Abstract
Merkel cell carcinoma (MCC) is an aggressive carcinoma with neuroendocrine features that arises preferentially in chronically sun-damaged skin of elderly and immunosuppressed individuals. As the annual yearly incidence steadily increases, likely due to increased detection in an aging population, our understating of MCC continues to improve with recent discoveries. One such breakthrough is the characterization of the Merkel cell polyomavirus (MCPyV), which is present in up to 80 % of tumors, shedding light on MCC pathogenesis. MCC is characteristically composed of a monomorphous population of small round blue cells that express epithelial and neuroendocrine markers by immunohistochemistry. Immunohistochemical stains are invaluable in the diagnosis of this tumor and aid in excluding other histologic mimics. Regarding MCC prognosis, the strongest predictor for outcome is tumor staging, chiefly size of the tumor. Other recently added prognostic factors include the MCPyV status of the tumor, tumor thickness, and regional nodal involvement, including sentinel lymph node status. In this chapter, we will provide an update regarding the latest developments in terms of pathogenesis, immunohistochemical evaluation, and prognostic factors of MCC.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Merkel cell carcinoma
- Primary neuroendocrine carcinoma of the skin
- Merkel cell polyomavirus
- Merkel cell carcinoma pathology
Introduction
Merkel cell carcinoma (MCC), also known as primary cutaneous neuroendocrine carcinoma and historically as trabecular carcinoma and as “APUDoma,” is a rare skin tumor with an aggressive clinical behavior and a predilection for the elderly and the immunosuppressed. Dr. Cyril Toker first described this entity in 1972 under the rubric of trabecular carcinoma [1]. Dr. Toker characterized this tumor as a primary cutaneous carcinoma composed of small round blue cells that could be potentially confused for metastatic carcinoma. In subsequent papers, he attributed sudoriferous and neurosecretory features to the tumor cells, speculating that the tumor may have originated from normal Merkel cells, sweat glands, or a pluripotential precursor [2]. The term Merkel cell carcinoma, the most widely accepted name for the entity, was coined by De Wolff-Peeters in 1980 [3]. The question of the “cell of origin” from which MCC arises has been a matter of much controversy. Since MCC tumor cells share many immunophenotypic and ultrastructural features with normal Merkel cells, it was long believed that these cells act as a precursor for MCC. This notion has been further reinforced by the fact that a fraction of MCC cases can exhibit a prominent intraepidermal component as well as by the existence of an in situ MCC variant.
Normal Merkel cells, first characterized by Friedrich Sigmund Merkel in 1875, are intraepidermal neural crest-derived cells that are thought to serve as slow-acting mechanoreceptors responsible for touch and hair movement sensitivity. They occur singly or in clusters in touch domes (haarscheibe) in close relationship with sensory axons and along the basilar layer of the epidermis, in hair disks, and in the bulge region of the follicle [4]. As far as anatomical distribution of Merkel cells is concerned, they are widely and numerously present in sensitive areas of the skin as well as of the oral and anal mucosal surfaces. Merkel cells can also be found in high numbers populating tongue taste buds, whisker pads, palatine mucosa rugae, and the vermillion border of the lips and in hairy and glabrous sensitive skin and even in sweat glands [5]. Interestingly enough, normal Merkel cells tend to be more abundant in sun-exposed areas [5].
Recently, the concept that MCC most likely originates from a pluripotential stem cell precursor with acquired immunophenotypic and ultrastructural characteristics akin to normal Merkel cells has gained more traction [6]. This concept is further supported by the presence of divergent differentiation in some MCC cases, a phenomenon to be discussed below. Some authors have recently proposed that a possible precursor of MCC could be represented by pre-/pro-B lymphoid stem cells [7], particularly after the observation that MCC cells can express primitive B-cell markers such as PAX5 and TdT [8, 9]. It is important to point out that the discovery of the Merkel cell polyomavirus (MCPyV) [10] has truly revolutionized our understating of MCC. MCPyV is a polyomavirus, first characterized in 2008, that is found in about 80 % of MCC cases. This finding prompted a classification of MCC into two groups: the MCPyV-positive MCCs and the MCPyV-negative MCCs. This dichotomous view of MCC will be expanded below.
Epidemiological and Clinical Features
Due to the combination of an aging population, increased disease awareness, and improved diagnostic tools and criteria, the yearly incidence rate of MCC in the United States has been steadily increasing from less than 0.1 cases per 100,000 in the 1980s to 0.24 cases per 10,000 toward the end of the twentieth century [11] to up to 0.6 cases per 10,000 in 2006 [12]. A similar trend has been observed in other industrialized nations [13]. Caucasian men are the most frequently affected, with MCC only rarely affecting blacks and other ethnic groups. MCC is a tumor of the elderly with average age at presentation being 76 years old for women and 72 years old for men [12]. This entity thrives in an immunosuppressed milieu [14], and patients with HIV/AIDS [15], hematologic conditions [16], and in the post-transplant status [17–19] are at increased risk of developing MCC. The frequent link between MCC and immunosuppression triggered a suspicion for an infective agent being an etiologic factor, ultimately leading to the discovery of the MCPyV [10]. Individuals afflicted by MCC are also at increased risk of developing additional malignancies [13, 20, 21], a finding likely related to the above-mentioned fact that these patients are frequently immunosuppressed. With regard to anatomic location, MCC frequently arises on chronically sun-damaged skin of the head and neck and extremities with rare cases arising from the trunk [12, 22–25]. However, MCC can arise in a variety of non-sun-exposed sites such as mucosal surfaces of the mouth [26–29], tongue [30], nasopharynx [31], and skin from the external genitalia [32, 33]. Lymph nodes are also mentioned as primary sites for MCC [34–37], probably representing nodal metastases from completely regressed cutaneous tumors. Rare cases of MCC-like tumors have been documented arising from the parotid gland [37–39], stomach [40], and vagina [41, 42]. Clinically, MCC often presents as a rapidly-growing painless and often firm nodule or mass with erythematous, pink, violaceous, or even bruise-like-colored overlying skin that is often non-ulcerated [14, 43] (Fig. 1). Some tumors can even exhibit a pigmented appearance [44, 45]. Due to this relatively nonspecific clinical presentation, MCC is more often included as part of a clinical differential diagnosis that includes basal cell carcinoma (BCC), squamous cell carcinoma (SCC), amelanotic melanoma, cyst, adnexal tumor, or lymphoma cutis [46]. Multicentric presentations [47] and in-transit metastatic spread [48, 49] have been reported. MCC is characterized by frequent regional nodal metastasis, and a positive lymph node status, particularly if clinically detected, is negatively correlated with outcome [50, 51]. Distant metastatic spread can affect a wide variety of organs and anatomical sites including the iris [52], brain [53–55], meninges [56, 57], gingiva [58],oropharynx [59], heart [60–63], gastrointestinal tract [64–66] (Fig. 2), pancreas [67, 68], genitourinary system [69–73], and soft tissue [74]. Cases of MCC exhibiting massive involvement of bone marrow [75] and leukemic dissemination [76] have been described, and although they are extremely rare events, such occurrences carry an obvious dismal prognosis. Spontaneous regression of MCC is an unusual but well-documented phenomenon [77–86] that may account for a good proportion of cases described as “primary” lymph node MCCs [34–36, 87–90] as well as for cases with unknown primary [91–94]. As is the case with other small cell carcinomas with neuroendocrine features, MCC can very rarely exhibit paraneoplastic manifestations such as thrombocytopenia [95], hyponatremia from ectopic ACTH production [96], and Lambert-Eaton syndrome [97].
Light Microscopic Findings
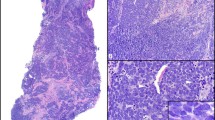
MCC is most often a dermal tumor, centered in the mid-dermis and exhibiting a Grenz zone. A fraction of the cases (up to 10 %) will display a prominent intraepidermal component, sometimes even more prominent than the dermal component [18, 98–102]. Pure in situ intraepidermal variants can be rarely encountered [103, 104]. On H&E-stained tissue sections, the presence of dark-blue dermal nodules “grossly” distinguishable on the glass slide section even before placing it under the microscope is a characteristic of this tumor. The tumor’s deeply basophilic hue reflects the fact that it represents a “small round blue cell” (SRBC) proliferation, thus composed of monomorphic small- to medium-sized cells bearing hyperchromatic nuclei and scant cytoplasm. These cells can be arranged in a variety of patterns: large sheets, nests, and cords or the characteristic trabeculae that prompted the tumor’s first moniker (Fig. 3). As is characteristic of small cell carcinomas and SRBC tumors, the lesional cells are often closely packed but lack intercellular cohesion, a consequence of dysfunctional E-cadherin/beta-catenin machinery, which mediates intercellular adhesion [105, 106]. This growth pattern can make evaluation for lymphovascular invasion (LVI) and margin status a quite difficult task. Nevertheless, Bona fide LVI is a frequent finding in MCC, particularly in thick tumors (Fig. 4). The tumor borders can be pushing or infiltrative, a distinction that might carry prognostic significance, as will be discussed below. Other features akin to SRBC and high-grade neuroendocrine tumors can be often seen in MCC, such as nuclear molding as well as signs of high cell turnover with numerous readily identifiable mitotic figures and apoptotic bodies. Classically, three morphologic patterns have been recognized: trabecular, intermediate, and small cell (Fig. 5). However, these morphologic variants have no correlation with clinical outcome [107] and are commonly found in combination in a single tumor. Furthermore, MCCs can exhibit a myriad of additional growth patterns, including organoid [108] and spindle cell patterns (Fig. 6), as well as tumors forming Homer-Wright rosettes [18, 98, 109] and some exhibiting a pseudo-follicular type of arrangement (Fig. 7). Cytologically distinctive tumor cell features include pleomorphic and plasmacytoid forms and cells with “washed-out” nuclear chromatin (Fig. 5) [110]. The so-called Azzopardi phenomenon, a feature frequently seen in SRBC and high-grade neuroendocrine tumors that relates to the increased cell fragility due to a practically absent cytoplasm, can be identified in MCCs [111, 112]. The stroma surrounding the tumor cells often shows a mucinous/myxoid appearance (Fig. 5), but it can also be sclerotic or show deposition of amyloid-like material [110]. Varying amounts of newly formed vessels, a product of reactive angiogenesis, can be identified surrounding MCCs [112]. In addition, it is not unusual to find prominent peri-tumoral lymphocytic infiltrates [110], sometimes adopting a follicular pattern [113]. Since MCC likely originates from a pluripotential stem cell precursor [6], this entity has the potential to exhibit quite varied types of divergent differentiation. Some of the described associated divergent phenotypes associated with MCC include squamous, glandular (Fig. 8), basal cell carcinoma, melanocytic, rhabdomyosarcomatous, leiomyosarcomatous, fibrosarcomatous, neuroblastic, and even atypical fibroxanthoma-like [114–128]. The most common associated neoplasm identified closely associated with MCC is squamous cell carcinoma (either in situ or invasive), which is recognized in approximately one-third of cases. Rare variants of MCC mimicking other skin neoplasms have been described, such as lymphoepithelioma-like [129, 130] and microcystic adnexal carcinoma-like [131] patterns.
