Abstract
Echolocation sounds of Rhinolophus ferrumequinum nippon as they approached a fluttering moth (Goniocraspidum pryeri) were investigated using an on-board telemetry microphone (Telemike). In 40 % of the successful moth-capture flights, the moth exhibited distinctive evasive flight behavior, but the bat pursued the moth by following its flight path. When the distance to the moth was approximately 3–4 m, the bats increased the duration of the pulses to 65–95 ms, which is 2–3 times longer than those during landing flight (30–40 ms). The mean of 5.8 long pulses were emitted before the final buzz phase of moth capture, without strengthening the sound pressure level. The mean duration of long pulses (79.9 ± 7.9 ms) corresponded to three times the fluttering period of G. pryeri (26.5 × 3 = 79.5 ms). These findings indicate that the bats adjust the pulse duration to increase the number of temporal repetitions of fluttering information rather than to produce more intense sonar sounds to receive fine insect echoes. The bats exhibited Doppler-shift compensation for echoes returning from large static objects ahead, but not for echoes from target moths, even though the bats were focused on capturing the moths. Furthermore, the echoes of the Telemike recordings from target moths showed spectral glints of approximately 1–1.5 kHz caused by the fluttering of the moths but not amplitude glints because of the highly acoustical attenuation of ultrasound in the air, suggesting that spectral information may be more robust than amplitude information in echoes during moth capturing flight.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aerial-insectivorous bats hunt insect prey using their sophisticated echolocation (Simmons et al. 1979; Schnitzler and Kalko 2001). From the perspective of sonar engineering, detection and tracking of such small and moving targets is a difficult challenge. Some bat researchers including Schnitzler and colleagues have addressed this issue for a long time. For example, quantitative analyses of echoes from insect prey have shown that amplitude fluctuations (“amplitude glints”) are created by the wing movement of fluttering insects (Schnitzler and Ostwald 1983; Kober and Schnitzler 1990; Schnitzler and Denzinger 2011). Simultaneously, insect fluttering also produces both positive and negative Doppler shifts in the echoes, referred to as “spectral glints” (Schuller 1984; Kober and Schnitzler 1990; Schnitzler and Denzinger 2011). Echolocating bats appear to use the acoustical information of insect fluttering that is encoded both in frequency and amplitude modulations of the echoes (acoustical glints; general term applied to both amplitude and spectral glints) to detect and even identify or classify the insects (Schnitzler and Flieger 1983; Schnitzler and Ostwald 1983; von der Emde and Menne 1989; von der Emde and Schnitzler 1991; Schnitzler and Kalko 2001; Schnitzler and Denzinger 2011).
Bats in the families of Rhinolophidae, Hipposideridae, and Mormoopidae emit biosonar pulses consisting of a long constant frequency (CF) component [i.e., Rhinolophus ferrumequinum 50–60 ms (Tian and Schnitzler 1997), Hipposideros terasensis 5–10 ms (Hiryu et al. 2005)] followed, and sometimes also preceded, by short (1–2 ms) initial upward and terminal downward frequency modulated (FM) components. These CF-FM bat species are known to compensate for Doppler shifts in the echo by adjusting their CF of the second harmonic (CF2) of the emitted pulse accordingly, thus maintaining the echo frequency within a narrow frequency range (reference frequency) that the bats can hear best (Doppler-shift compensation, Schnitzler 1968; Gustafson and Schnitzler 1979; Gaioni et al. 1990; Lancaster et al. 1992; Metzner et al. 2002; Hiryu et al. 2005). The auditory system of these CF-FM bats is extremely sharply tuned to the reference frequency of the bat so that CF-FM bats can detect fluttering information of insect prey that is delivered by the carrier frequency of Doppler-shifted compensated echo with fine frequency analysis (Schuller and Pollak 1979; Ostwald 1984; Schnitzler and Denzinger 2011). From an acoustical standpoint, the long CF components of bat ultrasound are suited for the delivery of insect information encoded in fine amplitude and frequency modulations rather than short FM components. CF-FM bats have adapted the acoustical characteristics of their emitted pulses as well as their auditory system to address a difficult task, i.e., detecting the presence of small and moving insect prey using ultrasound against high atmospheric attenuation in the air.
The foraging behaviors of CF-FM and FM bats have been described in several previous reports based on field recordings (Schnitzler et al. 1985; Neuweiler et al. 1987; Kalko 1995; Surlykke and Moss 2000; Jensen et al. 2001; Simmons 2005). For example, insectivorous bats generally decrease the duration and increase the interpulse interval (IPI) of emitted ultrasound signals as they approach a prey. Recent studies have examined the active control of echolocation behavior of bats in the field as well as flight maneuvers of foraging bats by acoustic monitoring using a microphone array (Brinkløv et al. 2010; Fujioka et al. 2011). However, laboratory experiments can be advantageous over field studies in several aspects because adjustments of acoustic behavior resulting from interaction between bats and moths can be investigated with high precision under controlled experimental conditions (Vogler and Neuweiler 1983; Hristov and Conner 2005; Jakobsen and Surlykke 2010). Furthermore, Henson and colleagues (1987) first described echoes from fluttering moths using a telemetry technique in CF-FM bats (Pteronotus p. parnellii) during flight in the chamber of the laboratory. In that experiment, echolocation sounds were recorded from flying bats by the microphone that was mounted on the bat, but the analog measurements of the bat’s ultrasonic signals were not sufficient to analyze echoes with precise resolution. In their next paper, changes in CF2 frequencies of emitted pulses were investigated for bats during flight, but the Doppler shift in the echoes was still estimated from the difference in CF2 frequencies of the bat’s vocalization between telemetry and stationary ground microphone recordings (Lancaster et al. 1992). To understand the bat’s echolocation maneuvers during insect pursuit, direct measurement of echoes that the bats listened to during flight are required with improved measurement accuracy.
