Abstract
Congenital cytomegalovirus infection is the most common congenital infection in neonates in the USA, affecting approximately 0.5–1.5% of all live births and 30,000–40,000 newborns annually. Congenital infections result from transplacental transmission of cytomegalovirus (CMV).
Access provided by CONRICYT-eBooks. Download reference work entry PDF
Similar content being viewed by others
Congenital cytomegalovirus infection is the most common congenital infection in neonates in the USA, affecting approximately 0.5–1.5% of all live births and 30,000–40,000 newborns annually. Congenital infections result from transplacental transmission of cytomegalovirus (CMV).
Synonyms and Related Disorders
Intrauterine cytomegalovirus infection of fetus
Genetics/Basic Defects
-
1.
Human cytomegalovirus (HCMV) (Griffiths et al. 2015)
-
1.
A DNA virus belonging to the herpesvirus group (herpes simplex, varicella zoster, Epstein-Barr) (Brown and Abernathy 1998).
-
2.
Ability of the virus (Pass 2002).
-
1.
To destroy host cells (lytic infection)
-
2.
To infect a wide range of cells and tissues
-
3.
To evade and interfere with host defense mechanisms
-
4.
To persist indefinitely in the host
-
5.
To infect and destroy cells during productive infection (with release of progeny virus)
-
1.
-
3.
HCMV: a recognized cause of disease in the fetus, the allograft recipient, and AIDS patients.
-
4.
More recently, it has been recognized as a pathogen for those admitted to intensive care units, for the elderly, and for the general population.
-
1.
-
2.
Transmission of CMV (Del Pizzo 2011)
-
1.
Horizontal transmission: close intimate contact with another person shedding the virus in body fluids (saliva, blood, cervical secretions, semen, urine, or respiratory droplets)
-
2.
Vertical transmission: from mother to infant
-
1.
In utero by transplacental passage of maternal blood-borne virus.
-
2.
At birth by passage through an infected maternal genital tract.
-
3.
Postnatally by ingestion of CMV-positive human milk from seropositive mothers.
-
4.
Mothers who have been exposed to CMV before pregnancy are still at risk for transmitting the infection to the fetus by way of reactivation or infection with a new strain. However, maternal infection before pregnancy and subsequent development of immunity significantly decrease the risk of congenital CMV.
-
1.
-
3.
Infected organ or bone marrow transplantation
-
4.
Infected blood transfusion from CMV-seropositive donors
-
1.
-
3.
Primary sources of CMV infection for women of childbearing age
-
1.
Young children
-
2.
Sexual contacts
-
1.
-
4.
Classifications of CMV (Mestas 2016)
-
1.
Primary CMV (first experience): primary maternal CMV infection during pregnancy presents the greatest danger to the fetus.
-
2.
Secondary CMV (recurrent or reactivated CMV infection): In secondary maternal CMV infection during pregnancy the presence of maternal anti-CMV antibodies offers substantial protection against congenital infection and presents much less danger to the fetus with much lower transmission rates (Guerra et al. 2000).
-
3.
Congenital CMV: CMV that has been transmitted from the mother to the fetus during pregnancy.
-
4.
Perinatal CMV: CMV acquired during the intrapartum period such as during delivery, via cervical secretions or maternal blood exposure.
-
5.
Postnatal CMV: CMV acquired after delivery, such as that acquired via maternal breast milk.
-
1.
-
5.
The most common vertically transmitted viral infection from mother to fetus in pregnancy
-
1.
Recurrent infection: 1% risk of vertical transmission
-
2.
Primary infection
-
1.
Pose a 30–40% risk of vertical transmission, and adverse outcome is more likely when infection occurs within the first half of gestation (Stagno et al. 1986).
-
2.
Congenital CMV infection resulting from primary maternal infection: more likely to be serious than that resulting from recurrent infection (Stagno et al. 1982).
-
3.
Congenital CMV infection acquired from primary maternal infection with normal fetal imaging: associated with a high rate of subtle signs and symptoms after birth (Amir et al. 2016).