Composite image demonstrating less frequent growth patterns in MCC, a spindle cell (a), an example with well-formed Homer-Wright rosettes (b), an example with a striking organoid arrangement (c), and a tumor exhibiting a “pseudo-follicular” pattern, in the manner of medulloblastoma (d) (H&E, a and b 200×, c and d: 100×)
As previously mentioned, MCC tends to arise in chronically sun-damaged skin. Thus, concomitant actinic keratosis, both invasive and in situ SCC (Fig. 9) and BCC, is often recognized [18, 103, 115, 128, 132–141]. In fact, similar UV-signature TP53 mutations can be found in both squamous cell carcinoma and MCC [142] suggesting a similar tumorigenesis pathway, and these MCC examples are often MCPyV negative [133, 141, 143]. Recent studies claim that there are somewhat specific morphologic features that will distinguish MCPyV-positive from MCPyV-negative MCCs, with a decent correlation when compared against immunohistochemical and molecular tests for the virus. The features associated with MCPyV-negative MCC include an association with SCC, cells with abundant cytoplasm, and larger pleomorphic and irregular nuclei, roughly corresponding to the “intermediate cell” type. On the other hand, MCPyV-positive MCC will have smaller cells with higher nuclear to cytoplasmic ratio and round nuclei, corresponding to the previously described “small cell variant” [144, 145]. Aside from the above-mentioned association between MCC and SCC, several other cutaneous lesions have been described to arise in conjunction with MCC including sebaceous carcinoma [146], atypical fibroxanthoma [147], and trichilemmal [148, 149] and infundibular follicular [150] cysts. Another well-documented and worth mentioning association is that with chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) (Fig. 10) [13, 16, 151–154]. As commented above this phenomenon is likely related to both the immunosuppressed milieu and the older population of patients in which MCC arises. Interestingly, although recent findings have suggested shared molecular events between CLL/SLL and MCC [16], it is likely that these two entities do not share the same pathogenesis [155].
The main morphologic differential diagnosis of MCC includes metastases from small cell carcinomas of other origins, particularly pulmonary, from which it can be practically indistinguishable by morphology. The possibility of a lymphoid neoplasm should also be ruled out. Other less frequently encountered differential diagnostic possibilities include other primary SRBC tumors that can rarely arise in the skin such as the superficial variant of Ewing sarcoma [156–159] and the rare small cell melanoma. In the unique and unusual occurrence of a purely in situ MCC, one should consider the differential of pagetoid entities such as superficial spreading melanoma and cutaneous T-cell lymphoma [98, 99, 103]. Fortunately, in the majority of the cases, immunohistochemical staining patterns differ between these entities, allowing for correct classification. Please refer to the immunohistochemistry section for a complete discussion.
Immunohistochemical Aspects of MCC
Just like their normal “counterparts,” MCC cells usually and characteristically express cytokeratins and neuroendocrine markers with the prototypical immunoprofile of CK7 negative, CK20 positive, synaptophysin, and chromogranin positive [160]. Albeit quite nonspecific and thus rarely used, another neuroendocrine/neural marker that is frequently described as positive in MCC is neuron-specific enolase (NSE). Regarding the cytokeratins, it is important to emphasize the quite distinctive staining pattern with CK20 found in MCC cells: a strong, paranuclear dot-like signal that either can be found in its “pure” form or accompanied by a diffuse perinuclear or membranous staining. In the latter case, the pattern is best characterized as paranuclear dot-like “accentuation” [107, 161, 162] (Fig. 11) (Importantly, peri is a Greek prefix for “around” or “about,” while the para prefix means “alongside” or “beside”). CK20 and keratin cocktails (CAM 5.2 and AE1:AE3) are particularly useful when evaluating lymph nodes for metastatic disease [163], a procedure that can be quite challenging if one were to use only morphology, particularly in small metastatic deposits (Fig. 12). The distinctive staining pattern with CK20 is replicated in staining for neurofilament [164, 165] and/or CAM 5.2 and AE1:AE3 keratin cocktails and translates to paranuclear accumulation of intermediate filaments, a constant ultrastructural feature of MCC tumor cells. Nevertheless, it is worth emphasizing that both of these immunohistochemical and ultrastructural features can also be observed in a wide variety of other SRBC tumors and small cell (high-grade neuroendocrine) carcinomas from other organs [166–170]. Another important caveat to take in account is that up to 38 % of MCCs can exhibit CK7 positivity [171] with some cases having a CK7+/CK20− immunophenotype [172, 173]. Interestingly enough, normal Merkel cells exhibit a strong, diffuse, and homogeneous cytoplasmic pattern when stained with CK20 and keratin cocktails, and they can also exhibit CK7 positivity [174]. Other epithelial markers expressed in MCC include BerEP4 [100] and p63. Expression of p63 in early-stage MCC has been correlated with progression of disease and worse prognosis [175–177]; however, subsequent studies yielded only a small fraction of MCC expressing p63 without correlation with survival, raising questions about the prognostic utility of this marker [176, 178]. Following the discovery of the MCPyV [10], an antibody clone directed to the large T antigen (LT-Ag) of the virus (CM2B4) was developed [179]. This antibody shows a diffuse nuclear signal by immunohistochemistry (Fig. 13) and performs fairly well and has been shown to have a sensitivity ranging from 77 to 95 % and specificity of 83 %, when compared to MCPyV detection via molecular techniques [133, 145, 179]. The Ab3 clone, a recently developed antibody directed to a different epitope of LT-Ag, has allegedly outperformed CM2B4, with a sensitivity of 97 % [180]. Importantly, these antibodies can certainly be used as diagnostic tools since it is usually not expressed in tumors within the differential diagnosis [133, 180]. Along the lines of the proposed dichotomous model of two tumor pathways (UV-damage TP53 mutated and MCPyV driven), expression of p53 has been more frequently found in the context of MCPyV-negative MCC tumors, with the p53-positive cases accounting for around 20 % [181] which corresponds to both the proportion of MCPyV-negative tumors and the TP53-mutated tumors [142, 182, 183]. Likewise, loss of expression of Rb protein by immunohistochemistry has been related to the MCPyV-negative group of tumors; however, recent data suggest that an underlying dysfunction of the RB gene is present in both MCC groups (MCPyV positive and negative) via two different mechanisms [184]. This interplay will be further discussed in the pathogenesis and prognosis sections. Several studies have demonstrated that MCC is positive for c-kit (CD117) immunohistochemical staining in approximately 50 % of patients [185–188], without a clear correlation with clinical outcome. Unfortunately, this feature is not related to activating KIT mutations; MCC is virtually unresponsive to small molecule tyrosine kinase inhibitor therapeutic agents such as imatinib [181, 189–191].
Cytokeratin immunohistochemistry for MCC, either CK20 or cytokeratin cocktails (AE1:AE3 and Cam 5.2) can potentially exhibit these patterns, the “globular” or “dot-like” paranuclear signal (a) or the “dot-like” paranuclear accentuation of the signal (b) (a: CK20 immunohistochemistry, 400×, b: AE1: AE3 immunohistochemistry, 400×)
Immunohistochemistry directed to the LT-Ag of MCPyV in an MCC case. Note the clean and strong nuclear signal denoting integration of the viral genome in the tumor cells (MCPyV immunohistochemistry (CM2B4 clone, 400×) (Image courtesy of Gauri Panse M.D. from Baystate Medical Center-Tufts University, Springfield, MA)
As previously mentioned, the morphologic differential diagnosis of MCC includes several completely unrelated entities requiring immunohistochemical evaluation to be solved. In the following section, we will describe these entities and their different staining patterns. Please see Table 1 for a concise review of the main immunohistochemical differential diagnosis of MCC. First and foremost, a crucial distinction to be made is between MCC and metastatic small cell carcinoma, particularly of pulmonary origin. In this setting, immunohistochemistry for thyroid transcription factor 1 (TTF-1) is of paramount importance. The great majority of MCC are negative for TTF-1, while a large proportion of pulmonary small cell carcinomas and other small cell carcinomas will be positive with this marker [167, 171, 192, 193]. There are rare but well-documented examples of TTF-1-positive MCC, with two cases in which the TTF-1 positivity was present in MCC metastases [55, 194–196]. These unusual examples comprise about 1.1 % of the MCC cases that were tested for TTF-1 according to a recent review [143]. A newly described marker that has been deemed as helpful in the MCC versus small cell lung carcinoma (SCLC) differential is the mammalian/human achaete-scute complex homolog 1 (MASH1/hASH1). This is a transcription factor related to neuroendocrine development that is retained in the majority of SCLCs tested, suggesting that it may be more specific than TTF-1 [195] in this differential diagnosis. Interestingly enough, Notch-1, which is a transcription factor that suppresses MASH1/hASH1, is expressed in MCC and is negative in other neuroendocrine tumors including SCLC [105], yet another available diagnostic tool for this differential diagnosis. Lymphoid neoplasms are also included in the differential diagnosis. Although MCC is consistently negative for CD45 (LCA) [8], it can exhibit considerable overlap with hematologic malignancies in terms of immunohistochemical markers, since MCCs can express a variety of lymphoid-related markers such as CD56 (NCAM) [171, 197], the B-cell-associated transcription factor PAX5 and the primitive lymphoid marker TdT [7–9, 194, 198]. Additionally, the majority of MCC will express the apoptosis-related marker bcl-2 [8, 106, 199], an apparently major player in MCC tumor cell survival [200]. Furthermore, another lymphoid-related marker that can be expressed by MCC cells is anaplastic lymphoma kinase (ALK), specifically with the D5F3 clone but less so with the ALK-1 clone [201]. Finally, consideration must be given to the SRBC group of tumors, particularly the superficial variant of Ewing sarcoma/primitive neuroectodermal tumor (PNET) group, since MCC can frequently express CD99 and Friend leukemia integration 1 (FLI1), while conversely up to 20 % of Ewing sarcoma/PNET can show expression of keratins, particularly with CAM 5.2 and AE1:AE3 cocktails [170]. In potential cases of PNET that are indistinguishable morphologically and immunohistochemically from MCC, one could resort to cytogenetic studies, since MCCs completely lack the cytogenetic aberrations found in those tumors [159].
Ultrastructural Features of MCC
As previously stated, MCC tumor cells exhibit many shared ultrastructural characteristics with normal Merkel cells, the main reason behind the tumor’s nomenclature. Under the transmission electron microscope, normal Merkel cells are identified as cells measuring about 15 μm size, oval shaped with their long axis oriented parallel to the basement membrane plane [4, 5]. Close relationship to a sensory axon is often evident. Normal Merkel cell bears a large, usually bilobulated nuclei, and their cytoplasm is scant, relatively scarcely populated by organelles but containing many free ribosomes. Numerous neurosecretory granules can be appreciated close to the area where the cell is in relationship with a sensory axon. Other salient ultrastructural features of normal Merkel cells are the abundant spikelike spinous cytoplasmic processes interdigitating with the surrounding keratinocytes to which they are attached by sparse, small, and poorly formed desmosomes [4, 5, 202]. Interestingly, melanosomes have been described to occur in normal Merkel cells.
