Abstract
Horseshoe bats (Rhinolophidae), Old World leaf-nosed bats (Hipposideridae), and several species of moustached bats (Mormoopidae), including Parnell’s moustached bat (Pteronotus parnellii), Paraguayan moustached bat (Pteronotus paraguayensis) and Mesoamerican moustached bats (Pteronotus mesoamericanus) emit pulses with long constant frequency (CF) components and have evolved mechanisms for separating the pulse and echo in frequency. This approach to echolocation—high duty cycle (HDC) echolocation—depends largely on Doppler shift compensation (DSC). In 1968, Schnitzler discovered that the greater horseshoe bat (Rhinolophus ferrumequinum) compensates for flight-induced Doppler shifts in the CF component of echoes by adjusting their call frequency, ensuring a stable echo frequency during flight. This significant behavioral adaptation is supported by an acoustic fovea, a striking morphological and physiological specialization occurring from the peripheral to the central auditory system in HDC bats. The auditory fovea has neurons with high sensitivity to the narrow frequency range of the CF components of echolocation calls of HDC bats. Doppler shift compensation of the frequency that dominates the echolocation calls maintains the frequency within the narrow range of the acoustic fovea. This combination of CF calls, HDC echolocation, and DSC allows fine-frequency analysis of acoustic glints in echoes from insects fluttering their wings, which makes these bats very effective at detecting flutter.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Acoustic glints
- CF-FM pulse
- CF2
- DSCF area
- Echo intensity compensation
- Foveal neurons
- Reference frequency
- Resting frequency (Frest)
9.1 Introduction
Unlike the large majority of echolocating bats studied to date, some bats (Rhinolophidae, Hipposideridae, and a few species of Mormoopidae) are high duty cycle (HDC) echolocators. They produce long calls dominated by a single frequency (constant frequency, CF) and separated by brief periods of silence. They separate pulse and echo in frequency while virtually all other echolocators, including most bats, separate pulse and echo in time (low duty cycle, LDC) (see review in Fenton et al. 2012). The species of bats using this approach to echolocation were initially referred to as CF-FM bats, reflecting the general structure of the call. To date only one HDC species, the East Asian tailless leaf-nosed bat (Coelops frithii, Hipposideridae) has been shown to use a low duty cycle echolocation strategy even when approaching fluttering targets (Ho et al. 2013).
HDC echolocation depends largely on Doppler shift compensation (DSC). HDC bats compensate for flight-induced Doppler shifts in echoes by adjusting the CFs in their outgoing calls, thereby stabilizing the CFs in returning echoes. By DSC, the echo CF can be maintained within the range of the “acoustic fovea,” which allows fine-frequency analysis in the auditory system of HDC bats.
9.2 General Principles of Doppler Shift Compensation
9.2.1 9.2.1 Doppler Effect
Figure 9.1a illustrates a sound source producing a constant frequency f s (Hz) while approaching a stationary observer at a constant moving velocity v s (m/s). The emitted sound waves propagate at a rate of c (m) per second (sound velocity c) and are accompanied by the movement of v s (m) of the sound source itself in the same direction per second; thus, the waves emitted from the sound source per second, f s, are distributed along a distance of \( c-{v}_{\mathrm{s}} \) (m). Therefore, the wavelength, λ o, at the observer is given by
The observed frequency at the observer f o is
As a result, the observer will receive the sound waves at a frequency higher than the original f s emitted by the sound source. In contrast, when the sound source moves away from the observer, the frequency at observer f o is lower than f s
In a case of an observer with constant moving velocity, v o, approaching a stationary sound source emitting a f s (wavelength λ s) (Figure 9.1b), the observed frequency by the observer f o is
Figure 9.1c depicts a situation where a flying bat is emitting a sound with f s and receives echoes from a large stationary object located in front of the bat. In this case, Eq. (9.2) and (9.4) should be combined. The frequency of echoes, f e, received at the bat is given by
where v b is the moving velocity of the bat. When the object is also moving, v b can be replaced by the relative moving velocity between the bat and the object. Because v b can be considered to be small compared to the sound velocity c, the Doppler shift Δf can be estimated by
Equation (9.6) indicates that the relative moving velocity can be obtained from the observed Doppler shift, which can be applied to various measurement techniques of target velocity in the field of engineering.
9.2.2 9.2.2 Ecology of Doppler Shift Compensation
9.2.2.1 9.2.2.1 High Duty Cycle Echolocation in Bats
The approximately 165 species of HDC bats use calls with a dominant, long CF component that begins and/or ends with a brief frequency-modulated (FM) component, referred to as the CF-FM pulse. The greater horseshoe bat, Rhinolophus ferrumequinum (10–50 ms), and Parnell’s mustached bat, Pteronotus parnellii (7–30 ms), use rather long CF component pulses, whereas hipposiderid bats emit shorter pulses (5–10 ms). HDC bats generally produce calls whose signal durations are ≥25 % of the time between the onset of successive calls (Fenton et al. 2012). Therefore, HDC echolocation results in overlap of the returning echo with the emitted pulse. To avoid masking of echoes by outgoing long-duration calls, HDC bats use DSC to separate the dominant CF components of the call and the echo in frequency. In contrast, LDC bats produce short-duration calls with long intervals between calls, allowing the bats to avoid forward masking by separating pulse and echo in time.
The CF-FM pulses emitted by HDC bats typically consist of harmonics in which the second harmonic has the highest energy because the fundamental component is attenuated by vocal tract filtering (Hartley and Suthers 1988, 1990). The frequency of the second CF component (CF2) of calls produced when at rest (resting frequency, Frest, e.g., when the bat is roosting and not compensating for Doppler-shifted echoes) differs among subspecies and among individuals (e.g., 81–85 kHz for R. ferrumequinum; 59–64 kHz for P. parnellii). In addition to physical constitution, sex, age, geography, and morphometrics, the Frest of an adult HDC bat also shows a slight but continual individual drift over several months or seasons or throughout its lifetime (Jones and Ransome 1993; Hiryu et al. 2006).
Among mormoopid bats, only Pteronotus parnellii, the Paraguayan mustached bat (P. paraguayensis), and the Mesoamerican mustached bat (P. mesoamericanus) use HDC echolocation, although Wagner’s moustached bat P. personatus (but not other mormoopids) performs DSC (Smotherman and Guillén-Servent 2008). The lesser bulldog bat (Noctilio albiventris) and the greater bulldog bat (N. leporinus) are LDC echolocators that sometimes use short, narrowband (quasi-CF) pulses, and the latter is considered to partially exhibit DSC (Wenstrup and Suthers 1984). For details, see Section 9.5 of this chapter.
9.2.2.2 9.2.2.2 Discovery of Doppler Shift Compensation
In 1968, Schnitzler reported that Rhinolophus ferrumequinum lowered the CF2 of the emitted pulse (pulse CF2) when flying from one place to another in a flight chamber. The bats maintained the CF2 of returning echoes (echo CF2) around the Frest. The Doppler shifts induced by the bat’s flight speed were compensated by lowering the pulse frequency. Schnitzler (1968) called this Doppler shift compensation (Figure 9.2). DSC was also confirmed in flying Pteronotus parnellii (then called Chilonycteris rubiginosa) (Schnitzler 1970). Schnitzler (1973) later demonstrated that R. ferrumequinum flying in a wind tunnel compensated for the Doppler shifts based on the ground speed, not the airspeed. Bats flying in a He-O2 gas mixture exhibited DSC based on the change in sound speed manipulated by the mixture rate of the gas (Schnitzler 1973). These findings demonstrated that the bats use feedback control for DSC involving the echo frequency, triggering a change in the pulse CF2 so that echo CF2 is maintained at a constant value.
Doppler shift compensation of a bat during flight in a flight chamber. Before the flight, the CF2 of the emitted pulse is maintained at a constant (Frest). During flight, the bat lowers the CF2 of the emitted pulse (Fpulse) so that the of the returning echo (Fecho) remains constant at about Frest. Fmic is the CF2 of the emitted pulse detected by the microphone. Fpulse and Fecho are determined based on the values of Fmic and the flight speed of the bat measured by a photoelectric detector (Adapted from Schnitzler 1968)
In flight experiments, the pulse and echo frequencies determined from remote recordings by a stationary microphone were corrected to eliminate flight-induced Doppler shifts. This required measurement of the bat’s flight speed with appropriate accuracy. Later experiments used a pendulum on which a stationary bat was mounted and swung toward a large target. Henson et al. (1980) first demonstrated that P. parnellii held on a pendulum lowered its pulse CF2 to keep the echo CF2 within a narrow frequency band near the frequency with the lowest threshold in a cochlear microphonic (CM) audiogram.
