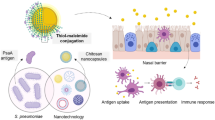
Abstract
Several evidences converge on the idea that among the mucosal administration routes, the nasal mucosa is the most attractive site for the delivery of vaccines. Mucoadhesive particulate adjuvants should be able to increase the residence time of antigens in nasal cavity in order to increase their probability of being taken up by nasopharynx-associated lymphoid tissue (NALT) cells and subsequently to initiate the innate and adaptive immune response. Focusing on chitosan, a mucoadhesive biopolymer, we describe in this chapter a method to prepare antigen loaded chitosan nanoparticles and a second method to prepare antigen loaded poly-ε-caprolactone/chitosan nanoparticles. Additionally the methodology for the assessment of mucoadhesivity of the delivery system is also described. The two critical procedures in mice intranasal immunization experiments include challenges in the intranasal administration itself due to the small mouse nose, and the other is related with the collection of mucosal secretions to assess the sIgA. The techniques are difficult to perform without advanced training. Therefore, protocols followed in our laboratory, as well as some tips, are described in this chapter.
Access provided by CONRICYT – Journals CONACYT. Download protocol PDF
Similar content being viewed by others
Keywords
1 Introduction
Mucosal surfaces, such as gastrointestinal tract, nasal and vaginal tract are the main entrance of some of the pathogenic microorganisms in the host. The easiest and most effective way to induce mucosal immune response in a particular tract, where secretory IgA (sIgA) is the main player, is to administer the vaccine through the same mucosal surface. Despite the existence of the common mucosal immune system, sIgA can be detected simultaneously in diverse mucosae. In particular, intranasal vaccination, in addition to the induction of antigen-specific serum IgG, normally induces good antigen-dose dependent sIgA titers, not only on nasal mucosa, but also on pulmonary and vaginal mucosae [1]. This feature constitutes an important advantage when compared with injectable vaccines. Other benefits of intranasal vaccine formulations include self-administrable opportunity and non-necessity of having sterile vaccine formulations. Nevertheless, the success of intranasal administration of vaccines is dependent on having good mucosal adjuvants, those that are able to activate innate immune system and a formulation that minimizes the effect of the mucociliary clearance and enzymatic degradation of antigens.
1.1 Design of Polymeric Delivery Systems
Among diverse strategies, mucoadhesive/biodegradable polymers are considered promising candidates to prepare microparticles or nanoparticles as antigen delivery systems.
1.1.1 Chitosan Nanoparticles
In particular, significant results have been obtained with chitosan, a mucoadhesive biodegradable polymer, recognized as a good adjuvant for mucosal surfaces. Despite the great effort made by adjuvant research groups, the mechanism of adjuvanticity is currently not completely understood. The diverse and sometimes opposite results produced do not allow drawing final conclusions since chitosan used in these studies may have different characteristics, like the molecular weight (MW) and deacetylation degree (DD). In fact, chitosan is a generic name for a wide family of biopolymers based on randomly distributed β-(1-4)-linked N-acetyl-d-glucosamine and d-glucosamine obtained by deacetylation of chitin from diverse sources like exoskeleton of crabs, shrimp, and fungi. One of the problems during generation of chitosan includes poor polymer characterization and possible contamination of the chitosan with other compounds, including lipopolysaccharides (LPS) [2]. In our laboratory we found that our chitosan batch (see Subheading 2) either, in solution or nanoparticle form, after a purification process (negative to LAL test), was able, in a dose-dependent manner and in a presence of CpGODN, to induce the production of IL-1β by mice bone marrow derived dendritic cells (BMDC) by a NLRP3 inflammasome activation dependent mechanism and, contrary to other published reports, in the concentrations tested in our laboratory, do not increase the production of TNF-α by same cells. We also found that chitosan nanoparticles are able to induce the production of β-hexosaminidase [3] by mast cells (HMC-1 cell line) which is a signal of mast cell activation. Finally, robust evidences obtained in our laboratory support the conclusion that chitosan nanoparticle-based formulations perform equally (subcutaneous) or even better (intranasal) than well know adjuvants like alum, present in commercial vaccines.
