Abstract
Ca2+ is a secondary messenger involved in early signaling events triggered in response to a plethora of biotic and abiotic stimuli. In plants, environmental cues that induce cytosolic Ca2+ elevation include touch, reactive oxygen species, cold shock, and salt or osmotic stress. Furthermore, Ca2+ signaling has been implicated in early stages of plant–microbe interactions of both symbiotic and antagonistic nature. A long-standing hypothesis is that there is information encoded in the Ca2+ signals (so-called Ca2+ signatures) to enable plants to differentiate between these stimuli and to trigger the appropriate cellular response. Qualitative and quantitative measurements of Ca2+ signals are therefore needed to dissect the responses of plants to their environment. Luminescence produced by the Ca2+ probe aequorin upon Ca2+ binding is a widely used method for the detection of Ca2+ transients and other changes in Ca2+ concentrations in cells or organelles of plant cells. In this chapter, using microbe-associated molecular patterns (MAMPs), such as the bacterial-derived flg22 or elf18 peptides as stimuli, a protocol for the quantitative measurements of Ca2+ fluxes in apoaequorin-expressing seedlings of Arabidopsis thaliana in 96-well format is described.
Justin Lee’s Researcher ID: B-6096-2012.
Access provided by CONRICYT – Journals CONACYT. Download protocol PDF
Similar content being viewed by others
Key words
1 Introduction
Ca2+ fluxes are elements in the signal transduction of many stimuli that are perceived by plant cells. These biotic or abiotic signals may be of endogenous or exogenous origin. For instance, Ca2+ fluxes are induced by bacterial and fungal microbe-associated molecular patterns (e.g., flagellin, elongation factor-Tu, peptidoglycan, chitin), by plant peptide hormones and by symbiosis signals. Similarly, abiotic stimuli such as osmotic stress , salinity, oxidative stress, and mechanical cues trigger Ca2+ fluxes [1–6]. Ca2+ signals are decoded by the cells in which they are produced, but may also be involved in the transduction of systemic signals throughout the plant as for example in response to changes in salinity or herbivore attacks [7, 8]. These manifold functions and important roles place Ca2+ signaling in the centre of attention when studying the responses of plants to their environment.
A variety of probes can be employed to examine changes in intracellular Ca2+ concentrations. One of the most widely employed is aequorin , the luciferase from Aequorea victoria, which can be heterologously expressed in plants [9, 4]. Aequorin contains three EF-hand motifs that bind Ca2+ ions with high affinity, leading to conformational changes, which result in the oxidation and subsequent decarboxylation of the prosthetic group coelenterazine (CTZ). The resulting coelenteramide is present in an excited state and will emit light at a wavelength of 468 nm when relaxing to the ground state. In order to reconstitute the holoprotein, CTZ needs to be added to plants expressing apoaequorin. Due to its hydrophobic nature, it will freely diffuse into cells and bind to the apoprotein. Differently mutagenized versions of aequorin and chemical derivatives of coelenterazine are available, enabling researchers to probe Ca2+ fluxes spanning five orders of magnitude. However, not all CTZ-aequorin combinations are suitable and absolute changes in Ca2+ concentrations can only be calculated when the combination of native CTZ and native aequorin is used in experiments, due to the lack of standard curves for the other combinations [10]. Therefore, this protocol is restricted to the measurements performed with the original pMAQ2 lines engineered by Knight et al. [9] in conjunction with native CTZ.
In the present protocol, whole seedlings are used for measurements. Readers are advised that the aequorin measurements presented in this protocol do not permit spatial resolution but report a “global” Ca2+ response from the whole plant. Nevertheless, measurements performed in this way are sensitive to detect even small differences, as demonstrated by reactive oxygen species feedback to Ca2+ signaling [11] and is amenable to high throughput measurements in 96-well or even 384-well plate format [12]. Thus, aequorin -dependent Ca2+ transient measurement of whole seedlings is a powerful tool for the analysis of plants’ reactions to environmental stimuli, such as MAMPs.