To evaluate the detailed responses of bats over a wide range of positive and negative Doppler shifts, the emitted pulses were electronically shifted in frequency so that artificial echoes could be played back to a stationary bat in real time (Schuller et al. 1974; Simmons 1974). Playback experiments offer substantial advantages for the quantitative analysis of DSC because arbitrary target motion can generate Doppler shifts. Some relevant findings are described in detail in Section 9.4.
9.2.2.3 9.2.2.3 Discovery of the Auditory Fovea
Schnitzler (1968) had pointed out that bats maintained the echo CF2 at a frequency approximately 150 Hz higher than the Frest. Schuller et al. (1974) referred to the echo CF2 maintained by DSC as the reference frequency. The compensated frequency difference between the Frest and the reference frequency varies among bat species and among studies, but it is usually 150–200 Hz above the Frest. The compensation offset is considered to be the DSC threshold, the point at which bats begin to exhibit DSC when the change in the echo CF2 exceeds the DSC threshold (Schuller et al. 1974; Smotherman and Metzner 2003b).
The most remarkable physiological features of HDC bats are specializations of the auditory system for fine-frequency analysis of the CF component dominating their echolocation sounds, particularly the CF2 used in DSC. As an analogy to the fovea in the visual system (maximal visual sharpness due to a high concentration of cones in the retina), Schuller and Pollak (1979) called this specialization in HDC bats the “auditory fovea” (or acoustic fovea). This specialization begins in the frequency place map of the basilar membrane (BM) in the cochlea where there are widely expanded representations around the echo CF2 (for a review see Kössl and Vater 1995), and an over-representation of sharply tuned neurons around the echo CF2 occurs in all stations along the auditory pathway from the peripheral to the central auditory system. Thus, the majority of neurons in the Doppler-shifted CF processing area (DSCF area) are tuned to frequencies between 61 and 63 kHz, corresponding to the frequency range of the echo CF2 modified by DSC in P. parnellii (for reviews see Suga 1984, 1990).
Schnitzler et al. (1976) demonstrated that the threshold minimum of the summated neural potentials (the N1-on response audiogram) in R. ferrumequinum was tuned to the individual reference frequency and that the individual reference frequency was 30–500 Hz higher than the Frest. In addition, the sharp minima of CM audiograms in rufous horseshoe bat Rhinolophus rouxi and P. parnellii are 200 Hz above the Frest (Henson et al. 1980). The specialized frequency in the auditory receiver of HDC bats corresponds to the reference frequency at which the echo CF2 is maintained by DSC. Anatomical and neurophysiological specializations found in HDC bats are described in detail in Section 9.3.
9.2.2.4 9.2.2.4 Impact of Doppler Shift Compensation on High Duty Cycle Echolocation
HDC echolocating bats are thought to hunt in clutter where their ability to detect fluttering targets permits them to detect and track a flying insect. A long CF component transmits fluttering information of insect prey because both periodic fluctuations of amplitude and frequency (acoustical glints) are encoded in the echo CF component (Figure 9.3). From an acoustical perspective, these acoustic glints are easily detected from background echoes in the cluttered environment. HDC bats use this acoustical information about fluttering insects while foraging. Bats that use HDC echolocation have better flutter detection ability than LDC bats (Lazure and Fenton 2011).
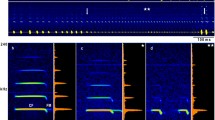
Spectrograms (above) and oscillograms (below) of acoustic frequency/amplitude glints generated by four different fluttering insects ensonified with an 83 kHz constant-frequency tone, which represents the main component of the echolocation call of Rhinolophus ferrumequinum. Each insect is facing in three different directions: 0°, 90°, and 180°. Notice that all insects are fluttering at 50 Hz, but glint structure and echo structure between the glints are species specific and orientation specific. Deilephila: sphingid moth, Deilephila elpenor, Lepidoptera; Scotia: noctuid moth, Scotia exclamations, Lepidoptera; Melolontha: scarabid beetle, Melolontha melolontha, Coleoptera; Tipula: crane fly, Tipula oleracea, Tipulidae, Diptera (Adapted from von der Emde and Schnitzler 1990)
What is the impact of DSC on echolocation by the HDC bats? The auditory receiver of HDC bats is highly sensitive to frequencies around the biologically most important frequency range, the reference frequency, where the echo CF2 is maintained by DSC (see Section 9.3). In contrast, in an echolocation pulse the CF2 is lower than the frequency of the sharp threshold minima in the audiograms. Thus the auditory receiver of HDC bats is sensitive to the compensated echo CF2 but relatively insensitive to the pulse CF2, suggesting that DSC reduces masking of weak echoes by intense emitted pulses. Furthermore, neurons highly tuned to the best frequency (BF) in the auditory fovea facilitate encoding information about fluttering insect prey. HDC bats increase the duration of a CF component or repetition rate when exposed to a fluttering target, further increasing the duty cycle so that they can repeatedly obtain the fluttering information within single echoes or over several successive echoes. DSC is a unique and important behavioral and physiological adaptation that supports flutter detection as a foraging strategy in HDC bats. Details of the neurophysiological aspects related to the processing of flutter information appear in the next Sec. 9.3.
9.3 Adaptations for Doppler Shift Compensation in the Auditory Receiver
The ears and the auditory pathway of HDC bats are an integral part of the echolocation system for bats that hunt flying insects in highly cluttered spaces. To process flutter information, the auditory receiver of HDC bats is strikingly specialized in the CF range that dominates the echolocation calls. The first evidence of such specializations was observed in the audiograms of HDC bats (Grinnell 1967).
Behavioral audiograms or neuronal audiograms (measured from single neurons of the auditory nerve or the auditory brain stem) are arguably the most reliable measurements of the threshold of hearing. In these audiograms, sharply tuned threshold minima and contrasting response maxima in the CF2 region were found for P. parnellii (Kössl and Vater 1996), for rhinolophids (Neuweiler et al. 1971; Long and Schnitzler 1975), and for hipposiderids (Neuweiler et al. 1984) (Figure 9.4). Maxima and minima in the CF2 ranges of neuronal audiograms in the hipposiderids, Schneider’s round-nosed bat (Hipposideros speoris) and bicoloured roundleaf bat (H. bicolor), however, are less pronounced than in rhinolophids and P. parnellii (Schuller 1980; Rübsamen et al. 1988). The sharp tuning of behavioral and neuronal audiograms in HDC bats is already apparent at the level of the CM and the N1 audiograms, and must be based on properties of the cochlea.
Mechanical audiogram of the cochlea in five bat species measured with Distortion Products Otoacoustic Emissions (DPOAEs). The DPOAE threshold curves represent the level of the f1 tone necessary to elicit a 2f1–f2 distortion product of −10 dB SPL; the level of f2 was 10 dB below that of f1 (for details see Kössl 1994; Kössl et al. 1999). The FM bat M. blainvillii and the short CF-FM bat P. macleayii do not employ DSC. H. lankadiva is a short CF-FM bat with incomplete DSC; P. parnellii and R. rouxi are CF-FM bats with DSC. Note the narrow threshold minimum and the distinct maximum a few kHz below in the threshold curves of the CF bats. Maxima and minima in the CF2 ranges of the DPOAE audiogram in the hipposiderid are less pronounced than in the rhinolophids and the moustached bat. The audiograms in M. blainvillii and P. macleayii are relatively smooth (Adapted from Foeller and Kössl 2000)
9.3.1 9.3.1 Auditory Fovea in the Cochlea of High Duty Cycle Echolocating Bats
Although the bat cochlea follows the common mammalian “bauplan” in structure and function, laryngeal echolocating bats have cochleae that are unusually large relative to body weight, in keeping with the importance of hearing for Chiroptera (Davies et al. 2013). The cochleae of HDC bats are larger relative to skull size than those of LDC bats (Habersetzer and Storch 1992). Among Pteronotus (Mormoopidae), the LDC echolocators have smaller cochleae than the HDC species. Among the HDC CF-FM bats (rhinolophids, hipposiderids, and P. parnellii) the smallest cochleae are found in hipposiderids, which use the shortest CF components in their echolocation (Fenton et al. 2011).
