 1 month after their arrival in the lab. Furthermore, the fish were habituated to the apparatus for 1 h a day in the week preceding the beginning of the experiments. During the experiments, the observer, who was blind to the treatment allocation, sat 2 m away from the tank.
Behavioral studies
Behavioral testing took place during the light phase between 0900 and 1400 hours. Tank water consisted of deionized H2O and sea salts (0.6 g/10 l of water; Instant Ocean, Aquarium Systems, Sarrebourg, France). The home tanks with groups of approximately 30 adult fish were maintained with constant filtration and aeration. Fish were fed daily with brine shrimp and flake fish food (Tropical fish food, Consorzio G5, Italy). All the fish were drug naive, and each fish was used only once. Ten fish per group were used. Experimental procedures were carried out in accordance with the European Community Council Directive No. 86/609/EEC and the subsequent Italian Law on the Protection of animals used for experimental and other scientific reasons.
The experimental protocol was approved by the Italian Governmental Decree No. 29/2010. All efforts were made to minimize the number of animals used and their discomfort.
Conditioned place preference
The fish were tested in a two-chamber tank (10 × 20 × 15 cm) as previously described (Braida et al. 2007). The tank was divided into two halves (10 × 10 cm) containing distinct visual cues (two black polka dots) with a perforated wall that allowed complete, albeit somewhat impeded, movement. On the first day, after a previous introduction to the apparatus, the fish were tested for baseline preference by calculating the percent time spent on a given side during a 15-min trial (preconditioning phase). Six hours later, the fish were given intramuscular (i.m.) injections with the different drugs and then restricted to the least preferred side for 30 min. Twenty-four hours after, fish receiving vehicle were confined in the opposite compartment for 30 min. Drug–texture pairings were always counterbalanced. On the third day, the fish were free to access to two sides for 15 min, and the time spent in each compartment was recorded. The change in preference, obtained by subtracting the baseline value from the final value in the drug-paired compartment, reflected rewarding or aversive properties.
Swimming behavior
Immediately after treatment, each subject was placed in a transparent observation chamber (20 × 10 × 15 cm) containing home tank water filled at a level of 12 cm. The floor of the chamber was divided into ten equal-sized 2 × 10 cm rectangles. Using a time-sampling procedure, swimming activity was monitored by counting the number of lines crossed in a 30-s observation period every 5 min, for a total of six observation bins over 30 min (Swain et al. 2004).
Treatment
Zebrafish body weight was measured as previously described (Braida et al. 2007). Briefly, fish were removed from their tank using a net and placed in a container containing tank water, positioned on a digital balance. Fish weight was determined as the weight of the container plus the fish minus the weight of the container before the fish was added. The mean of three measurements was recorded. Each fish was injected i.m. in the caudal musculature along the posterior axis. The site of injection was constantly maintained at the area below the caudal fin on the left side of each fish. Each volume, depending on the fish’s weight (2 μl/g), was given using a Hamilton syringe (Hamilton Bonaduz AG, Bonaduz, Switzerland). Fish were individually pulled out of the water tank with the use of a net. Then, each fish was kept immobilized through the net with two fingers of the left hand. Immediately after, the needle was positioned at a 45° angle in relation to the back of the fish with the needle pointing towards the head. The injection was in the largest portion of the caudal muscle, immediately posterior to the caudal fin. The needle was inserted into the muscle just beyond the bevel of the needle. Total time out of water was approximately 10 s. Each fish was removed from the net and immediately dipped in the tank water.
NIC determination in zebrafish brain and tissues
LC-MS/MS analysis
NIC was determined by liquid chromatography–tandem mass spectrometry (LC-MS/MS) as previously described (Vieira-Brock et al. 2011), with minor modifications. Liquid chromatography was performed using an HP 1200 system consisting of a binary pump, an automatic sampler, and a column oven (Agilent Technologies, Germany), which was equipped with a Discovery HS-F5 column (150 mm × 2.1 mm, 3 μm) (Supelco, Milan, Italy). The column temperature was 25 °C. The mobile phase was 10 mM ammonium acetate (A) and acetonitrile (B). The elution conditions were as follows: 5 % B to 95 % B (0–10 min), 95 % B (10–13 min), 95 to 5 % B (13–13.1 min) and the flow rate was 0.3 ml/min. An Agilent 6410 triple quadrupole–mass spectrometer with an electrospray ion source operated in positive mode was used for detection. Flow injection analysis was used to optimize the fragmentor and source parameters. The optimized source parameters for MS analysis were as follows: drying gas temperature 350 °C, gas flow 12 l/min, nebulizer gas flow pressure 35 psi, and capillary voltage 3,500 V. The optimized fragmentor voltage was 90 V and the collision energy was 20 eV. The SRM pairs were 163.2– > 130.2 for NIC and 166.2– > 130.2 for NIC -d 3 (IS). The retention time of NIC was 5.8 min. The calibration curve was constructed using nine calibration standards (viz. 10–100 ng/ml). The best fitting calibration curve was obtained by linear regression analysis with a 1 / x 2 weighting factor (r 2 > 0.993). The calibration curve equation was y = 0.003284 + 0.01235, using the peak area ratios of the analyte and IS (NIC -d 3 ). Linearity was confirmed by statistical analysis (P < 0.05). The lower limit of quantification (LLOQ) was 10 ng/ml. The limit of detection (LOD) at a signal-to-noise ratio of 3:1 was 5 ng/ml.
Sample preparation
The fish brains were homogenized in deionized water (2 ml/g brain tissue) and treated in accordance with a standard protein precipitation protocol. Briefly, the homogenate was diluted 1:4 in acetonitrile and centrifuged at 10,000 rpm for 10 min at 4 °C. The supernatant was evaporated, reconstituted in 10 mM ammonium acetate/acetonitrile 70:30, and then analyzed using the liquid chromatography/mass spectrometry. Deuterated NIC was used as the internal standard.
