Abstract
Milk fat-based whipping cream is primarily comprised of cream and whole milk. It has melt-in-the-mouth texture and unique milk flavor. However, milk fat-based whipping cream suffers from poor emulsion stability and foam firmness. The effects of monoacylglycerols (MAGs) with different saturation degrees (M1: 98% saturation, M2: 70% saturation and M3: 30% saturation) on emulsion properties (average particle size, viscosity, and emulsion stability) and whipping properties (overrun, firmness, shape retention ability, and foam stability) of milk fat-based whipping creams were investigated in this study. MAGs significantly decreased particle sizes (from 2.84 to 1.16 μm) and enhanced viscosity (from 350 to 490 cP) of the milk fat-based emulsions (emulsion without MAGs: M0, 5.01 μm, 298 cP) (P < 0.05). MAGs increased the stability of the milk fat-based emulsions with lesser phase separation during centrifugation tests and lower changes in particle sizes and viscosities during temperature cycling tests. Emulsion M1 with highest degree of saturation is less likely to destabilize and phase inverse. The decrease sharply in conductivity can be attributed to the entrapment of large amounts of air. Following that, the conductivity of M1 with low variation indicating high whipping resistance and less likely to coalescence and phase separation. Adding MAGs can significantly enhance overrun (M1: 205.3%, M2: 198.5%, M3: 141.4%) as compared to the control sample (M0: 97.9%) (P < 0.05). In emulsions containing MAGs with high degree of saturation (M1 and M2), firmness (M1: 95 g, M2: 109 g) and shape retention ability of the whipped creams were reduced as compared to control emulsion without MAG (M0: 173 g), but the foam stability (M1: 89%, M2: 91%) was enhanced (M0: 81%); M3 (firmness: 507 g; foam stability: 66%) has the contrasted effects. Whipping cream M2 demonstrated the best whipping properties with high overrun (198.46%), good firmness (109 g), shape retention ability and foam stability (91%). Good quality whipping creams can be obtained by selecting suitable MAGs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Whipping cream is a type of aeratable oil-in-water emulsion containing high-fat content (30–40%) (Zhao et al. 2009). It is frequently used as toppings for many food products, such as pastries, desserts and ice cream. Milk fat-based whipping creams can be prepared by centrifuging fresh milk or mixing recombining milk products (mostly milk fat and low-fat milk powder) with water. The former is known as native cream and the latter as recombined cream. Recombined cream is normally made by homogenizing a mixture of milk fat and aqueous containing dairy or non-dairy proteins stabilizers to improve the whipping process and prolong product shelf-life (Li et al. 2020a; Singh et al. 2008). Recombined creams also have unique milk flavor. They can be stored and transported more easily; and can be adjusted or standardized to reduce the effects of production seasonality (Fredrick 2011).
Whipping normally cause transformation of the two-phase system into a three-phase system. During whipping, large air bubbles are incorporated into creams. Protein in the emulsions will rapidly absorb onto the air-water interface. Following that, the large air bubbles will break into smaller bubbles. Fat globules will then displace some proteins from the bubble interface. The continued whipping causes partial coalescence of fat globules and stiffening of the cream. At the end of the whipping process, air bubbles are surrounded and stabilized by a network of coalesced fat globules to prevent collapse of the whipped cream (Tamime 2009). A desirable whipping cream should be stable during distribution and storage; and able to destabilize forming a network of coalesced fat globules for better whipping (Li et al. 2020b).
One of the limitations of milk fat-based whipping cream is its tendency to form large clumps due to damage of coalesced fat globules under excessive whipping. This will result in reduced ability to retain shape for the bakery decoration. Oil-water separation or fat coalescence during storage is a common problem for whipped cream due to the instability. One of the common solutions is to add stabilizers, which lead to enhance the viscosity of whipping cream. Kováčová et al. (2010) found that the increase of the carrageenan concentration minimized the release of milk plasmas, and the stability of whipping cream was significantly improved. Li et al. (2020b) compared the effects of three casein i.e. micellar casein concentrate, calcium caseinate, and sodium caseinate on the stability of whipping cream. A similar tendency was found that increasing the concentration of micellar casein concentrate provided better stability for whipping cream. At the same time, the micellar casein concentrated whipping cream showed the smaller particle size, higher viscosity, and larger steric repulsion compared to the others. Emulsifiers as additives also can be added to improve the physical (particle sizes and viscosity) and whipping properties of whipped creams. Some of the commonly used emulsifiers including monoacylglycerols (MAGs), sorbitan fatty acid ester, and polyoxyethylene sorbitan fatty acid ester etc. (Fredrick et al. 2013a).