Among HDC bats, the morphology of the basilar membrane (BM) shows two common features: (1) abrupt changes or discontinuities in thickness and width that might play a role in enhancing tuning in a narrow-frequency band and (2) expanded areas with very little change in morphology and probably a very slight stiffness gradient, thus leading to expanded frequency mapping (Kössl and Vater 1995). These two special features of the BM both filter and largely over-represent the biologically important frequency range for flutter detection, the CF2 component in the stabilized echo, the reference frequency.
Cochlear frequency maps in HDC bats clearly show that a narrow-frequency range around the reference frequency is expanded to about 30 % of the BM length (Kössl and Vater 1995). The area on the BM representing the reference frequency has the highest afferent innervation. The abrupt thickening of the BM could provide a reflection zone for incoming waves, allowing standing waves to be set up in the region between the BM discontinuity and the stapes, which would then implement a passive and highly tuned resonator. The resonator would ensure the high sensitivity and sharp tuning apical to the BM discontinuity and into the reference frequency region (reviewed in Kössl and Vater 1995; Neuweiler 2003).
In addition to low threshold and sharp tuning, an active cellular component also may account for spontaneous otoacoustic emissions in the region of the reference frequency. Still under investigation, the cellular force generator (electromotility) that amplifies the sound energy of the CF echo could be established by fast movements of the bodies or stereocilia of outer hair cells (OHCs) (review in Kössl and Vater 1995). The electromotility of OHCs has been found up to at least 79 kHz (Frank et al. 1999). Interestingly, a number of the observed macro and micro-mechanical properties of the cochlear fovea differ among species of HDC bats (Kössl and Vater 1995; Vater 1998).
9.3.2 9.3.2 Auditory Fovea in the Higher Auditory Nuclei
Foveal areas with overrepresented neurons with best frequencies near the reference frequency characterize the entire auditory system of HDC bats. These correspond with the cochlear frequency expansion in the cochlear nucleus of HDC bats, where about half of all recorded auditory neurons are tuned to frequencies around the species-specific CF2 component. Sharp tuning of foveal neurons is evidenced in extremely narrow tuning curves with a Q10dB value (best frequency divided by bandwidth of the tuning curve at 10 dB above minimal threshold) well above 20 and often as high as 400 (Covey and Casseday 1995). The frequency ranges expanded at the cochlea and cochlear nucleus are further expanded at the level of the superior olivary complex (SOC) and the lateral lemniscus (LL).
The inferior colliculus (IC) of horseshoe bats and P. parnellii shows the typical tonotopic organization but with a distorted general arrangement of the isofrequency layers due to the overrepresentation of the CF2 range (Pollak and Park 1995). In the hipposiderid bat H. speoris, there is a less developed foveal area in the IC (Rübsamen et al. 1988; Fu et al. 2010).
The overrepresentation of a very narrow frequency band around CF2 also characterizes the tonotopically organized primary auditory cortex (AC) (O’Neill 1995). In P. parnellii, one-third of the tonotopic region within the primary AC represents frequencies between 60 and 63 kHz. In R. ferrumequinum, there is also significant magnification of the CF2 representation in the primary AC relative to the cochlear representation (Ostwald 1984). The foveal area in the primary AC is “personalized” in that the expanded frequencies vary among individual Frest and reference frequency in P. parnellii (Suga et al. 1987). This is called the “Doppler-shifted CF” area.
9.3.3 9.3.3 The Processing of Flutter Information in the Auditory Pathway
Both in the IC and in the AC of R. ferrumequinum, neurons processing information about fluttering accurately encode natural species-specific glint patterns (Schuller 1984; Ostwald 1988), perhaps allowing HDC bats to precisely identify prey. Because natural echoes are complex, in the laboratory sinusoidally amplitude-modulated (SAM) and frequency-modulated (SFM) stimuli were used to simulate flutter information from flying insects. In studies with P. parnellii and with horseshoe bats, foveal neurons show response selectivity to specific parameters of the modulating waveform, such as carrier frequency, modulation rate, modulation depth, and intensity. Already in the peripheral auditory system of HDC bats, foveal cochlear neurons show clear phase-locked responses to frequency modulations as small as ±0.01 to ±0.02 % of the carrier frequency.
Modulation rate reflects the wingbeat frequencies of different insects. Unlike peripheral neurons, filter neurons in the central auditory pathway respond preferentially to a limited range of modulation frequencies. In AC neurons, synchronization occurred up to 100–150 Hz with the range of maximal activity between 40 and 70 Hz (Ostwald 1988). The activity of most filter neurons in the higher auditory centers covers the wingbeat frequencies of the insects that HDC bats perhaps preferred as prey. There are also high sensitivity and selectivity for specific ranges of amplitude modulations in the foveal areas of the central auditory nuclei. Neurons sensitive to small amplitude variations of 10–20 % are able to encode the fine structure of the echoes created by wingbeat patterns (Vater 1982; Reimer 1987).
Many SFM-sensitive foveal units exhibit the most vigorous response and sharpest locking at low intensities. They reduce or lose their modulation encoding capabilities for stimuli with sound pressure levels above 50–70 dB SPL. This may be an adaptation for detecting faint echoes (Figure 9.5) (Pollak and Schuller 1981). In the auditory cortex, the DSCF area of P. parnellii and the CF2 area of R. ferrumequinum are populated with flutter processing neurons segregated by their best amplitudes. This may support insect discrimination tasks according to echo strength. The influence of sound pressure level on the processing of flutter information is of relevance also in light of echo intensity compensation. HDC bats maintain the intensity of the echoes returning from approaching targets at an optimal range (Kobler et al. 1985; Hiryu et al. 2008).
Effect of stimulus intensity on the locked discharges to sinusoidal frequency-modulated signals in four neurons of the inferior colliculus of Rhinolophus ferrumequinum . The neuron on the right shows tightly locked firings at all intensities above threshold; the three other units each locked best to only a small range of low intensities. Stimulus frequency was set at the neuron’s best frequency (BF) as indicated. All signals were 80 ms long (Adapted from Pollak and Schuller 1981)
Combination-sensitive neurons in nontonotopic areas show response selectivity to flutter information in the CF2 range. CF1/CF2 sensitive neurons, for example, are sensitive to small periodic modulations in the CF2 echo-frequency range if there is also stimulation in the CF1 range (Suga et al. 1983).
Foveal neurons all along the auditory pathway show preferences for selective ranges of frequency and intensity as well as modulation depth and rate. These foveal neurons may play a significant role in the dynamic neural representation of target attributes due to changes in position, orientation, and speed of either the bat or its prey.
9.4 Ethology of Doppler Shift Compensation
DSC is the result of behaviors as well as specialized anatomical and neurophysiological functions. Bats using HDC echolocation use DSC primarily to detect fluttering target prey. In this section, additional significant features of DSC are discussed in the context of ethology.
9.4.1 9.4.1 Acoustical Measurements of Doppler Shift Compensation Behaviors
Schuller et al. (1974) pointed out that the observed maximum compensation for positive Doppler shift in playback echoes ranges from 4,400 to 6,000 Hz, corresponding to the Doppler shift induced by flight at approximately 9 m/s in R. ferrumequinum (Schnitzler 1973). Later, Schnitzler (1978) reported that R. ferrumequinum compensated for positive Doppler shift of up to 8,000 Hz. Playback experiments have revealed that bats do not respond to negative Doppler shifts (downward frequency shifts) in echoes that would occur when a simulated target moves away from the bat (Figure 9.6) (Gaioni et al. 1990). This significant difference in behavioral responses between positive and negative Doppler shifts suggests that DSC is more important when bats approach targets (prey) than when the distance between the bat and the target is increasing.
Changes in the pulse CF2 during pendulum experiments in Pteronotus parnellii. The solid line indicates estimated Doppler shift of the echo CF2 if the bats do not exhibit DSC. The bats lower the pulse CF2 on the forward swing of the pendulum but do not compensate echoes on the backward swing (Adapted from Gaioni et al. 1990)
Metzner et al. (2002) used playbacks to demonstrate that R. ferrumequinum increased the pulse CF2 response to negative Doppler shifts, although the magnitude of compensation was small compared to the response to positive Doppler shifts. Negative Doppler shifts may also occur when the flying bats slow down. Then the echo CF2 will fall below the reference frequency, requiring the bats to increase the pulse CF2.