2 Materials
2.1 Plant Material
Seeds from A. thaliana pMAQ2 plants (Col-0 background, cytoplasmatic aequorin under control of CaMV 35S promoter) [9] or other lines expressing aequorin [13].
2.2 Seed Sterilization Components
-
1.
Cell culture plates for plant culture: e.g., 24-well suspension culture plates (Greiner Bio-One, Frickenhausen, Germany).
-
2.
Gas-permeable plate sealing membranes 80 × 140 mm: e.g., BREATHseal® (Greiner Bio-One) or breathe-EASIER® (Diversified Biotech, Dedham, Massachusetts, USA).
-
3.
Filter paper (thin).
-
4.
Plastic racks for cell culture plates.
-
5.
Vacuum desiccator with socket, approximately 18.5 L volume, with fitting ceramic plate (Fig. 1).
Fig. 1 Seed sterilization with chlorine gas. (a) Setup for gas-phase seed sterilization. (b) Air inlet/outlet for the desiccator can be fitted with any standard 0.2 μm filter to maintain sterility. Note this may be integrated in the covers of smaller desiccator (as shown here) or in the side of larger desiccators (as shown in a). (c) Placement of beaker in the bottom of the desiccator. Note position of outlet tubing next to beaker. (d) Pipetting of sodium hypochlorite solution with the ceramic plate removed. (e) Pipetting of HCl (into the beaker below) through central opening of ceramic plate
-
6.
Two gas-washing bottles, 1 L volume (Fig. 1).
-
7.
Rubber tubing.
-
8.
Vacuum pump.
-
9.
Sodium hypochlorite solution (with 12 % available chlorine).
-
10.
Hydrochloric acid, fuming, 37 %.
2.3 Plant Culture Components
-
1.
Half-strength Murashige and Skoog (MS) medium, 0.25 % (w/v) sucrose: Weigh in 2.207 g of MS medium salt mixture (e.g., from Duchefa Biochemie, Haarlem, The Netherlands), 2.5 g of sucrose, and 0.1952 g of MES and dissolve in 900 mL ultrapure water. Adjust to pH 5.7 with potassium hydroxide (KOH). Adjust volume to 1 L and sterilize medium by autoclaving.
-
2.
Gas-permeable adhesive tape: e.g., Leukopor® (BSN medical, catalog number 0248200).
2.4 Seedling Transfer and Reconstitution Components
-
1.
Ultrapure water.
-
2.
Pipetting reservoir.
-
3.
Cushioned forceps (see Note 1 ) (Fig. 2a).
Fig. 2 Plant culture and seedling transfer materials. (a) Cushioned forceps. Note plastic pipette tips fitted on tips of forceps to reduce damage when handling delicate seedlings. (b) 24-well cell culture plate sealed with gas-permeable sealing membrane. (c) 24-well culture plate with germinating seedlings. Note gas-permeable tape was used to seal and reduce microbial contamination. (d) 96-well microplate with transferred seedlings
-
4.
200 μL pipette tips.
-
5.
96-well microplates for luminescence measurements (see Note 2 ).
-
6.
Coelenterazine stock solution: CTZ stocks are prepared by dissolving solid coelenterazine in methanol to achieve a 10 mM stock solution. Different CTZ variants are available from Biosynth, PJK, Invitrogen, or Sigma (e.g., native CTZ, PJK GmbH, Kleinblittersdorf, Germany; CTZ-h, Biosynth, Staad, Switzerland). Store CTZ stock solutions in opaque (light protection) vessels at −20 °C for short time storage or −80 °C for long time storage.
2.5 Ca2+ Measurement Components
-
1.
MAMP stock solutions: AtPep-1, flg-22, and elf-18 synthetic peptides can be stored as 1 mM stock solutions in ultrapure water. Chitin from ground shrimp shells (Sigma-Aldrich, catalog number C9752) can be stored as 20 mg/mL in ultrapure water. All elicitor stock solutions should be stored as aliquots at −20 °C in vessels with low protein binding characteristics (e.g., Protein LoBind tubes®, Eppendorf, Hamburg, Germany). Prepare 3× concentrated working solutions from these stocks in ultrapure water immediately before use.
-
2.