As an oil-soluble small-molecule surfactant, MAGs play an essential role in controlling fat crystallization, emulsion stability, and whipping properties (Wu et al. 2016). It also can prompt the displacement of adsorbed protein by fat globules, and establish a firmer structure by fat aggregates to enhance the foam stability (Wang et al. 2022). MAGs, rich in oleic acid (MAG-O), have no significant effects on the crystallization growth, but promote crystallized fat to form spiky spherulites. The crystal ruptures the fat globule membrane between two colliding fat globules causing reduced emulsion stability during aging and shearing. Thus, the whipped cream containing MAG-O showed the high firmness, foam stability, and low overrun. However, MAGs, rich in stearic acid (MAG-S), demonstrated a slow crystal growth rate and formation of smaller spherulites with poor retention ability and optimal overrun for the whipped cream (Fredrick et al. 2013a, 2013b). However, few studies have focused on the influence of MAGs saturation degree on the emulsion stability and whipping properties of whipping creams.
The present study aims to investigate the effects of MAGs with different saturation degrees on emulsion physical (particle size, apparent viscosity and emulsion stability) and whipping properties (overrun, conductivity, firmness, shape retention ability and foam stability) of the whipped cream. In addition, the impacts of MAGs on whipping cream stability against temperature fluctuations and centrifugation during storage are also elucidated. Findings from this study will be useful to guide production of recombined whipping cream with improved functionality.
Materials and methods
Materials
Cream (40% fat) and whole cow milk (3.8% fat) were purchased from Bright Dairy Food and Yili Dairy, Shanghai, China. MAG-98% (Dimodan® HP-C, 43% stearic and 55% palmitic acid monoglycerides), MAG-70% (Dimodan® PH 320/B-M, 11% stearic, 59% palmitic, 23% oleic and 5% linoleic acid monoglycerides), and MAG-30% (Dimodan® UP/B SG, contain 5% stearic, 23% palmitic, 35% oleic and 35% linoleic acid monoglycerides) were kindly donated by IFF, China.
Preparation of the whipping cream
Standardized whipping cream was prepared according to previously reported work with slight modifications by Gafour and Aly (2020). Firstly, whole milk (93.2 g) and MAGs (1.4 g) were mixed at 65 °C by stirring at 200 rpm for 10 min, then sheared at 7000 rpm for 1 min (Silverson, L5t, England). Cream (600 g) was added to the above mixture to reach the fat content of 36%. The mixture was stirred at 200 rpm for 10 min at room temperature before homogenization. Finally, the mixture was heated to 65 °C and subjected two-stage homogenization (the first stage at 30 bar/the second stage at 20 bar) by a APV homogenizer (APV-1000, Albertslund, Denmark). The homogenized cream was filled into a sterile bottle, cooled down quickly in the ice-water bath, and then stored in the refrigerator at 5 °C overnight before usage. Four whipping creams were prepared with MAGs at different saturated degrees of M0 (without MAG), M1 (with MAG-98%), M2 (with MAG-70%) and M3 (with MAG-30%), respectively.
Average particle size of the whipping creams
Particle size distributions of the whipping creams were measured by LS13320 laser diffraction particle size analyzer (Beckman coulter, INC, Kraemer Blvd, Brea, CA, USA). Wet analysis was used according to Zhao et al. (2009). The emulsion was diluted 1000-fold with distilled water, and then added to the laser diffraction instrument. Measurements were performed at ambient temperatures with triplicates.