Neoplastic Merkel cells replicate the majority of the above-cited ultrastructural features of normal Merkel cells, including the very characteristic spinous processes and the neurosecretory granules. However, MCC tumor cells exhibit a prominent perinuclear ball-like collection of intermediate filaments contrasting to the sparse collections of simple keratin-intermediate filaments found in the cytoplasm of normal Merkel cells [4, 5]. This cytoplasmic ball of intermediate filaments is an ultrastructural feature common among neuroendocrine tumors and small cell carcinomas. This accumulation of intermediate filaments translates to the immunohistochemical pattern that is observed in MCC with CK20, keratin cocktails, and neurofilament immunostains.
Molecular Aspects, Pathogenesis, and Association with the Merkel Cell Polyomavirus (MCPyV)
The discovery of the MCPyV has indeed completely changed our understanding of MCC pathogenesis and behavior. MCPyV is a novel polyomavirus that belongs to the same family as the JC, BK, KI, and WU as well as the recently discovered trichodysplasia-associated polyomavirus (TSPyV) [203]. However, so far, only MCPyV has an associated oncogenic potential. Several recent studies have demonstrated that MCPyV is quite prevalent in the healthy adult population, and it is detected in peripheral blood both by molecular DNA amplification techniques and serology. Evidence of the virus has also demonstrated from cutaneous swabs [143, 204–208]. A recent prospective study has correlated high serum MCPyV antibody titers in healthy individuals with an increased risk of subsequent MCC development in older age [209]. Transmission is likely to occur in early childhood, especially between family members [210], and the virus remains dormant in a wide variety of tissues [211] until immunosuppression-triggered reactivation, as is characteristic of this viral group. The importance of MCPyV resides in that it is part of the two proposed models of MCC pathogenesis, the other being a UV-damage-induced pathway, which involves TP53 mutations and is to MCPyV. The causative oncogenic role of MCPyV in MCC is nowadays widely accepted, since the viral genetic material is integrated and clonally expanded in viral-positive MCC tumor cells [10]. Numerous studies on the subject have shown that MCPyV can be detected in about 70–80 % of MCC tumors [133, 145, 205, 207, 211–218]. However, a recent study claims that if one were to use sensitive enough molecular techniques, all MCC tumors will show integrated MCPyV genome [180]. Along this line of thought, some authors claim that the two pathways could be unified into one that is driven by mutagenic effects of preexisting MCPyV, which has been reactivated itself in an immunosuppressed host that has been accumulating deleterious UV-induced mutations [219]. As we discussed before in this chapter, there is a well-documented and clear link between MCC and various types of immunosuppressive states, including chronic solar damage. The main oncogenic mechanism of MCPyV is exerted via the interaction between LT-Ag and the retinoblastoma (Rb) group of proteins. It is important to understand the fact that the oncogenic gene LT-Ag present in the infected MCC tumor cells has undergone a signature truncating mutation [179, 220]. The product of this mutated LT-Ag, through its LxCxE motif, binds and sequesters hypophosphorylated Rb leading to its accumulation and thus allowing E2F-mediated transcription that leads to the entry of the cell into S-phase [221]. The p53 protein system also becomes affected by LT-Ag, albeit in an indirect fashion [220, 222]. Additionally, the LT-Ag protein also serves to allow detection of MCPyV by immunohistochemistry. Regarding the MCPyV-negative MCC group, Rb function is also impaired but through different mechanisms that include hypermethylation and heterozygous deletions [184]. Characteristically, p53 is more often suppressed and immunohistochemically overexpressed (accumulated) in this particular group. These alterations in Rb are reflected on immunohistochemistry with LT-Ag-positive MCC tumors expressing the accumulated Rb and conversely with 87 % of LT-Ag-negative tumors being predominantly Rb negative due to the deletions and silencing hypermethylation [212, 223]. Likewise, it seems that there is an inverse relationship between p53 overexpression and MCPyV viral abundance [181, 224] with overexpression of p53 being also negatively related to survival. Furthermore, recent studies have correlated both tumor positivity for MCPyV and an effective viral-directed immune response with better survival [15, 181, 223–225]. After reviewing these models, one could draw comparisons with what occurs in other organ systems such as in the lower female genital tract SCC (HPV driven versus p53 driven) and head and neck SCC (p16-positive/HPV-positive tumors having better prognosis than HPV-negative tumors).
Prognostic Factors, Staging, and Management
Several clinical and histopathologic features in MCC are associated with clinical outcome. In terms of clinical features, a large Surveillance, Epidemiology, and End Results (SEER)-based National Cancer Institute (NCI) study [12] demonstrated that women have a 10-year survival advantage compared to men with the rates being 64.8 % and 50.5 %, respectively. Additionally, tumor location yielded survival differences, and if one were to align the anatomical sites from best to worse prognosis, it would show the following pattern: upper limbs, head and neck, lower limbs, and trunk. Furthermore, this study added tumor diameter to the prognostic factors, demonstrating that tumors equal or less than 2 cm have statistically significant better survival (61 %) than tumors larger than 2 cm (39.6 %). In fact, tumor size is the main focus of tumor staging [14, 226]. In the 2010 American Joint Committee on Cancer (AJCC) guidelines, a four-tier staging system is used for MCC: tumors are designated as stage I or stage II if they are localized to the skin at the primary site (stage I/T1 is less or equal than 2 cm in diameter, and stage II/T2 is more than 2 cm in size); stage III denotes spread to regional lymph nodes; and stage IV indicates spread beyond the regional lymph nodes. According to some recent studies, tumor thickness measured utilizing the Breslow technique has emerged as a strong and important independent prognostic factor. Studies indicate that thicker tumors are related to advanced stage [185], predict positive sentinel lymph node (SLN) status [227, 228], and are associated with both higher recurrence rate and worse survival [178, 229, 230]. A suggested cutoff of equal or greater than 10 mm in thickness [178] was proposed to indicate increased risk for the mentioned complications. Tumor growth pattern and tumor borders have also been reported to be associated with prognosis with both infiltrative tumor borders [229] and diffuse growth pattern [230] being negatively associated with survival. It is likely that the development of thick tumors with irregular borders is correlated with higher expression of several types of metalloproteinases by the tumor cells, which has been found to impact prognosis by itself [186, 231]. The presence of LVI, a rather frequent finding in MCC, is also considered a strong adverse prognostic factor [228, 229, 232]. Unsurprisingly, a high proliferative rate, represented by either an elevated Ki-67 proliferation index of equal or more than 35 or 50 % and/or a high mitotic rate, has also been correlated with both disease progression and worse survival [24, 185, 233, 234].
While a comprehensive discussion of management is beyond the scope of this chapter, the mainstay of treatment, particularly in localized disease, is surgical. The data related to the role of the sentinel lymph node biopsy is somewhat less clear in MCC in comparison to melanoma and breast carcinoma. Current National Comprehensive Cancer Network (NCCN) treatment recommendations include surgical excision of the primary tumor with wide margins (1–2 cm) followed by sentinel lymph node biopsy (SLNB) or elective lymphadenectomy (for clinically node-negative cases), as 25–30 % patients have regional nodal disease at presentation [22, 227, 235]. Chemotherapy and radiation therapy are reserved for advanced stage patients. A recent study from a large cohort of patients underscored the beneficial impact of SLNB and completion lymphadenectomy on survival while finding that chemotherapy and radiotherapy had no effect on survival in node-negative patients [235]. Other studies pointed out that clinically detectable metastatic lymph node deposits were more predictive of outcome than lymph nodes with microscopic deposits [232]. More data need to be accumulated in this regard to be able to make stronger evidence-based management recommendations.
MCC tumors can be accompanied by a brisk immune response. However, as is the case with other highly immunogenic tumors such as melanoma, the relationship between this immune host response and survival has had conflicting results in the literature, with some studies attributing a worse prognosis to either the presence [230] or absence [185] of immune response, while in other study the presence of an immune response was associated with good prognosis particularly in lymph node-negative tumors with nodular growth pattern [229]. It appears that MCPyV might play a role in this issue since it has been recently suggested that the MCPyV status of the tumor is reflected in patient survival with patients with a higher viral load and mounting an appropriate immune response having better clinical outcome [15, 181, 223, 224]. Another interesting new finding is that in MCPyV-positive tumors, there is evidence of tumor-driven immune suppression, as a high proportion of PD-L1-positive tumor-infiltrating lymphocytes (TILs) and macrophages found particularly at the tumor-stroma interface [236]. Furthermore, the same study showed that expression of PD-L1 in MCC tumor cells is correlated with better survival. The innate immune system is apparently also affected by the tumor [237]. In addition, it has been shown that specific T-cell populations directed to MCPyV are detected peripherally in MCC patients and also express the immune exhaustion markers PD-1 and Tim-3 [238]. Other studies demonstrated that presence of specific T-cell [239] TIL subpopulations without impaired function might also predict improved survival. These observations open exciting opportunities for potential immune modulatory therapy with recently developed monoclonal antibodies [219] in this particular group of virus-driven MCC.
References
Toker C. Trabecular carcinoma of the skin. Arch Dermatol. 1972;105(1):107–10.
Toker C. Trabecular carcinoma of the skin. A question of title. Am J Dermatopathol. 1982;4(6):497–500.
De Wolff-Peeters C, Marien K, Mebis J, Desmet V. A cutaneous APUDoma or Merkel cell tumor? A morphologically recognizable tumor with a biological and histological malignant aspect in contrast with its clinical behavior. Cancer. 1980;46(8):1810–6.
Sidhu GS, Chandra P, Cassai ND. Merkel cells, normal and neoplastic: an update. Ultrastruct Pathol. 2005;29(3–4):287–94.
Boulais N, Misery L. Merkel cells. J Am Acad Dermatol. 2007;57(1):147–65.
Lemasson G, Coquart N, Lebonvallet N, Boulais N, Galibert MD, Marcorelles P, et al. Presence of putative stem cells in Merkel cell carcinomas. J Eur Acad Dermatol Venereol. 2012;26(6):789–95.
Zur Hausen A, Rennspiess D, Winnepenninckx V, Speel EJ, Kurz AK. Early B-cell differentiation in Merkel cell carcinomas: clues to cellular ancestry. Cancer Res. 2013;73(16):4982–7.
Sur M, AlArdati H, Ross C, Alowami S. TdT expression in Merkel cell carcinoma: potential diagnostic pitfall with blastic hematological malignancies and expanded immunohistochemical analysis. Mod Pathol. 2007;20(11):1113–20.
Dong HY, Liu W, Cohen P, Mahle CE, Zhang W. B-cell specific activation protein encoded by the PAX-5 gene is commonly expressed in Merkel cell carcinoma and small cell carcinomas. Am J Surg Pathol. 2005;29(5):687–92.
Feng H, Shuda M, Chang Y, Moore PS. Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science. 2008;319(5866):1096–100.
Agelli M, Clegg LX. Epidemiology of primary Merkel cell carcinoma in the United States. J Am Acad Dermatol. 2003;49(5):832–41.
Albores-Saavedra J, Batich K, Chable-Montero F, Sagy N, Schwartz AM, Henson DE. Merkel cell carcinoma demographics, morphology, and survival based on 3870 cases: a population based study. J Cutan Pathol. 2010;37(1):20–7.