PCR 12-tube strips.
-
3.
Discharge solution (2 M CaCl2, 20 % (v/v) ethanol): Weigh in 294.04 g of CaCl2 × 2H2O and dissolve in 700 mL ultrapure water. Make up volume to 800 mL with ultrapure water and add 200 mL 100 % ethanol (p.a.).
-
4.
Luminometer microplate reader with automatic reagent dispenser: e.g., Luminoskan® Ascent, Varioskan® Flash multimode reader (Thermo Fisher Scientific, Waltham, Massachusetts, USA), GloMax®-96 microplate Luminometer (Promega, Madison, Wisconsin, USA).
3 Methods
3.1 Seed Sterilization with Chlorine Gas
-
1.
Under a fume hood, prepare the following setup (Fig. 1a): Connect the vacuum desiccator to two gas-washing bottles each filled with 800 mL of water and connect the whole setup to a vacuum pump. The outlet of the desiccator should be fashioned in a way so that chlorine gas with a higher density compared to air may be removed from the desiccator; i.e., a rubber tube should be fixed to the outlet that reaches to the bottom of the desiccator. This is to promote complete removal of chlorine gas with the pump. Furthermore, the influx of fresh air to the desiccator has to be enabled, so that there is no formation of vacuum while pumping out the chlorine gas (see Note 3 and Fig. 1a, b).
-
2.
Remove the lids of 24-well cell culture plates under sterile conditions under a laminar flow bench. The lids should be stored in the bench or any other sterile environment until ready to be reused in Subheading 3.2 below.
-
3.
Load 15–20 seeds per well: Place some seeds on thin filter paper and slightly tap paper to load the seeds into the wells. Take care to avoid carryover of plant debris, as this will hamper sterilization and allow fungal/bacterial growth during liquid culture (see Note 4 ).
-
4.
Seal plates with gas-permeable sealing membranes, load plates to racks.
-
5.
Put a small beaker (at least 70 mL volume) into the bottom of the desiccator. Fill it with 45 mL of sodium hypochlorite (see Note 5 and Fig. 1c, d). Put the ceramic plate into the desiccator so that cell culture plates can be positioned above the beaker. Thread the outlet tube through the central opening of the ceramic plate (Fig. 1e). Position the tube in a way so that it is next to the beaker with sodium hypochlorite (Fig. 1c–e).
-
6.
Through the central opening of the ceramic plate, add 15 mL of 37 % hydrochloric acid into the beaker (Fig. 1e). Be careful, the reaction starts immediately! Quickly put racks with cell culture plates into the desiccator and close it. Close the fume hood and sterilize for 3–4 h.
-
7.
After sterilization, start the pump to remove chlorine gas (Fig. 1a). Let it run for at least 3 h to remove most of the chlorine gas. Keep the fume hood closed during pumping.
-
8.
Stop the pump and open the desiccator. Let it stand open overnight under the closed fume hood to let remaining chlorine gas dissipate.
-
9.
Remove plates. Discard solution in beaker and the water from the gas-washing bottles according to your local waste disposal regulations.
3.2 Liquid Culture
-
1.
Under a laminar flow bench, remove sealing membranes (Fig. 2b) and discard.
-
2.
Add 2 mL of half-strength MS, 0.25 % sucrose medium to each well.
-
3.
Put lids onto plates and secure in place with gas-permeable adhesive tape (Fig. 2c).
-
4.
Incubate plates at 4 °C in the dark for at least 2 days to stratify seeds.
-
5.
Put plates into growth cabinet under long day conditions (16 h light), 21 °C for 8–10 days.
3.3 Transfer of Seedlings and Holoaequorin Reconstitution
-
1.
With a multichannel pipette, add 75 μL of ultrapure water to each well of a 96-well plate.
-
2.
Transfer the seedlings one by one into the 96-well plate (see Note 6 , Fig. 2d) and make sure they are completely (including the roots) covered with water.
-
3.