Apparent viscosity of the whipping creams
Viscosity of the whipping cream was measured at 5 °C after taken out from refrigerator using a Brookfield digital rotational viscometer (Model DV2T-LV, Brookfield Engineering Laboratories Inc., Middleboro, MA, USA) according to Athari et al. (2021). Spindle No. 63 was used at a speed of 20 rpm. Result was recorded in centipoises (cP) after 1 min of shearing.
Stability of the milk-fat based whipping cream emulsions
Stability against centrifugation
The prepared emulsion (15 g) from Sect. “Preparation of the whipping cream” was centrifuged at 4 × g for 10 min. Emulsion stability was defined as the ratio of separated lipid to the total mass of the emulsion according to the method of Szymańska et al. (2019).
Stability against temperature fluctuation
Whipping cream was placed at room temperature for 8 h and stored in the refrigerator at 5 °C for 16 h. This temperature cycling was repeated twice. After that, the average particle size and viscosity of the whipping cream were measured according to methods described in Sects. “Average particle size of the whipping creams” and “Apparent viscosity of the whipping creams”. Changes in particle size and viscosity after the temperature cycling are calculated according to the following equations:
where particle size0 and viscosity0 represent the particle size and viscosity of the oil-in-water emulsion before temperature cycling; particle size1 and viscosity1 represent the particle size and viscosity of the oil-in-water emulsion after temperature cycling, respectively.
Whipping properties of the milk fat-based whipped creams
Overrun measurement of the milk fat-based whipped creams
The whipping process was carried out with a Kenwood chef mixer (KMC510, Kenwood Let., United Kingdom) at a maximum rotational velocity of 1200 W. Time to achieve oil-water separation was defined as the total whipping time. Overrun was defined as the volume of air trapped in the whipped cream. Overrun measurement was conducted at 30 s intervals. The measurement will be stopped at the event of phase separation according to Cao et al. (2020). Overrun is calculated according to the following equation:
where M1 (g) and M2 (g) represented the mass of the cream before and after whipping, respectively. The measurements were done in triplicate.
Conductivity measurement of the milk fat-based whipped creams
Conductivity of the cream was determined by using a pH meter (SevenMulti S40, Mettler Toledo, China). It was conducted at 30 s intervals. Measurement of the conductivity is stopped at phase separation. The measurements were done three times for each sample.
Firmness measurement of the milk fat-based whipped creams
The cream was whipped until it was stiff enough to form a standing peak shape. Firmness of the whipped creams was then measured by texture analyzer (TA-XT plus, Stable Micro Systems, UK). Compression mode and A/BE probe (diameter 35 mm) were used. Puncture tests were performed at a rate of 2.0 mm/s over a distance of 25 mm through the whipped cream. The trigger value was set at 20 g. Force (g) required to reach this depth was defined as the firmness of the whipped cream. Measurements were carried out in triplicate.
Shape retention ability of the milk fat-based whipped creams
Shape retention ability was evaluated according to Liu et al. (2021) with slight modifications. Whipped creams were extruded into rosette shape using pastry tube set and placed on cardboard at room temperature for 2 h. The shape of the foam structure for the cream were observed and photographed.
Foam stability of the milk fat-based whipped creams
Serum loss is used as a measurement of foam stability. Whipped cream (10 g) was put on a sieve (60 mesh) and the amount of serum passing through the sieve (room temperature and 3 h) was recorded as indicator of foam stability. The foam stability was calculated according to the following equation:
where M1 represented the initial mass of whipped cream placed on the sieve and M2 was the weight of serum collected.
Statistical analysis
Statistical data analyses were conducted using SPSS 23.0 and Origin X 9.1 software. All tests were performed in triplicate, and the analyses were reported as means ± standard. Analyses of variance (one-way ANOVA) were employed to determine significant differences between results. All tests were carried out at a 95% significance level.
