Abstract
This chapter describes the role of single-cell recordings in understanding the mechanisms underlying human cognition. Cognition is a function of the brain, a complex computational network, whose most elementary nodes are made up out of individual neurons. These neurons encode information and influence each other through a dynamically changing pattern of action potentials. For this reason, the activity of neurons in the awake, behaving brain constitutes the most fundamental form of neural data for cognitive neuroscience. This chapter discusses a number of technical issues and challenges of single-cell neurophysiology using a recent project of the authors as an example. We discuss issues such as the choice of an appropriate animal model, the role of psychophysics, technical challenges surrounding the simultaneous recording of multiple neurons, and various methods for perturbation experiments. The chapter closes with a consideration of the challenge that the brain’s complexity poses for fully understanding any realistic nervous circuit, and of the importance of conceptual insights and mathematical models in the interpretation of single-cell recordings.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Action potentials
- Nervous circuit
- Electrophysiological recording
- Behavior
- Perturbation experiment
- Animal models
- Primate
- Frontal cortex
- Decision making
1 Introduction
The fundamental goal of cognitive neuroscience is the explanation of psychological processes by their underlying neural mechanisms. This explanatory goal is reductionist and operates under the assumption that some form of identity hypothesis is correct, i.e., that specific mental events or processes are identical or intimately linked to specific neuronal events and processes. An explanation therefore only starts with a description of the processes on the neuronal level that give rise to the processes on the psychological level. A full explanation also requires a specification of the exact causal link between the two levels, i.e., a hypothesis about which of the many physical phenomena in the brain is thought to correspond with a specific mental phenomenon.
Thus, the first question is the adequate level of description of the brain at which this causal (or explanatory) link can be established. In general, there are three broad levels at which brain activity can be described, which relate to three different sets of measurement technologies that are currently available. The first level encompasses all the subcellular, molecular processes that explain the behavior of a neuron. This level of description includes for example biochemical and biophysical investigations of receptors, G-proteins, ion channels, and other building blocks that determine the internal organization and workings of neurons.
The second level encompasses the electrophysiological activity of individual neurons or circuits of individual neurons. This level includes experiments in which the temporal pattern of action potentials of individual neurons is recorded, while behaviorally relevant sensory, motor, or cognitive variables are changed, and is the primary topic of this chapter. Experiments on individual neurons allow one to investigate whether neuronal activity (the temporal pattern of action potentials, or spikes) represents (is correlated with) behaviorally relevant information. This level of description also includes the connection and interaction between individual neurons across different brain areas. Importantly, perturbation experiments in which neuronal activity is either suppressed or enhanced, allow one to go a step further and to establish causal links between spiking activity and behavioral functions.
The third level encompasses experiments aimed at recording mass action of large numbers of neurons. Human imaging experiments (fMRI, PET) fall into this category, as does electrophysiological recordings of field potentials at varying scales (LFP, ECoG, EEG). This third level somewhat overlaps with the second level, insomuch as the second level of description includes simultaneous recordings of many individual neurons within a local circuit, or across different parts of the brain. The main distinction is essentially methodological; level two descriptions are of identified, individual neurons, while level three descriptions are of unidentified, averaged neurons. This summing up over many neurons is due to technical constraints of the measurement techniques used, and leads to lower spatial and temporal resolution. Consequently, recordings of mass activity are likely to be most accurate in cases in which most neurons in a particular location have similar functions and activity patterns. However, recent research has shown that even in primary sensory areas, but particularly in associate brain regions, such as frontal and parietal cortex, individual neurons with different functional roles are often located in close vicinity to each other. We will see an example of this later in this chapter (see Fig. 6.2). Here mass action recordings of brain activity will likely have a lower resolution in the identification of functionally relevant signals. These disadvantages are balanced by two great advantages: the ability to record activity from many—or, indeed, all—parts of the brain, and the non-invasive nature of the measurement methods, which permits their routine use in humans.
2 Single Neurons Provide the Critical Link Between Brain and Cognition
It is clear that we can learn from all available techniques and their respective usefulness will depend on the specific question at hand, as well as technical constraints. Thus, pragmatic considerations will lead scientists always towards using all sources of information. Nevertheless, we can ask from a theoretical point of view at which of the three levels we can best articulate the relationship between particular mental (or cognitive) states and neural states [1–3]. Ever since the pioneering work of Adrian and Hartline [4, 5], individual neurons are seen as the elementary units of the nervous system that represent information and perform computations on these representations [2, 3, 6–10]. There is general agreement that the temporal structure of action potentials encodes the information. However, the exact nature of this code is still under active investigation [11–14]. Mountcastle was the first to formulate a research program centered on a systematic comparison of psychophysical measures in conscious subjects and recordings of individual neurons [7, 15]. At present, this program has resulted in multiple examples of individual neurons, whose firing patterns match to a stunning degree with mental states, such as perceptions or decisions, as measured using psychophysical methods [16–18].
A particularly impressive example of such a match between neural activity and perception are results from recordings in single somatosensory nerve fibers in humans during stimulation of the skin [19]. Near-threshold skin indentations resulted in a variable response of the nerve fiber. During some trials, an action potential was generated, while on other trials no electrophysiological response was observed. Astonishingly, on trials in which an action potential was registered, the human subjects reported the subjective experience of a light touch. On trials with an identical mechanical stimulation, but without an action potential, the humans reported no touch perception. This finding implies a very strong linking hypothesis, according to which a single action potential in a peripheral nerve elicits a particular mental state. Similar findings linking changes in the activity of individual neurons to changes in mental state have been observed in cortical neurons of animals [20, 21].
All of these findings point towards the spiking activity of single cells in awake, behaving animals (including humans) as the key level for understanding how physical events in the brain underlie mental events and cognition [2, 3]. So, what is then the best way in which we can get these critical experimental data, and what are the technical requirements? Some of the technical requirements are described by Crist and Lebedev [22]. They include the choice of an appropriate animal model, useful behavioral tasks, methods for electrophysiological recordings of one or more single neurons, methods for perturbing spiking activity in the brain, and data analysis. In the following section of this chapter we will go, one by one, over these different requirements, using an ongoing research project from our laboratory as an example. We chose our own work mainly because we are most familiar with it. Wherever appropriate, we will refer to the works of others to illustrate different approaches than the ones we used.
3 Choice of Animal Model
Traditionally, the majority of the electrophysiological investigations of the sensory, motor and cognitive functions of the brain have been done in primates. Techniques for recording from individual neurons in awake, behaving primates were pioneered by Evarts [23, 24] and then further developed by Mountcastle, Wurtz and others [25, 26]. This is in contrast to the majority of modern biomedical research in which rodents, in particular mice and rats, are the dominant animal models. The reasons for this preference are the greater number of genetic and other molecular biological tools that are available in these animals, because of the much shorter generational span of rodents compared to primates. More recently, rodents have been used increasingly to study the neural mechanisms of cognitive functions, such as decision-making under uncertainty [27, 28]. The fact that rats can be trained in sophisticated behavioral tasks opens up the question, to what extent they might not be a superior animal model relative to monkeys. This is a particularly pressing question, since neuroscience is at the moment in the middle of a technical revolution. New tools for observing neural activity of large numbers of neurons optically, such as two photon imaging [29], and the automation of anatomical methods [30] allows for an unprecedented level of insight into the activity of large numbers of neurons, and their internal connection. The functional relevance of identified types of neurons can be probed using optogenetic tools [31]. All of these new tools have been developed in rodents, in particular mice.
We used macaque monkeys in our study, and we feel that there are still strong reasons that support the continued use of this animal model, in particular in cognitive neuroscience. The most important reason is the fact that there are radical anatomical and structural differences between the brains of rodents and primates [32]. This is particularly true for the frontal cortex, which is generally believed to be essential for higher cognitive function in humans and other mammals [33, 34].