Here, we examined the echolocation sounds of Japanese CF-FM bats (R. ferrumequinum nippon) as they approached a fluttering moth (Goniocraspidum pryeri), using a telemetry microphone (Telemike) that was mounted on the bats (Riquimaroux and Watanabe 2000). We investigated how the bats changed their emitted pulse structures as they approached the moth, by monitoring both of the sounds (pulse and echo) and the flight trajectories of bats and moths.
To study the echolocation of CF-FM bats, it is essential to measure changes in the CF2 frequency of pulse–echo pairs in the context of the Doppler-shift compensation without errors induced by flight. The on-board Telemike recordings enabled us to directly evaluate the Doppler-shift compensation behavior while the flying bats pursued fluttering insects.
Materials and methods
Subjects
Four adult Japanese horseshoe bats (R. ferrumequinum nippon, body mass 20–30 g) were used. The bats were captured from a natural cave in Hyogo prefecture in Japan under license and in compliance with current Japanese laws. The animals were housed in a temperature- and humidity-controlled colony room (3 × 4 × 2 m) at Doshisha University in Kyoto, Japan. The bats were allowed to fly freely and were provided access to food (mealworms) and water. The day and night cycle of the room was set to 12-h dark:12-h light. On the day before the experiment, the amount of food provided to the bats was reduced by 50 % to ensure motivation for capturing moths during the experiment.
R. ferrumequinum nippon use compound echolocation signals, each consisting of a CF component with the second harmonic around 68–70 kHz being strongest, plus an accompanying initial short upward FM sweep (2–8 kHz, ending at 68–70 kHz) and a terminal short downward FM sweep (beginning at 68–70 kHz and extending to 8–12 kHz lower) (Hiryu et al. 2008).
Noctuid moths (G. pryeri) were caught as adults from a cave in Osaka prefecture in Japan. G. pryeri are widely distributed in Japan, and they diapause from summer to the next spring in caves that are used by R. ferrumequinum nippon as a day roost (Sano 2006). G. pryeri have ears, but their auditory characteristics with respect to the echolocation sounds of bats have not been investigated. The average body length is approximately 20 mm with the maximum wing span of 40–44 mm. The moths were housed in a rearing cage in the bat room under the same light cycle.
Moth-capture flight
The experiments were conducted in a flight chamber 8 (L) × 3 (W) × 2 m (H) under long wavelength lighting with red filters (>650 nm) to avoid visual effects on the bat. The experimenter released an individual bat from one end of the flight chamber. The flight chamber was constructed of steel plates to minimize the interference from external electromagnetic noise and waves used by commercial FM radio stations. A thin polyester tether (the diameter of the tether was 0.1–0.2 mm; Fujix, Schappespun, Kyoto, Japan) was carefully attached to the dorsal part of the moth body with a drop of beeswax (Tree of Life Co., Ltd., Tokyo, Japan) so that the fluttering moths were tethered 60–90 cm from the ceiling 4–6 m from the bat release point in the flight chamber. For each successive trial, moths were placed at different heights and distances from the bat’s release point.
For comparison, we recorded the echolocation behaviors of the bats during a stereotyped landing flight. The bats were released at one end of the flight chamber and allowed to fly freely to the opposite end, where a landing mesh [1 m (W) × 0.7 m (H)] was attached to the wall 1.8 m above the floor. This wall was referred to as the front wall during the landing flight task.
Sound recording
The echolocation sounds emitted during each flight were recorded using a custom-made telemetry microphone (Telemike) mounted on the bat. The recording procedure was the same as that used in a previous study (Hiryu et al. 2008). The Telemike consisted of a 1/8-inch omnidirectional condenser microphone (Knowles, Model FG-3329, Itasca, IL, USA), a miniature custom-designed FM transmitter unit, a 1.5 V hearing aid battery (Sony, Type SR421SW, Tokyo, Japan), and a transmitting antenna. Because the Telemike weighed 0.6 g (including the battery), it was small and light enough to be carried by the bats. The Telemike was attached to the back of the bat with a piece of double-sided adhesive tape, with the microphone pointed forward and positioned approximately 1 cm above the noseleaf, in the center of the right and left pinnae of the bat. Each experimental session lasted less than an hour, and the bats did not exhibit any fatigue during the experiments as verified by continuous visual inspection and vigor of flight. The removal of the Telemike from the back of the bat after each experiment was facilitated by the use of a parting agent to avoid skin irritation.
The Telemike’s transmitter produced radio signals that were received by an FM antenna (RadioShack Corporation, Model 15-1859, TX, USA) that was hung from the ceiling of the flight chamber. The received signals were demodulated to recover the bat’s ultrasonic broadcasts using a custom-made FM receiver. The signals from the receiver were then band-pass filtered from 20 to 150 kHz (NF Corporation, Model 3625, Yokohama, Japan), digitized by a DAT recorder (SONY, Model SIR-1,000 W, Tokyo, Japan, 16-bit, 384 kHz), and stored as files on the hard disk of a personal computer. The total frequency response of the Telemike system was flat within ±4 dB between 20 and 100 kHz.
