Abstract
Milk proteins are known to possess a wide range of functional properties, such as emulsification, thickening, gelling and foaming. Milk proteins facilitate the formation and stabilisation of oil droplets in emulsions or of air bubbles in foams in formulated foods. These functional properties of milk proteins are exploited in the manufacture of dairy and other products, such as recombined milk, cream, butter, yoghurt, ice cream, cream liqueurs, dressings, mayonnaise, sauces and desserts. This chapter provides an overview of the emulsifying and foaming properties of milk proteins, focusing on the adsorption of milk proteins at oil–water and air–water interfaces with emphasis on the preferential adsorption among milk proteins and the stability of milk-protein-based emulsions and foams. Highlights on the behaviour of milk-protein-stabilised emulsions after consumption that have recently attracted a great deal of research interest are discussed briefly.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Milk-protein-stabilised emulsions
- Foam stability
- Emulsion stability
- Emulsion digestion
- Gastrointestinal tract
5.1 Introduction
Milk proteins are generally classified into caseins and whey proteins. Caseins are flexible proteins that have no rigid α-helix and β-pleated sheet structure and comprise four distinct proteins, α s1-, α s2-, β- and κ-casein, all of which are phosphoproteins (Fox, 2009; Singh, 2011). In contrast, whey proteins are globular in nature and possess high levels of secondary, tertiary and, in most cases, quaternary structure. Whey proteins can be fractionated into β-lactoglobulin (β-lg), α-lactalbumin (α-la), bovine serum albumin, lactoferrin, immunoglobulins and several minor proteins. Most commercially available milk protein ingredients are mixtures of various caseins or whey proteins, e.g., caseinates, whey protein concentrates (WPCs), whey protein isolates (WPIs) and milk protein concentrates (MPCs). These ingredients are widely used in the preparation of a broad range of food emulsions and foams.
Emulsions (milk, cream, butter, mayonnaise, coffee whiteners, whipped toppings, cream liqueurs and low fat spreads) and foams (whipped cream and ice cream) are dispersed oil–water and air–water systems, respectively, and represent a major proportion of processed food formulations. During the emulsification or foaming process, both caseins and whey proteins adsorb rapidly at oil–water or air–water interfaces, forming a film around the oil droplets or air bubbles (Damodaran, 1997; Dickinson and Patino, 1999). This adsorbed layer or film protects the oil droplets or air bubbles against various physicochemical processes of instability. Knowledge of protein structures at the interfaces and their mechanical and rheological properties is essential for controlling the stability of these dispersed systems. Protein interfacial structures and properties are affected by changes in pH, ionic strength, temperature, shear and pressure, which in turn alter the stability of these dispersed systems. The aim of this chapter is to provide readers with an overview of the formation and properties of emulsions and foams stabilised by various forms of milk proteins, focusing mainly on recent studies.
In the case of emulsions, major advances have been made in understanding the adsorption process, the composition and structure of adsorbed layers of proteins and how these proteins influence their physical and chemical properties (Dickinson and Stainsby, 1988; Dickinson, 1998, 1999a; Damodaran, 2005; McClements, 2005). Interestingly, in recent years, the physical and biochemical stability of emulsions after consumption has generated a great deal of research interest (McClements et al., 2009; Singh et al., 2009; Golding and Wooster, 2010; Le Révérend et al., 2010; Singh, 2011; Singh and Sarkar, 2011; Singh and Ye, 2013). Some progress has been made on understanding how the adsorbed layers and the physical structures of food emulsions influence the rates of lipid digestion. Knowledge of these complex interactions between the emulsion droplets and the physiological components, such as mucin, gastric and intestinal enzymes (e.g., pepsin, trypsin and lipases) and bile salts, is key to understanding the physiological behaviour of emulsions during their transit through the gastrointestinal tract. Hence, in this chapter, we discuss current advances in our understanding of the physiological behaviour of emulsions, particularly those stabilised by milk proteins, from a physicochemical viewpoint.
5.2 Formation and Stability of Protein-Stabilised Emulsions
Generally, emulsions can be prepared using a wide range of high shear apparatus, such as colloid mills, high speed blenders, high pressure valve homogenisers and ultrasonic equipment, that mix an oil phase and an aqueous phase together in the presence of a surfactant (McClements, 2005). During high pressure valve homogenisation a coarse mixture of the oil and aqueous phases is forced through a narrow slit under the action of high pressure, resulting in cavitation, intense laminar shear flow and turbulence. Consequently, the structurally amphiphilic emulsifier molecules, such as proteins, are adsorbed at the interface, creating a stabilising interfacial layer at the droplet surface and leading to the generation of fine, uniformly dispersed droplets (Dickinson, 2003).
The physicochemical properties and the stability of emulsions depend on a number of factors such as the types and concentrations of the dispersed phase and the continuous phase, the nature of the stabilising layer, temperature, pH, the viscosity of both phases, the homogenisation conditions and other processing parameters employed, such as heat treatment, high pressure processing and enzymatic hydrolysis (McClements, 2005). The stability of an emulsion therefore refers to its ability to resist any alteration in its properties and structure over the time scale of observation. Interfacial layers of different structures, compositions and charges can be carefully designed using specific proteins to meet the physicochemical demands and the required stability of food emulsions. Interestingly, as long as sufficient surfactant to cover the newly-created interface is present during homogenisation, emulsions are generally very stable to coalescence over prolonged storage periods. However, these emulsions are susceptible to different types of instability as a result of various types of physical and chemical processes, which in turn lead to enhanced creaming or serum separation. Generally, physical instability refers to modifications in the spatial arrangement or size distribution of the emulsion droplets, such as creaming, flocculation and coalescence, whereas chemical instability includes changes in the composition of the emulsion droplets themselves, such as oxidation and hydrolysis (McClements, 2005).
Stokes’ Law can be used to describe creaming, which involves the movement of oil droplets under gravity or an applied centrifugal force to form a concentrated cream layer at the top of the emulsion without any change in the droplet size distribution. The rate of creaming can be calculated using the following mathematical expression (Hunter, 1989; McClements, 2005; Singh et al., 2009):
where ν stokes = velocity of creaming, r = radius of the emulsion droplets, ρ 1 and ρ 2 = densities of the continuous and dispersed phases, respectively, and η = shear viscosity of the continuous phase.
Hence, the kinetic stability of an emulsion can be increased or the creaming rate can be decreased by lowering the radius of the droplets, by increasing the viscosity of the continuous phase or by decreasing the difference in density between the two phases.
However, instabilities other than creaming, such as flocculation or coalescence, cannot be described by this law. Emulsion flocculation is an aggregation process that arises when droplets associate because of unbalanced inter-atomic attractive and repulsive forces (Dalgleish, 1997). Commonly, there are two types of droplet–droplet interaction, i.e., depletion flocculation and bridging flocculation. Generally, depletion flocculation occurs because of the presence of a non-adsorbing biopolymer in the continuous phase of the emulsion, which can promote the association of emulsion droplets by inducing an osmotic pressure gradient within the continuous phase surrounding the droplets. In contrast, bridging flocculation occurs when a high molecular weight biopolymer at a sufficiently low concentration adsorbs on to two or more emulsion droplets, resulting in bridges (McClements, 2005).
In contrast to flocculation, coalescence refers to a completely irreversible increase in droplet size by the accretion of two or more primary emulsion droplets, gradually leading to the separation of the oil phase and the aqueous phase. Coalescence generally occurs when the stabilising film surrounding the emulsion droplets is thinned to a certain critical thickness, resulting in film breakage, thus joining emulsion droplets (van Aken et al., 2003; van Aken, 2004). Generally, emulsions are stable to coalescence as the proteins or other biopolymer molecules adsorb at the droplet surfaces, forming a dense viscoelastic interfacial layer (Dickinson and Stainsby, 1988). However, any extreme processing conditions, such as high shear or enzymatic hydrolysis, that lead to significant attrition of the interfacial film can give rise to gradual agglomeration of bare emulsion droplets, resulting in coalescence and oiling-off. For instance, coalescence has been widely reported in emulsions stabilised by whey protein hydrolysates because of the formation of a thinner interfacial film and the reduced surface viscosity of an interface formed with predominantly short peptides as opposed to intact proteins (Agboola et al., 1998 Singh and Dalgleish, 1998).
5.2.1 Aspects of Emulsions Stabilised by Milk Proteins
Milk proteins such as caseins, caseinates, WPIs, β-lg and bovine serum albumins are known to be excellent emulsifiers because of their amphiphilic nature (Morr, 1982; Mulvihill and Fox, 1989). In most food emulsions, the oil droplets are coated by a continuous film of adsorbed material, such as caseins and/or whey proteins. They reduce the interfacial tension between the oil and aqueous phases, form films with different rheological properties and, thus, stabilise the emulsion droplets. The structure and the composition of the adsorbed layers can be quite complicated because foods in general contain a variety of surface-active agents; all are possibly adsorbed at the interface either individually as monolayers or aggregates or in combination, resulting in complex multi-layered interfacial layers (Singh et al., 2009), as illustrated in Fig. 5.1. The nature of the interfacial layers formed depends largely on the type, concentration, charge and conformation of the adsorbed milk protein, and on the types of interaction and competition that occur between the adsorbed species (Dickinson, 2003; McClements, 2005).
Schematic illustration of the possible changes in milk protein-stabilised emulsions as they pass through the in vitro physiological model (Singh and Ye, 2013: reproduced with the permission of Elsevier Inc.)
The role of the caseins and whey proteins in stabilising emulsions has been thoroughly investigated (see reviews by Kinsella, 1984; Morr and Ha, 1993; Dalgleish, 1995, 2006; Wong et al., 1996; Dickinson, 1999b, 2001; Singh, 2005). The most commonly used forms of milk protein in food emulsions are sodium caseinate and whey proteins (WPIs or WPCs). Because of their highly surface-active properties, it is possible to make stable emulsions at a relatively low ratio of milk protein to oil (about 1:60). In these emulsions, the surface protein coverage is a function of increasing protein concentration until it reaches a plateau value of about 2.0–3.0 mg/m2 (Euston and Hirst, 1999; Srinivasan et al., 2001). Because of the flexible structures of caseins, they adsorb rapidly at the interface, forming extended adsorbed layers up to about 10 nm thick (Holt and Sawyer, 1988; Dalgleish, 1990, 1995, 1996a; Mackie et al., 1993; Dickinson and McClements, 1995; Fang and Dalgleish, 1998). In contrast, globular whey proteins such as β-lg unfold partially, somewhere intermediate between the native state and the fully denatured conformation, resulting in compact adsorbed layers that are only about 2 nm thick (Dickinson, 1998). The sequence of surface activity reported for milk proteins is β-casein > monodispersed casein micelle > serum albumin > α-la > αs-casein = κ-casein > β-lg > euglobulins (Ennis and Mulvihill, 2000).