Based on cytoarchitectonical and structural differences between different areas in the frontal lobe of rodents and primates, Wise suggested that primates have evolved certain new areas that do not exist in rodents [32, 35]. Recent support for this hypothesis comes from fMRI experiments in humans that show a regional specialization in the representations of primary and secondary, abstract reward in the orbitofrontal cortex [36]. Whereas the anterior lateral orbitofrontal cortex, a phylogenetically recent structure (only present in primates), processes monetary gains, the posterior lateral orbitofrontal cortex, phylogenetically and ontogenetically older (and shared with rodents), processes erotic stimuli, a more basic reward. Interestingly, the phylogenetically newer parts make up the majority of the frontal cortex in primates [32].
These differences in frontal architecture and their unknown functional consequences can lead to difficulties in the interpretation of neuroscientific findings. For example, reports in monkeys have claimed that the activity of certain neurons in the orbitofrontal cortex represents uncertainty and risk (defined as outcome variability) [37]. This finding is in agreement with human neuroimaging studies [38]. Recently, a very clever study in rats suggests that this neuronal activity pattern might not represent risk per se, but instead acquired salience [28]. However, while this finding is intriguing, it will need to be replicated in primates, simply to make sure that the functional differences revealed in these two studies are not the result of differences in the functional architecture and overall function of orbitofrontal cortex in monkeys and rats.
Another reason to use primates is related to the potential for what might be called ‘behavioral mimicry’. Organisms with completely different internal architectures can generate behavior that looks similar, but is produced for entirely different reasons. The formal mathematical proof of this possibility was derived in the theory of finite automata [2, 39]. In such a case of mimicry, the behavioral similarity is likely to be only superficial and strongly context-dependent. A real-world example is the response of rodents, macaques and humans in reward-reversal tasks. In such tasks, one of two options is consistently rewarded and, therefore, almost exclusively chosen. If, however, the reward contingencies are unexpectedly switched without notice, so that the previously unrewarded option will now lead consistently to reward, rodents, monkeys, and humans will all learn to switch their preferences to the newly rewarded option. From this qualitative similarity, one might conclude that very similar, perhaps even identical, choice and learning mechanisms underlie this behavior in all of these organisms. However, such a conclusion does not take into account some intriguing differences in the time course of the switch. Human subjects need typically only one error trial to switch [40]. In contrast, rodents switch their behavior only after 20–40 trials [41]. This is (at least from a human point of view) a staggering amount of exposure to a clear-cut, non-probabilistic change in reward contingencies. This seems to imply that at least humans represent the task contingencies in a different way and might use different learning or choice mechanisms than rodents. Thus, the picture that emerges is complex. Monkeys need at least 10–15 trials [42], which is still different from humans, but closer to them than the behavior of rodents.
Obviously, primates are not superior animal models with regards to all possible research questions. In general, the choice of rodents as models for human behavioral, neural, and even mental processes is likely to be most appropriate if the object of study is an aspect of behavior and the brain that is common among all mammals. An example is the role of the neural circuits in the hypothalamus in the control of hunger and food consumption [43]. However, even for something as seemingly primitive as appetite and food consumption there exist important behavioral differences between humans and other mammals with less complex brains. For instance, humans show reliable behavioral and neural differences while consuming the same wine, when given different information about its price [44]. Thus, the choice of appropriate animal model ultimately depends on the research question. Within the domain of cognitive neuroscience, it seems to us that non-human primates are still the obvious choice, given their overall similarity with humans, and the fact that many of the new techniques first developed in rodents are now applied to primates [45–49].
Of course, there are also large differences between humans and other non-human primates [50]. These differences will likely forever preclude the study of certain human abilities, such as language, in animal models. This is important, because language, and the abstract, symbol-operating cognitive abilities that go along with it, pervade every other aspect of human mental and cognitive life, from memory to decision-making. In addition, there are likely to be other, potentially more subtle differences in the way cognitive mechanisms operate in humans and other primates. It is therefore of great interest to use every opportunity to study single-unit responses in awake humans [51]. This approach has already led to some insights into language [52], representation of objects [53], and cognitive control [54]. In addition, singleunit recordings can provide new insights into mental diseases, such as obsessivecompulsive disorders [55]. This resource should be used more widely by cognitive neuroscientists.
4 Behavioral Tasks and Psychophysics
The ability to link neuronal activity and cognitive function depends critically on our ability to vary the cognitive signals of interest in a controlled and measurable fashion. This, in turn, depends entirely on the behavioral task design, and the psychophysical methods used to analyze behavior and deduce cognitive states from it. Thus, single unit electrophysiology, and mathematical psychology and modeling are critically linked [Forstmann, Wagenmakers, chapter of this book]. Mathematical psychology provides formal models of cognitive processes, which afford quantifiable variables that are related to behavior in an operational manner and that can be compared to measures of neuronal activity. Of course in practice, the hypothesized link might turn out not to exist, because the model might not have been an appropriate description of the underlying cognitive and neuronal mechanism. However, this form of model falsification is exactly how science progresses.
In the case of the specific project that we chose as an example, we were interested in the neuronal mechanism underlying value-based decision making. Decision-making involves the selection of a particular behavioral response from a set of two or more possible responses. Our understanding of the neural processes that underlie this selection process is most advanced in the case of perceptual decisions [56]. These decisions are guided by external sensory stimuli and reflect the beliefs of the decision maker about the external world. Value-based decisions, on the other hand, are much less well understood.
Value-based decision making is the process of selecting an action among several alternatives based on the subjective value of their outcomes. This requires the brain to first estimate the value of the outcome of each possible response, and then to select one of them on the basis of those values [57–60]. This raises two fundamental questions: (1) Where in the brain are the values of different types of outcomes and actions represented and how are these value signals computed? and (2) How and where does the brain compare those value signals to generate a choice?
With respect to the first question, a rapidly growing number of studies have found neural responses that are correlated with some form of value signals [57, 61–63]. Several studies found orbitofrontal cortex (OFC) and amygdala encoding the value of different goals [62, 64–67]. These signals are stimulus-based and independent of the actions required to obtain them. These option value signals represent therefore predicted future states of the world. To allow the selection of an appropriate action, these goal representations need to be associated with the actions that are most likely to bring them about. This type of value signal is known as action value. Action-value signals for hand and eye movements have been found in the striatum [68, 69], in the dorsolateral prefrontal cortex (DLPFC) [70], and in the medial frontal cortex [71, 72], including the supplementary eye field (SEF) [73].
With respect to the second question, there are currently two major theories of how the brain compares value signals and uses them to select an action [74]. One theory is the goods- or goal-based theory of decision making [62], according to which the brain computes the subjective value of each offer, selects between these option value signals, and then prepares the appropriate action plan. This theory in its purest form predicts that motor areas should only represent the chosen action. The other theory is the action-based theory of decision making [18, 75–78], according to which potential actions are simultaneously represented in the brain and compete against each other. This competition is biased by a variety of factors including the subjective value of each offer (i.e., their action values). This theory in its purest form predicts that option value signals should not predict the chosen option, before an action is chosen, since these signals are only precursors to the decision. A third alternative is that competition occurs at both levels in parallel [74].
In order to study value-based decision making, we needed to design a task in which the monkey was forced to select actions based on its internal estimation about the worth of various options. In our case, we kept the number of alternatives binary to start with the simplest condition. In addition, we were also interested in creating a task in which identical task conditions would elicit different choices. This would allow us to differentiate between the representation of the decision process itself that should co-vary with the behavioral choice, and the representation of other factors that should stay invariant across trials (e.g., the representation of a particular option and its attributes, independent of whether it is chosen or not).