Kaae J, Hansen AV, Biggar RJ, Boyd HA, Moore PS, Wohlfahrt J, et al. Merkel cell carcinoma: incidence, mortality, and risk of other cancers. J Natl Cancer Inst. 2010;102(11):793–801.
Heath M, Jaimes N, Lemos B, Mostaghimi A, Wang LC, Penas PF, et al. Clinical characteristics of Merkel cell carcinoma at diagnosis in 195 patients: the AEIOU features. J Am Acad Dermatol. 2008;58(3):375–81.
Paulson KG, Iyer JG, Blom A, Warton EM, Sokil M, Yelistratova L, et al. Systemic immune suppression predicts diminished Merkel cell carcinoma-specific survival independent of stage. J Invest Dermatol. 2013;133(3):642–6.
Tadmor T, Aviv A, Polliack A. Merkel cell carcinoma, chronic lymphocytic leukemia and other lymphoproliferative disorders: an old bond with possible new viral ties. Ann Oncol. 2011;22(2):250–6.
Penn I, First MR. Merkel’s cell carcinoma in organ recipients: report of 41 cases. Transplantation. 1999;68(11):1717–21.
Walsh NM. Primary neuroendocrine (Merkel cell) carcinoma of the skin: morphologic diversity and implications thereof. Hum Pathol. 2001;32(7):680–9.
Kanitakis J, Euvrard S, Chouvet B, Butnaru AC, Claudy A. Merkel cell carcinoma in organ-transplant recipients: report of two cases with unusual histological features and literature review. J Cutan Pathol. 2006;33(10):686–94.
Brenner B, Sulkes A, Rakowsky E, Feinmesser M, Yukelson A, Bar-Haim E, et al. Second neoplasms in patients with Merkel cell carcinoma. Cancer. 2001;91(7):1358–62.
Gass JK, Chan SK, Rytina E, Greenberg DC, Burrows NP. Multiple primary malignancies in patients with Merkel cell carcinoma. J Eur Acad Dermatol Venereol. 2010;24(5):601–3.
Hitchcock CL, Bland KI, Laney 3rd RG, Franzini D, Harris B, Copeland 3rd EM. Neuroendocrine (Merkel cell) carcinoma of the skin. Its natural history, diagnosis, and treatment. Ann Surg. 1988;207(2):201–7.
Gollard R, Weber R, Kosty MP, Greenway HT, Massullo V, Humberson C. Merkel cell carcinoma: review of 22 cases with surgical, pathologic, and therapeutic considerations. Cancer. 2000;88(8):1842–51.
Skelton HG, Smith KJ, Hitchcock CL, McCarthy WF, Lupton GP, Graham JH. Merkel cell carcinoma: analysis of clinical, histologic, and immunohistologic features of 132 cases with relation to survival. J Am Acad Dermatol. 1997;37(5 Pt 1):734–9.
Hohaus K, Kostler E, Schonlebe J, Klemm E, Wollina U. Merkel cell carcinoma – a retrospective analysis of 17 cases. J Eur Acad Dermatol Venereol. 2003;17(1):20–4.
Inoue T, Shimono M, Takano N, Saito C, Tanaka Y. Merkel cell carcinoma of palatal mucosa in a young adult: immunohistochemical and ultrastructural features. Oral Oncol. 1997;33(3):226–9.
Longo F, Califano L, Mangone GM, Errico ME. Neuroendocrine (Merkel cell) carcinoma of the oral mucosa: report of a case with immunohistochemical study and review of the literature. J Oral Pathol Med. 1999;28(2):88–91.
Baker P, Alguacil-Garcia A. Moderately differentiated neuroendocrine carcinoma in the floor of the mouth: a case report. J Oral Maxillofac Surg. 1999;57(9):1143–7.
Prabhu S, Smitha RS, Punnya VA. Merkel cell carcinoma of the alveolar mucosa in a young adult: a rare case report. Br J Oral Maxillofac Surg. 2010;48(1):48–50.
Yom SS, Rosenthal DI, El-Naggar AK, Kies MS, Hessel AC. Merkel cell carcinoma of the tongue and head and neck oral mucosal sites. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101(6):761–8.
Snow SN, Larson PO, Hardy S, Bentz M, Madjar D, Landeck A, et al. Merkel cell carcinoma of the skin and mucosa: report of 12 cutaneous cases with 2 cases arising from the nasal mucosa. Dermatol Surg. 2001;27(2):165–70.
Hierro I, Blanes A, Matilla A, Muñoz S, Vicioso L, Nogales FF. Merkel cell (neuroendocrine) carcinoma of the vulva. Pathol Res Pract. 2000;196(7):503–9.
Tomic S, Warner TF, Messing E, Wilding G. Penile Merkel cell carcinoma. Urology. 1995;45(6):1062–5.
Eusebi V, Capella C, Cossu A, Rosai J. Neuroendocrine carcinoma within lymph nodes in the absence of a primary tumor, with special reference to Merkel cell carcinoma. Am J Surg Pathol. 1992;16(7):658–66.
Fotia G, Barni R, Bellan C, Neri A. Lymph nodal Merkel cell carcinoma: primary or metastatic disease? A clinical case. Tumori. 2002;88(5):424–6.
Kim EJ, Kim HS, Kim HO, Jung CK, Ko YH, Kim TH, et al. Merkel cell carcinoma of the inguinal lymph node with an unknown primary site. J Dermatol. 2009;36(3):170–3.
de Biase D, Ragazzi M, Asioli S, Eusebi V. Extracutaneous Merkel cell carcinomas harbor polyomavirus DNA. Hum Pathol. 2012;43(7):980–5.
Fornelli A, Eusebi V, Pasquinelli G, Quattrone P, Rosai J. Merkel cell carcinoma of the parotid gland associated with Warthin tumour: report of two cases. Histopathology. 2001;39(4):342–6.
Chernock RD, Duncavage EJ, Gnepp DR, El-Mofty SK, Lewis Jr JS. Absence of Merkel cell polyomavirus in primary parotid high-grade neuroendocrine carcinomas regardless of cytokeratin 20 immunophenotype. Am J Surg Pathol. 2011;35(12):1806–11.
Capella C, Marando A, Longhi E, Bernasconi B, Finzi G, Parravicini C, et al. Primary gastric Merkel cell carcinoma harboring DNA polyomavirus: first description of an unusual high-grade neuroendocrine carcinoma. Hum Pathol. 2014;45(6):1310–4.
Bing Z, Levine L, Lucci JA, Hatch SS, Eltorky MA. Primary small cell neuroendocrine carcinoma of the vagina: a clinicopathologic study. Arch Pathol Lab Med. 2004;128(8):857–62.
Coleman NM, Smith-Zagone MJ, Tanyi J, Anderson ML, Coleman RL, Dyson SW, et al. Primary neuroendocrine carcinoma of the vagina with Merkel cell carcinoma phenotype. Am J Surg Pathol. 2006;30(3):405–10.
Ratner D, Nelson BR, Brown MD, Johnson TM. Merkel cell carcinoma. J Am Acad Dermatol. 1993;29(2 Pt 1):143–56.
Perdikis G, TerKonda SP. Pigmented skin lesions of the upper extremity. Hand Clin. 2004;20(3):283–91. vi.
McKee PH, Calonje E, Granter SR. Pathology of the skin: with clinical correlations/[edited by] Phillip H. McKee, Eduardo Calonje, Scott R. Granter. 3rd ed. Edinburgh: Philadelphia Elsevier Mosby; 2005. v <1 > p.
Smith PD, Patterson JW. Merkel cell carcinoma (neuroendocrine carcinoma of the skin). Am J Clin Pathol. 2001;115(Suppl):S68–78.
Satter EK, Derienzo DP. Synchronous onset of multiple cutaneous neuroendocrine (Merkel cell) carcinomas localized to the scalp. J Cutan Pathol. 2008;35(7):685–91.
Grandpeix C, Bonvalot S, Petrow P, Fraitag S, Gounod N, Avril MF. Continued complete remission of Merkel cell carcinoma with in-transit metastasis after treatment with isolated limb perfusion regional chemotherapy. Ann Dermatol Venereol. 2006;133(8–9 Pt 1):700–3.
Grotz TE, Tarantola TI, Otley CC, Weaver AL, McGree ME, Jakub JW. Natural history of Merkel cell carcinoma following locoregional recurrence. Ann Surg Oncol. 2012;19(8):2556–62.
Howle JR, Veness MJ. Outcome of patients with microscopic and macroscopic metastatic nodal Merkel cell carcinoma: an Australian experience. Dermatol Surg. 2014;40(1):46–51.
Iyer JG, Storer BE, Paulson KG, Lemos B, Phillips JL, Bichakjian CK, et al. Relationships among primary tumor size, number of involved nodes, and survival for 8044 cases of Merkel cell carcinoma. J Am Acad Dermatol. 2014;70(4):637–43.
Kirwan C, Carney D, O’Keefe M. Merkel cell carcinoma metastasis to the iris in a 23 year old female. Ir Med J. 2009;102(2):53–4.
Ikawa F, Kiya K, Uozumi T, Yuki K, Takeshita S, Hamasaki O, et al. Brain metastasis of Merkel cell carcinoma. Case report and review of the literature. Neurosurg Rev. 1999;22(1):54–7.
Feletti A, Marton E, Rossi S, Canal F, Longatti P, Billeci D. Pituitary metastasis of Merkel cell carcinoma. J Neurooncol. 2010;97(2):295–9.
Shalin SC, Cifarelli CP, Suen JY, Gao L. Loss of cytokeratin 20 and acquisition of thyroid transcription factor-1 expression in a Merkel cell carcinoma metastasis to the brain. Am J Dermatopathol. 2014.
Chang DT, Mancuso AA, Riggs Jr CE, Mendenhall WM. Merkel cell carcinoma of the skin with leptomeningeal metastases. Am J Otolaryngol. 2005;26(3):210–3.
Abul-Kasim K, Soderstrom K, Hallsten L. Extensive central nervous system involvement in Merkel cell carcinoma: a case report and review of the literature. J Med Case Rep. 2011;5:35.
Schmidt-Westhausen A, Reichart PA, Gross UM. Gingival metastasis of Merkel cell carcinoma: a case report. J Oral Pathol Med. 1996;25(1):44–7.
Reichel OA, Mayr D, Issing WJ. Oropharyngeal metastasis of a Merkel cell carcinoma of the skin. Eur Arch Otorhinolaryngol. 2003;260(5):258–60.
Jongbloed MR, Kanen BL, Visser M, Niessen H, Flens MJ, Loffeld RJ. Case 2. Intracardiac metastasis from a Merkel cell carcinoma. J Clin Oncol. 2004;22(6):1153–6.
Yamana N, Sueyama H, Hamada M. Cardiac metastasis from Merkel cell skin carcinoma. Int J Clin Oncol. 2004;9(3):210–2.
Fong LS, Mathur M, Bhindi R, Figtree GA. Right atrial Merkel cell tumour metastasis characterization using a multimodality approach. Eur Heart J. 2012;33(17):2205.
Suttie CF, Hruby G, Horvath L, Thompson J. Cardiac metastasis in Merkel cell carcinoma. J Clin Oncol. 2014;32(13):e52–3.