An overview of milk protein layers adsorbed at oil–water interfaces and their relationship to the physicochemical stabilisation of emulsions is given in the following subsections.
5.2.1.1 Emulsions Stabilised by Caseins and Caseinates
Caseins, because of their surface activity, are known to adsorb strongly at an oil–water interface during emulsification, thus protecting the emulsion droplets against physicochemical instability (Dickinson, 1999b). The long-term stability of emulsions against coalescence can be attributed to both electrostatic and steric stabilisation effects (Dickinson, 2006). The relative absence of tertiary and secondary structure (Holt and Sawyer, 1988) and the presence of distinct hydrophobic and hydrophilic domains in the primary structure (Swaisgood, 1992) contribute to the relatively high surface activity of the caseins. However, as there is a lack of clarity of the native structure of caseins, the conformational changes upon adsorption at the oil droplet surface are not completely understood (Dalgleish, 2004).
Generally, α s1-casein and β-casein, which contribute almost 75 % of the total casein of milk, provide similar emulsifying properties based on their amino acid sequences (Swaisgood, 1982). Both α s1-casein and β-casein carry a net negative charge at neutral pH, are distinctly amphiphilic, have similarities in terms of linear disordered chains of around 200 residues with phosphoserine chains and have strong abilities to adsorb at oil–water interfaces. However, β-casein is reported to have higher surface activity and is more flexible in nature, because of its numerous proline residues, little ordered structure and negligible intermolecular cross-links, than α s1-casein (Swaisgood, 1982; Dickinson, 1994). Experimental analysis has shown that β-casein adsorbs to the droplet surface with its hydrophobic region strongly anchored to the oil phase and its hydrophilic region (4–50 residues at the N-terminal) protruding into the aqueous phase (Dalgleish, 1996a; Dickinson, 1999b). In contrast to the tail-like anchoring phenomenon of β-casein, α s1-casein has a loop-like conformation that binds to the droplet surface via peptides towards the middle of the sequence (compared with the end of the sequence in the case of β-casein).
Both β-casein and α s1-casein have been used to prepare stable oil-in-water emulsions (Dickinson, 1989, 1999b; Swaisgood, 1992). Both caseins lower interfacial tension at the oil–water interface but the rate of lowering of the interfacial tension is greater for β-casein than for α s1-casein. Moreover, α s1-casein-stabilised emulsion droplets have higher negative charge at neutral pH and are relatively more susceptible to flocculation at high ionic strengths than β-casein-stabilised emulsion droplets (Dickinson et al., 1988). Based on a few experimental studies, it can be inferred that α s1-casein accounts for less surface coverage, resulting in thinner interfacial films, than β-casein (Brooksbank et al., 1993; Dalgleish, 1993, 1996b). β-Casein, because of its relatively higher surface activity, also adsorbs preferentially at the oil–water interface compared to α s1-casein, and appears to displace α s1-casein from the droplet surface (Dickinson and Stainsby, 1988).
In the food industry, individual caseins are generally not used to prepare emulsions because of the cost of pure fractions and their sparse availability. Instead, various types of caseinate, such as sodium caseinate, are widely used in the preparation of emulsion-type products. Caseinates are produced from skim milk by lowering the pH to 4.6, by adding either lactic or hydrochloric acid or microbial cultures to precipitate the casein, then resolubilising it with alkali or alkaline salts of sodium, potassium or calcium at neutral pH followed by spray drying (Mulvihill, 1989). Sodium caseinate comprises not only α s1- and β-caseins but also κ- and αs2-caseins and small quantities of lipids and inorganic salts. The stability of an oil-in-water emulsion made with sodium caseinate largely depends on the composition of the adsorbed layer, the quantities of proteins and the conformation of the casein in the continuous phase. In sodium caseinate-stabilised oil-in-water emulsions, all forms of caseins (α s1-, α s2-, β- and κ-caseins) are adsorbed at the emulsion droplet surface (Robson and Dalgleish, 1987; Hunt and Dalgleish, 1994a; Srinivasan et al., 1996), providing stability against coalescence and flocculation.
In contrast to pure caseins, the competitive adsorption of β-casein rather than the other casein fractions in sodium caseinate appears to be driven by the total protein content or the volume ratio of caseinate to oil in the emulsion. It has been shown that β-casein adsorbs preferentially at the interface only at lower caseinate concentrations (<2.0 %) and/or when the ratio of caseinate to oil is low, i.e., when the caseins are predicted to exist as monomers (Srinivasan et al., 1996, 1999). However, at higher total caseinate concentrations (caseinate:oil ratio of > 1:60), α s1-casein adsorbs preferentially and β-casein loses its competitive adsorption ability; this has been attributed to the self-aggregating tendency of β-casein to form micelles or to complex with other casein fractions, such as α s1-casein, via hydrophobic interactions (Lucey et al., 2000). Furthermore, these aggregated complexes appear to have less emulsifying capability, as the hydrophobic areas are mutually blocked in the process of complex formation (Lorient et al., 1989). Irrespective of the caseinate concentration, κ-casein from sodium caseinate has been found to be least adsorbed at droplet surfaces.
The creaming stability of sodium caseinate emulsions (20–30 % w/w oil) shows a complex dependence on the caseinate content. At lower caseinate concentrations, the emulsion is destabilised by bridging flocculation because there is insufficient protein to fully cover all the droplets in the emulsion. At an intermediate caseinate concentration of about 2.0 % w/w, the emulsion is stabilised against flocculation, coalescence and creaming for several weeks as the protein content is sufficient to cover the droplet surface. However, when the caseinate concentration is increased to above 3.0 % w/w, unadsorbed caseinate gives rise to depletion flocculation (Dickinson and Golding, 1997; Srinivasan et al., 2001). Further increasing the protein concentration above 6.0 % w/w results in a very high degree of depletion flocculation, leading to a strong emulsion droplet network, which is stable to creaming.
Interestingly, concentration-dependent depletion flocculation is not common in whey-protein-stabilised emulsions. It appears that depletion flocculation in sodium caseinate-stabilised emulsions is caused by the presence of casein aggregates (sub-micelles) formed from the self-assembly of casein molecules in the aqueous phase of the emulsion at concentrations above 2 % w/w. The addition of moderate amounts of calcium chloride to emulsions containing excess sodium caseinate has been shown to eliminate depletion flocculation and to improve the creaming stability (Ye and Singh, 2001). This effect can be attributed to an increase in the average size of the casein aggregates in the aqueous phase, resulting in a large increase in the molecular mass of the caseins (Dickinson et al., 2001). In addition, there is a reduction in the concentration of unadsorbed caseinate.
5.2.1.2 Emulsions Stabilised by Whey Proteins
Whey proteins (β-lg, α-la, bovine serum albumin, lactoferrin and immunoglobulins) are characterised by three-dimensional structures that are held together by disulphide bridges (Kinsella, 1984). They are soluble over a wide pH range. Whey proteins in general are highly susceptible to thermal denaturation above 70 °C because of their globular nature (Kinsella and Whitehead, 1989; Hunt and Dalgleish, 1995; Singh, 2005). The most important whey protein fractions include β-lg and α-la, which account for ~70–80 % of the total whey protein and possess excellent emulsifying properties. These proteins adsorb on to oil–water interfaces and form stable emulsions, although the emulsions formed are slightly less stable than casein-stabilised emulsions under the same conditions (Hunt and Dalgleish, 1994a; Dalgleish, 1995).
Structurally, β-lg is a compact, folded, globular protein, containing 162 amino acids along with two disulphide bonds and one free thiol group (Swaisgood, 1982). The three-dimensional structure of β-lg comprises nine strands of anti-parallel β-sheets, joined together into a conical β-hydrophobic barrel unit, and a flanking three-turn α-helix (Sawyer et al., 1985; Papiz et al., 1986; Oliveira et al., 2001). Under ambient temperatures (~25 °C) and at neutral pH, β-lg exists mostly as a non-covalently linked dimer with a molecular weight of ~36 kDa (McKenzie and Sawyer, 1967; Ziegler and Foegeding, 1990). Because of its amphiphilic nature, β-lg shows good emulsifying properties by adsorbing at the interfacial layer, where it partially unfolds and forms a continuous interfacial film through intermolecular β-pleated sheet interactions. The exposed reactive free thiol groups at the interface lead to slow polymerisation of the adsorbed protein via sulphydryl–disulphide interchange mechanisms (Dickinson and Matsumura, 1991; McClements et al., 1993; Lefèvre and Subirade, 2003). β-Lg has been the most extensively studied of all food proteins for its role in stabilising oil-in-water emulsions, because of its well-defined structure and properties (McKenzie, 1971; Kinsella and Whitehead, 1989).
α-La, another major whey protein, is a globular, calcium metallo-protein, which is stabilised by four intra-chain disulphide bonds (Swaisgood, 1982). In contrast to β-lg, α-la does not contain a free thiol group. These two whey proteins also differ in their amino acid composition, with β-lg having more proline residues than α-la (eight and two, respectively), resulting in higher hydrophobicity, and α-la having more cysteine residues than β-lg (eight and five, respectively), resulting in more internal disulphide bridges (Ng-Kwai-Hang, 2003). α-La denatures at a relatively low temperature (~66 °C) compared with β-lg (~73 °C) but does not aggregate because of the absence of a free thiol group (Dalgleish et al., 1997; Schokker et al., 2000; Considine et al., 2007). Native α-la has good emulsifying capabilities, but has poor gelation properties.