Both of these conditions were fulfilled in a gambling task, in which the monkeys had to choose between gambles with different probabilities to win reward of varying amounts (Fig. 6.1a, b). We used targets that consisted of two colors corresponding to the two possible reward amounts. The portion of a color within the target corresponded to the probability of receiving that reward amount (Fig. 6.1b). The minimum reward amount for the gamble option was always 1 unit of water, while the maximum reward amount varied between 3, 5 and 9 units, with three different probabilities of receiving the larger reward (20, 40, and 80 %). This resulted in a set of 7 gambles. The colors and differences in area size were easy to discriminate for the monkey. Thus, in presenting two targets to the monkey, the problem for the animal was not one of perceptual uncertainty. Instead, the problem of selecting the better of the two options was related to the uncertainty about the actual outcome that would follow from each choice. A decision-maker that is indifferent to risk should base his decision on the sum of values of the various outcomes weighted by their probabilities, i.e., the expected value of the gamble. However, humans and animals are not indifferent to risk and their actual decisions deviate from this prediction in a systematic fashion. Thus, the subjective value of a gamble depends on the risk attitude of a decision-maker.
Gambling task and estimate of subjective value. a The gambling task consisted of two types of trials, choice trials and no-choice trials. All the trials started with the appearance of a fixation point at the center of the screen, which the monkeys were required to fixate for 500–1000 ms. After that, in choice trial, two targets appeared on two locations that were randomly chosen among the four quadrants. Simultaneously, the fixation point disappeared and within 1000 ms the monkeys had to choose between the gambles by making a saccade toward one the targets. Following the choice, the nonchosen target disappeared from the screen. The monkeys were required to keep fixating the chosen target for 500–600 ms, after which the target changed color. The two-colored square then changed into a single-colored square associated with the final reward amount. This indicated the result of the gamble to the monkeys. The monkeys were required to continue to fixate the target for another 300 ms until the reward was delivered. In the choice trial, each gamble option was paired with all other six gamble options. The sequence of events in no-choice trial was the same as in choice trial except that only one target was presented. In those trials, the monkeys were forced to make a saccade to the given target. All 7 gamble options were presented during no-choice trials. We presented no-choice and choice trials interleaved in blocks of trials that consisted of all twenty one different choice trials and eight onset different trials and seven different no-choice trials. Within a block, the order of trials was randomized. The locations of the targets in each trial were also randomized, which prevented the monkeys from preparing a movement toward a certain direction before the target appearance. b Four different colors indicated four different reward amounts (increasing from 1, 3, 5 to 9 units of water, where 1 unit equaled 30 µl). Note that the expected value of the gambles along the diagonal axis was the same. c The mean subjective value of the 7 gamble options for one of the monkeys. The subjective value ranges between 0 for the least and 1 for the most valuable option
In addition, there is another interesting feature that can be seen in everyday life, as well as in our laboratory task. For certain combinations of gambles, the monkeys were very certain which one they preferred. However, for a large range of other combinations they varied in their choice, even after they were exposed to the different gamble options daily for many months. One of the reasons for this persistent uncertainty about the value of the gamble options might be the fact that gambles vary across two independent dimensions, reward amount and probability, both of which affect the overall value. Options can be attractive for different reasons, e.g., either because of low risk or high payoff. Assessing the value of a gamble option requires, therefore, a trade-off between the different attributes that have to be integrated in a weighted fashion in order to generate a one-dimensional decision variable, the subjective value of the option. This process has no obvious best solution and agents can remain ambivalent with respect to which of the options is optimal. In addition, there might also be other sources of variance, such as changes in attention or motivation or recent outcome history. In any case, the monkeys showed behavioral variance in our gambling task, which was important for us. Combined, the two features of our task produce a situation that is almost a perfect inversion of the classic perceptual decision-making task, in which sensory stimuli are very ambiguous, but the correct response can easily be selected, given a particular belief about the state of the world [16, 18]. In contrast, in our gambling task, the state of the world is easy to perceive, but the appropriate response is unclear.
In terms of the link between the neural and the mental level, we are faced in our research with the problem of comparing a subjective, internal variable (the subjective value of the various options) to an objective, measurable variable (the firing rate of neurons). Here the behavioral variance is likewise of great importance, since it allows us to use psychophysical scaling techniques [79, 80] to estimate the subjective value of different targets (Fig. 6.1c). These techniques go back to Fechner, who was the first to suggest that a psychophysical experiment could be conducted in which an observer makes judgments along a psychological dimension having no obvious physical correlate (here, subjective value) [81, 82]. Thurstone further developed the theoretical method for analyzing such data from paired comparison judgments [83]. In his model, Thurstone assumed that the judgment process was based on the comparison of noisy internal representations. The differences across the compared distributions lead to systematic differences in the probability of particular judgments. Similar ideas within economics lead to the development of random utility models [84], in which it is presumed that choices are based on noisy internal utility estimations [85]. Importantly, this scaling method gave us an estimate of the subjective value that went beyond a mere ordinal ranking. Instead, we could order the subjective value of the various gambles on an interval scale [82, 86] that allowed us to estimate not only which gamble is preferred over another, but also by how much (Fig. 6.1c).
5 Electrophysiological Recordings of One or More Identified Neurons
In our experiment, we used an in-house built system of electrode microdrives that allowed us to independently control the position of up to 6 different electrodes. Our recording setup required us to advance electrodes acutely during each recording session into the brain. This allowed us to cover a wide range of different cortical locations and, more importantly, to position the electrode close to neurons, whose activity was task-related. In this regard, apart from the fact that we did this with multiple electrodes, our approach was very similar to the traditional single electrode recording approach. However, both approaches introduce mechanical disturbances within the brain tissue during advancement of the electrode. These mechanical instabilities, together with pulsations introduced by heart rate and breathing generated instabilities in the position of the neuron with respect to the tip of the electrode. These instabilities influenced our long-term recording stability. To keep the signal to noise ratio of spike identification stable required constant monitoring and minute adjustments of the electrode position by the researcher. This is a well-known problem for single unit electrophysiology, but it is exacerbated in the case of multiple electrodes. Ultimately, for human researchers one reaches very soon an attentional bottleneck. Overcoming these limitations would be a major breakthrough that would allow us to record simultaneously from large numbers of neurons [87].
There are a number of ways to achieve this goal. One possibility is the use of a series of electrode drives that can move electrodes independently operated by multiple researchers working in conjunction [88]. Each researcher is responsible for a few electrodes. This acute recording approach is in some sense the most conservative, insomuch as it requires the least dramatic change relative to traditional methods of single-unit recordings. Because of this, it is easy to implement in principle. However, it is not clear how scalable this approach is, given the increasing demands in welltrained man power.
Another possibility is the use of microelectrode arrays [89–91]. In this approach the electrodes are not advanced acutely for each recording session. Instead, an array consisting of multiple electrodes (with as many recording contacts as desired and technically feasible) is chronically implanted into the brain [92–94]. Due to its better mechanical stability, neuronal spikes can be recorded typically for extended time periods. The signal to noise ratio of microelectrode arrays and of conventional electrodes is comparable [91]. One disadvantage of the microarray recording setup is the inability to actively search for task-relevant neuronal activity. Once implanted, the electrodes cannot be moved and the researcher has to be content with whatever signal he or she can get. To some extent, this disadvantage can even be seen as strength, since pre-selection of ‘interesting’ neurons introduces severe sampling biases in traditional recording studies. This has made it very hard to directly compare the results of single unit studies in different brain areas. Since microarrays sample neurons in different parts of the brain in a more random fashion, they allow a more unbiased comparison [95]. A straightforward and unbiased way to increase the likelihood to record from task-relevant neurons using chronic microarrays would be a strategy of recording from as many parts of the electrode as possible to increase the number and extent of neurons that can be sampled [90, 94]. This strategy relies on the use of modern lithographic techniques to fabricate electrodes. There has been a lot of progress in the manufacture and use of such polytrodes [96] and there exists a large design space that can be explored for further improvements [97].
A third approach that combines aspects of the acute and chronic recording methods is semichronic recording [98, 99]. In this approach, miniature mechanical microdrives are implanted into the brain, each containing a number of independently movable microelectrodes. Recordings are made by slowly advancing a subset of electrodes in each chamber each day. This procedure has been used very successfully during the investigation of neurons in the rodent hippocampus [100]. Semichronic recording provides the ability to move electrodes into brain areas that are of particular interest, and the possibility of recording from many individual neurons simultaneously. However, this type of recording device is still not commonly used in primate experiments and requires further development.
