Abstract
Many of the underlying causes of human disease result from the effects of physical/mechanical forces acting on living cells. However, the constant overriding force of gravity precludes our ability to identify the full spectrum of cellular responses to mechanical forces that dictate the transition between homeostasis and disease. Cell and tissue culture studies in true spaceflight or in the Rotating Wall Vessel (RWV) spaceflight analogue bioreactor offer dynamic approaches to engineer high fidelity, physiologically relevant 3-D tissue models with a vast array of biomedical applications. These organotypic models have furthered our understanding of structure–function relationships and design principles of the cellular microenvironment and cellular biomechanics that are critical in establishment of in vitro models that better recapitulate in vivo responses as compared to conventional flat 2-D cultures, and have complemented and advanced the knowledge being gained from other 3-D cell culture approaches.
The applications of tissue engineering research in true spaceflight and the RWV are as diverse as the number of cell types that can be cultured using these platforms, and hold the potential to help us better understand organogenesis and normal tissue development using cell lines, stem cells, and primary cells, as well as disease pathologies, including infectious disease, immunological disorders, and cancer. Accordingly, these studies have shown tremendous potential to accelerate our understanding of human physiology and susceptibility to disease and hold translational promise to benefit mankind on Earth. In addition, studying the response of mammalian cells to culture under microgravity and microgravity analogue conditions provides the opportunity to unveil underpinning mechanisms regulating spaceflight-induced alterations in human physiology, adaptation during long duration missions, and associated clinical problems for astronauts.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Spaceflight cell culture
- Spaceflight analogue cell culture
- Tissue engineering
- Cellular biomechanics
- Three-dimensional (3-D) tissue models
- Organotypic models
- Organoids
1984—Activation of human T lymphocytes is profoundly depressed during in vitro culture in spaceflight microgravity [1].
1995–2002—Ground-based experiments in the RWV bioreactor suggested that microgravity may facilitate engineering advanced three-dimensional (3-D) human surrogate tissue models from individual cells. To test this hypothesis, a spaceflight experiment aboard the Mir space station showed that cells formed cartilage that was significantly more compressible than the control on Earth, due in part to a decrease in production of glycosaminoglycan [2–4].
1997—Functional cardiac tissues engineered in the RWV bioreactor [5].
1997—Primary myoblasts cultured in the RWV demonstrate myotube formation [6].
1997—Pellis et al. use the RWV to analyze microgravity-induced inhibition of lymphocyte locomotion and investigate mechanisms related to blunted lymphocyte movement [7].
1998—Cooper and Pellis use the RWV to characterize suppression of T cell activation observed in microgravity and microgravity analogue culture and demonstrate that signaling pathways upstream of protein kinase C are sensitive to these conditions [8].
1999—3-D skeletal muscle organoids constructed from myoblasts demonstrate that microgravity exposure can induce muscle atrophy even without the removal of extrinsic mechanical load [9].
1998–2008—First applications of RWV-derived 3-D cell culture models to study host–pathogen interactions and infectious disease mechanisms using viral, bacterial, and parasitic pathogens [10–12].
2000—Mathematical model of cartilage development in the RWV [13].
2001—Permanent and non-random phenotypic and genotypic changes observed in prostate cancer epithelial cells upon co-culture in 3-D in the RWV with either prostate or bone stromal cells [14].
2001—Use of the RWV in quantitative studies of cartilage healing [15].
2003—Fluid-mechanic analysis of cartilage development in the RWV [16].
2005—3-D skeletal muscle organoids created from myoblasts, fibroblasts, and endothelial cells formed a functional microvascular network as well as contractile myotubes when transplanted into the whole animal [17].
2005—Wang et al. report 3-D co-culture of prostate cancer epithelial cells and bone stromal cells in space [18].
2005—Impaired induction of gene signaling pathways contributes to T cell dysfunction in microgravity [19].
2006—RWV culture of human bone marrow stem cells produced tissue constructs resembling those of trabecular bone [20].
2006–2013—Cartilage formation from bone marrow cells in the RWV [21–23].
2008—Sung et al. use the RWV to demonstrate the co-evolution of cancer and the interacting stromal cells during prostate cancer progression and metastasis [24].