Idowu MO, Contos M, Gill S, Powers C. Merkel cell carcinoma: a report of gastrointestinal metastasis and review of the literature. Arch Pathol Lab Med. 2003;127(3):367–9.
Syal NG, Dang S, Rose J, Chen C, Aduli F. Gastric metastasis of Merkel cell cancer – uncommon complication of a rare neoplasm. J Ark Med Soc. 2012;109(7):134–6.
Parikh MP, Samo S, Ganipisetti V, Krishnan S, Dhandha M, Yungbluth M, et al. Gastric metastasis of Merkel cell carcinoma, a rare cause of gastrointestinal bleeding: case report and review of the literature. J Gastrointest Oncol. 2014;5(4):E68–72.
Ouellette JR, Woodyard L, Toth L, Termuhlen PM. Merkel cell carcinoma metastatic to the head of the pancreas. JOP. 2004;5(2):92–6.
Bachmann J, Kleeff J, Bergmann F, Shrikhande SV, Hartschuh W, Buchler MW, et al. Pancreatic metastasis of Merkel cell carcinoma and concomitant insulinoma: case report and literature review. World J Surg Oncol. 2005;3:58.
Woo HH, Kencian JD. Metastatic Merkel cell tumour to the bladder. Int Urol Nephrol. 1995;27(3):301–5.
Santis WF, Billings EJ, DeWolf WC. Metastatic Merkel cell tumor to bladder presenting as an encroachment tumor with gross hematuria. Urology. 1999;54(1):163.
Mack DP, Moussa M, Cook A, Izawa JI. Metastatic Merkel cell tumor to the prostate and bladder. Urology. 2004;64(1):156–8.
Gleason JM, Kohler TS, Monga M. Merkel cell carcinoma metastatic to testis. Urology. 2006;67(2):423.e13–e14.
Strasser H, Amann K, Schrott KM, Krause FS. Solitary metastasis of a Merkel cell tumor to the urinary bladder. Anticancer Res. 2008;28(2b):1361–4.
Wang W, Veness M. Metastatic Merkel cell carcinoma to the soft tissues of the lower back. Australas J Dermatol. 2004;45(1):38–41.
Lentz SR, Krewson L, Zutter MM. Recurrent neuroendocrine (Merkel cell) carcinoma of the skin presenting as marrow failure in a man with systemic lupus erythematosus. Med Pediatr Oncol. 1993;21(2):137–41.
Nemoto I, Sato-Matsumura KC, Fujita Y, Natsuga K, Ujiie H, Tomita Y, et al. Leukaemic dissemination of Merkel cell carcinoma in a patient with systemic lupus erythematosus. Clin Exp Dermatol. 2008;33(3):270–2.
Kayashima K, Ono T, Johno M, Kojo Y, Yamashita N, Matsunaga W. Spontaneous regression in Merkel cell (neuroendocrine) carcinoma of the skin. Arch Dermatol. 1991;127(4):550–3.
Duncan WC, Tschen JA. Spontaneous regression of Merkel cell (neuroendocrine) carcinoma of the skin. J Am Acad Dermatol. 1993;29(4):653–4.
Yanguas I, Goday JJ, Gonzalez-Guemes M, Oleaga JM, Lozano M, Soloeta R. Spontaneous regression of Merkel cell carcinoma of the skin. Br J Dermatol. 1997;137(2):296–8.
Connelly TJ, Cribier B, Brown TJ, Yanguas I. Complete spontaneous regression of Merkel cell carcinoma: a review of the 10 reported cases. Dermatol Surg. 2000;26(9):853–6.
Maruo K, Kayashima KI, Ono T. Regressing Merkel cell carcinoma-a case showing replacement of tumour cells by foamy cells. Br J Dermatol. 2000;142(6):1184–9.
Sais G, Admella C, Soler T. Spontaneous regression in primary cutaneous neuroendocrine (Merkel cell) carcinoma: a rare immune phenomenon? J Eur Acad Dermatol Venereol. 2002;16(1):82–3.
Junquera L, Torre A, Vicente JC, Garcia-Consuegra L, Fresno MF. Complete spontaneous regression of Merkel cell carcinoma. Ann Otol Rhinol Laryngol. 2005;114(5):376–80.
Missotten GS, de Wolff-Rouendaal D, de Keizer RJ. Merkel cell carcinoma of the eyelid review of the literature and report of patients with Merkel cell carcinoma showing spontaneous regression. Ophthalmology. 2008;115(1):195–201.
Karkos PD, Sastry A, Hampal S, Al-Jafari M. Spontaneous regression of Merkel cell carcinoma of the nose. Head Neck. 2010;32(3):411–4.
Wooff JC, Trites JR, Walsh NM, Bullock MJ. Complete spontaneous regression of metastatic Merkel cell carcinoma: a case report and review of the literature. Am J Dermatopathol. 2010;32(6):614–7.
Warnick M, Singh S, Boisvert ME, Peck GL. Merkel cell carcinoma presenting as lymphadenopathy without a primary cutaneous lesion: a report of 2 cases. Arch Dermatol. 2008;144(10):1397–8.
Cozzolino I, Zeppa R, Zeppa P. Lymph nodal Merkel cell carcinoma: primary tumor or metastasis from unknown primary site? J Cutan Pathol. 2011;38(10):836–7.
de Zeeuw S, Schouten van der Velden AP, de Wilt HJ, Wetzels CT, Bonenkamp HJ. A Merkel cell carcinoma presenting as a solitary lymph node metastasis without a primary lesion. Report of a case and review of the literature. Acta Chir Belg. 2012;112(4):317–21.
Deneve JL, Messina JL, Marzban SS, Gonzalez RJ, Walls BM, Fisher KJ, et al. Merkel cell carcinoma of unknown primary origin. Ann Surg Oncol. 2012;19(7):2360–6.
Boghossian V, Owen ID, Nuli B, Xiao PQ. Neuroendocrine (Merkel cell) carcinoma of the retroperitoneum with no identifiable primary site. World J Surg Oncol. 2007;5:117.
Nazarian Y, Shalmon B, Horowitz Z, Bedrin L, Pfeffer MR, Talmi YP. Merkel cell carcinoma of unknown primary site. J Laryngol Otol. 2007;121(4), e1.
Tarantola TI, Vallow LA, Halyard MY, Weenig RH, Warschaw KE, Weaver AL, et al. Unknown primary Merkel cell carcinoma: 23 new cases and a review. J Am Acad Dermatol. 2013;68(3):433–40.
Rossini D, Caponnetto S, Lapadula V, De Filippis L, Del Bene G, Emiliani A, et al. Merkel cell carcinoma of the retroperitoneum with no identifiable primary site. Case Rep Oncol Med. 2013;2013:131695.
Haider K, Hu Q, McFadden AJ, Shoker A, Barton JW, Ahmed S. Thrombocytopenia: an unusual manifestation of advanced composite Merkel cell carcinoma and in situ squamous cell carcinoma. Am J Clin Oncol. 2007;30(4):442–3.
Anzai S, Sato T, Takayasu S, Asada Y, Terashi H, Takasaki S. Postoperative hyponatremia in a patient with ACTH-producing Merkel cell carcinoma. J Dermatol. 2000;27(6):397–400.
Eggers SD, Salomao DR, Dinapoli RP, Vernino S. Paraneoplastic and metastatic neurologic complications of Merkel cell carcinoma. Mayo Clin Proc. 2001;76(3):327–30.
Rocamora A, Badia N, Vives R, Carrillo R, Ulloa J, Ledo A. Epidermotropic primary neuroendocrine (Merkel cell) carcinoma of the skin with Pautrier-like microabscesses. Report of three cases and review of the literature. J Am Acad Dermatol. 1987;16(6):1163–8.
LeBoit PE, Crutcher WA, Shapiro PE. Pagetoid intraepidermal spread in Merkel cell (primary neuroendocrine) carcinoma of the skin. Am J Surg Pathol. 1992;16(6):584–92.
Smith KJ, Skelton 3rd HG, Holland TT, Morgan AM, Lupton GP. Neuroendocrine (Merkel cell) carcinoma with an intraepidermal component. Am J Dermatopathol. 1993;15(6):528–33.
Ball NJ, Tanhuanco-Kho G. Merkel cell carcinoma frequently shows histologic features of basal cell carcinoma: a study of 30 cases. J Cutan Pathol. 2007;34(8):612–9.
D’Agostino M, Cinelli C, Willard R, Hofmann J, Jellinek N, Robinson-Bostom L. Epidermotropic Merkel cell carcinoma: a case series with histopathologic examination. J Am Acad Dermatol. 2010;62(3):463–8.
Al-Ahmadie HA, Mutasim DF, Mutema GK. A case of intraepidermal Merkel cell carcinoma within squamous cell carcinoma in-situ: Merkel cell carcinoma in-situ? Am J Dermatopathol. 2004;26(3):230–3.
Ferringer T, Rogers HC, Metcalf JS. Merkel cell carcinoma in situ. J Cutan Pathol. 2005;32(2):162–5.
Panelos J, Batistatou A, Paglierani M, Zioga A, Maio V, Santi R, et al. Expression of Notch-1 and alteration of the E-cadherin/beta-catenin cell adhesion complex are observed in primary cutaneous neuroendocrine carcinoma (Merkel cell carcinoma). Mod Pathol. 2009;22(7):959–68.
Knapp CF, Sayegh Z, Schell MJ, Rawal B, Ochoa T, Sondak VK, et al. Expression of CXCR4, E-cadherin, Bcl-2, and survivin in Merkel cell carcinoma: an immunohistochemical study using a tissue microarray. Am J Dermatopathol. 2012;34(6):592–6.
Pilotti S, Rilke F, Bartoli C, Grisotti A. Clinicopathologic correlations of cutaneous neuroendocrine Merkel cell carcinoma. J Clin Oncol. 1988;6(12):1863–73.
Wick MR, Goellner JR, Scheithauer BW, Thomas 3rd JR, Sanchez NP, Schroeter AL. Primary neuroendocrine carcinomas of the skin (Merkel cell tumors). A clinical, histologic, and ultrastructural study of thirteen cases. Am J Clin Pathol. 1983;79(1):6–13.
Silva EG, Mackay B, Goepfert H, Burgess MA, Fields RS. Endocrine carcinoma of the skin (Merkel cell carcinoma). Pathol Annu. 1984;19(Pt 2):1–30.
Plaza JA, Suster S. The Toker tumor: spectrum of morphologic features in primary neuroendocrine carcinomas of the skin (Merkel cell carcinoma). Ann Diagn Pathol. 2006;10(6):376–85.
Vazmitel M, Michal M, Kazakov DV. Merkel cell carcinoma and Azzopardi phenomenon. Am J Dermatopathol. 2007;29(3):314–5.
Vazmitel M, Michal M, Shelekhova KV, Sima R, Mukensnabl P, Kazakov DV. Vascular changes in Merkel cell carcinoma based on a histopathological study of 92 cases. Am J Dermatopathol. 2008;30(2):106–11.
Vazmitel M, Michal M, Kempf W, Mukensnabl P, Kazakov DV. Merkel cell carcinoma with a follicular lymphocytic infiltrate: report of 2 cases. Am J Dermatopathol. 2008;30(4):389–91.