6 Relationship Between Neural Activity and Decision Variables
Decision-making under risk is very common in everyday life, where practically every action can have more than one possible outcome. Value-based decision making requires the translation of internal value signals related to the different options into the selection of unique motor signals necessary to obtain the desired option. Where and how this is achieved is still debated [101]. Therefore, we concentrated our initial research on brain areas that receive input from motivational and cognitive systems and provide output to the motor system. One such region is the supplementary eye field (SEF). SEF receives input from areas that represent option value, such as the orbitofrontal cortex and the amygdala [102, 103]. SEF forms a cortico-basal ganglia loop with the caudate nucleus, which is known to contain saccadic action value signals [104, 105]. SEF projects to oculomotor areas, such as frontal eye field, lateral intraparietal cortex, and superior colliculus [102]. Neurons in SEF become active before value-based saccades, much earlier than neurons in frontal eye field and lateral intraparietal cortex [106]. SEF might therefore participate in the process of value-based decision making in the case of eye movements.
Our initial recordings confirmed that SEF neurons represent three major functional signals during decision-making [107]. One group of neurons encoded the value of reward options, but not the type of eye movement necessary to obtain it. Such option value signals are similar to signals found in the orbitofrontal cortex. These signals appeared first in the SEF. Next, a group of neurons became active that combined information about the value of an option with information about the direction of the saccade necessary to receive the reward. Such action value signals are ideally suited to select the action that will most likely maximize reward. Lastly, pure motor-related neurons became active that only carried eye movement related signals. This succession of value- to motor-related signals is in line with our working hypothesis that SEF serves as a bridge between the value and the motor systems. We presume that it takes value information and translates it into action value representations. The learning of appropriate action value signals requires a system that can evaluate the outcome of actions that are taken. Interestingly, SEF neurons also carry various monitoring signals [108]. Altogether, these earlier findings suggested that SEF participates in value-based decision making by computing an action value map of the existing choice options. Competition within this map could select the action associated with the highest value, which in turn could be used to guide the selection and execution of the appropriate eye movement.
In order to test this hypothesis, we recorded from SEF neurons, while monkeys chose between gambles of varying subjective values. The histograms in Fig. 6.2 show the activity of multiple identified single neurons during saccades to four different targets recorded in one of these sessions. The activity differences indicate the preferred direction and the strength of the tuning of the different cells. This directional tuning (or lack thereof in the time period preceding the saccade) is of course only one of the functional dimensions along which the SEF neurons can vary [73]. The other major dimension, sensitivity to subjective value of the target, is not shown in Fig. 6.2. However, even while ignoring this other potential source of functional variability; a comparison of the neurons is enough to make clear, why it is important to record from individual neurons. The SEF neurons that were recorded from each of the three electrodes were, as a group, in very close anatomical proximity to each other. Otherwise, it would not have been possible to separate their action potentials from the background modulation of all the other neurons surrounding the electrode tip. Nevertheless, there is a marked difference in directional tuning among these neurons. In particular, one of the neurons recorded by the second electrode (middle column, first row) is most active for saccades to target T3 and least active for saccades to target T4. This is in contrast to two other neurons recorded by the same electrode (middle column, second and fourth row) that show an exactly opposite pattern of activity: these are most active for saccades to target T4 and least active for saccades to target T3. Any form of mass-activity recording would have simply averaged over these differences. In the best case, this would have increased the noise of the recording, and in the worst case it would have led to a failure to detect an important functional difference among the neurons forming the local network.
Recording of multiple identified single neurons. An example of numerous individual neurons recorded simultaneously from three different electrodes inserted into different parts of SEF during one recording session. Each panel shows the the average spike rate of one neuron aligned on saccade onset for movements towards each of the four different target locations (T1: blue, T2: black, T3: violet, T4: red line). The panel in each of the three columns represents the activity of one individual neuron that was recorded from one of the three electrodes., We were able to isolate four different neurons in the first two electrodes, and two more neurons from the third electrode
If a framework based on anatomical proximity is inadequate to functionally understand the SEF neurons, what kind of alternative works better? In our case, it turns out that the functional framework of the action value map works well to give us some insights on the pattern of activity in SEF during decision-making. Figure 6.3 shows the population activity in SEF as a time-direction map of neuronal activity. Here, we sort the neurons according to their preferred direction relative to the position of the chosen and unchosen target. Since the monkey made saccades in four different directions, each neuron contributed four different activity traces to the time-direction map. To avoid a bias introduced by neurons with higher activity levels, we normalized the activity of each neuron across all conditions. To smooth over inevitable differences with which the preferred directions were represented in our neuronal sample, we binned the neuronal activity. It should be understood that this simple act itself represents a form of interpretation or hypothesis regarding the function of the neurons. We presume that each neuron represents the action value of saccades directed towards its preferred direction. Thus, as a whole the activity distribution across the entire neuronal population encodes the combined estimation of the relative values of the various saccades that the monkey can make. Each vertical line in the map represents the state of this activity distribution in the action value map at one moment in time. Since in our experiment, all targets were presented with the same distance from the center, we can presume that our map here is one-dimensional. Thus, the time-direction map shows the development of action value-related activity over the course of decision-making.
Time-direction map of normalized neuronal activity in SEF. Each of the two maps shows the normalized population activity distribution of directionally SEF neurons as a function of time. We averaged neuronal activity over all different subjective target values. The vertical axis shows the activity distribution of neurons sorted by the orientation of their preferred direction relative to the direction of the chosen target (at 1.57 radians) and the non-chosen target (at 4.71 radians). The horizontal axis shows the change of this activity distribution across time relative to saccade onset. The time-direction map on the left shows the SEF activity during no-choice trials, in which only one target was presented. The time-direction map on the right shows the activity of the same neurons during choice trials. The upper band of activated neurons corresponds to neurons representing the chosen target direction, while the lower band of activated neurons represents the non-chosen target location
The map on the left shows the simple no-choice case, in which only one target is presented. In response to the target presentation, activity in a broad set of neurons increases. Activity centered on the target direction reaches a maximum around the time of saccade initiation. The map on the right shows the more complex case, in which two targets are presented. There are a number of differences. First, activity starts to rise in two parts of the map. One is centered on the target that will be chosen, while the other is centered on the non-chosen target. The initial rise in activity relative to saccade onset starts earlier, in keeping with the fact that reaction times are longer when the monkey has to choose between two response options. In the beginning, the activity associated with both possible targets is of similar strength, but around 50 ms before saccade onset, a difference develops between these two different groups of cells. The activity centered on the chosen target becomes much larger than the one centered on the non-chosen target, and increases until saccade onset. In fact, the peak activity associated with the chosen target is much larger than the peak activity associated with the same saccade during no-choice trials. This increased activity for increased number of targets is very unusual and differs from the behavior of neurons in other oculomotor regions. However, it might allow SEF to reliably encode the best action value even in the face of distractors. In sum, the SEF population activity seems to represent first both equally possible alternatives, before in a second step reflecting the selection of the chosen target.
These observations then beg the question, whether SEF activity actively takes part in the decision process. To answer this, we have to establish at least two links [2, 3]. The first link is between the variations of neuronal activity and the behavioral choices of the monkey. To establish this link, we have to show that we can decode (i.e., predict) the behavioral choice from the neuronal activity to some statistically significant degree on any given trial. Traditionally, with single units, such a link was established using techniques derived from signal detection theory [109, 110]. These techniques rely on a comparison of the neuron’s activity across trials that never occurred simultaneously. For example, all trials, in which the two options A and B are presented, are divided into trials in which option A was chosen and trials in which option B was chosen. By comparing the activity of a neuron across these two types of trials one can hope to see if there are differences related to behavioral choice. However, this entails that the trials recorded from an individual neuron are treated as if they belonged to a pair of neurons. Underlying this analysis is therefore the assumption of a fictitious ‘antineuron’ that behaves as the mirror image of the recorded neuron, but that was never actually recorded (and most likely does not exist). Apart from these issues, there is the deeper question about the extent to which the different trial repetitions are actually identical. In light of these conceptual problems, it would be better to use the activity of many neurons on a single trial to do what, in the traditional approach, is done with the activity of a single neuron on many trials [111].