Biochemical studies
Binding assays and pharmacological experiments
Before the brain extraction, each fish was anesthetized in 0.2 % Tricaine and then euthanized by incubation in ice water for 15 min according to Gupta and Mullin (2010). After dissection, brains were immediately frozen until later use. In each experiment, the tissues from 40 to 60 zebrafish were pooled and homogenized in 5 ml of 50 mM Na phosphate pH 7.4, 1 M NaCl, 2 mM EDTA, 2 mM EGTA, and 2 mM phenylmethylsulfonyl fluoride (PMSF) with a Potter homogenizer. The homogenates were then diluted and centrifuged for 1.5 h at 60,000 × g. The procedures of homogenization, dilution, and centrifugation of the total membranes were performed twice, after which the pellets were collected, rapidly rinsed with 50 mM Tris–HCl pH 7, 120 mM NaCl, 5 mM KCl, 1 mM MgCl2, 2.5 mM CaCl2, and 2 mM PMSF, and then resuspended in phosphate buffer saline (PBS) buffer containing a mixture of 10 μg/ml of each of the following protease inhibitors: leupeptin, bestatin, pepstatin A, and aprotinin.
[3H]-Epibatidine binding
In order to ensure that the α7-nAChRs did not contribute to [3H]-Epibatidine (Epi) binding to the membrane, the binding was performed in the presence of 1 μM αBgtx, which specifically binds to α7*-nAChR and thus prevents [3H]-Epi binding to these sites. Saturation binding to the membrane homogenates was carried out for 3 h at RT or overnight at 4 °C by incubating aliquots of the membrane with increasing concentration of [3H]-Epi (0.005–10 nM). Nonspecific binding (averaging 5–10 % of total binding) was determined in parallel samples containing 100 nM unlabeled Epi. At the end of the incubation, the samples were filtered on a GFC filter soaked in 0.5 % polyethylenimine, washed with 15 ml of buffer (Na phosphate, 10 mM, pH 7.4; NaCl, 50 mM), and counted in a γ counter.
[125I]-αBungarotoxin binding
Binding experiments were performed by incubating zebrafish membranes with increasing concentration (0.01–10 nM) of [125I]-α bungarotoxin (αBgtx) overnight at 20 °C in the presence of 2 mg/ml bovine serum albumin (BSA). Specific radioligand binding was defined as total binding minus nonspecific binding determined in the presence of 1 μM cold αBgtx. After incubation, the samples were filtered as described above and the bound radioactivity directly counted in a γ counter.
Pharmacological experiments
The inhibition of [3H]-Epi and [125I]-αBgtx binding by the test compounds was measured by pre-incubating zebrafish membrane homogenates with increasing concentrations (10 pM–10 mM) of the drug to be tested for 30 min at room temperature, followed by overnight incubation with a final concentration of 0.1 nM of [3H]-Epi at 0 °C or 1 nM [125I]-αBgtx at room temperature.
Immunoprecipitation studies
The immunoprecipitation studies were performed as previously described (Grady et al. 2009). Triton X-100 at a final concentration of 2 % was added to the washed brain zebrafish membranes, which were extracted for 2 h at 4 °C. The membrane extracts were centrifuged for 1.5 h at 60,000 × g and recovered. For the immunoprecipitation experiments, the extracts obtained from the zebrafish membranes preincubated with 1 μM αBgtx were labeled with 2 nM [3H]-Epi and incubated with a saturating concentration (20 μg) of anti-α4 antibodies. The extracts labeled with [125I]-αBgtx were incubated with a saturating concentration (20 μg) of anti-α7 antibodies. After overnight incubation, the samples were recovered by incubating samples with beads containing bound anti-rabbit goat IgG (Technogenetics, Milan, Italy). The level of antibody immunoprecipitation was expressed as fentomole of immunoprecipitated receptors per milligram of protein.
Both α4 (PPWLAGMI) and α7 (SAPNFVEAVSKDFA) peptide sequences used to raise antibodies against the subunits are located at the C-terminus of the subunits, which have 100 % amino acid sequence identity conserved between zebrafish and mammals.
Electrophysiological studies
Expression of zebrafish nAChRs in oocytes
Zebrafish cDNAs were obtained from R. Thomas Boyd (Ohio State University, Columbus, OH) as previously described (Papke et al. 2012). Mature (>9 cm) female Xenopus laevis African frogs (Nasco, Ft. Atkinson, WI) were used as a source of oocytes. Prior to surgery, the frogs were anesthetized by placing the animal in a 0.7 g/l solution of MS222 (3-aminobenzoic acid ethyl ester) buffered with sodium bicarbonate for 15–30 min. Oocytes were removed from an incision made in the abdomen. To remove the follicular cell layer, harvested oocytes were treated with 1.25 mg/ml type 1 collagenase (Worthington Biochemical Corporation, Freehold, NJ) for 3–4 h at room temperature in calcium-free Barth's solution (88 mM NaCl, 1 mM KCl, 0.33 mM MgSO4, 2.4 mM NaHCO3, 10 mM HEPES (pH 7.6), 12 mg/l tetracycline chloride). Subsequently, stage 5 oocytes were isolated and injected with 50 nl (5–20 ng) each of the appropriate subunit cRNAs. The RNAs injected for α-β pairs were injected at 1:1 ratios. Recordings were made 1 to 10 days after injection.
Experiments were conducted using OpusXpress 6000A (Molecular Devices, Union City CA) as previously described (Papke and Stokes 2010). OpusXpress is an integrated system that provides automated impalement and voltage clamp of up to eight oocytes in parallel. Cells were automatically perfused with bath solution, and drug solutions were delivered from a 96-well plate. Both the voltage and current electrodes were filled with 3 M KCl. The agonist solutions were applied via disposable tips, which eliminated any possibility of cross-contamination. Drug applications alternated between acetylcholine (ACh) controls and experimental applications. Flow rates were set at 2 ml/min for experiments with α7 receptors and 4 ml/min for other subtypes. Cells were voltage-clamped at a holding potential of −60 mV. Data were collected at 50 Hz and filtered at 20 Hz. ACh applications were 12 s in duration followed by 181-s washout periods with α7 receptors and 6 s with 241-s wash periods for other subtypes. Fresh ACh stock solutions were made daily in Ringer's solution and diluted.