Dickinson et al. (1989) studied competitive adsorption at the oil–water interface in emulsions stabilised by β-lg and α-la model systems using classical exchange measurements. They showed that competitive displacement between β-lg and α-la was rather slow and very limited, in contrast to that between caseins, which was shown to be much faster and essentially reversible in character (Dickinson et al., 1988). For instance, β-lg was not displaced from the interface in β-lg-stabilised emulsions when α-la was added at a level of 1:1 % w/w until a prolonged time period of 32 h. On increasing the proportion of α-la to β-lg (to 10:1), only 15 % of the β-lg was displaced to the serum phase. In contrast, on increasing the proportion of β-lg in an α-la-stabilised emulsion (to 10:1), nearly 30 % of the α-la was displaced. This suggests that, for β-lg and α-la model systems, the interfacial adsorption is relatively irreversible (in comparison with casein systems) and the protein that is initially introduced to the interface will probably dominate at the interface, irrespective of its relative surface activity (Dickinson et al., 1988, 1989). Both these whey proteins are highly structured globular proteins and undergo conformational changes upon adsorption at an interface (Fang and Dalgleish, 1997, 1998). However, of the two globular proteins, β-lg is even more difficult to displace than α-la. This can be attributed to the sulphydryl–disulphide interchange reactions that occur in β-lg-stabilised emulsions, but not in pure α-la-stabilised emulsions because of the absence of a free thiol group in α-la (Dickinson and Matsumura, 1991; Monahan et al., 1995; Damodaran and Anand, 1997). Upon adsorption, β-lg undergoes partial unfolding, stretches and becomes densely packed at the interface, enabling the free thiol group on each molecule of β-lg to link via intermolecular covalent disulphide bridges at the droplet interface. As the extent of polymerisation increases during storage, the interfacial film continues to strengthen irreversibly with time, resulting in high surface rheology (Dickinson, 1989; Damodaran and Anand, 1997; Dalgleish, 2004). Thus, its displacement from the interface by α-la becomes highly unlikely.
Whey proteins also contain low levels of lactoferrin, a glycoprotein of molecular weight ~80 kDa, which has about 700 amino acid residues and is well known for its iron-binding capacity (Baker and Baker, 2005). Unlike most milk proteins, which have isoelectric points (pIs) ranging from 4.5 to 5.5, lactoferrin has a relatively high pI of ~8.0 and thus has the unique property of possessing a high positive surface charge at neutral pH (almost +50 mV) (Ye and Singh, 2006a). This high positive charge density of lactoferrin has been predicted to allow the formation of cationic emulsion droplets over wide pH ranges. The adsorption behaviour of lactoferrin in oil-in-water emulsions was explored by Ye and Singh (2006a). Similar to other milk proteins such as caseinates and β-lg, lactoferrin adsorbed to the oil–water interface, producing stable emulsion droplets with a net positive charge.
In contrast to caseinates and β-lg, emulsions stabilised by lactoferrin were stable over a wide range of pH from 7.0 to 3.0. The droplet sizes of lactoferrin emulsions were reported to be very similar to those of β-lg emulsions prepared under the same conditions of pH, oil:protein ratio and homogenisation pressure. However, lactoferrin-stabilised emulsions had a comparatively higher surface coverage because of the higher molecular weight of lactoferrin. As lactoferrin in solution is highly positively charged, lactoferrin has been shown to exhibit electrostatic complexation with anionic β-lg at neutral pH (Wahlgren et al., 1993). Using this theory, multi-layered oil-in-water emulsions were produced from the electrostatic interactions of oppositely charged milk proteins, i.e., lactoferrin and β-lg, at neutral pH at the droplet surface, resulting in stable emulsion droplets with thick multi-layered interfacial layers and greater amounts of protein adsorbed at the oil–water interface (Ye and Singh, 2007). The primary emulsion, containing either cationic (lactoferrin-coated) or anionic (β-lg-coated) droplets, was produced initially. The secondary emulsion was then formed by adding either lactoferrin or β-lg solution to the primary emulsion based on opposite charges. Interestingly, the overall charge of emulsion droplets stabilised using the binary protein mixtures was close to zero at some concentrations. However, the multi-layered emulsions were protected against flocculation because of the strong steric effects of the dense interfacial film at the droplet surface.
In addition to pure proteins, the commercially available forms of whey protein that are widely used as emulsifiers in food industries are WPCs (comprising 25–80 % protein) and WPIs (comprising >90 % protein). These concentrated forms of whey protein are produced by ultrafiltration, diafiltration or ion exchange followed by drying steps to obtain protein levels of ~80–95 % (Morr and Ha, 1993; Mulvihill and Ennis, 2003). Both WPC and WPI are widely used in processed food applications because of their water-binding, gelling, foaming and surface-active properties (Mulvihill and Ennis, 2003; Singh, 2005). Processing treatments during the manufacture of WPC and WPI tend to denature some of the whey proteins because globular proteins are highly susceptible to conformational changes (denaturation) and aggregation when the pH, ionic strength or temperature is changed and this generally affects their functional properties.
It is interesting to note here that, when WPC or WPI is used to stabilise emulsions, similar in quantities to pure fractions of the individual proteins, there is little preferential adsorption of β-lg over α-la or vice versa at the droplet surface regardless of the proportion of protein to oil (Euston et al., 1996; Ye and Singh, 2000, 2006b). Ye and Singh (2000) showed that in WPC-stabilised emulsions (30 % w/w oil, 0.5 % w/w WPC), the proportions of adsorbed α-la and β-lg were ∼18 % or ∼82 %, respectively, compared with those in the original WPC solution (α-la ∼ 25 %; β-lg ∼ 75 %), suggesting that β-lg was adsorbed slightly in preference to α-la under these conditions.
Preferential adsorption of α-la and β-lg was more clearly demonstrated when WPI-stabilised emulsions were subjected to pH changes. Shimizu et al. (1981) showed that the total protein adsorption was highest at pH 5 in whey-protein-stabilised emulsions, possibly because of the dense network formed at a pH close to the pI. Interestingly, they also observed that the adsorption of β-lg decreased as a function of decreasing pH from 9 to 3, whereas the adsorption of α-la increased in the same pH range. This decrease in the adsorption of β-lg was attributed to pH-dependent conformational changes of tertiary and quaternary structures (Shimizu et al., 1985; Hunt and Dalgleish, 1994b).
In emulsions formed with both caseinate and whey protein, Hunt and Dalgleish (1994b) reported that the preferential adsorption depended on the protein concentration. This was further validated by a study by Ye (2008), which suggested that in emulsions made with mixtures of sodium caseinate and WPC, caseins adsorb preferentially at the oil–water interface at high protein concentrations whereas whey proteins adsorb preferentially at low protein concentrations (<3 %).
5.3 Formation and Stability of Protein-Stabilised Foams
Because of their surface-active properties, proteins are known to contribute to the formation of foams and to the physical stability of foam-based food formulations such as whipped cream, mousse and ice cream. Proteins are less effective than low molecular weight surfactants in reducing the air–water interfacial tension but they form an interfacial film that exhibits viscoelastic properties and that enables the foam to resist destabilisation (Murray and Ettelaie, 2004).
In general, foams can be generated by two mechanical methods, i.e., bubbling and stirring (Bikerman, 1973; Prud’homme and Khan, 1996; Exerowa and Kruglyakov, 1998; Weaire and Hutzler, 1999). In the bubbling method, the foam is produced by bubbling gas or air through the aqueous phase containing the foaming agent (protein, surfactants, etc.) using a single capillary, a set of capillaries or a porous plate. The size of the foam bubbles thus generated depends on the pore size of the capillaries or the porous plate, the properties of the surfactant solution, such as dynamic surface tension, surface elasticity and bulk viscosity, and the conditions of foam formation, i.e., rate of gas flow, temperature, pressure, etc. In contrast, the stirring method involves mixing the gaseous phase and the aqueous phase, which contains the foaming agent, mechanically using a stirrer or shaker or allowing simultaneous flow of the gas and the liquid in a tube.
The foaming capacity (or foamability) is defined either as the volume of foam generated under fixed conditions of temperature and intensity of mechanical agitation or by the time needed to generate a certain volume of foam. Both the foam formation process and foamability depend largely on the physicochemical properties of the stabilising substances. From a protein perspective, foam formation depends on the rate at which the proteins can transfer to the air–liquid interface and the stability of the foam generated depends on the ability of the adsorbed proteins to form a cohesive viscoelastic film via intermolecular bonds (Damodaran, 1997).
Similar to emulsions, foams also require high energy and subsequent thermodynamic instability makes them liable to separate into their two original phases over time. Thus, foams are also kinetically stable colloidal dispersions and undergo destabilisation over different time scales mainly by three mechanisms, i.e., liquid drainage, bubble coalescence and disproportionation of individual bubbles (Ivanov, 1988; Prud’homme and Khan, 1996; Exerowa and Kruglyakov, 1998; Weaire and Hutzler, 1999; Pereira et al., 2003; Denkov, 2004; Murray and Ettelaie, 2004; Saint-Jalmes et al., 2005; Denkov and Marinova, 2006). Drainage is driven mainly by gravity and involves a gradual rise of bubbles through the foam mass, while the aqueous phase drains through the lamellae and the plateau borders between the foam bubbles. In contrast, bubble coalescence involves thinning and rupturing of the isolated liquid interfacial films separating two neighbouring bubbles. Foam bubbles are stabilised against coalescence by the generation of strong colloidal forces that act between the film surfaces and the adsorption of surface-active molecules such as proteins to form a dense film. The third type of foam destabilisation is disproportionation, which involves bubble coarsening because of the diffusion of gas through the foam films, from the smaller bubbles to the larger bubbles.
5.3.1 Aspects of Foams Stabilised by Milk Proteins
Milk proteins are widely known for their foam-forming and foam-stabilising properties (Anderson and Brooker, 1988). Despite their high molecular weight and (in the case of whey proteins) their complex secondary and tertiary structures, milk proteins are able to diffuse from the aqueous phase and adsorb at the air–water interface during foam formation because of the compatibility of the hydrophobic regions of their structure with the hydrophobic nature of the gaseous phase. The different molecular structures of flexible and globular milk proteins generally lead to different structures of the adsorbed layers at the air–water interface. Milk proteins are well known to alter their charge and surface activity with pH and accordingly their foamability is also affected.