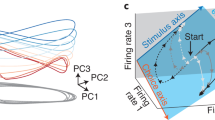
A promising new technique for decoding neural activity is the use of modern pattern classification methods to analyze activity recorded simultaneously from multiple neurons [112–115]. An interesting new approach for visualizing the pattern of activity within a large number of neurons is the state space representation [116]. In this general framework, the activity of a group of neurons is represented as a particular point in an N-dimensional space, where N is equal to the number of neurons. The activity of each neuron at a given moment in time is represented numerically along one of the dimensions forming the space. Thus, the entire population forms a vector pointing to the part of the state space that represents the momentary state of the set of neurons. Changes in neuronal activity lead to shifts in the state space that form a trajectory. The direct visualization of this state space is obviously not possible for groups of neurons larger than three. However, it is possible to visualize the main changes in state space following dimensionality-reduction through methods, such as principal component analysis. The mean trajectories describing the shifts of population activity in SEF during decision making are shown in Fig. 6.4a for the set of 10 neurons depicted in Fig. 6.2. The trajectories associated with the choice of the four saccade directions all start in the same part of the state space projection spanned by the first two principal components (PC1, PC2), before moving in four different directions in this state space. The trajectories shown in Fig. 6.4a indicate the mean positions of the state vector. The state vectors associated with the individual trials form a cloud around the mean trajectory.
Neuronal dynamics during decision-making within a neuronal state space and results of linear classifier. a The activity of the simultaneously recorded neurons shown in Fig. 6.2 defined a 10-dimensional state space. A projection of this state space onto a 2-dimensional subspace is shown. The subspace is defined by the first two principal components (PC1, PC2) explaining variance in the neuronal state vector distribution. The temporal succession of the mean state vector location between target and saccade onset is shown separately for trials in which one of four saccade directions was chosen by the monkey. The mean state vector locations form a trajectory (T1: blue, T2: black, T3: violet, T4: red line). The green dot on the trajectories indicates the moment of saccade initiation. b A linear discriminant analysis of the distribution of state vectors for all combination of saccade directions was performed. The percentage of correctly predicted choices based on this analysis is plotted as a function of the time bin during which the state vectors were defined. The red bar indicates those time periods, in which the percentage of correct predictions was significantly larger than chance, as determined through a permutation test
We can now ask if there is a linear boundary that optimally divides the state space, so that we can distinguish between the state vectors that are associated with particular choices (of options or saccade direction). Next, we can ask how well we can decode the monkey’s choices based on this approach, and at what point in time our predictive power is better than chance. The result of this analysis for the same set of 10 SEF neurons is shown in Fig. 6.4b. As we can see, the neuronal assembly does not allow us to predict the chosen saccade direction up until 60 ms before saccade onset. However, after this point the predictive power rapidly increases until it reaches a choice probability of ~ 75 % just before saccade onset. This ‘decision point’ matches the estimate derived from the time-direction map for the moment at which the activity map differentiates (Fig. 6.3).
7 Perturbing Spiking Activity in the Brain
Showing that SEF activity is correlated with decisions in the gambling task is a good beginning towards establishing a link between neuronal activity and mental processes. However, the critical step is clearly the establishment of causality. This requires perturbation experiments, to show that changes in neuronal activity cause changes in behavior.
To this end, we used a cooling probe to temporarily inactivate the SEF in both hemispheres, while a monkey performed the gambling task [117]. It has been known for some time that cooling suppresses the generation of action potentials. This inactivation is fast (with 1–5 min.), reversible, and causes no damage to the affected tissue. All these factors make this technique easier to use than pharmacological inactivation. At the same time, the size and extent of the affected area can be easily controlled by the shape of the cooling probe. Thus, large brain areas can be influenced simultaneously, a relative advantage over optogenetic techniques that are limited by the ability to spread light evenly. On the downside, cooling affects all neurons near the probe, and therefore does not allow the specific manipulation of functionally or anatomically defined neuronal sub-groups.
The behavioral effects of the inactivation are shown in Fig. 6.5. We plot the probability of choosing the less valuable target as a function of the difference in subjective values. Behavior under normal conditions is shown by the blue bars. As one would expect, the probability of choosing the less valuable option is largest when the difference is small and the discrimination of the value difference is hard. For larger value differences (> 20 %), the monkey typically picks the less valuable target only rarely. If SEF plays a causal role in value-based decision making, we would expect the monkey to show an increased rate of sub-optimal choices when SEF can no longer guide motor selection. This is indeed the case, as shown by the red bars. The effect is largest for intermediate value differences. This is probably due to the fact that there is a ceiling effect for very small value differences, while for very large value differences the decision is so easy that other brain regions beside the SEF are sufficient to pick the better option. Nevertheless, the overall effect of cooling on behavior is significant (p < 0.01), and the size of the effect is comparable to the effect of permanent lesions of the orbitofrontal cortex through ablation [118]. Importantly, the fact that inactivation of SEF has an immediate effect on value-based decisions establishes a causal link between SEF single unit activity and the monkey’s choice based on subjective preferences (at least with regards to eye movements).
Behavioral effects of bilateral inactivation of SEF through cooling. The probability of choosing the smaller of two options was plotted as a function of relative difference in subjective value of the options as determined behaviorally (see Fig. 6.1 ). The behavior under normal conditions is shown by the blue bars (normal temperature; 37 ºC). The behavior, when SEF is inactivated in both hemispheres, is shown by the red bars (~10 ºC)
SEF is clearly part of a larger network that is involved in value-based decision making. An important future direction will therefore be the exploration of the other brain areas in the network. One group of areas, such as dorsolateral prefrontal cortex, orbitofrontal cortex and amygdala, provide inputs to the SEF. Another set of areas including frontal eye field, superior colliculus and dorsal striatum, in turn, receive inputs from the SEF. Understanding the entire circuit responsible for value-based decision making will require us to describe the types of signals and their temporal order within this network of brain areas.
8 Future Developments
Recently, very ambitious proposals for large-scale projects have been suggested within neuroscience. Many prominent and accomplished neuroscientists have suggested that we should try to reconstruct the neuronal network for large parts if not the entire, brain of a small mammal, a ‘structural’ connectome [30]. Other researchers have suggested that we should attempt to record every action potential from every neuron within a circuit, ultimately again within the entire brain, a ‘functional’ connectome [119]. Such ideas are very ambitious and attractive, since they promise to tackle directly one of the biggest problems in our current understanding of the brain. While we can observe the spiking activity of individual neurons, and can establish links between their activity and behavior or even mental states, we mostly do not know why the neurons show the activity pattern that we observe. The activity of neurons in the brain is ultimately an emergent property of the interactions between the different elements that make up the circuit that they belong to. Thus, only knowledge about the fine structure of this circuit and the distributed activity of the various elements that make up the circuit will provide us with a true mechanistic understanding of the brain.
However, there is reason to be cautious. It is easy to underestimate the true complexity of the brain [2, 120]. Moore proved that the number of steps necessary to learn about the internal structure of a computing machine is at least the same order of magnitude as the number of states of the machine [39]. It is easy to see the consequences of this relationship for a task such as fully characterizing the visual cortex of the mouse, which contains about 2 million neurons [120]. Any realistic hope of progress relies on our ability to discover hierarchical structures in the network, which would allow us to simplify the level of complexity of the circuit that needs to be understood in order to explain the behavior of the entire network [120, 121]. Such insights ultimately require further conceptual breakthroughs, and the input of theorists, such as mathematical psychologists or computational neuroscientists.