In R. ferrumequinum, the returning echoes always overlap with outgoing pulses because of the long pulse duration (Tian and Schnitzler 1997). Pulse-echo overlap is a prerequisite for DSC (Schuller 1974, 1977). In contrast, hipposiderids (the Taiwanese leaf-nosed bat, Hipposideros terasensis, and trident leaf-nosed bat, Asellia tridens) do not exhibit this overlap because they compensate for flight-induced frequency shifts in echoes by emitting pulses of short duration (Gustafson and Schnitzler 1979; Hiryu et al. 2005), suggesting a fundamental difference between rhinolophids and hipposiderids.
Hipposiderids are considered to have lower DSC abilities than horseshoe bats, and P. parnellii, Hipposideros speoris, and Hipposideros bicolor showed incomplete DSC, decreasing the pulse CF2 by only about half of the full Doppler shift (Habersetzer et al. 1984). However, some hipposiderid bats compensate for Doppler shifts in echoes during free flight (Gustafson and Schnitzler 1979; Hiryu et al. 2005). This suggests that certain experimental conditions, such as being retained on a swinging pendulum with an unnatural, greater rate of change in echo frequency, may result in lower DSC than under free-flight conditions (Schnitzler and Denzinger 2011). In fact, when the rate of change in the frequency of playback echoes is very fast, bats cannot keep pace with it and DSC becomes incomplete (Smotherman and Metzner 2003a). In sum, compensation performance varies among bat species and among studies, which may in part be due to unnatural conditions of some experimental procedures to induce Doppler shifts without flight.
9.4.2 9.4.2 Telemetry Recordings of Bats During Flight
For precise acoustical measurements of the calls of flying bats, microphones should be attached to the bats themselves. One option is a telemetry device consisting of a microphone, transmitter, and battery that is light enough to be mounted on a bat’s head or body. Henson and his colleagues developed a telemetry device for P. parnellii (∼11 g body mass) so that the echolocation signals emitted by flying bats were recorded without correcting for flight-induced Doppler shift. Their recordings demonstrated that flying bats lowered their pulse CF2; the echo CF2 was estimated to be maintained within 150 Hz around the reference frequency (Lancaster et al. 1992).
Riquimaroux and Watanabe (2000) developed an onboard telemetry microphone (Telemike), and confirmed DSC in free-flying Hipposideros terasensis (Hiryu et al. 2005). In another study, the CF2 of returning echoes was observed directly and was compensated by DSC in flying Japanese horseshoe bats R. ferrumequinum nippon (Hiryu et al. 2008) (Figure 9.7). In that study, the echo CF2 was maintained at the reference frequency, which is approximately 60 Hz higher than the Frest of each individual (with the standard deviation of 80–90 Hz). This indicates that flying bats compensate for the echo CF2 with an accuracy of regulation equivalent to bats at rest (Hiryu et al. 2008).
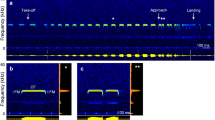
Echolocation signals of Rhinolophus ferrumequinum nippon recorded by the telemetry microphone (Telemike) mounted on the back of the bat during free flight in an experimental flight chamber. Spectrograms show the sequence of pulse-echo pairs during U-turn (U) and landing (L) events (top) and the magnified view of recorded sounds before landing (bottom). The bat dynamically changes the pulse CF2 while the echo CF2 remains relatively stable (Adapted from Hiryu et al. 2008)
In addition to acoustical measurements, Henson and his colleagues used telemetry to record CM potentials from flying bats (Henson et al. 1982, 1987). Interestingly, telemetry-recorded CM responses of echoes are usually greater than pulse-evoked CM responses, although the pulses are considerably louder than the returning echoes (Henson 1967; Henson et al. 1982). In one study, when tethered fluttering moths were presented to bats restrained on a swinging pendulum, the recorded sounds of echoes from insects did not show a prominent amplitude pattern, whereas the CM potentials were often prominent with acoustic glints caused by the fluttering moths (Henson et al. 1987). Such “amplified” echo-evoked CM potentials may indicate specialization of the auditory periphery in HDC bats, which likely plays an important role in detecting weak echoes from fluttering insects. An important challenge for future biosonar research is to combine telemetry recording of physiological data with acoustical measurements.
9.4.3 Flutter Detection by Doppler Shift Compensation
Some horseshoe bats hunt from perches in the wild and make short flights out to intercept prey. Before and after takeoff, the bats extend the duration of the CF component of their emitted pulse. The increase in pulse duration has also been observed in HDC bats at the beginning of the approach phase in the capture sequence for a fluttering moth in laboratory recordings (Mantani et al. 2012). HDC bats extend the pulse duration to increase the number of temporal repetitions of fluttering information.
HDC bats maintain the echo CF2 at the reference frequency range within their own acoustic fovea. Hence, they perform DSC for echoes from their prey. However, Trappe and Schnitzler (1982) reported that R. ferrumequinum performs DSC not on insect echoes but rather on echoes from stationary objects in the surroundings. Telemetry recordings have also provided direct evidence for this (Figure 9.8a) (Mantani et al. 2012). In this scenario, the echo from a moving target would be above or below the reference frequency. Such differences could be used by the bat to perceive the direction of the moth’s flight, either toward or away from the bat.
Echolocation behavior of Rhinolophus ferrumequinum nippon in pursuit of fluttering prey. (a) Changes in the CF2 of pulses and echoes as a function of time to capture during moth-capture flights in an experimental flight chamber. The bats compensate for echoes returned from the large static object in front of them (changing from the front wall to the right wall of the chamber during flight) but not for echoes from target moths, even though the bats were focused on capturing. (b) Sound sequence recorded by the Telemike while the bat was approaching a fluttering moth for capture; amplitude pattern (top) and spectrogram (bottom). Spectral glints caused by moth fluttering can be observed every 26–27 ms in the CF2 component of echoes (dashed box) (Adapted from Mantani et al. 2012)
Doppler shifts in echoes from moving insects consist of flight-induced Doppler shift and acoustical glints caused by insect fluttering. In fact, telemetry recordings of flying bats capturing moths have revealed periodic spectral glints of 1–1.5 kHz that are synchronized with wing fluttering (Figure 9.8b) (Mantani et al. 2012). By exhibiting DSC on echoes from objects ahead of the bat’s flight direction, the extent of Doppler shift in target insects is estimated as ±2–3 kHz from the reference frequency at a maximum. This indicates that fine-frequency analysis for fluttering information is necessary in the range of ±2–3 kHz from the reference frequency, which covers the acoustic fovea found in HDC bats (reviewed in Schnitzler and Denzinger 2011).
9.4.4 9.4.4 Effect of Echo Intensity on Doppler Shift Compensation
Schuller et al. (1974) reported that the ability to perform DSC is not affected by attenuation of playback echoes between 20 and 60 dB relative to emitted pulses. More recently, Smotherman and Metzner showed that the rapidity of DSC responses actually decreases with attenuation of playback echoes relative to that of emitted pulses (Metzner et al. 2002; Smotherman and Metzner 2003b).
Echolocating bats decrease the intensity of their emitted pulses as they approach a prey or an obstacle. This is considered to be echo intensity compensation, in which pulse intensity is adjusted with respect to the distance to a target, resulting in maintenance of echo intensity within the optimal sensitivity range (Kobler et al. 1985; Hiryu et al. 2007). Telemetry recordings of R. ferrumequinum nippon indicate that bats gradually decrease pulse amplitude as they approach a landing site so that observed echoes from the target are compensated for at a stable level (Hiryu et al. 2008). Thus, the bats compensate not only for increases in echo frequency but also for echo amplitude as the range to the target decreases.
The DSCF area of P. parnellii is tonotopic for the best frequency and amplitopic for the best amplitude in different axes. The delay tuning of FM-FM neurons in P. parnellii is affected by echo amplitude, suggesting that echo intensity compensation also helps to stabilize range estimations (Edamatsu and Suga 1993). HDC bats adjust their call frequency and amplitude together to maintain both within an optimal sensitivity range, which can help them to sustain consistent, fine analyses of returning echoes.
