Abstract
Experiments with a variety of plant and animal tissues over the past 250 years have given rise to concepts of osmosis, osmotic pressure and permeability that are used to this day. The frog skin and mammalian red blood cells proved particularly useful in establishing that water transport across membranes was mediated by pores rather than simple diffusion. Comparative studies identified membrane intrinsic proteins (MIPs) in a variety of tissues that included red blood cells, bovine lens, plant cell membranes, fruit fly brain and microbial membranes. From these studies emerged the concept of water conducting channels called aquaporins (Aqps) that were present in virtually all living organisms. The discovery of Aqps and methodology for identifying them by RT-PCR cloning resulted in an enormous literature that provided mechanistic bases for physiological functions related to ionic and osmotic regulation. Among the vertebrates mammalian Aqps are the best studied due to their importance for biomedical issues such as diabetes insipidus and the availability of knock out (KO) models where specific deficiencies can be studied. From an evolutionary perspective, fish have multiple copies of most of the canonical Aqps that have been characterized in mammals. The development of genomic technologies is beginning to identify episodes of whole genome duplication, beginning in the earliest chordates, that resulted in the variety of Aqps that serve osmoregulatory functions in living vertebrate taxa. Comparative studies are beginning to describe how different Aqps have been coopted to osmoregulatory epithelia but more research is needed to understand the coordination of apical and basolateral membrane function.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
2.1 Introduction
The study of water transport across biological membranes has a long history of experimentation with plant and animal tissues that established basic principles of osmosis, the role of membrane integral proteins and ultimately the identification of aquaporins (Aqps) as water conducting membrane channels. The first objective of this chapter is to use selected studies to give a linear time line for these events. Techniques for cloning Aqps have generated an enormous literature that makes it difficult if not impossible to address a topic as broad as the chapter title within the time and space limitations for its preparation. Given the context of this volume, the second objective will focus on vertebrate Aqps but even then it is likely there will be omissions that some may find fault with. There is an equally interesting literature on Aqps in plants [summarized by Maurel et al. (2008)] and invertebrates [summarized by Campbell et al. (2008)].
The literature on the structure and function of vertebrate Aqps is dominated by research with mammalian tissues [summarized by Ishibashi et al. (2009)] and the numerical terminology for mammals is applied to Aqps in other vertebrate classes. Of particular interest are recent studies that reveal the expression of homologs to mammalian Aqps in elasmobranch, actinopterigian and sarcopterigian fishes, suggesting the diversity of Aqps associated with mammalian evolution dates from the earliest chordates (Cutler et al. 2012; Cerda and Finn 2010; Konno et al. 2010). The invasion of land is associated with unique amphibian Aqps in the skin and bladder (Suzuki and Tanaka 2009, 2010). Mammalian-like Aqps have been cloned from osmoregulatory tissues of reptiles and birds, including the avian kidney that has nephrons with loops of Henle and modest concentrating capability (Nishimura and Yang 2013). Despite the broad evolutionary aspects of vertebrate Aqps surprisingly few physiological conclusions can be made. The presence of mRNA used for cloning doesn’t necessarily indicate the Aqp is translated or even expressed in a membrane. Antibodies used to immunolocalize Aqps often show expression in the apical or basolateral membrane of osmoregulatory epithelia but not both. Furthermore, many Aqps perform functions other than simply water channels including cell adhesion and motility in adult and embryonic tissues (Ishibashi et al. 2011). Phylogenies based on Aqp sequence identities, in combination with comparative physiological studies of ionic and osmotic regulation (Evans 2009), raise interesting questions regarding the role of gene duplication and co-optation of Aqps in the evolution of water balance strategies of many species.
2.2 Historical Perspectives
2.2.1 Osmosis and Water Permeability
The movement of water across biological structures has been studied for many years, using different plant and animal tissues for experiments. Among the earliest was Nollet (1752, 1995 translation) who stretched a pig bladder over the opening of a flask filled with an ethanol solution and placed the bladder into a vase of water. Water was taken into the solution, a process he termed ebullition, leading to the conclusion that ethanol exerted a force attracting water across the tissue. Anecdotal reference was made to similar results obtained by another researcher in 1688 which is only a short time after the term “cell” was coined by Hooke (1664) and the description of red blood cells by Malphighi (1666, translated by Forrester 1995). Hewson (1773) observed changes in the shape of red blood cells of many species as they rolled down a tilted microscope slide. He noted that cells bathed in serum were disc shaped and when placed in water they swelled and became spherical. It was suggested that cells were contained within a membrane and that water was taken up from dilute pond water [Reviewed by Kleinzeller (1996)].
The term “osmosis” is attributed to Dutrochet (1827) who used a device similar to that of Nollet (1752) with the caecum of a chicken separating an upper chamber containing a solution from a container of pure water (Fig. 2.1a) Movement from water in the lower chamber into the solution in the upper chamber was termed endosmosis. Over time solute permeated the tissue resulting in an outward movement of water that he called exosmosis. A similar Dutrochet-like osmometer was used by Matteucci and Cima (1845) and Reid (1890) to demonstrate inward water movement across isolated frog skin. The pressure generated by osmotic water movement across a membrane was quantified by the German botanist Wilhelm Pfeffer (1877) who constructed a similar osmometer using an artificial membrane of copper ferrocyanide precipitated on a porous ceramic surface. A mercury manometer was used to measure the pressure generated as water flowed across the membrane into the solution (Fig. 2.1b). The osmotic pressure was taken as the hydrostatic pressure at which osmotic water flow ceased. The relationship between solute concentration and osmotic pressure (denoted as π) was quantified by van’t Hoff (1887, 1901) who applied the ideal gas law to this relationship and derived the famous equation:
(a) Osmometers used by Dutrochet (1827) to measure osmotic water movement across chicken cecum tied over the opening of a flask and immersed into a container of water. (b) Osmometer constructed by Pfeffer (1877) in which an artificial membrane made of copper ferrocyanide precipitated on a ceramic plate (r) separated water in the lower chamber (z) from solutions of different concentration in the upper chamber (t). Osmotic flow into the upper chamber displaced a mercury column (m). The pressure measured when osmotic flow was balanced by the mercury column was the osmotic pressure
in which Cs is the concentration of an ideal solute, R the appropriate gas constant and T the absolute temperature. For this synthesis he was awarded the first Nobel Prize in chemistry in 1901.
In practice, the osmolar concentration (Cosm) is generally lower than the molar concentration and can be corrected for by an osmotic coefficient for a particular solute. For example the osmotic coefficient for NaCl is assumed to be about 0.93 at physiological concentrations (Stadie and Sunderman 1931). Further, many biological membranes may be permeable to the solute as well. This may be compensated for by correcting Eq. (2.1) for solute permeability with the reflection coefficient (δs) for a given solute. A reflection coefficient of 1 indicates complete solute impermeability while a value of 0 indicates a solute permeability equal to that of water. The osmotic pressure generated by a concentration difference across a water permeable barrier is then:
Osmotic pressure is generally expressed as a positive value since it is measured as the force exerted as water moves from a more dilute to a more concentrated solution. The free energy of water can be determined from the equation (Marshall 1978),
where Vw is the partial molal volume of water (18 cm3 mol−1) and Xa is the mole fraction of water [(moles water)/(moles water + moles solute)]. In this notation the free energy of pure water (Xa = 1) is taken to be zero and the addition of solute results in a progressively more negative value (Xa < 1) so osmotic water movement occurs down a favorable free energy gradient. Note that corrections from ideality are also required for accurate evaluation of osmotic gradients. A more rigorous description of the thermodynamics of osmotic water movement is provided by Larsen et al. (2014). The osmotic permeability coefficient P f , (cm s−1) is often used for comparisons among biological structures.
In this equation Lp is the hydraulic conductivity (the rate of water movement across a given area per unit of osmotic concentration gradient.
2.2.2 The Amphibian Skin and Urinary Bladder
The English naturalist Robert Townson (1795) observed living frogs (Rana temporaria) and tree frogs Hyla (then Rana) arborea to absorb water across their skin when water was made available, rapidly regaining water lost by evaporation. He also noted the storage of water in the urinary bladder as a reservoir to offset evaporation. Edwards (1824) made similar observations that frogs dehydrated by 15 % of their hydrated weight recovered 2/3 of the loss in 15 min. These and other studies on amphibian water balance during the nineteenth and twentieth centuries were reviewed by Jørgensen (1997) who noted most of the research was directed at the application of basic principles of water transport to human physiology (e.g. Matteucci and Cima 1845; Reid 1890). Comparative physiology as an independent discipline had not yet been established. Frogs were often used because they were readily available and “tenacious of life” (attributed to Claude Bernard). A significant advancement in understanding the regulation of osmotic water permeability was the finding by Brunn (1921) that neurohypophyseal hormones (Pituitrin) were able to stimulate water absorption by living frogs (Fig. 2.2a). The “Brunn effect” or hydroosmotic response established antidiuretic hormone as major factor in the regulation of amphibian water balance and the utility of frog skin for studying mechanisms of regulation. The availability of D2O as an isotopic tracer for water movement was investigated by Hevesy et al. (1935) who found osmotic flow was three to five times more rapid than the theoretical value calculated from heavy water flux. They concluded: “The large difference between the observed rate for heavy and ordinary water came as a surprise to us” and that the discrepancy “limits the practical applicability of heavy water or any other indicator to comparative experiments.” These concerns were addressed by Koefoed-Johnsen and Ussing (1953) who formulated a mathematical analysis of osmotic flow vs diffusion of isotopic water. They observed that the stimulation of osmotic flow by treatment of isolated skin from frogs (Rana temporaria) and toads (Bufo bufo) with neurohypophyseal extracts was increased by 100–200 % while heavy water diffusion “…had only slightly increased.” They concluded, “The results are consistent with the assumption that neurohypophyseal hormones increase the pore size in some layer of the skin without increasing the total area available for diffusion.”
(a) The increase of body mass of three frogs each injected with two doses of pituitrin (from Brunn (1921) with permission). (b) Osmotic water flow across isolated toad urinary bladder following treatment with antidiuretic hormone, is much greater than diffusional flow measured with tritiated water (plotted from tabular data in Hays and Leaf (1962) in Hillman et al. (2009), with permission)
The anuran urinary bladder, long known as a storage organ for dilute urine formed when water is available (Steen 1929), also became a valuable tool for studying the effects of neurohypophyseal peptides on epithelial water permeability (Bentley 1958). Similar increases in water permeability could be obtained following treatment either with the mammalian antidiuretic hormone, arginine vasopressin (AVP), or the amphibian antidiuretic hormone, arginine vasotocin (AVT). Hays and Leaf (1962) compared the increase in water flux calculated from D2O and tritiated water with that determined from osmotic flow and, like Koefoed-Johnsen and Ussing (1953), found a large increase in osmotic flow compared with that calculated from isotopic flux (Fig. 2.2b). Further experiments with the toad bladder showed the increase in water permeability to correspond with an increase in membrane area, measured as capacitance (Stetson et al. 1982) and was inhibited by colchicine, a disruptor of microtubules (Taylor et al. 1973) both suggesting the insertion of vesicles containing putative water channels. This concept was further supported by the identification of clusters of intramembranous particles that could be seen in freeze fracture electron micrographs of toad bladders stimulated by vasopressin or vasotocin (Chevalier et al. 1974; Kachadorian et al. 1975).