Marinova et al. (2009) compared the foaming behaviours of caseins and WPC as a function of pH and over a range of ionic strengths. As expected, both caseins and whey proteins showed a rapid increase in foam volume as a function of protein concentration until a plateau value was reached; there was a corresponding increase in protein adsorption at the air–water interface and a decrease in dynamic surface tension with increasing protein concentration. Both types of protein led to the stabilisation of foams against bubble coalescence. However, there were significant differences between foams stabilised with WPC and foams stabilised with caseins. For example, a relatively lower concentration of sodium caseinate (>0.3 % w/w) than of WPC (>1 % w/w) was needed to generate stable foam. Also, the volume of foam generated by WPC (10–11 mL) was nearly half of that generated by sodium caseinate (22–23 mL). When the pH was varied, WPC had maximum foamability near the pI whereas sodium caseinate had minimum foamability near the pI.
Marinova et al. (2009) explained these differences based on the molecular structures of the adsorbed milk protein layers and the different aggregation behaviours, as schematically presented in Fig. 5.2. At the natural pH (pH 6.5–6.8), i.e., far from the pI (pH 4.6), flexible casein molecules allowed the formation of denser adsorbed layers comprising hydrophobic amino acid residues in a “loop”-like configuration and the hydrophilic chain extending farther away as a “tail” within the serum phase, thus ensuring better foam stabilisation (Dickinson et al., 1993) (Fig. 5.2). In contrast, WPC could not anchor strongly at the surface of the foam bubbles because of its intact globular conformation, at least initially (Gurkov et al., 2003; Freer et al., 2004). The minimum foamability of sodium caseinate near the pI can be explained on the basis of the unavailability of sufficient quantities of casein for adsorption at the surfaces because of the precipitation of casein at the pI. However, in the case of whey proteins at pH 4.5, slightly positively charged β-lg (pI 5.1), and slightly negatively charged α-la (pI 4.5), may have interacted and strengthened the compaction of the molecules at the air–water interface via electrostatic interactions and thus allowed stronger interfacial films, ensuring better bubble coverage and thus high foamability. This is in line with the results obtained by other authors; it was found that, even in mixed protein systems, globular whey proteins, such as β-lg, and flexible random coil caseins, such as β-caseins, typically have different adsorption rates and also different foaming abilities because of their different abilities to adapt their conformation at the air–serum interface (Martin et al., 2002; Ridout et al., 2004).
Schematic presentation of sodium caseinate (a) and WPC (b) molecules adsorbed at the water–air interface (Marinova et al., 2009: reproduced with the permission of Elsevier Inc.)
Interestingly, the foaming properties of β-lg and WPI can be improved by increasing the ionic strength in the range 0–0.1 M NaCl at pH levels above or below their pI, as evidenced by dynamic surface tension measurements (Davis et al., 2004; Zhang et al., 2004). This is because of the charge screening effect of the salt, which allows the proteins to be compacted at the interface, resulting in increased protein adsorption, and the formation of a strong viscoelastic network, which provides stabilisation of the foam against bubble coalescence.
Furthermore, certain chemical (Enomoto et al., 2007; Wooster and Augustin, 2007), physical (Phillips et al., 1990; Yang et al., 2001) or enzymatic (Davis et al., 2005) treatments are also known to improve the foaming properties of whey proteins. These treatments generally influence the conformation of whey proteins, especially β-lg, by modifying the protein structure, and thus alter the kinetics of protein adsorption at the interface, the time needed for the whey protein to rearrange upon adsorption at the interface and the ability to interact with other interfacial proteins, in the case of mixed systems.
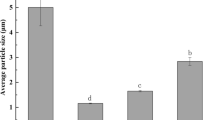
The effects of heat treatment on the foaming properties of β-lg have been studied because heat treatment is commonly used in food processing to make many food foams. When native β-lg was compared with thermally treated β-lg, it was demonstrated that heated β-lg had higher surface hydrophobicity, adsorbed at a much faster rate at the air–water interface and had better foaming properties with respect to the initial rheology of the interfacial film (Phillips et al., 1995; Kim et al., 2005; Croguennec et al., 2006). It has been shown that β-lg aggregates produced by heat treatment of β-lg solution at 85 °C for 15 min can significantly improve the foaming properties (Schmitt et al., 2007; Unterhaslberger et al., 2007; Rullier et al., 2008). Moro et al., (2011) showed that preheating a 5.5 % (w/v) β-lg solution at 85 °C for 3 min generated a considerable change in its aggregation profile, producing non-native monomers (51 %) and dimers (33 %) and trimers (16 %). Because of the formation of these polymeric aggregates, the surface hydrophobicity was increased dramatically, which in turn improved the foamability; the foamability was of the order of ~800 % higher than that of the corresponding foam formed with unheated β-lg. This greater foam stability against disproportionation or collapse was attributed to an increase in the viscosity of the protein solution because of the presence of aggregates, which slowed the rate of liquid drainage because of compaction of the interfacial film.
As well as the individual proteins, the interactions between β-lg and α-la during the thermal treatment of WPI have been found to play a significant role in the foaming properties and the stability of foams. Zhu and Damodaran (1994) showed that the heat treatment of WPI at neutral pH generated aggregates that improved foamability (at a monomeric:polymeric ratio of 60:40) or foam stability (at a monomeric:polymeric ratio of 40:60). Davis and Foegeding (2004) further confirmed these findings by showing similar foam stability improvements even when native whey protein was mixed with whey protein polymers generated by heat treatment at a similar monomeric:polymeric ratio of 40:60. This can be explained on the basis of rapid movement of monomeric whey protein at the interface upon foaming to decrease the surface tension followed by the formation of a viscoelastic interfacial network (mainly driven by disulphide bond formation and hydrophobic interactions) at the interface by the soluble aggregates. Nicorescu et al. (2009) showed that an optimal heat treatment of 2 % w/v WPI at 80 °C at ionic strength of 50 mM NaCl and at neutral pH was effective in obtaining an improved firmness of the interfacial films because of the simultaneous formation of a cohesive network of protein aggregates (the generation of approximately 10 % soluble whey protein aggregates) at the interface and throughout the foam lamellae, which enhanced stability against liquid drainage. However, at temperatures of 80–100 °C, the generation of more than 50 % soluble aggregates caused weakening of the interfacial network because the presence of a large number of heavy clusters of aggregates, which behaved as a “solid”, led to more rapid drainage.
Our understanding of the role of the molecular structure and the processing of milk proteins on the formation and stability of foams has advanced significantly over recent years. For the future, it is crucial to understand how factors such as protein–protein interactions, interactions with other ingredients such as sugar, the emulsifiers in food foams and the interfacial composition of mixed interfaces contribute to foam stability holistically and the generation of “novel” foam properties.
5.4 Interactions of Milk-Protein-Stabilised Emulsions Under Physiological Conditions
For the last decade or so, the effects of processing (e.g., heat, high pressure and shear) on the properties of food emulsions (e.g., viscosity, droplet size distribution and phase stability) have been studied extensively. Interestingly, much of this work has involved the use of milk proteins and has focused on understanding the functionality of milk proteins in stabilising emulsions and exploiting their unique properties to produce novel structures and sensory perceptions. In contrast, efforts to elucidate the fate of milk-protein-stabilised emulsions following consumption during in vitro gastrointestinal digestion are relatively recent and are now generating a great deal of interest.
When a complex food emulsion is consumed, the properties of each of its components, together with its interactions with physiological factors, including mucin, pepsin, lipase, gastric mucins and phospholipids, should be considered. These biochemical agents may interact with the emulsion and result in modification of the adsorbed protein layers and the droplet characteristics, affecting the stability of the emulsion and the digestibility of its components (Fig. 5.1). Although emulsions can be carefully manipulated using various physical or chemical processes before they are consumed, understanding of the interactions during transit through the gastrointestinal tract is of great importance to gain insights into the post-consumption structural and physicochemical changes in these emulsions. As this chapter is concerned with milk proteins, we briefly describe the behaviour of milk-protein-stabilised emulsions in physiological environments and discuss how the milk protein-based interfacial layer influences the various steps involved in the digestibility of emulsified lipids.
When a milk-protein-stabilised food emulsion is consumed, it resides for a short period in the mouth and is exposed to a wide range of biochemical conditions, such as dilution effects, because of mixing with saliva, and access to salivary enzymes such as amylases, biopolymers such as mucins and different electrolytes in the saliva, as well as physicochemical conditions, such as moderate changes in pH and temperature (to around 37 °C) and shear forces between the tongue and the oral palate (Malone et al., 2003a, b; de Wijk et al., 2004; de Wijk and Prinz, 2005; Vingerhoeds et al., 2005). Interestingly, there is some evidence to show that the behaviour of milk-protein-stabilised emulsions in the mouth is largely driven by the non-covalent interactions of salivary components with the adsorbed milk protein layer at the oil droplet surface (van Aken et al., 2005; Sarkar et al., 2009a; Vingerhoeds et al., 2009). Emulsions formed with WPI, sodium caseinate and lactoferrin showed flocculation of the droplets when mixed with human saliva. This flocculation was predominantly driven by the highly glycosylated negatively-charged mucin present in human saliva. The emulsion flocculation in the presence of saliva was considered to be regulated by depletion forces, van der Waals’ forces and/or electrostatic interactions between emulsion droplets and salivary proteins, and was largely dependent on the initial charge of the milk-protein-stabilised emulsion droplets (Silletti et al., 2007; Sarkar et al., 2009a).
Sarkar et al. (2009a) investigated the behaviour of negatively and positively charged oil-in-water emulsions stabilised by milk proteins in the presence of artificial human saliva. At neutral pH, negatively-charged β-lg-stabilised emulsions underwent some degree of depletion flocculation because of strong repulsive forces with anionic mucin. In contrast, positively charged lactoferrin-stabilised emulsions interacted with mucin via electrostatic interactions, which led to bridging-type flocculation under certain conditions. These kinds of emulsion–saliva electrostatic interactions might occur when emulsions are consumed in real situations and could result in different sensory and textural perceptions in vivo.