2010 – First study using RWV-derived 3-D intestinal cell culture model that mechanistically confirmed clinical data showing that the Salmonella type three secretion system (T3SS) is not required for enteric infection in humans [35].2010—First RWV-derived 3-D immunocompetent co-culture model used for studying infectious disease [25].
2013—Brinley et al. reported RWV culture decreased DNA repair in cells infected with Epstein Barr virus (EBV) and caused greater DNA damage from radiation compared to EBV negative cells [26].
2014—First transcriptomic study to reveal molecular genetic differences between epithelial cells grown in 2-D versus RWV-derived 3-D cultures in response to infection [27].
2015—Barrila et al. report first human cell infection conducted in spaceflight aboard Space Shuttle mission STS-131 [28].
1 Introduction
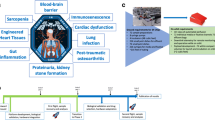
Many of the underlying causes of human disease result from the effects of physical/mechanical forces acting on living cells ( cellular biomechanics ) [29–31]. However, the constant overriding force of gravity precludes our ability to identify the full spectrum of cellular responses to mechanical forces that dictate the transition between homeostasis and disease. One method to elucidate these previously undetected responses is to exploit the quiescent environment of spaceflight as a unique platform to profile cellular responses to microgravity that are directly relevant to normal cellular development and disease progression that cannot otherwise be observed using traditional experimental approaches. Garnering information about cellular structure–function relationships in this environment is not unreasonable, as many breakthroughs in biological research and translational advancements have been achieved through studying the response of biological systems to extreme environments. Accordingly, mammalian cell culture studies using spaceflight platforms like the International Space Station (ISS) are no exception to this rule, and provide a unique opportunity to see life in a new adaptational mode that has not been seen before—adaptation to sustained low gravity.
As discussed in Chap. 1, mammalian cells may not “sense” changes in gravity directly, but rather by sensing indirect mechanical stresses (or the relief from such stresses) associated with the reduced force of gravity during spaceflight via mechanotransductive responses [32]. These responses effectively convert physical force changes at the cell surface (like those induced by mechanical signals exerted on the tension-dependent support of the cytoskeletal architecture) into biochemical responses that orchestrate coordinated cellular behaviors. Accordingly, the response of mammalian cells to culture in the microgravity environment must undergo a careful dissection to understand the direct contribution of microgravity as opposed to the indirect effect of physical changes invoked by cell culture in this unique environment, including alterations in fluid shear, mass transfer, hydrostatic pressure, and lack of sedimentation.
Since access to spaceflight experimental platforms is not yet easily accessible and affordable, NASA engineers designed ground-based spaceflight analogue bioreactor systems, RWVs, to allow scientists on Earth to culture cells in the lab under conditions that simulate aspects of the microgravity environment (Fig. 4.1) (see Chap. 2 for the operational principles of the RWV bioreactor). The RWV has allowed major advances in bioengineering research and our understanding of the mechanisms regulating the structure–function relationship of normal tissues, as well the pathogenesis of a variety of important human diseases, including infectious disease, cancer, immunological disorders, and bone and muscle wasting diseases [3, 14, 15, 18, 20–25, 33–45].
2 Culture of Three-Dimensional Mammalian Tissue Aggregates during Spaceflight
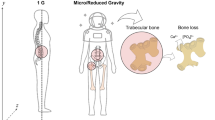
Mammalian cell culture in microgravity has been shown to promote spontaneous aggregation, which is associated with cellular assembly into high-density 3-D growth of differentiated human cells under these conditions [34, 46–48]. Since cellular assembly and 3-D growth is a prerequisite for functional tissue development, microgravity cell culture has proven beneficial in advancing our understanding of (1) the development and function of tissues and organs, (2) the progressive diversification of cells and tissues during the differentiation process, and (3) the mechanisms underlying transition between normal homeostasis and disease [34, 46–48]. Indeed, reduced gravity constitutes a physical perturbation that alters cytoskeletal organization, cellular morphology, signal transduction, gene expression, metabolism, and differentiation in mammalian cells [32, 34, 46, 47]. Moreover, culturing cells under the microgravity environment of spaceflight has enhanced our understanding of cellular biomechanics, leading to major advances in bioengineering, including development of innovative bioreactor technology (the RWV) that allows establishment of 3-D organotypic (organoid) models of tissue-like assemblies from a variety of cells and their practical application as human surrogate models for disease research, therapeutic development, and regenerative medicine [33, 34, 46, 47].