-
1.
-
1.
-
6.
Greatest risk occurring with infection during the first 22 weeks of gestation
-
7.
Congenitally infected newborns: may shed the virus for many years
-
8.
Perinatal CMV infection acquired during birth or from mother’s milk: not associated with newborn illness or CNS sequelae, except perhaps in very preterm newborns who have very low levels of passively acquired CMV antibody at the time of infection
Clinical Features
-
1.
Symptomatic at birth in 5–10% of congenital CMV infections (Enders et al. 2001).
-
1.
Approximately 20% of these will die.
-
2.
Ninety percent of survivors will develop major neurological sequelae.
-
1.
-
2.
Asymptomatic or “silent” congenital infections at birth in vast majority (85–90%) of cases. Ten to fifteen percent of the asymptomatic newborns will be afflicted by late sequelae such as mental retardation, deafness, or hearing defects, usually during the first 2 years of life (Enders et al. 2001).
-
3.
Non-neurologic symptoms at birth.
-
1.
Prematurity
-
2.
Small size for gestation
-
3.
Petechiae (most common non-neurologic symptom)
-
4.
Blueberry muffin rash
-
5.
Thrombocytopenia
-
6.
Purpura
-
7.
Ecchymoses
-
8.
Hepatosplenomegaly
-
9.
Jaundice
-
10.
Intrauterine growth retardation
-
11.
Chorioretinitis
-
12.
Pneumonitis
-
13.
Anemia
-
14.
Nonimmune hydrops
-
1.
-
4.
Neurologic symptoms at birth.
-
1.
Hypotonia
-
2.
Lethargy
-
3.
Jitteriness
-
4.
Split sutures
-
5.
Immature primitive reflexes
-
6.
Feeding difficulties
-
7.
Microcephaly: the most specific predictor of mental retardation and major motor disability
-
8.
Seizures
-
1.
-
5.
Eye manifestations (Coats et al. 2000; Metz 2001).
-
1.
Chorioretinitis resulting in a chorioretinal scar
-
2.
Corneal opacities
-
3.
Bilateral anterior polar cataracts
-
4.
Optic nerve hypoplasia and optic nerve coloboma
-
5.
Strabismus
-
6.
Visual impairment
-
7.
Cyclopia (Byrne et al. 1987) and anophthalmia
-
1.
-
6.
Later neurologic symptoms.
-
1.
Sensorineural hearing loss
-
2.
Learning disability
-
3.
Mental retardation
-
4.
Cerebral palsy
-
1.
-
7.
Atypical findings in preterm infants rarely reported in term infants (Perlman and Argyle 1992).
-
1.
Hypotonia
-
2.
Multiple contractures
-
3.
Periventricular leukomalacia
-
4.
Optic atrophy
-
1.
-
8.
Prognosis.
-
1.
Neonatal clinical abnormalities expected to resolve spontaneously within weeks, except for those involving the CNS and hearing.
-
2.
Neonates with symptomatic congenital CMV infection have a multisystem disease with significant morbidity and mortality (Boppana et al. 1992).
-
3.
A leading cause of mental retardation and sensory impairment (50–90% of symptomatic newborns) and sensorineural hearing loss (7–15% of asymptomatic infants).
-
4.
An important cause of cerebral palsy and retinal damage.
-
5.
Children with postnatal microcephaly, postnatal seizures, and an abnormal central nervous system imaging study: more likely to have severe developmental sequelae (Bale et al. 1990).
-
1.
-
9.
Long-term sequelae of congenital cytomegalovirus infection in children with and without symptoms at birth (Sharon and Schleiss 2007; Schleiss 2008).
-
1.
Overall incidence
-
1.
Symptomatic: 50–90%
-
2.
Asymptomatic: 10–15%
-
1.
-
2.
Hearing loss
-
1.
Symptomatic: 50–60%
-
2.
Asymptomatic: 7–15%
-
1.
-
3.
Cognitive deficits
-
1.
Symptomatic: 50–70%
-
2.