9.4.5 9.4.5 Jamming Avoidance Behavior of High Duty Cycle Echolocating Bats
The Frest of HDC bats differs slightly among individuals. However, if the Frest (or more precisely, the reference frequency) overlaps or comes into very close range with the calls of conspecifics, how would a HDC bat avoid or manage acoustic interference (i.e., a jamming avoidance response, JAR)?
By using telemetry, Furusawa et al. (2012) demonstrated that R. ferrumequinum nippon flying in pairs or flying alone made DSCs of identical accuracy. Interestingly, although the reference frequencies of individuals in that study were not significantly different, the bats did not shift their frequencies away from each other. Instead, most bat pairs actually shifted their frequencies slightly toward each other, decreasing the difference between them, the opposite of what is done by electric fish (Watanabe and Takeda 1963). Such paradoxical frequency shift was also observed in Noctilio albiventris during discrimination experiments; bats shifted the CF of emitted pulses toward that of the artificial jamming CF sounds (Roverud and Grinnell 1985). In contrast, non-DSC LDC bats adaptively change the characteristics of emitted FM signals to minimize acoustical interference from conspecific sounds (Habersetzer 1981; Chiu et al. 2009).
P. parnellii can detect frequency differences as small as 50 Hz in an echo CF2 due to the high sensitivity of their auditory system (Suga 1984; Riquimaroux et al. 1991). Therefore, the inherent inter-individual variation in reference frequency may be sufficient to allow HDC bats to discriminate between each other without shifting their reference frequencies while flying in groups. In hipposiderids there is no strong evidence of an active shift of the frequencies in echolocation calls to avoid jamming (Jones et al. 1993, 1994).
9.5 Evolution of Doppler Shift Compensation
Wing morphology, cochlear size, and a variety of other characters clearly demonstrate that bats from the early Eocene already featured powered flight and echolocation (Habersetzer et al. 1992). Because no “pre-bats” have been found to answer the question about the timing of the origin of flight and echolocation, several hypotheses have been proposed to explain the sequence in which these two main bat traits have evolved (Fenton 2010). Some hypotheses agree that the putative first echolocation call used by bats may have been a short, broadband multi-harmonic call emitted with long inter-call intervals and low duty cycles. CF echolocation and HDC, both of which depend largely on DSC, are considered to be derived behaviors that evolved more recently from LDC bats (Fenton et al. 1995; Maltby et al. 2009).
9.5.1 9.5.1 Doppler Shift Compensation in the Bat Phylogenetic Tree
Within Yinpterochiroptera, DSC appears to characterize the echolocation behavior of about 77 species of horseshoe bats (Rhinolophidae) and about 81 species of roundleaf bats (Hipposideridae) (Altringham 2011). DSC is not known from other families in the suborder (e.g., Craseonycteridae, Rhinopomatidae, Megadermatidae). Species in Craseonycteridae (e.g., Kitti’s hog-nosed bat, Craseonycteris thonglongyai; Surlykke et al. 1993) and in Rhinopomatidae (e.g., lesser mouse-tailed bat, Rhinopoma hardwickei; Habersetzer 1981) emit relatively long CF or narrowband signals with non-overlapping multiple harmonics of which the second harmonic is the most powerful. In the evolution of DSC, R. hardwickei may represent an intermediate evolutionary step because it emits long CF calls of about 50 ms and high duty cycles up to about 40 % (Habersetzer 1981). In addition, R. hardwickei shows a prominent sensitivity peak in its audiogram in the frequency of the dominant second harmonic (Simmons et al. 1984). The ancestor of Rhinopoma, Rhinolophus, and Hipposideros was probably in the process of evolving an acoustic fovea as a prerequisite for DSC (Neuweiler 1990).
In the suborder Yangochiroptera, just three species of Mormoopidae are HDC echolocators and one other, Pteronotus personatus, uses DSC (Smotherman and Guillén-Servent 2008). Recent phylogenetic evidence indicates that P. parnellii stems from the most basal node in the Pteronotus lineage and that P. personatus stems after P. parnellii from the second most basal node (Van den Bussche and Weyandt 2003). DSC has been reported in two species of Noctilio, suggesting that DSC may have occurred in the common ancestor of Noctilionidae and Mormoopidae.
9.5.2 9.5.2 Doppler Shift Compensation: CF and HDC in Bat Echolocation
It seems safe to state that the CF components of bat calls are a requisite to operate DSC. Long (>20 ms) CF components and calls are distinctive of rhinolophids, and within the family Mormoopidae, P. parnellii is the only species to use a particularly long CF component (Figure 9.9). Hipposiderids, the other recognized “DSC bats,” emit short CF-FM calls. Among LDC bats, the two species of Noctilio and Pteronotus personatus employ DSC; the three of them show a short CF component in their calls. Outside the four bat families known to have “DSC species,” CF components have been recorded in bats from Rhinopomatidae (Habersetzer 1981), Molossidae (Mora et al. 2004), and Phyllostomidae (Mora and Brinklov, personal observations). None of these species are known to employ DSC.
Spectrograms of typical search echolocation calls of the eight bat species of the family Mormoopidae. Notice that despite the similarities in call design (signals with multiple harmonics without overlap in which most energy is concentrated in the 2nd harmonic), only one species (Pper) emits long CF calls at HDC and only two species (Ppar and Pper) perform DSC. Ppar, Pteronotus parnellii; Mm, Mormoops megalophylla; Pg, Pteronotus gymnonotus; Mb, Mormoops blainvillei; Pd, Pteronotus davyi; Pm, Pteronotus macleayii; Pper, Pteronotus personatus; Pq, Pteronotus quadridens (Adapted from Mora et al. 2013)
The same duty cycle implies the same amount of available information as prey-generated amplitude and frequency glints. Therefore, HDC due to longer call durations may have assisted the development of a more precise DSC. This last assumption seems difficult to prove. The DSC of P. parnellii is indeed more precise than that of hipposiderids, but it performs as well as that of Pteronotus personatus, a congeneric LDC species (Smotherman and Guillén-Servent 2008). The precise DSC behavior of P. personatus shows that HDC doesn’t seem to be a requirement for the evolutionary acquisition of DSC. So far, narrowband calls appear to be a fact of life for echolocating bats while DSC and HDC are not.
9.5.3 9.5.3 Ecological and Behavioral Factors in the Evolution of Doppler Shift Compensation
In both the Old and the New Worlds, several “DSC bat species” developed similar echolocation behaviors and auditory systems, which reveal similarities in early echolocation tasks. The hunting of flying insects in cluttered habitat was undoubtedly among the primeval tasks leading to DSC.
Because most airborne targets encountered by bats flying in the open are insects, there is no clear advantage for bats in the acquisition of a sophisticated echolocation based on DSC and an auditory fovea. On the other hand, DSC and flutter detection are of great value for hunting insects in cluttered environments. The ability to extract information from Doppler-shifted echoes of fluttering insects may have allowed pre-bats exploiting DSC to detect and approach prey in dense vegetation and thus forage in areas with little competition from other bats species without DSC (Lazure and Fenton 2011). There are several species without DSC (e.g., Myotis nattereri, Murina spp.) that effectively separate prey from background clutter, which is evidence that echolocation strategies based on FM calls can also support foraging in highly cluttered environments (Siemers and Schnitzler 2004; Lazure and Fenton 2011).
Outside of the forest understory, DSC and flutter detection also assist noctilionid bats in hunting over water. Both species of Noctilio produce pure CF signals interspersed with CF-FM signals. However, there are many other bat species known t o capture prey from, or near, water surfaces; none of these perform DSC.
The specialized calling behavior and auditory receiver of “DSC bats” in both Yinpterochiroptera and Yangochiroptera are arguably the best examples of convergent evolution among echolocators. Because of large phylogenetic and geographic distances between Old World rhinolophids and New World mormoopids, the evolution of DSC clearly demonstrates that perceptual challenges imposed by the environment can override phylogenetic constraints.
9.6 Summary
DSC is achieved through behavioral and neurophysiological specializations in HDC bat species. These findings have advanced the understanding of biosonar systems considerably, and, therefore, DSC is among the most successful research topics in bat echolocation.