In conclusion, we are living in exciting times for neuroscientists. Important technological breakthroughs have been made and there is the potential for the development of even more advanced methods for recording neural activity from hundreds, if not thousands, neurons simultaneously and to reconstruct nervous circuits in unprecedented detail [122, 123]. Without any doubt, these attempts at technical innovation will move neuroscience forward. This is true, not least, because work towards achieving these goals might lead to much-needed improvements in measurement technology, even if the ultimate goal should remain elusive. However, it is important not to ignore the main source of most of the real insights into the brain that have been acquired up to now, namely, the establishment of a functional and explanatory link between neural activity and mental phenomena using psychophysics and mathematical models. When the newly available techniques are combined with these established approaches, we will truly see great steps forward in our understanding of the brain.
Exercises
-
1.
Given the fact that, in humans, for the foreseeable future we will have to rely on mass-activity measures of brain activity, the relationship between single-unit activity and mass-action recordings is of interest. What do you think is the relationship between individual neurons producing action potentials and fMRI or EEG?
-
2.
New generations of neuroprobes will allow extreme miniaturization. Currently available probes allow the construction of devices with 456 electrodes. Within a few years we will likely have neuroprobes available that have up to 2000 electrodes [123]. Also currently already available are microchips that can be injected into the brain and that allow the recording of electrical fields, temperature, and the local emission of light, which would allow the spatially precise control of neural activity through optogenetics [124]. More futuristic approaches envision “nanometer-scale light-sensitive devices that could embed themselves in the membrane of neurons, power themselves from cellular energy, and wirelessly convey the activity of millions of neurons simultaneously” [123, 125]. If we assume for a moment that all these technical advances come to fruition, what are questions that could be answered using these new techniques?
Further Reading
-
1.
Wise [32] provides a provocative review about the function of prefrontal cortex. It includes a discussion of the differences between the frontal cortex of primates and rodents.
-
2.
In a seminal paper, Teller [1] lays out the internal logic that allows establishing a link between a set of physical events in the brain to psychological events or a functional concept. A more in depth discussion of these requirements is also provided by Parker & Newsome [3] in the sensory domain and by Schall [2] in the motor domain.
-
3.
In a highly amusing and interesting book, Passingham [50] describes what is known about the specific characteristics of human brain anatomy and physiology, as opposed to the brains of other primates.
-
4.
Glimcher [85] lays out, how psychology, economics, and neuroscience can be related in a new reductionist framework.
-
5.
In this classic description, Gescheider [82] gives an overview over modern psychophysics. This topic is of utmost importance to anyone interested in connecting brain activity with behavior and mental states.
-
6.
Modern approaches for the recording of multiple individual neurons are discussed by Kipke et al. [89] and Buzsaki [94]. An interesting new method for analyzing the resulting multi-neuron data is described in Yu, et al. [116]. These papers are of course just highlights out of a vast literature.
-
7.
Denk et al. [30] and Alivisatos et al. [119] describe ambitious new proposals of obtaining structural and functional ‘connectomes’, that is, a complete description of a neuronal circuit.
-
8.
Koch [120] discusses the difficulties for explaining the mammalian brain related to its astonishing complexity.
References
Teller DY (1984) Linking propositions. Vision Res 24(10):1233–1246
Schall JD (2004) On building a bridge between brain and behavior. Ann Rev Psychol 55:23–50
Parker AJ, Newsome WT (1998) Sense and the single neuron: probing the physiology of perception. Ann Rev Neurosci 21:227–277
Adrian ED (1928) The basis of sensation: the action of the sense organs. W. W. Norton, New York
Hartline HK, Milne LJ, Wagman IH (1947) Fluctuation of response of single visual sense cells. Fed Proc 6(1 Pt 2):124
Barlow HB (1995) The neuron doctrine in perception. In: Gazzaniga MS (ed) The cognitive neurosciences. MIT Press, Cambridge, pp 415–435
Mountcastle VB, Talbot WH, Darian-Smith I, Kornhuber HH (1967) Neural basis of the sense of flutter-vibration. Science 155(762):597–600
Rieke F, Warland DK, de Ruyter van Steveninck R, Bialek W (1997) Spikes: exploring the neural code. MIT Press, Cambridge
Koch C (1999) Biophysics of computation: information processing in single neurons. In: Computational Neuroscience Series, Stryker M (ed) Oxford University Press, Oxford
Silver RA (2010) Neuronal arithmetic. Nat Rev Neurosci 11(7):474–489
Softky WR, Koch C (1993) The highly irregular firing of cortical cells is inconsistent with temporal integration of random EPSPs. J Neurosci 13(1):334–350
Shadlen MN, Newsome WT (1998) The variable discharge of cortical neurons: implications for connectivity, computation, and information coding. J Neurosci 18(10):3870–3896
Singer W, Gray CM (1995) Visual feature integration and the temporal correlation hypothesis. Ann Rev Neurosci 18:555–586
London M, Roth A, Beeren L, Hausser M, Latham PE (2010) Sensitivity to perturbations in vivo implies high noise and suggests rate coding in cortex. Nature 466(7302):123–127
Werner G, Mountcastle VB (1965) Neural activity in mechanoreceptive cutaneous afferents: stimulus-response relations, weber functions, and information transmission. J Neurophysiol 28:359–397.
de Lafuente V Romo R (2005) Neuronal correlates of subjective sensory experience. Nature Neuroscience. [Research Support, Non-U.S. Gov't]. 8(12):1698–1703
Newsome WT, Britten KH, Salzman CD, Movshon JA (1990) Neuronal mechanisms of motion perception. Cold Spring Harb Symp Quant Biol 55:697–705
Shadlen MN, Newsome WT (2001) Neural basis of a perceptual decision in the parietal cortex (area LIP) of the rhesus monkey. J Neurophysiol 86(4):1916–1936
Vallbo AB, Johansson RS (1984) Properties of cutaneous mechanoreceptors in the human hand related to touch sensation. Hum Neurobiol 3(1):3–14
Houweling AR, Brecht M (2008) Behavioural report of single neuron stimulation in somatosensory cortex. Nature 451(7174):65–68
Li CY, Poo MM, Dan Y.(2009) Burst spiking of a single cortical neuron modifies global brain state. Science 324(5927):643–646
Crist RE, Lebedev MA (2007) Multielectrode recording in behaving monkeys. In: Nicolelis MAL (ed) Methods for neural ensemble recordings. CRC, Boca Raton
Evarts EV (1966) Pyramidal tract activity associated with a conditioned hand movement in the monkey. J Neurophysiol 29(6):1011–1027
Evarts EV (1968) Relation of pyramidal tract activity to force exerted during voluntary movement. J Neurophysiol 31(1):14–27
Mountcastle VB, Talbot WH, Sakata H, Hyvarinen J (1969) Cortical neuronal mechanisms in flutter-vibration studied in unanesthetized monkeys. Neuronal periodicity and frequency discrimination. J Neurophysiol 32(3):452–484
Wurtz RH (1968) Visual cortex neurons: response to stimuli during rapid eye movements. Science 162(858):1148–1150
Kepecs A, Uchida N, Zariwala HA, Mainen ZF (2008) Neural correlates, computation and behavioural impact of decision confidence. Nature 455(7210):227–231
Ogawa M, van der Meer MA, Esber GR, Cerri DH, Stalnaker TA, Schoenbaum G (2013) Risk-responsive orbitofrontal neurons track acquired salience. Neuron 77(2):251–258
Ohki K, Chung S, Kara P, Hubener M, Bonhoeffer T, Reid RC (2006) Highly ordered arrangement of single neurons in orientation pinwheels. Nature 442(7105):925–958
Denk W, Briggman KL, Helmstaedter M.(2012) Structural neurobiology: missing link to a mechanistic understanding of neural computation. Nat Rev Neurosci.13(5):351–358
Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K (2005) Millisecond-timescale, genetically targeted optical control of neural activity. Nat Neurosci 8(9):1263–1268
Wise SP (2008) Forward frontal fields: phylogeny and fundamental function. Trends Neurosci 31(12):599–608
Tanji J, Hoshi E (2008) Role of the lateral prefrontal cortex in executive behavioral control. Physiol Rev 88(1):37–57
Fuster JM (2008) The prefrontal cortex, 4th edn. Academic Press, Amsterdam
Uylings HB, Groenewegen HJ, Kolb B (2003) Do rats have a prefrontal cortex? Behav Brain Res 146(1–2):3–17
Sescousse G, Redoute J, Dreher JC (2010) The architecture of reward value coding in the human orbitofrontal cortex. J Neurosci 30(39):13095–13104
O'Neill M, Schultz W (2010) Coding of reward risk by orbitofrontal neurons is mostly distinct from coding of reward value. Neuron 68(4):789–800
Tobler PN, O'Doherty JP, Dolan RJ, Schultz W (2007) Reward value coding distinct from risk attitude-related uncertainty coding in human reward systems. J Neurophysiol 97(2):1621–1632
Moore EF (1956) Gedanken-experiments on sequential machines. In: Shannon CE, McCarthy J (eds) Automata studies. Princeton University Press, Princeton pp. 129–153
Ridley RM, Haystead TA, Baker HF (1981 Mar) An analysis of visual object reversal learning in the marmoset after amphetamine and haloperidol. Pharmacol Biochem Behav 14(3):345–351
Kruzich PJ, Grandy DK (2004) Dopamine D2 receptors mediate two-odor discrimination and reversal learning in C57BL/6 mice. BMC Neurosci 5:12
Mehta MA, Swainson R, Ogilvie AD, Sahakian BJ, Robbins TW (2001) Improved short-term spatial memory but impaired following the dopamine D-2 agonist bromocriptine reversal learning in human volunteers. Psychopharmacology 159(1):10–20.