There are several methods to prepare chitosan particles. There is substantial literature available to generate chitosan particles published since the last 20 years. Among them, ionic gelation technique has attracted considerable attention. The method is based on the property that in acidic medium, primary amine groups of chitosan become protonated and easily interact with small anionic molecules, such as tripolyphosphate (TPP), citrate or sulfate ions. The nanohydrogels obtained by this method result from inter and intra-crosslink ionic interactions. The advantage of the ionic gelation technique is that it is a simple and fast method, easily controllable and organic solvent free. Therefore, the method is adequate to encapsulate biomolecules like proteins or DNA which could be destroyed by organic solvents or by strong shear stress, two conditions used in several particle preparation methods. In opposition to the use of chemical cross-linking agents like glutaraldehyde, that confers a certain toxicity degree to the particles, the physical cross-linking method with the polyanion produce particles with less cytotoxicity. However, the physical stability of chitosan/polyanion particles is poor and thus particles are normally prepared immediately before to be used in both, in vitro and in vivo assays. Otherwise, the use of chemical crosslinkers or the design of polymeric nanoparticles with more than one polymer, in addition to display particle additional properties, has been described as an attempt to obtain better particle stability. For example, the association of chitosan to poly-ε-caprolactone nanoparticles, theoretically, confers to these particles the properties of chitosan, like mucoadhesivity and immunostimulatory effects and inherits from the pure PCL particles their hydrophobic nature which can be advantageous to adsorb on their surface certain biomolecules like therapeutic proteins, peptides or antigens.
1.1.2 Poly-ε-caprolactone/Chitosan Nanoparticles
Poly-ε-caprolactone (PCL) is a biocompatible polyester that is widely used in drug delivery applications. It is a highly hydrophobic crystalline polymer that degrades very slowly in the absence of enzymes (in vitro) and presents a low cytotoxicity profile [4]. Different methods have been reported in the literature for the preparation of drug entrapped PCL nanoparticles. Among them, interfacial polymer disposition method is a simple and fast procedure. In this method the polymer is first dissolved in an organic solvent, usually acetone and then poured with stirring into water containing the surfactant. To perform blend nanoparticles a second polymer can be dissolved either in organic solvent or in water. In this chapter we use the chitosan dissolved in diluted acetic acid with the surfactant.
1.2 Antigen Loading
Depending on the preparation method, antigens can either be entrapped in the polymer matrix or bound to particle surface by adsorption (Fig. 1). In the first case the incorporation of the antigens into the particle matrix is performed during particle preparation and it implies that high shear forces or organic solvents, conditions that could decrease the bioactivity of the antigens, are not part of the preparation method. The chitosan nanoparticle preparation methods satisfy with this indication but the chitosan/PCL nanoparticle methods do not. Whereas, the adsorption of the antigens to preformed nanoparticles is simple, normally made with a gentle agitation of the particle suspension containing the antigen previously solubilized in water or in a buffer. The success to obtain antigen adsorption depends on antigen-nanoparticle interaction. Particularly, electrostatic interactions, hydrophobic interaction, and specific chemical interaction between the protein and the nanoparticle play important roles [5]. In this chapter, two kinds of nanoparticles are described and the interactions between protein (antigens) and nanoparticles are different since polymer-based nanoparticles, chitosan and poly-ε-caprolactone/chitosan have different degrees of hydrophobicity. Chitosan is a hydrophilic polymer and the interactions between chitosan nanoparticles and the antigens are mainly electrostatic. Therefore, in the present case, isoelectric point (IEP) of the protein, ionic strength and pH of the buffer are extremely important in order to obtain a high adsorption efficacy. By contrast, the same factors are less important in the case of poly-ε-caprolactone/chitosan nanoparticles since protein–nanoparticle interaction is predominantly hydrophobic.
Schematic representation of antigen location at the two described nanoparticle types. (1) Encapsulation of the antigen—antigen is distributed in the chitosan matrix. (2) Adsorption of the antigen—antigen is located on the surface of chitosan particles. (3) Adsorption of the antigen—antigen is located on the surface of blend (PCL/chitosan) nanoparticles
1.3 Mucoadhesivity Assessment
Mucoadhesive particulate adjuvants should be able to increase the residence time of antigens in nasal cavity. This feature would increase antigen loaded particle probability of being taken up by NALT cells and most probably the intensity and quality of the immune response. Therefore the nanoparticle mucoadhesivity is an important quality attribute that can be evaluated. The method described in this chapter is slightly modified compared to the references [6–9]. Briefly, mucin is placed in contact with particles. In subsequent step, particle suspension is centrifuged and the free mucin assessed using periodic acid:Schiff (PAS) colorimetric method.
1.4 Nasal Administration
Taking into account the relative small size of the mouse nose, the volumes administered should range between 4 μL and 10 μL per nostril. Large volumes may not be technically easy to administer without any formulation pass to the stomach or to the lungs. A second critical point to consider during the administration is using a good mouse restrainer to immobilize the head of the mouse to place the formulation in the nostrils. These steps are critical and would influence the results.
1.5 Biological Sampling
The evaluation of the immune response generated by vaccine formulation is performed throughout the experiment. Blood samples and mucosal secretions are collected periodically and analyzed. Exceptions are nasal secretion and spleen cells which are only collected at the end of the experiment. Although IgA constitutes only 10 % to 15 % of the total serum immunoglobulin, it is the predominant immunoglobulin class in external secretions such as from the nasopharynx, genitourinary and digestive tracts. Serum immunoglobulin transudation into mucosal associated lymphoid tissues has been investigated. This feature has been associated with serum IgG, however serum IgA seems to be able to experience same phenomenon too, although to a lesser extent [10].