After oral transit, emulsions are swallowed, are subjected to shear effects in the oesophagus and are finally exposed to a highly acidic pH (typically between 1 and 3) and shear forces because of peristaltic movements of the stomach (Weisbrodt, 2001; Kalantzi et al., 2006). During the gastric phase, emulsions are exposed to digestive juices, containing proteolytic (pepsin) and lipolytic (gastric lipase) enzymes, mucins and salts. It is obvious that milk-protein-stabilised emulsions would undergo major changes in the stomach because of the possible action of pepsin on the protein layer at the interface, the effects of low pH and ionic strength on the droplet charge and the interactions of gastric mucin with interfacial protein. For example, flexible caseins are highly susceptible to hydrolysis by pepsin in aqueous solutions (Guo et al., 1995). However, globular whey proteins, particularly β-lg, are known to be largely resistant to peptic hydrolysis in their native state (Schmidt and Poll, 1991).
Studies by Macierzanka et al. (2009) and Sarkar et al. (2009b), which are in agreement with other reports, suggest that β-lg becomes highly prone to hydrolysis by pepsin when present as the interfacial layer in an emulsion. This is due to a conformational change of the β-lg molecules upon adsorption at the droplet surface, which opens up the peptic cleavage sites for enzymatic attack by pepsin. Interestingly, in milk-protein-stabilised emulsions, generally not all the protein is present at the droplet surface, i.e., in the adsorbed state; a considerable proportion remains in the aqueous phase. As the adsorbed and unadsorbed proteins are likely to exist in different conformational states, their susceptibilities to pepsin could be different under gastric conditions; the rate of hydrolysis of β-lg, from sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE) gels, is shown in Fig. 5.3. Sarkar et al. (2009b) showed that adsorbed β-lg was more susceptible to pepsin hydrolysis (85 % decrease in the intact protein) than unadsorbed protein (about 50 % decrease in the intact protein). In contrast, β-lg in solution in its native state was largely resistant to pepsin digestion (about 20 % decrease in the intact protein) under the same simulated gastric conditions. This suggested that the conformation of unadsorbed β-lg is significantly altered during emulsion formation, possibly as a result of the high turbulence during homogenisation, as mentioned in the previous section.
Sodium dodecyl sulphate polyacrylamide gel electrophoretograms of β-lg emulsions: (a) cream phase; (b) continuous phase; (c) native β-lg solutions [containing 0.36 % β-lg (the same as the concentration of β-lg in the continuous phase of the emulsion)] after mixing with simulated gastric fluid as a function of incubation time (Sarkar et al., 2009b: reproduced with the permission of Elsevier Inc.)
The hydrolysis of the adsorbed layers by pepsin also results in a loss of positive charge on the droplet surface as well as a reduction in the thickness of the adsorbed layer (Sarkar et al., 2009b). The peptides that remain at the interface are unable to provide sufficient electrostatic and steric stabilisation effects. As a result, these emulsions with hydrolysed interfaces are highly susceptible to flocculation and coalescence (Fig. 5.1). Such a phenomenon has been demonstrated for lactoferrin- and β-lg-stabilised emulsions, which undergo flocculation followed by some degree of coalescence on exposure to simulated gastric conditions (Sarkar et al., 2009b; Sarkar, 2010).
In addition to the important effect of gastric enzymes, highly glycosylated mucin, which forms a self-associated gel-like structure at gastric pH and protects the stomach from digesting itself, also has an important role in its interaction with milk-protein-stabilised emulsions (Bansil and Turner, 2006). The work of Sarkar et al. (2010a) suggests that the addition of a low level of soluble mucin promotes the flocculation of β-lg-stabilised emulsions, possibly through a bridging mechanism, but does not significantly affect the action of pepsin on the adsorbed β-lg layer. Overall, when they reach the gastric tract, emulsions stabilised by milk proteins show a significant level of instability, which is predominantly driven by the proteolytic effect of pepsin.
Following transit through the gastric tract, emulsions enter the human intestinal tract, which is a very complex environment, as it contains various salts, pancreatic enzymes, coenzymes, bile salts, phospholipids, remnants of oral and gastric digestion and various microbial species at neutral to alkaline pH (6.0–7.5) (McClements et al., 2009; Singh et al., 2009; Singh and Ye, 2013). As milk protein might be susceptible to the actions of pancreatic enzymes such as trypsin and chymotrypsin, both the adsorbed proteins/peptides and the unadsorbed proteins/peptides might be further hydrolysed into smaller peptides and amino acids. Over the last few decades, several studies have reported the effects of the hydrolysis of milk-protein-stabilised oil-in-water emulsions by trypsin on their physical stability (Kaminogawa et al., 1987; Leaver and Dalgleish, 1990). As expected, extensive hydrolysis of adsorbed protein layers results in rupturing of the interfacial layer, leading to the coalescence of emulsion droplets and subsequent oiling-off (Agboola and Dalgleish, 1996). However, the behaviour of milk protein-based emulsions in a more complex intestinal environment, simulating intestinal conditions, has not been reported until very recently, by Sarkar et al. (2010b, c).
In addition to intestinal enzymes, the interaction of surface-active bile salts with the adsorbed protein layer are important. Bile salts, which originate from the liver via the gall bladder, consist mainly of sodium salts of taurocholic, taurodeoxycholic, taurochenodeoxycholic, glycocholic and glycodeoxycholic acids. These surface-active compounds displace the adsorbed proteins/peptides from the surface of emulsion droplets because of their relatively higher surface activity, thus promoting the accessibility of the active site of lipase to the hydrophobic lipid core (Wickham et al., 1998; Fave et al., 2004; Mun et al., 2007; Sarkar et al., 2010b). The nature and, in particular, the charge of the adsorbed milk protein layer seem to drive the preferential adsorption of bile salts and the subsequent displacement of the protein layer (Singh et al., 2009). For instance, bile salts have been shown to displace whey proteins more readily than caseinates from the interface of emulsion droplets during storage (Mun et al., 2007). In emulsions stabilised by negatively charged β-lg, displacement of protein was observed even at the lowest concentration of bile salts (Sarkar et al., 2010b), but the bile salts did not displace positively-charged lactoferrin from the emulsion droplets. The bile salts appeared to bind to the adsorbed lactoferrin layer via an electrostatic mechanism.
Upon entering the intestine, pancreatic lipase adsorbs to the droplet interface, usually via complexation with colipase and/or bile salts (Bauer et al., 2005). Colipase is a short polypeptide with a molecular weight of 10 kDa, which forms a stoichiometric complex with lipase in a 1:1 w/w ratio, enabling the water-soluble pancreatic lipase to attach firmly to the hydrophobic lipid core at the oil droplet surface (Erlanson-Albertsson, 1992). Bile salts may either facilitate or inhibit the activity of pancreatic lipase depending on their concentration (Lowe, 2002; Bauer et al., 2005). At low concentrations, bile salts promote pancreatic lipase activity, mainly by allowing the adsorption of lipase to the oil–water interface (Gargouri et al., 1983; Mun et al., 2007) as well as solubilising and removing the inhibitory reaction products from the oil–water interface. However, at high concentrations, bile salts generally compete with lipases for adherence to the droplet surface, thus inhibiting the point of contact between the hydrophobic lipid core and the lipase (Gargouri et al., 1983) and retarding lipase activity. Pancreatic lipase cleaves triacylglycerols to form 2-monoacylglycerols and fatty acids; some of these digestion products are surface active and could potentially displace the initial adsorbed material from the droplet surface (McClements et al., 2009; Singh et al., 2009; Sarkar et al., 2010c).
Most studies of lipid digestion in milk protein-based emulsions have used in vitro intestinal models containing pancreatic lipase and bile salts. The extent of lipid hydrolysis was found to be similar in caseinate- and whey protein-stabilised emulsions, although the oil droplets in the whey protein-stabilised emulsions were less stable (Mun et al., 2007). Our study showed that lactoferrin- and β-lg-stabilised emulsions underwent a significant degree of coalescence on the addition of physiological concentrations of pancreatin and bile salts (Sarkar et al., 2010c). For both emulsions, destabilisation in simulated intestinal fluid was largely attributed to the lipolysis of the hydrophobic lipid core by the lipase fractions of the pancreatin as well as the proteolysis of the adsorbed protein layer by the trypsin or other proteolytic fractions present in pancreatin.
In addition to pure whey protein systems, studies on the in vitro digestion of WPI emulsions showed that they did not undergo pronounced structural changes during simulated gastric digestion although the α-la and a portion of the β-lg adsorbed at the interface were hydrolysed by pepsin. However, during the subsequent intestinal phase of digestion, the partially digested WPI-stabilised emulsion droplets underwent coalescence (Li et al., 2013). In contrast, in the case of sodium caseinate-stabilised emulsions, the droplets underwent droplet flocculation with some degree of coalescence during the gastric phase itself (Li et al., 2012). Because of its open flexible structure, casein was easily hydrolysed by pepsin, which in turn led to coalescence of droplets under gastric conditions. Overall, at both sodium caseinate- and whey-protein-stabilised interfaces, digestion in the gastric fluid containing pepsin apparently accelerated the coalescence of the emulsion droplets during subsequent exposure to intestinal fluid containing pancreatic lipase. However, for both milk-protein-stabilised emulsions, the rate and the extent of lipid digestion in the intestinal environment were found not to be influenced by the previous structural changes that may have occurred during the gastric phase.
Recently, a number of researchers (Hur et al., 2009; Li et al., 2012; Kenmogne-Domguia et al., 2013) have studied the effect of proteolysis of the adsorbed milk protein layer and subsequent physicochemical changes of the emulsion droplets during the entire physiological transit. Emulsions stabilised by casein and bovine serum albumin were treated under in vitro gastric conditions at various pH values and at various concentrations of pepsin, as a function of incubation time. The adsorbed protein was hydrolysed to different degrees by pepsin, which resulted in droplet flocculation and coalescence in the emulsions. When these gastric-treated samples were exposed to in vitro intestinal digestion, the results showed that gastric conditions could modify the kinetics of lipolysis, but had limited impact on the final extent of lipolysis in the intestinal step of digestion.