Measurement of functional responses
Responses of cells expressing α4β2 receptors were measured as the peaks in the currents evoked by ACh or ACh co-applied with the experimental compounds. For α7 receptors, the peak currents are associated with synchronization of channel activation that occurs well in advance of the full agonist application (Papke and Porter Papke 2002). Therefore, we measured the net charge of the α7 agonist-evoked responses.
Experimental protocols and data analysis
Each oocyte received two initial control applications of 60 μM ACh, then 60 s experimental drug (1 μM) pre-applications followed by co-application of the test compounds at 1 μM plus 60 μM ACh and additional follow-up control applications of ACh. Responses to each drug application were calculated relative to the average of the two preceding ACh control responses to normalize the data, compensating for the varying levels of channel expression among the oocytes.
Drugs
For the in vivo studies 1,2-bis(cytisin-12-yl)ethane (CC4), 1,4-bis(cytisin-12-yl)-2-butyne (CC26), and cystine (CYT) were synthesized as described by Braida et al. (2014); Carbonnelle et al. (2003). NIC bi-tartrate (0.0001–0.1 mg/kg) was purchased from Sigma-Aldrich (St. Louis, MO, USA). Due to the lack of data on zebrafish and nicotinic compounds injected and not dissolved in the tank water, a pilot study was previously undertaken on selective nAChRs antagonists alone or in combination with NIC on CPP. CC4 (5 mg/kg), CC26 (1 mg/kg), CYT (2.5 mg/kg), varenicline (VAR, 7.5 mg/kg), mecamylamine hydrochloride (MEC, 0.1 mg/kg), α-conotoxin MII (MII, 0.01–1 mg/kg), methyllycaconitine (MLA, 0.01–1 mg/kg), and dihydro-β-erythroidine (DhβE, 0.001–0.1 mg/kg) (Sigma-Aldrich, St. Louis MO, USA) were given alone or combined with the maximal rewarding dose of NIC. For CC4 and CC26 antagonism, NIC was used at different doses. The rewarding and maximal employed dose of each compound was tested on swimming activity. The dose of MEC to antagonize NIC-induced CPP was chosen according to the dose of the other selective antagonists. All the drugs were dissolved in sterile saline and administered i.m. 20 min before the test. When multiple treatments were needed, the drug solutions were put in the same syringe to avoid potential tissue trauma. Vehicle group received saline (2 μl/g). All the fish were drug naive, and each fish was used only once. Ten fish per treatment were used. The doses of the drugs were calculated as salt. All drugs were prepared fresh daily. α-Conotoxin MII was synthesized as described by Pucci et al. (2011).
For the in vitro studies ACh, Epi, PMSF, Tris, Triton X-100, NaCl, KCl, MgSO4, CaCl2, KH2PO4, NaHCO3, and protease inhibitors were purchased from Sigma-Aldrich (St. Louis, MO, USA). Cold α-Bgtx was supplied by Tocris (Bristol, UK). (±)-[3H]-Epi (specific activity 45–65 Ci/mmol) and [125I]-αBgtx (150 Ci/mmol) were purchased from PerkinElmer (USA). Receptor nomenclature is conformed to BJP’s Guide to Receptors and Channels (Alexander et al. 2011).
Statistical analysis
Behavioral data were analyzed by means of one-way analysis of variance (ANOVA). Tukey post hoc tests were used to follow up significant F values found in the primary analysis.
The LIGAND program was used to calculate K i values of all the tested compounds using data obtained from at least three to four independent saturation and competition binding experiments. For electrophysiological studies, means (±SEM) were calculated from the normalized responses of at least four oocytes for each experimental condition. All the statistical analysis was performed using Prism 5 software (GraphPad Inc, La Jolla, CA).
Results
Behavioral studies
NIC induces CPP, which is antagonized by selective and nonselective nicotinic antagonists
The zebrafish spent an equivalent average amount of time in each of the two outer compartments during the conditioning phase (453 ± 17.76 s in one with the polka dots and 447 ± 17.25 s in the one without the polka dots, P = 0.81, not significant). The CPP induced by different doses of NIC is shown in Fig. 1. One-way ANOVA revealed a significant treatment effect [F(4,45) = 4.55, P < 0.005] when comparing the time in the drug-paired compartment during the pre- and post-conditioning period. Post hoc analysis showed that NIC increased the time spent in the drug-paired compartment on the post-conditioning day at a dose of 0.001 mg/kg, while at the highest dose NIC appeared slightly aversive (even if no significant difference was shown compared to saline group), exhibiting an inverted U-shaped dose–response curve.
Effect of NIC on CPP. NIC elicited CPP following an inverted U shape. Increasing doses (mg/kg) of NIC were given i.m. 20 min before the conditioning session. Preference was calculated by subtracting the time (mean ± SEM) spent in the drug-paired compartment before drug conditioning from the time spent after drug conditioning. (n = 10 zebrafish for each group). ***P < 0.01 significantly different from the corresponding saline group during post-conditioning; $$ P < 0.01 significantly different from NIC (0.001 mg/kg) (ANOVA followed by a post hoc Tukey’s test)
Given the lack of data concerning zebrafish injected with nicotinic compounds, a pilot study of CPP was undertaken using selective nAChR antagonists alone or in combination with NIC (Table 1). The three selective antagonists had a treatment effect: MLA [F(7,72) = 13.93, P < 0.0001], MII [F(7,72) = 5.86, P < 0.0001], and DHβE [F(7,72) = 8.08, P < 0.0001]. Post hoc tests revealed that MLA (0.01 mg/kg), MII (1 mg/kg), and DHβE (0.01 mg/kg) had slight but significant reinforcing effects per se, but when combined with NIC (0.001 mg/kg), MLA (1 mg/kg) and DHβE (0.001 and 0.01 mg/kg) antagonized NIC-induced CPP, whereas MII was ineffective at doses up to 1 mg/kg.
Nicotinic partial agonists evoke a CPP, but attenuate NIC-induced CPP
Figure 2 shows the CPP induced by different doses of CYT, VAR, CC4, and CC26. One-way ANOVA revealed a significant treatment effect when comparing the time in the drug-paired compartment during the pre- and post-conditioning period: CYT [F(3,36) = 4.95, P < 0.005] (panel a), VAR [F(5,54) = 14.77, P < 0.0001] (panel b), CC4 [F(6,63) = 5.12, P < 0.0002] (panel c), CC26 [F(4,45) = 3.44, P < 0.02] (panel d). Post hoc analysis showed that all of the compounds had a dose–response curve with an inverted U shape. The most potent partial agonist to evoke CPP was CC26 (0.01 mg/kg), followed by CC4 (0.01–1 mg/kg), CYT (1 mg/kg), and VAR (2.5 mg/kg). The highest doses of all of the compounds were ineffective.