Man-made sonar systems are generally designed to transmit sonar sounds with fixed frequency and amplitude. Thus, target information, such as target velocity, is obtained by measuring deviations in frequency and amplitude of the echo. In contrast, HDC bats adjust the frequency of emitted sonar sounds to maintain the echo frequency within their auditory fovea. As a consequence, these bats can analyze the resulting echo within a narrow, sensitive range, allowing them to reduce computational effort by limiting the frequency and dynamic range being processed. To facilitate fine and stable analysis of fluctuating echoes, various compensation mechanisms may also underlie the fundamental processes of bat echolocation.
Doppler shift compensation may seem simple, but some of its behavioral and physiological features remain unexplained. The following are some open questions related to DSC:
-
1.
In highly cluttered environments, it is difficult to detect the weak echo returning from small insect prey, even though DSC adjusts the carrier frequency of the echo to the foveal range of the auditory receiver. What are the acoustic characteristics of the compensated echoes to which the bats actually respond, and how do they change through DSC? Furthermore, as indicated by the early work by Henson, unrevealed specializations likely function in the auditory periphery to facilitate the extraction of information from target prey under cluttered conditions.
-
2.
Thus far, the behavioral and physiological ontogeny of DSC has not been well studied. Furthermore, the evolution of DSC remains to be elucidated, as do the origin of flight and the origin of echolocation. Structured comparative studies of the mormoopids would help to reveal the evolutionary history of DSC. A more interesting question is why HDC bats diversified in the Old World and not in the New World. This also can give new leads to elucidate the evolution of DSC.
-
3.
HDC bats can perform DSC under conspecific-jammed conditions. Further investigation is needed to understand how bats adapt their echolocation both behaviorally and physiologically to overcome unexpected jamming sounds while flying with conspecifics.
Again, we should consider the adaptive benefits of DSC, and the effects of DSC on echolocation (e.g., in finding prey) should also be examined experimentally. DSC, which is a unique strategy for echolocation in HDC bats, will provide new perspectives not only for animal neuroethology but also for various design concepts in the technology and engineering fields.
References
Altringham, J. D. (2011). Bats: From evolution to conservation. Oxford, UK: Oxford University Press.
Chiu, C., Xian, W., & Moss, C. F. (2009). Adaptive echolocation behavior in bats for the analysis of auditory scenes. Journal of Experimental Biology, 212, 1392–1404.
Covey, E., & Casseday, J. H. (1995). The lower brainstem auditory pathways. In A. N. Popper & R. R. Fay (Eds.), Hearing by bats (pp. 235–295). New York: Springer-Verlag.
Davies, K. T. J., Maryanto, I., & Rossiter, S. J. (2013). Evolutionary origins of ultrasonic hearing and laryngeal echolocation in bats inferred from morphological analyses of the inner ear. Frontiers in Zoology, 10, 1–15.
Edamatsu, H., & Suga, N. (1993). Differences in response properties of neurons between two delay-tuned areas in the auditory cortex of the mustached bat. Journal of Neurophysiology, 69, 1700–1712.
Fenton, M. B. (2010). Convergences in the diversification of bats. Current Zoology, 56, 454–468.
Fenton, M. B., Audet, D., Obrist, M. K., & Rydell, J. (1995). Signal strength, timing and self-deafening: The evolution of echolocation in bats. Paleobiology, 21, 229–242.
Fenton, M. B., Skowronski, M. D., McGuire, L. P., & Faure, P. A. (2011). Variation in the use of harmonics in the calls of laryngeally echolocating bats. Acta Chiropterologica, 13, 169–178.
Fenton, M. B., Faure, P. A., & Ratcliffe, J. M. (2012). Evolution of high duty cycle echolocation in bats. Journal of Experimental Biology, 215, 2935–2944.
Foeller, E., & Kössl, M. (2000). Mechanical adaptations for echolocation in the cochlea of the bat, Hipposideros lankadiva. Journal of Comparative Physiology A: Neuroethology, Sensory, Neural, and Behavioral Physiology, 186, 859–870.
Frank, G., Hemmert, W., & Gummer, A. W. (1999). Limiting dynamics of high-frequency electromechanical transduction of outer hair cells. Proceedings of the National Academy of Sciences of the USA, 96, 4420–4425.
Fu, Z. Y., Tang, J., Jen, P. H., & Chen, Q. C. (2010). The auditory response properties of singe-on and double-on responders in the inferior colliculus of the leaf-nosed bat, Hipposideros armiger. Brain Research, 1306, 39–52.
Furusawa, Y., Hiryu, S., Kobayashi, I. K., & Riquimaroux, H. (2012). Convergence of reference frequencies by multiple CF-FM bats (Rhinolophus ferrumequinum nippon) during paired flights evaluated with onboard microphones. Journal of Comparative Physiology A: Neuroethology, Sensory, Neural, and Behavioral Physiology, 198, 683–693.
Gaioni, S. J., Riquimaroux, H., & Suga, N. (1990). Biosonar behavior of mustached bats swung on a pendulum prior to cortical ablation. Journal of Neurophysiology, 64, 1801–1817.
Grinnell, A. D. (1967). Mechanisms of overcoming interference in echolocating animals. In R.-G. Bushel (Ed.), Animal sonar systems (pp. 451–481). Jouy-en-Josas, France: Laboratorie de Physiologie Acoustique.
Gustafson, Y., & Schnitzler, H.-U. (1979). Echolocation and obstacle avoidance in the hipposiderid bat Asellia tridens. Journal of Comparative Physiology A: Neuroethology, Sensory, Neural, and Behavioral Physiology, 131, 161–167.
Habersetzer, J. (1981). Adaptive echolocation in the bat Rhinopoma hardwickei. Journal of Comparative Physiology A: Neuroethology, Sensory, Neural, and Behavioral Physiology, 144, 559–566.
Habersetzer, J., & Storch, G. (1992). Cochlea size in extant Chiroptera and middle Eocene microchiropterans from Messel. Naturwissenschaften, 79, 462–466.
Habersetzer, J., Schuller, G., & Neuweiler, G. (1984). Foraging behavior and Doppler shift compensation in echolocating hipposiderid bats, Hipposideros bicolor and Hipposideros speoris. Journal of Comparative Physiology A: Neuroethology, Sensory, Neural, and Behavioral Physiology, 155, 559–567.
Habersetzer, J., Richter, G., & Storch, G. (1992). Bats: Already highly specialized insect predators. In S. Schaal & W. Ziegler (Eds.), Massel: An insight into the history of life and of the earth (pp. 179–191). Oxford, UK: Clarendon Press.
Hartley, D. J., & Suthers, R. A. (1988). The acoustics of the vocal tract in the horseshoe bat, Rhinolophu hildebrandti. Journal of the Acoustical Society of America, 84, 1201–1213.
Hartley, D. J., & Suthers, R. A. (1990). Sonar pulse radiation and filtering in the mustached bat, Pteronotus parnellii rubiginosus. Journal of the Acoustical Society of America, 87, 2756–2772.
Henson, O. W., Jr., Henson, M. M., Kobler, J. B., & Pollak, G. D. (1980). The constant frequency component of the biosonar signals of the bat, Pteronotus parnellii parnellii. In R.-G. Busnel & F. F. James (Eds.), Animal sonar systems (pp. 913–916). New York: Plenum Press.
Henson, O. W., Jr., Pollak, G. D., Kobler, J. B., Henson, M. M., & Goldman, L. J. (1982). Cochlear microphonic potentials elicited by biosonar signals in flying bats, Pteronotus p. parnellii. Hearing Research, 7, 127–147.
Henson, D. W., Jr., Bishop, A. L., Keating, A. W., Kobler, J. B., Henson, M. M., Wilson, B. S., & Hansen, R. (1987). Biosonar imaging of insects by Pteronotus p. parnellii, the mustached bat. National Geographic Research, 3, 82–101.
Henson, O. W. J. (1967). The perception and analysis of biosonar signals by bats. In R.-G. Busnel (Ed.), Animal sonar systems (pp. 949–1002). Jouy-en-Josas, France: Laboratorie de Physiologie Acoustique.
Hiryu, S., Hagino, T., Riquimaroux, H., & Watanabe, Y. (2007). Echo-intensity compensation in echolocating bats (Pipistrellus abramus) during flight measured by a telemetry microphone. Journal of the Acoustical Society of America, 121, 1749–1757.