Prepare and load CTZ working solution (3 mL of CTZ working solution are required per 96-well plate, including spare volume). To prepare 3 mL of 40 μM CTZ working solution, add 3 mL of ultrapure water to a pipetting reservoir. While slightly tilting the reservoir to collect the water on one side, add 12 μL of 10 mM CTZ stock solution and mix immediately by slightly shaking the reservoir. Directly transfer 25 μL of this mix to each well of the microplate with a multichannel pipette (hence, the end concentration of CTZ we routinely use is 10 μM, see Note 7 ). Cover the plate and incubate in the dark at ambient temperature for 4–12 h (see Note 7 ).
3.4 Ca2+ Measurements in 96-Well Plates with Manual Elicitor Injection
-
1.
Prepare elicitor working solutions: Dilute thawed stock solutions to achieve 3× concentrated working solutions in ultrapure water. Equilibrate to room temperature to avoid “cold shock” reaction when adding to seedlings. Peptide elicitors are usually used at final working concentrations of 100 nM to 1 μM, whereas shrimp shell chitin is used at a concentration of 50–200 μg/mL.
-
2.
Switch on plate reader and computer, open the software and select the area to be measured (see Note 8 ).
-
3.
Fill a 50-mL vessel (e.g., a Greiner tube) with discharge solution and place in vessel carrier, put aspirate tubing into vessel. Place dispenser head into external waste container and prime the dispenser with 1500 μL discharge solution. Gently wipe the dispenser head, remove blind plug, and inject into correct dispenser port (see Notes 9 and 10 ). Depending on your machine, make sure that the dispenser height is adjusted correctly to the height of the microplate you are using; otherwise the machine may be severely damaged (see Note 11 ).
-
4.
Pipet 60 μL of MAMP working solutions into single tubes of a PCR 12-tube strip. Thus, a 12-channel pipette can be used to conveniently load different elicitors at any desired combination while minimizing dead volume (see Note 12 ).
-
5.
Put the microplate into the plate reader. Remove the lid; otherwise, some machines may be severely damaged! You may want to seal the remaining wells not chosen for measurements with tape to avoid evaporation of liquid.
-
6.
Start the measurement. Perform at least ten measurements per well at 6-s intervals with 300 ms measuring time to record background luminescence. Let the machine eject the plate. Add 50 μL of your MAMP working solution to each well of a row with a multichannel pipette and immediately resume the measurement. Perform 300 measurements per well at 6-s intervals with 300 ms measuring time. This covers a 30 min (300 × 6 s) overall measurement time per sample; other desired time periods can be achieved by adjusting the number of measurements per well. After elicitor measurement, program the machine to inject 150 μL of discharge solution and measure the discharge well by well in “monitor” mode (continuous measurement) for 1–2 min. Perform the measurement of the whole plate in this way row by row (see Notes 9 and 13 ).
-
7.
After the run, take out the microplate. Remove the dispenser head from the port and place it into an external waste container. Close the port with the blind plug. Rinse aspirate tubing with distilled water and put it into a vessel with distilled water. Flush the pump, tubing, and dispenser with at least 50 mL of distilled water (see Note 10 ). Finally, place aspirate tubing in 20 % ethanol and fill tubing with ethanol. Dry the dispenser head carefully and place it into the dispensing head holder. Switch off the machine.