Studies in our laboratory on lipid droplets initially coated with lactoferrin (cationic) and β-lg (anionic) and sequentially treated with simulated oral, gastric and intestinal fluids in an in vitro physiological model further validated that milk-protein-stabilised interfaces, irrespective of their high original electrostatic charges, offer little protection to the droplets against pepsin- and pancreatin-induced destabilisation and thus cannot help, individually, in controlling the rate and the extent of lipid digestion (Sarkar, 2010; Singh and Sarkar, 2011). The mechanism of destabilisation and re-stabilisation in intestinal fluid following pre-processing in oral and gastric fluids could not be interpreted reliably because of interference from one or more of the factors in the chemically complex, simulated physiological media used. There is a clear need for further research in this area to have a better understanding of the different competitive displacement mechanisms and hydrolytic reactions occurring in the intestine and to characterise the final state of the droplets and the products of lipid hydrolysis. More complete in vitro digestion models to simulate various physiological processes occurring in the mouth, stomach and small intestine need to be developed and then validated by in vivo and clinical studies. Further research in this area is likely to lead to new knowledge that can be used in designing food matrices by manipulating milk proteins effectively at the oil–water interface during physiological transit for controlled lipid delivery applications.
5.5 Conclusions
Milk proteins in both soluble and dispersed forms have excellent surface-active, foaming and emulsion-stabilising properties. Differences in structure, flexibility and state of aggregation of the different milk proteins give rise to differences in their emulsion- and foam-stabilising properties. These attributes of milk proteins have been exploited to manufacture various prepared foods. For decades, research has been performed on oil-in-water emulsions and foams using purified or simple mixtures of caseins and whey proteins to manufacture a wide range of products and there is now a great deal of understanding on the conformation of proteins at oil–water interfaces, competitive exchange reactions and factors controlling the rheology and stability of emulsions under different environmental conditions (temperature, pH and ionic conditions). However, much less is known about the further processing of emulsions after they have been consumed, i.e., during oral processing in the mouth and during the digestion processes. This area of research needs to be developed further before the interactions between milk-protein-stabilised emulsions and physiological factors can be carefully utilised to develop novel products with sensory and/or health benefits.
References
Agnboola SO, Dalgleish DG (1996) Enzymatic hydrolysis of milk proteins used for emulsion formation. 1 Kinetics of protein breakdown and storage stability of the emulsions. J Agric Food Chem 44:3631–3636
Agboola SO, Singh H, Munro PA, Dalgleish DG, Singh AM (1998) Destabilization of oil-in-water emulsions formed using highly hydrolyzed whey proteins. J Agric Food Chem 46:84–90
Anderson M, Brooker BE (1988) Dairy foams. In: Stainsby G, Dickinson E (eds) Advances in food emulsions and foams. Elsevier Applied Science, London, pp 221–255
Baker EN, Baker HM (2005) Molecular structure, binding properties and dynamics of lactoferrin. Cell Mol Life Sci 62:2531–2539
Bansil R, Turner BS (2006) Mucin structure, aggregation, physiological functions and biomedical applications. Curr Opin Colloid Interface Sci 11:164–170
Bauer E, Jakob S, Mosenthin R (2005) Principles of physiology of lipid digestion. Asian Aust J Anim Sci 18:282–295
Bikerman JJ (1973) Foams. Springer, Berlin
Brooksbank DV, Leaver J, Horne DS (1993) Adsorption of milk-proteins to phosphatidyl-glycerol and phosphatidyl choline liposomes. J Colloid Interface Sci 161:38–42
Considine T, Patel HA, Anema SG, Singh H, Creamer LK (2007) Interactions of milk proteins during heat and high hydrostatic pressure treatments—a review. Innovat Food Sci Emerg Technol 8:1–23
Croguennec T, Renault A, Bouhallab S, Pezennec S (2006) Interfacial and foaming properties of sulfydryl-modified bovine β-lactoglobulin. J Colloid Interface Sci 302:32–39
Dalgleish DG (1990) The conformations of proteins on solid/water interfaces—caseins and phosvitin on polystyrene latices. Colloids Surf 46:141–155
Dalgleish DG (1993) The sizes and conformations of the proteins in adsorbed layers of individual caseins on latices and in oil-in-water interfaces. Colloids Surf B 1:1–8
Dalgleish DG (1995) Structures and properties of adsorbed layers in emulsions containing milk proteins. In: Lorient D, Dickinson E (eds) Food macromolecules and colloids. Royal Society of Chemistry, Cambridge, pp 23–34
Dalgleish DG (1996a) Conformations and structures of milk proteins adsorbed to oil–water interfaces. Food Res Int 29:541–547
Dalgleish DG (1996b) Food emulsions. In: Sjöblom J (ed) Emulsions and emulsion stability. Marcel Dekker, New York, pp 287–325
Dalgleish DG (1997) Adsorption of protein and the stability of emulsions. Trends Food Sci Technol 8:1–6
Dalgleish DG (2004) Food emulsions: their structures and properties. In: Friberg SE, Larsson K, Sjöblom J (eds) Food emulsions. Marcel Dekker, New York, pp 1–44
Dalgleish DG (2006) Food emulsions—their structures and structure-forming properties. Food Hydrocoll 20:415–422
Dalgleish DG, Senaratne V, Francois S (1997) Interactions between α-lactalbumin and β-lactoglobulin in the early stages of heat denaturation. J Agric Food Chem 45:3459–3464
Damodaran S (1997) Protein-stabilized foams and emulsions. In: Paraf A, Damodaran S (eds) Food proteins and their application. Marcel Dekker, New York, pp 57–110
Damodaran S (2005) Protein stabilization of emulsions and foams. J Food Sci 70:R54–R66
Damodaran S, Anand K (1997) Sulfhydryl–disulfide interchange-induced interparticle protein polymerization in whey protein-stabilized emulsions and its relation to emulsion stability. J Agric Food Chem 45:3813–3820
Davis JP, Foegeding EA (2004) Foaming and interfacial properties of polymerized whey protein isolate. J Food Sci 69:C404–C410
Davis JP, Foegeding EA, Hansen FK (2004) Electrostatic effects on the yield stress of whey protein isolate foams. Colloids Surf B 34:13–23
Davis JP, Doucet D, Foegeding EA (2005) Foaming and interfacial properties of hydrolyzed β-lactoglobulin. J Colloid Interface Sci 288:412–422
de Wijk RA, Prinz JF (2005) The role of friction in perceived oral texture. Food Qual Prefer 16:121–129
de Wijk RA, Prinz JF, Engelen L, Weenen H (2004) The role of a-amylase in the perception of oral texture and flavour in custards. Physiol Behav 83:81–91
Denkov ND (2004) Mechanisms of foam destruction by oil-based antifoams. Langmuir 20:9463–9505
Denkov ND, Marinova KG (2006) Antifoam effects of solid particles, oil drops and oil-solid compounds in aqueous foams. In: Binks BP, Horozov TS (eds) Colloidal particles at liquid interfaces. Cambridge University Press, Cambridge, pp 383–444
Dickinson E (1989) Surface and emulsifying properties of caseins. J Dairy Res 56:471–477
Dickinson E (1994) Protein-stabilized emulsions. J Food Eng 22:59–74
Dickinson E (1998) Proteins at interfaces and in emulsions: stability, rheology and interactions. J Chem Soc Faraday Trans 94:1657–1669
Dickinson E (1999a) Adsorbed protein layers at fluid interfaces: interactions, structure and surface rheology. Colloids Surf B 15:161–176
Dickinson E (1999b) Caseins in emulsions: interfacial properties and interactions. Int Dairy J 9:305–312
Dickinson E (2001) Milk protein interfacial layers and the relationship to emulsion stability and rheology. Colloids Surf B 20:197–210
Dickinson E (2003) Hydrocolloids at interfaces and the influence on the properties of dispersed systems. Food Hydrocoll 17:25–39
Dickinson E (2006) Structure formation in casein-based gels, foams, and emulsions. Colloids Surf A 288:3–11
Dickinson E, Golding M (1997) Depletion flocculation of emulsions containing unadsorbed sodium caseinate. Food Hydrocoll 11:13–18
Dickinson E, Matsumura Y (1991) Time-dependent polymerization of β-lactoglobulin through disulfide bonds at the oil–water interface in emulsions. Int J Biol Macromol 13:26–30
Dickinson E, McClements DJ (1995) Advances in food colloids. Blackie Academic and Professional, London
Dickinson E, Patino JMR (1999) Food emulsions and foams—interfaces, interactions and stability. Royal Society of Chemistry, London
Dickinson E, Stainsby G (1988) Emulsion stability. In: Stainsby G, Dickinson E (eds) Advances in food emulsions and foams. Elsevier Applied Science, London, pp 1–44
Dickinson E, Rolfe SE, Dalgleish DG (1988) Competitive adsorption of α s1 -casein and β-casein in oil-in-water emulsions. Food Hydrocoll 2:397–405
Dickinson E, Rolfe SE, Dalgleish DG (1989) Competitive adsorption in oil in water emulsions containing alpha lactalbumin and beta lactoglobulin. Food Hydrocoll 3:193–203
Dickinson E, Horne DS, Phipps JS, Richardson RM (1993) A neutron reflectivity study of the adsorption of beta-casein at fluid interfaces. Langmuir 9:242–248
Dickinson E, Semenova MG, Belyakova LE, Antipova AS, Il’in MM, Tsapkina EN, Ritzoulis C (2001) Analysis of light scattering data on the calcium ion sensitivity of caseinate solution thermodynamics: relationship to emulsion flocculation. J Colloid Interface Sci 239:87–97