2 Materials
2.1 Preparation of Polymeric Delivery Systems
2.1.1 Chitosan Nanoparticles
-
1.
ChitoClear™: chitosan with 95 % DD and 8 mPa s viscosity (e.g., Primex Biochemicals AS, Avaldsnes, Norway) (seeNote1).
-
2.
Sodium acetate buffer solution (AcB); 25 mM; pH 5.0: Weigh 1.36 g of sodium acetate anhydrous. Add deionized water to a final volume of 1 L. Adjust the pH to 5.0 with 1 M acetic acid solution.
-
3.
0.625 % (w/v) sodium sulfate aqueous solution.
-
4.
15 mL centrifuge tubes.
-
5.
Vortex (e.g., Vortex Mixer, Labnet).
-
6.
Centrifuge (e.g., Sigma 3K15, Rotor 11133).
2.1.2 PCL/Chitosan Nanoparticles
-
1.
ChitoClear™: chitosan with 95 % DD and 8 mPa s viscosity (e.g., Primex Biochemicals AS, Avaldsnes, Norway) (seeNote1).
-
2.
PCL (average MW 14,000) (e.g., Sigma-Aldrich Corporation, St Louis, MO, USA).
-
3.
Aqueous solution of 1 % acetic acid (v/v) with 5 % Tween 80™ (w/v).
-
4.
Acetone (Analytical grade).
-
5.
Beaker stand.
-
6.
50 mL beaker.
-
7.
High speed homogenizer with a 7 mm probe.
-
8.
Magnetic stirrer and magnetic stir bar.
-
9.
Glycerol (Analytical grade).
-
10.
Beckman J-26 XPI centrifuge with JA 25.50 rotor and 50 ml centrifuge tubes (e.g., Oak Ridge, Nalgene®, 50 ml PPCO tubes with PP screw closure).
-
11.
Disposable Pasteur pipette.
-
12.
2 L beaker and dialysis clamps.
-
13.
Spectra/Por® cellulose ester dialysis membrane, MWCO 300,000 (Spectrum Laboratories, Inc., CA, USA).
2.2 Antigen Loading
-
1.
Recombinant antigen (e.g., recombinant hepatitis B surface antigen (rHBsAg) adw, Aldevron or other recombinant proteins).
-
2.
Sodium acetate buffer solution (AcB); 25 mM; pH 5.0: Weigh 1.36 g of sodium acetate anhydrous. Add deionized water to a final volume of 1 L. Adjust the pH to 5.0 with 1 M acetic acid solution.
2.3 Mucoadhesivity Assessment
-
1.
Mucin from porcine stomach Type III (e.g., Sigma-Aldrich Corporation, St Louis, MO, USA).
-
2.
Schiff reagent: Add 500 mg of basic fuchsin (Pararosaniline) to 80 mL of water and heat until dissolution (≈80 °C). When temperature decreases to 50 °C add 10 mL of HCl (1 M). Make up to 100 mL with deionized water and let it cool to room temperature. Add 0.1 g of sodium metabisulfite to every 6 mL of the previous solution incubated at 37 °C until the Schiff reagent becomes pale yellow (several hours).
-
3.
Periodic acid reagent: Add 0.14 mL of 50 % (w/v) periodic acid solution to 10 mL of 7 % (v/v) acetic acid solution.
-
4.
UV–visible spectrometer.
-
5.
Disposable plastic cuvettes.
2.4 Nasal Administration
-
1.
Isoflurane.
-
2.
Low volume micropipette.
-
3.
Mice (seeNote2).
2.5 Biological Sampling
-
1.
Anesthetic saturation chamber.
-
2.
Isoflurane.
-
3.
Goldenrod animal lancets (e.g., 5 mm point length for 2–6 month old mice).
-
4.
1.5 mL centrifuge tubes.
-
5.
Centrifuge (e.g., Sigma 3K15, rotor 12154H).
-
6.
Phosphate Buffer Saline (PBS): add 8 g of sodium chloride, 1.44 g of sodium phosphate dibasic, 0.24 g of potassium dihydrogen phosphate and 0.2 g of potassium chloride to 1 L of deionized water. Mix and adjust the pH to 7.4 with HCl or with NaOH. Sterilize by autoclave. Store at 4 °C.
-
7.
100 mM phenylmethanesulfonyl fluoride (PMSF) stock solution: add 87.1 mg of PMSF to 5 mL of absolute ethanol. Stable between 2 and 8 °C for at least 9 months.
-
8.
1 % sodium azide stock solution: add 0.01 g of sodium azide to 1 mL of deionized water. Store at 4 °C.
-
9.
Mice (seeNote2).