3.5 Data Analysis
Analysis of data acquired in Ca2+ measurements is conveniently achieved with the FlagScreen script for R available on the homepage of the Leibniz Institute of Plant Biochemistry (http://www.ipb-halle.de/datenbanken/flagscreen/) [12]. Basically, it offers two modes of analysis. The program can be used to plot the rate k (or L/L max), which is the ratio of luminescence counts per second at a given measurement point i (L i ), and the sum of the total remaining counts from point i + 1 including the end of the measurement and discharge (L max) (Fig. 3a). For experimental setups performed with measurement intervals other than 1 s, k needs to be corrected by multiplying L max with the measuring interval. In the case of the current protocol, L max has to be multiplied with the 6 s interval applied in the measurements. Therefore, for the protocol at hand, k is calculated in the following way:
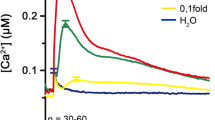
Calcium transients recorded with pMAQ2 lines. (a) Calcium transients can be plotted as the rate constant k (i.e., L/L max) or, if calibration curves are available, as actual cellular calcium concentrations [Ca2+]. (b) Different elicitors generate distinct calcium signatures in pMAQ2 seedlings. All measurements were performed with 1 μM of the indicated elicitors. (c) Feedback of ROS on Ca2+ signature can be analyzed with calcium measurements. Flg22 -induced calcium transients were compared between wild type (pMAQ2) and rbohD (respiratory burst oxidase homolog D) mutant seedlings. For further information on measurements performed in (b) and (c), see [11, 13]. Measurements in (a) and (c) were performed with 1 μM flg22, n ≥ 25. Error bars represent 2× SE
Alternatively, the program can be used to calculate the Ca2+ concentration (Fig. 3a) directly. However, it has to be noted that until now, a calibration curve for the calculation of Ca2+ concentrations has only been developed for native coelenterazine used with native aequorin [10]. Therefore, values of Ca2+ concentrations calculated in the fashion described above will only be meaningful if the correct aequorin-CTZ combination has been used in the measurements.
The calculation of the Ca2+ concentration is performed as described by Rentel and Knight [14]:
We recommend measuring multiple seedlings to obtain the average Ca2+ concentrations. Values calculated in this way can be plotted conveniently by using the FlagScreen script or in any spreadsheet calculation software. Thus, the method described here can reveal distinct cytosolic Ca2+ signatures induced by various microbe-derived or damage-derived elicitor signals (Fig. 3b). Alternatively, when the apoaequorin transgene is introduced into selected mutant backgrounds, it can reveal the contribution of the mutated genetic component in Ca2+ signaling. For instance, reactive oxygen species produced through the respiratory burst oxidase homolog D, RBOHD, is required for the second peak of the flg22-induced Ca2+ signature (Fig. 3c).
4 Notes
-
1.
To prepare cushioned forceps, take a pair of style 5 forceps (e.g., from Rubis® or A. Dumont & Fils®) and put the cut points of 1–10 μL pipette tips (preferably long tips; e.g., Gel Saver II tips from Kisker Biotech®) onto the tips of the tweezers. Carefully tap the forceps points down onto a hard surface in order to secure the pipette tips in place. Cushioned forceps reduce damage inflicted from handling of the seedlings (Fig. 2a).
-
2.
Generally, all microplates specified for luminescence measurements can be used but low protein binding properties are desirable to reduce elicitor sequestration. We recommend Lumitrac® 200 medium binding F-bottom chimney well 96-well white plates (Greiner Bio-One). While half-area microplates can be used, seedling transfer is easier with full-area plates. All volumes cited in the Ca2+ measurement protocol are calculated for full-area plates and will have to be adjusted accordingly when using half-area plates.
-
3.
Chlorine gas sterilization is a simple and convenient way to sterilize seeds directly inside the vessel in which they will be cultured. Cell culture plates with sterilized seeds that have been sealed with gas-permeable sealing membranes can also be stored for some time. However, due to the strongly oxidizing properties of chlorine gas, precautions need to be taken to avoid injuries. Sterilization is therefore performed in a desiccator under a fume hood and the chlorine gas is removed before the desiccator is being opened. Proper protective clothing has to be worn when performing seed sterilization.
-
4.
Seeds should be already pre-sieved to remove most of the siliques and other plant debris. Using blotting paper for seed transfer helps reducing husk in the culture. If husk falls into a well of the culture plate, it can be easily removed with a pipette tip. Do not load too many seeds, as this will hamper sterilization success and favor fungal growth. Use well-dried seeds. Freshly harvested seeds can be loaded into the 24-well plates, sealed with gas-permeable seal and dried overnight at 37–50 °C. If seeds are not dry, HCl will develop upon contact with chlorine gas on the seed surface and damage seeds.
-
5.
The amount of hypochlorite and HCl needed depends on the size of the desiccator and may have to be adjusted accordingly. Generally, the ratio is 3 parts hypochlorite and 1 part HCl. Hypochlorite needs to be in slight excess, as otherwise HCl vapor will damage the seeds. Be sure to let the chlorine gas dissipate completely overnight under a fume hood as chlorine traces sticking on the plastic will acidify the culture medium and affect seedling growth.