Asymptomatic: ~4%
-
1.
-
4.
Microcephaly
-
1.
Symptomatic: 35–40%
-
2.
Asymptomatic: ~2%
-
1.
-
5.
Ocular abnormalities
-
1.
Symptomatic: 25–50%
-
2
Asymptomatic: ~3%
-
1.
-
6.
Seizures
-
1
Symptomatic: 15–20%
-
2.
Asymptomatic: ~1%
-
1
-
7.
Mild to moderate motor deficits
-
1.
Symptomatic: 25–30%
-
2.
Asymptomatic: <1%
-
1.
-
8.
Severe motor deficits
-
1.
Symptomatic: 15–25%
-
2.
Asymptomatic: <1%
-
1.
-
1.
-
10.
Maternal primary CMV infection.
-
1.
Asymptomatic: vast majority of pregnant women
-
2.
Symptomatic (mononucleosis-like syndrome) in approximately 10% of infected pregnant patients
-
1.
Fever
-
2.
Fatigue/malaise
-
3.
Myalgia
-
4.
Pharyngitis
-
5.
Cough
-
6.
Nausea
-
7.
Headache
-
1.
-
1.
Diagnostic Investigations
-
1.
Children with symptomatic congenital CMV infection (Istas et al. 1995; Pass 2002; Bonalumi et al. 2011)
-
1.
Virus detection by PCR amplification in the urine or saliva samples (Joseph et al. 2013)
-
2.
Elevated alanine aminotransferase (ALT >80 IU/mL)
-
3.
Thrombocytopenia (<100,000 cells/mcL)
-
4.
Conjugated hyperbilirubinemia (direct bilirubin >2 mg/dL)
-
5.
Anemia
-
6.
Elevated CSF protein (>120 mg/dL)
-
1.
-
2.
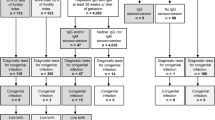
Diagnostic tests for identification of CMV infection in mother, fetus, and newborn infants (Naing et al. 2016)
-
1.
Prenatal
-
1.
Maternal CMV: IgM positivity
-
2.
IgM/IgG serology: IgG seroconversion, low IgG avidity
-
3.
Qualitative CMV-PCR (amniotic fluid): CMV-DNA positive
-
4.
Real-time PCR (amniotic fluid): CMV-DNA positive, with high viral load (>104 copies/mL)
-
5.
Fetal ultrasound: fetal abnormalities (cerebral ventriculomegaly, echogenic bowel, intrauterine growth restriction)
-
1.
-
2.
At birth
-
1.
Maternal CMV-IgM/IgG serology: IgM detection, IgG seroconversion, and low IgG avidity
-
2.
Qualitative CMV-PCR (cord blood, infant urine, placenta/infant saliva): CMV-DNA positive
-
3.
Real-time PCR (cord blood, infant urine, placenta/infant saliva): CMV-DNA positive, with high viral load (>104 copies/mL)
-
1.
-
1.
-
3.
Ultrasonography (Crino 1999; Lipitz et al. 2002; Malinger et al. 2003, 2011; Bonalumi et al. 2011)
-
1.
Fetal growth restriction
-
2.
Cerebral ventriculomegaly
-
3.
Increased periventricular echogenicity
-
4.
Periventricular pseudocysts and intraventricular synechiae
-
5.
Ascites
-
6.
Intracranial calcifications
-
7.
Abnormality of amniotic fluid volume (usually oligohydramnios)
-
8.
Microcephaly
-
9.
Hyperechogenic bowel
-
10.
Hydrops fetalis
-
11.
Pleural effusion
-
12.
Liver calcifications
-
1.
-
4.
Radiography
-
1.
Intracranial calcification: usually periventricular (Roach et al. 1983)
-
2.
Microcephaly
-
1.
-
5.
CT scan
-
1.
Intracerebral calcification: the most frequent finding (Boppana et al. 1997)
-
2.
Microcephaly: the most specific predictor of poor cognitive outcome in children with symptomatic congenital CMV infection (Noyola et al. 2001)
-
3.