3 Culture of 3-D Mammalian Tissue Aggregates in the RWV Bioreactor Ground-Based Spaceflight Analogue
The development of the dynamic RWV spaceflight analogue culture system has provided the opportunity to optimize experiments before spaceflight and validate findings after spaceflight, as well as enable translational advances in biomedical research for the general public. Perhaps the most widely used spaceflight analogue cell culture system is the RWV bioreactor (Synthecon, Houston, TX), which is arguably the best mimic of cellular spaceflight responses [33, 49]. The design and theory of the RWV bioreactor is thoroughly described in Chap. 2, and is based on the principle that organs and tissues function in a 3-D environment. This optimized form of suspension culture is used for growing 3-D cell cultures in vitro that recapitulate key aspects of the differentiated form and function of parental tissues in vivo, including 3-D spatial organization/polarity, cellular differentiation, multicellular complexity, and functionality [33, 34, 46, 48]. Key factors influencing the physiologically relevant cellular differentiation and development in the RWV include efficient mass transfer of nutrients and wastes in combination with culture conditions that provide a low fluid shear growth environment similar to that encountered in certain regions of the body [33, 34, 46, 47]. The dynamic RWV culture conditions allow cells to grow in three dimensions, aggregate based on natural cellular affinities (facilitating co-culture of multiple cell types), and to differentiate into 3-D tissue-like assemblies [25, 33, 34, 46, 47, 50].
While 3-D cells cultured in the RWV require more time to grow and are initially more costly as compared to monolayers, the inherent experimental flexibility and reproducibility of these human surrogate tissues are ideal for the design of hierarchical models with a modular functionality and multicellular complexity, such that different individual cell types can be mixed and matched to explore fundamental biological questions [25, 33, 34, 46, 47, 50]. RWV-derived 3-D tissue models can easily be exposed to infectious agents and external chemical compounds, thereby facilitating studies related to host–pathogen interactions or incorporation of models into drug, adjuvant, toxin, and vaccine screens [33, 51, 52] (also see patents in Chap. 1). A variety of RWV-derived 3-D organotypic cell culture models have been extensively characterized, and many different cell and tissue types from both cell lines and primary cells have been developed. This includes, but is not limited to, 3-D models of human small intestine, colon, lung, bladder, liver, placenta, neuronal, vaginal, tonsil, and lymphoma that have been used to investigate mechanisms of cellular differentiation, tissue morphogenesis, and transition to disease (e.g., infectious disease and cancer) [10–12, 33, 35, 46, 51, 53–70]. In addition, these studies have also contributed insight toward advancing our understanding of mechanisms leading to tissue loss and deconditioning as well as immune system dysfunction during prolonged stays in space [3, 45].
4 Conclusion
Cellular studies in true spaceflight or in the RWV spaceflight analogue bioreactor offer dynamic approaches to engineer high fidelity, physiologically relevant 3-D tissue models with a vast array of biomedical applications. Research findings generated from these organotypic in vitro cell culture models also reflect the benefits of architectural context previously described by Bissell and others that are necessary for the establishment of predictive models of human disease [31, 71–74]. In particular, these models have furthered our understanding of structure–function relationships and design principles of the cellular microenvironment and cellular biomechanics that are critical in establishment of in vitro models that better recapitulate in vivo responses as compared to conventional flat 2-D cultures, and have complemented the knowledge being gained from other 3-D cell culture approaches. The applications of this tissue engineering research are as diverse as the number of cell types that can be cultured using these platforms. The following chapters in this section of the book describe the use of spaceflight and the RWV bioreactor to better understand organogenesis and normal tissue development using cell lines, stem cells, and primary cells, as well as disease pathologies, including infectious disease, immunological disorders, and cancer. These studies have shown tremendous potential to accelerate our understanding of human physiology and susceptibility to disease and hold translational promise to benefit mankind on Earth. In addition, studying the response of mammalian cells to culture under microgravity and microgravity analogue conditions provides the opportunity to unveil underpinning mechanisms regulating spaceflight-induced alterations in human physiology, adaptation during long duration missions, and associated clinical problems for astronauts.