Atasoy D, Betley JN, Su HH, Sternson SM (2012) Deconstruction of a neural circuit for hunger. Nature 488(7410):172–177
Plassmann H, O'Doherty J, Shiv B, Rangel A (2008) Marketing actions can modulate neural representations of experienced pleasantness. Proc Natl Acad Sci U S A 105(3):1050–1054
Cavanaugh J, Monosov IE, McAlonan K, Berman R, Smith MK, Cao V et al (2012) Optogenetic inactivation modifies monkey visuomotor behavior. Neuron 76(5):901–907
Nauhaus I, Nielsen KJ, Disney AA, Callaway EM (2012) Orthogonal micro-organization of orientation and spatial frequency in primate primary visual cortex. Nat Neurosci 15(12):1683–1690
Han X, Qian X, Bernstein JG, Zhou HH, Franzesi GT, Stern P et al (2009) Millisecondtimescale optical control of neural dynamics in the nonhuman primate brain. Neuron 62(2):191–198
Ozden I, Wang J, Lu Y, May T, Lee J, Goo W et al (2013) A coaxial optrode as multifunction write-read probe for optogenetic studies in non-human primates. J Neurosci Methods 219:142–154
Diester I, Kaufman MT, Mogri M, Pashaie R, Goo W, Yizhar O et al (2011) An optogenetic toolbox designed for primates. Nat Neurosci 14(3):387–397
Passingham R (2008) What is special about the human brain? Oxford University Press, Oxford
Suthana N, Fried I (2012 Aug) Percepts to recollections: insights from single neuron recordings in the human brain. Trends Cogn Sci 16(8):427–436
Tankus A, Fried I, Shoham S (2012) Structured neuronal encoding and decoding of human speech features. Nat Commun 3:1015
Quiroga RQ, Reddy L, Kreiman G, Koch C, Fried I (2005) Invariant visual representation by single neurons in the human brain. Nature 435(7045):1102–1107
Cerf M, Thiruvengadam N, Mormann F, Kraskov A, Quiroga RQ, Koch C et al (2010) On-line, voluntary control of human temporal lobe neurons. Nature 467(7319):1104–1108
Burbaud P, Clair AH, Langbour N, Fernandez-Vidal S, Goillandeau M, Michelet T et al (2013) Neuronal activity correlated with checking behaviour in the subthalamic nucleus of patients with obsessive-compulsive disorder. Brain 136(Pt 1):304–317
Gold JI, Shadlen MN (2007) The neural basis of decision making. Ann rev neurosci 30:535–574
Rangel A, Camerer C, Montague PR (2008) A framework for studying the neurobiology of value-based decision making. Nat Rev Neurosci 9(7):545–556
Balleine BW, Dickinson A (1998) Goal-directed instrumental action: contingency and incentive learning and their cortical substrates. Neuropharmacology 37(4–5):407–419
Yin HH, Knowlton BJ (2006) The role of the basal ganglia in habit formation. Nat rev Neurosci 7(6):464–476
Daw ND, Niv Y, Dayan P (2005) Uncertainty-based competition between prefrontal and dorsolateral striatal systems for behavioral control. Nat Neurosci 8(12):1704–1711
Vickery TJ, Chun MM, Lee D (2011) Ubiquity and specificity of reinforcement signals throughout the human brain. Neuron 72(1):166–177
Padoa-Schioppa C (2011) Neurobiology of economic choice: a good-based model. Annu Rev Neurosci 34:333–359
Kable JW, Glimcher PW (2009) The neurobiology of decision: consensus and controversy. Neuron 63(6):733–745
Plassmann H, O'Doherty J, Rangel A (2007) Orbitofrontal cortex encodes willingness to pay in everyday economic transactions. J Neurosci 27(37):9984–9988
Plassmann H, O'Doherty JP, Rangel A (2010) Appetitive and aversive goal values are encoded in the medial orbitofrontal cortex at the time of decision making. J Neurosci 30(32):10799–10808
Bermudez MA, Schultz W (2010) Reward magnitude coding in primate amygdala neurons. J Neurophysiol 104(6):3424–3432
Grabenhorst F, Hernadi I, Schultz W (2012) Prediction of economic choice by primate amygdala neurons. Proc Natl Acad Sci U S A 109(46):18950–18955
Lau B, Glimcher PW (2007) Action and outcome encoding in the primate caudate nucleus. J Neurosci 27(52):14502–14514
Samejima K, Ueda Y, Doya K, Kimura M (2005) Representation of action-specific reward values in the striatum. Science 310(5752):1337–1340
Kim S, Hwang J, Lee D (2008) Prefrontal coding of temporally discounted values during intertemporal choice. Neuron 59(1):161–172
Hernandez A, Nacher V, Luna R, Zainos A, Lemus L, Alvarez M et al (2010) Decoding a perceptual decision process across cortex. Neuron 66(2):300–314
Seo H, Barraclough DJ, Lee D (2007) Dynamic signals related to choices and outcomes in the dorsolateral prefrontal cortex. Cereb Cortex 17(suppl 1):i110–i117
So NY, Stuphorn V (2010) Supplementary eye field encodes option and action value for saccades with variable reward. J Neurophysiol 104(5):2634–2653
Cisek P (2012) Making decisions through a distributed consensus. Curr Opin Neurobiol 22(6):927–936
Cisek P, Kalaska JF (2010) Neural mechanisms for interacting with a world full of action choices. Ann Rev Neurosci 33:269–298
Platt ML, Glimcher PW (1999) Neural correlates of decision variables in parietal cortex. Nature 400(6741):233–238
Sugrue LP, Corrado GS, Newsome WT (2004) Matching behavior and the representation of value in the parietal cortex. Science 304(5678):1782–1787
Shadlen MN, Kiani R, Hanks TD, Churchland AK (2008) Neurobiology of decision making, an intentional framework. In: Engel C, Singer W (eds) Better than conscious? MIT Press, Cambridge, pp. 71–101
Kingdom FAA, Prins N (2010) Psychophysics: a practical introduction. Academic Press, Amsterdam
Maloney LT, Yang JN (2003) Maximum likelihood difference scaling. J Vis 3(8):573–585
Fechner GT (1876) Vorschule der Aesthetik. Breitkopf & Haerterl, Leibzig
Gescheider GA (1997) Psychophysics: the fundamentals. 3rd edn. Lawrence Erlbaum Assoc., Mahwah
Thurstone LL (1927) A law of comparative judgment. Psychol Rev 34:273–286
McFadden D (1974) Conditional logit analysis of qualitative choice behavior. In: Zarembka P (ed) Frontier in econometrics. Academic Press, NewYork, pp. 105–142
Glimcher P (2011) Foundations of neuroeconomic analysis. Oxford University Press, Oxford
Stevens SS (1951) Handbook of experimental psychology. Wiley, NewYork
Einevoll GT, Franke F, Hagen E, Pouzat C, Harris KD (2012) Towards reliable spike-train recordings from thousands of neurons with multielectrodes. Curr Opin Neurobiol 22(1):11–17
Hernandez A, Nacher V, Luna R, Alvarez M, Zainos A, Cordero S et al (2008) Procedure for recording the simultaneous activity of single neurons distributed across cortical areas during sensory discrimination. Proc Natl Acad Sci U S A 105(43):16785–16790