-
6.
Seedlings have to be transferred in a healthy state to the microplates they will be measured in. Therefore, infliction of stress by handling should be minimized. In order to reduce possible bacterial contaminations, seedlings should be transferred under a laminar flow bench. When loading seedlings to microplates it is advisable to handle the seedlings with cushioned tweezers in one hand and untangling the roots with a yellow pipette tip (200 μL) in the other hand. Seedlings can be gripped carefully at the hypocotyl. Seedlings are thus transferred into microplates one by one. Generally, seedlings of the same size should be chosen for analysis, although normalization during data analysis will compensate signal variations, which are based on different seedling sizes. For best results, seedlings that have their first rosette leaves just emerging should be chosen.
-
7.
Holoprotein reconstitution can be performed with different amounts of CTZ. The final concentration for reconstitution should be between 2 and 20 μM. In experimental setups with manual elicitor injections it is usually desirable to perform the holoprotein reconstitution overnight before the measurement. In setups with automated injection, the reconstitution can be performed for 4–6 h and measurement can be performed overnight. Keep in mind that CTZ is not stable for prolonged periods of time in the presence of oxygen and reconstituted aequorin will be constantly consumed. Therefore, reconstitution should not be prolonged excessively.
-
8.
For measurements with manual injection of elicitors, it is best to perform measurements row by row. Thus, the time which elapses for each row between elicitor injection and resumption of measurements is the same for each seedling . Furthermore, the time which elapses between elicitor treatment and discharge is reduced, which is favorable for accuracy. In setups with automatic elicitor injection, 2 rows (24 wells) can generally be measured at the same time. When more rows are measured at the same time, the measurement interval has to be changed accordingly at the expense of accuracy. Whereas background and transient measurements can be performed in short intervals, the discharge response is so fast that the peak can easily be missed by interval measurements. However, the peak height is essential for the normalization procedure during data analysis. Therefore, discharge measurements should be performed one well at a time in one continuous measurement.
-
9.
Due to the speed of the discharge response (see Note 8 ), the dispenser injecting the discharge solution should be inserted into the port directly beneath the measuring position of the microplate reader . Thus, the measurement can start at the moment of injection without the necessity of an additional moving step. In Luminoskan® instruments, this is port M, whereas in Varioskan® instruments, both L1 and L2 can be employed, depending on which dispenser is used. Consult the manual of your machine to find out which port to use.
-
10.
Discharge solution has a low surface tension due to the high ethanol content. Therefore, drops of solution may spill from the dispenser head at times. Regrettably, CaCl2 causes metallic corrosion and can therefore cause damage in the machine. In order to avoid this, the instrument should therefore be checked and cleaned at regular intervals and possible spills removed immediately.
-
11.
All safety guidelines applying for your instrument must be followed. Make sure that the plate layout fits the microplate you are using. Make sure you prime the dispensers before starting a measurement with the specified volume of solution. Make sure dispensers and tubing are certified for the solutions you are using.
-
12.
Peptide MAMPs are extremely sticky and containers for MAMPs should not be reused for another MAMP. Ca2+ transients in plants may be triggered at very low concentrations. Therefore, precautions should be taken not to contaminate the elicitor solutions: As an example, pipetting from (narrow) 15-mL reaction vessels can lead to the contamination of the neck of the pipette, causing carryover of elicitors into other tubes. This may result in the misinterpretation of Ca2+ transients.
-
13.
Luminescence as a function of Ca2+ influx can easily be measured in plate readers. Generally, the measurement can be divided into three phases: The background measurement, the MAMP treatment with measurement of the Ca2+ transient, and the discharge step, in which all aequorin is released to determine its total amount for subsequent data normalization. The whole measurement can be automated when plate readers with two or more dispensers are available to inject the elicitor and discharge solution. For reasons of time constraints, background and discharge measurements may have to be shortened in automated measurement setups.