Gould E, Albores-Saavedra J, Dubner B, Smith W, Payne CM. Eccrine and squamous differentiation in Merkel cell carcinoma. An immunohistochemical study. Am J Surg Pathol. 1988;12(10):768–72.
Iacocca MV, Abernethy JL, Stefanato CM, Allan AE, Bhawan J. Mixed Merkel cell carcinoma and squamous cell carcinoma of the skin. J Am Acad Dermatol. 1998;39(5 Pt 2):882–7.
Cooper L, Debono R, Alsanjari N, Al-Nafussi A. Merkel cell tumour with leiomyosarcomatous differentiation. Histopathology. 2000;36(6):540–3.
Foschini MP, Eusebi V. Divergent differentiation in endocrine and nonendocrine tumors of the skin. Semin Diagn Pathol. 2000;17(2):162–8.
Boutilier R, Desormeau L, Cragg F, Roberts P, Walsh N. Merkel cell carcinoma: squamous and atypical fibroxanthoma-like differentiation in successive local tumor recurrences. Am J Dermatopathol. 2001;23(1):46–9.
Fernandez-Figueras MT, Puig L, Gilaberte M, Gomez-Plaza Mdel C, Rex J, Ferrandiz C, et al. Merkel cell (primary neuroendocrine) carcinoma of the skin with nodal metastasis showing rhabdomyosarcomatous differentiation. J Cutan Pathol. 2002;29(10):619–22.
Hwang JH, Alanen K, Dabbs KD, Danyluk J, Silverman S. Merkel cell carcinoma with squamous and sarcomatous differentiation. J Cutan Pathol. 2008;35(10):955–9.
Tan KB, Murali R, Karim RZ, Dutta B, Dutta R, McCarthy SW, et al. Merkel cell carcinoma with fibrosarcomatous differentiation. Pathology. 2008;40(3):314–6.
Lau PP, Ting SH, Ip YT, Tsang WY, Chan JK. Merkel cell carcinosarcoma: Merkel cell carcinoma with embryonal rhabdomyosarcoma-like component. Ann Diagn Pathol. 2012;16(5):388–91.
Adhikari LA, McCalmont TH, Folpe AL. Merkel cell carcinoma with heterologous rhabdomyoblastic differentiation: the role of immunohistochemistry for Merkel cell polyomavirus large T-antigen in confirmation. J Cutan Pathol. 2012;39(1):47–51.
Martin B, Poblet E, Rios JJ, Kazakov D, Kutzner H, Brenn T, et al. Merkel cell carcinoma with divergent differentiation: histopathological and immunohistochemical study of 15 cases with PCR analysis for Merkel cell polyomavirus. Histopathology. 2013;62(5):711–22.
Koba S, Misago N, Nagase K, Tsuruta N, Inoue T, Ikeda S, et al. Triphasic differentiations of Merkel cell carcinoma in primary and metastatic lesions. J Cutan Pathol. 2014;41(5):469–74.
Roncaroli F, Mauri F, Pasquinelli G. Primary neuroendocrine carcinoma with ganglion cell differentiation in a crural lymph node. Virchows Arch. 2000;437(6):675–9.
Vanchinathan V, Marinelli EC, Kartha RV, Uzieblo A, Ranchod M, Sundram UN. A malignant cutaneous neuroendocrine tumor with features of Merkel cell carcinoma and differentiating neuroblastoma. Am J Dermatopathol. 2009;31(2):193–6.
Iwasaki T, Kodama H, Matsushita M, Kuroda N, Yamasaki Y, Murakami I, et al. Merkel cell polyomavirus infection in both components of a combined Merkel cell carcinoma and basal cell carcinoma with ductal differentiation; each component had a similar but different novel Merkel cell polyomavirus large T antigen truncating mutation. Hum Pathol. 2013;44(3):442–7.
Rosso R, Paulli M, Carnevali L. Neuroendocrine carcinoma of the skin with lymphoepithelioma-like features. Am J Dermatopathol. 1998;20(5):483–6.
Ben Abdelkrim S, Dhouibi A, Moussa A, Hadhri R, Njim L, Mighri K, et al. Merkel cell carcinoma with lymphoepithelioma-like pattern: a case report of an exceedingly rare variant of Merkel cell carcinoma with lymph node metastases at presentation. Case Rep Pathol. 2011;2011:840575.
Li N, Wolgamot G, Argenyi Z. Primary cutaneous neuroendocrine cell carcinoma (Merkel cell carcinoma) with prominent microcystic features, mimicking eccrine carcinoma. J Cutan Pathol. 2007;34(5):410–4.
Aydin A, Kocer NE, Bekerecioglu M, Sari I. Cutaneous undifferentiated small (Merkel) cell carcinoma, that developed synchronously with multiple actinic keratoses, squamous cell carcinomas and basal cell carcinoma. J Dermatol. 2003;30(3):241–4.
Busam KJ, Jungbluth AA, Rekthman N, Coit D, Pulitzer M, Bini J, et al. Merkel cell polyomavirus expression in Merkel cell carcinomas and its absence in combined tumors and pulmonary neuroendocrine carcinomas. Am J Surg Pathol. 2009;33(9):1378–85.
Cerroni L, Kerl H. Primary cutaneous neuroendocrine (Merkel cell) carcinoma in association with squamous- and basal-cell carcinoma. Am J Dermatopathol. 1997;19(6):610–3.
Gomez LG, DiMaio S, Silva EG, Mackay B. Association between neuroendocrine (Merkel cell) carcinoma and squamous carcinoma of the skin. Am J Surg Pathol. 1983;7(2):171–7.
Hewitt JB, Sherif A, Kerr KM, Stankler L. Merkel cell and squamous cell carcinomas arising in erythema ab igne. Br J Dermatol. 1993;128(5):591–2.
Iwafuchi M, Watanabe H, Ishihara N, Takahashi Y, Yoshimura M. A neuroendocrine (Merkel) cell carcinoma with coexisting intraepidermal squamous cell carcinoma of the skin. Its growth accelerated by an extrinsic factor. Acta Pathol Jpn. 1986;36(7):1099–108.
Jones CS, Tyring SK, Lee PC, Fine JD. Development of neuroendocrine (Merkel cell) carcinoma mixed with squamous cell carcinoma in erythema ab igne. Arch Dermatol. 1988;124(1):110–3.
Sirikanjanapong S, Melamed J, Patel RR. Intraepidermal and dermal Merkel cell carcinoma with squamous cell carcinoma in situ: a case report with review of literature. J Cutan Pathol. 2010;37(8):881–5.
Vieites B, Suarez-Penaranda JM, Delgado V, Vazquez-Veiga H, Varela J, Forteza J. Merkel cell carcinoma associated with in situ and invasive squamous cell carcinoma. Acta Derm Venereol. 2009;89(2):184–6.
Tan BH, Busam KJ, Pulitzer MP. Combined intraepidermal neuroendocrine (Merkel cell) and squamous cell carcinoma in situ with CM2B4 negativity and p53 overexpression(*). J Cutan Pathol. 2012;39(6):626–30.
Popp S, Waltering S, Herbst C, Moll I, Boukamp P. UV-B-type mutations and chromosomal imbalances indicate common pathways for the development of Merkel and skin squamous cell carcinomas. Int J Cancer. 2002;99(3):352–60.
Kuwamoto S. Recent advances in the biology of Merkel cell carcinoma. Hum Pathol. 2011;42(8):1063–77.
Iwasaki T, Matsushita M, Kuwamoto S, Kato M, Murakami I, Higaki-Mori H, et al. Usefulness of significant morphologic characteristics in distinguishing between Merkel cell polyomavirus-positive and Merkel cell polyomavirus-negative Merkel cell carcinomas. Hum Pathol. 2013;44(9):1912–7.
Kuwamoto S, Higaki H, Kanai K, Iwasaki T, Sano H, Nagata K, et al. Association of Merkel cell polyomavirus infection with morphologic differences in Merkel cell carcinoma. Hum Pathol. 2011;42(5):632–40.
Tanahashi J, Kashima K, Daa T, Yada N, Fujiwara S, Yokoyama S. Merkel cell carcinoma co-existent with sebaceous carcinoma of the eyelid. J Cutan Pathol. 2009;36(9):983–6.
Youker SR, Billingsley EM. Combined Merkel cell carcinoma and atypical fibroxanthoma. J Cutan Med Surg. 2005;9(1):6–9.
Ivan D, Bengana C, Lazar AJ, Diwan AH, Prieto VG. Merkel cell tumor in a trichilemmal cyst: collision or association? Am J Dermatopathol. 2007;29(2):180–3.
Su W, Kheir SM, Berberian B, Cockerell CJ. Merkel cell carcinoma in situ arising in a trichilemmal cyst: a case report and literature review. Am J Dermatopathol. 2008;30(5):458–61.
Requena L, Jaqueti G, Rutten A, Mentzel T, Kutzner H. Merkel cell carcinoma within follicular cysts: report of two cases. J Cutan Pathol. 2008;35(12):1127–33.
Craig PJ, Calonje JE, Harries M, Stefanato CM. Incidental chronic lymphocytic leukaemia in a biopsy of Merkel cell carcinoma. J Cutan Pathol. 2009;36(6):706–10.
Koljonen V, Kukko H, Pukkala E, Sankila R, Bohling T, Tukiainen E, et al. Chronic lymphocytic leukaemia patients have a high risk of Merkel-cell polyomavirus DNA-positive Merkel-cell carcinoma. Br J Cancer. 2009;101(8):1444–7.
Li Z, Yang JJ, Wu M. Collision tumor of primary Merkel cell carcinoma and chronic lymphocytic leukemia/small lymphocytic lymphoma, diagnosed on ultrasound-guided fine-needle aspiration biopsy: A unique case report and review of literature. Diagn Cytopathol. 2015;43:66–71.
Papalas JA, McKinney MS, Kulbacki E, Dave SS, Wang E. Merkel cell carcinoma with partial B-cell blastic immunophenotype: a potential mimic of cutaneous richter transformation in a patient with chronic lymphocytic lymphoma. Am J Dermatopathol. 2014;36(2):148–52.
Tolstov YL, Arora R, Scudiere SC, Busam K, Chaudhary PM, Chang Y, et al. Lack of evidence for direct involvement of Merkel cell polyomavirus (MCV) in chronic lymphocytic leukemia (CLL). Blood. 2010;115(23):4973–4.
Shingde MV, Buckland M, Busam KJ, McCarthy SW, Wilmott J, Thompson JF, et al. Primary cutaneous Ewing sarcoma/primitive neuroectodermal tumour: a clinicopathological analysis of seven cases highlighting diagnostic pitfalls and the role of FISH testing in diagnosis. J Clin Pathol. 2009;62(10):915–9.
Terrier-Lacombe MJ, Guillou L, Chibon F, Gallagher G, Benhattar J, Terrier P, et al. Superficial primitive Ewing’s sarcoma: a clinicopathologic and molecular cytogenetic analysis of 14 cases. Mod Pathol. 2009;22(1):87–94.
Machado I, Llombart B, Calabuig-Farinas S, Llombart-Bosch A. Superficial Ewing’s sarcoma family of tumors: a clinicopathological study with differential diagnoses. J Cutan Pathol. 2011;38(8):636–43.