2.2.3 Discovery of Aquaporins
In parallel with research on amphibian epithelial tissues, experiments with red blood cells showed a similar discrepancy between osmotic water flow and diffusion of tritiated water (Paganelli and Solomon 1957). This study cited Koefoed-Johnsen and Ussing (1953) and similarly hypothesized water transport to be mediated by pores in the membrane. It was later found that osmotic water permeability of red blood cells was inhibited by HgCl2 and organomercurial compounds (Macey and Farmer 1970). Benga et al. (1986) subsequently utilized (chloromercuri) benzene sulfonate (PCMBS) with 130Hg as a label to identify proteins associated with inhibition of water permeability of red blood cell ghosts. Two labeled bands were identified with gel electrophoresis that the authors concluded “….have to be considered as playing a role in water transport.” During this same period, studies on proteins associated with junctional complexes in the bovine lens revealed a “main intrinsic polypeptide”, abbreviated as MIP, with a molecular weight of about 27 kD (Broekhuyse et al. 1976). Takemoto et al. (1983) were able to purify the MIP with a molecular weight of 26 kDa (called the major intrinsic polypeptide, MIP26) and obtain its amino acid sequence. From this sequence, Gorin et al. (1984) were able to construct an array of 20 mer oligonucleotide probes to screen a cDNA library established for bovine lens. The derived amino acid sequence obtained from the resulting cDNA revealed a protein with six transmembrane domains “…and that it has at least one amphiphilic transmembrane segment, as expected if the protein were to participate in the formation of an aqueous channel.” In this regard MIP26 (now called Major Intrinsic Protein) was proposed to be a candidate for a gap junction protein and was identified in the lens of many mammalian species (Kistler and Bullivant 1980).
Alternative approaches also supported the existence of water channel proteins in the kidney that are known to reabsorb a considerable amount of water from the glomerular filtrate. In one study water permeability of brush border vesicles from proximal tubules in rat kidney was evaluated by measuring changes in volume by light scattering and radiation inactivation. Because absorption of radiation is a linear function of molecular weight, the radiation dose that inactivates water permeability could be used to estimate the mass of the water conducting protein as 30 ± 5 kDa (van Hoek et al. 1991). The discovery that mRNA for membrane proteins from mammalian tissues was able to be expressed in Xenopus oocytes made it possible to use this assay for water channels. Zhang et al. (1990) micro injected poly A mRNA from rat and rabbit kidney into oocytes and showed a greater increase in water permeability compared with water injected oocytes.
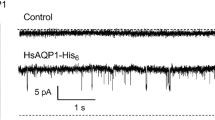
While isolating a 32 kDa Rh protein from red blood cells, Agre and co-workers discovered a novel 28 kDa protein that was also present in rat kidney (Agre et al. 1987; Denker et al. 1988). This protein was further characterized by Smith and Agre (1991) who obtained its amino acid sequence and observed the first 35 NH2-terminal amino acids had 37 % homology with the 26 kDa MIP described in bovine lens by Gorin et al. (1984). The 28 kDa protein was shown to occur as a tetramer. Oligonucleotide primers corresponding to the N-terminal amino acid sequence were used to screen a cDNA library from erythroid tissue and create a cDNA for the 28 kDa protein (Preston and Agre 1991). The deduced amino acid sequence was highly homologous with other members of the MIP family that had been identified in wide range of organisms (Pao et al. 1991). Because these proteins were collectively proposed to form membrane channels, the term channel-like integral membrane protein of 28 kDa (CHIP28) was applied to this protein. When in vitro transcribed CHIP28 RNA was injected into Xenopus oocytes the water permeability was increased from 27.9 × 10−4 cm s−1 to 210 × 10−4 cm s−1 and inhibited by exposure to HgCl2 (Preston et al. 1992). This was further demonstrated by the incorporation of highly purified CHIP28 protein into proteoliposomes and showing similar increase in water permeability (Zeidel et al. 1994). Thus, a specific protein was identified as a water channel and Peter Agre was awarded the Nobel Prize in chemistry in 2003, a century after the pioneering work of van’t Hoff.
2.2.4 Diversity of MIP Proteins
The MIP family of integral membrane transport proteins examined by Pao et al. (1991) included a neurogenic protein in Drosophila (Big Brain, Rao et al. 1990), plant tissues including the cell membrane and that of the tonoplast (PIP and TIP, Johnson et al. 1990) and in the inner membrane of a bacterial cell (Escherichia coli, glycerol facilitator protein, GlpF) where it formed a pore to facilitate glycerol uptake (Miramatsu and Mizuno 1989). Alignment of the consensus amino acid sequences for these proteins revealed six transmembrane helical domains connected by extracellular and intracellular loops A–E. Domains 1–3 (segment 1) resembled domains 4–6 (segment 2) but their orientation in the membrane (inward vs outward facing) was reversed. Comparison of segments 1 and 2 with corresponding segments 1 and 2 of several species showed a 32 % average identity whereas comparison of segment 1 with segment 2 of these species showed a 23 % average identity. It was hypothesized that MIP proteins arose by internal gene duplication of a three transmembrane protein in an ancestral organism. Preston and Agre (1991) found similar sequence similarities and membrane orientation for CHIP28. Further examination of CHIP 28 revealed four intron sequences with intron-exon boundaries that were identical with human MIP26 despite there being only a 44 % overall sequence identity between the two and different sizes of introns (Moon et al. 1993). The gene for human CHIP28 was localized to chromosome 7p14 and found to exist as a single copy.
Research during the following decade revealed a plethora of MIP-related proteins in virtually all living organisms. Given the similarities among many members of the MIP family, Agre et al. (1993) suggested, “We believe that other sequence-related water pores will be identified and feel that the functional name, aquaporin, will be useful for communication among scientists…”. With this designation CHIP28 became Aqp1, MIP26 became Aqp0 and an aquaporin that had been recently described in the mammalian collecting duct (WCH-CD, Fushimi et al. 1993) became Aqp2. In prokaryotic cells, Calamita et al. (1995) characterized a water channel in E. coli that was termed AqpZ. Phylogenetic comparisons of AqpZ and GlpF indicated an ancient gene divergence. At about the same time, Ishibashi et al. (1994) cloned an aquaporin (Aqp3) from rat kidney that was also permeable to glycerol and urea in addition to water (see also Yamaguchi et al. 1994). Thus, the proposed internal gene duplication that gave rise to an ancestral MIP family and the divergence of water vs glycerol conducting isoforms occurred very early in the evolution of cellular life and persists in extant species. In this context, aquaporins that are also permeable to glycerol and other small organic molecules are termed aquaglyceroporins although the term aquaporin is often applied to both.
The characteristic feature of Aqps and aquaglyceroporins is the presence of corresponding asparagine-proline-alanine (NPA) sequences in intracellular loop B and extracellular loop E (Fig. 2.3a). Comparing osmotic water permeability of CHIP28 (Aqp1) with site-directed mutations in the B and E loops Jung et al. (1994) proposed an “hourglass model” in which loops B and E fold into the membrane in such a way that the NPA sequences align to form a single narrow aqueous pore surrounded by the six transmembrane helices (Fig. 2.3b). Resolution of Aqp1 structure was made at 3.8 Å resolution with electron crystallographic data (Murata et al. 2000) and 2.2 Å resolution with X-ray diffraction data (Sui et al. 2001). Further insight into the structure and function of Aqps was made with molecular dynamic simulation (Fujiyoshi et al. 2002). These studies revealed that loops B and E contain α-helical domains each extending half way across the membrane to form a seventh “broken” half membrane helix with the NPA motifs at the center of the channel (reviewed by Törnroth-Horsefield et al. 2010). The positive dipole of the complimentary helices aligns with that of water molecules as they pass by the NPA motifs and restricts proton movement. A selectivity filter for water permeation is provided by a constriction at the extracellular face of the channel containing an “aromatic arginine” motif in which an arginine residue from the E loop interacts with aromatic groups of phenylalanine and histidine residues in transmembrane helices (Fig. 2.3c). Aquaglyceroporins have a different selectivity filter in which arginine is paired opposite phenylalanine and tryptophan (Wang et al. 2005; Wang and Tajkhorshid 2007). A variety of other amino acid residues have been implicated in the regulation of permeation, selectivity and gating of both Aqps and aquaglyceroporins as has the observation that Aqps invariably occur as homotetramers (Wang and Tajkhorshid 2007; Törnroth-Horsefield et al. 2010).
(a) The structure of Aqp1 illustrating the two repeats that are inversely oriented in the cell membrane and the NPA sequences of loops B and E that characterize aquaporins and aquaglyceroporins (From Heymann et al. 1998 with permission). Note also a cysteine residue that confers sensitivity to mercury. (b) The membrane orientation of the six transmembrane segments and the formation of a water conducting channel by the infolding of loops B and E with helical domains that restrict ion permeation. (c) The juxtaposition of arginine with phenylalanine and histidine constitute a size filter for water passing entering the channel (b and c from Törnroth-Horsefield et al. 2010, with permission)
2.2.5 Phylogenetic Considerations
Evolutionary relationships are often expressed as phylogenetic trees produced by computer based analyses of amino acid sequence identities. Figure 2.4 (from Calamita et al. 1995) provides an example of phylogenetic relationships among Aqps that were known at that time. It can be seen that Aqp1 has 37 % sequence identity with AqpZ. However, water permeability and selectivity in oocytes is increased to almost 200 × 10−4 cm s−1 when injected with AqpZ cRNA, i.e. both homologs are able to stimulate water permeability by a similar amount despite their long established divergence from one another. In contrast, AqpZ lacks a Hg2+ sensitive cysteine residue while water permeability resulting from the expression of Aqp1 is inhibited by Hg2+ (Yang et al. 1996). It is evident that many functional properties of Aqps and aquaglyceroporins are conserved despite the differences in amino acid sequences used to establish phylogenies and that some, but not all amino acid substitutions result in functional differences. In an examination of the NCBI data base, Zardoya (2005) identified over 450 non-redundant amino acid sequences for members of the MIP family in eubacteria, archaea and eucaryia. Computer programs were used to align amino acid sequences and construct phylogenetic trees. The diversity of Aqps in plants and animals is the result of gene duplication and cooptation for different physiological functions in different tissues. This can be seen in Table 2.1 showing Aqps in mammals, plants, yeast and a bacterium [compiled from Hillyard (2012)]. E. coli has a single Aqp and aquaglyceroporin, yeast (Saccharomyces cervevisiae) has two copies of each while the model plant (Arabidopsis thaliana) has 35 integral membrane proteins including those expressed in the plasma membrane (PIPs), tonoplast (TIP) and root nodules (NIPs). Thirteen mammalian Aqps have been described that include three classes: class 1 are water selective Aqps (Aqp 0, 1, 2, 4, 5, 6, 8), class 2 are aquaglyceroporins (Aqp 3, 7, 9, 10), class 3 are unorthodox or “superaquaporins ” (Aqp 11, 12) characterized by variations in the amino acid sequences of the characteristic NPA sequence in the transmembrane pore.
Phylogenetic analysis of AqpZ with other aquaporins and homologous MIPs whose amino acid sequences were known at the time. The numbers refer to the percent sequence identity with AqpZ. Fps1 and Aqp3 are aquaglyceroporins, Nod 26 is a root nodule Aqp, γTIP and TobRB7 are tonoplast Aqps, MIP 26 was the contemporary term for Aqp0 (Calamita et al. 1995)
The diversity of Aqps and aquaglyceroporins can arise from tandem gene duplication or whole genome duplication. Terminology for these patterns of gene duplication is important for interpreting these patterns of Aqp evolution. Differences in sequence identities between homologous Aqps that resulted from a speciation event are termed orthologs while differences that result from gene duplication are termed paralogs. In general (but not always), paralogs assume a different function or become expressed in a different tissue than the parent form. For example, microbial AQPZ and GlpF can be seen as paralogs. Now that genomic sequences are available, it is possible to determine the position of paralogous genes in the same chromosomal regions, a condition termed synteny and such regions are termed paralogons. Patterns of gene duplication and synteny seen in aquaporins provide examples for the evolution of osmoregulatory function at the cellular and organismal level.