Video recording
The flight behavior of the bats was also recorded using two digital high-speed video cameras (IDT Japan, Inc., MotionPro X3, Tokyo, Japan) located on the left and right sides of the flight chamber, behind the bat so as not to interfere with the flying bat’s paths. The video cameras recorded 125 frames per second, and three-dimensional (3D) coordinates of the flying bat paths were reconstructed from the video images using motion analysis software (Ditect Corporation, DIPPMotionPro Ver.2.2.1.0, Tokyo, Japan). Prior to recording the bat flights, a 3D reference frame with known coordinates was positioned in the center of the flight chamber which was briefly recorded by the two video cameras. The analysis software calibrated the 3D flight path reconstruction system using the cameras’ stereo view of the reference frame. Based on a direct linear transformation technique from the reference frame’s coordinates, successive positions of the flying bat as well as the locations of other objects were reconstructed from video-scene coordinates measured from the pair of 2D video images. The control signal that triggered and synchronized the frames of the video cameras with one another was digitally stored using the DAT recorder so that flight coordinates could then be synchronized with Telemike sound recordings. Using 3D coordinate data, the flight trajectories of the bat and the moth were determined in conjunction with the acoustic characteristics of the bat’s echolocation sounds.
Sound analysis
The acoustic characteristics of the flying bat’s broadcast sounds and reflected echoes were analyzed from spectrograms of the Telemike recordings using custom Matlab routines on a personal computer (Hiryu et al. 2008). Each pulse or echo was extracted from the recording, and then the second harmonic component of the pulse-echo pair was analyzed. Pulse duration was determined from the spectrogram at −25 dB relative to the peak intensity of the pulse. In this study, IPI was defined as the time from the beginning of one pulse to the beginning of the subsequent pulse. The spectrogram exhibited a peak in energy at the CF2 component in each sound, and this maximum energy was measured to quantify changes in sound pressure level of pulses and echoes. For each flight session, the measured energy values were normalized relative to the maximum energy across all pulses for that session. The CF2 frequency of each sound was determined from the frequency at the peak energy location in the extracted spectrogram with a frequency resolution of 46 Hz using a fast Fourier transform over 8,192 sample points identified from the spectrogram.
We analyzed the values of echo delay for each pulse–echo pair, which was determined from spectrograms of the terminal FM portion of the Telemike recorded sounds while the bat approached the tethered moth. The echo delay (t) was calculated from the distance (d) between the flying bat and the target (the moth) or different objects (the frontal or side wall and the ceiling of the chamber) calculated from the 3D coordinate data of the flying bat (video reconstruction data) using the formula t = 2d/c, where c is the sound velocity in the air (344 m/s). By comparing the echo delays shown from the acoustic data to the expected delays for the surrounding walls of the flight chamber and the moths from the video reconstruction, the reflector of each echo could be identified for the analysis of CF2 frequency (Hiryu et al. 2008).
Measurements of moth fluttering
We measured the fluttering period of three G. pryeri using high-speed video cameras (Integrated Design Tools Inc., MotionPro X3, Tokyo, Japan). The tip of a thin steel wire (0.5 mm diameter, 10 cm length) was carefully fastened to the dorsal part of the moth body with a drop of beeswax, which did not interfere with fluttering so that the moth remained in place but kept fluttering. The opposite end of the wire was attached to a fixed pole stand so that the fluttering moth was suspended at the center of the video image. The frontal images of the moth during fluttering were recorded at 1,000 frames per second. The 3D coordinates of the position of the tip of the moth wing were reconstructed from the video image using motion analysis software (Ditect, DippMotion 2D v 2.1), following the procedure described previously in this paper. Using 3D coordinate data, the fluttering period and speed of the tip of the moth wing were also measured.
To record the returning echo from the moth, the moth was suspended 0.5 m in front of a loudspeaker (Pioneer Corporation, PT-R7 III, Kanagawa, Japan), which was positioned in the center of the flight chamber. The thin wire was attached to the moth body. The walls of the chamber were coated with sound-absorbing materials to adequately reduce surrounding echoes. An ultrasonic microphone (Titley Electronics, Ltd., Anabat II, Ballina, Australia) was mounted on the top of the loudspeaker in the same orientation to point toward the moth (top right panel in Fig. 7a).
The CF sounds (100 ms duration, 70 kHz), which were generated using a function generator (Agilent Technologies, 33220A, Tokyo, Japan), were emitted from the loudspeaker with 114 dB SPL (peak-to-peak) measured 0.5 m in front of the loudspeaker. Echoes from the moth were recorded by the microphone, then band-pass filtered from 20 to 150 kHz (NF Corporation, Model 3625, Yokohama, Japan), digitized by a DAT recorder (SONY, Model SIR-1,000 W, Tokyo, Japan, 16-bit, 384 kHz) and stored as files on the hard disk of a personal computer.