White matter lucencies
-
4.
Ventriculomegaly
-
5.
Destructive encephalopathy
-
6.
Brain atrophy
-
7.
Neuronal migration disorders
-
1.
-
6.
Fetal MRI (Doneda et al. 2010; Averill et al. 2015)
-
1.
Anterior temporal cysts and occipital horn septations, as dilation of these areas may decrease later in development.
-
2.
Cortical migration abnormalities.
-
3.
White matter abnormalities.
-
4.
Cerebellar dysplasia.
-
5.
Periventricular calcifications.
-
6.
Fetal MR imaging can show abnormalities in the fetal brain after CMV infection, even when US results are normal. The early detection of some brain abnormalities, such as microencephaly and cortical anomalies, may substantially influence the prognosis of fetal infection.
-
1.
-
7.
Neonatal auditory screening (Hicks et al. 1993)
-
8.
Evidence of infection with CMV
-
1.
Fourfold rise in anti-CMV IgG titers.
-
2.
Seroconversion from negative to positive.
-
3.
Sensitivity of the CMV-IgM assays (50–90%). The IgM titers may not become positive during acute infection.
-
1.
-
9.
Viral isolation
-
1.
The most sensitive method to diagnose CMV infection
-
2.
Culture of CMV from virtually all body fluids, including saliva and urine of the newborn, semen, and cervicovaginal secretions
-
3.
Detection of CMV within the first 3 weeks of life: considered proof of congenital CMV infection
-
1.
-
10.
Identification of CMV-DNA through PCR on amniotic fluid (best sensitivity and 100% specificity) (Lazzarotto et al. 2000; Liesnard et al. 2000)
-
11.
Newborn screening for congenital cytomegalovirus infection (Bale 2010)
-
1.
Universal screening methods by using polymerase chain reaction (PCR) analysis of newborn dried blood spots (Boppana et al. 2010).
-
2.
Cytomegalovirus infection.
-
1.
Accounts for as much or more disability over the past 50 years than was associated with congenital rubella syndrome
-
2.
Represents the most common nongenetic cause of permanent hearing loss among children in the USA
-
1.
-
3.
The most effective strategy for reducing CMV-induced sensorineural hearing loss, as well as for eliminating CMV-associated neurodevelopmental disability, will not be universal screening but prevention of congenital CMV infection (Pass et al. 2009).
-
1.
Genetic Counseling
-
1.
Recurrence risk
-
1.
Preconceptional immunity to CMV provides substantial protection against intrauterine transmission and severe fetal infection.
-
2.
Presence of maternal humoral antibody: conferring no fetal protection in subsequent reinfection or reactivation.
-
1.
-
2.
Prenatal diagnosis
-
1.
Prenatal ultrasonography.
-
1.
Intrauterine growth retardation
-
2.
Microcephaly
-
3.
Ventriculomegaly
-
4.
Periventricular calcifications
-
5.
Intrahepatic calcifications
-
6.
Nonimmune hydrops
-
7.
Fetal ascites
-
8.
Pericardial effusion
-
9.
Hepatosplenomegaly
-
10.
Echogenic bowel
-
11.
Cardiomegaly
-
12.
Oligohydramnios
-
13.
Placentomegaly
-
1.
-
2.
Prenatal diagnosis of congenital CMV infection by combined detection of CMV-DNA and CMV-IgM in fetal blood or by combined testing of AF and fetal blood for CMV-DNA and IgM antibodies (sensitivity of 100%) (Enders et al. 2001) or isolating the virus from amniotic fluid (Plosa et al. 2012).
-
3.
Prenatal diagnosis: established by serological tests in umbilical cord blood and confirmed by detection of viral DNA in fetal blood and tissues from the postmortem specimen after termination of pregnancy (Beksaç et al. 2001).
-
4.
Negative results of CMV culture or PCR in the amniotic fluid cannot formally exclude intrauterine infection (Bodéus et al. 1999).
-
5.