Ennis MP, Mulvihill DM (2000) Milk proteins. In: Phillips GO, Williams PA (eds) Handbook of hydrocolloids. Woodhead, Cambridge, pp 189–217
Enomoto H, Li CP, Morizane K, Ibrahim HR, Sugimoto Y, Ohki S, Ohtomo H, Aoki T (2007) Glycation and phosphorylation of β-lactoglobulin by dry-heating: effect on protein structure and some properties. J Agric Food Chem 55:2392–2398
Erlanson-Albertsson C (1992) Pancreatic colipase. Structural and physiological aspects. Biochim Biophys Acta 1125:1–7
Euston SR, Hirst RL (1999) Comparison of the concentration-dependent emulsifying properties of protein products containing aggregated and non-aggregated milk protein. Int Dairy J 9:693–701
Euston SE, Singh H, Munro PA, Dalgleish DG (1996) Oil-in-water emulsions stabilized by sodium caseinate or whey protein isolate as influenced by glycerol monostearate. J Food Sci 61:916–920
Exerowa D, Kruglyakov PM (1998) Foams and foam films: theory, experiment, application. Elsevier, Amsterdam
Fang Y, Dalgleish DG (1997) Conformation of β-lactoglobulin studied by FTIR: effect of pH, temperature, and adsorption to the oil–water interface. J Colloid Interface Sci 196:292–298
Fang Y, Dalgleish DG (1998) The conformation of α-lactalbumin as a function of pH, heat treatment and adsorption at hydrophobic surfaces studied by FTIR. Food Hydrocoll 12:121–126
Fave G, Coste TC, Armand M (2004) Physicochemical properties of lipids: new strategies to manage fatty acid bioavailability. Cell Mol Biol 50:815–831
Fox PF (2009) Milk: an overview. In: Thompson A, Boland M, Singh H (eds) Milk proteins: from expression to food. Academic, New York, pp 1–44
Freer EM, Yim KS, Fuller GG, Radke CJ (2004) Interfacial rheology of globular and flexible proteins at the hexadecane/water interface: comparison of shear and dilatation deformation. J Phys Chem B 108:3835–3844
Gargouri Y, Julien R, Bois AG, Verger R, Sarda L (1983) Studies on the detergent inhibition of pancreatic lipase activity. J Lipid Res 24:1336–1342
Golding M, Wooster TJ (2010) The influence of emulsion structure and stability on lipid digestion. Curr Opin Colloid Interface Sci 15:90–101
Guo MR, Fox PF, Flynn A, Kindstedt PS (1995) Susceptibility of β-lactoglobulin and sodium caseinate to proteolysis by pepsin and trypsin. J Dairy Sci 78:2336–2344
Gurkov TD, Russev SC, Danov KD, Ivanov IB, Campbell B (2003) Monolayers of globular proteins on air/water interface: applicability of the Volmer equation of state. Langmuir 19:7362–7369
Holt C, Sawyer L (1988) Primary and predicted secondary structures of the caseins in relation to their biological functions. Protein Eng 24:251–259
Hunt JA, Dalgleish DG (1994a) Adsorption behaviour of whey protein isolate and caseinate in soya oil-in-water emulsions. Food Hydrocoll 8:175–187
Hunt JA, Dalgleish DG (1994b) Effect of pH on the stability and surface composition of emulsions made with whey protein isolate. J Agric Food Chem 42:2131–2135
Hunt JA, Dalgleish DG (1995) Heat stability of oil-in-water emulsions containing milk proteins: effect of ionic strength and pH. J Food Sci 60:1120–1123
Hunter RJ (1989) Foundations of colloid science, vol 2. Oxford University Press, Oxford
Hur SJ, Decker EA, McClements DJ (2009) Influence of initial emulsifier type on microstructural changes occurring in emulsified lipids during in vitro digestion. Food Chem 114:253–262
Ivanov IB (1988) Thin liquid films: fundamentals and applications. Marcel Dekker, New York
Kalantzi L, Goumas K, Kalioras V, Abrahamsson B, Dressman J, Reppas C (2006) Characterization of the human upper gastrointestinal contents under conditions simulating bioavailability/bioequivalence studies. Pharm Res 23:165–176
Kaminogawa S, Shimizu M, Ametai A, Lee S, Yamauchi K (1987) Proteolysis in structural analysis of αs1-casein adsorbed onto oil surfaces of emulsions and improvement of the emulsifying properties of protein. J Am Oil Chem Soc 64:1688–1691
Kenmogne-Domguia HB, Meynier A, Viau M, Llamas G, Genot C (2013) Gastric conditions control both the evolution of the organization of protein-stabilized emulsions and the kinetic of lipolysis during in vitro digestion. Food Funct 3:1302–1309
Kim DA, Cornec M, Narsimhan G (2005) Effect of thermal treatment on interfacial properties of β-lactoglobulin. J Colloid Interface Sci 285:100–109
Kinsella JE (1984) Milk proteins: physical and functional properties. Crit Rev Food Sci Nutr 21:197–262
Kinsella JE, Whitehead DM (1989) Proteins in whey: chemical and physical and functional properties. Adv Food Nutr Res 33:343–438
Le Révérend BJD, Norton IT, Cox PW, Spyropoulos F (2010) Colloidal aspects of eating. Curr Opin Colloid Interface Sci 15:84–89
Leaver J, Dalgleish DG (1990) The topography of bovine beta-casein at an oil/water interface as determined from the kinetics of trypsin-catalysed hydrolysis. Biochim Biophys Acta 1041:217–222
Lefèvre T, Subirade M (2003) Formation of intermolecular β-sheet structures: a phenomenon relevant to protein film structure at oil–water interfaces of emulsions. J Colloid Interface Sci 263:59–67
Li J, Ye A, Lee SJ, Singh H (2012) Influence of gastric digestive reaction on subsequent in vitro intestinal digestion of sodium caseinate-stabilized emulsions. Food Funct 3:320–326
Li J, Ye A, Lee SJ, Singh H (2013) Physicochemical behaviour of WPI-stabilized emulsions in in vitro gastric and intestinal conditions. Colloids Surf B 1:80–87
Lorient D, Closs B, Courthaudon JL (1989) Surface properties of the bovine casein components: relationships between structure and foaming properties. J Dairy Res 56:495–502
Lowe ME (2002) The triglyceride lipases of the pancreas. J Lipid Res 43:2007–2016
Lucey JA, Srinivasan M, Singh H, Munro PA (2000) Characterization of commercial and experimental sodium caseinates by multiangle laser light scattering and size-exclusion chromatography. J Agric Food Chem 48:1610–1616
Macierzanka A, Sancho AI, Mills ENC, Rigby NM, Mackie AR (2009) Emulsification alters simulated gastrointestinal proteolysis of β-casein and β-lactoglobulin. Soft Matter 5:538–550
Mackie AR, Mingins J, Dann R (1993) Preliminary studies of β-lactoglobulin adsorbed on polystryrene latex. In: Dickinson E, Walstra P (eds) Food colloids and polymers: stability and mechanical properties. Royal Society of Chemistry, Cambridge, UK, pp 96–112
Malone ME, Appelqvist IAM, Norton IT (2003a) Oral behaviour of food hydrocolloids and emulsions. Part 1. Lubrication and deposition considerations. Food Hydrocoll 17:763–773
Malone ME, Appelqvist IAM, Norton IT (2003b) Oral behaviour of food hydrocolloids and emulsions. Part 2. Taste and aroma release. Food Hydrocoll 17:775–784
Marinova KG, Basheva ES, Nenova B, Temelska M, Mirarefi AY, Campbell B, Ivanov IV (2009) Physicochemical factors controlling the foamability and foam stability of milk proteins: sodium caseinate and whey protein concentrates. Food Hydrocoll 23:1864–1876
Martin AH, Grolle K, Bos MA, Stuart MA, van Vliet T (2002) Network forming properties of various proteins adsorbed at the air/water interface in relation to foam stability. J Colloid Interface Sci 254:175–183
McClements DJ (2005) Food emulsions: principles, practices and techniques, 2nd edn. CRC, Boca Raton
McClements DJ, Monahan FJ, Kinsella JE (1993) Disulfide bond formation affects stability of whey protein isolate emulsions. J Food Sci 58:1036–1039
McClements DJ, Decker EA, Park Y (2009) Controlling lipid bioavailability through physicochemical and structural approaches. Crit Rev Food Sci Nutr 49:48–67
McKenzie HA (1971) β-lactoglobulin. In: McKenzie HA (ed) Milk proteins, vol 2. Academic, New York, pp 257–330
McKenzie HA, Sawyer WH (1967) Effect of pH on β-lactoglobulin. Nature 214:1101–1104
Monahan FJ, McClements DJ, Kinsella JE (1995) Polymerization of whey proteins in whey protein-stabilized emulsions. J Agric Food Chem 41:1826–1829
Moro A, Báez GD, Busti PA, Ballerini GA, Delorenzi NJ (2011) Effects of heat-treated β-lactoglobulin and its aggregates on foaming properties. Food Hydrocoll 25:1009–1015
Morr CV (1982) Functional properties of milk proteins and their use as food ingredients. In: Fox PF (ed) Developments in dairy chemistry—I. Elsevier Applied Science, London, pp 375–399
Morr CV, Ha EYW (1993) Whey protein concentrates and isolates: processing and functional properties. Crit Rev Food Sci Nutr 33:431–476
Mulvihill DM (1989) Caseins and caseinates: manufacture. In: Fox PF (ed) Developments in dairy chemistry—IV. Elsevier Applied Science, London, pp 97–130
Mulvihill DM, Ennis MP (2003) Functional milk proteins: production and utilization. In: Fox PF, McSweeney PLH (eds) Advanced dairy chemistry: proteins, vol 1. Kluwer Academic, New York, pp 1175–1228
Mulvihill DM, Fox PF (1989) Physicochemical and functional properties of milk proteins. In: Fox PF (ed) Developments in dairy chemistry. Elsevier Applied Science, London, pp 131–172
Mun S, Decker EA, McClements DJ (2007) Influence of emulsifier type on in vitro digestibility of lipid droplets by pancreatic lipase. Food Res Int 40:770–781
Murray BS, Ettelaie R (2004) Foam stability: proteins and nanoparticles. Curr Opin Colloid Interface Sci 9:314–320
Ng-Kwai-Hang KF (2003) Milk proteins/heterogeneity, fractionation and isolation. In: Fuquay J, Fox P, Roginsky H (eds) Encyclopedia of dairy sciences. Academic, Amsterdam, pp 1881–1894
Nicorescu I, Loisel C, Riaublanc A, Vial C, Djelveh G, Cuvelier G, Legrand J (2009) Effect of dynamic heat treatment on the physical properties of whey protein foams. Food Hydrocoll 23:1209–1219