3 Methods
3.1 Preparation of Polymeric Delivery Systems (Fig. 2)
Schematic overview of the experimental setup used to produce nanoparticles and outcome. (a1) Chitosan nanoparticles. (a2) Transmission electron microscopy (TEM) photo of freeze-dried chitosan nanoparticles after resuspension in water. (b1) PCL/chitosan nanoparticles. (b2) TEM photo of freeze-dried PCL/chitosan nanoparticles after resuspension in water
3.1.1 Chitosan Nanoparticles
-
1.
Dissolve chitosan in AcB (pH 5.0) to a final concentration of 0.1 % (w/v) (seeNote3).
-
2.
Place 2 mL of chitosan in AcB solution in a 15 mL centrifuge tube.
-
3.
Under high speed vortexing, add dropwise 2 mL of the sodium sulfate solution to the same tube.
-
4.
Let the nanoparticles formed maturate 1 h at room temperature (seeNote4).
-
5.
Centrifuge 25 min at 4500 × g.
-
6.
Discard the supernatant.
-
7.
Resuspend the resultant pellet in 2 mL of deionized water.
-
8.
Repeat steps 5 and 6 to completely remove exceeding compounds.
-
9.
Resuspend in the desired volume of deionized water or other suitable buffer for further steps (seeNote5).
-
10.
After nanoparticle preparation some parameters should be monitored to guarantee successful execution and reproducibility (seeNote6).
3.1.2 PCL/Chitosan Nanoparticles
-
1.
Prepare a 0.2 % (w/v) PCL solution in acetone (seeNote7).
-
2.
Prepare a 0.1 % (w/v) chitosan solution in diluted acetic acid (with Tween) (seeNote8).
-
3.
Place the high speed homogenizer probe into a beaker containing 13.5 mL of chitosan aqueous solution.
-
4.
Start the high speed homogenization and add 4.5 mL PCL solution dropwise. Keep the homogenization for 1 min more after complete PCL solution addition.
-
5.
Remove the suspension from the beaker holder and introduce a magnetic stirrer bar for additional 45 min magnetic stirring.
-
6.
To concentrate the delivery system, slowly add the resulting 18 mL nanoparticle suspension to a centrifuge tube with a 200 μL glycerol bed. Centrifuge at 16,000 × g for 75 min at 4 °C.
-
7.
Carefully remove the tube from the centrifuge rotor and with a Pasteur pipette aspirate the supernatant without disturbing the high concentrated nanoparticle layer on the bottom of the tube.
-
8.
To remove the glycerol and other remaining constituents from the nanoparticle concentrate perform a 48 h dialysis against water. Follow the dialysis membrane manufacturer washing instructions before filling it with the suspension. Fill the dialysis membrane, clamp the both ends, and place it in a 2 L water beaker under magnetic stirring. Change the dialysis water twice during the 48 h.
-
9.
Considering the final application dilute the suspension with deionized water or other suitable buffer, or concentrate the particles by centrifuging at 21,000 × g for 15 min.
-
10.
After nanoparticle preparation some parameters should be monitored to guarantee successful execution and reproducibility (seeNotes5 and 6).
3.2 Antigen Loading
3.2.1 Encapsulation—Chitosan Nanoparticles
-
1.
Perform the method described in Subheading 3.1 with a slight modification. Add the desired amount of the recombinant antigen to the sodium sulfate solution (e.g., considering a yield of 3.2 mg chitosan nanoparticles per batch, the encapsulation of 80 μg of the hepatitis B surface antigen (HBsAg) would allow a vnasal administration of eight mice that corresponds to 10 μg HBsAg loaded into 400 μg of nanoparticles per animal).
-
2.
Use the supernatant discarded in step 6 described in Subheading 3.1—chitosan nanoparticles, to determine the antigen loading efficacy of the recombinant antigen used (seeNotes9 and 10).
-
3.
After washes resuspend in AcB for further nasal administration (e.g., 120 μL for eight animals, 15 μL per animal) (seeNote11).
3.2.2 Adsorption—Chitosan and PCL/Chitosan Nanoparticles
-
1.
Perform either methods described in Subheading 3.1—chitosan nanoparticles or—PCL/chitosan nanoparticles, depending of which delivery system you wish to adsorb the recombinant antigen and start the adsorption having the nanoparticles concentrated in pellet.
-
2.
Add recombinant antigen solution (AcB pH 5.0 or other) to the nanoparticle pellet at room temperature and incubate (e.g., in a rotor mixer) for 30 min (e.g., for chitosan nanoparticles, considering a pellet of 3.2 mg add 80 μg of HBsAg suspended in 120 μL AcB for a total of 8 animals: 15 μL per animal containing 400 μg of nanoparticles and 10 μg of HBsAg; for PCL/chitosan nanoparticles considering a pellet of 10 mg, in order to immunize eight animals each with 400 μg nanoparticles and 10 μg HBsAg, the pellet can be resuspended in 100 μL AcB, use 32 μL of the resulting suspension and mix with 80 μg HBsAg in 88 μL AcB) (seeNotes5 and 10–12).