2.3 Comparative and Evolutionary Relationships
2.3.1 Mammals
Although vertebrate evolution began long before the appearance of mammals the use of the term aquaporin arose in the context of mammalian aquaporins and the numerical assignments of mammalian aquaporins are commonly used for comparisons with other vertebrates (Crane and Goldstein 2007). For this reason it is useful to begin with discussion of the mammalian aquaporins and compare their structure and function with other vertebrates in an evolutionary context. The following description of mammalian aquaporins (with chromosomal locus) is compiled from Ishibashi et al. (2009). This review also notes that the above three classes have characteristic intron-exon boundaries: class 1 are aquaporins with four exons, class 2 are aquaglyceroporins with six exons and class 3 are the unorthodox aquaporins with three exons. Primary literature references from this review are selected to provide additional information.
Aqp0 (12q13) was the first MIP described in the lens in the context of junctions that adhere cells closely together to maintain organization of the cellular structure of the lens (Gorin et al. 1984). Humans with defective Aqp0 and Aqp0 knock out (KO) mice both develop cataracts. These junctions were shown to be alignments of Aqp0 homotetramers of adjacent cells forming an octomer (Gonen et al. 2004). X-ray diffraction studies indicated proteolytic cleavage of a C-terminal peptide segment that made the junctional form impermeable to water while the non-junctional homotetramer expressed in oocytes showed water permeability only one tenth of Aqp1. More recently, Jensen et al. (2008) used molecular dynamic simulation of Aqp0 function in the tetrameric and junctional configuration and found both to have similar low water permeabilities. The low permeability of the junctional form was suggested to allow water movement within cells of the lens as it changes shape in the course of focusing images on the retina. Aqp0 is found in the lens of other vertebrates and, importantly, exists as paralogs that are implicated in the evolution of Aqps 2, 5 and 6 that are syntenic in the amphibian and mammalian genomes.
Aqp1 (7p14) was discovered in red blood cells and is the most characterized of the Aqps. It is widespread in mammalian tissues including the endothelium of non-fenestrated capillaries on the serosal side of epithelia where it may facilitate water absorption and secretion. Humans with defective Aqp1 and Aqp1-KO mice show little or no loss of function for most of the potential tissues where AQP1 has been described although pulmonary edema can be induced in some individuals (Agre 2006). An exception is in the kidney where AQP1 is expressed in the apical and basolateral membrane of epithelial cells of the proximal tubule and descending loop of Henle. Aqp1-null humans and Aqp-1-KO mice both show concentration defects that are not life threatening when water is available. Aqp1 has also been implicated in angiogenesis associated with tumor growth. Melanoma cells implanted in Aqp1-KO mice show less growth than those implanted in wild type mice. Further, cultured proximal tubule cells from Aqp1-KO mice show a reduced capacity for migration in vitro and a reduced capacity for healing of ischemia-reperfusion injury in vivo. It was suggested that cell movements associated with wound healing and morphogenesis requires osmotic water movement in conjunction with changes in the actin cytoskeleton and ionic transport along the leading edge of lammelipodia (reviewed by Belge and Devuyst 2006).
Aqp2 (12q13) was initially described as WCH-CD in collecting duct principal cells (Fushimi et al. 1993). It is translocated into the apical membrane by exocytosis from a subapical pool of vesicles during vasopressin stimulation to increase water reabsorption and urine concentration. Activation of V2 receptors promotes phosphorylation of serine 256 by way of an adenylate cyclase-cyclic AMP-protein kinase A pathway (Fushimi et al. 1997). Aqp2-null humans display diabetes insipidus that is lethal in Aqp2-KO mice. Although a common model for ADH stimulation is from a subapical pool, Aqp2 also occurs in the basolateral membrane of collecting duct cells (reviewed by Brown 2003). Various studies with different species and cell cultures have implicated increased basolateral expression with ADH stimulation and hyperosmolality (van Balkom et al. 2003; Hasler 2009). Aqp2 is also an integrin binding protein (Chen et al. 2012). These authors note that Aqp2-KO mice show abnormalities in basolateral integrin binding and abnormalities in tubular structure. They propose Aqp2 expressed in the basolateral membrane plays a role in cell migration and epithelial morphogenesis.
Aqp3 (9p13) is an aquaglyceroporin that was cloned from rat kidney and is expressed in the basolateral membrane of principal cells in the medullary collecting duct (Echevarria et al. 1994). Permeability of Aqp3 to water and small organic molecules was inhibited by the organomercurial compound PCMBS. Ecelbarger et al. (1995) subsequently showed Aqp3 to be localized in the basolateral membrane of cells in the cortical and outer medullary collecting duct with greatest expression in the inner medulla. Water deprivation for 48 h doubled the expression of Aqp3 in the inner medulla and appears to serve an important role in transepithelial water transport in conjunction with Aqp2 in the apical membrane. Aqp3 is also present in basolateral membrane of secretory epithelia including airway, salivary glands, lacrimal glands and sweat glands. In this capacity water from the serosal fluid enters the cells and is expelled via Aqp5 located in the apical membrane, generally in response to an osmotic gradient generated by serosa-mucosa ion transport. Another phenotype of Aqp3-KO mice is dry skin due to the role of Aqp3 in transporting water and glycerol to the basal layer of keratinocytes (Hara-Chikuma and Verkman 2008). Aqp3-KO mice also show delayed proliferation of enterocytes of the colon following an episode of colitis (Thiagarajah et al. 2007).
Aqp4 (18q22) was first described as a mercurial insensitive water channel (MIWC) that was co-localized with Aqp3 (then called glycerol intrinsic protein, GLIP ) in the basolateral membrane of several epithelial tissues, including principal cells of the collecting duct (Frigeri et al. 1995). Aqp4-KO mice show a mild urinary concentration defect while the review of Ishibashi et al. (2009) report that no phenotype has been reported for Aqp4-null humans. Aqp4 is expressed to a great degree in glial and ependymal cells of the brain and has opposing roles in the formation or reduction of edema depending on whether it results from cytotoxic or vasogenic sources (Papadopoulos and Verkman 2007). Aqp4 also affects cell migration and neural excitability with reduced hearing, vision and olfaction observed in Aqp4-KO mice.
Aqp5 (12q13) is expressed in the apical membrane of many exocrine glands where it serves to mediate water movement associated with glandular secretion and often functions with AQP3 in the basolateral membrane. Aqp5-KO mice show reduced sweating (mice have sweat glands in their feet) and salivary secretions. Stimulation of muscarinic (M3) receptors causes Aqp5 to become translocated from intracellular lipid rafts into the apical membrane of cells in the interlobular ducts of rat parotid gland (Ishikawa et al. 2005). Aqp5-KO mice also show a lower level of paracellular transport than males indicating a possible connection with tight junction proteins (Kawedia et al. 2007). This was more prominent in female mice but a reason for this difference was not obvious. In the lung, Aqp5 mediates fluid secretion into the alveolar air space by type 1 alveolar pneumocytes (Nielsen et al. 1997).
Aqp6 (12q13) is of interest because it is activated by mercury. It has been identified in a number of tissues including the kidney where it is localized in the endosome of type A intercalated cells of the collecting duct. Aqp6-KO mice appear normal and no phenotypes are noted by Ishibashi et al. (2009) in humans.
Aqp7 (9p13) is an aquaglyceroporin described initially in testis and sperm although Aqp7-KO mice have normal reproductive function. It is localized in brush border epithelia of proximal tubules and Aqp7-KO mice show an increase in urinary glycerol loss. Aqp7-KO mice and Aqp7-null humans also show a reduced capacity for glycerol mobilization from adipose tissue. Dumas et al. (2007) observed humans are unique among primates in that there are five copies of Aqp7 gene in the human genome that resulted from segmental gene duplication. These authors suggest this is related to the large capacity for endurance running relative to other primates.
Aqp8 (16p12), initially described in testis and pancreatic acinar cells, is unique among class 1 aquaporins in having six exons and a sequence identity that is similar to that of tonoplast integral proteins (TIPs) of plants. Aqp8 has been identified in numerous tissues including the inner mitochondrial membrane of liver hepatocytes (Calamita et al. 2005). Aqp8 is also permeable to urea but its role in urea transport may be considered to be redundant because more specific urea transport proteins are expressed in the liver and the role of Aqp8 is not clear.
Aqp9 (15q22) is an aquaglyceroporin initially described in leucocytes and also in liver. Aqp9-KO mice have a slightly reduced capacity for glycerol uptake but the lack of Aqp9 may be compensated by other aquaglyceroporins (e.g. Aqp7). Aqp9 mRNA levels are increased in osteoclasts but no bone defects are seen in Aqp9-KO mice.
Aqp10 (1q21) is a pseudogene in mice. In humans it is an aquaglyceroporin that is expressed in a variety of tissues however its physiological function remains poorly understood.
Aqp11 (11q 13) KO mice suffer fatal polycystic kidney disease with renal cysts forming from proximal tubule cells beginning shortly after birth. Vacuoles appear to have originated from the endoplasmic reticulum and it is suggested Aqp11 serves a role in intracellular water transport.
Aqp 12 (2q37.2) in the human genome exists as two genes denoted as Aqp12A and Aqp12B that appear to have resulted from local gene duplication that is not apparent in the mouse genome. Although expressed in the pancreas its physiological role remains to be identified.
2.3.2 Ray Finned Fish (Actinopterygii)
The presence of a diverse genome among the vertebrates has been ascribed to two rounds of whole genome duplication (ca 600 mya), prior to that seen in the teleosts, at the very base of vertebrate evolution (Nakatain et al. 2007; Vandepoele et al. 2004). The increase in genetic diversity, in particular Hox genes and related anatomical complexity, has been ascribed to whole genome duplication (Ohno 1970). An increase in Aqp paralogs from genomic duplication and in orthologs from evolutionary changes in homologous Aqps could account for their varied functions in tissues of the diverse array of vertebrate species. Figure 2.5 provides an approximate time line for vertebrate evolution based largely on the fossil record. What stands out is the divergence of the tetrapod lineage from bony fishes in the upper Devonian, ca 350–400 million years ago (mya). Among the teleosts, examination of aquaporin transcripts from the zebrafish (Danio rerio) genome revealed 18 sequences related to aquaporins, aquaglyceroporins and unorthodox aquaporins that are also present in the mammalian genome, with the exception of Aqps 2, 5, and 6 (Tingaud-Sequeira et al. 2010). Of these, one is a pseudogene with one exon having similarity to the tetrapod Aqps 5 and 2 and another to tetrapod Aqp1. The deduced amino acid sequences revealed paralogs for many of the Aqps. Thus, the genes that express most of the mammalian Aqps were present in the vertebrate genome long before the divergence of land vertebrates and persist as multiple paralogs in extant fishes. Three of the duplicated Aqp genes have unlinked genetic loci, two are linked and a third, DrAqp8, is expressed in triplicate with isoforms encoded on three different linkage groups. The mechanism for multiple gene copies spread among different linkage groups has been ascribed to an additional round of whole genome duplication (WGD) at the base of the teleost crown group ca 320 mya (Vandepoele et al. 2004) with subsequent “fusion and and/or loss of gene segments” (reviewed by Cerdà and Finn 2010). In some cases, Aqp genes remain syntenic suggesting tandem gene duplication.
A diagram of vertebrate evolution illustrating the time course for the divergence of the major vertebrate clades (Benton 1998). Arrows indicate two proposed gene duplications at the base of the vertebrate lineage and the single gene duplication at the base of the teleost crown group
Functional expression of the different paralogs in Xenopus oocytes usually showed similar increases in water and glycerol permeability as seen in mammalian orthologs with the exception of DrAqp8. DrAqp8aa has a particularly high permeability to water and urea while the DrAqp8 ab and Dr.Aqp8bb have much lower permeabilities to urea and water (Fig. 2.6).
Zebrafish express multiple paralogs of mammalian Aqps except for Aqps 2, 5 and 6. Aqp4 is expressed a single copy. When expressed in Xenopus oocytes, the permeabilities to water, glycerol and urea are similar to the mammalian orthologs except for DrAqp8ab and b (Tingaud-Sequeira et al. 2010)
From a physiological perspective, osmotic regulation at the whole organism level requires regulated water permeability in the gills, gut and kidney. Fish in fresh water face osmodilution, and absorb salts across their gills. They drink little and form large volumes of dilute urine while fish in salt water drink copiously and absorb water across the gut while the gills eliminate excess salt and the kidneys form a small volume of isoosmotic urine (Evans and Claiborne 2009). The following is a summary of the putative roles for Aqps in these tissues taken from a recent review by Cerdà and Finn (2010).