In acknowledgement of the significance and importance of the scientific potential for these platforms to advance human health, federal funding agencies including the National Aeronautics and Space Administration (NASA), National Institutes of Health (NIH), and the Defense Advanced Research Projects Agency (DARPA) have funded spaceflight and spaceflight analogue biomedical research. As stated by the NIH, “the microgravity environment of spaceflight affords a new tool to investigate the influences of various forces on life” that are often obscured on Earth by the presence of gravity and to understand how these forces are “manifest in structural and functional processes in cells, tissues, and organ systems” (http://grants.nih.gov/grants/guide/pa-files/PAR-09-120.html). Accordingly, cellular and molecular mechanisms that underlie disease are studied in the spaceflight environment, offering new opportunities to understand how cells operate in these conditions, and giving new fundamental mechanistic insight into the disease process that will lead to new strategies for treatment and prevention.
With the ISS, commercial spaceflight entities, and increasing international participation from spacefaring nations, we are in a Renaissance period for spaceflight (and spaceflight analogue) biomedical research that has tremendous potential for breakthrough advances to benefit life on Earth and exploration of space. It is indeed an exciting time to be a biomedical scientist!
References
Cogoli, A., Tschopp, A., & Fuchs-Bislin, P. (1984). Cell sensitivity to gravity. Science, 225(4658), 228–230.
Freed, L. E., Hollander, A. P., Martin, I., Barry, J. R., Langer, R., & Vunjak-Novakovic, G. (1998). Chondrogenesis in a cell-polymer-bioreactor system. Experimental Cell Research, 240(1), 58–65.
Freed, L. E., Langer, R., Martin, I., Pellis, N. R., & Vunjak-Novakovic, G. (1997). Tissue engineering of cartilage in space. Proceedings of the National Academy of Sciences of the United States of America, 94(25), 13885–13890.
Freed, L. E., & Vunjak-Novakovic, G. (1995). Cultivation of cell-polymer tissue constructs in simulated microgravity. Biotechnology and Bioengineering, 46(4), 306–313.
Carrier, R. L., Papadaki, M., Rupnick, M., Schoen, F. J., Bursac, N., Langer, R., et al. (1999). Cardiac tissue engineering: Cell seeding, cultivation parameters, and tissue construct characterization. Biotechnology and Bioengineering, 64(5), 580–589.
Molnar, G., Schroedl, N. A., Gonda, S. R., & Hartzell, C. R. (1997). Skeletal muscle satellite cells cultured in simulated microgravity. In Vitro Cellular & Developmental Biology Animal, 33(5), 386–391.
Pellis, N. R., Goodwin, T. J., Risin, D., McIntyre, B. W., Pizzini, R. P., Cooper, D., et al. (1997). Changes in gravity inhibit lymphocyte locomotion through type I collagen. In Vitro Cellular & Developmental Biology Animal, 33(5), 398–405.
Cooper, D., & Pellis, N. R. (1998). Suppressed PHA activation of T lymphocytes in simulated microgravity is restored by direct activation of protein kinase C. Journal of Leukocyte Biology, 63(5), 550–562.
Vandenburgh, H., Chromiak, J., Shansky, J., Del Tatto, M., & Lemaire, J. (1999). Space travel directly induces skeletal muscle atrophy. FASEB Journal, 13(9), 1031–1038.
Alcantara Warren, C., Destura, R. V., Sevilleja, J. E., Barroso, L. F., Carvalho, H., Barrett, L. J., et al. (2008). Detection of epithelial-cell injury, and quantification of infection, in the HCT-8 organoid model of cryptosporidiosis. Journal of Infectious Diseases, 198(1), 143–149.
Long, J. P., Pierson, S., & Hughes, J. H. (1998). Rhinovirus replication in HeLa cells cultured under conditions of simulated microgravity. Aviation, Space, and Environmental Medicine, 69(9), 851–856.