3.3 Mucoadhesivity Assessment
-
1.
Incubate 1 mg of nanoparticles with mucin at several different concentrations (50–400 μg/mL) to a final volume of 1 mL (samples).
-
2.
Prepare 1 mL mucin standards in water (from 0 to 250 μg/mL).
-
3.
Incubate the samples and the standards under agitation (e.g., rotational) at room temperature for 60 min.
-
4.
Centrifuge the samples and the standards at 21,460 × g for 15 min and collect 900 μL of each supernatant.
-
5.
Add 90 μL of the periodic acid to each 900 μL of supernatant and incubate for 75 min at 37 °C.
-
6.
Add 90 μL of Schiff reagent and incubate for 30 min at room temperature.
-
7.
Transfer the solution to a disposable plastic cuvette and read the optical density (OD) at 550 nm (seeNote13).
-
8.
Built a calibration curve with the ODs obtained for mucin standards vs standard mucin concentrations.
-
9.
Calculate the free mucin present in the supernatants interpolating the ODs obtained for samples in the equation obtained in 8.
-
10.
Calculate the mucin adsorbed onto the nanoparticles surface by subtracting the amount of free mucin from the total mucin content in the test mixture.
-
11.
Interpret data using the following equations which describe adsorption isotherms:
Freudlich equation
Langmuir equation
where x/m is mucin adsorbed, K and n are Freudlich isotherms constants, a and b are Langmuir isotherms constants, and Ce is the free mucin (seeNote14).
3.4 Nasal Administration
-
1.
Restrain the mouse using double hand method and ensure the mouse is well immobilized (seeNote15).
-
2.
With a low volume pre-calibrated micropipette place a drop of the formulation into the mouse nostril and wait for it to be dry. Repeat the procedure alternating from one nostril to the other until the total of 15 μL is administered (seeNote16).
3.5 Biological Sampling
3.5.1 Serum
-
1.
Place the mouse in a saturated isoflurane chamber until it loses responsiveness to manipulations and rear foot reflexes.
-
2.
Collect blood from the mandibular vein by venipuncture with an animal lancet to a 1.5 mL microcentrifuge tube (seeNote17).
-
3.
Let the blood coagulate over 6 h.
-
4.
Centrifuge for 10 min at 4500 × g.
-
5.
Carefully transfer the serum (supernatant) to another tube (do not aspirate the bottom erythrocytes).
-
6.
Store at −20 °C until further analysis (seeNote18).
3.5.2 Vaginal Washes
-
1.
Restrain the mouse using one hand method.
-
2.
Collect vaginal mucosa using a micropipette by flushing in and out the vagina surface with 100 μL of ice-cold PBS (e.g., 10 flushes per wash) to a 1.5 mL centrifuge tube.
-
3.
Add 1 μL of sodium azide and 1 μL of PMSF stock solutions to each 100 μL of collected vaginal wash (seeNote19).
-
4.
Incubate at room temperature for 15 min.
-
5.
Centrifuge for 15 min at 3300 × g.
-
6.
Collect the supernatant and store at −80 °C until further analysis (seeNotes20 and 21).
3.5.3 Nasal Washes (Fig. 3)
Representative photographs of the nasal wash technique performed in order to evaluate mucosal immune response in mice nasal mucosa (described in Subheading 3.5). Steps leading to the collection of the nasal wash are illustrated. (1) After the animal euthanasia the jaw is carefully removed by cutting the mouth sideways until the trachea shows up accessible without disruption. The separated jaw and tongue are transversally cut for better approach. (2) Blood present in the oral cavity is cleaned using PBS and medical compresses or absorbent paper. (3) A small hole in the trachea is done with a 19 G needle in order to further insert a mouse oral gavage needle; (4) The mice is positioned vertically to the collection tube, while the mouse oral gavage needle is inserted cautiously in the animal trachea. PBS is then flushed through the needle, passing the mice nasal cavity and collected at nostrils into the collection tube
-
1.
Place the mouse in a saturated isoflurane chamber until it loses responsiveness to manipulations and rear foot reflexes.
-
2.
Euthanize the mice by cervical dislocation.
-
3.
Carefully remove the jaw, cutting the mouth sideways until the trachea shows up accessible without disruption.
-
4.
Clean the open cavity with some cold PBS and soak it with medical compresses (seeNote22).
-
5.
Use a 19 G needle to make a hole in the trachea.
-
6.
Insert a mouse oral gavage needle in the hole previously made.
-
7.
Flush 200 μL of PBS into the trachea collecting the outcoming wash fluid through the nose to a 1.5 mL centrifuge tube.
-
8.
Add 2 μL of sodium azide and 2 μL of PMSF stock solutions to each 200 μL nasal wash.
-
9.
Incubate at room temperature for 15 min.
-
10.
Centrifuge for 20 min at 15,700 × g.
-
11.