Gills
The gills of various teleost species that have been examined express Aqp3a, Aqp3b or both at levels that are generally lower in salt water adapted fish. They have been localized in the apical and basolateral membrane of chloride cells in the European eel but only the basolateral membrane of chloride cells of other species. It has been proposed that these paralogs play a role in osmoreception of chloride cells but “the physiological roles for Aqp3a or -3b in the gill epithelium of teleosts remains speculative”. Branchial expression of Aqp1a and -b paralogs has also been reported in teleost gills. Aqp1a has been localized in epithelial cells of gill filaments of the gilthead seabream (Sparus auratus) and suggested to function with Aqp3 paralogs in promoting transepithelial water transport. However, no consistent expression of these paralogs has been reported for the European eel. Aqp4 exists as a single gene in fishes and has been localized in the basolateral membranes of gill pavement cells that are involved with mucous secretion. Freeze fracture electron microscopy reveals orthogonal arrays of particles that suggest Aqp4 may also function in cell adhesion and migration. Finally, various studies have detected mRNAs for virtually all of the other known piscene Aqps in the gills but their functional significance remains to be determined.
Intestine
Marine and euryhaline fish in saline environments drink large amounts of water. Monovalent salts are removed by ion transport in the esophagus and along the gastrointestinal tract with water following osmotically. Transcripts for Aqp1a, 1b and Aqp3b are upregulated in the freshwater eels treated with the steroid hormone cortisol, which is known to be elevated in salt water-adapted fish. However, the esophagus is primarily a site of ion transport with low water permeability so the role of these Aqps in this region is not clear. Aqp1a and 1b are variably expressed by different species along segments of the intestine, the pyloric ceca and the rectum. This is consistent with earlier observations that isolated intestine from cortisol-treatment of freshwater eels resulted in an increase in intestinal water permeability. Cerdà and Finn (2010) caution that the presence of Aqps in the basolateral membrane of enterocytes remains to be shown so the role of Aqps in coordination of epithelial transport in these tissues is unresolved.
Kidney
Although mRNAs for different paralogs of Aqp 1, 3 and 10 have been reported from the kidneys of various fish species, their cellular localization is not well described. The European eel expresses Aqp1a and -3b in the apical membrane of some renal tubules. Aqp1a is seen in the endothelia and more proximal segments while Aqp3b is seen in the more distal tubular segments. During salt water acclimation Aqp1a is upregulated while Aqp3b is downregulated. These and other observations stimulate hypotheses regarding the roles of Aqps in renal function but “…definitive conclusions remain elusive.” (Cerdà and Finn 2010).
Reproduction
Radiation of teleosts into the oceans required the capacity of eggs to survive in hyperosmotic sea water and, for pelagic species, for eggs to be buoyant so they can disperse. This is accomplished by rapid hydration during oocyte maturation. Prior to ovulation oocytes of the sea bream (Sparus aurata) were found to express an Aqp1-like Aqp that was translocated to the membrane as hydration occurred during egg maturation (Fabra et al. 2005). This Aqp was termed SaAqp1o with a sequence identity of 45–54 % relative to other vertebrate orthologs while a second SaAqp1 was identified that was closer to that of the mammalian Aqp1. Further characterization resulted in reclassification of these paralogs as SaAqp1a (mammalian type) and SaAqp1b that is proposed to have resulted from gene duplication after the divergence of teleosts from the tetrapod lineage (Tingaud-Sequiera et al. 2010).
2.3.3 Chondrichthys and Primitive Chordates
Sharks and rays are descendents of a more primitive piscine ancestor and provide intriguing clues about Aqp evolution in vertebrates. The first Aqp cloned from an elasmobranch was Aqp1e from the bullshark (Charcharhinus leucas) that had similar amino acid sequence identities with mammalian Aqps 1, 2 and 5 (Cutler et al. 2005). A similar Aqp was cloned from a more primitive chordate, the hagfish (Myxine glutinosa). This was suggested to be a putative gene that duplicated to give rise to the Aqps expressed in tetrapods and may be represented as introns of the pseudogene described in the teleost genome. With improved PCR techniques Cutler (2007) found two Aqp1 paralogs in S. acanthius, one with a sequence identity closer to the mammalian Aqp1 and another more similar to Aqp1e. This study also identified orthologs of the human Aqp4 (78.8 % sequence identity) and the aquaglyceroprotein Aqp3 (69.9 % sequence homology). More recently, Aqp4 in S. acanthius was shown to be expressed in many tissues including the gills and rectal gland (Cutler et al. 2012). While acclimation to 75 % vs. 120 % seawater resulted in no difference in Aqp4 mRNA levels in the rectal gland, a significant reduction was seen in the gills. As with other fish Aqps, additional research is needed to describe their contribution to regulatory mechanisms for water transport across osmoregulatory epithelia.
2.3.4 Lobe Finned Fish (Sarcopterygii)
The evolution of terrestriality in vertebrates began with the divergence of the sarcopterigii (lobe finned) from the actinopterigii (ray finned) with the oldest sarcopterigian fossils dating from the upper Silurian, ca 420 mya. The sarcopterigii gave rise to the earliest tetrapods such as panderichthys and ichthyostega that appear as fossils in fresh water deposits in the upper Devonian ca 375 mya (Carroll 2001) and ultimately to extant amphibians and amniotes (Fig. 2.7a). That the mammalian and teleost genomes both express orthologs of Aqp 0, 1, 3, 4, 8, 10, 11 and 12 indicates these isoforms were present at the time of this divergence. With the invasion of land, new strategies for osmoregulation evolved, including the regulation of epithelial water permeability by antidiuretic hormone. This can be seen in lungfish (subclass Dipnoi) that are primitive survivors of the sarcopterigii and the closest living relatives of extant land vertebrates. Lungfish species are found in fresh water habitats in Africa, South America and Australia. They burrow in mud when water sources dry up and endure dry seasons with reduced metabolic rate. Konno et al. (2010) cloned an aquaporin (Aqp0p) from the kidney of an aestivating African species (Protopterus annectans) that had sequence identity similar to tetrapod Aqp0 (75–78 %), Aqp2 (74–78 %) Aqp5 (74–77 % and Aqp6 (68–70 %). An Aqp cloned from the eye had sequence identity that was 84–89 % of tetrapod Aqp0 and was a distinct ortholog of the lens Aqp0. It was further demonstrated that Aqp0p in aestivating lungfish was localized in the apical membrane of the late distal tubule and co-localized with a vasopressin type 2 (V2) receptor in the basolateral membrane. Protein and mRNA levels for Aqp0p were reduced when animals were kept in water. The consensus peptide sequence identified a putative site (ser 263) for phosphorylation by protein kinase A and when expressed in Xenopus oocytes an increase in water permeability was further stimulated by cyclic AMP. Taken together, these results suggest Aqp0p to be ancestral to the Aqp that regulates reabsorption by the kidneys of terrestrial vertebrates. Duplication of Aqp0 is consistent with the observation that Aqps 0 2, 5 and 6 are syntenic in the mammalian genome. Given that amniotes diverged from amphibians in the upper carboniferous, the gene duplication that gave rise to these Aqps must have occurred in a primitive amphibian prior to this divergence. The similarity between Aqp0p and the teleost pseudogene suggests the existence of a putative Aqp0p—like Aqp that was functionally lost in teleosts but retained in species that exploited terrestrial habitats.
(a) Divergence of actinopterigian and sarcopterigian and the emergence of tetrapods (from Romer 1962). Note the position of lungfish and the divergence of stem amphibians. (b) Phylogenetic tree for extant amphibians showing the estimated time course for evolution of the three orders relative to the breakup of the Pangea supercontinent. The estimated times for the divergence of anurans and urodeles (?) and amniotes (??) are rough estimates but still predate the breakup of the supercontinent (San Mauro et al. 2005)
2.3.5 Amphibians
While amphibians have been used as an example for the adaptations required for the initial colonization of terrestrial habitats, there is a large gap in the fossil record between the earliest amphibious tetrapods and the radiation of amphibians in the Carboniferous and Permian (Carroll 2001). There is another gap between the Permian species and fossil evidence of extant species in the Mesozoic so the relationships between the physiological adaptations of the earliest tetrapods and living species is speculative. None-the-less, the expression of Aqps in living amphibians provides insight into strategies that may have been retained from these earliest species. The three orders of living amphibians, anura, caudata and gymnophiona all have species that occupy a range of habitats that range from highly aquatic to purely terrestrial (Hillman et al. 2009). A phylogeny for the three orders has been proposed by San Mauro et al. (2005) based on a combination of nuclear and mitochondrial gene sequences (Fig. 2.7b). Note the earlier divergence of the gymnophiona relative to that of the anura and caudata. The figure also shows the juxtaposition of the continental land masses of the Pangea supercontinent at the time of these divergences. The taxonomic and physiological similarities of amphibians worldwide was likely established prior to the breakup of Pangea and the subsequent tectonic events that resulted in the current distribution of amphibians in present day continents.
Despite their very different body plans, all three orders are often viewed as monophyletic and are unique among vertebrates in that they utilize their skin as a primary surface for osmotic water absorption (reviewed by Hillman et al. 2009 and Hillyard et al. 2009). Because of their greater abundance and availability anuran species are most commonly used for experiments. When water is available they are able to rehydrate quickly and form dilute urine that can be stored in a urinary bladder, which is a diverticulum off of the cloaca. When they venture on land or burrow during dry periods stored bladder water is reabsorbed to maintain osmotic balance. As noted earlier amphibian skin and bladder played an important role in the understanding of epithelial water permeability and its regulation by antidiuretic hormone. Given the similarity with the response seen in the mammalian kidney, an Aqp2-like protein would be expected.
Examination of skin from the Japanese tree frog (Hyla japonica) revealed two Aqps that became inserted into the apical membrane of the outer most living epithelial cell layer of dehydrated animals or when the tissue was treated with AVT (Tanii et al. 2002; Hasegawa et al. 2003). These homologs, labeled Aqp-h3 and Aqp-h2, had serine residues that were protein kinase A phosphorylation sites but were sufficiently different from the mammalian Aqp2 that they were termed anuran specific “Aqpa2” paralogs. They are suggested to have resulted from duplication of an Aqp2 precursor that remained a single gene in amniotes. Similar Aqp2as were expressed in the skin of Bufo species while only an Aqp-h3-like Aqp was expressed in the skin of Rana species . This was seen as an adaptation for the more terrestrial habitats occupied by Hyla and Bufo species that also have larger regions specialized for water absorption, extending from the ventral surface of the skin to the lower limb (Suzuki et al. 2007; Suzuki and Tanaka 2009; Ogushi et al. 2010a). The apical insertion of these Aqps and the associated increase in water permeability was also stimulated by beta-adrenergic agonists in conjunction with an increase in vascular perfusion of the skin (Ogushi et al. 2010b). This more closely resembles the response of dehydrated animals in which vascular perfusion is more prominent than observed in AVT-injected animals (Viborg and Rosenkilde 2004). Thus, the sympathetic nervous system in addition to neurohypophyseal hormones regulates water absorption by terrestrial species. As with Aqp2 in the mammalian nephron, Aqp2a paralogs are frequently observed in the basolateral membrane of the outermost living cell layer of the anuran skin in addition to the apical membrane (Ogushi et al. 2010a; Shibata et al. 2011). Anuran skin undergoes a regular cycle of cell division in the basal cell layer and constant replacement of the outermost living cell layer as cells cornify and are shed. It is possible that basolateral expression of Aqp2as is involved in cell adhesion and migration as has been suggested for Aqp2 in the mammalian kidney (Chen et al. 2012).