Effects of different nAChR partial agonists on CPP. Dose–response curves (mg/kg) of cytisine (CYT) (a), varenicline (VAR) (b), CC4 (c), and CC26 (d) given i.m. 20 min before the conditioning session, and all elicited CPP. e Effects of CC4 (5 mg/kg) and CC26 (1 mg/kg) on CPP induced by increasing doses of NIC. f Effect of CYT (2.5 mg/kg), VAR (1–10 mg/kg), or MEC (0.1 mg/kg) given alone or in combination with the maximal reinforcing dose of NIC (0.001 mg/kg). Preference was calculated by subtracting the time (mean ± SEM) spent in the drug-paired compartment before drug conditioning from the time spent after drug conditioning. (n = 10 zebrafish for each group). *P < 0.05, **P < 0.01, ***P < 0.001 significantly different from the corresponding saline group during post-conditioning; $ P < 0.05 compared to VAR 2.5; ## P < 0.001, #### P < 0.0001 compared with corresponding saline + NIC(ANOVA followed by a post hoc Tukey’s test)
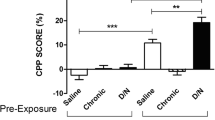
When different doses of NIC were combined with the most potent compound, CC4 and CC26 (panel e), there was a significant treatment effect [F(8,81) = 41.19, P < 0.0001, one-way ANOVA] on the time spent in the drug-paired compartment during the pre-and post-conditioning periods. Both the partial agonists significantly blocked either the reinforcing or the aversive effect of NIC. A treatment effect was also shown when CYT, VAR, and MEC before NIC [F(5,54) = 7.27, P < 0.001, one-way ANOVA ]. Post hoc analysis showed that CYT (2.5 mg/kg), VAR (7.5 mg/kg), and MEC (0.1 mg/kg) given alone did not change the mean time (between the pre- and post-conditioning period) spent in the drug-paired side compared with the saline-treated zebrafish. However, when nicotinic partial agonists and MEC were co-administered with the maximally effective dose of NIC (0.001 mg/kg), they significantly blocked NIC-induced CPP.
Nicotinic compounds did not affect swimming behavior
Combined treatment with the maximal rewarding dose of NIC and the various partial agonists or antagonists given at the maximal or rewarding doses used for the antagonism studies did not induce any change in swimming behavior [F(13,116) = 1.04, P < 0.44, one-way ANOVA, ns) (Fig. 3).
Effect of NIC and nicotinic compounds on swimming behavior. None of the compounds modified swimming activity. NIC (0.001 mg/kg), cytisine (CYT, 1 and 2.5 mg/kg), varenicline (VAR, 2.5–7.5 mg/kg), CC4 (1 and 5 mg/kg) and CC26 (0.01–1 mg/kg), mecamylamine (MEC, 0.1 mg/kg), methyllycaconitine (MLA, 1 mg/kg), α-conotoxin MII (MII, 1 mg/kg), dihydro-beta-erythroidine (DHβE, 0.1 mg/kg) were given i.m. 20 min before the test. Swimming behavior was evaluated in terms of the number of lines crossed in a 30-s observation period every 5 min, for a total of six observations bins (n = 10 zebrafish for each group)
Biochemical studies
NIC can be determined in zebrafish brain and tissues
The level of NIC was measured by means of LC-MS/MS in the brain of zebrafish that had received the same NIC doses as those used to induce CPP. Twenty minutes after the injection of 0.001, 0.01, or 0.1 mg/kg of NIC base in the tail, the brains, and tissues (liver and kidney) were dissected and the samples extracted for NIC detection as described in “Materials and methods.” As shown in Table 2, there was a dose-dependent increase in NIC levels in the brain, comparable with that found in the brain of smokers (Matta et al. 2007), which was detectable even at the lowest dose of 0.001 mg/kg. Parallel quantification of NIC also showed a dose-dependent increase of NIC in the other tissues, this was much greater than in the brain. This is probably due to differences in the pharmacokinetic and pharmacodynamic properties of NIC in different tissues.
Identification of native zebrafish nAChR subtypes
In order to identify the major classes of nicotinic receptor subtypes expressed in zebrafish brain, we used binding studies and two radioligands: [125I]-αBgtx, which binds to a class of homomeric receptors containing the α7 subunits, and [3H]-Epi, which binds a number of nAChR subtypes with high affinity.
Preliminary binding studies, using increasing concentrations of [3H]-Epi, revealed the presence of two classes of sites with apparent dissociation constants (K d) of 20 pM and 5 nM. As it has been reported that, albeit with low affinity, [3H]-Epi binds to α7-containing receptors in vertebrates (Marks et al. 2010), we incubated membrane homogenates with an excess of cold αBgtx (1 μM) and performed saturation binding experiments using [3H]-Epi. Under these conditions, we detected only a single high affinity site with a K d of 26 pM (coefficient of variation (CV) 23 %) and a B max of 252.4 ± 23 fmol/mg of protein (n = 3).
Saturation binding studies of zebrafish membrane homogenates using [125I]-αBgtx determined a single class of site with a K d of 0.42 nM (CV 31 %) and a B max of 122.4 ± 10.9 fmol/mg of protein (n = 3).
In order to identify the subtypes that bind [3H]-Epi and [125I]-αBgtx, we used quantitative immunoprecipitation studies and antibodies directed against peptides located at the C-terminus of the mammalian α4 and α7 subunits, which have 100 % amino acid sequence identity with the zebrafish subunits (see “Materials and methods”). The immunoprecipitation of membrane-solubilized [125I]-αBgtx-labeled receptors showed that more than 95 % were immunoprecipitated by anti-α7 antibodies. There was no anti-α4 antibody specific immunoprecipitation of the [3H]-Epi-labeled receptors, although the antibodies immunoprecipitated more than 90 % of the [3H]-Epi labeled receptors from rat brain.