Results
Hunting behavior
A total of 56 flight recordings of successful moth captures were taken from the four bats. When the experimenter released the bat in the flight chamber, the bat started to approach the fluttering, tethered moth. The bat eventually grasped in the wings. The bats never directly captured moths with their mouth. In some cases, the bat initially flew back and forth in front of the moth without capturing it, but usually the moth was captured within 5 s.
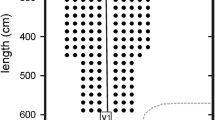
Figure 1 shows representative flight paths of the bats and the moths during the final approach. When a bat came within approximately 1 m of a moth, the insect often changed its flight direction, an evasive behavior in response to a bat’s approach. In 40 % of the successful moth-capture flights (22 of the 56 fights), the moth exhibited distinctive evasive flight behavior, but the bat was able to pursue the moth by following its flight path (Fig. 1a, b, c). In rest of the cases (34 of the 56 flights, 60 %), the moth remained in place and fluttered even when the bat was approaching (no evasive flight), and the bat flew directly toward the moth (Fig. 1d). G. pryeri never stopped fluttering while being pursued by the bat whereas some moth species have previously been reported to stop fluttering as an evasive action responding to the echolocation sounds of approaching bats (Miller and Surlykke 2001).
Top views of flight trajectories of bats during moth-capturing flights. Data were taken from representative capturing flights of four bats. The red solid circle represents a vocalization. The position of the moth at the timing of the bat’s vocalization is marked with a blue open circle. Each arrow indicates the flight direction of the bat (red) and moth (blue). The last circle of each flight sequence of the moth indicates the capturing point by the bat. a–d The bat pursued and successfully captured the moth even when it was exhibiting evasive flight. When the moth remained stationary but kept fluttering, the bat flew directly toward the moth and captured it (d)
To conduct further detailed analyses, we used a total of 16 flight recordings (four recordings from each of the four bats) that had good signal-to-noise ratios.
Echolocation pulses for fluttering moths
Figure 2a (top panel) shows a representative sequence of a spectrogram of echolocation sounds recorded by the Telemike mounted on the bat during the direct approach to the moth (the flight paths of the bat and the moth were shown in Fig. 2b). For comparison, echolocation sound sequences during a stereotyped landing flight of the same bat when the moth was not present are shown in bottom panel in Fig. 2a. In both cases, the bats decreased their pulse duration with decreasing distance to the moth and the front wall, respectively. Compared to the landing flight (Fig. 2c), the echolocation sounds emitted by the bat while approaching the fluttering moth were characterized by an extended duration of the CF portions of the pulses emitted before the final buzz phase (asterisks in Fig. 2b). When a bat was released while the tethered G. preyeri remained stationary without fluttering (we put the drop of beeswax on the dorsal part of the moth body including the junction between the wing and the body), this bat did not emit these long duration pulses. As shown in Fig. 4, pulses with the duration >65 ms were referred to as the long pulse in this study.
Comparison of echolocation sounds between moth-capture and landing flights. a Spectrograms of the second harmonic components of echolocation sounds of R. ferrumequinum nippon recorded using a Telemike during moth-capture flight (top) and landing flight (bottom). b Flight trajectories of the bat (red) and the moth (blue) shown in top panel (a). Each arrow indicates the flight direction of the bat and moth. The asterisk shows the long duration pulse (see text). c Changes in the duration of pulses emitted by the bat during moth-capture flight (solid circles) as a function of time to capture (top axis) and distance to capturing point (top axis). The dashed line indicates the mean of the fluttering period of the moth (26.5 ms). Circles represent changes in pulse duration during a stereotyped landing flight as a function of time to land. d Changes in the interpulse interval (IPI) during the moth-capture flight (top) and the landing flight (bottom)
Throughout the experiments, the bats consistently emitted the long duration pulses when the distance to the moth was approximately 3–4 m (asterisks in Fig. 2b and top axis of 2c), corresponding to approximately 1.5 s before the capture. The number of long duration pulses ranged from 2 to 10 pulses and the mean was 5.8 pulses (n = 95). The duration increased to 65–95 ms (mean ± SD 79.9 ± 7.9 ms, n = 95). The long pulse was 2–3 times longer than the mean of the maximum duration of 30–40 ms during the landing flight. The horseshoe bat usually emitted sounds in pairs and occasionally triplets during the landing flight (bottom panels in Fig. 2a, d). Although the bats sometimes emitted sound in groups during the moth-capture flights, the long pulse was usually emitted as a single pulse in all moth-capture flights (the bat emitted single pulse per respiration with almost constant IPI. See the top panel of Fig. 2d). The mean IPI of the long pulses was 105.8 ± 16.5 ms, and the mean of the duty cycle for emissions of the long pulses (ratio of pulse duration to IPI) was 73.8 ± 7.0 %.
The mean of the measured fluttering period of G. pryeri was 26.5 ± 0.6 ms (dashed lines in Figs. 2c, 3). Before the consecutive emissions of long pulses, the bats periodically increased and decreased their pulse duration so that the duration of some emissions exceeded the fluttering period of the moth (Fig. 3). The distribution of pulse duration during moth-capture flights shows bimodal peaks (Fig. 4). Beside the main peak at around 20 ms, the second peak appeared at around 80 ms that resulted from long duration pulses. The mean duration of long pulses (79.9 ± 7.9 ms) corresponded to three times the fluttering period of G. pryeri (the third solid triangle in Fig. 4; 26.5 × 3 = 79.5 ms), indicating that the bats adjusted the duration of long pulses to receive information on moth fluttering three times per pulse emission on average.