Fernandez-Flores A, Suarez-Penaranda JM, Alonso S. Study of EWS/FLI-1 rearrangement in 18 cases of CK20+/CM2B4+ Merkel cell carcinoma using FISH and correlation to the differential diagnosis of Ewing sarcoma/peripheral neuroectodermal tumor. Appl Immunohistochem Mol Morphol. 2013;21(5):379–85.
Lloyd RV, Cano M, Rosa P, Hille A, Huttner WB. Distribution of chromogranin A and secretogranin I (chromogranin B) in neuroendocrine cells and tumors. Am J Pathol. 1988;130(2):296–304.
Leong AS, Milios J. Cytokeratin distribution in Merkel cell tumor of the skin. Hum Pathol. 1987;18(3):308–9.
Battifora H, Silva EG. The use of antikeratin antibodies in the immunohistochemical distinction between neuroendocrine (Merkel cell) carcinoma of the skin, lymphoma, and oat cell carcinoma. Cancer. 1986;58(5):1040–6.
Allen PJ, Busam K, Hill AD, Stojadinovic A, Coit DG. Immunohistochemical analysis of sentinel lymph nodes from patients with Merkel cell carcinoma. Cancer. 2001;92(6):1650–5.
Miettinen M. Synaptophysin and neurofilament proteins as markers for neuroendocrine tumors. Arch Pathol Lab Med. 1987;111(9):813–8.
Narisawa Y, Hashimoto K, Kohda H. Immunohistochemical demonstration of the expression of neurofilament proteins in Merkel cells. Acta Derm Venereol. 1994;74(6):441–3.
Arnold MA, Schoenfield L, Limketkai BN, Arnold CA. Diagnostic pitfalls of differentiating desmoplastic small round cell tumor (DSRCT) from Wilms tumor (WT): overlapping morphologic and immunohistochemical features. Am J Surg Pathol. 2014;38(9):1220–6.
Jerome Marson V, Mazieres J, Groussard O, Garcia O, Berjaud J, Dahan M, et al. Expression of TTF-1 and cytokeratins in primary and secondary epithelial lung tumours: correlation with histological type and grade. Histopathology. 2004;45(2):125–34.
Rund CR, Fischer EG. Perinuclear dot-like cytokeratin 20 staining in small cell neuroendocrine carcinoma of the ovary (pulmonary-type). Appl Immunohistochem Mol Morphol. 2006;14(2):244–8.
Schmidt U, Muller U, Metz KA, Leder LD. Cytokeratin and neurofilament protein staining in Merkel cell carcinoma of the small cell type and small cell carcinoma of the lung. Am J Dermatopathol. 1998;20(4):346–51.
Gu M, Antonescu CR, Guiter G, Huvos AG, Ladanyi M, Zakowski MF. Cytokeratin immunoreactivity in Ewing’s sarcoma: prevalence in 50 cases confirmed by molecular diagnostic studies. Am J Surg Pathol. 2000;24(3):410–6.
Bobos M, Hytiroglou P, Kostopoulos I, Karkavelas G, Papadimitriou CS. Immunohistochemical distinction between Merkel cell carcinoma and small cell carcinoma of the lung. Am J Dermatopathol. 2006;28(2):99–104.
Calder KB, Coplowitz S, Schlauder S, Morgan MB. A case series and immunophenotypic analysis of CK20-/CK7+ primary neuroendocrine carcinoma of the skin. J Cutan Pathol. 2007;34(12):918–23.
Beer TW. Merkel cell carcinomas with CK20 negative and CK7 positive immunostaining. J Cutan Pathol. 2009;36(3):385–6; author reply 7.
Lundquist K, Kohler S, Rouse RV. Intraepidermal cytokeratin 7 expression is not restricted to Paget cells but is also seen in Toker cells and Merkel cells. Am J Surg Pathol. 1999;23(2):212–9.
Asioli S, Righi A, de Biase D, Morandi L, Caliendo V, Picciotto F, et al. Expression of p63 is the sole independent marker of aggressiveness in localised (stage I-II) Merkel cell carcinomas. Mod Pathol. 2011;24(11):1451–61.
Dabner M, McClure RJ, Harvey NT, Budgeon CA, Beer TW, Amanuel B, et al. Merkel cell polyomavirus and p63 status in Merkel cell carcinoma by immunohistochemistry: Merkel cell polyomavirus positivity is inversely correlated with sun damage, but neither is correlated with outcome. Pathology. 2014;46(3):205–10.
Fleming KE, Ly TY, Pasternak S, Godlewski M, Doucette S, Walsh NM. Support for p63 expression as an adverse prognostic marker in Merkel cell carcinoma: report on a Canadian cohort. Hum Pathol. 2014;45(5):952–60.
Lim CS, Whalley D, Haydu LE, Murali R, Tippett J, Thompson JF, et al. Increasing tumor thickness is associated with recurrence and poorer survival in patients with Merkel cell carcinoma. Ann Surg Oncol. 2012;19(11):3325–34.
Shuda M, Arora R, Kwun HJ, Feng H, Sarid R, Fernandez-Figueras MT, et al. Human Merkel cell polyomavirus infection I. MCV T antigen expression in Merkel cell carcinoma, lymphoid tissues and lymphoid tumors. Int J Cancer. 2009;125(6):1243–9.
Rodig SJ, Cheng J, Wardzala J, DoRosario A, Scanlon JJ, Laga AC, et al. Improved detection suggests all Merkel cell carcinomas harbor Merkel polyomavirus. J Clin Invest. 2012;122(12):4645–53.
Waltari M, Sihto H, Kukko H, Koljonen V, Sankila R, Bohling T, et al. Association of Merkel cell polyomavirus infection with tumor p53, KIT, stem cell factor, PDGFR-alpha and survival in Merkel cell carcinoma. Int J Cancer. 2011;129(3):619–28.
Van Gele M, Kaghad M, Leonard JH, Van Roy N, Naeyaert JM, Geerts ML, et al. Mutation analysis of P73 and TP53 in Merkel cell carcinoma. Br J Cancer. 2000;82(4):823–6.
Lassacher A, Heitzer E, Kerl H, Wolf P. p14ARF hypermethylation is common but INK4a-ARF locus or p53 mutations are rare in Merkel cell carcinoma. J Invest Dermatol. 2008;128(7):1788–96.
Sahi H, Savola S, Sihto H, Koljonen V, Bohling T, Knuutila S. RB1 gene in Merkel cell carcinoma: hypermethylation in all tumors and concurrent heterozygous deletions in the polyomavirus-negative subgroup. APMIS. 2014;122:1157–66.
Llombart B, Monteagudo C, Lopez-Guerrero JA, Carda C, Jorda E, Sanmartin O, et al. Clinicopathological and immunohistochemical analysis of 20 cases of Merkel cell carcinoma in search of prognostic markers. Histopathology. 2005;46(6):622–34.
Fernandez-Figueras MT, Puig L, Musulen E, Gilaberte M, Lerma E, Serrano S, et al. Expression profiles associated with aggressive behavior in Merkel cell carcinoma. Mod Pathol. 2007;20(1):90–101.
Su LD, Fullen DR, Lowe L, Uherova P, Schnitzer B, Valdez R. CD117 (KIT receptor) expression in Merkel cell carcinoma. Am J Dermatopathol. 2002;24(4):289–93.
Feinmesser M, Halpern M, Kaganovsky E, Brenner B, Fenig E, Hodak E, et al. c-kit expression in primary and metastatic Merkel cell carcinoma. Am J Dermatopathol. 2004;26(6):458–62.
Andea AA, Patel R, Ponnazhagan S, Kumar S, DeVilliers P, Jhala D, et al. Merkel cell carcinoma: correlation of KIT expression with survival and evaluation of KIT gene mutational status. Hum Pathol. 2010;41(10):1405–12.
Swick BL, Ravdel L, Fitzpatrick JE, Robinson WA. Merkel cell carcinoma: evaluation of KIT (CD117) expression and failure to demonstrate activating mutations in the C-KIT proto-oncogene – implications for treatment with imatinib mesylate. J Cutan Pathol. 2007;34(4):324–9.
Kartha RV, Sundram UN. Silent mutations in KIT and PDGFRA and coexpression of receptors with SCF and PDGFA in Merkel cell carcinoma: implications for tyrosine kinase-based tumorigenesis. Mod Pathol. 2008;21(2):96–104.
Ordonez NG. Value of thyroid transcription factor-1 immunostaining in distinguishing small cell lung carcinomas from other small cell carcinomas. Am J Surg Pathol. 2000;24(9):1217–23.
Byrd-Gloster AL, Khoor A, Glass LF, Messina JL, Whitsett JA, Livingston SK, et al. Differential expression of thyroid transcription factor 1 in small cell lung carcinoma and Merkel cell tumor. Hum Pathol. 2000;31(1):58–62.
Buresh CJ, Oliai BR, Miller RT. Reactivity with TdT in Merkel cell carcinoma: a potential diagnostic pitfall. Am J Clin Pathol. 2008;129(6):894–8.
Ralston J, Chiriboga L, Nonaka D. MASH1: a useful marker in differentiating pulmonary small cell carcinoma from Merkel cell carcinoma. Mod Pathol. 2008;21(11):1357–62.
Sierakowski A, Al-Janabi K, Dam H, Sood M. Metastatic Merkel cell carcinoma with positive expression of thyroid transcription factor-1 – a case report. Am J Dermatopathol. 2009;31(4):384–6.
McNiff JM, Cowper SE, Lazova R, Subtil A, Glusac EJ. CD56 staining in Merkel cell carcinoma and natural killer-cell lymphoma: magic bullet, diagnostic pitfall, or both? J Cutan Pathol. 2005;32(8):541–5.
Kolhe R, Reid MD, Lee JR, Cohen C, Ramalingam P. Immunohistochemical expression of PAX5 and TdT by Merkel cell carcinoma and pulmonary small cell carcinoma: a potential diagnostic pitfall but useful discriminatory marker. Int J Clin Exp Pathol. 2013;6(2):142–7.
Feinmesser M, Halpern M, Fenig E, Tsabari C, Hodak E, Sulkes J, et al. Expression of the apoptosis-related oncogenes bcl-2, bax, and p53 in Merkel cell carcinoma: can they predict treatment response and clinical outcome? Hum Pathol. 1999;30(11):1367–72.
Verhaegen ME, Mangelberger D, Weick JW, Vozheiko TD, Harms PW, Nash KT, et al. Merkel cell carcinoma dependence on bcl-2 family members for survival. J Invest Dermatol. 2014;134(8):2241–50.
Filtenborg-Barnkob BE, Bzorek M. Expression of anaplastic lymphoma kinase in Merkel cell carcinomas. Hum Pathol. 2013;44(8):1656–64.
Rickelt S, Moll I, Franke WW. Intercellular adhering junctions with an asymmetric molecular composition: desmosomes connecting Merkel cells and keratinocytes. Cell Tissue Res. 2011;346(1):65–77.
Kazem S, van der Meijden E, Wang RC, Rosenberg AS, Pope E, Benoit T, et al. Polyomavirus-Associated Trichodysplasia Spinulosa Involves Hyperproliferation, pRB Phosphorylation and Upregulation of p16 and p21. PLoS One. 2014;9(10):e108947.