Nickerson, C. A., Goodwin, T. J., Terlonge, J., Ott, C. M., Buchanan, K. L., Uicker, W. C., et al. (2001). Three-dimensional tissue assemblies: Novel models for the study of Salmonella enterica serovar Typhimurium pathogenesis. Infection and Immunity, 69(11), 7106–7120.
Martin, I., Obradovic, B., Treppo, S., Grodzinsky, A. J., Langer, R., Freed, L. E., et al. (2000). Modulation of the mechanical properties of tissue engineered cartilage. Biorheology, 37(1–2), 141–147.
Rhee, H. W., Zhau, H. E., Pathak, S., Multani, A. S., Pennanen, S., Visakorpi, T., et al. (2001). Permanent phenotypic and genotypic changes of prostate cancer cells cultured in a three-dimensional rotating-wall vessel. In Vitro Cellular & Developmental Biology Animal, 37(3), 127–140.
Obradovic, B., Martin, I., Padera, R. F., Treppo, S., Freed, L. E., & Vunjak-Novakovic, G. (2001). Integration of engineered cartilage. Journal of Orthopaedic Research, 19(6), 1089–1097.
Lappa, M. (2003). Organic tissues in rotating bioreactors: Fluid-mechanical aspects, dynamic growth models, and morphological evolution. Biotechnology and Bioengineering, 84(5), 518–532.
Levenberg, S., Rouwkema, J., Macdonald, M., Garfein, E. S., Kohane, D. S., Darland, D. C., et al. (2005). Engineering vascularized skeletal muscle tissue. Nature Biotechnology, 23(7), 879–884.
Wang, R., Xu, J., Juliette, L., Castilleja, A., Love, J., Sung, S. Y., et al. (2005). Three-dimensional co-culture models to study prostate cancer growth, progression, and metastasis to bone. Seminars in Cancer Biology, 15(5), 353–364.
Boonyaratanakornkit, J. B., Cogoli, A., Li, C. F., Schopper, T., Pippia, P., Galleri, G., et al. (2005). Key gravity-sensitive signaling pathways drive T cell activation. FASEB Journal, 19(14), 2020–2022.
Marolt, D., Augst, A., Freed, L. E., Vepari, C., Fajardo, R., Patel, N., et al. (2006). Bone and cartilage tissue constructs grown using human bone marrow stromal cells, silk scaffolds and rotating bioreactors. Biomaterials, 27(36), 6138–6149.
Ohyabu, Y., Kida, N., Kojima, H., Taguchi, T., Tanaka, J., & Uemura, T. (2006). Cartilaginous tissue formation from bone marrow cells using rotating wall vessel (RWV) bioreactor. Biotechnology and Bioengineering, 95(5), 1003–1008.
Wu, X., Li, S. H., Lou, L. M., & Chen, Z. R. (2013). The effect of the microgravity rotating culture system on the chondrogenic differentiation of bone marrow mesenchymal stem cells. Molecular Biotechnology, 54(2), 331–336.
Sakai, S., Mishima, H., Ishii, T., Akaogi, H., Yoshioka, T., Ohyabu, Y., et al. (2009). Rotating three-dimensional dynamic culture of adult human bone marrow-derived cells for tissue engineering of hyaline cartilage. Journal of Orthopaedic Research, 27(4), 517–521.
Sung, S. Y., Hsieh, C. L., Law, A., Zhau, H. E., Pathak, S., Multani, A. S., et al. (2008). Coevolution of prostate cancer and bone stroma in three-dimensional coculture: Implications for cancer growth and metastasis. Cancer Research, 68(23), 9996–10003.
Crabbe, A., Sarker, S. F., Van Houdt, R., Ott, C. M., Leys, N., Cornelis, P., et al. (2010). Alveolar epithelium protects macrophages from quorum sensing-induced cytotoxicity in a three-dimensional co-culture model. Cellular Microbiology, 13, 469–481.
Brinley, A. A., Theriot, C. A., Nelman-Gonzalez, M., Crucian, B., Stowe, R. P., Barrett, A. D., et al. (2013). Characterization of Epstein-Barr virus reactivation in a modeled spaceflight system. Journal of Cellular Biochemistry, 114(3), 616–624.