Prenatal diagnosis of CMV infection remains a “long-standing problem still seeking a solution” as long as no assistance (treatment or prevention) can be offered to the pregnant women.
-
6.
Amniotic fluid peptidome analysis: effectively predict the severity of congenital CMV infection (Desveaux et al. 2016).
-
1.
-
3.
Management
-
1.
Prevention.
-
1.
Complicated because both primary and secondary maternal infections give rise to disease in the offspring and absence of characteristic symptoms in CMV-infected mothers excludes clinical recognition of at-risk pregnancies
-
2.
Routine screening of pregnant patients for CMV status: not currently recommended because no effective antiviral therapy is available during gestation and also there is no means to predict the outcome in an infected fetus
-
3.
Risk to CMV infection for women in high-risk environments (day-care centers, nurseries, elementary schools, or health-care facilities)
-
4.
Strict hygiene practices for seronegative women to reduce the risk for the infection
-
1.
-
2.
Prospects of intervention (Cheeran et al. 2009).
-
1.
Antiviral drugs (Ganciclovir, Valganciclovir, Foscarnet, Cidofovir, CMV immune globulin) are available for treatment of congenital CMV infection (Plosa et al. 2012), and there is evidence that therapy ameliorates the severity of one of the CNS complications of infection, sensorineural hearing loss. Long-term neurodevelopmental follow-up studies should further clarify the value of antiviral therapy in congenitally infected infants.
-
2.
Uncontrolled studies of therapy in utero with CMV immune globulin have suggested an impact on neuropathogenesis, and controlled trials should be conducted with pregnant women.
-
3.
CMV vaccines (Pass et al. 2009; Bale 2010) may hold the greatest promise in reducing the neurodevelopmental consequences of congenital infection, although the immune correlates of protection of the fetus remain incompletely defined.
-
1.
-
3.
Recent progress in developing novel antiviral drugs and vaccines suggests the possibility that the diverse effects of HCMV may soon become controllable at the individual and population level, respectively (Griffiths et al. 2015).
-
1.
(a, b) A neonate with blue berry muffin skin lesions (generalized purpura) due to cytomegalovirus infection. He died 20 h after birth. CMV inclusions were noted in the kidneys, lungs, liver, pancreas, thyroid, brain, and eyes and also in the urine. Photomicrograph of the kidney shows many tubular epithelial cells containing large cytomegalic inclusion bodies (Courtesy of Dr. Samuel Yang)
(a, b) Photomicrograph of the kidney (macerated fetus, 16-week gestation). Even though the tissue is macerated, many cytomegalic inclusion bodies are demonstrable. Coronal section of the brain (frontal lobe) showing ventricular hemorrhage and focal encephalomalacia (chalky white discoloration) due to cytomegalovirus infection
References
Amir, J, Atias, J., Linder, N., et al. (2016). Follow-up of infants with congenital cytomegalovirus and normal fetal imaging. Archives of Disease in Childhood. Fetal and Neonatal Edition, January 18. F1-F5. [Epub ahead of print].
Averill, L. W., Kandula, V. V. R., Akyol, Y., et al. (2015). Fetal brain magnetic resonance imaging findings in congenital cytomegalovirus infection with postnatal imaging correlation. Seminars in Ultrasound, CT, and MRI, 36, 476–486.
Bale, J. F. (2010). Screening newborns for congenital cytomegalovirus infection (Editorial). JAMA, 303, 1425–1426.
Bale, J. F., Jr., Blackman, J. A., & Sato, Y. (1990). Outcome in children with symptomatic congenital cytomegalovirus infection. Journal of Child Neurology, 5, 131–136.
Beksaç, M. S., Saygan-Karamürsel, B., Ustaçelebi, S., et al. (2001). Prenatal diagnosis of intrauterine cytomegalovirus infection in a fetus with non-immune hydrops fetalis. Acta Obstetricia et Gynecologica Scandinavica, 80, 762–765.