Surprisingly, mRNA for an Aqp-h3 homolog (Aqp-x3) was identified in the skin of the aquatic frog, Xenopus laevis, but was not expressed at the protein level because additional nucleotides at the C-terminal tail prevented its translation (Ogushi et al. 2010a). In its native habitat, Xenopus species encounter dry seasons when they seek moist substrates to avoid desiccation. At this time cutaneous water absorption could be important for survival. Regulation at the translational or post-translational level according to seasonal or husbandry conditions need to be considered in the interpretation of aquatic vs terrestrial adaptations of the amphibians.
Aqp-h2 homologs were found in the urinary bladder of Hyla, Bufo, Rana and even Xenopus species. Syntenic analysis of the genome of X. tropicalis, recently sequenced by Hellsten et al. (2010) showed them to be localized between the same genes (Fas apoptotic inhibitory molecule 2, FAIM2 and Rac GTPase-activating protein 1, RACGAP1) that flank Aqps2 and 5 on chromosome 12 of the human genome. Suzuki and Tanaka (2009) suggest a sequence of events whereby Aqp0 in an ancestral tetrapod duplicated to give rise to Aqps 2 and 5 that remained as single copies in amniotes but duplicated further in the course of amphibian evolution (Fig. 2.8).
A proposed sequence for the evolution of Aqps 2 and 5 from gene duplication in an early amphibian ancestor that persists in mammals. Two subsequent gene duplications are required to produce the Aqpa-2 paralogs (Aqp-h2 and Aqp-h3 first described in the tree frog, Hyla japonica) (From Suzuki and Tanaka 2009, with permission)
Of interest, an Aqp homologous with the mammalian Aqp2 (Aqp-h2K) was localized in the apical membrane of the collecting segment of kidneys of Hyla japonica and a related Aqp, HC-2 has been identified in H. chrysoscelis (Ogushi et al. 2007; Zimmerman et al. 2007; Crane and Goldstein 2007) indicating that expression of this gene has been retained in the kidney in addition to duplication and cooptation of the Aqp2a paralogs in the skin and bladder. When amphibians become dehydrated, glomerular filtration and urine production is greatly reduced via AVT activation of V1 receptors. The glomerular filtration rate of H. chrysocelis dehydrated by 20 % declined by almost 84 % relative to hydrated controls but fractional water clearance declined from 62 to 2 %. Amphibians are not able to concentrate their urine but elevated water permeability in the collecting duct could allow the tubular fluid to become iso-osmotic with the body fluids (Fig. 2.9).
(a) Schematic diagram of a nephron from an anuran kidney that is not able to concentrate urine. (b) Immunolabeling shows dispersal of Aqp-h2K in principal cells of the collecting duct of unstimulated kidney (green FITC). Aqp3 in the basolateral membrane is labeled red. (c) When stimulated with AVT Aqp2K is translocated to the apical membrane showing the pattern seen in the mammalian kidney predates the capacity to form a concentrated urine. (a, b and c from Suzuki and Tanaka (2009), with permission)
Aqp5 has been immunolocalized in the apical membrane of granular glands in the skin of all anurans studied. In terrestrial species, glandular secretion has been related to thermoregulation, mucus secretion and the need to keep the skin moist as a respiratory surface (Lillywhite 2006). In this regard Aqp5 may serve a function similar to that seen in the alveoli of the mammalian lung. Recently, Shibata et al. (2014) identified two Aqp5 paralogs (Aqp-xt5a and -5b) in the genome of X. tropicalis. Both are syntenic with Aqpa2 paralogs in the X. tropicalis genome. Molecular phylogenetic analysis showed Aqp-xt5a to be orthologous with mammalian Aqp5 and also to be expressed in the urinary bladder. Dehydration resulted in increased expression of Aqp-xt5a in the apical membrane of granular cells suggesting it might facilitate reabsorption of water as opposed to fluid secretion as seen in glands. AVT did not increase apical expression of Aqp-xt5a. The bladder also expresses a homolog of the urinary bladder type Aqp2a that is stimulated by AVT in more terrestrial species with larger bladder capacities. The physiological importance of this observation remains to be determined. The genomic events that resulted in the evolution of amphibian and mammalian Aqp2 and Aqp5-related Aqps, their ability to regulate epithelial water transport and their relationship to a putative ancestral Aqp0p in the early sarcopterygian lineage remain intriguing questions that can be addressed by a more extensive examination of Aqps in living amphibian species.
In order for apically expressed Aqp2 or Aqp5 related Aqps to function in epithelial water transport it is essential that a basolateral route of permeation occur as well. As with mammalian epithelia, an amphibian aquaglyceroporin (Aqp3) homolog has been identified in the basolateral membrane of absorptive and secretory epithelia (Suzuki and Tanaka 2009; Zimmerman et al. 2007). Other functions of basolateral Aqps could be to maintain the hydration status of the skin of terrestrial species as they forage on land in a manner similar to that suggested for Aqp3 in mammalian skin (Hara et al. 2002). At the same time increased expression of Aqp3 could accommodate a greater rate of rehydration in conjunction with Aqp2a expression in the apical membrane. Exchange between the vascular and extracellular fluid compartments at the serosal face of these epithelia is required for fluid absorption and secretion. This is mediated by Aqp1 homologs that are expressed in capillary endothelia (Fig. 2.10).
(a) Immunolabeling of Aqp-h2 and Aqp-h3 in the apical membrane of the outer most living cell layer of Hyla japonica skin that has been stimulated by AVT. (b) Light micrograph of toad skin with Aqp1 immunolabeled in endothelial cells of subepithelial capillaries [a and b from Suzuki and Tanaka (2007), with permission]. (c) Schematic drawing of water absorption across anuran skin showing the apical entry step with Aqp-h2 and h3 stimulated by AVT, water transport across the basolateral membrane mediated by Aqp3 (not labeled) and transfer into the vasculature via Aqp1 in the subepithelial capillaries (from Hillman et al. (2009), with permission)
A unique function for an aquaglyceroporin is seen in the freeze-tolerant frogs, Hyla chrysoscelis (Zimmerman et al. 2007). These anurans accumulate glycerol as a cryoprotectant when cold acclimated. Plasma glycerol concentration increased from 0.18 to 51 mM when cold acclimated while tubular reabsorption increased from 64 to 82 %. Tissue glycerol levels similarly increased from non-detectable levels in liver and muscle to 187 and 131 μM, respectively, indicating a greater synthesis, tissue distribution and renal reabsorption that would protect the tissues if freezing occurred. An aquaglyceroporin, HC-3 was cloned from these tissues and found to have high sequence identity with mammalian and amphibian AQP3. Oocyte expression of HC-3 resulted in a greater uptake of glycerol. HC-3 cDNA was used as a template to quantify HC-3 expression in a variety of tissues and showed marked increases in the liver and muscle of cold acclimated frogs. Kidney levels were relatively high in both warm and cold acclimated animals but increased in the urinary bladder of cold acclimated frogs.
These results suggest an important role for HC-3 in cryoprotection but the authors caution that correlation between mRNA levels in tissues may not be an accurate measure of protein expression because of regulation at the post-translational level. As noted above, mRNA for ventral skin type Aqp-x3 was identified in skin of Xenopus laevis but the protein was not expressed. In an earlier study, expression of an Aqp from toad bladder in Xenopus oocytes did not result in an increase in water permeability (Siner et al. 1996). It was suggested this was due to intracellular sequestration because of a YXRF sequence motif in the carboxy terminal domain. These observations suggest mechanisms for regulation at the translational and post-translational level that are important for amphibian water balance physiology in the face of their exposure to variable habitats.
Cope’s grey tree frog complex includes H. chrysoscelis and its tetraploid sister species H. versicolor. Both accumulate glycerol during cold acclimation. Polyploidy is relatively common among amphibians, for example the commonly used species Xenopus laevis is tetraploid while its sister species, X. tropicalis is diploid. Mabel et al. (2011) note that among vertebrates fish and amphibians have a greater tendency to have polyploidy populations but found no evidence in the zoogeographic and conservation literature that additional genetic material in polyploid species has resulted in any selective advantage. How species level polyploidy in existing populations might affect Aqp gene distribution, in addition to that attributed to whole genome duplication events early in vertebrate evolution remains an interesting question.
2.3.6 Reptiles
Modern reptiles are descended from stem tetrapods that, based on homologies among teleost and mammalian species, could potentially express all 13 Aqp homologs identified in these taxa. In a comprehensive review of osmotic and ionic regulation in reptiles, Dantzler and Bradshaw (2009) observed that the three most abundant orders of reptiles: Crocodilia (alligators and crocodiles) Squamata (lizards, snakes and amphisbaenids) and Testunides (turtles and tortoises) have species that occupy habitats that range from terrestrial to aquatic to marine. A fourth order, Rynchocephalia contains a single terrestrial species, Sphenodon punctatus from New Zealand (a second species S. guntheri was recognized in 1989). Reported values for the relative proportions of extracellular, intracellular and vascular fluid volumes vary among taxa as does the regulation of ionic composition of the “milieu interieur”. Physiological studies of ionic and osmotic regulation are limited to a small number of species. The genome of the lizard, Anolis carolinensis (Aföldi et al. 2011) contains many of the tertrapod Aqps associated with osmoregulation in mammalian and amphibian tissues but their identification in osmoregulatory tissues is limited. An overview of regulatory structures characterized in reptiles provides opportunities for future research. These include the skin, kidneys, cloaca-colon and, in some species a urinary bladder.
Skin
The colonization of terrestial habitats by amniotes involved evolution of a skin that is resistant to evaporative water loss (Lillywhite 2006). Indeed, skin of desert species fulfills this requirement. It might be expected that basolateral expression of Aqp3 might be present to keep the epithelial cells hydrated, as per that seen in the skin of mice by Hara et al. (2002).
Kidneys
As with the amphibia, the reptilian kidney is unable to form concentrated urine and AVT is the antidiuretic hormone. Antidiuresis is mediated to a great extent by a reduction in glomerular filtration in response to AVT binding to a V1-type receptor. Plasma AVT concentrations correlate with plasma osmolality in the lizard, Varanus gouldii but levels in other reptile species have been variable. Adenylate cyclase activity in nephron segments of the lizard, Ctenophorus ornatus was significantly stimulated by AVT in the intermediate segment and collecting duct regions (Bradshaw and Bradshaw 1996). This would indicate the presence of an Aqp2 homolog but this remains to be demonstrated. While investigating the role of extra-renal ion regulation by salt glands, Babonis et al. (2011) cloned an Aqp3 from transcripts in the kidney of aquatic and sea snakes without salt glands. This Aqp3 was immunolocalized in the connecting segments and collecting ducts. Taken together, these observations suggest a similar function to that of amphibians for the formation of iso-osmotic urine . It should be remembered that many reptiles are uricotelic so the volume of water that must be voided to maintain nitrogen balance is less than ureotelic species.
Cloaca and Colon
Urine flows in ureters to the cloaca. Muscular contraction of the cloaca forces cloacal fluid antegrade into the colon. Together they form a functional complex where solute linked fluid reabsorption is believed to occur. Dantzler and Bradshaw (2009) note, “The transport mechanism underpinning the reabsorption of water and electrolytes from the cloacal-colonic complex are the subject of some debate.” Babonis et al. (2012) used an antibody made against an amino acid sequence of the amphibian Aqp3 to immunolocalize this aquaporin in the basolateral membrane of the ureters and cloaca suggesting a role for fluid reabsorption or possibly mucus secretion. An apical Aqp and its regulation remain to be described.