David, J., Sayer, N. M., & Sarkar-Tyson, M. (2014). The use of a three-dimensional cell culture model to investigate host-pathogen interactions of Francisella tularensis in human lung epithelial cells. Microbes and Infection, 16(9), 735–745.
Barrila, J., Sarkar, S. F., Hansmeier, N., Briones, N., Park, J., Ott, C. M., et al. (2015). Microgravity uniquely alters the host-pathogen interaction between human intestinal epithelial cells and Salmonella enterica serovar Typhimurium. In 115th American Society for Microbiology General Meeting. New Orleans, LA.
Nelson, C. M., & Bissell, M. J. (2005). Modeling dynamic reciprocity: Engineering three-dimensional culture models of breast architecture, function, and neoplastic transformation. Seminars in Cancer Biology, 15(5), 342–352.
Xu, R., Boudreau, A., & Bissell, M. J. (2009). Tissue architecture and function: Dynamic reciprocity via extra- and intra-cellular matrices. Cancer Metastasis Reviews, 28(1–2), 167–176.
Ingber, D. E. (2003). Mechanobiology and diseases of mechanotransduction. Annals of Medicine, 35(8), 564–577.
Ingber, D. (1999). How cells (might) sense microgravity. FASEB Journal, 13(Suppl), S3–S15.
Barrila, J., Radtke, A. L., Crabbe, A., Sarker, S. F., Herbst-Kralovetz, M. M., Ott, C. M., et al. (2010). Organotypic 3D cell culture models: Using the rotating wall vessel to study host-pathogen interactions. Nature Reviews Microbiology, 8(11), 791–801.
Duray, P. H., Hatfill, S. J., & Pellis, N. R. (1997). Tissue culture in microgravity. Science and Medicine, 4(3), 46–55.
Radtke, A. L., Wilson, J. W., Sarker, S., & Nickerson, C. A. (2010). Analysis of interactions of Salmonella type three secretion mutants with 3-D intestinal epithelial cells. PLoS One, 5(12), e15750.
De Weirdt, R., Crabbe, A., Roos, S., Vollenweider, S., Lacroix, C., van Pijkeren, J. P., et al. (2012). Glycerol supplementation enhances L. reuteri’s protective effect against S. Typhimurium colonization in a 3-D model of colonic epithelium. PLoS One, 7(5), e37116.
O’Connor, K. C. (1999). Three-dimensional cultures of prostatic cells: Tissue models for the development of novel anti-cancer therapies. Pharmaceutical Research, 16(4), 486–493.
Margolis, L., Hatfill, S., Chuaqui, R., Vocke, C., Emmert-Buck, M., Linehan, W. M., et al. (1999). Long term organ culture of human prostate tissue in a NASA-designed rotating wall bioreactor. Journal of Urology, 161(1), 290–297.
O’Connor, K. C., Enmon, R. M., Dotson, R. S., Primavera, A. C., & Clejan, S. (1997). Characterization of autocrine growth factors, their receptors and extracellular matrix present in three-dimensional cultures of DU 145 human prostate carcinoma cells grown in simulated microgravity. Tissue Engineering, 3(2), 161–171.
Zhau, H. E., Goodwin, T. J., Chang, S. M., Baker, T. L., & Chung, L. W. (1997). Establishment of a three-dimensional human prostate organoid coculture under microgravity-simulated conditions: Evaluation of androgen-induced growth and PSA expression. In Vitro Cellular & Developmental Biology Animal, 33(5), 375–380.
Sambandam, Y., Townsend, M. T., Pierce, J. J., Lipman, C. M., Haque, A., Bateman, T. A., et al. (2014). Microgravity control of autophagy modulates osteoclastogenesis. Bone, 61, 125–131.
Capulli, M., Rufo, A., Teti, A., & Rucci, N. (2009). Global transcriptome analysis in mouse calvarial osteoblasts highlights sets of genes regulated by modeled microgravity and identifies a “mechanoresponsive osteoblast gene signature”. Journal of Cellular Biochemistry, 107(2), 240–252.
Qiu, Q. Q., Ducheyne, P., & Ayyaswamy, P. S. (2001). 3D bone tissue engineered with bioactive microspheres in simulated microgravity. In Vitro Cellular & Developmental Biology Animal, 37(3), 157–165.