Collect the supernatant and store at −80 °C until further analysis (seeNotes20 and 21).
4 Notes
-
1.
The viscosity of 1 % chitosan in 1 % acetic acid solution was measured by the supplier. The value of 8 mPa s corresponds to a low molecular weight (LMW) chitosan. Chitosan from other sources can be used; however, make sure to follow the exact protocol described in this chapter and the characteristics (DD and LMW) of chitosan have to be similar. Slight differences in properties of chitosan by different manufacturers may be the reason that it is difficult to reproduce the methods involving chitosan [11–13]. Besides commercial differences, chitosan may be further purified to remove contaminants and endotoxins by a technique already described by us [14]. Nevertheless, after this process it is important to evaluate the resulting polymer in order to verify if its characteristics were altered.
-
2.
If you intend to evaluate vaginal mucosal immunity following a nasal administration of a recombinant antigen you need do acquire female mice. C57BL/6J and BALB/c are animal models widely used for immunology studies.
-
3.
Chitosan suffers from a poor solubility in water or in organic solvents. Charge density depends on the degree of acetylation and pH [12]. Chitosan is readily soluble in dilute acidic solutions below pH 6.0 because it possesses primary amino groups with a pKa value of 6.3 [15], which is the case of AcB (pH 5.0) used in the present work. Chitosan takes more than 4 h to solubilize by magnetic stirring at room temperature.
-
4.
Laboratory temperature should be carefully monitored during the preparation of the nanoparticles for reproducibility. For temperatures higher than 20 °C the particles normally aggregate.
-
5.
In this phase of the preparation process, nanoparticle yield can be estimated. To calculate the mass of nanoparticles obtained, do not resuspend the particles in buffer and instead the pellet can be freeze-dry and weighed. Nanoparticle yield is an important data to predict the number of batches that could be produced for a particular experiment.
-
6.
To validate the preparation method with a new chitosan batch, size and zeta potential of the particles should be measured. Dynamic light scattering and electrophoretic light scattering methods can be used with this aim (e.g., Delsa™ Nano C particle analyzer, at 25 °C and 165° angle). Nanoparticles can be suspended in water or any other suitable buffer according to their final use. Please note that all measurements should be performed at same concentration and in the same buffer for direct comparison between delivery systems, as buffer pH and ionic strength might change nanoparticle surface charge and hydrodynamic size [16] (e.g., zeta potential should be measured in sodium acetate buffer pH 5.0 if the nasal administration will be performed in the same buffer for a more realistic characterization). In fact, for PCL/chitosan nanoparticles zeta potential is highly influenced by the diluent used. When they are suspended in acetate buffer their zeta potential is neutral, but when suspended in water, values around +25 mV are observed.
-
7.
PCL solubility depends on the organic solvent chosen (e.g., high solubility in chloroform, low solubility in acetone, and no solubility in diethyl ether) [17, 18]. To achieve total solubilization in acetone you may warm the solution until 45–50 °C and then place it in a rotor mixer. Make sure to properly seal the tube cap with Parafilm® to prevent evaporation because acetone is a volatile solvent. Use the solution only when temperature is around 20 °C.
-
8.
Take into consideration what was mentioned in Note3. In this situation, to dissolve chitosan in the diluted acetic acid solution you will need approximately 2 h with magnetic stirring.
-
9.
The recombinant antigen loading efficacy is affected by several factors including the recombinant antigen isoelectric point (IEP) and pH of the medium. In case of chitosan nanoparticles we have tested the encapsulation of several model proteins with different isoelectric points and observed that using the AcB (pH 5.0), as the solvent, during particle preparation and antigen encapsulation, proteins with a IEP lower than 5.0 (carrying a strong negative surface charge at pH higher than its IEP) present a higher loading efficacy and a slower release profile, as described by others [19]. On the contrary, at the same loading conditions, recombinant antigens with IEP higher than 5.0 normally present lower loading efficacies. This is explained by the fact that if their isoelectric point is higher than the pH of the encapsulation solution (acetate buffer pH 5.0) the protein is carrying a net positive charge, hindering the interaction with the also positively charged chitosan. For each recombinant antigen, encapsulation conditions (e.g., antigen concentration in sodium sulfate solution) should be optimized. With this aim, a model protein resembling its molecular weight and isoelectric point, can be used due to the high cost of most recombinant antigens. The assessment of the loading efficacy (seeNote10) will help you optimize the protocol so that all antigens added stay encapsulated and nothing is wasted.
-
10.
Recombinant antigen/protein loading efficacy (LE) or loading capacity (LC) is calculated by an indirect way, quantifying the unbound protein remaining in the supernatant. Recombinant antigen/protein concentration can be determined using a BCA protein assay kit for general protein quantification (be aware of possible interferences) or an antigen-specific ELISA (e.g., HBsAg ELISA Kit). The loading percentages are obtained using the following equations:
Loading efficacy
Loading capacity
For recombinant protein encapsulation in chitosan nanoparticles, the unbound protein is measured in the supernatants resulting from step 6 (see Subheading 3.2). For adsorbed recombinant protein in chitosan nanoparticles or in PCL/chitosan nanoparticles, the unbound protein is measured in supernatants obtained by centrifugation of the final formulations for 25 min at 4500 × g, or for 15 min at 21,000 × g, respectively.