Hiryu, S., Katsura, K., Lin, L. K., Riquimaroux, H., & Watanabe, Y. (2005). Doppler-shift compensation in the Taiwanese leaf-nosed bat (Hipposideros terasensis) recorded with a telemetry microphone system during flight. Journal of the Acoustical Society of America, 118, 3927–3933.
Hiryu, S., Katsura, K., Nagato, T., Yamazaki, H., Lin, L. K., Watanabe, Y., & Riquimaroux, H. (2006). Intra-individual variation in the vocalized frequency of the Taiwanese leaf-nosed bat, Hipposideros terasensis, influenced by conspecific colony members. Journal of Comparative Physiology A: Neuroethology, Sensory, Neural, and Behavioral Physiology, 192, 807–815.
Hiryu, S., Shiori, Y., Hosokawa, T., Riquimaroux, H., & Watanabe, Y. (2008). On-board telemetry of emitted sounds from free-flying bats: Compensation for velocity and distance stabilizes echo frequency and amplitude. Journal of Comparative Physiology A: Neuroethology, Sensory, Neural, and Behavioral Physiology, 194, 841–851.
Ho, Y.-Y., Fang, Y.-P., Chou, C.-H., Cheng, H.-C., & Chang, H.-W. (2013). High duty cycle to low duty cycle: Echolocation behaviour of the Hipposiderid bat Coelops frithii. PloS ONE, 8, e62938.
Jones, G., & Ransome, R. D. (1993). Echolocation calls of bats are influenced by maternal effects and change over a lifetime. Proceedings of the Royal Society of London B: Biological Sciences, 252, 125–128.
Jones, G., Morton, M., Hughesand, P. M., & Buden, R. M. (1993). Echolocation, flight morphology and foraging strategies of some West African hipposiderid bats. Journal of Zoology, 230, 385–400.
Jones, G., Sripathi, K., Waters, D. A., & Marimuthu, G. (1994). Individual variation in the echolocation calls of three sympatric Indian hipposiderid bats, and an experimental attempt to jam bat echolocation. Folia Zoologica, 43, 347–361.
Kobler, J. B., Wilson, B. S., Henson, O. W., Jr., & Bishop, A. L. (1985). Echo intensity compensation by echolocating bats. Hearing Research, 20, 99–108.
Kössl, M. (1994). Otoacoustic emissions from the cochlea of the ‘constant frequency’ bats, Pteronotus parnellii and Rhinolophus rouxi. Hearing Research, 72, 59–72.
Kössl, M., & Vater, M. (1995). Cochlear structure and function in bats. In A. N. Popper & R. R. Fay (Eds.), Hearing by bats (pp. 191–234). New York: Springer-Verlag.
Kössl, M., & Vater, M. (1996). Further studies on the mechanics of the cochlear partition in the mustached bat. I. Ultrastructural observations on the tectorial membrane and its attachments. Hearing Research, 94, 63–77.
Kössl, M., Mayer, F., Frank, G., Faulstich, M., & Russell, I. J. (1999). Evolutionary adaptations of cochlear function in Jamaican mormoopid bats. Journal of Comparative Physiology A: Neuroethology, Sensory, Neural, and Behavioral Physiology, 185, 217–228.
Lancaster, W. C., Keating, A. W., & Henson, O. W., Jr. (1992). Ultrasonic vocalizations of flying bats monitored by radiotelemetry. Journal of Experimental Biology, 173, 43–58.
Lazure, L., & Fenton, M. B. (2011). High duty cycle echolocation and prey detection by bats. Journal of Experimental Biology, 214, 1131–1137.
Long, G. R., & Schnitzler, H.-U. (1975). Behavioural audiograms from the bat, Rhinolophus ferrumequinum. Journal of Comparative Physiology, A: Neuroethology, Sensory, Neural, and Behavioral Physiology, 100, 211–219.
Maltby, A., Jones, K. E., & Jones, G. (2009). Understanding the evolutionary origin and diversification of bat echolocation. In S. M. Brudzynski (Ed.), Handbook of mammalian vocalization (pp. 37–48). Oxford, UK: Academic Press.
Mantani, S., Hiryu, S., Fujioka, E., Matsuta, N., Riquimaroux, H., & Watanabe, Y. (2012). Echolocation behavior of the Japanese horseshoe bat in pursuit of fluttering prey. Journal of Comparative Physiology A: Neuroethology, Sensory, Neural, and Behavioral Physiology, 198, 741–751.
Metzner, W., Zhang, S., & Smotherman, M. (2002). Doppler-shift compensation behavior in horseshoe bats revisited: Auditory feedback controls both a decrease and an increase in call frequency. Journal of Experimental Biology, 205, 1607–1616.
Mora, E. C., Macias, S., Vater, M., Coro, F., & Kössl, M. (2004). Specializations for aerial hawking in the echolocation system of Molossus molossus (Molossidae, Chiroptera). Journal of Comparative Physiology A: Neuroethology, Sensory, Neural, and Behavioral Physiology, 190, 561–574.
Mora, E., Macías, S., Hechavarria, J., Vater, M., & Kössl, M. (2013). Evolution of the heteroharmonic strategy for target-range computation in the echolocation of Mormoopidae. Frontiers in Physiology, doi: 10.3389/fphys.2013.00141
Neuweiler, G. (1990). Auditory adaptations for prey capture in echolocating bats. Physiological Reviews, 70, 615–641.
Neuweiler, G. (2003). Evolutionary aspects of bat echolocation. Journal of Comparative Physiology A: Neuroethology, Sensory, Neural, and Behavioral Physiology, 189, 245–256.
Neuweiler, G., Schuller, G., & Schnitzler, H.-U. (1971). On- and off-responses in the inferior colliculus of the greater horseshoe bat to pure tones. Zeitschrift für vergleichende Physiologie, 74, 57–63.
Neuweiler, G., Singh, S., & Sripathi, K. (1984). Audiograms of a South Indian bat community. Journal of Comparative Physiology A: Neuroethology, Sensory, Neural, and Behavioral Physiology, 154, 133–142.
O’Neill, W. E. (1995). The bat auditory cortex. In A. N. Popper & R. R. Fay (Eds.), Hearing by bats (pp. 416–480). New York: Springer-Verlag.
Ostwald, J. (1984). Tonotopical organization and pure tone response characteristics of single units in the auditory cortex of the greater horseshoe bat. Journal of Comparative Physiology A: Neuroethology, Sensory, Neural, and Behavioral Physiology, 155, 821–834.
Ostwald, J. (1988). Encoding of natural insect echoes and sinusoidally modulated stimuli by neurons in the auditory cortex of the greater horseshoe bat, Rhinolophus ferrumequinum. In P. E. Nachtigall & P. W. B. Moore (Eds.), Animal sonar processes and performance (pp. 483–487). New York: Plenum Press.
Pollak, G. D., & Schuller, G. (1981). Tonotopic organization and encoding features of single units in inferior colliculus of horseshoe bats: Functional implications for prey identification. Journal of Neurophysiology, 45, 208–226.
Pollak, G. D., & Park, T. J. (1995). The inferior colliculus. In A. N. Popper & R. R. Fay (Eds.), Hearing by bats (pp. 296–367). New York: Springer-Verlag.
Reimer, K. (1987). Coding of sinusoidally modulated acoustic stimuli in the inferior colliculus of the rufous horseshoe bat, Rhinolophus rouxi. Journal of Comparative Physiology A: Neuroethology, Sensory, Neural, and Behavioral Physiology, 161, 305–313.
Riquimaroux, H., & Watanabe, Y. (2000). Characteristics of bat sonar sounds recorded by a telemetry system and a fixed ground microphone. Seventh Western Pacific Regional Acoustics Conference (WESTPRACVII), 233–238.
Riquimaroux, H., Gaioni, S. J., & Suga, N. (1991). Cortical computational maps control auditory perception. Science, 251, 565–568.
Roverud, R. C., & Grinnell, A. D. (1985). Frequency tracking and Doppler shift compensation in response to an artificial CF/FM echolocation sound in the CF/FM bat, Noctilio albiventris. Journal of Comparative Physiology A: Neuroethology, Sensory, Neural, and Behavioral Physiology, 156, 471–475.