Bodéus, M., Hubinont, C., Bernard, P., et al. (1999). Prenatal diagnosis of human cytomegalovirus by culture and polymerase chain reaction: 98 pregnancies leading to congenital infection. Prenatal Diagnosis, 19, 314–317.
Bonalumi, S., Trapanese, A., Santamaria, A., et al. (2011). Cytomegalovirus infection in pregnancy: Review of the literature. Journal of Prenatal Medicine, 5, 1–8.
Boppana, S. B., Pass, R. F., Britt, W. J., et al. (1992). Symptomatic congenital cytomegalovirus infection: Neonatal morbidity and mortality. The Pediatric Infectious Disease Journal, 11, 93–99.
Boppana, S. B., Fowler, K. B., Vaid, Y., et al. (1997). Neuroradiographic findings in the newborn period and long-term outcome in children with symptomatic congenital cytomegalovirus infection. Pediatrics, 99, 409–414.
Boppana, S. B., Ross, S. A., Novak, Z., et al. (2010). Dried blood spot real-time polymerase chain reaction assays to screen newborns for congenital cytomegalovirus infection. JAMA, 303, 1375–1382.
Brown, H. L., & Abernathy, M. P. (1998). Cytomegalovirus infection. Seminars in Perinatology, 22, 260–266.
Byrne, P. J., Silver, M. M., Gilbert, J. M., et al. (1987). Cyclopia and congenital cytomegalovirus infection. American Journal of Medical Genetics, 28, 61–65.
Cheeran, M. C.-J., Lokensgard, J. R., & Schleiss, M. R. (2009). Neuropathogenesis of congenital cytomegalovirus infection: Disease mechanisms and prospects for intervention. Clinical Microbiology Reviews, 22, 99–126.
Coats, D. K., Demmler, G. J., Paysse, E. A., et al. (2000). Ophthalmologic findings in children with congenital cytomegalovirus infection. American Association for Pediatric Ophthalmology and Strabismus, 4, 110–116.
Crino, J. P. (1999). Ultrasound and fetal diagnosis of perinatal infection. Clinical Obstetrics and Gynecology, 42, 71–80.
Del Pizzo, J. (2011). Congenital infections (TORCH). Pediatrics in Review, 32, 537–542.
Desveaux, C., Klein, J., Leruez-Ville, M., et al. (2016). Identification of symptomatic fetuses infected with cytomegalovirus using amniotic fluid peptide biomarkers. PLoS Pathogens, 12, 1–21.
Doneda, C., Parazzini, C., Righini, A., et al. (2010). Early cerebral lesions in cytomegalovirus infection: Prenatal MR imaging. Radiology, 255, 613–621.
Enders, G., Bäder, U., Lindemann, L., et al. (2001). Prenatal diagnosis of congenital cytomegalovirus infection in 189 pregnancies with known outcome. Prenatal Diagnosis, 21, 362–377.
Fowler, K. B., McCollister, F. P., Dahle, A. J., et al. (1997). Progressive and fluctuating sensorineural hearing loss in children with asymptomatic congenital cytomegalovirus infection. Journal of Pediatrics, 130, 624–630.
Goderis, J., De Leenheer, E. D., Smets, K., et al. (2014). Hearing loss and congenital CMV infection: A systematic review. Pediatrics, 134, 972–982.
Griffiths, P., Baraniak, I., & Reeves, M. (2015). The pathogenesis of human cytomegalovirus. Journal of Pathology, 235, 288–297.
Guerra, B., Lazzarotto, T., Quarta, S., et al. (2000). Prenatal diagnosis of symptomatic congenital cytomegalovirus infection. American Journal of Obstetrics and Gynecology, 183, 476–482.
Hicks, T., Fowler, K., Richardson, M., et al. (1993). Congenital cytomegalovirus infection and neonatal auditory screening. Journal of Pediatrics, 123, 779–782.
Istas, A. S., Demmler, G. J., Dobbins, J. G., et al. (1995). Surveillance for congenital cytomegalovirus disease: A report from the National Congenital Cytomegalovirus Disease registry. Clinical Infectious Diseases, 20, 665–670.