Martin, I., Shastri, V. P., Padera, R. F., Yang, J., Mackay, A. J., Langer, R., et al. (2001). Selective differentiation of mammalian bone marrow stromal cells cultured on three-dimensional polymer foams. Journal of Biomedical Materials Research, 55(2), 229–235.
Gueguinou, N., Huin-Schohn, C., Bascove, M., Bueb, J. L., Tschirhart, E., Legrand-Frossi, C., et al. (2009). Could spaceflight-associated immune system weakening preclude the expansion of human presence beyond Earth’s orbit? Journal of Leukocyte Biology, 86(5), 1027–1038.
Unsworth, B. R., & Lelkes, P. I. (1998). Growing tissues in microgravity. Nature Medicine, 4(8), 901–907.
Freed, L. E., & Vunjak-Novakovic, G. (2002). Spaceflight bioreactor studies of cells and tissues. Advances in Space Biology and Medicine, 8, 177–195.
Vunjak-Novakovic, G., Searby, N., De Luis, J., & Freed, L. E. (2002). Microgravity studies of cells and tissues. Annals of the New York Academy of Sciences, 974, 504–517.
Wolf, D. A., & Schwarz, R. P. (1991). Analysis of gravity-induced particle motion and fluid perfusion flow in the NASA-designed rotating zero-head space tissue culture vessel.
Nickerson, C. A., & Ott, C. M. (2004). A new dimension in modeling infectious disease. ASM News, 70(4), 169–175.
Hjelm, B. E., Berta, A. N., Nickerson, C. A., Arntzen, C. J., & Herbst-Kralovetz, M. M. (2010). Development and characterization of a three-dimensional organotypic human vaginal epithelial cell model. Biology of Reproduction, 82(3), 617–627.
Durand, R. E., & Olive, P. L. (2001). Resistance of tumor cells to chemo- and radiotherapy modulated by the three-dimensional architecture of solid tumors and spheroids. Methods in Cell Biology, 64, 211–233.
Carterson, A. J., Honer zu Bentrup, K., Ott, C. M., Clarke, M. S., Pierson, D. L., Vanderburg, C. R., et al. (2005). A549 lung epithelial cells grown as three-dimensional aggregates: Alternative tissue culture model for Pseudomonas aeruginosa pathogenesis. Infection and Immunity, 73(2), 1129–1140.
Carvalho, H. M., Teel, L. D., Goping, G., & O’Brien, A. D. (2005). A three-dimensional tissue culture model for the study of attach and efface lesion formation by enteropathogenic and enterohaemorrhagic Escherichia coli. Cellular Microbiology, 7(12), 1771–1781.
Duray, P. H., Yin, S. R., Ito, Y., Bezrukov, L., Cox, C., Cho, M. S., et al. (2005). Invasion of human tissue ex vivo by Borrelia burgdorferi. Journal of Infectious Diseases, 191(10), 1747–1754.
Goodwin, T. J., Jessup, J. M., & Wolf, D. A. (1992). Morphologic differentiation of colon carcinoma cell lines HT-29 and HT-29KM in rotating-wall vessels. Vitro Cellular & Developmental Biology, 28A(1), 47–60.
Guo, P., Weinstein, A. M., & Weinbaum, S. (2000). A hydrodynamic mechanosensory hypothesis for brush border microvilli. American Journal of Physiology Renal Physiology, 279(4), F698–F712.
Hughes, J. H., & Long, J. P. (2001). Simulated microgravity impairs respiratory burst activity in human promyelocytic cells. In Vitro Cellular & Developmental Biology Animal, 37(4), 209–215.
Ingram, M., Techy, G. B., Saroufeem, R., Yazan, O., Narayan, K. S., Goodwin, T. J., et al. (1997). Three-dimensional growth patterns of various human tumor cell lines in simulated microgravity of a NASA bioreactor. In Vitro Cellular & Developmental Biology Animal, 33(6), 459–466.
Jessup, J. M., Frantz, M., Sonmez-Alpan, E., Locker, J., Skena, K., Waller, H., et al. (2000). Microgravity culture reduces apoptosis and increases the differentiation of a human colorectal carcinoma cell line. In Vitro Cellular & Developmental Biology Animal, 36(6), 367–373.