Pharmacological characterization of native subtypes
In order to obtain a complete pharmacological profile of the [3H]-Epi and [125I]-αBgtx receptors, we used competition binding experiments with a series of known nicotinic ligands and the CYT derivatives CC4 and CC26, the affinities of which are shown in Table 3 and Fig. 4. The order of affinity of the agonists of [3H]-Epi-labeled receptors was VAR>CYT>CC26>NIC>CC4, whereas the order of the antagonists was MII>MLA>αBgtx>DHβE>and MEC. The order of potency of the agonists of the [125I]-αBgtx receptors was VAR>CYT>CC26>NIC>CC4, whereas the order of the antagonists was αBgtx>MLA>MII>DHβE>MEC.
Ligand binding competition profiles in zebrafish brain membrane homogenates from competition binding assays were carried out as described in “Materials and methods” using [3H]-Epi concentration of 200 pM and [125I]-αBgtx of 2 nM. The data were analyzed using nonlinear least square regression and the LIGAND program. The data shown are representative of the ligands tested in one experiment. See Table 2 for a summary of the K i values in all of the competition experiments
In line with other findings in vertebrates, VAR was the compound with the highest affinity for both classes of receptors, whereas CYT, CC26, NIC, and CC4 had, respectively, 25, 23, 18, and 21 times higher affinity for [3H]-Epi receptors than [125I]-αBgtx receptors.
We also found that αBgtx and MLA had, respectively, 3,750 and 1,422 times higher affinity for [125I]-αBgtx receptors than for [3H]-Epi receptors, thus indicating that these compounds are selective for α7 containing receptors and that MII has 118 times higher affinity for [3H]-Epi receptors than for [125I]-αBgtx receptors. These binding studies showed that zebrafish have two major classes of nicotinic subtypes (Epi and αBgtx receptors) and that the binding selectivity of most, but not all, of the nicotinic ligands is conserved.
Evaluation of the functional activity of CYT derivatives on zebrafish subtypes
In order to identify the nAChR subtypes involved in CPP, we electrophysiologically tested the antagonists MLA and DHβE and the CYT derivatives on oocytes expressing α7 and α4β2 zebrafish subtypes as previously described (Papke et al. 2012). As shown in Table 4, a 1 μM concentration of MLA completely blocked (98 %) the 60 μM ACh-evoked responses of the α7 subtype, and the same concentration also reduced the response of the α4β2 subtype, although to a lesser extent (79 %). The antagonist DHβE blocked 84 % of the ACh-induced current in the α4β2 subtype, but had very little effect on the α7 subtype (5 %). On the basis of these data, we can conclude that DHβE clearly antagonized the ACh response of the α4β2 subtype but not that of the α7 subtype, whereas MLA was very active on the α7 subtype and just as active as DHβE on the α4β2 subtype.
When tested on the same subtypes, the CYT derivatives CC4 and CC26 compounds blocked more than 85 % of the response of the α4β2 ACh-induced currents but did not significantly reduce the currents in the α7 subtype.
Discussion
We used the zebrafish model to identify candidate molecules for treating tobacco addiction. by pharmacologically and electrophysiologically characterizing the native and heterologously expressed zebrafish nAChR subtypes involved in NIC-induced CPP. Our data showing that the i.m. injection of NIC elicits CPP confirm previous CPP findings obtained using NIC dissolved in water (Kedikian et al. 2013; Kily et al. 2008). The detection of NIC in the brain indicate that zebrafish are very sensitive to much lower concentrations of NIC, than previously reported (Kedikian et al. 2013). One possible explanation for this sensitivity is that i.m. injections lead to more precise and accurate NIC concentrations in the brain, although both methods indicate that the rewarding effect of NIC has an inverted U-shaped dose–response curve similar to that of many drugs of abuse (Kily et al. 2008).
Our findings also confirm that CC4 blocks NIC-induced CPP in zebrafish as it does in rats (Sala et al. 2013), and that the new CYT derivative CC26 (which has previously been shown to improve spatial memory) (Braida et al. 2014) is more potent in blocking CPP than CC4.
When administered alone, CC4, CC26, CYT, and VAR induced CPP in a manner that was similar to that induced by NIC. However, when co-administered with NIC they abolished its reinforcing effect. Moreover, when CC4 (5 mg/kg) or CC26 (1 mg/kg) were combined with different doses of NIC they reversed both the reinforcing and the slight aversive effect of NIC.
Surprisingly, the highest dose of NIC became reinforcing when combined with CC4 or CC26. Our binding study clearly show that CC4 or CC26 and nicotine are all competitive drugs, so due to the direct competition between CC4 or CC26 and nicotine doses there is a decrease of the reinforcing and aversive effects of low and high doses of nicotine. In conclusion at the highest dose of NIC, in the presence of competitive partial agonists, the concentration of nicotine bound to the receptors is lower and probably in the reinforcing effect range
The maximal reinforcing effects of CC26 and CC4 were reached at doses that were, respectively, 10 times (0.01 mg/kg) and 100 times (0.1 mg/kg) higher than that of NIC (0.001 mg/kg), and CYT and VAR elicited their maximal effect at doses that were, respectively, 1,000 and 2,500 times higher. The findings obtained using CC4 and CYT are in line with those recently obtained in rats when both compounds were self-administered icv (Sala et al. 2013). The rewarding effect of CYT is also in line with results showing that CYT is self-administered by drug-naive mice in a manner similar to NIC (Rasmussen and Swedberg 1998) and that rats receiving ventral tegmental (VTA) injections of CYT spend significantly more time in the drug-paired than in the saline-paired compartment (Museo and Wise 1994). Although no data are available concerning VAR-induced CPP, it is known that the compound can produce rewarding effects in rats as it replaces NIC in a self-administration paradigm and fully replaces NIC as the training stimulus in the drug discrimination in rats (Rollema et al. 2007; Smith et al. 2007). Accordingly, VAR dose-dependently decreases NIC self-administration in rats allowed either limited (1 h/day) or extended access to it (23 h/day) (George et al. 2011). The systemic administration of VAR has a dose-dependent biphasic effect on rewarding electrical brain stimulation in rats (Spiller et al. 2009). The range of VAR doses that are effective in blocking NIC-induced CPP as well as the reinstatement of NIC CPP in rat is similar to ours (0.5–2 mg/kg subcutaneous) (Biala et al. 2010). It is important to note that none of the doses of any of the nAChR ligands had any significant effect on swimming behavior.