The distribution of the duration of CF-FM pulses emitted by the bat during moth-capture flight. Data were taken from 16 flights of 4 bats. The solid triangle indicates the multiple of the mean of the fluttering period of G. pryeri (26.5 ms). Beside the main peak at around 20 ms, the second peak was appeared at around 80 ms that resulted from long duration pulses. The mean duration of the long duration pulse (79.9 ± 7.9 ms, n = 95) corresponded to three times that of the fluttering period of the moth (26.5 × 3 = 79.5 ms)
The bats usually started to decrease the sound pressure level of the pulse as well as the pulse duration during the final approach phase of moth-capture flight (Fig. 5a), which appeared to be echo-intensity compensation behavior (Kobler et al. 1985; Hiryu et al. 2008). The sound pressure level of the pulse decreased when the pulse duration was shorter than 30 ms (Fig. 5b). On the other hand, there was no significant change in the sound pressure level of pulses where the duration was longer than 30 ms (one-way factorial ANOVA: F6,277 = 213, P = 0.91, Fig. 5b). This means that the long pulses (the pulse duration ranged from 65 to 95 ms) were produced by the bats without strengthening the sound intensity of vocalizations.
Representative changes in the sound pressure level and duration of the pulse emitted by bats during moth-capture flight. The sound pressure level was normalized relative to the maximum of the pulse measured across each flight session. a Changes in the relative sound pressure level of the pulse and pulse duration as a function of time to capture. b Relationship between the relative sound pressure level of the pulse and the pulse duration. Data were taken from 16 flight sessions of 4 bats. The mean and the standard deviation of the relative sound pressure level were analyzed for every 10 ms time bins of pulse duration over all flight sessions. There was no significant increase in the sound pressure level of the long duration pulse that ranged from 65 to 95 ms (one-way factorial ANOVA: F6, 277 = 213, P = 0.91)
Doppler-shift compensation
We examined the Doppler-shift compensation behavior of bats as they pursued the moths. Figure 6a shows the estimated echo delays (recorded by the Telemike) from the reflectors during the moth-capture flight shown in Fig. 1a. Comparing target distances determined from the 3D video reconstruction, we identified the reflectors of returning echoes that were recognized from the spectrogram. For example, in the flight shown in Fig. 1a, intense echoes were identified from the left wall (the wall on the bat’s left; see purple open circles in Fig. 6a) instead of the front wall (red open circles) within approximately 0.5 s before capture, according to a change in the flight direction of the bat. Figure 6b shows changes in measured CF2 frequencies of these recognized echoes and emitted pulses. Because the echoes from fluttering moths were only recorded by the Telemike within approximately 0.5 s before capture (1 m from the moths) in this study, the CF2 frequencies of moth echoes were estimated using the pulse CF2 frequency recorded by the Telemike and the 3D flight speed of the bat relative to the moth. As shown in Fig. 6b, the CF2 frequencies of the echoes from the front and left walls remained constant at around 67.3 kHz, whereas those from the target moth were not compensated for the Doppler shift during moth-capture flight. This behavior was consistently observed throughout our experiment (Fig. 6c); bats exhibited the Doppler-shift compensation for echoes that returned from the large static object in front of them, but not for echoes from target moths even though the bats were focused on capturing the moth.
Doppler-shift compensation of bats during moth-capture flight. a Changes in echo delay determined from sound data as a function of time to capture. Data are taken from Fig. 1a. Circle size indicates the relative variation in echo intensity, where bigger circles designate sounds of greater intensity relative to smaller circles. The four solid lines represent the estimated echo delays between the flying bat and four objects, namely, the front wall, ceiling, left wall and the moth which were calculated using three-dimensional coordinate data of the bat and the moth. b Changes in the CF2 frequencies of pulses (triangle) and echoes (circles) as a function of time to capture for the flight shown in Fig. 6a. Red solid, purple open circles and blue cross indicate the measured CF2 frequencies of returning echoes from the front, left wall and ceiling of the chamber, respectively. Green solid square indicates the estimated CF2 frequency of the moth from the relative velocity of the bat and the moth (see text). c Changes in the CF2 frequencies of pulses and echoes during moth-capture flight of bat B
Moth fluttering recorded by the Telemike
To investigate the extent of the frequency and amplitude of the modulated echoes that bats received from fluttering moths (we recorded echoes from fluttering G. pryeri using an artificial CF sound at 70 kHz), 114 dB SPL emitted from an ultrasound loudspeaker that was located at 0.5 m in front of the moth (top panel in Fig. 7a). Echoes from the fluttering G. pryeri were amplitude-modulated, showing periodic peaks (amplitude glints: middle panel in Fig. 7a). In addition, positive and negative Doppler shifts (spectral glints) of approximately ±1 kHz were repeated in synchronization with the wing beat cycle of the moth (bottom panel in Fig. 7a). Figure 7b shows a representative sound sequence of the Telemike recording during moth-capture flight. Whereas negative Doppler shifts were masked by the emitted pulses, positive Doppler shifts can be observed every 26–27 ms in the CF component of echoes in the last 0.3 s of the flight (dashed box in Fig. 7b). The extent of the Doppler shift was approximately 1–1.5 kHz; these spectral glints were caused by moth fluttering. Given that the echoes were temporally overlapped with the emitted pulse, amplitude glints caused by moth fluttering could not be observed on the Telemike recordings during moth capturing flight.