Joseph, A., Mahida, N., Irving, W., et al. (2013). Congenital cytomegalovirus infection. Paediatrics and Child Health, 24, 255–259.
Lazzarotto, T., Varani, S., Guerra, B., et al. (2000). Prenatal indicators of congenital cytomegalovirus infection. Journal of Pediatrics, 137, 90–95.
Liesnard, C., Donner, C., Brancart, F., et al. (2000). Prenatal diagnosis of congenital cytomegalovirus infection: Prospective study of 237 pregnancies at risk. Obstetrics and Gynecology, 95, 881–888.
Lipitz, S., Achiron, R., Zalel, Y., et al. (2002). Outcome of pregnancies with vertical transmission of primary cytomegalovirus infection. Obstetrics & Gynecology, 100, 428–433.
Malinger, G., Lev, D., Zahalka, N., et al. (2003). Fetal cytomegalovirus infection of the brain: The spectrum of sonographic findings. AJNR. American Journal of Neuroradiology, 24, 28–32.
Malinger, G., Lev, D., & Lerman-Sagie, T. (2011). Imaging of fetal cytomegalovirus infection. Fetal Diagnosis and Therapy, 29, 117–126.
Mestas, E. (2016). Congenital cytomegalovirus. Advances in Neonatal Care, 16, 60–65.
Metz, M. B. (2001). Eye manifestations of intrauterine infections. Ophthalmology Clinics of North America, 14, 521–531.
Naing, Z. W., Scott, G. M., Shand, A., et al. (2016). Congenital cytomegalovirus infection in pregnancy: A review of prevalence, clinical features, diagnosis and prevention. Australian and New Zealand Journal of Obstetrics and Gynaecology, 56, 9–18.
Noyola, D. E., Demmler, G. J., Griesser, C., et al. (2001). Early predictors of neurodevelopmental outcome in symptomatic. Journal of Pediatrics, 138, 325–331.
Pass, R. F. (2002). Cytomegalovirus infection. Pediatrics in Review, 23, 163–169.
Pass, R. F., Zhang, C., Evans, A., et al. (2009). Vaccine prevention of maternal cytomegalovirus infection. The New England Journal of Medicine, 360, 1191–1199.
Perlman, J. M., & Argyle, C. (1992). Lethal cytomegalovirus infection in preterm infants: Clinical, radiological, and neuropathological findings. Annals of Neurology, 31, 64–68.
Plosa, E. J., Esbenshade, J. C., Fuller, M. P., et al. (2012). Cytomegalovirus infection. Pediatrics in Review, 33, 156–163.
Roach, E. S., Sumner, T. E., Volverg, F. M., et al. (1983). Radiological case of the month. American Journal of Diseases of Children, 137, 799.
Schleiss, M. R. (2008). Congenital cytomegalovirus infection: Update on management strategies. Current Treatment Options in Neurology, 10, 186–192.
Sharon, B., & Schleiss, M. R. (2007). Congenital cytomegalovirus infection: An unrecognized epidemic. Infections in Medicine, 24, 402–415.
Stagno, S., Pass, R. F., Dworsky, M. E., et al. (1982). Congenital cytomegalovirus infection: The relative importance of primary and recurrent maternal infection. The New England Journal of Medicine, 306, 945–949.
Stagno, S., Pass, R. F., Cloud, G., et al. (1986). Primary cytomegalovirus infection in pregnancy. Incidence, transmission to fetus and clinical outcome. JAMA, 256, 1904–1908.
Author information
Authors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer Science+Business Media LLC
About this entry
Cite this entry
Chen, H. (2017). Congenital Cytomegalovirus Infection. In: Atlas of Genetic Diagnosis and Counseling. Springer, New York, NY. https://doi.org/10.1007/978-1-4939-2401-1_50
Download citation
DOI: https://doi.org/10.1007/978-1-4939-2401-1_50
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4939-2400-4
Online ISBN: 978-1-4939-2401-1
eBook Packages: MedicineReference Module Medicine