NIC is the major psychoactive ingredient in tobacco, and its addictive properties are due to its interaction with nAChRs, especially those expressed in the mesocorticolimbic pathway, one of the main reinforcement circuits of the brain, in which they modulate dopamine (DA) release. In line with their partial agonist mechanism, CYT and VAR increase rat mesolimbic DA turnover in a dose-dependent manner (Rollema et al. 2011). The mammalian mesolimbic dopaminergic system consists of DA neurons whose cell bodies are in the midbrain VTA and whose projections reach the ventral striatum, prefrontal cortex, and amygdala. Zebrafish have no DA neurons in the midbrain, but dye tracing experiments have identified a conserved ascending DA system essential for reward responses in which DA neurons of the posterior tuberculum of the dorsal hypothalamus project to the dorsal and ventral (limbic) striatum. It has been suggested that these projections, respectively, represent the meso-striatal and meso-limbic system (Rink and Wullimann 2002). The neuroanatomical organization of DA neurons in zebrafish and mammals therefore seems to be similar (Klee et al. 2011; Panula et al. 2010) and the increase in DOPAC (a dopamine metabolite) levels during NIC-enhanced learning tasks indicates that NIC also increases DA activity in zebrafish (Eddins et al. 2009).
In order to investigate the subtypes involved in the rewarding effects of NIC, we tested the NIC-induced CPP in the presence of different nAChR antagonists. We found that at low doses the antagonists per se have a modest reinforcing effect probably due to the fact that the antagonists may reduce a physiological inhibitory effect of Ach acting on nAChRs present in the rewarding circuit. At high doses, MEC, DHβE (more efficiently), and MLA fully blocked the reinforcing effect of NIC, whereas MII, the α6β2* nAChR antagonist (Pucci et al. 2011), had no effect on NIC-induced CPP. We believe that the block of the nicotine rewarding effects are due to the competition at the receptor level between nicotine and the antagonists.
As there is little published information concerning the zebrafish, we used binding and immunoprecipitation studies to test the affinities of these compounds for native zebrafish nAChRs. The results indicate that zebrafish brain expresses two distinct classes of nicotinic receptors: one containing the α7 subunit that binds [125I]-αBgtx with high affinity and another that binds [3H]-Epi and various nicotinic ligands with high affinity, presumably including several subtypes. We believe that one of these is the α4β2 subtype, although our immunoprecipitation studies could not clearly demonstrate this, possibly because our anti-α4 antibodies are directed against a short C-terminus peptide that may be hidden in the zebrafish subtype.
As our binding studies indicated that DHβE cannot distinguish between the two classes, we further characterized them with the α7 selective compound MLA and the CYT derivatives CC4 and CC26 by means of electrophysiology in oocytes expressing the α4β2 and α7 subtypes. Functional assays showed that DHβE, CC4, and CC26 block the 1-μM ACh-induced current in the α4β2 subtype and have little effect on the α7 subtype, thus behaving as selective α4β2 antagonists. Although our binding studies indicated that MLA was selective for the α7 subtype, it did not discriminate between the α7 and α4β2 subtypes.
On the basis of the results of our binding and functional studies, we conclude that heteromeric receptors that bind [3H]-Epi with high affinity and are functionally blocked by DHβE, CC4, CC26, and MEC (presumably α4β2-containing receptors) are involved in mediating CPP in zebrafish. The MLA-induced block of CPP is puzzling because, although our binding studies showed that it had 2,000 times greater affinity for α7-containing receptors, the electrophysiological findings clearly show that it is similarly active in blocking ACh-induced currents in oocytes expressing α4β2 or α7 subtypes. The effect of MLA on the α4β2 is in line with previously published data demonstrating that ligands selective for mammalian α7 receptors have a broader range of activity in zebrafish than in mammalian CNS (Papke et al. 2012).
Our finding that the α4β2 subtype is involved in zebrafish CPP is in line with the fact that pretreating C57BL/6 mice with the α4β2 antagonists mecamylamine or DHβE (2.0 mg/kg, s.c.) blocks the NIC-induced CPP and that mice lacking the β2 nAChR do not show NIC-induced CPP, whereas α7 knock-out mice do (Grabus et al. 2006; Walters et al. 2006).
Previous findings indicated an important role of nAChRs in other behavioral effects of NIC such as locomotor sensitization, improved learning, and anxiety (Bencan and Levin 2008; Eddins et al. 2009; Levin et al. 2007; Levin et al. 2006; Papke and Porter Papke 2002; Petzold et al. 2009; Svoboda et al. 2002)
In conclusion, we used the zebrafish model to characterize CC4 further and identify a new compound CC26 that may be active in inducing tobacco smoking cessation. This demonstrates that the model can be very useful for screening new compounds that can affect the rewarding properties of NIC.