Urinary Bladder
The urinary bladder is absent in crocodilians and snakes but is found in a number of lizards, the tuatara and to varying degrees in all chelonians. Historically, Darwin (1839) noted that the Galapagos tortoises held large volumes of water in their bladders and suggested they might act as a water storage organ. The use of bladder water to offset dehydration has been demonstrated in the desert tortoise (Gopherus agassizii) that is able to spend months without water or even succulent vegetation (Nagy and Medica 1986). Bladder water has similarly been shown to offset dehydration in a desert lizard, the Gila monster, Heloderma suspectum (Davis and DeNardo 2007). The presence of Aqps in these tissues and the regulation of water permeability remain to be determined. Other reptilian species have a urinary bladder but its use as a water reservoir, in an ecological context is not well established.
Salt Glands
Salt glands in reptiles are derived from a variety of embryonic sources and a variety of mechanisms have been identified that allow them to regulate the secretion of salt solutions that can become highly concentrated. The degree to which Aqps are involved in water transport associated with these secretions and interactions between transcellular and paracellular pathways is poorly understood.
2.3.7 Birds
Birds (class Aves) diverged from theropod reptiles beginning in the Jurassic and appear prominently in the Cretaceous. Current species inhabit virtually all habitats on earth from the polar regions to the tropics, oceans and the extreme deserts. As such they require a suite of osmoregulatory mechanisms that have been inherited from their reptilian heritage including the cloaca-colon complex and in many species nasal salt glands. Unlike reptiles birds have evolved a kidney capable of forming concentrated urine. The concentration of ureteral urine in the domestic fowl may be twice that of the plasma while other species may void a final urine that is 2.5 times that of the plasma which is small compared to that of desert mammals that may concentrate urine by a factor of over 20 times that of the plasma. These values and mechanisms for osmotic and ionic regulation by avian species are reviewed by Braun (2009).
Kidney
The avian kidney has features similar to that of reptiles and amphibians in that glomerular circulation is fed by higher pressure arterial circulation while peritubular circulation is mediated by a lower pressure renal portal system. The avian kidney is divided into multiple lobes with an outer cortical region that has reptilian-like nephrons without loops of Henle. The inner cortical region has nephrons with highly convoluted proximal segments and loops of Henle that extend to varying degrees into a medullary cone. The distribution and function of Aqps in the avian kidney has been reviewed by Nishimura and Yang (2013).
Aqp1
A full length cDNA cloned from quail kidney has 82 % sequence identity with rat Aqp1. qAqp1 was immunolocalized in the apical brush border membrane of proximal tubule cells but not in the basolateral membrane. Unlike mammalian nephrons, qAqp1 was not localized in the descending loop of Henle. In the house sparrow, Passer domesticus, an Aqp1 homolog was shown to be expressed in both the proximal and distal tubules in addition to podocytes of the glomerulus (Casotti et al. 2007). Nishimura and Yang (2013) note “that AQP1 may have a role in osmoregulation in sparrows, although the biological function of Aqp1 has not been tested.” Further, there are no knock-out animals available to test this hypothesis.
Aqp2
A full-length Aqp2 from quail kidney has a 76 % amino acid sequence identity with rat Aqp2 and serine residues that are potential sites for phosphorylation by protein kinase A. qAqp2 mRNA was localized in branches of the collecting duct in the medulla and cortex. Using real time PCR greater levels of mRNA for qAqp2 were found in the medullary cones but water deprivation resulted in increased expression in both superficial and looped nephrons. The interpretation is an increase in Aqp2 expression resulting from antidiuretic hormone as is seen in the mammalian kidney. It is noted that the increase in expression in the cortical nephrons indicates the role of antidiuretic hormone in water retention by the kidney predates the evolution of the loop of Henle, which is consistent with what has been observed in amphibian and lungfish kidneys.
Aqp3
Aqp3 cloned from quail kidney has an 81 % sequence identity with rat and human Aqp3. mRNA for qAqp3 was localized in collecting tubules and ducts suggesting it plays a role in water exit from collecting duct cells as is seen in the mammalian kidney.
Aqp4
Two cDNAs for quail Aqp4 resulting from long and short reading frames have been cloned from medullary cones of quail kidney. Both have high degrees of homology with human Aqp4s. Orthogonal arrays of intramembranous particles are not seen in quail collecting ducts and in situ hybridization failed to show mRNA for qAqp4 in collecting ducts suggesting “their physiological function may be different” from that of the mammalian kidney. Aqp4 homologs have also been cloned from a variety of avian tissues including the hypothalamus and circumventricular organs where they may serve a role in osmosensation .
Cloaca-Colon
Ureteral urine deposited into the cloaca is forced by muscular contraction into the distal rectum. Casotti et al. (2007) were able to clone an Aqp1 homolog in from the distal rectum and immunolocalize it in the epithelium. However, whether it is distributed at the basolateral membrane or within the cells remains to be described so a route for transepithelial water absorption is not evident.
Salt Glands
Many birds utilize nasal salt glands to excrete excess salt taken in the diet and thereby conserve water. Salt glands of ducks given hypertonic salt show a down regulation of Aqp1 in the endothelial cells and Aqp5 in the epithelial cells (Müller et al. 2006). These results suggest decreased capillary ultrafiltration and epithelial water loss in the course of forming a hypertonic secretion.
In reviewing the avian literature, Nishimura (2008) observes, “….recent evidence indicates that AQPs show functions other than water channel or glycerol transport. Comparative analysis of AQPs in primitive animals may provide useful information as to the fundamental function. The importance of AQP2 to fluid homeostasis in developing kidneys needs to be pursued. Such studies will provide insight for understanding possible mechanisms of developmental programming in adults.” These comments mirror those of Ishibashi et al. (2011) on the different functions served by aquaporins in mammals.
2.4 Perspectives
The expression of mammalian Aqps is the result of gene duplication and co-optation for tissue-specific functions that date at least from the earliest chordates. The phylogenetic tree developed by Zardoya and Villalba (2001) using amino acid sequences from insect Aqps as outgroups provides a useful framework for interpreting comparative and evolutionary aspects of vertebrate Aqps (Fig. 2.11). The data set was analyzed with maximum parsimony (MP), neighbor joining (NJ) and maximum likelihood (ML) phylogenetic methods. In this analysis, the first branch point gave rise to Aqp4 as the most basal Aqp in eukaryotic evolution. A paralog of that duplication underwent a second duplication that resulted in Aqp1 and an ancestral Aqp for Aqps 0, 2, 5 and 6. This phylogeny is consistent with the observation that Aqp4 serves a number of functions in various vertebrate tissues, as early as the elasmobranchs (Cutler et al. 2012). Aqp1 is widely expressed in tissues of many vertebrates including elasmobranchs that express the paralog, Aqp1e. The sequence of Aqp1e is similar to mammalian Aqps 1, 2 and 5. As such, it could be representative of the putative Aqp at the branch point that gave rise to Aqps 0, 2, 5, and 6, which are syntenic in the mammalian genome. Further up the evolutionary scale, Aqp0p expressed in the collecting duct of lungfish kidney is suggested to be ancestral to Aqp2 in the tetrapod kidney. There is a branch point in the derived phylogeny in which Aqp0p, as a paralog of Aqp0, could be ancestral to Aqps 2 and 5, which are important for fluid reabsorption and secretion in the kidney and exocrine glands. Note the gene duplications proposed to have resulted amphibian-specific Aqps are not shown in this figure. Shortly after submission of this chapter, Finn et al. (2014) published an extensive analysis of aquaporins in deuterostomes and created a more complete phylogeny than depicted in Fig. 2.11. In some cases the assignment of amphibian Aqp-2a paralogs is modified from the earlier literature (Suzuki and Tanaka 2010).
Phylogenetic tree of metazoan Aqps. The branch points indicate gene duplications and the branch lengths are proportional to evolutionary distances. In this analysis, the first duplication gave rise to Aqp4 that remains widely distributed in metazoans. A paralog of that branch further duplicated to give Aqp1 and an ancestral gene for Aqps 0, 2, 5, and 6 that are paralogous in mammals and potentially other vertebrates. Arrows indicate putative roles for an Aqp1e-like and Aqp0p-like Aqps during the course of vertebrate evolution (Zardoya and Villalba 2001)
On a broader evolutionary scale, it is important to note that six of the canonical Aqp genes (Aqps 1, 2, 3, 4, 6, 7) have been characterized in the nematode worm Caenorhabditis elegans (Huang et al. 2007) indicating these molecules were present earlier in metazoan evolution and have provided a “toolbox” of membrane proteins that serve a variety of physiological functions related to water transport, cell adhesion and cell motility. Four of the canonical Aqps identified in C. elegans were found to be expressed in osmoregulatory tissues. Quadruple KO animals displayed a normal phenotype under normal and hypertonic culture conditions. Recovery from hypotonic conditions was slower than controls but survivorship was the same. The authors suggested that these Aqps may confer a selective advantage to animals in their natural soil habitat where the osmotic conditions “are likely to vary dramatically both in time and space”. A similar argument can be made that Aqp1-null mice that are viable when provided water may face conditions in t heir natural habitat where the inability to concentrate urine would be maladaptive.
The concept that the diversity of Aqps is related to natural selection in the face of environmental stresses [reviewed by Ishibashi et al. (2011)] is consistent with conditions associated with genome duplication in amphibians and fish (Mabel et al. 2011). For example, temperature fluctuations that interfere with spindle formation and external fertilization that may result in polyspermy. These and other factors likely occurred earlier in eukaryotic evolution and could contribute to the diversity yet the structural similarity of Aqps and aquaglyceroporins that serve a variety of functions in different tissues of living species. Ishibashi et al. (2011) conclude, “Such interdisciplinary works as comparative physiology and endocrinology in particular should be encouraged and rewarded.”