Tolstov YL, Pastrana DV, Feng H, Becker JC, Jenkins FJ, Moschos S, et al. Human Merkel cell polyomavirus infection II. MCV is a common human infection that can be detected by conformational capsid epitope immunoassays. Int J Cancer. 2009;125(6):1250–6.
Touze A, Gaitan J, Maruani A, Le Bidre E, Doussinaud A, Clavel C, et al. Merkel cell polyomavirus strains in patients with Merkel cell carcinoma. Emerg Infect Dis. 2009;15(6):960–2.
Touze A, Le Bidre E, Laude H, Fleury MJ, Cazal R, Arnold F, et al. High levels of antibodies against Merkel cell polyomavirus identify a subset of patients with Merkel cell carcinoma with better clinical outcome. J Clin Oncol. 2011;29(12):1612–9.
Foulongne V, Kluger N, Dereure O, Mercier G, Moles JP, Guillot B, et al. Merkel cell polyomavirus in cutaneous swabs. Emerg Infect Dis. 2010;16(4):685–7.
Pancaldi C, Corazzari V, Maniero S, Mazzoni E, Comar M, Martini F, et al. Merkel cell polyomavirus DNA sequences in the buffy coats of healthy blood donors. Blood. 2011;117(26):7099–101.
Faust H, Andersson K, Ekstrom J, Hortlund M, Robsahm TE, Dillner J. Prospective study of Merkel cell polyomavirus and risk of Merkel cell carcinoma. Int J Cancer. 2014;134(4):844–8.
Martel-Jantin C, Pedergnana V, Nicol JT, Leblond V, Tregouet DA, Tortevoye P, et al. Merkel cell polyomavirus infection occurs during early childhood and is transmitted between siblings. J Clin Virol. 2013;58(1):288–91.
Loyo M, Guerrero-Preston R, Brait M, Hoque MO, Chuang A, Kim MS, et al. Quantitative detection of Merkel cell virus in human tissues and possible mode of transmission. Int J Cancer. 2010;126(12):2991–6.
Bhatia K, Goedert JJ, Modali R, Preiss L, Ayers LW. Merkel cell carcinoma subgroups by Merkel cell polyomavirus DNA relative abundance and oncogene expression. Int J Cancer. 2010;126(9):2240–6.
Garneski KM, Warcola AH, Feng Q, Kiviat NB, Leonard JH, Nghiem P. Merkel cell polyomavirus is more frequently present in North American than Australian Merkel cell carcinoma tumors. J Invest Dermatol. 2009;129(1):246–8.
Kassem A, Schopflin A, Diaz C, Weyers W, Stickeler E, Werner M, et al. Frequent detection of Merkel cell polyomavirus in human Merkel cell carcinomas and identification of a unique deletion in the VP1 gene. Cancer Res. 2008;68(13):5009–13.
Becker JC, Houben R, Ugurel S, Trefzer U, Pfohler C, Schrama D. MC polyomavirus is frequently present in Merkel cell carcinoma of European patients. J Invest Dermatol. 2009;129(1):248–50.
Andres C, Puchta U, Flaig MJ. Detection of Merkel cell polyomavirus DNA in atypical fibroxanthoma in correlation to clinical features. Am J Dermatopathol. 2010;32(8):799–803.
Sastre-Garau X, Peter M, Avril MF, Laude H, Couturier J, Rozenberg F, et al. Merkel cell carcinoma of the skin: pathological and molecular evidence for a causative role of MCV in oncogenesis. J Pathol. 2009;218(1):48–56.
Sihto H, Kukko H, Koljonen V, Sankila R, Bohling T, Joensuu H. Clinical factors associated with Merkel cell polyomavirus infection in Merkel cell carcinoma. J Natl Cancer Inst. 2009;101(13):938–45.
Bhatia S, Afanasiev O, Nghiem P. Immunobiology of Merkel cell carcinoma: implications for immunotherapy of a polyomavirus-associated cancer. Curr Oncol Rep. 2011;13(6):488–97.
Borchert S, Czech-Sioli M, Neumann F, Schmidt C, Wimmer P, Dobner T, et al. High-affinity Rb binding, p53 inhibition, subcellular localization, and transformation by wild-type or tumor-derived shortened Merkel cell polyomavirus large T antigens. J Virol. 2014;88(6):3144–60.
Houben R, Adam C, Baeurle A, Hesbacher S, Grimm J, Angermeyer S, et al. An intact retinoblastoma protein-binding site in Merkel cell polyomavirus large T antigen is required for promoting growth of Merkel cell carcinoma cells. Int J Cancer. 2012;130(4):847–56.
Houben R, Dreher C, Angermeyer S, Borst A, Utikal J, Haferkamp S, et al. Mechanisms of p53 restriction in Merkel cell carcinoma cells are independent of the Merkel cell polyoma virus T antigens. J Invest Dermatol. 2013;133(10):2453–60.
Sihto H, Kukko H, Koljonen V, Sankila R, Bohling T, Joensuu H. Merkel cell polyomavirus infection, large T antigen, retinoblastoma protein and outcome in Merkel cell carcinoma. Clin Cancer Res. 2011;17(14):4806–13.
Bhatia K, Goedert JJ, Modali R, Preiss L, Ayers LW. Immunological detection of viral large T antigen identifies a subset of Merkel cell carcinoma tumors with higher viral abundance and better clinical outcome. Int J Cancer. 2010;127(6):1493–6.
Paulson KG, Carter JJ, Johnson LG, Cahill KW, Iyer JG, Schrama D, et al. Antibodies to Merkel cell polyomavirus T antigen oncoproteins reflect tumor burden in Merkel cell carcinoma patients. Cancer Res. 2010;70(21):8388–97.
Allen PJ, Bowne WB, Jaques DP, Brennan MF, Busam K, Coit DG. Merkel cell carcinoma: prognosis and treatment of patients from a single institution. J Clin Oncol. 2005;23(10):2300–9.
Schwartz JL, Griffith KA, Lowe L, Wong SL, McLean SA, Fullen DR, et al. Features predicting sentinel lymph node positivity in Merkel cell carcinoma. J Clin Oncol. 2011;29(8):1036–41.
Fields RC, Busam KJ, Chou JF, Panageas KS, Pulitzer MP, Kraus DH, et al. Recurrence and survival in patients undergoing sentinel lymph node biopsy for Merkel cell carcinoma: analysis of 153 patients from a single institution. Ann Surg Oncol. 2011;18(9):2529–37.
Andea AA, Coit DG, Amin B, Busam KJ. Merkel cell carcinoma: histologic features and prognosis. Cancer. 2008;113(9):2549–58.
Mott RT, Smoller BR, Morgan MB. Merkel cell carcinoma: a clinicopathologic study with prognostic implications. J Cutan Pathol. 2004;31(3):217–23.
Suomela S, Koljonen V, Skoog T, Kukko H, Bohling T, Saarialho-Kere U. Expression of MMP-10, MMP-21, MMP-26, and MMP-28 in Merkel cell carcinoma. Virchows Arch. 2009;455(6):495–503.
Fields RC, Busam KJ, Chou JF, Panageas KS, Pulitzer MP, Allen PJ, et al. Five hundred patients with Merkel cell carcinoma evaluated at a single institution. Ann Surg. 2011;254(3):465–73; discussion 73–5.
Fernandez-Figueras MT, Puig L, Musulen E, Gilaberte M, Ferrandiz C, Lerma E, et al. Prognostic significance of p27Kip1, p45Skp2 and Ki67 expression profiles in Merkel cell carcinoma, extracutaneous small cell carcinoma, and cutaneous squamous cell carcinoma. Histopathology. 2005;46(6):614–21.
Koljonen V, Tukiainen E, Haglund C, Bohling T. Proliferative activity detected by Ki67 correlates with poor outcome in Merkel cell carcinoma. Histopathology. 2006;49(5):551–3.
Tseng J, Dhungel B, Mills JK, Diggs BS, Weerasinghe R, Fortino J, et al. Merkel cell carcinoma: what makes a difference? Am J Surg. 2015;209:342–6.
Lipson EJ, Vincent JG, Loyo M, Kagohara LT, Luber BS, Wang H, et al. PD-L1 expression in the Merkel cell carcinoma microenvironment: association with inflammation, Merkel cell polyomavirus and overall survival. Cancer Immunol Res. 2013;1(1):54–63.
Shahzad N, Shuda M, Gheit T, Kwun HJ, Cornet I, Saidj D, et al. The T antigen locus of Merkel cell polyomavirus downregulates human Toll-like receptor 9 expression. J Virol. 2013;87(23):13009–19.
Afanasiev OK, Yelistratova L, Miller N, Nagase K, Paulson K, Iyer JG, et al. Merkel polyomavirus-specific T cells fluctuate with Merkel cell carcinoma burden and express therapeutically targetable PD-1 and Tim-3 exhaustion markers. Clin Cancer Res. 2013;19(19):5351–60.
Sihto H, Bohling T, Kavola H, Koljonen V, Salmi M, Jalkanen S, et al. Tumor infiltrating immune cells and outcome of Merkel cell carcinoma: a population-based study. Clin Cancer Res. 2012;18(10):2872–81.
Fan R. PAX immunoreactivity in poorly differentiated small round cell tumors of childhood. Fetal Pediatr Pathol. 2014;33(4):244–52.
Desouki MM, Post GR, Cherry D, Lazarchick J. PAX-5: a valuable immunohistochemical marker in the differential diagnosis of lymphoid neoplasms. Clin Med Res. 2010;8(2):84–8.
Abbreviations
AJCC American Joint Committee on Cancer
APUD Amine precursor uptake and decarboxylation
BCC Basal cell carcinoma
CK Cytokeratin
CLL Chronic lymphocytic leukemia
ACTH Adrenocorticotropic hormone
HIV/AIDS Human immunodeficiency virus/acquired immunodeficiency syndrome
LT-Ag Large T antigen
LVI Lymphovascular invasion
MASH1/hASH1 Mammalian/human achaete-scute complex homolog 1
MCC Merkel cell carcinoma
MCPyV Merkel cell polyomavirus
NCCN National Comprehensive Cancer Network
NSE Neuron-specific enolase
PNET Peripheral neuroectodermal tumor
Rb Retinoblastoma
SCC Squamous cell carcinoma
SCLC Small cell lung carcinoma
SEER Surveillance, Epidemiology, and End Results
SLN Sentinel lymph node
SRBC Small round blue cell
TILs Tumor-infiltrating lymphocytes
TTF-1 Thyroid transcription factor 1
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer Science+Business Media, LLC
About this chapter
Cite this chapter
Prieto-Granada, C.N., Messina, J.L. (2016). Pathology of Merkel Cell Carcinoma (Primary Neuroendocrine Carcinoma of the Skin). In: Nasir, A., Coppola, D. (eds) Neuroendocrine Tumors: Review of Pathology, Molecular and Therapeutic Advances. Springer, New York, NY. https://doi.org/10.1007/978-1-4939-3426-3_21
Download citation
DOI: https://doi.org/10.1007/978-1-4939-3426-3_21
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4939-3424-9
Online ISBN: 978-1-4939-3426-3
eBook Packages: MedicineMedicine (R0)