References
Ackerman KM, Nakkula R, Zirger JM, Beattie CE, Boyd RT (2009) Cloning and spatiotemporal expression of zebrafish neuronal nicotinic acetylcholine receptor alpha 6 and alpha 4 subunit RNAs. Dev Dyn Off Publ Am Assoc Anatomists 238:980–992
Alexander SP, Mathie A, Peters JA (2011) Guide to receptors and channels (GRAC), 5th edition. Br J Pharmacol 164(Suppl 1):S1–S324
Bencan Z, Levin ED (2008) The role of alpha7 and alpha4beta2 nicotinic receptors in the nicotine-induced anxiolytic effect in zebrafish. Physiol Behav 95:408–412
Biala G, Staniak N, Budzynska B (2010) Effects of varenicline and mecamylamine on the acquisition, expression, and reinstatement of nicotine-conditioned place preference by drug priming in rats. Naunyn Schmiedeberg's Arch Pharmacol 381:361–370
Braida D, Limonta V, Pegorini S, Zani A, Guerini-Rocco C, Gori E, Sala M (2007) Hallucinatory and rewarding effect of salvinorin A in zebrafish: kappa-opioid and CB1-cannabinoid receptor involvement. Psychopharmacology 190:441–448
Braida D, Ponzoni L, Martucci R, Sparatore F, Gotti C, Sala M (2014) Role of neuronal nicotinic acetylcholine receptors (nAChRs) on learning and memory in zebrafish. Psycopharmacol 231:1975–1985
Cahill K, Stead LF, Lancaster T (2011) NIC receptor partial agonists for smoking cessation. The Cochrane database of systematic reviews: CD006103
Carbonnelle E, Sparatore F, Canu-Boido C, Salvagno C, Baldani-Guerra B, Terstappen G, Zwart R, Vijverberg H, Clementi F, Gotti C (2003) Nitrogen substitution modifies the activity of cytisine on neuronal nicotinic receptor subtypes. Eur J Pharmacol 471:85–96
Eddins D, Petro A, Williams P, Cerutti DT, Levin ED (2009) NIC effects on learning in zebrafish: the role of dopaminergic systems. Psychopharmacology 202:103–109
Foulds J (2006) The neurobiological basis for partial agonist treatment of NIC dependence: varenicline. Int J Clin Pract 60:571–576
Freedman R (2007) Exacerbation of schizophrenia by varenicline. Am J Psychiatry 164:1269
George O, Lloyd A, Carroll FI, Damaj MI, Koob GF (2011) Varenicline blocks NIC intake in rats with extended access to NIC self-administration. Psychopharmacology 213:715–722
Gonzales D, Rennard SI, Nides M, Oncken C, Azoulay S, Billing CB, Watsky EJ, Gong J, Williams KE, Reeves KR (2006) Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: a randomized controlled trial. JAMA J Am Med Assoc 296:47–55
Gotti C, Clementi F, Fornari A, Gaimarri A, Guiducci S, Manfredi I, Moretti M, Pedrazzi P, Pucci L, Zoli M (2009) Structural and functional diversity of native brain neuronal nicotinic receptors. Biochem Pharmacol 78:703–711
Grabus SD, Martin BR, Brown SE, Damaj MI (2006) NIC place preference in the mouse: influences of prior handling, dose and strain and attenuation by nicotinic receptor antagonists. Psychopharmacology 184:456–463
Grady SR, Moretti M, Zoli M, Marks MJ, Zanardi A, Pucci L, Clementi F, Gotti C (2009) Rodent habenulo-interpeduncular pathway expresses a large variety of uncommon nAChR subtypes, but only the alpha3beta4* and alpha3beta3beta4* subtypes mediate acetylcholine release. J Neurosci Off J Soc Neurosci 29:2272–2282
Gupta T, Mullin MC (2010) Dissection of organs from the adult zebrafish. J Vis Exp 37:1717
Hughes JR, Stead LF, Lancaster T (2007) Antidepressants for smoking cessation. The Cochrane database of systematic reviews: CD000031
Jorenby DE, Hays JT, Rigotti NA, Azoulay S, Watsky EJ, Williams KE, Billing CB, Gong J, Reeves KR (2006) Efficacy of varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained-release bupropion for smoking cessation: a randomized controlled trial. JAMA J Am Med Assoc 296:56–63
Kedikian X, Faillace MP, Bernabeu R (2013) Behavioral and molecular analysis of nicotine-conditioned place preference in zebrafish. PLoS One 8:e69453
Kily LJ, Cowe YC, Hussain O, Patel S, McElwaine S, Cotter FE, Brennan CH (2008) Gene expression changes in a zebrafish model of drug dependency suggest conservation of neuro-adaptation pathways. J Exp Biol 211:1623–1634
Klee EW, Ebbert JO, Schneider H, Hurt RD, Ekker SC (2011) Zebrafish for the study of the biological effects of nicotine. NIC Tob Res Off J Soc Res NIC Tob 13:301–312
Levin ED, Limpuangthip J, Rachakonda T, Peterson M (2006) Timing of NIC effects on learning in zebrafish. Psychopharmacology 184:547–552
Levin ED, Bencan Z, Cerutti DT (2007) Anxiolytic effects of NIC in zebrafish. Physiol Behav 90:54–58
Marks MJ, Laverty DS, Whiteaker P, Salminen O, Grady SR, McIntosh JM, Collins AC (2010) John Daly’s compound, epibatidine, facilitates identification of nicotinic receptor subtypes. J Mol Neurosci MN 40:96–104
Mathur P, Guo S (2010) Use of zebrafish as a model to understand mechanisms of addiction and complex neurobehavioral phenotypes. Neurobiol Dis 40:66–72
Matta SG, Balfour DJ, Benowitz NL, Boyd RT, Buccafusco JJ, Caggiula AR et al (2007) Guidelines on nicotine dose selection for in vivo research. Psychopharmacol (Berl) 190:269–319
Mihalak KB, Carroll FI, Luetje CW (2006) Varenicline is a partial agonist at alpha4beta2 and a full agonist at alpha7 neuronal nicotinic receptors. Mol Pharmacol 70:801–805
Moore TJ, Furberg CD, Glenmullen J, Maltsberger JT, Singh S (2011) Suicidal behavior and depression in smoking cessation treatments. PLoS One 6:e27016
Museo E, Wise RA (1994) Place preference conditioning with ventral tegmental injections of cytisine. Life Sci 55:1179–1186
Panula P, Chen YC, Priyadarshini M, Kudo H, Semenova S, Sundvik M, Sallinen V (2010) The comparative neuroanatomy and neurochemistry of zebrafish CNS systems of relevance to human neuropsychiatric diseases. Neurobiol Dis 40:46–57