-
11.
Particularly for chitosan nanoparticles, highly concentrated suspensions may have an increased viscosity that makes it difficult to use during pipetting and intranasal administration of the formulation.
-
12.
Recombinant antigen adsorption on both chitosan and PCL/chitosan nanoparticles is based on electrostatic interactions and is affected by the same factors referred to in Note9. Regarding PCL/chitosan nanoparticles, hydrophobic interactions between PCL and protein occur and may be the main forces involved. Antigen loading efficacy should be assessed using a model protein resembling its molecular weight and isoelectric point, regarding the high cost of most recombinant antigens. This will help you to choose the adsorption conditions that result in the best loading efficacy and to predict the amount of antigen that will be administered free or bound to the nanoparticles since in this method, unbound antigen should not be separated from the formulation that will be administered to mice.
-
13.
The colorimetric method described here was first developed for glycoprotein quantification by Mantle and Alan [6]. Once the method is based in the oxidation of mucin glycols into aldehydes by periodic acid and further reaction of these groups with Schiff reagent, caution must be taken concerning interferences. When analyzing supernatants for mucin unable to bind the nanoparticles, interferences from nanoparticles may also be present. Therefore it is advisable to perform the same test without mucin in order to evaluate method interferences. In fact, PCL/chitosan nanoparticles blanks supernatants (without mucin) generate high ODs that must be subtracted from the mucin containing sample ODs.
-
14.
In order to better analyze mucoadhesion of polymeric nanoparticles Freudlich and Langmuir isotherms may be calculated. The goodness of the linear regressions fitting obtained are the parameter used to estimate which isotherm better fits each delivery system. Langmuir equation represents a monolayer limited adsorption isotherm for a finite number of sites, while Freudlich equation fits the adsorption to a heterogeneous surface supporting sites with varied affinities [20].
-
15.
If some difficulties are encountered during the immobilization process anesthetizing the animals may be a solution. Place the mice in a saturated isoflurane chamber until it lose responsiveness to manipulation and foot reflexes which may simplify the subsequent nasal administration process. Nevertheless, caution should be made once volatile anesthesia may interfere with some experiments. The advantages and disadvantages of using anesthetics may be considered for specific experiments.
-
16.
Formulation volume to deposit in each mouse nostril should be as smaller as possible. However, issues like high viscosity of very concentrated suspensions or even the inability to obtain higher concentration formulations, implicate the administration of greater volumes (until 10 μL per nostril). Other factors may also be relevant for the administered volume. For PCL/chitosan nanoparticles to present a positive zeta potential when adsorbed with protein, a higher nanoparticles–antigen ratio than 40:1 (as exemplified) should be used, as well as the diluent should be preferably water. This positive zeta potential would be beneficial for the nasal administration, but presents difficulties to obtain. We quadruplicated the amount of nanoparticles for the same amount of antigen (ratio 160:1, in water) and we reached weak positive zeta potentials. As increasing nanoparticle concentration was not viable, diminishing the amount of antigen in this formulation (e.g., 160:0.5 and 160:0.15) resulted in more positive zeta potential values. With this we pretend to exemplify a situation, where if no more concentrated particles may be obtained, a reduction in the antigen ratio may increase the zeta potential and an increased volume may be considered per mouse, to give an optimal antigen dose.
-
17.
In our laboratory we perform a single blood draw, repeated multiple times with 2-week interval to monitor the systemic immune response over time. We usually collect about 2–3 drops (about 150 μL of blood) representing 10 % of circulating blood volume of a 6–8 week old mice, which is the sample volume recommended for this sampling frequency (NIH Guidelines for Survival Bleeding of Mice and Rats).
-
18.
Antigen-specific immunoglobulins (IgG, IgM, IgE, and IgA) are frequently analyzed on serum samples. It is also possible to analyze IgG subtypes to explore the Th1/Th2 immune response profile.
-
19.
Sodium azide can interfere with some ELISA antibody measurements. However, we tested that possibility and we found that in the concentrations used in each sample, it does not interfere using the Mouse IgA ELISA Quantitation Kit (Bethyl Laboratories).
-
20.
Cytokines and interleukins should be ideally stored at −80 °C, but they can also be stored at −20 °C for a maximum of 6 months.
-
21.