Echo recordings from fluttering G. pryeri. a Echoes from the moth using the artificial CF sound (100 ms duration, 70 kHz) emitted from an ultrasound loudspeaker located 0.5 m in front of the moth. The sound pressure level (peak-to-peak) was 114 dB SPL at 0.5 m from the loudspeaker. The moth was oriented at 0° relative to the sound source (top panel). Positive and negative Doppler shifts of approximately ±1 kHz were repeated in synchronization with the wing beat cycle of the moth (marked with arrows, 26–27 ms in middle panel). b Representative sound sequence recorded by the Telemike on the bat during moth-capture flight. Top amplitude pattern, bottom spectrogram. Spectral glints were boxed with dashed line
Discussion
Extension of pulse duration during target search
We confirmed that the bat greatly extended the pulse duration when moth fluttering was detected. In the absence of the moth (or when the moth was not fluttering), the bats did not emit long pulses. In some CF-FM bat species, the beginning of the approach phase for capturing a fluttering moth was reported to be characterized by an increase in pulse duration [Pteronotus parnellii (Novick 1963); R. ferrumequinum (Vogler and Neuweiler 1983; Schnitzler et al. 1985; Neuweiler et al. 1987)]. Schnitzler and Flieger (1983) demonstrated that R. ferrumequinum produced pulses with a duration of 90–100 ms while discriminating an oscillating target in a two-alternative forced-choice experiment in a resting state, which was significantly longer than that at rest without the task (50–60 ms). In that experiment, stationary bats were tasked to perceive the difference of the extent of the Doppler shifts in the echoes caused by the movement of an oscillating target. In our study, flying bats produced long pulses without increasing the sound pressure level. The present evidence found on consistency in the sound pressure level suggests that the bats extended the duration of the emitted pulse to increase the number of temporal repetitions of fluttering information (spectral glints) in the echo rather than to produce more physically intense sonar sounds to increase the gain of fine echoes returning from a small target. The mean duration of long pulses corresponded exactly to three wing beat cycles of the fluttering moths (Fig. 4), which appear to be a common characteristic of Rhinolophus bats [the bats adjusted the pulse duration to contain 2–4 spectral glints (Schnitzler and Ostwald 1983; von der Emde and Menne 1989)]. The bats can repeatedly detect the fluttering period of target moths by an increase in pulse duration, which may provide the bats with a cue for classification of insects (Schnitzler and Denzinger 2011).
The maximum fluttering speed of G. pryeri as determined by the high-speed video cameras was approximately ±5 m/s (Fig. 8). When the CF sound was aimed toward the fluttering moth to produce the maximum Doppler shift, we estimated ±2 kHz of a Doppler shift at a maximum of 70 kHz in the echo. The Doppler shift in the echoes observed by the Telemike was approximately 1–1.5 kHz (Fig. 7b), which is slightly smaller than that estimated. It was probably because the extent of such spectral glints, which are caused by wing movements during fluttering, depends on the incident angle of the CF sounds that were delivered from the front of the moth (Schnitzler and Ostwald 1983; Kober and Schnitzler 1990; Schnitzler and Denzinger 2011).
Wing tip velocity of G. pryeri. The speed of the tip of the moth wing (fluttering speed) was measured using the 3D coordinate data of the position of the tip of the moth wing that was reconstructed from the high-speed video image (1,000 frames per second). For estimation of Doppler shift caused by wing movement, we assumed that the CF sound was directed at the fluttering moth to produce the maximum Doppler shift
Conversely, an amplitude peak (amplitude glints) was clearly observed in the echoes using the artificial CF sound emitted from an ultrasound loudspeaker (middle panel in Fig. 7a). However, it was difficult to observe the acoustical glints in the amplitude patterns of the sound recordings by the Telemike even in conditions where spectral glints clearly appeared in the spectrogram (bottom panel in Fig. 7b). Because the echo is heavily attenuated in the air, it appears to make it more difficult to analyze the amplitude modulation caused by moth fluttering than spectral glints during flight. Therefore, we suggest that spectral information may be more robust than amplitude information for bats’ echolocation.