References
Aföldi J et al (2011) The genome of the green anole lizard and a comparative analysis with birds and mammals. Nature 477:587–591
Agre P (2006) The aquaporin water channels. Proc Am Thorac Soc 3:5–13
Agre P, Saboori AM, Asimos A, Smith BL (1987) Purification and partial characterization of the Mr 30,000 integral membrane protein associated with the erythrocyte Rh(D) antigen. J Biol Chem 262:17497–17503
Agre P, Sasaki S, Chrispeels MJ (1993) Aquaporins: a family of water channel proteins. Am J Physiol 265:F461
Babonis LS, Miller SN, Evans DH (2011) Renal responses to salinity change in snakes with and without salt glands. J Exp Biol 214:2140–2156
Babonis LS, Womack MC, Evans DH (2012) Morphology and putative function of the colon and cloaca of marine and freshwater snakes. J Morphol 273:88–102
Belge H, Devuyst O (2006) Aquaporin-1—a water channel on the move. Nephrol Dial Transplant 21:2069–2071
Benga G, Popescu O, Pop VI (1986) p-(Chloromercuri)benzenesulfonate binding by membrane proteins and the inhibition of water transport in human erythrocytes. Biochemistry 25:1535–1538
Bentley PJ (1958) The effects of neurohypophyseal extracts on water transfer across the wall of the isolated urinary bladder of the toad Bufo marinus. J Endocrinol 17:201–209
Benton MJ (1998) The quality of the fossil record of vertebrates. In: Donovan SK, Paul CRC (eds) The quality of the fossil record. Wiley, Hoboken, NJ, p 312
Bradshaw FJ, Bradshaw SD (1996) Arginine vasotocin: locus of action along the nephron of the ornate dragon lizard, Ctenophorus ornatus. Gen Comp Endocrinol 103:281–289
Braun EJ (2009) Osmotic and ionic regulation in birds. In: Evans DH (ed) Osmotic and Ionic regulation cells and animals. CRC, Boca Raton, FL
Broekhuyse RM, Kuhlmann ED, Stols ALH (1976) Lens membranes II. Isolation and characterization of the main intrinsic polypeptide (MIP) of bovine lens fiber membranes. Exp Eye Res 23:365–371
Brown D (2003) The ins and outs of aquaporin-2 trafficking. Am J Physiol 284:F893–F901
Brunn F (1921) Beitrag zur Kenntnis der Wirkung yon hypophysenextrakten auf den Wasserhaushalt des Frosches. Zeit f Gesam Exp Med 25:120–125
Calamita G, Bishai WR, Preston GM, Guggino WB, Agre P (1995) Molecular cloning and characterization of AqpZ, a water channel from Escherichia coli. J Biol Chem 270:29063–29066
Calamita G, Ferri D, Gena P, Liquori GE, Cavalier A, Thomas D, Svelto M (2005) The inner mitochondrial membrane has aquaporin-8 water channels and is highly permeable to water. J Biol Chem 280:17149–17153
Campbell EM, Ball A, Hoppler S, Bowman AS (2008) Invertebrate aquaporins: a review. J Comp Physiol B 178:35–955
Carroll RL (2001) The origin and early radiation of terrestrial vertebrates. J Paleontol 75:1202–1213
Casotti G, Waldron T, Misquith G, Powers D, Slusher L (2007) Expression and localization of an aquaporin-1 homolog in the avian kidney and lower intestinal tract. Comp Biochem Physiol A Mol Integr Physiol 147:355–362
Cerdà J, Finn RN (2010) Piscine aquaporins: an overview. J Exp Zool 313A:623–650
Chen Y, Rice W, Gu Z, Li J, Huang J, Brenner MB, Van Hoek A, Xiong J, Gunderson GG, Norman JC, Hsu VW, Fenton RA, Brown D, Lu HAJ (2012) Aquaporin 2 promotes cell migration and epithelial morphogenesis. J Am Soc Nephrol 23:1506–1517
Chevalier J, Bourget J, Hugon JS (1974) Membrane associated particles: distribution in frog urinary bladder epithelium at rest and after oxytocin treatment. Cell Tissue Res 152:129–140
Crane CM, Goldstein DL (2007) Comparative functional analysis of aquaporins/glyceroporins in mammals and anurans. Mamm Genome 18:452–462
Cutler CP (2007) Cloning and identification of four aquaporin genes in the dogfish shark (Squalus acanthias). Bull Mt Desert Isl Biol Lab 46:19–20
Cutler CP, Meischke L, Cramb G (2005) Evolutionary and comparative analysis of aquaporin water channels in fish. Bull Mt Desert Isl Biol Lab 44:55
Cutler CP, Maclver B, Cramb G, Zeidel M (2012) Aquaporin 4 is ubiquitously expressed in the dogfish (Squalus acanthias) shark. Front Physiol 2:107
Dantzler WH, Bradshaw SD (2009) Osmotic and ionic regulation in reptiles. In: Evans DH (ed) Osmotic and Ionic regulation cells and animals. CRC, Boca Raton, FL
Darwin C (1839) Journal of researches into the natural history and geography of the countries visited during the voyage of the HMS Beagle, around the world. Routledge, London
Davis JR, DeNardo DF (2007) The urinary bladder as a physiological reservoir that moderates dehydration in a large desert lizard, the Gila monster Heloderma suspectum. J Exp Biol 210:1472–1480
Denker BM, Smith BL, Kuhajda FP, Agre P (1988) Identification, purification, and partial characterization of a novel Mr 28,000 integral membrane protein from erythrocytes and renal tubules. J Biol Chem 263:15634–15642
Dumas L, Kim YH, Karimpour-Fard A, Cox M, Hopkins J, Pollack JR, Sikela JM (2007) Gene copy number variation spanning 60 million years of human and primate evolution. Genome Res 17:1266–1277
Dutrochet RJR (1827) Nouvelles observations sur l’endosmos et l’exosmos, et sur la cause de ce double phenomene. Anal Chim Phys 35:393–400
Ecelbarger CA, Terris J, Frindt G, Echevarria M, Marples D, Nielsen S, Knepper M (1995) Aquaporin-3 water channel localization and regulation in rat kidney. Am J Physiol 269:F663–F672
Echevarria M, Windhager EE, Tate SS, Frindt G (1994) Cloning and expression of AQP3, a water channel from the medullary collecting duct of rat kidney. Proc Natl Acad Sci U S A 91:10997–11001
Edwards WF (1824) De l’influence des agens physiques sur la vie. Crochard, Paris
Evans DH (ed) (2009) Osmotic and ionic regulation cells and animals. CRC, Boca Raton, FL
Evans DH, Claiborne JB (2009) Osmotic and ionic regulation in fishes. In: Evans DH (ed) Osmotic and Ionic regulation cells and animals. CRC, Boca Raton, FL
Fabra M, Raldua D, Power DM, Deen PM, Cerdà J (2005) Marine fish egg hydration is aquaporin-mediated. Science 307:545
Finn RN, Chauvigne F, Baldur Hlidberg J, Cutler CP, Cerda J (2014) The lineage-specific evolution of aquaporin gene clusters facilitated tetrapod terrestrial adaptation. PLoS One 9(11):e113686
Forrester JM (1995) Malpighi’s De Polypo Cordis: an annotated translation. Med Hist 39:477–492
Frigeri A, Gropper MA, Turck CW, Verkman AS (1995) Immunolocalization of the mercurial-insensitive water channel and glycerol intrinsic membrane protein in epithelial cell plasma membranes. Proc Natl Acad Sci U S A 92:4328–4331
Fujiyoshi Y, Mitsuoka A, de Groot BL, Phillippsen A, Grubmuller H, Agre P, Engel A (2002) Structure and function of water channels. Curr Opin Struct Biol 12:509–515
Fushimi K, Uchida S, Hara Y, Hirata Y, Marumo F, Sasaki S (1993) Cloning and expression of apical membrane water channel of rat collecting tubule. Nature 361:549–552
Fushimi K, Sasaki S, Marumo F (1997) Phosphorylation of serine 256 is required for cAMP-dependent exocytosis of the aquaporin 2 water channel. J Biol Chem 272:14800–14804
Gonen T, Sliz P, Kistler J, Cheng YF, Waltz T (2004) Aquaporin-0 membrane junctions reveal the structure of a closed water pore. Nature 429:193–197
Gorin WB, Yancey SB, Cline J, Revel JP, Horwitz J (1984) The major intrinsic protein (MIP) of the bovine lens fiber membrane: characterization and structure based on cDNA cloning. Cell 39:49–59
Hara M, Ma T, Verkman AS (2002) Selectively reduced glycerol in skin of aquaporin-3-deficient mice may account for impaired skin hydration, elasticity and barrier recovery. J Biol Chem 277:46616–46621
Hara-Chikuma M, Verkman AS (2008) Prevention of skin tumorigenesis and impairment of epidermal cell proliferation by targeted aquaporin-3 gene disruption. Mol Cell Biol 28:326–332
Hasegawa T, Tanii H, Suzuki M, Tanaka S (2003) Regulation of water absorption in the frog skins by two vasotocin-dependent water channel aquaporins, AQP-h2 and AQP-h3. Endocrinology 144:4087–4096
Hasler U (2009) Controlled aquaporin-2 expression in the hypertonic environment. Am J Physiol 296:C641–C653
Hays RM, Leaf A (1962) Studies on the movement of water through the isolated toad bladder and its modification by vasopressin. J Gen Physiol 45:905–919
Hellsten U et al (2010) The genome of the western clawed frog, Xenopus tropicalis. Science 328:633–636
Hevesy GV, Hofer E, Krogh A (1935) The permeability of the skin of frogs to water as determined by D2O and H2O. Skand Arch Physiol 72:199–214
Hewson R (1773) On the figure and composition of the red particles of the blood, commonly called the red globules. Philos Tran R Soc Lond 63:306–324
Heymann JB, Agre P, Engel A (1998) Progress on the structure and function of aquaporin 1. J Struct Biol 121:191–206
Hillman SS, Withers PC, Drewes RC, Hillyard SD (2009) Ecological and environmental physiology of amphibians. Oxford University Press, New York, p 469
Hillyard SD (2012) Sensing meets separation: water transport across biological membranes. In: Nielsen CH (ed) Biomimetic membranes for sensor and separation applications, biological and medical physics, biomedical engineering. Springer, Amsterdam
Hillyard SD, Møbjerg N, Tanaka S, Larsen EH (2009) Osmotic and ionic regulation in amphibians. In: Evans DH (ed) Osmotic and Ionic regulation cells and animals. CRC, Boca Raton, FL
Hooke R (ed) (1664) Micrographia some physiological descriptions of minute bodies made by magnifying glasses with observations and inquiries thereupon. Martyn and Allestry, London
Huang CG, Lamitina T, Agre P, Strange K (2007) Functional analysis of the aquaporin gene family in Caenorhabditis elegans. Am J Physiol 292:C1867–C1873
Ishibashi K, Sasaki S, Fushimi K, Uchida S, Kuwahara M, Saito H, Furukawa T, Nakajima K (1994) Molecular cloning and expression of a member of the aquaporin family with permeability to glycerol and urea in addition to water expressed at the basolateral membrane of kidney collecting duct cells. Proc Natl Acad Sci U S A 91:6269–6273
Ishibashi K, Hara S, Kondo S (2009) Aquaporin water channels in mammals. Clin Exp Nephrol 13:107–117
Ishibashi K, Kondo S, Hara S, Morishita Y (2011) The evolutionary aspects of aquaporin family. Am J Physiol 300:R566–R576
Ishikawa Y, Yuan Z, Inuoe N, Skowronski MT, Nakae Y, Shono M, Cho G, Yasui M, Agre P, Nielsen S (2005) Identification of AQP5 in lipid rafts and its translocation to apical membranes by activation of M3 mAChRs in interlobular ducts of rat parotid gland. Am J Physiol Cell Physiol 289:C1303–C1311
Nollet (Abbé) JA (1752) Investigations on the causes for the ebullition of liquids. English translation, J Membr Sci 100:1–3 (1995)
Jensen MØ, Dror RO, Xu H, Borhani DW, Arkin IT, Eastwood MP, Shaw DE (2008) Dynamic control of slow water transport by aquaporin 0: Implications for hydration and junction stability in the eye lens. Proc Natl Acad Sci U S A 105:14430–14435
Johnson KD, Höfte H, Chrispeels MJ (1990) An intrinsic tonoplast protein of protein storage vacuoles in seeds is structurally related to a bacterial solute transporter (GlpF). Plant Cell 2:525–532
Jørgensen CB (1997) 200 years of amphibian water economy: from Robert Townson to the present. Biol Rev 72:153–237
Jung JS, Preston GM, Smith BL, Guggino WB, Agre P (1994) Molecular structure of the water channel through aquaporin CHIP28. The hourglass model. J Biol Chem 269:14648–14654
Kachadorian WA, Wade JB, Di Scala VA (1975) Vasopressin induced structural change in toad bladder luminal membrane. Science 190:67–69
Kawedia JD, Nieman ML, Bolvin GP, Melvin JE, Kikuchi K-I, Hand AR, Lorenz JN, Menon AG (2007) Interaction between transcellular and paracellular water transport pathways through aquaporin 5 and the tight junction complex. Proc Natl Acad Sci 104:3621–3626