Results and discussion
Physicochemical properties
Effects of MAGs on average particle size of the whipping cream emulsions
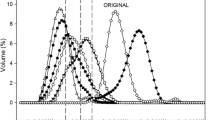
Figure 1 shows the average particle size of whipping cream emulsions prepared with different MAGs. MAGs significantly decreased the average particle sizes (M1: 1.16 μm; M2: 1.65 μm; M3: 2.84 μm) compared to M0 (without MAGs: 5.01 μm) (P < 0.05). Whipping cream emulsion is composed mainly of two immiscible liquid phases (water and oil). Therefore, it is a thermodynamically unstable system. Emulsifiers such as MAGs can be added to increase the stability of whipping cream emulsions. MAGs can preferentially be adsorbed at the fat droplet interface which efficiently lowered the interfacial tension, decreased the average particle sizes and enhanced the stability of the emulsion (Rouimi et al. 2005).
Saturation degree of the MAGs also affects the particle sizes of the whipping cream emulsions. Particle sizes of the whipping cream emulsions show an increasing trend with decreasing saturation degree of MAGs. Unsaturated MAGs (M3: 2.84 μm) promoted formation of spiky spherulites which easily pierce the oil-water interface of fat globules resulted in increased droplet size compared with high saturated MAGs (M1: 1.16 μm and M2: 1.65 μm) (Daviesa et al. 2001).
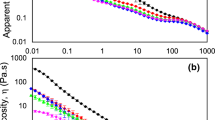
Effects of MAGs on apparent viscosity of the whipping cream emulsions
Viscosity is defined as a force of resistance to the flow of a liquid and measured by viscometers. Figure 2 shows the apparent viscosity of the whipping cream emulsions with MAGs (M1, M2 and M3) and without MAGs (M0). MAGs increased the viscosity (M1: 350 cP; M2: 428 cP; M3: 492 cP) of the emulsions (emulsion without MAG, M0: 298 cP). As described in the aforementioned findings on the particle sizes, the addition of MAGs resulted in formation of high amounts of smaller fat globules. The interactions between these fat globules were enhanced and resulted in the increased viscosity for the emulsions (Jiang et al. 2018).
Stability of the whipping cream emulsions
Stability against centrifugation
Centrifugation is used to evaluate the effects of emulsifiers on the stability of the oil-in-water emulsion. Fat droplets are forced to collide under centrifugal force resulting in fat aggregation (Liang et al. 2020; Li et al. 2020b). Table 1 shows the stability of the whipping creams against centrifugation. Whipping cream emulsion prepared without MAG had a high fat separation rate of 31.78%.
Adding MAGs significantly decrease fat separation rate and increase stability of the whipping cream emulsions (P < 0.05) against centrifugation. As MAGs can be effectively adsorbed on the oil-water interface, reduced interfacial tension and enhanced the stability of the oil-in-water emulsions (Mcclements 2011). Among the oil-in-water emulsions containing MAGs, the emulsion containing M1 (highest saturation) had the lowest fat separation rate which demonstrated the highest stability against centrifugation. This indicated saturated MAGs have higher ability to crystallize upon cooling and then provide solid-like property at the oil-water interface. This crystalline state and sufficiently rigid oil-water interface layer contribute to the enhanced interfacial stability which conferred greater protection against oil-water separation during the centrifugation (Fredrick et al. 2013a; Kim et al. 2013).
Stability against temperature fluctuation
Temperature fluctuations affect fat crystals state, solid fat content and speed of demulsification which result in changes in physical characteristics of whipping creams (Sugimoto et al. 2001; Rousseau 2000). Table 2 shows the changes in particle size and viscosity of the emulsions following temperature fluctuations.
Among all the samples, emulsion M1 demonstrated small changes in particle size following two temperature cycles indicating high stability of the oil-in-water emulsion stabilized by saturated MAGs. In terms of viscosity, emulsion M1 (98% saturation) demonstrated the smallest changes in terms of viscosity following two temperature cycles. M3 showed the greatest viscosity change. This indicated saturated MAGs played a role in resisting change in viscosity following temperature fluctuations. This is agreed with aforementioned findings, which the saturated MAGs formed a thick and viscoelastic interfacial layer to prevent droplets agglomeration during temperature changing (Li et al. 2020a).