References
Dodd AN, Kudla J, Sanders D (2010) The language of calcium signalling. Annu Rev Plant Biol 61:593–620. doi:10.1146/annurev-arplant-070109-104628
Haruta M, Sabat G, Stecker K, Minkoff BB, Sussman MR (2014) A peptide hormone and its receptor protein kinase regulate plant cell expansion. Science 343(6169):408–411. doi:10.1126/science.1244454
Kudla J, Batistic O, Hashimoto K (2010) Calcium signals: the lead currency of plant information processing. Plant Cell 22:541–563. doi:10.1105/tpc.109.072686
Mithofer A, Mazars C (2002) Aequorin-based measurements of intracellular Ca2+-signatures in plant cells. Biol Proced Online 4:105–118. doi:10.1251/bpo40
Sanders D, Brownlee C, Harper JF (1999) Communicating with calcium. Plant Cell 11(4):691–706
Seybold H, Trempel F, Ranf S, Scheel D, Romeis T, Lee J (2014) Ca2+ signalling in plant immune response: from pattern recognition receptors to Ca2+ decoding mechanisms. New Phytol 204:782–790. doi:10.1111/nph.13031
Choi WG, Toyota M, Kim SH, Hilleary R, Gilroy S (2014) Salt stress-induced Ca2+ waves are associated with rapid, long-distance root-to-shoot signaling in plants. Proc Natl Acad Sci U S A 111(17):6497–6502. doi:10.1073/pnas.1319955111
Mousavi SA, Chauvin A, Pascaud F, Kellenberger S, Farmer EE (2013) GLUTAMATE RECEPTOR-LIKE genes mediate leaf-to-leaf wound signalling. Nature 500(7463):422–426. doi:10.1038/nature12478
Knight MR, Campbell AK, Smith SM, Trewavas AJ (1991) Transgenic plant aequorin reports the effects of touch and cold-shock and elicitors on cytoplasmic calcium. Nature 352(6335):524–526. doi:10.1038/352524a0
Bonora M, Giorgi C, Bononi A, Marchi S, Patergnani S, Rimessi A, Rizzuto R, Pinton P (2013) Subcellular calcium measurements in mammalian cells using jellyfish photoprotein aequorin-based probes. Nat Protoc 8(11):2105–2118. doi:10.1038/nprot.2013.127
Ranf S, Eschen-Lippold L, Pecher P, Lee J, Scheel D (2011) Interplay between calcium signalling and early signalling elements during defence responses to microbe- or damage-associated molecular patterns. Plant J 68(1):100–113. doi:10.1111/j.1365-313X.2011.04671.x
Ranf S, Grimmer J, Pöschl Y, Pecher P, Chinchilla D, Scheel D, Lee J (2012) Defense-related calcium signaling mutants uncovered via a quantitative high-throughput screen in Arabidopsis thaliana. Mol Plant 5(1):115–130. doi:10.1093/mp/ssr064
Mehlmer N, Parvin N, Hurst CH, Knight MR, Teige M, Vothknecht UC (2012) A toolset of aequorin expression vectors for in planta studies of subcellular calcium concentrations in Arabidopsis thaliana. J Exp Bot 63(4):1751–1761. doi:10.1093/jxb/err406
Rentel MC, Knight MR (2004) Oxidative stress-induced calcium signaling in Arabidopsis. Plant Physiol 135(3):1471–1479. doi:10.1104/pp. 104.042663
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer Science+Business Media New York
About this protocol
Cite this protocol
Trempel, F., Ranf, S., Scheel, D., Lee, J. (2016). Quantitative Analysis of Microbe-Associated Molecular Pattern (MAMP)-Induced Ca2+ Transients in Plants. In: Duque, P. (eds) Environmental Responses in Plants. Methods in Molecular Biology, vol 1398. Humana Press, New York, NY. https://doi.org/10.1007/978-1-4939-3356-3_27
Download citation
DOI: https://doi.org/10.1007/978-1-4939-3356-3_27
Published:
Publisher Name: Humana Press, New York, NY
Print ISBN: 978-1-4939-3354-9
Online ISBN: 978-1-4939-3356-3
eBook Packages: Springer Protocols