Kipke DR, Shain W, Buzsaki G, Fetz E, Henderson JM, Hetke JF et al (2008) Advanced neurotechnologies for chronic neural interfaces: new horizons and clinical opportunities. J Neurosci 28(46):11830–11838
Du J, Riedel-Kruse IH, Nawroth JC, Roukes ML, Laurent G, Masmanidis SC (2009) High-resolution three-dimensional extracellular recording of neuronal activity with microfabricated electrode arrays. J Neurophysiol 101(3):1671–1678
Kelly RC, Smith MA, Samonds JM, Kohn A, Bonds AB, Movshon JA et al (2007) Comparison of recordings from microelectrode arrays and single electrodes in the visual cortex. J Neurosci 27(2):261–264
Nicolelis MA, Dimitrov D, Carmena JM, Crist R, Lehew G, Kralik JD et al (2003) Chronic, multisite, multielectrode recordings in macaque monkeys. Proc Natl Acad Sci U S A 100(19):11041–11046
McNaughton BL, O'Keefe J, Barnes CA (1983) The stereotrode: a new technique for simultaneous isolation of several single units in the central nervous system from multiple unit records. J Neurosci Methods 8(4):391–397
Buzsaki G (2004) Large-scale recording of neuronal ensembles. Nat Neurosci 7(5):446–451
Miller EK, Wilson MA (2008) All my circuits: using multiple electrodes to understand functioning neural networks. Neuron 60(3):483–488
Fujisawa S, Amarasingham A, Harrison MT, Buzsaki G (2008) Behavior-dependent short-term assembly dynamics in the medial prefrontal cortex. Nat neurosci 11(7):823–833
Seymour JP, Kipke DR (2007) Neural probe design for reduced tissue encapsulation in CNS. Biomaterials 28(25):3594–3607
Salazar RF, Dotson NM, Bressler SL, Gray CM (2012) Content-specific fronto-parietal synchronization during visual working memory. Science 338(6110):1097–1100
Hoffman KL, McNaughton BL (2002) Coordinated reactivation of distributed memory traces in primate neocortex. Science 297(5589):2070–2073
Wilson MA, McNaughton BL (1994) Reactivation of hippocampal ensemble memories during sleep. Science 265(5172):676–679
Cisek P (2012) Making decisions through a distributed consensus. Curr Opin Neurobiol 22(6):927–936
Huerta MF, Kaas JH (1990) Supplementary eye field as defined by intracortical microstimulation: connections in macaques. J Comp Neurol 293:299–330
Ghashghaei HT, Hilgetag CC, Barbas H (2007 Feb 1) Sequence of information processing for emotions based on the anatomic dialogue between prefrontal cortex and amygdala. Neuroimage 34(3):905–923
Lau B, Glimcher PW (2008) Value representations in the primate striatum during matching behavior. Neuron 58(3):451–463
Shook BL, Schlag-Rey M, Schlag J (1991) Primate supplementary eye field. II. Comparative aspects of connections with the thalamus, corpus striatum, and related forebrain nuclei. J Comp Neurol 307(4):562–583
Coe B, Tomihara K, Matsuzawa M, Hikosaka O (2002) Visual and anticipatory bias in three cortical eye fields of the monkey during an adaptive decision-making task. J Neurosci 22(12):5081–5090
So NY, Stuphorn V (2010) Supplementary eye field encodes option and action value for saccades with variable reward. J Neurophysiol 104(5):2634–2653
So NY, Stuphorn V (2012) Supplementary eye field encodes reward prediction error. J Neurosci 32(9):2950–2963
Barlow HB, Levick WR, Yoon M (1971) Responses to single quanta of light in retinal ganglion cells of the cat. Vision Res Suppl 3:87–101
Thompson KG, Hanes DP, Bichot NP, Schall JD (1996) Perceptual and motor processing stages identified in the activity of macaque frontal eye field neurons during visual search. J Neurophysiol 76(6):4040–4055
Churchland MM, Yu BM, Sahani M, Shenoy KV (2007) Techniques for extracting single-trial activity patterns from large-scale neural recordings. Curr Opin Neurobiol 17(5):609–618
Duda RO, Hart PE, Stork DG (2000) Pattern classification, 2nd edn. Wiley, NewYork
Broome BM, Jayaraman V, Laurent G (2006) Encoding and decoding of overlapping odor sequences. Neuron 51(4):467–482
Briggman KL, Abarbanel HD, Kristan WB, Jr (2005) Optical imaging of neuronal populations during decision-making. Science 307(5711):896–901
Harvey CD, Coen P, Tank DW (2012) Choice-specific sequences in parietal cortex during a virtual-navigation decision task. Nature 484(7392):62–68
Yu BM, Cunningham JP, Santhanam G, Ryu SI, Shenoy KV, Sahani M (2009) Gaussianprocess factor analysis for low-dimensional single-trial analysis of neural population activity. J Neurophysiol 102(1):614–635
Lomber SG, Payne BR, Horel JA (1999) The cryoloop: an adaptable reversible cooling deactivation method for behavioral or electrophysiological assessment of neural function. J Neurosci Methods 86(2):179–194
Noonan MP, Walton ME, Behrens TE, Sallet J, Buckley MJ, Rushworth MF (2010) Separate value comparison and learning mechanisms in macaque medial and lateral orbitofrontal cortex. P Natl Acad Sci USA 107(47):20547–20552
Alivisatos AP, Chun M, Church GM, Greenspan RJ, Roukes ML, Yuste R (2012)The brain activity map project and the challenge of functional connectomics. Neuron 74(6):970–974.
Koch C (2012) Systems biology. Modular biological complexity. Science 337(6094):531–532
Mountcastle VB (1997) The columnar organization of the neocortex. Brain 120(Pt 4):701–722
Kim TI, McCall JG, Jung YH, Huang X, Siuda ER, Li Y et al (2013) Injectable, cellular-scale optoelectronics with applications for wireless optogenetics. Science 340(6129):211–216
Abbott A (2013) Neuroscience: solving the brain. Nature 499(7458):272–274
Kim TI et al (2013) Injectable, cellular-scale optoelectronics with applications for wireless optogenetics. Science 340:211–216
Alivisatos AP et al (2013) Nanotools for neuroscience and brain activity mapping. ACS Nano 7:1850–1866
Acknowledgements
We are grateful to K. Nielsen, D. Sasikumar and E. Emeric for comments on the manuscript. This work was supported by the National Eye Institute through grant R01-EY019039 to VS.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer Science+Business Media, LLC
About this chapter
Cite this chapter
Stuphorn, V., Chen, X. (2015). An Introduction to Neuroscientific Methods: Single-cell Recordings. In: Forstmann, B., Wagenmakers, EJ. (eds) An Introduction to Model-Based Cognitive Neuroscience. Springer, New York, NY. https://doi.org/10.1007/978-1-4939-2236-9_6
Download citation
DOI: https://doi.org/10.1007/978-1-4939-2236-9_6
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4939-2235-2
Online ISBN: 978-1-4939-2236-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)