Whipping properties of the milk fat-based whipping creams
Effects of MAGs on overrun and conductivity of the milk fat-based whipped creams
Figure 3a depicts the effects of MAGs on the overrun of milk fat-based whipped creams. For whipped cream, MAGs normally increase the overrun during the early stage of whipping. MAGs, as low-molecular-weight emulsifiers, can display proteins at the oil-water interface. The displayed proteins were then released into the serum phase which increased the foamability and overrun of the whipped creams (Zhao et al. 2013).
a Changes in overrun of whipped creams with and without MAGs during whipping. b Changes in conductivity of whipped creams with and without MAGs during whipping. c Foam firmness of whipped cream with and without MAGs (Different superscript letters on the top of columns represent significant differences at P < 0.05). d Images of whipped creams with a rosette shape after preparation. Each sample contains 3 parallel cases. e Foam stability of whipped cream with and without MAGs (Different superscript letters on the top of columns represent significant differences at P < 0.05)
Unsaturation decreased the overrun of the whipped creams (overrunM1 > overrunM2 > overrunM3). Unsaturated MAGs resulted in the formation of larger fat globules due to coalesce during whipping. The coalesced fat globules will be enlarged and easily rupture the interface membrane of air bubbles, thereby resulting in the collapse of foam, the decline of overrun and rough mouth feeling (Zhao et al. 2013; Fredrick et al. 2013a). In short, MAGs with high saturation degree resulted in higher overrun and longer whipping times for the whipped creams. Similar results were found for recombined dairy cream and plant-based cream (Liu et al. 2021; Moens 2018).
During whipping, demulsification and phase inversion are taken place. The emulsion transformed into water-in-oil foam, and the conductivity was decreased. Whipping cream M1 had a large conductivity (1488 μs/cm) which reduced significantly during the first min of whipping. The conductivity of M1 decreased sharply. It is attributed to the entrapment of large amounts of air. Following that, the low variation conductivity during whipping indicates high whipping resistance, less coalescence, and less phase separation.
Effects of MAGs on firmness and shape-retention ability of the milk fat-based whipped creams
Firmness refers to the ability of aerated emulsions to form stiff shaped toppings. Figure 3c shows the firmness of the whipped creams with and without MAGs. Firmness of the whipped creams decreased in the following order: FM3 (507 ± 93 g) > FM0 (173 ± 10 g) > FM2 (109 ± 7 g) > FM1 (95 ± 3 g).
Firmness and shape retention ability have a positive relationship. Figure 3d shows the appearance of the whipped cream in the form of rosette which is left for 2 h at the room temperature. Besides the whipped cream M1, all the other whipped creams (M0, M2 and M3) form rosette shape with sharp edges, hard peaks and smooth surfaces. The width and height of all samples (M0, M2 and M3) did not show significant changes within two hours. As shown in the conductivity measurements (Fig. 3b), the conductivity of M1changes slowly during whipping, which indicates that the emulsion is less likely to have the phase reverse in order to achieve enough coalesced fat globules. Without sufficient of partial coalesced fat globules, M1 cream was unable to have strong structure to prevent collapse and form stiff foam during whipping (Smiddy et al. 2009). On the other hand, a higher partial coalescence is taken place with the increase of the unsaturation (M2 < M3) which is also correlated with the changes of conductivity (M2 < M3). Therefore, the conductivity is a very simple and useful tool to monitor the variation during the whipping. At the same time, a high degree of partial coalescence of fat droplets contributes to better shape-retention ability and firmness was also observed by other studies which is agreed with our results (Fredrick et al. 2013a; Wang et al. 2022).
Effects of MAGs on foam stability of the milk fat-based whipped creams
Figure 3e indicates the effects of MAGs on foam stability of the milk fat-based whipped creams. Higher saturated MAGs (M1 and M2) improve foam stability, while the lower one (M3) significantly decrease the foam stability. According to Fig. 3b, the conductivities of M1 and M2 changes slowly in the later of whipping, which demonstrate the separation of oil and water was prevented in the foam. M3 sample showed the expulsion of aqueous phase with the increased conductivity after 3 h. This implied the collapse of the space-filling network composed of aggregated oil droplets were unable to entrap serum. This kind of observation was also found in Liu’s study. (Liu et al. 2022). Therefore, the whipped cream containing unsaturated MAGs (30% saturation) has the lowest foam stability.