Doppler-shift compensation during moth capturing flight
Trappe and Schnitzler (1982) studied the Doppler-shift compensation of the CF-FM bats during moth-capture flight, and demonstrated that while pursuing insects, R. ferrumequinum maintained the CF2 frequency of the echoes from the stationary object ahead, suggesting that these bats might compensate for the Doppler shift caused by their own flight movement. This is the only study about Doppler-shift compensation behavior of CF-FM bats pursuing insects. In their experiment, echolocation pulses (but not echoes) were recorded by a stationary microphone on the ground while flight paths and velocities of the bats and insects were constructed by taking photographs with strobe light. This means that the CF2 frequencies of the echoes were not measured but estimated. On the other hand, the Telemike in our study recorded echoes that the bats listened to during flight. This allowed us to directly measure which object the bat compensated for while pursuing the target moth. We concluded that the bats exhibited Doppler-shift compensation for echoes that returned from the large static object ahead, not from the target moth, even though the bat’s goal in this task was to capture moths. The estimated CF2 frequencies of the echoes from target moths were below (Fig. 6b) or above (Fig. 6c) the reference frequency of the bats, consistent with the estimation in a previous study (Trappe and Schnitzler 1982). Schnitzler and his co-authors have discussed the interpretation of such behavioral adaptation of CF-FM bats in several reports (Trappe and Schnitzler 1982; Schnitzler and Kalko 2001; Schnitzler and Denzinger 2011), suggesting that echoes from a fluttering moth are not loud enough to activate the Doppler-shift compensation behavior (Schnitzler and Denzinger 2011). Therefore, CF-FM bats were thought to exhibit the Doppler-shift compensation for intense echoes such as echoes from large objects. In the present study, bats usually exhibited the Doppler-shift compensation for echoes from the large static object ahead of the bat, which were louder than those from target moths. However, we found that the bats did not always exhibit Doppler-shift compensation for the most intense echoes at the time [i.e., bats compensated for echoes not from the ceiling (blue cross in Fig. 6b) but left wall (purple open circle in Fig. 6b), while the echo intensities of the left wall (purple open circle in Fig. 6a) were weaker than those from the ceiling (blue open circle in Fig. 6a) at around 0.5–0.3 s before capture]. In the flight pattern shown in Fig. 1a, bats turned left while pursuing the moth with a constant flying height (vertical flight trajectory was not shown). Therefore, the static object ahead of bats changed from the front wall to the left wall of the chamber. The bats exhibited Doppler-shift compensation so for echoes returning from the static object ahead, which appeared to be applied regardless of the echo intensity (the echo from a large static object ahead of bats is usually the most intense, but sometimes, it is not due to the bat’s pulse direction). When the CF2 frequency of the echo from a large static object in a bat’s traveling direction was maintained at the reference frequency, the echo from the moving target moth was above or below the reference frequency. Therefore, the frequency deviation of the direct echo from the moth could be used as a cue for a bat to perceive the moth’s flight direction, either toward or away from the bat. In addition, separating the frequency ranges of an echo CF2 of the moth echo from the other objects may be useful for bats to detect fine echoes from small moving targets in a frequency domain, which is more robust than an amplitude domain.
Abbreviations
- BF:
-
Best frequency
- CF:
-
Constant frequency
- FM:
-
Frequency modulated
- IC:
-
Inferior colliculus
- IPI:
-
Interpulse interval
- TF:
-
Terminal frequency
References
Brinkløv S, Kalko EKV, Surlykke A (2010) Dynamic adjustment of biosonar intensity to habitat clutter in the bat Macrophyllum macrophyllum (Phyllostomidae). Behav Echol Sociobiol 64:1867–1874
Fujioka E, Mantani S, Hiryu S, Riquimaroux H, Watanabe Y (2011) Echolocation and flight strategy of Japanese house bats during natural foraging, revealed by a microphone array system. J Acoust Soc Am 129:1081–1088
Gaioni SJ, Riquimaroux H, Suga N (1990) Biosonar behavior of mustached bats swung on a pendulum prior to cortical ablation. J Neurophysiol 64:1801–1817
Gustafson Y, Schnitzler HU (1979) Echolocation and obstacle avoidance in the hipposiderid bat Asellia tridens. J Comp Physiol A 131:161–167
Henson DW Jr, Bishop AL, Keating AW, Kobler JB, Henson MM, Wilson BS, Hansen R (1987) Biosonar imaging of insects by Pteronotus p. parnellii, the mustached bat. Natl Geogr Res 3:82–101
Hiryu S, Katsura K, Lin LK, Riquimaroux H, Watanabe Y (2005) Doppler-shift compensation in the Taiwanese leaf-nosed bat (Hipposideros terasensis) recorded with a telemetry microphone system during flight. J Acoust Soc Am 118:3927–3933
Hiryu S, Shiori Y, Hosokawa T, Riquimaroux H, Watanabe Y (2008) On-board telemetry of emitted sounds from free-flying bats: compensation for velocity and distance stabilizes echo frequency and amplitude. J Comp Physiol A 194:841–851
Hristov NI, Conner WE (2005) Sound strategy: acoustic aposematism in the bat-tiger moth arms race. Naturwissenschaften 92:164–169
Jakobsen L, Surlykke A (2010) Vespertilionid bats control the width of their biosonar sound beam dynamically during prey pursuit. Proc Natl Acad Sci USA 107:13930–13935