Rübsamen, R., Neuweiler, G., & Sripathi, K. (1988). Comparative collicular tonotopy in two bat species adapted to movement detection, Hipposideros speoris and Megaderma lyra. Journal of Comparative Physiology A: Neuroethology, Sensory, Neural, and Behavioral Physiology, 163, 271–285.
Schnitzler, H.-U. (1968). Die Ultraschallortungslaute der Hufeisen-Fledermäuse (Chiroptera-Rhinolophidae) in verschiedenen Orientierungssituationen [The ultrasonic sounds of horseshoe bats (Chiroptera-Rhinolophidae) in different orientation situations]. Zeitschrift für Vergleichende Physiologie, 57, 376–408.
Schnitzler, H.-U. (1970). Echoortung bei der Fledermaus Chlonycteris rubiginosa. Zeitschrift für Vergleichende Physiologie, 68, 25–38.
Schnitzler, H.-U. (1973). Control of Doppler shift compensation in the greater horsehoe bat Rhinolophus ferrumequinum. Journal of Comparative Physiology A: Neuroethology, Sensory, Neural, and Behavioral Physiology, 82, 79–92.
Schnitzler, H.-U. (1978). Die Detektion von Bewegungen durch Echoortung bei Fledermäusen. Verhandlungen der deutschen Zoologischen Gesellschaft, 71, 16–33.
Schnitzler, H.-U., & Denzinger, A. (2011). Auditory fovea and doppler shift compensation: adaptations for flutter detection in echolocating bats using CF-FM signals. Journal of Comparative Physiology A: Neuroethology, Sensory, Neural, and Behavioral Physiology, 197, 541–559.
Schnitzler, H.-U., Suga, N., & Simmons, J. A. (1976). Peripheral auditory tuning for fine frequency analysis by the CF-FM bat, Rhinolophus ferrumequinum. III Cochlear microphonic and N1 responses. Journal of Comparative Physiology A: Neuroethology, Sensory, Neural, and Behavioral Physiology, 82, 93–102.
Schuller, G. (1974). The role of overlap of echo with outgoing echolocation sound in the bat Rhinolophus ferrumequinum. Naturwissenschaften, 61, 171–172.
Schuller, G. (1977). Echo delay and overlap with emitted orientation sounds and Doppler-shift compensation in the bat, Rhinolophus ferrumequinum. Journal of Comparative Physiology A: Neuroethology, Sensory, Neural, and Behavioral Physiology, 114, 103–114.
Schuller, G. (1980). Hearing characteristics and Doppler shift compensation in South Indian CF-FM bat. Journal of Comparative Physiology A: Neuroethology, Sensory, Neural, and Behavioral Physiology, 139, 349–356.
Schuller, G. (1984). Natural ultrasonic echoes from wing beating insects are encoded by collicular neurons in the CF-FM bat, Rhinolophus ferrumequinum. Journal of Comparative Physiology A: Neuroethology, Sensory, Neural, and Behavioral Physiology, 155, 121–128.
Schuller, G., & Pollak, G. D. (1979). Disproportionate frequency representation in the inferior colliculus of Doppler-compensating greater horseshoe bats: Evidence for an acoustic fovea. Journal of Comparative Physiology A: Neuroethology, Sensory, Neural, and Behavioral Physiology, 132, 47–54.
Schuller, G., Beuter, K., & Schnitzler, H.-U. (1974). Response to frequency shifted artificial echoes in the bat Rhinolophus ferrumequinum. Journal of Comparative Physiology A: Neuroethology, Sensory, Neural, and Behavioral Physiology, 89, 275–286.
Siemers, B. M., & Schnitzler, H.-U. (2004). Echolocation signals reflect niche differentiation in five sympatric congeneric bat species. Nature, 429, 657–661.
Simmons, J. A. (1974). Response of the Doppler echolocation system in the bat, Rhinolophus ferrumequinum. Journal of the Acoustical Society of America, 56, 672–682.
Simmons, J. A., Kick, S. A., & Lawrence, B. D. (1984). Echolocation and hearing in the mouse-tailed bat, Rhinopoma hardwickei: Acoustic evolution of echolocation in bats. Journal of Comparative Physiology A: Neuroethology, Sensory, Neural, and Behavioral Physiology, 154, 347–356.
Smotherman, M., & Metzner, W. (2003a). Fine control of call frequency by horseshoe bats. Journal of Comparative Physiology A: Neuroethology, Sensory, Neural, and Behavioral Physiology, 189, 435–446.
Smotherman, M., & Metzner, W. (2003b). Effects of echo intensity on Doppler-shift compensation behavior in horseshoe bats. Journal of Neurophysiology, 89, 814–821.
Smotherman, M., & Guillén-Servent, A. (2008). Doppler-shift compensation behavior by Wagner’s mustached bat, Pteronotus personatus. Journal of the Acoustical Society of America, 123, 4331–4339.
Suga, N. (1984). The extent to which biosonar information is represented in the bat auditory cortex. In G. M. Edelman, W. E. Gall & W. M. Cowan (Eds.), Dynamic aspects of neocortical function (pp. 315–373). New York: John Wiley & Sons.
Suga, N. (1990). Biosonar and neural computation in bats. Scientific American, 60–68.
Suga, N., Niwa, H., & Taniguchi, I. (1983). Representation of biosonar information in the auditory cortex of the mustached bat, with emphasis on representation of target velocity information. Advances in Vertebrate Neuroethology, 56, 829–867.
Suga, N., Niwa, H., Taniguchi, I., & Margoliash, D. (1987). The personalized auditory cortex of the mustached bat: Adaptation for echolocation. Journal of Neurophysiology, 58, 643–654.
Surlykke, A., Miller, L. A., Møhl, B., Andersen, B. B., Christensen-Dalsgaard, J., & Jørgensen, M. B. (1993). Echolocation in two very small bats from Thailand: Craseonycteris thonglongyai and Myotis siligorensis. Behavioral Ecology and Sociobiology, 33, 1–12.
Tian, B., & Schnitzler, H.-U. (1997). Echolocation signals of the greater horseshoe bat (Rhinolophus ferrumequinum) in transfer flight and during landing. Journal of the Acoustical Society of America, 101, 2347–2364.
Trappe, M., & Schnitzler, H.-U. (1982). Doppler-shift compensation in insect-catching horseshoe bats. Naturwissenschaften, 69, 193–194.
Van den Bussche, R. A., & Weyandt, S. E. (2003). Mitochondrial and nuclear DNA sequence data provide resolution to sister-group relationships within Pteronotus (Chiroptera: Mormoopidae). Acta Chiropterologica, 5, 1–13.
Vater, M. (1982). Single unit responses in cochlear nucleus of horseshoe bats to sinusoidal frequency and amplitude modulated signals. Journal of Comparative Physiology A: Neuroethology, Sensory, Neural, and Behavioral Physiology, 149, 369–388.
Vater, M. (1998). Adaptation of the auditory periphery of bats for echolocation. In T. H. Kunz & P. A. Racey (Eds.), Bat biology and conservation (pp. 231–245). Washington, DC: Smithonian Institution Press.
von der Emde, G., & Schnitzler, H.-U. (1990). Classification of insects by echolocating greater horseshoe bats. Journal of Comparative Physiology A: Neuroethology, Sensory, Neural, and Behavioral Physiology, 167, 423–430.
Watanabe, A., & Takeda, K. (1963). The change of discharge frequency by A. C. stimulus in a weakly electric fish. Journal of Experimental Biology, 40, 57–66.
Wenstrup, J. J., & Suthers, R. A. (1984). Echolocation of moving targets by the fish-catching bats, Noctilio leporinus. Journal of Comparative Physiology A: Neuroethology, Sensory, Neural, and Behavioral Physiology, 155, 75–89.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer Science+Business Media New York
About this chapter
Cite this chapter
Hiryu, S., Mora, E.C., Riquimaroux, H. (2016). Behavioral and Physiological Bases for Doppler Shift Compensation by Echolocating Bats. In: Fenton, M., Grinnell, A., Popper, A., Fay, R. (eds) Bat Bioacoustics. Springer Handbook of Auditory Research, vol 54. Springer, New York, NY. https://doi.org/10.1007/978-1-4939-3527-7_9
Download citation
DOI: https://doi.org/10.1007/978-1-4939-3527-7_9
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4939-3525-3
Online ISBN: 978-1-4939-3527-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)