Long, J. P., Pierson, S., & Hughes, J. H. (1999). Suppression of Epstein-Barr virus reactivation in lymphoblastoid cells cultured in simulated microgravity. In Vitro Cellular & Developmental Biology Animal, 35(1), 49–54.
Margolis, L. B., Fitzgerald, W., Glushakova, S., Hatfill, S., Amichay, N., Baibakov, B., et al. (1997). Lymphocyte trafficking and HIV infection of human lymphoid tissue in a rotating wall vessel bioreactor. AIDS Research and Human Retroviruses, 13(16), 1411–1420.
Pei, M., He, F., Kish, V. L., & Vunjak-Novakovic, G. (2008). Engineering of functional cartilage tissue using stem cells from synovial lining: A preliminary study. Clinical Orthopaedics and Related Research, 466(8), 1880–1889.
Sainz, B., Jr., TenCate, V., & Uprichard, S. L. (2009). Three-dimensional Huh7 cell culture system for the study of hepatitis C virus infection. Virology Journal, 6, 103.
Schwarz, R. P., Goodwin, T. J., & Wolf, D. A. (1992). Cell culture for three-dimensional modeling in rotating-wall vessels: An application of simulated microgravity. Journal of Tissue Culture Methods, 14(2), 51–57.
Smith, Y. C., Grande, K. K., Rasmussen, S. B., & O’Brien, A. D. (2006). Novel three-dimensional organoid model for evaluation of the interaction of uropathogenic Escherichia coli with terminally differentiated human urothelial cells. Infection and Immunity, 74(1), 750–757.
Straub, T. M., Honer zu Bentrup, K., Orosz-Coghlan, P., Dohnalkova, A., Mayer, B. K., Bartholomew, R. A., et al. (2007). In vitro cell culture infectivity assay for human noroviruses. Emerging Infectious Diseases, 13(3), 396–403.
Honer zu Bentrup, K., Ramamurthy, R., Ott, C. M., Emami, K., Nelman-Gonzalez, M., Wilson, J. W., et al. (2006). Three-dimensional organotypic models of human colonic epithelium to study the early stages of enteric salmonellosis. Microbes and Infection, 8(7), 1813–1825.
Lamarca, H. L., Ott, C. M., Honer Zu Bentrup, K., Leblanc, C. L., Pierson, D. L., Nelson, A. B., et al. (2005). Three-dimensional growth of extravillous cytotrophoblasts promotes differentiation and invasion. Placenta, 26(10), 709–720.
Myers, T. A., Nickerson, C. A., Kaushal, D., Ott, C. M., Honer zu Bentrup, K., Ramamurthy, R., et al. (2008). Closing the phenotypic gap between transformed neuronal cell lines in culture and untransformed neurons. Journal of Neuroscience Methods, 174(1), 31–41.
Schmeichel, K. L., & Bissell, M. J. (2003). Modeling tissue-specific signaling and organ function in three dimensions. Journal of Cell Science, 116(Pt 12), 2377–2388.
Griffith, L. G., & Swartz, M. A. (2006). Capturing complex 3D tissue physiology in vitro. Nature Reviews Molecular Cell Biology, 7(3), 211–224.
Weigelt, B., & Bissell, M. J. (2008). Unraveling the microenvironmental influences on the normal mammary gland and breast cancer. Seminars in Cancer Biology, 18(5), 311–321.
Ingber, D. E. (1998). The architecture of life. Scientific American, 278(1), 48–57.
Author information
Authors and Affiliations
Corresponding authors
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer Science+Business Media New York
About this chapter
Cite this chapter
Nickerson, C.A., Mark Ott, C. (2016). Biomedical Advances in Three Dimensions: An Overview of Human Cellular Studies in Space and Spaceflight Analogues. In: Nickerson, C., Pellis, N., Ott, C. (eds) Effect of Spaceflight and Spaceflight Analogue Culture on Human and Microbial Cells. Springer, New York, NY. https://doi.org/10.1007/978-1-4939-3277-1_4
Download citation
DOI: https://doi.org/10.1007/978-1-4939-3277-1_4
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4939-3276-4
Online ISBN: 978-1-4939-3277-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)