Papke RL, Porter Papke JK (2002) Comparative pharmacology of rat and human alpha7 nAChR conducted with net charge analysis. Br J Pharmacol 137:49–61
Papke RL, Stokes C (2010) Working with OpusXpress: methods for high volume oocyte experiments. Methods 51:121–133
Papke RL, Ono F, Stokes C, Urban JM, Boyd RT (2012) The nicotinic acetylcholine receptors of zebrafish and an evaluation of pharmacological tools used for their study. Biochem Pharmacol 84:352–365
Petzold AM, Balciunas D, Sivasubbu S, Clark KJ, Bedell VM, Westcot SE, Myers SR, Moulder GL, Thomas MJ, Ekker SC (2009) NIC response genetics in the zebrafish. Proc Natl Acad Sci U S A 106:18662–18667
Picciotto MR, Kenny PJ (2013) Molecular mechanisms underlying behaviors related to NIC addiction. Cold Spring Harb Perspect Med 3:a012112
Pucci L, Grazioso G, Dallanoce C, Rizzi L, De Micheli C, Clementi F, Bertrand S, Bertrand D, Longhi R, De Amici M, Gotti C (2011) Engineering of alpha-conotoxin MII-derived peptides with increased selectivity for native alpha6beta2* nicotinic acetylcholine receptors. FASEB J Off Publ Fed Am Soc Exp Biol 25:3775–3789
Rasmussen T, Swedberg MD (1998) Reinforcing effects of nicotinic compounds: intravenous self-administration in drug-naive mice. Pharmacol Biochem Behav 60:567–573
Raupach T, van Schayck CP (2011) Pharmacotherapy for smoking cessation: current advances and research topics. CNS Drugs 25:371–382
Rink E, Wullimann MF (2002) Connections of the ventral telencephalon and tyrosine hydroxylase distribution in the zebrafish brain (Danio rerio) lead to identification of an ascending dopaminergic system in a teleost. Brain Res Bull 57:385–387
Rollema H, Chambers LK, Coe JW, Glowa J, Hurst RS, Lebel LA, Lu Y, Mansbach RS, Mather RJ, Rovetti CC, Sands SB, Schaeffer E, Schulz DW, Tingley FD 3rd, Williams KE (2007) Pharmacological profile of the alpha4beta2 nicotinic acetylcholine receptor partial agonist varenicline, an effective smoking cessation aid. Neuropharmacology 52:985–994
Rollema H, Wilson GG, Lee TC, Folgering JH, Flik G (2011) Effect of co-administration of varenicline and antidepressants on extracellular monoamine concentrations in rat prefrontal cortex. Neurochem Int 58:78–84
Sala M, Braida D, Pucci L, Manfredi I, Marks MJ, Wageman CR, Grady SR, Loi B, Fucile S, Fasoli F, Zoli M, Tasso B, Sparatore F, Clementi F, Gotti C (2013) CC4, a dimer of cytisine, is a selective partial agonist at alpha4beta2/alpha6beta2 nAChR with improved selectivity for tobacco smoking cessation. Br J Pharmacol 168:835–849
Singh S, Loke YK, Spangler JG, Furberg CD (2011) Risk of serious adverse cardiovascular events associated with varenicline: a systematic review and meta-analysis. CMAJ Can Med Assoc J = J Assoc Med Can 183:1359–1366
Smith JW, Mogg A, Tafi E, Peacey E, Pullar IA, Szekeres P, Tricklebank M (2007) Ligands selective for alpha4beta2 but not alpha3beta4 or alpha7 nicotinic receptors generalise to the NIC discriminative stimulus in the rat. Psychopharmacology 190:157–170
Spiller K, Xi ZX, Li X, Ashby CR Jr, Callahan PM, Tehim A, Gardner EL (2009) Varenicline attenuates nicotine-enhanced brain-stimulation reward by activation of alpha4beta2 nicotinic receptors in rats. Neuropharmacology 57:60–66
Streisinger G (2000) The zebrafish book. Oregon Press, Eugene
Svoboda KR, Vijayaraghavan S, Tanguay RL (2002) Nicotinic receptors mediate changes in spinal motoneuron development and axonal pathfinding in embryonic zebrafish exposed to nicotine. J Neurosci Off J Soc Neurosci 22:10731–10741
Swain HA, Sigstad C, Scalzo FM (2004) Effects of dizocilpine (MK-801) on circling behavior, swimming activity, and place preference in zebrafish (Danio rerio). Neurotoxicol Teratol 26:725–729
Tonstad S, Tonnesen P, Hajek P, Williams KE, Billing CB, Reeves KR (2006) Effect of maintenance therapy with varenicline on smoking cessation: a randomized controlled trial. JAMA J Am Med Assoc 296:64–71
Vieira-Brock PL, Miller EI, Nielsen SM, Fleckenstein AE, Wilkins DG (2011) Simultaneous quantification of nicotine and metabolites in rat brain by liquid chromatography–tandem mass spectrometry. J Chromatogr B Anal Technol Biomed Life Sci 879:3465–3474
Walters CL, Brown S, Changeux JP, Martin B, Damaj MI (2006) The beta2 but not alpha7 subunit of the nicotinic acetylcholine receptor is required for nicotine-conditioned place preference in mice. Psychopharmacology 184:339–344
Zierler-Brown SL, Kyle JA (2007) Oral varenicline for smoking cessation. Ann Pharmacother 41:95–99
Zirger JM, Beattie CE, McKay DB, Boyd RT (2003) Cloning and expression of zebrafish neuronal nicotinic acetylcholine receptors. Gene Expr Patterns GEP 3:747–754
Acknowledgments
We thank Prof. Fabio Sparatore for the generous gift of CC4 and CC26. All the authors declare that they have no conflict of interest or financial disclosures to make. Funding was received from the Italian PRIN 2009R7WCZS; the European Union grant Eranet; the CNR Research Project on Aging, Regione Lombardia Projects NUTEC ID 30263049 and MbMM-convenzione no.18099/RCC; and James and Esther King Biomedical Research 1KG12.
Author information
Authors and Affiliations
Corresponding author
Additional information
Luisa Ponzoni and Daniela Braida contributed equally in this study.
Luisa Ponzoni is a recipient of a fellowship from the Fondazione Fratelli Confalonieri, Milano.
Rights and permissions
About this article
Cite this article
Ponzoni, L., Braida, D., Pucci, L. et al. The cytisine derivatives, CC4 and CC26, reduce nicotine-induced conditioned place preference in zebrafish by acting on heteromeric neuronal nicotinic acetylcholine receptors. Psychopharmacology 231, 4681–4693 (2014). https://doi.org/10.1007/s00213-014-3619-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-014-3619-x