Oliveira KMG, Valente-Mesquita VL, Botelho MM, Sawyer L, Ferreira ST, Polikarpov I (2001) Crystal structures of bovine β-lactoglobulin in the orthorhombic space group C2221. Structural differences between genetic variants a and B and features of the Tanford transition. Eur J Biochem 268:477–483
Papiz MZ, Sawyer L, Eliopoulos EE, North ACT, Findley JBC, Sivapradadarao R, Jones TA, Newcomer ME, Kraulis PJ (1986) The structure of β-lactoglobulin and its similarity to plasma retinol-binding protein. Nature 324:383–385
Pereira LGC, Johansson C, Radke CJ, Blanch HW (2003) Surface forces and drainage kinetics of protein-stabilized aqueous films. Langmuir 19:7503–7513
Phillips LG, Schulman W, Kinsella JE (1990) pH and heat-treatment effects on foaming of wheyprotein isolate. J Food Sci 55:1116–1119
Phillips LG, Hawks SE, German JB (1995) Structural characteristics and foaming properties of β-lactoglobulin: effects of shear rate and temperature. J Agric Food Chem 43:613–619
Prud’homme RK, Khan SA (1996) Foams: theory, measurements, and applications. Marcel Dekker, New York
Ridout MJ, Mackie AR, Wilde PJ (2004) Rheology of mixed β-casein/β-lactoglobulin films at the air–water interface. J Agric Food Chem 52:3930–3937
Robson EW, Dalgleish DG (1987) Interfacial composition of sodium caseinate emulsions. J Food Sci 52:1694–1698
Rullier B, Novales B, Axelos MAV (2008) Effect of protein aggregates on foaming properties of β-lactoglobulin. Colloids Surf A 330:96–102
Saint-Jalmes A, Peugeot M-L, Ferraz H, Langevin D (2005) Differences between protein and surfactant foams: microscopic properties, stability and coarsening. Colloids Surf A 263:219–225
Sarkar A (2010) Behaviour of milk protein-stabilized oil-in-water emulsions in simulated physiological fluids. Ph.D. Thesis, Massey University, Palmerston North
Sarkar A, Goh KKT, Singh H (2009a) Colloidal stability and interactions of milk-protein-stabilized emulsions in an artificial saliva. Food Hydrocoll 23:1270–1278
Sarkar A, Goh KKT, Singh RP, Singh H (2009b) Behaviour of an oil-in-water emulsion stabilized by β-lactoglobulin in an in vitro gastric model. Food Hydrocoll 23:1563–1569
Sarkar A, Goh KKT, Singh H (2010a) Properties of oil-in-water emulsions stabilized by β-lactoglobulin in simulated gastric fluid as influenced by ionic strength and presence of mucin. Food Hydrocoll 24:534–541
Sarkar A, Horne DS, Singh H (2010b) Interactions of milk protein stabilized oil-in-water emulsions with bile salts in a simulated upper intestinal model. Food Hydrocoll 24:142–151
Sarkar A, Horne DS, Singh H (2010c) Pancreatin-induced coalescence of oil-in-water emulsions in an in vitro duodenal model. Int Dairy J 20:589–597
Sawyer L, Papiz MZ, North ACT, Eliopoulos EE (1985) Structure and function of bovine β-lactoglobulin. Biochem Soc Trans 13:265–266
Schmidt DG, Poll JK (1991) Enzymatic hydrolysis of whey proteins. Hydrolysis of α-lactalbumin and β-lactoglobulin in buffer solutions by proteolytic enzymes. Neth Milk Dairy J 45:225–240
Schmitt C, Bovay C, Rouvet M, Shojaei-Rami S, Kolodziejczyk E (2007) Whey protein soluble aggregates from heating with NaCl: physicochemical, interfacial, and foaming properties. Langmuir 23:4155–4166
Schokker EP, Singh H, Creamer LK (2000) Heat-induced aggregation of β-lactoglobulin a and B with α-lactalbumin. Int Dairy J 10:843–853
Shimizu M, Kamiya T, Yamauchi K (1981) The adsorption of whey proteins on the surface of emulsified fat. Agric Biol Chem 45:2491–2496
Shimizu M, Saito M, Yamauchi K (1985) Emulsifying and structural-properties of beta-lactoglobulin at different pHs. Agric Biol Chem 49:189–194
Silletti E, Vingerhoeds MH, Norde W, van Aken GA (2007) The role of electrostatics in saliva-induced emulsion flocculation. Food Hydrocoll 21:596–606
Singh AM, Dalgleish DG (1998) The emulsifying properties of hydrolyzates of whey proteins. J Dairy Sci 81:918–924
Singh H (2005) Milk protein functionality in food colloids. In: Dickinson E (ed) Food colloids: interactions, microstructure and processing. Royal Society of Chemistry, Cambridge, pp 179–193
Singh H (2011) Aspects of milk protein-stabilised emulsions. Food Hydrocoll 25:1938–1944
Singh H, Sarkar A (2011) Behaviour of protein-stabilised emulsions under various physiological conditions. Adv Colloid Interface Sci 165:47–57
Singh H, Ye A (2013) Structural and biochemical factors affecting the digestion of protein-stabilized emulsions. Curr Opin Colloid Interface Sci 18:360–370
Singh H, Ye A, Horne D (2009) Structuring food emulsions in the gastrointestinal tract to modify lipid digestion. Prog Lipid Res 48:92–100
Srinivasan M, Singh H, Munro PA (1996) Sodium caseinate-stabilized emulsions: factors affecting coverage and composition of surface proteins. J Agric Food Chem 44:3807–3811
Srinivasan M, Singh H, Munro PA (1999) Adsorption behaviour of sodium and calcium caseinates in oil-in-water emulsions. Int Dairy J 9:337–341
Srinivasan M, Singh H, Munro PA (2001) Creaming stability of oil-in-water emulsions formed with sodium and calcium caseinates. J Food Sci 66:441–446
Swaisgood HE (1982) Chemistry of milk protein. In: Fox PF (ed) Developments in dairy chemistry—I. Elsevier Applied Science, London, pp 1–59
Swaisgood HE (1992) Chemistry of the caseins. In: Fox PF (ed) Advanced dairy chemistry—I. Elsevier Applied Science, Barking, pp 63–110
Unterhaslberger G, Schmitt C, Shojaei-Rami S, Sanchez C (2007) β-lactoglobulin aggregates from heating with charged cosolutes: formation, characterization and foaming. In: Colloids F (ed) Self assembly and material science. Royal Society of Chemistry, Cambridge, pp 175–192
van Aken GA (2004) Coalescence mechanisms in protein-stabilized emulsions. In: Friberg SE, Larsson K, Sjöblom J (eds) Food emulsions. Marcel Dekker, New York, pp 299–325
van Aken GA, Blijdenstein TBJ, Hotrum NE (2003) Colloidal destabilisation mechanisms in protein-stabilised emulsions. Curr Opin Colloid Interface Sci 8:371–379
van Aken GA, Vingerhoeds MH, De Hoog EHA (2005) Colloidal behaviour of food emulsions under oral conditions. In: Dickinson E (ed) Food colloids: interactions, microstructure and processing. Royal Society of Chemistry, Cambridge, pp 356–366
Vingerhoeds MH, Blijdenstein TBJ, Zoet FD, van Aken GA (2005) Emulsion flocculation induced by saliva and mucin. Food Hydrocoll 19:915–922
Vingerhoeds MH, Silletti E, de Groot J, Schipper RG, van Aken GA (2009) Relating the effect of saliva-induced emulsion flocculation on rheological properties and retention on the tongue surface with sensory perception. Food Hydrocoll 23:773–785
Wahlgren MC, Arnebrant T, Paulsson MA (1993) The adsorption from solutions of β-lactoglobulin mixed with lactoferrin or lysozyme onto silica and methylated silica surfaces. J Colloid Interface Sci 158:46–53
Weaire D, Hutzler S (1999) The physics of foams. Clarendon, Oxford
Weisbrodt NW (2001) Swallowing. In: Johnson LR (ed) Gastrointestinal physiology. Mosby, St. Louis, pp 27–35
Wickham M, Garrood M, Leney J, Wilson PDG, Fillery-Travis A (1998) Modification of a phospholipid stabilized emulsion interface by bile salt: effect on pancreatic lipase activity. J Lipid Res 39:623–632
Wong DWS, Camirand WM, Pavlath AE (1996) Structures and functionalities of milk proteins. Crit Rev Food Sci Nutr 36:807–844
Wooster TJ, Augustin MA (2007) Rheology of whey protein–dextran conjugate films at the air/water interface. Food Hydrocoll 21:1072–1080
Yang J, Dunker AK, Powers JR, Clark S, Swanson BG (2001) β-lactoglobulin molten globule induced by high pressure. J Agric Food Chem 49:3236–3243
Ye A (2008) Interfacial composition and stability of emulsions made with mixtures of commercial sodium caseinate and whey protein concentrate. Food Chem 110:946–952
Ye A, Singh H (2000) Influence of calcium chloride addition on the properties of emulsions stabilized by whey protein concentrate. Food Hydrocoll 14:337–346
Ye A, Singh H (2001) Interfacial composition and stability of sodium caseinate emulsions as influenced by calcium ions. Food Hydrocoll 15:195–207
Ye A, Singh H (2006a) Adsorption behaviour of lactoferrin in oil-in-water emulsions as influenced by interactions with β-lactoglobulin. J Colloid Interface Sci 295:249–254
Ye A, Singh H (2006b) Heat stability of oil-in-water emulsions formed with intact or hydrolysed whey proteins: influence of polysaccharides. Food Hydrocoll 20:269–276
Ye A, Singh H (2007) Formation of multilayers at the interface of oil-in-water emulsion via interactions between lactoferrin and β-lactoglobulin. Food Biophys 2:125–132
Zhang Z, Dalgleish DG, Goff HD (2004) Effect of pH and ionic strength on competitive protein adsorption to air/water interfaces in aqueous foams made with mixed milk proteins. Colloids Surf B 34:113–121
Zhu H, Damodaran S (1994) Heat-induced conformational changes in whey protein isolate and its relation to foaming properties. J Agric Food Chem 42:846–855
Ziegler GR, Foegeding EA (1990) The gelation of proteins. Adv Food Nutr Res 34:203–208
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer Science+Business Media New York
About this chapter
Cite this chapter
Sarkar, A., Singh, H. (2016). Emulsions and Foams Stabilised by Milk Proteins. In: McSweeney, P., O'Mahony, J. (eds) Advanced Dairy Chemistry. Springer, New York, NY. https://doi.org/10.1007/978-1-4939-2800-2_5
Download citation
DOI: https://doi.org/10.1007/978-1-4939-2800-2_5
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4939-2799-9
Online ISBN: 978-1-4939-2800-2
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)