Conclusion
Phase transformation (from oil-in-water to water-in-oil) was measured using conductivity and used to indicate stability of the milk fat-based whipping cream. The effects of MAGs’ saturation on physical properties and whipping behavior of whipping cream were studied. Present results showed saturated MAGs provide superior stability to the emulsion by hindering coalescence of fat globules. It is worth noting that particle size and viscosity are inversely related. In terms of whipping properties, saturated MAGs produced whipped cream with high overrun and foam stability. However, the low saturated MAGs demonstrated the exact opposite effects. The obtained decrease in emulsion stability provoked a higher firmness and sharp-retention ability for the whipped cream. Each MAG has a specifically positive effect on stability and whipping properties. A moderately saturated MAGs are more suitable to be used as emulsifiers for whipping cream since they present adequate stability for both emulsion and whipping properties. In the future, more work should focus on improving the quality of whipping cream by using a mixture of different MAGs and other emulsifiers.
Data availability statement
All data generated or analysed during this study are included in this published article (and its supplementary information files).
Abbreviations
- MAGs:
-
Monoacylglycerols
References
Athari B, Nasirpour A, Saeidy S, Esehaghbeygi A (2021) Physicochemical properties of whipped cream stabilized with electrohydrodynamic modified cellulose. J Food Process Preserv 45(9):1–8
Cao ZY, Liu ZL, Zhang HJ, Wang J, Ren SC (2020) Protein particles ameliorate the mechanical properties of highly polyunsaturated oil-based whipped cream: a possible mode of action. Food Hydrocoll 99:105350
Daviesa E, Dickinsona E, Bee RD (2001) Orthokinetic destabilization of emulsions by saturated and unsaturated monoglycerides. Int Dairy J 11(10):827–836
Fredrick E (2011) Fat crystallization and partial coalescence in dairy creams: role of monoacylglycerols. PhD Dissertation, Ghent University, Ghent, Belgium. https://www.researchgate.net/publication/292335230
Fredrick E, Heyman B, Moens K, Fischer S, Verwijlen T, Moldenaers P, Van Der Meeren P, Dewettinck K (2013a) Monoacylglycerols in dairy recombined cream: II. The effect on partial coalescence and whipping properties. Food Res Int 51(2):936–945
Fredrick E, Moens K, Heyman B, Fischer S, Van Der Meeren P, Dewettinck K (2013b) Monoacylglycerols in dairy recombined cream: I. The effect on milk fat crystallization. Food Res Int 51(2):892–898
Gafour W, Aly E (2020) Organoleptic, textural and whipping properties of whipped cream with different stabilizer blends. Acta Sci Pol Technol Aliment 19(4):425–433
Jiang J, Jin Y, Liang XY, Piatko M, Campbell S, Lo SK, Liu YF (2018) Synergetic interfacial adsorption of protein and low-molecular-weight emulsifiers in aerated emulsions. Food Hydrocoll 81:15–22
Kim H-J, Bot A, De Vries ICM, Golding M, Pelan EG (2013) Effects of emulsifiers on vegetable-fat based aerated emulsions with interfacial rheological contributions. Food Res Int 53(1):342–351
Kováčová R, Štětina J, Čurda L (2010) Influence of processing and κ-carrageenan on properties of whipping cream. J Food Eng 99(4):471–478
Li ML, Li Y, Wang RC, Wang YN, Li Y, Zhang LB (2020a) Effects of triglycerol monostearate on physical properties of recombined dairy cream. Int Dairy J 103:104622
Li Y, Li Y, Yuan DD, Wang YN, Li ML, Zhang LB (2020b) The effect of caseins on the stability and whipping properties of recombined dairy creams. Int Dairy J 105:104658
Liang EL, Siva SP, Yong KH, Chan ES, Tey BT (2020) Recent advances of characterization techniques for the formation, physical properties and stability of Pickering emulsion. Adv Colloid Interface Sci 277:102117
Liu PL, Huang LH, Liu TX, Cai YJ, Zeng D, Zhou FB, Zhao MM, Deng XL, Zhao QZ (2021) Whipping properties and stability of whipping cream: the impact of fatty acid composition and crystallization properties. Food Chem 347:128997