Jensen ME, Miller LA, Rydell J (2001) Detection of prey in a cluttered environment by the northern bat Eptesicus nilssonii. J Exp Biol 204:199–208
Kalko E (1995) Insect pursuit, prey capture and echolocation in pipistrelle bats (Microchiroptera). Anim Behav 50:861–880
Kober R, Schnitzler H-U (1990) Information in sonar echoes of fluttering insects available for echolocating bats. J Acoust Soc Am 87:882–896
Kobler JB, Wilson BS, Henson OW Jr, Bishop AL (1985) Echo intensity compensation by echolocating bats. Hear Res 20:99–108
Lancaster WC, Keating AW, Henson OW Jr (1992) Ultrasonic vocalizations of flying bats monitored by radiotelemetry. J Exp Biol 173:43–58
Metzner W, Zhang S, Smotherman M (2002) Doppler-shift compensation behavior in horseshoe bats revisited: auditory feedback controls both a decrease and an increase in call frequency. J Exp Biol 205:1607–1616
Miller LA, Surlykke A (2001) How some insects detect and avoid being eaten by bats: tactics and countertactics of prey and predator. BioScience 51:570–581
Neuweiler G, Metzner W, Heilmann U, Rubsamen R, Eckrich M, Costa HH (1987) Foraging behaviour and echolocation in the rufous horseshoe bat (Rhinolophus rouxi) of Sri Lanka. Behav Echol Sociobiol 20:53–67
Novick A (1963) Pulse duration in the echolocation of insects by the bats, Pteronotus. Ergebnisse Biol 26:21–26
Ostwald J (1984) Tonotopical organization and pure tone response characteristics of single units in the auditory cortex of the greater horseshoe bat. J Comp Physiol A 155:821–834
Riquimaroux H, Watanabe Y (2000) Characteristics of bat sonar sounds recorded by a telemetry system and a fixed ground microphone. 7th Western Pacific Regional Acoustics conference (WESTPRACVII), pp 233–238
Sano A (2006) Impact of predation by a cave-dwelling bat, Rhinolophus ferrumequinum, on the diapausing population of a troglophilic moth, Goniocraspidum preyeri. Ecol Res 21:321–324
Schnitzler HU (1968) Die Ultraschallortungslaute der Hufeisen-Fledermäuse (Chiroptera-Rhinolophidae) in verschiedenen Orientierungssituationen [The ultrasonic sounds of horseshoe bats (Chiroptera-Rhinolophidae) in different orientation situations]. Z Vergl Physiol 57:376–408
Schnitzler HU, Denzinger A (2011) Auditory fovea and Doppler-shift compensation: adaptations for flutter detection in echolocating bats using CF-FM signals. J Comp Physiol A 197:541–559
Schnitzler H-U, Flieger E (1983) Detection of oscillating target movements by echolocation. J Comp Physiol A 153:385–391
Schnitzler HU, Kalko EKV (2001) Echolocation by insect-eating bats. BioScience 51:557–569
Schnitzler HU, Ostwald J (1983) Adaptations for the detection of fluttering insects by echolocation in Horseshoe bats. In: Ewert JP, Capranica RR, Ingle DJ (eds) Advances in vertebrate neuroethology. Plenum, New York, pp 801–827
Schnitzler HU, Hackbath H, Heilmann U, Herbert H (1985) Echolocation behavior of rufous horseshoe bats hunting for insects in the flycatcher-style. J Comp Physiol A 157:39–46
Schuller G (1984) Natural ultrasonic echoes from wing beating insects are encoded by collicular neurons in the CF-FM bat, Rhinolophus ferrumequinum. J Comp Physiol A 155:121–128
Schuller G, Pollak GD (1979) Disproportionate frequency representation in the inferior colliculus of Doppler-compensating greater horseshoe bats: evidence for an acoustic fovea. J Comp Physiol A 132:47–54
Simmons JA (2005) Big brown bats and June beetles: multiple pursuit strategies in a seasonal acoustic predator–prey system. Acoust Res Lett Online 6:238–242
Simmons JA, Fenton MB, O’Farrell MJ (1979) Echolocation and pursuit of prey by bats. Science 203:16–21
Surlykke A, Moss CF (2000) Echolocation behavior of big brown bats, Eptesicus fuscus, in the field and the laboratory. J Acoust Soc Am 108:2419–2429
Tian B, Schnitzler HU (1997) Echolocation signals of the greater horseshoe bat (Rhinolophus ferrumequinum) in transfer flight and during landing. J Acoust Soc Am 101:2347–2364
Trappe M, Schnitzler HU (1982) Doppler-shift compensation in insect-catching horseshoe bats. Naturwissenschaften 69:193–194
Vogler B, Neuweiler G (1983) Echolocation in the noctule (Nyctalus noctula) and horseshoe bat (Rhinolophus ferrumequinum). J Comp Physiol A 152:421–432
von der Emde G, Menne D (1989) Discrimination of insect wingbeat-frequencies by the bat Rhinolophus ferrumequinum. J Comp Physiol A 164:663–671
von der Emde G, Schnitzler HU (1991) Classification of insects by echolocating greater horeseshoe bats. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 167:423–430
Acknowledgments
These experiments complied with the Principles of Animal Care, publication no. 86-23, revised in 1985, of the National Institutes of Health, and with current Japanese laws. All experiments were approved by the Animal Experiment Committee at Doshisha University. We thank Dr. Takuma Takanashi and Dr. Ryo Nakano for their valuable support launching this study. We also thank James A Simmons and Andrea M Simmons for comments on previous versions of the manuscript, and Nobutaka Urano for assistance in capturing bats in the field. This work was partly supported by a Grant-in-Aid for Scientific Research on Innovative Areas (Grant No. 20200055), a Grant-in-Aid for Young Scientists (B) (Grant No. 21760318) of Japan Society for the Promotion of Science (JSPS), and an Office of Naval Research (ONR) grant (Grant No. 00014-07-1-0858).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mantani, S., Hiryu, S., Fujioka, E. et al. Echolocation behavior of the Japanese horseshoe bat in pursuit of fluttering prey. J Comp Physiol A 198, 741–751 (2012). https://doi.org/10.1007/s00359-012-0744-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00359-012-0744-z