Mucosal samples in the case of nasal vaccination are of extreme importance. One of the advantages in nasal immunization is the possibility to induce mucosal antibodies, particularly specific secretory IgA. Nevertheless, sample collection has conditioned reproducibility leading to variability between animals. Instead of only analyzing specific IgA, normalization may be performed with the total IgA present in the sample. For nasal samples 200 μL is enough for both immunoglobulin ELISA measurements, using 80 μL concentrated sample for each. For vaginal washes, a pool of two consecutive sampling days may be a solution to achieve enough volume for analysis.
-
22.
It is important to ensure a blood free cavity (from trachea to nose) for an optimal nasal wash. Results from samples contaminated with blood may be misleading since antibodies detected may be from blood source.
References
Holmgren J, Czerkinsky C (2005) Mucosal immunity and vaccines. Nat Med 11:S45–S53
Vasiliev YM (2015) Chitosan-based vaccine adjuvants: incomplete characterization complicates preclinical and clinical evaluation. Expert Rev Vaccines 14:37–53
Bento D, Staats HF, Goncalves T, Borges O (2015) Development of a novel adjuvanted nasal vaccine: C48/80 associated with chitosan nanoparticles as a path to enhance mucosal immunity. Eur J Pharm Biopharm 93:149–164
Chawla JS, Amiji MM (2002) Biodegradable poly(epsilon -caprolactone) nanoparticles for tumor-targeted delivery of tamoxifen. Int J Pharm 249:127–138
Lynch I, Dawson KA (2008) Protein-nanoparticle interactions. Nanotoday 3:40–47
Mantle M, Allen A (1978) A colorimetric assay for glycoproteins based on the periodic acid/Schiff stain [proceedings]. Biochem Soc Trans 6:607–609
Pawar D, Mangal S, Goswami R, Jaganathan KS (2013) Development and characterization of surface modified PLGA nanoparticles for nasal vaccine delivery: effect of mucoadhesive coating on antigen uptake and immune adjuvant activity. Eur J Pharm Biopharm 85(3 Pt A):550–559
Dhawan S, Singla AK, Sinha VR (2004) Evaluation of mucoadhesive properties of chitosan microspheres prepared by different methods. AAPS PharmSciTech 5(4), e67
Martinac A, Filipovic-Grcic J, Voinovich D, Perissutti B, Franceschinis E (2005) Development and bioadhesive properties of chitosan-ethylcellulose microspheres for nasal delivery. Int J Pharm 291:69–77
Meckelein B, Externest D, Schmidt MA, Frey A (2003) Contribution of serum immunoglobulin transudate to the antibody immune status of murine intestinal secretions: influence of different sampling procedures. Clin Diagn Lab Immunol 10:831–834
Sonia TA, Sharma CP (2011) Chitosan and its derivatives for drug delivery perspective. Adv Polym Sci 243:23–53
Rinaudo M (2006) Chitin and chitosan: properties and applications. Prog Polym Sci 31:603–632
Dutta PK, Dutta J, Tripathi VS (2004) Chitin and chitosan: chemistry, properties and applications. J Sci Ind Res 63:20–31
Jesus S, Borchard G, Borges O (2013) Freeze dried chitosan/poly-e-caprolactone and poly-e-caprolactone nanoparticles: evaluation of their potential as DNA and antigen delivery systems. J Genet Syndr Gene Ther 4:164
Pillai CKS, Paul W, Sharma CP (2009) Chitin and chitosan polymers: chemistry, solubility and fiber formation. Prog Polym Sci 34:641–678
Jiang J, Oberdörster G, Biswas P (2009) Characterization of size, surface charge, and agglomeration state of nanoparticle dispersions for toxicological studies. J Nanoparticle Res 11:77–89
Sinha VR, Bansal K, Kaushik R, Kumria R, Trehan A (2004) Poly-epsilon-caprolactone microspheres and nanospheres: an overview. Int J Pharm 278:1–23
Labet M, Thielemans W (2009) Synthesis of polycaprolactone: a review. Chem Soc Rev 38:3484–3504
Koppolu BP et al (2014) Controlling chitosan-based encapsulation for protein and vaccine delivery. Biomaterials 35:4382–4389
Chuah LH, Billa N, Roberts CJ, Burley JC, Manickam S (2013) Curcumin-containing chitosan nanoparticles as a potential mucoadhesive delivery system to the colon. Pharm Dev Technol 18:591–599
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer Science+Business Media New York
About this protocol
Cite this protocol
Jesus, S., Soares, E., Borges, O. (2016). Poly-ε-caprolactone/Chitosan and Chitosan Particles: Two Recombinant Antigen Delivery Systems for Intranasal Vaccination. In: Thomas, S. (eds) Vaccine Design. Methods in Molecular Biology, vol 1404. Humana, New York, NY. https://doi.org/10.1007/978-1-4939-3389-1_45
Download citation
DOI: https://doi.org/10.1007/978-1-4939-3389-1_45
Published:
Publisher Name: Humana, New York, NY
Print ISBN: 978-1-4939-3388-4
Online ISBN: 978-1-4939-3389-1
eBook Packages: Springer Protocols