Liu ZL, Cao ZY, Zhao MM, Zhang HJ, Wang J, Sun BG (2022) Synergistic influence of protein particles and low-molecular-weight emulsifiers on the stability of a milk fat-based whippable oil-in-water emulsion. Food Hydrocoll 127:107520
Mcclements DJ (2011) Edible nanoemulsions: fabrication, properties, and functional performance. Soft Matter 7(6):2297–2316
Moens K (2018) Fat crystal networks in relation to partial coalescence. PhD. Dissertation, Ghent University, Ghent, Belgium. https://www.researchgate.net/publication/328091809
Rouimi S, Schorsch C, Valentini C, Vaslin S (2005) Foam stability and interfacial properties of milk protein–surfactant systems. Food Hydrocoll 19(3):467–478
Rousseau D (2000) Fat crystals and emulsion stability—a review. Food Res Int 33(1):3–14
Singh P, Kumar R, Sabapathy SN, Bawa AS (2008) Functional and edible uses of soy protein products. Compr Rev Food Sci Food Saf 7(1):14–28
Sugimoto T, Mori T, Mano JI, Mutoh TA, Shiinoki Y, Matsumura Y (2001) Effects of fat crystallization on the behavior of proteins and lipids at oil droplet surfaces. J Am Oil Chem Soc 78(2):183–188
Szymańska I, Żbikowska A, Marciniak-Łukasiak K (2019) Effect of addition of a marine algae (Chlorella protothecoides) protein preparation on stability of model emulsion systems. J Dispers Sci Technol 41(5):699–707
Tamime AY (2009) Dairy fats and related products. Wiley, Ayr, pp 61–85. https://doi.org/10.1002/9781444316223.ch4
Wang ZJ, Liang GJ, Chen WP, Qie XJ, Fu LW, Li X, He ZY, Zeng MM, Goff HD, Chen J (2022) Effects of soy proteins and hydrolysates on fat globule coalescence and whipping properties of recombined low-fat whipped cream. Food Biophys. https://doi.org/10.1007/s11483-021-09714-7
Wu S, Wang G, Lu Z, Li Y, Zhou X, Chen L, Cao J, Zhang L (2016) Effects of glycerol monostearate and Tween 80 on the physical properties and stability of recombined low-fat dairy cream. Dairy Sci Technol 96(3):377–390
Zhao QZ, Kuang WM, Long Z, Fang M, Liu DL, Yang B, Zhao MM (2013) Effect of sorbitan monostearate on the physical characteristics and whipping properties of whipped cream. Food Chem 141(3):1834–1840
Zhao QZ, Zhao MM, Yang B, Cui C (2009) Effect of xanthan gum on the physical properties and textural characteristics of whipped cream. Food Chem 116(3):624–628
Acknowledgements
The author would like to acknowledge the Wilmar Biotechnology Research and Development Center (Shanghai) Co., Ltd., for kind support and providing a facility for the study.
Funding
The authors gratefully acknowledge the financial support provided by the Fundamental Research Funds for the Wilmar Biotechnology Research and Development Center (Shanghai) Co., Ltd. (Project Nos.WRD-02-C-22-019).
Author information
Authors and Affiliations
Contributions
Xueli Wei: Conceptualization, Formal analysis, Data curation, Writing—original draft. Lingzhi Cheong: Conceptualization, Writing—review and editing. Jingjing Gong: Methodology, Formal analysis. Hong Zhang: Conceptualization, Formal analysis, Writing—review and editing. Xuebing Xu and Yanlan Bi: Conceptualization, Methodology and resources.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interest or personal relationships that could have appeared to influence the work reported in this paper.
Ethical approval
Compliance with ethics approval.
Consent to participate
All authors have seen and agreed with the contents of the manuscript.
Consent for publication
All authors are aware of its submission to JFST.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wei, X., Zhang, H., Cheong, L. et al. Effects of monoacylglycerols with different saturation degrees on physical and whipping properties of milk fat-based whipping creams. J Food Sci Technol 60, 2468–2476 (2023). https://doi.org/10.1007/s13197-023-05769-1
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-023-05769-1