Kistler J, Bullivant S (1980) Lens gap junctions and orthogonal arrays are unrelated. FEBS Lett 111:73–78
Kleinzeller A (1996) William Hewson’s studies of red blood corpuscles and the evolving concept of a cell membrane. Am J Physiol 271:C1–C8
Koefoed-Johnsen V, Ussing HH (1953) The contributions of diffusion and flow to the passage of D2O through living membranes. Acta Physiol Scand 28:60–76
Konno N, Hyodo S, Yamaguchi Y, Matsuda K, Uchiyama M (2010) Vasotocin/V2-type receptor/aquaporin axis exists in African lungfish kidney but is functional only in terrestrial condition. Endocrinology 151:1089–1096
Larsen EH, Deaton LE, Onken H, O’Donnell M, Grosell M, Dantzler WH, Weihrauch D (2014) Osmoregulation and excretion. Compr Physiol 4:405–473
Lillywhite H (2006) Water relations of tetrapod integument. J Exp Biol 202:222–226
Mabel BK, Alexandrou MA, Taylor MI (2011) Genome duplication in amphibians and fish: an extended synthesis. J Zool 284:151–182
Macey RI, Farmer REI (1970) Inhibition of water and solute permeability in human red cells. Biochim Biophys Acta 211:104–106
Marshall AG (1978) Biophysical chemistry principles, techniques and applications. Wiley, New York
Matteucci C, Cima A (1845) Memoire sur l’endosmose. Annal Chim et Phys 13:63–86
Maurel C, Verdoucq L, Luu D-T, Santoni V (2008) Plant aquaporins: membrane channels with multiple integrated functions. Annu Rev Plant Biol 59:595–624
Miramatsu S, Mizuno T (1989) Nucleotide sequence of the region encompassing the glpKF operon region containing a bent DNA sequence of Escherichia coli. Nucleic Acids Res 17:4378
Moon C, Preston GM, Griffin CA, Jabs EW, Agre P (1993) The human aquaporin gene. Structure, organization and chromosomal localization. J Biol Chem 21:15772–15778
Müller C, Sendler M, Hildebrandt JP (2006) Downregulation of aquaporins 1 and 5 in nasal gland by osmotic stress in ducklings, Anas platyrhynchos: implications for the production of hypertonic fluid. J Exp Biol 209:4067–4076
Murata K, Mitsuoka K, Hirai T, Walz T, Agre P, Heymann JB, Engel A, Fujiyoshi Y (2000) Structural determinants of water permeation through aquaporin-1. Nature 407:599–605
Nagy KA, Medica PA (1986) Physiological ecology of desert tortoises in southern Nevada. Herpetologica 42:73–92
Nakatain Y, Takeda H, Kohara Y (2007) Reconstruction of the vertebrae ancestral genome reveals dynamic genome reorganization in early vertebrates. Genome Res 17:1254–1265
Nielsen S, King LS, Christensen BM, Agre P (1997) Aquaporins in complex tissues. II. Subcellular distribution in respiratory and glandular tissues of rat. Am J Physiol 273:C1549–C1561
Nishimura H (2008) Urine concentration and avian aquaporin water channels. Pfleugers Arch 456:755–768
Nishimura H, Yang Y (2013) Aquaporins in avian kidneys: function and perspectives. Am J Physiol 305:R1201–R1214
Ogushi Y, Mochida H, Nakakura T, Suzuki M, Tanaka S (2007) Immunocytochemical and phylogenetic analysis of vasotocin-dependent aquaporin, AQP-h2K, specifically expressed in the kidney of the tree frog, Hyla japonica. Endocrinology 148:5891–5901
Ogushi Y, Tsuzuki A, Sato M, Mochida M, Okada R, Suzuki M, Hillyard SD, Tanaka S (2010a) The water-absorption region of ventral skin of several semiterrestrial and aquatic anuran amphibians identified by aquaporins. Am J Physiol 299:R1150–R1162
Ogushi Y, Kitagawa D, Hasegawa T, Suzuki M, Tanaka S (2010b) Correlation between aquaporin and water permeability in response to vasotocin, hydrin, and beta-adrenergic effectors in the pelvic skin of the tree frog, Hyla japonica. J Exp Biol 213:288–294
Ohno S (1970) Evolution by gene duplication. Springer, New York
Paganelli CV, Solomon AK (1957) The rate of exchange of tritiated water across the human red cell membrane. J Gen Physiol 41:259–277
Pao GM, Wu LF, Johnson KD, Hofte H, Chrispeels MJ, Sweet G, Sandal NN, Saier MH (1991) Evolution of the MIP family of integral membrane proteins. Mol Microbiol 5:33–37
Papadopoulos MC, Verkman AS (2007) Aquaporin-4 and brain edema. Pediatr Nephrol 22:778–784
Pfeffer W (ed) (1877) Osmotische Untersuchungen. Studien zur Zellmechanic. Engelmann, Leipzig
Preston GM, Agre P (1991) Isolation of the cDNA for erythrocyte integral membrane protein of 28 kilodaltons: member of an ancient channel family. Proc Natl Acad Sci U S A 88:11110–11114
Preston GM, Carroll TP, Guggino WB, Agre P (1992) Appearance of water channels in Xenopus oocytes expressing red cell CHIP28 protein. Science 256:385–387
Rao Y, Jan LY, Jan YN (1990) Similarity of the product of the Drosophila neurogenic gene big brain to transmembrane channel proteins. Nature 345:163–167
Reid EW (1890) Osmosis experiments with living and dead membranes. J Physiol 11:312–351
Romer AS (1962) The vertebrate body. WB Saunders, Philadelphia, PA, p 627
San Mauro D, Vences M, Alcobendes M, Zardoya R, Meyer A (2005) Initial diversification of living amphibians predated the breakup of Pangaea. Am Nat 165:590–599
Shibata Y, Takeuchi H-A, Hasegawa T, Suzuki M, Tanaka S, Hillyard SD, Nagai T (2011) Location of water channels in the skin of two species of desert toads, Anaxyrus (Bufo) punctatus and Incilius (Bufo) alvarius. Zool Sci 28:664–670
Shibata Y, Sano T, Tsuchiya N, Okada R, Mochida H, Tanaka S, Suzuki M (2014) Gene expression and localization of two types of AQP5 in Xenopus tropicalis under hydration and dehydration. Am J Physiol 307:R44–R56
Siner J, Paredes A, Hosselet C, Hammond T, Strange K, Harris HW (1996) Cloning of an aquaporin homolog present in water channel containing endosomes of toad urinary bladder. Am J Physiol 270:C372–C381
Smith BL, Agre P (1991) Erythrocyte 2Mr 28,000 transmembrane protein exists as a multisubunit oligomer similar to channel proteins. J Biol Chem 266:6407–6415
Stadie WC, Sunderman FW (1931) The osmotic coefficient of sodium in sodium hemoglobinate and of sodium chloride in hemoglobin solution. J Biol Chem 91:227–241
Steen WB (1929) On the permeability of the frog’s bladder to water. Anat Rec 43:215–220
Stetson DL, Lewis SA, Alles W, Wade JB (1982) Evaluation by capacitance measurements of antidiuretic hormone induced membrane area changes in toad bladder. Biochim Biophys Acta 689:267–274
Sui H, Han BG, Lee JK, Wilian P, Jap BK (2001) Structural basis of water-specific transport through the AQP1 water channel. Nature 414:872–878
Suzuki M, Tanaka S (2009) Molecular and cellular regulation of water homeostasis in anuran amphibians by aquaporins. Comp Biochem Physiol A Mol Integr Physiol 153:231–241
Suzuki M, Tanaka S (2010) Molecular diversity of vasotocin-dependent aquaporins closely associated with water adaptation strategy in anuran amphibians. J Neuroendocrinol 22:407–412
Suzuki M, Hasegawa T, Ogushi Y, Tanaka S (2007) Amphibian aquaporins and adaptation to terrestrial environments: a review. Comp Biochem Physiol 148A:72–81
Takemoto LJ, Hansen JS, Nicholson BJ, Hunkapiller M, Revel JP, Horwitz J (1983) Major intrinsic polypeptide of lens membrane biochemical and immunological characterization of the major cyanogen bromide fragment. Biochim Biophys Acta 731:267–274
Tanii H, Hasegawa T, Hirakawa N, Suzuki M, Tanaka S (2002) Molecular and cellular characterization of a water channel protein AQP-h3, specifically expressed in ventral frog skin. J Mambr Biol 188:43–53
Taylor A, Mamelak M, Reaven E, Maffly R (1973) Vasopressin: possible role of microtubules and microfilaments in its action. Science 181:347–350
Thiagarajah JR, Zao D, Verkman AS (2007) Impaired enterocyte proliferation in aquaporin-3 deficiency in mouse models of colitis. Gut 56:1529–1535
Tingaud-Sequeira A, Calusinska M, Finn RN, Chauvigné F, Lozano J, Cerdà J (2010) The zebrafish genome encodes the largest vertebrate repertoire of functional aquaporins with dual paralogy and substrate specificities similar to mammals. BMC Evol Biol 10:38–56
Törnroth-Horsefield S, Hedfalk K, Fischer G, Lindkvist-Petersson K, Neutze R (2010) Structural insights into eukaryotic aquaporin regulation. FEBS Lett 584:2580–2588
Townson R (1795) Physiological observations on the amphibia. Dissertation the second. Respiration continued with a fragment upon the subject of absorption, University of Edinburgh
van Balkom BWM, van Raak M, Breton S, Pastor-Soler N, Bouley R, van der Sluijs P, Brown D, Deen PMT (2003) Hypertonicity is involved in redirecting the aquaporin-2 water channel into the basolateral, instead of the apical, plasma membrane of renal epithelial cells. J Biol Chem 278:1101–1107
van Hoek AN, Horn ML, Luthjens LH, de Jong MD, Dempstert JA, van Os CH (1991) Functional unit of 30 kDa for proximal tubule water channels as revealed by radiation inactivation. J Biol Chem 266:16633–16635
Vandepoele K, De Vos W, Taylor JS, Meyer A, Van d Peer Y (2004) Major events in the genome evolution of vertebrates: paranome age and size differ considerably between ray-finned fishes and land vertebrates. Proc Natl Acad Sci 101:1638–1643
van’t Hoff JH (1887) Die Rolle des osmotischen Druckesin der Analogie zwischen Losungenund Gasen. Z Phys Chem 1:481–508
van’t Hoff JH (1901) Osmotic pressure and chemical equilibrium. Nobel Lecture, 13 December 1901
Viborg A, Rosenkilde P (2004) Water potential receptors in the skin regulate blood perfusion in the ventral pelvic skin of toads. Physiol Biochem Zool 77:39–49
Wang Y, Tajkhorshid E (2007) Molecular mechanisms of conduction and selectivity in aquaporin water channels. J Nutr 137:1509S–1515S
Wang Y, Schluten K, Tajkhorshid E (2005) What makes an aquaporin a glycerol channel? A Comparative study of AqpZ and GlpF. Structure 13:1107–1118
Yamaguchi Y, Gojobori T, Marumo F (1994) Molecular cloning and expression of a member of the aquaporin family with permeability to glycerol and urea in addition to water expressed at the basolateral membrane of kidney collecting duct cells. Proc Natl Acad Sci 91:6269–6273
Yang B, Brown D, Verkman AS (1996) The mercurial insensitive water channel (AQP4) forms orthogonal arrays in stably transfected Chinese hamster ovary cells. J Biol Chem 271:4577–4580
Zardoya R (2005) Phylogeny and evolution of the major intrinsic protein family. Biol Cell 97:397–414
Zardoya R, Villalba S (2001) A phylogenetic framework for the aquaporin family in eukaryotes. J Mol Evol 52:391–404
Zeidel ML, Nielsen S, Smith BL (1994) Ultrastructure, pharmacologic inhibition, and transport selectivity of aquaporin channel-forming integral protein in proteoliposomes. Biochemistry 33:1606–1615
Zhang RB, Logee KA, Verkman AS (1990) Expression of mRNA coding for kidney and red cell water channels in Xenopus oocytes. J Biol Chem 265:15375–15378
Zimmerman SL, Frisbie J, Goldstein DL, West J, Rivera K, Krane CM (2007) Ecretion of glycerol, and expression of aquaporins and glyceroporins, during cold acclimation in Cope’s gray tree frog Hyla chrysoscelis. Am J Physiol 292:R544–R555
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 American Physiological Society
About this chapter
Cite this chapter
Hillyard, S.D. (2015). Comparative and Evolutionary Physiology of Water Channels. In: Hyndman, K., Pannabecker, T. (eds) Sodium and Water Homeostasis. Physiology in Health and Disease. Springer, New York, NY. https://doi.org/10.1007/978-1-4939-3213-9_2
Download citation
DOI: https://doi.org/10.1007/978-1-4939-3213-9_2
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4939-3212-2
Online ISBN: 978-1-4939-3213-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)